There is a widespread acceptance in the literature that plasma nonesterified fatty acids (NEFA), also called free fatty acids (FFA), can mediate many adverse metabolic effects, most notably insulin resistance. Elevated NEFA concentrations in obesity are thought to arise from an increased adipose tissue mass. It is also argued that the process of fatty acid mobilization from adipose tissue, normally suppressed by insulin, itself becomes insulin resistant—thus, lipolysis is further increased, potentially leading to a vicious cycle. Although we have also accepted this model for many years (1,2), recently there has been a steady accumulation of data, both in the literature and from our own research, that has forced us to realize that this simple story is not always true. Here we review the background to the idea of “fatty acids as metabolic villains,” together with data from the literature and from our own studies, which tend to show another side to the fatty acids/insulin resistance story.
We will first examine the relationship between systemic concentrations of NEFA and obesity/insulin resistance and then study adipose tissue in the obese state with regard to its adaptation for NEFA release.
FATTY ACIDS AND METABOLIC PHYSIOