Leucine is the only physiologic amino acid that can stimulate insulin release by itself, and a great deal of evidence suggests that leucine does this by allosterically activating glutamate dehydrogenase (GDH). GDH catalyzes the oxidative deamination of endogenous glutamate, which is present at a high concentration in the pancreatic β-cell. Studies that support this role of leucine include the fact that leucine and 2-aminobicyclo[2.2.1]heptane-2-carboxylic acid (BCH), a nonmetabolizable leucine analog, are activators of GDH and promote insulin release from pancreatic islets (1–4). Although the addition to pancreatic islets of glutamine alone—which by its conversion to glutamate enormously increases the intracellular concentration of glutamate—does not stimulate insulin release, adding glutamine in the presence of leucine or BCH causes a robust stimulation of insulin release. Patients with mutations in the region of the GDH gene that encodes the part of the GDH protein where the allosteric inhibitor guanosine triphosphate (GTP) binds to the enzyme suffer from hyperinsulinism and hypoglycemia (5), and this indicates that GDH is involved in insulin secretion in humans. In addition, recent studies showed that short-chain 3-hydroxyacyl-CoA dehydrogenase (SCHAD) deficiency causes hyperinsulinism secondary to a loss of inhibition of GDH by SCHAD (6). Antischizophrenic drugs can produce hyperglycemia in patients (7,8) perhaps due to their ability to inhibit GDH. Both insulin release and GDH activity are decreased by SIRT4 (9), a mitochondrial ADP-ribosyl transferase, and deletion of GDH in β-cells partially abolishes the insulin secretory response (10).
THE GDH REACTION
GDH catalyzes the reaction NAD(P) + Glutamate ⇆ NAD(P)H + α-ketoglutarate + NH4+ (Fig. 1). The catalytically active form of the enzyme is six identical 5.7 × 104 molecular weight monomers configured as a hexamer (11–13). When the concentration of the hexamer is high, it polymerizes (12,13). In addition to possessing binding sites for substrates and products, the enzyme has allosteric sites (1,11,13). Ligands are not altered chemically at allosteric sites, but specific ligands, when bound to these sites, either inhibit or activate the catalytic reaction at the active sites. Binding of leucine, ADP, succinyl-CoA, or BCH to the allosteric sites increases GDH enzyme activity and polymerization of its polypeptide chain, while binding of GTP or palmitoyl-CoA to these sites decreases GDH enzyme activity and causes dissociation of the polypeptide chains from one another (1,2,12,13). The activator and inhibitor sites are overlapping (1,12,13). Consequently, for example, leucine can displace GTP from the allosteric sites and activate the enzyme (1).
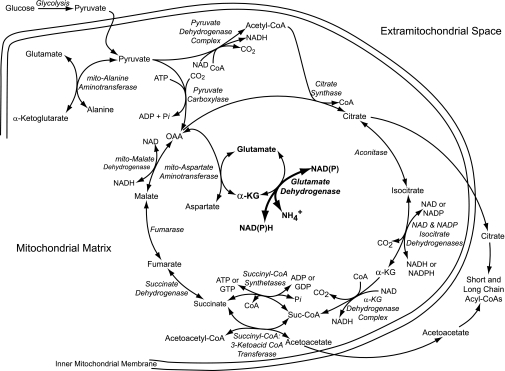
FIG. 1.
Reactions associated with oxidative deamination of glutamate by glutamate dehydrogenase to stimulate insulin secretion. Leucine’s allosteric activation of GDH causes the oxidative deamination of glutamate to α-ketoglutarate in β-cell mitochondria to lead to increased production of citrate. This could be because reduced pyridine nucleotides and α-ketoglutarate, which are products of the GDH reaction, are also product inhibitors of isocitrate dehydrogenases. Inhibition of isocitrate dehydrogenases should raise the level of isocitrate and, via the conversion of isocitrate to citrate in the aconitase reaction, increase the level of citrate. In addition, the generation of α-ketoglutarate by GDH should promote production of oxaloacetate and pyruvate by mitochondrial aspartate aminotransferase and mitochondrial alanine aminotransferase, respectively. Consequently, oxaloacetate could be used in the citrate synthase reaction to generate citrate. Pyruvate, via the pyruvate dehydrogenase complex reaction, could supply citrate synthase with acetyl-CoA and, via the pyruvate carboxylase reaction, supply oxaloacetate to citrate synthase. In addition, α-ketoglutarate produced by GDH should be converted to succinyl-CoA catalyzed by the α-ketoglutarate dehydrogenase complex reaction. The higher level of succinyl-CoA, which is also an activator of GDH, should further enhance GDH activity. The formation of citrate in the mitochondrial matrix should be followed by its transfer to the extramitochondrial space where it can be used for the synthesis of short-chain acyl-CoAs, which are believed to be signals for insulin secretion. The reactions described above would be supplemented by the direct synthesis from leucine by itself of acetoacetate by succinyl-CoA:3-ketoacid-CoA transferase and hydroxymethylglutaryl-CoA lyase in mitochondria, followed by the transport of acetoacetate into the extramitochondrial space and its utilization for the synthesis of short-chain acyl-CoAs. Some of the relevant enzymes that can associate with GDH are mitochondrial aspartate aminotransferase, mitochondrial malate dehydrogenase, and also (not shown) short-chain 3-hydroxyacyl-CoA dehydrogenase. GDP, guanosine diphosphate; α-KG, α-ketoglutarate; OAA, oxaloacetate; Pi, inorganic phosphate; Suc-CoA, succinyl-CoA.
In 1973 we found that GDH can associate with mitochondrial aspartate aminotransferase, (14) and in 1988 we found that GDH can associate with both mitochondrial malate dehydrogenase and mitochondrial aspartate aminotransferase. Furthermore, binding of mitochondrial aspartate aminotransferase to GDH enhanced binding of mitochondrial malate dehydrogenase to GDH (15). For these and other reasons it was concluded that the three enzymes form a trienzyme complex (3,16). SCHAD can also bind to GDH (6). SCHAD is similar to mitochondrial malate dehydrogenase with respect to molecular weight, being a dimer, amino acid composition, isoelectric point, keto substrate inhibition, and immunological cross-reactivity (17). However, unlike the mitochondrial malate dehydrogenase, which can activate GDH (15), SCHAD inhibits GDH (6). We also found that GDH can form complexes with the α-ketoglutarate dehydrogenase complex and succinyl-CoA synthetase (1,16). Detailed discussions of the specificities of these interactions are given in references 1, 3, and 16.
MECHANISM OF ENHANCEMENT OF INSULIN RELEASE BY GDH
In β-cell mitochondria, the oxidative deamination of glutamate by GDH can stimulate insulin release (18,19) via indirect effects on other enzymes. We propose that this is because oxidative deamination of glutamate (Fig. 1), besides raising the mitochondrial concentration of α-ketoglutarate, also raises the NADH/NAD and NADPH/NADP ratios (18,19). In addition to being products of the GDH reaction, α-ketoglutarate and reduced pyridine nucleotides are also products of the mitochondrial NAD isocitrate dehydrogenase and both the mitochondrial and the cytosolic NADP isocitrate dehydrogenase. The increases in α-ketoglutarate, NADH, and NADPH will raise the level of isocitrate secondary to product inhibition of the isocitrate dehydrogenases (Fig. 1). Reduced pyridine nucleotides further enhance the inhibition of isocitrate dehydrogenases by enhancing binding of α-ketoglutarate to isocitrate dehydrogenases (20). A higher level of isocitrate should, through a mass action effect via the aconitase reaction (Fig. 1), convert isocitrate into citrate. Citrate should in turn be converted into short-chain acyl-CoAs (21,22). Indeed, activation of GDH by BCH raises the level of citrate in pancreatic islets and stimulates insulin release (23). However, activation of other dehydrogenases or other α-ketoglutarate–generating enzymes does not stimulate insulin release (24). To summarize, GDH can play a special role in regulating insulin secretion because it can generate α-ketoglutarate to inhibit isocitrate dehydrogenases, NADPH to inhibit NADP-isocitrate dehydrogenases, and NADH to inhibit NAD-isocitrate dehydrogenase. The resulting high level of citrate in the cytosol stimulates the synthesis of short- and long-chain acyl-CoAs, which are believed to be coupling factors for insulin secretion (21,22).
We have demonstrated that BCH can produce over a 12-fold increase in the level of citrate in islets (23), and others have shown that BCH can raise malate from 5.5 to 8.9 pmol/islet and pyruvate from 8.5 to 12.2 pmol/islet (19). BCH also lowers islet aspartate by 65% and glutamate by 28% (25). These results are consistent with BCH activating α-ketoglutarate production by GDH, which increases production of 1) pyruvate by mitochondrial alanine aminotransferase; 2) oxaloacetate by mitochondrial aspartate aminotransferase; 3) oxaloacetate by pyruvate carboxylase; and 4) acetyl-CoA by the pyruvate dehydrogenase complex (Fig. 1). The oxaloacetate generated is then used along with acetyl-CoA (Fig. 1) to produce citrate via the citrate synthase reaction in the mitochondrion. Citrate is then exported to the cytosol where it can be used for synthesis of the short- and long-chain acyl-CoAs. Malate derived from oxaloacetate produced by aspartate aminotransferase and pyruvate carboxylase in the mitochondria can then enter the cytosol where it provides reduced pyridine nucleotides. These sets of reactions start with GDH, which adds α-ketoglutarate to the citric acid cycle, and culminate in the production of citrate catalyzed by citrate synthase. A rise in citrate levels also coincides with the initiation of insulin secretion when glucose instead of BCH is the secretagogue (26), and the dose dependence of glucose-stimulated insulin release correlates with the cellular contents of citrate and malate.
EFFECT OF GLUTAMINE
Glutamine, after its conversion to glutamate by glutaminase, can also increase α-ketoglutarate production by GDH (18,19); however, as mentioned above, adding even a high concentration of glutamine alone to islets does not stimulate insulin release (18,19). Glutamine alone probably does not promote insulin release because glutamate derived from glutamine would lower the level of oxaloacetate and pyruvate via reversing the mitochondrial alanine aminotransferase and aspartate aminotransferase reactions. Lowering the levels of oxaloacetate and pyruvate would diminish insulin release because there would be insufficient levels of these metabolites for their conversion into citrate and acyl-CoAs. The concept that adding glutamine alone to islets leads to depletion of oxaloacetate and pyruvate by reversing these aminotransferase reactions is consistent with the fact that adding glutamine alone increases the level of alanine (from 14.3 to 26.3 pmol/islet) and the level of aspartate (from 17.2 to 28.4 pmol/islet) (18,19). When islets are incubated with glutamine in the presence of BCH to activate GDH, there is stimulation of insulin release (19): the α-ketoglutarate produced by GDH enables the production of pyruvate catalyzed by mitochondrial alanine aminotransferase and oxaloacetate catalyzed by mitochondrial aspartate aminotransferase.
When glutamine alone is incubated with islets, the glutamate that is generated can also be decarboxylated to γ-aminobutyrate (GABA) via the glutamate decarboxylase reaction (27). Although GABA can be used for the production of succinate via the GABA shunt, and succinate is insulinotropic in fresh pancreatic islets (28), the GABA pathway by itself is apparently not sufficiently active to promote insulin release (27). When leucine is added with glutamine to activate α-ketoglutarate production by GDH, however, there is insulin release. This could be because sufficient α-ketoglutarate is generated for the transamination of GABA by GABA aminotransferase (27).
CITRATE
As previously cited above, activating GDH leads to an increased production of citrate, which promotes insulin release. Incubation of pyruvate with insulinoma cells and islet mitochondria results in a progressive increase in the level of citrate and oscillations in citrate levels (29). Opposing an increase in the level of citrate would be substrate inhibition of citrate synthase by citrate and the export of citrate from the mitochondria; thus, an increase in citrate to a high level in mitochondria would be followed by its decrease causing the level of citrate to oscillate (29). Oscillations of this type are either not found with other Krebs cycle intermediates or are only found with a lower amplitude (29). In islets, citrate oscillations could correlate with oscillations in insulin release.
DIRECT SUBSTRATE TRANSFERS THROUGH ENZYME:ENZYME INTERACTION
As mentioned above, we have found with studies of pure enzymes that GDH has a high affinity for mitochondrial aspartate aminotransferase and mitochondrial malate dehydrogenase (4,13–15). The catalytically active GDH hexamer (342 kDa) is a considerably larger enzyme than the 90 kDa mitochondrial aspartate aminotransferase dimer (30) or the 68 kDa mitochondrial malate dehydrogenase dimer (17). Consequently, heteroenzyme complexes can be formed with both mitochondrial aspartate aminotransferase and mitochondrial malate dehydrogenase bound to GDH (3,4,13–16). In these trienzyme complexes, the active sites of each enzyme could be in close proximity with one another. NADH, therefore, can be directly transferred from GDH to mitochondrial malate dehydrogenase such that: GDH-NADH + mitochondrial malate dehydrogenase ⇆ GDH-NADH-mitochondrial malate dehydrogenase ⇆ GDH + mitochondrial malate dehydrogenase-NADH. This would not only eliminate the necessity of NADH having to diffuse from GDH to mitochondrial malate dehydrogenase, but it would also retard interception of NADH by other dehydrogenases (3,4,13,16,31). The net effect of this enzyme:enzyme interaction would be the facilitation of oxidative deamination of glutamate and consequently insulin secretion because dissociation of NADH from GDH is the rate-limiting step in the GDH reaction (32). These interactions between GDH, malate dehydrogenase, and NADH can take place because GDH has B chirality with respect to NADH binding while malate dehydrogenase has A chiral specificity (31,33) enabling NADH to be directly transferred from one enzyme to the other.
The direct transfer of oxaloacetate from mitochondrial malate dehydrogenase to mitochondrial aspartate aminotransferase might not take place unless the active sites of these two enzymes are in close proximity (3,13,15). The mitochondrial aspartate aminotransferase-GDH-mitochondrial malate dehydrogenase trienzyme complex therefore might serve as a segregated source of malate for the transfer of reducing equivalents to the cytosol when the demand for NADH is high. If the demand for NADH is in part met by fatty acid oxidation, then synthesis of malate within this heteroenzyme complex, which has GDH in close proximity to mitochondrial malate dehydrogenase, protects mitochondrial malate dehydrogenase from inhibition by fatty acyl-CoAs such as palmitoyl-CoA (4,13,15).
The ability of GDH to generate NADH for the mitochondrial malate dehydrogenase can also be enhanced by the known abilities of GDH to associate with either the α-ketoglutarate dehydrogenase complex or succinyl-CoA synthetase (4,13,15). The complexes between GDH and the latter two enzymes can serve to remove inhibitors, such as α-ketoglutarate or GTP, from GDH. Removal of α-ketoglutarate from mitochondrial aspartate aminotransferase would be facilitated by the higher reactivity of α-ketoglutarate bound to the pyridoxamine-P form of the mitochondrial aspartate aminotransferase than the lower reactivity of the free α-ketoglutarate with the α-ketoglutarate dehydrogenase complex (34). The production by the α-ketoglutarate dehydrogenase complex of succinyl-CoA, an activator of GDH, would therefore be enhanced (13).
By using previously described methods (3), we have found that the concentrations of GDH (hexamer), mitochondrial aspartate aminotransferase (dimer), and mitochondrial malate dehydrogenase (dimer) in islet mitochondria are about 7.6 μmol/L, 26 μmol/L, and 43 μmol/L, respectively (L.A.F., unpublished observations). There is thus ample mitochondrial aspartate aminotransferase and mitochondrial malate dehydrogenase so that all of the GDH in islet mitochondria could be associated with these two enzymes. In addition, there is evidence that a stable trienzyme complex of these enzymes is one in which one mitochondrial malate dehydrogenase dimer plus one mitochondrial aspartate aminotransferase dimer is bound to one GDH hexamer (3). The formation of the trienzyme complex is favored by activation of GDH by Mg2+ plus leucine or Mg2+ plus ATP, which in turn enhances binding of GDH to mitochondrial aspartate aminotransferase (1,13). Inhibitors of GDH, such as citrate, malate, and high levels of ATP, decrease binding of mitochondrial aspartate aminotransferase to GDH (3,4). Mitochondrial aspartate aminotransferase also has a high affinity for a specific site on the inner mitochondrial membrane, which we have purified and characterized (35). This site could facilitate organization of mitochondrial aspartate aminotransferase, GDH, and mitochondrial malate dehydrogenase on the inner mitochondrial membrane. (Most of the experiments described above were performed with bovine GDH [36]. Unlike bovine GDH, rat GDH does not polymerize, but the interactions between rat GDH and mitochondrial aspartate aminotransferase are similar to those of bovine GDH with aspartate aminotransferase [36–38]. This indicates that polymerization of GDH is not necessary for GDH to associate with the mitochondrial aspartate aminotransferase.) SCHAD also associates with and inhibits GDH (6,39). This is quite significant in islet mitochondria where the level of SCHAD is higher than the level of GDH (17). SCHAD is quite similar in many ways to mitochondrial malate dehydrogenase. As previously suggested (17), a difference between the two could be attributed to 180° rotation in the binding orientation of NAD.
ALLOSTERIC MODIFIERS
Allosteric modifiers are bound in the region of a site on GDH, which is different from the binding site for its substrates and products. The allosteric activators leucine, BCH, ADP, and succinyl-CoA (1,4,13) can almost completely replace the allosteric inhibitors GTP, palmitoyl-CoA, and Zn2+ on the allosteric site (1,13,40–42). Consequently, succinyl-CoA and other GDH activators can produce a marked increase in GDH activity not only by directly activating GDH, but also by enhancing dissociation of inhibitors from GDH.
Inhibition of GDH can also depend on the level of GDH in the mitochondria relative to the concentration of the inhibitor in the mitochondria. For example, the level of GDH in rat liver mitochondria is about 80 μmol/L with respect to GDH hexamers or 430 μmol/L with respect to individual GDH polypeptide chains (3); therefore, even if the dissociation constant of the enzyme-inhibitor complex is quite low, complete inhibition of GDH in liver mitochondria would require a concentration of at least 430 μmol/L inhibitor. In rat islet mitochondria, the level of GDH is low and ∼8 μmol/L with respect to hexamer. This considerably lowers the level of an inhibitor required for complete inhibition of islet GDH. GDH has a higher affinity than any other known protein for palmitoyl-CoA (40,41). The Ki of palmitoyl-CoA (0.03 μmol/L) is lower than the mitochondrial level of palmitoyl-CoA (1,13,40,41), and thus palmitoyl-CoA is likely a potent inhibitor of the GDH reaction in pancreatic islet mitochondria.
Long-chain acyl-CoAs, such as palmitoyl-CoA, are synthesized in the cytosol. The first committed step in fatty acid synthesis is utilization of acetyl-CoA for production of malonyl-CoA via the acetyl-CoA carboxylase reaction. Long-chain acyl-CoAs do not readily traverse the inner mitochondrial membrane; therefore, in order for a long-chain acyl-CoA to inhibit GDH, which is located within the mitochondrial matrix, the long-chain acyl group must first form a complex with carnitine followed by the transport of this complex across the mitochondrial membranes. On the matrix side (inside) of the mitochondrial membrane, the acyl group is transferred back to CoA. This transport mechanism is inhibited by malonyl-CoA (43); therefore, malonyl-CoA can decrease the mitochondrial level of long-chain acyl-CoAs and thereby decrease inhibition of GDH by long-chain acyl-CoAs and increase insulin release. The reduced ability of GTP to inhibit the mutant GDH found in some patients accounts for the high levels of insulin and hypoglycemia found in these patients (5). The level of Zn2+, which is a potent inhibitor of GDH (Ki = 0.5 μmol/L [4,44]), can be quite high in islet mitochondria (45); therefore, Zn2+ would also be expected to inhibit GDH in islets.
Although leucine allosterically activates GDH and stimulates insulin secretion similarly to BCH (2,18,19), leucine alone is a more potent insulin stimulant than BCH (46,47). The additional insulinotropic action of leucine probably results from its conversion to acetoacetate plus acetyl-CoA (46,47). This would be followed by the utilization of these two metabolites for the synthesis of the short- and long-chain acyl-CoA, which are believed to enhance insulin release as previously described (1,21,22).
Antischizophrenic drugs, such as haloperidol, have anti-insulin actions. They can produce hyperglycemia, which in turn can result in type 2 diabetes, metabolic acidosis, ketosis, and hyperglycemia-related deaths (7). The diabetogenic action of these drugs could be because these drugs are potent inhibitors of GDH (8) in the β-cell and therefore inhibit insulin secretion. The effect of these drugs on GDH is similar to that of the other allosteric inhibitors of GDH discussed above and is decreased by ADP (1,8).
ACKNOWLEDGMENTS
This work was supported in part by National Institutes of Health Grant DK28348 and the Nowlin Family Trust administered by the Lutheran Community Foundation to M.J.M.
No potential conflicts of interest relevant to this article were reported.
L.A.F. researched data and wrote the manuscript. M.J.M. contributed data and reviewed and wrote the manuscript.
REFERENCES
- 1.Fahien LA, Teller JK, Macdonald MJ, Fahien CM. Regulation of glutamate dehydrogenase by Mg2+ and magnification of leucine activation by Mg2+. Mol Pharmacol 1990;37:943–949 [PubMed] [Google Scholar]
- 2.Gylfe E. Comparison of the effects of leucines, non-metabolizable leucine analogues and other insulin secretagogues on the activity of glutamate dehydrogenase. Acta Diabetol Lat 1976;13:20–24 [DOI] [PubMed] [Google Scholar]
- 3.Fahien LA, Teller JK. Glutamate-malate metabolism in liver mitochondria: a model constructed on the basis of mitochondrial levels of enzymes, specificity, dissociation constants, and stoichiometry of hetero-enzyme complexes. J Biol Chem 1992;267:10411–10422 [PubMed] [Google Scholar]
- 4.Fahien LA, Kmiotek EH, Woldegiorgis G, Evenson M, Shrago E, Marshall M. Regulation of aminotransferase-glutamate dehydrogenase interactions by carbamyl phosphate synthase-I, Mg2+ plus leucine versus citrate and malate. J Biol Chem 1985;260:6069–6079 [PubMed] [Google Scholar]
- 5.Stanley CA, Lieu YK, Hsu BY, et al. Hyperinsulinism and hyperammonemia in infants with regulatory mutations of the glutamate dehydrogenase gene. N Engl J Med 1998;338:1352–1357 [DOI] [PubMed] [Google Scholar]
- 6.Li C, Chen P, Palladino A, et al. Mechanism of hyperinsulinism in short-chain 3-hydroxyacyl-CoA dehydrogenase deficiency involves activation of glutamate dehydrogenase. J Biol Chem 2010;285:31806–31818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Llorente MD, Urrutia VJ. Diabetes, psychiatric disorders, and the metabolic effects of antipsychotic medications. Clin Diabetes 2006;24:18–24 [Google Scholar]
- 8.Shemisa OA, Fahien LA. Modifications of glutamate dehydrogenase by various drugs which affect behavior. Mol Pharmacol 1971;7:8–25 [PubMed] [Google Scholar]
- 9.Haigis MC, Mostoslavsky R, Haigis KM, et al. SIRT4 inhibits glutamate dehydrogenase and opposes the effects of calorie restriction in pancreatic β cells. Cell 2006;126:941–954 [DOI] [PubMed] [Google Scholar]
- 10.Carobbio S, Frigerio F, Rubi B, et al. Deletion of glutamate dehydrogenase in β-cells abolishes part of the insulin secretory response not required for glucose homeostasis. J Biol Chem 2009;284:921–929 [DOI] [PubMed] [Google Scholar]
- 11.Cassman M, Schachman HK. Sedimentation equilibrium studies on glutamic dehydrogenase. Biochemistry 1971;10:1015–1024 [DOI] [PubMed] [Google Scholar]
- 12.Frieden C. Glutamic dehydrogenase. II. The effect of various nucleotides on the association-dissociation and kinetic properties. J Biol Chem 1959;234:815–820 [PubMed] [Google Scholar]
- 13.Fahien LA, MacDonald MJ, Teller JK, Fibich B, Fahien CM. Kinetic advantages of hetero-enzyme complexes with glutamate dehydrogenase and the α-ketoglutarate dehydrogenase complex. J Biol Chem 1989;264:12303–12312 [PubMed] [Google Scholar]
- 14.Fahien LA, Smith SE. Interaction between glutamate dehydrogenase and transaminase. In Metabolic Interconversion of Enzymes. Fischer FH, Krebs EG, Neurath H, Stadtman ER, Eds. Berlin, Springer Verlag, 1974, p. 369–378 [Google Scholar]
- 15.Fahien LA, Kmiotek EH, MacDonald MJ, Fibich B, Mandic M. Regulation of malate dehydrogenase activity by glutamate, citrate, α-ketoglutarate, and multienzyme interaction. J Biol Chem 1988;263:10687–10697 [PubMed] [Google Scholar]
- 16.Fahien LA, Chobanian MC. Kinetic advantages of multienzyme complexes involving aminotransferases. In Channelling in Intermediary Metabolism. Agius L, Sherratt HAS, Eds. London, Portland Press, 1996, p. 219–236 [Google Scholar]
- 17.Noyes BE, Glatthaar BE, Garavelli JS, Bradshaw RA. Structural and functional similarities between mitochondrial malate dehydrogenase and L-3-hydroxyacyl CoA dehydrogenase. Proc Natl Acad Sci USA 1974;71:1334–1338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sener A, Malaisse-Lagae F, Malaisse WJ. Stimulation of pancreatic islet metabolism and insulin release by a nonmetabolizable amino acid. Proc Natl Acad Sci USA 1981;78:5460–5464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Malaisse-Lagae F, Sener A, Garcia-Morales P, Valverde I, Malaisse WJ. The stimulus-secretion coupling of amino acid-induced insulin release: influence of a nonmetabolized analog of leucine on the metabolism of glutamine in pancreatic islets. J Biol Chem 1982;257:3754–3758 [PubMed] [Google Scholar]
- 20.Cleland WW. Steady state kinetics. In The Enzymes. Vol II, 3rd ed. Boyer P, Ed. New York, Academic Press, 1970, p. 28–29 [Google Scholar]
- 21.Corkey BE, Glennon MC, Chen KS, Deeney JT, Matschinsky FM, Prentki M. A role for malonyl-CoA in glucose-stimulated insulin secretion from clonal pancreatic beta-cells. J Biol Chem 1989;264:21608–21612 [PubMed] [Google Scholar]
- 22.Prentki M, Vischer S, Glennon MC, Regazzi R, Deeney JT, Corkey BE. Malonyl-CoA and long chain acyl-CoA esters as metabolic coupling factors in nutrient-induced insulin secretion. J Biol Chem 1992;267:5802–5810 [PubMed] [Google Scholar]
- 23.Hasan NM, Longacre MJ, Stoker SW, et al. Impaired anaplerosis and insulin secretion in insulinoma cells caused by small interfering RNA-mediated suppression of pyruvate carboxylase. J Biol Chem 2008;283:28048–28059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fahien LA, MacDonald MJ, Kmiotek EH, Mertz RJ, Fahien CM. Regulation of insulin release by factors that also modify glutamate dehydrogenase. J Biol Chem 1988;263:13610–13614 [PubMed] [Google Scholar]
- 25.Gylfe E. Glucose oxidation and contents of free amino acids in pancreatic beta-cells stimulated by a non-metabolizable leucine analogue. Biochim Biophys Acta 1974;343:584–589 [DOI] [PubMed] [Google Scholar]
- 26.Farfari S, Schulz V, Corkey B, Prentki M. Glucose-regulated anaplerosis and cataplerosis in pancreatic β-cells: possible implication of a pyruvate/citrate shuttle in insulin secretion. Diabetes 2000;49:718–726 [DOI] [PubMed] [Google Scholar]
- 27.Pizarro-Delgado J, Braun M, Hernández-Fisac I, Martín-Del-Río R, Tamarit-Rodriguez J. Glucose promotion of GABA metabolism contributes to the stimulation of insulin secretion in β-cells. Biochem J 2010;431:381–389 [DOI] [PubMed] [Google Scholar]
- 28.MacDonald MJ, Fahien LA. Insulin release in pancreatic islets by a glycolytic and a Krebs cycle intermediate: contrasting patterns of glyceraldehyde phosphate and succinate. Arch Biochem Biophys 1990;279:104–108 [DOI] [PubMed] [Google Scholar]
- 29.MacDonald MJ, Fahien LA, Buss JD, Hasan NM, Fallon MJ, Kendrick MA. Citrate oscillates in liver and pancreatic beta cell mitochondria and in INS-1 insulinoma cells. J Biol Chem 2003;278:51894–51900 [DOI] [PubMed] [Google Scholar]
- 30.Feliss N, Martinez-Carrion M. The molecular weight and subunits of the isozymes of glutamic aspartic transaminase. Biochem Biophys Res Commun 1970;40:932–940 [DOI] [PubMed] [Google Scholar]
- 31.Srivastava DK, Bernhard SA. Enzyme-enzyme interactions and the regulation of metabolic reaction pathways. Curr Top Cell Regul 1986;28:1–68 [DOI] [PubMed] [Google Scholar]
- 32.Iwatsubo M, Pantaloni D. Regulation of the activity of glutamate dehydrogenase by effectors GTP and ADP: study by means of “stopped flow”. Bull Soc Chim Biol (Paris) 1967;49:1563–1572 [in French] [PubMed] [Google Scholar]
- 33.Fahien LA, MacDonald MJ. The succinate mechanism of insulin release. In Commentaries on Perspectives in Diabetes Volume 3 (1998–2002) Robertson RP, Ed. New York, McGraw-Hill, 2007, p. 2669-2676 [DOI] [PubMed] [Google Scholar]
- 34.Smith BC, Clotfelter LA, Cheung JY, LaNoue KF. Differences in 2-oxoglutarate dehydrogenase regulation in liver and kidney. Biochem J 1992;284:819–826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Teller JK, Fahien LA, Valdivia E. Interactions among mitochondrial aspartate aminotransferase, malate dehydrogenase, and the inner mitochondrial membrane from heart, hepatoma, and liver. J Biol Chem 1990;265:19486–19494 [PubMed] [Google Scholar]
- 36.Fahien LA, Strmecki M, Smith S. Studies of gluconeogenic mitochondrial enzymes. I. A new method of preparing bovine liver glutamate dehydrogenase and effects of purification methods on properties of the enzyme. Arch Biochem Biophys 1969;130:449–455 [DOI] [PubMed] [Google Scholar]
- 37.King KS, Frieden C. The purification and physical properties of glutamate dehydrogenase from rat liver. J Biol Chem 1970;245:4391–4396 [PubMed] [Google Scholar]
- 38.Fahien LA, Hsu SL, Kmiotek E. Effect of aspartate on complexes between glutamate dehydrogenase and various aminotransferases. J Biol Chem 1977;252:1250–1256 [PubMed] [Google Scholar]
- 39.Pepin E, Guay C, Delghingaro-Augusto V, Joly E, Madiraju SR, Prentki M. Short-chain 3-hydroxyacyl-CoA dehydrogenase is a negative regulator of insulin secretion in response to fuel and non-fuel stimuli in INS832/13 β-cells. J Diabetes 2010;2:157–167 [DOI] [PubMed] [Google Scholar]
- 40.Fahien LA, Kmiotek E. Regulation of glutamate dehydrogenase by palmitoyl-coenzyme A. Arch Biochem Biophys 1981;212:247–253 [DOI] [PubMed] [Google Scholar]
- 41.Kawaguchi A, Bloch K. Inhibition of glutamate dehydrogenase and malate dehydrogenases by palmitoyl coenzyme A. J Biol Chem 1976;251:1406–1412 [PubMed] [Google Scholar]
- 42.Pal PK, Colman RF. Affinity labeling of an allosteric GTP site of bovine liver glutamate dehydrogenase by 5′-p-fluorosulfonylbenzoylguanosine. Biochemistry 1979;18:838–845 [DOI] [PubMed] [Google Scholar]
- 43.McGarry JD, Foster DW. Interrelations between fatty acid synthesis, fatty acid oxidation and ketogenesis in liver. In Microenvironments and Metabolic Compartmentation. Srere PA, Estabrook RW, Eds. New York, Academic Press, 1978, p. 245–260 [Google Scholar]
- 44.Colman RF, Foster DS. The absence of zinc in bovine liver glutamate dehydrogenase. J Biol Chem 1970;245:6190–6195 [PubMed] [Google Scholar]
- 45.Anderson TPO, Berggren A, Flatt PR. Subcellular distribution of zinc in islet beta-cell fractions. Horm Metab Res 1980;12:275–276 [DOI] [PubMed] [Google Scholar]
- 46.MacDonald MJ, Hasan NM, Longacre MJ. Studies with leucine, beta-hydroxybutyrate and ATP citrate lyase-deficient beta cells support the acetoacetate pathway of insulin secretion. Biochim Biophys Acta 2008;1780:966-972 [DOI] [PMC free article] [PubMed]
- 47.MacDonald MJ, Smith AD, 3rd, Hasan NM, Sabat G, Fahien LA. Feasibility of pathways for transfer of acyl groups from mitochondria to the cytosol to form short chain acyl-CoAs in the pancreatic beta cell. J Biol Chem 2007;282:30596–30606 [DOI] [PubMed] [Google Scholar]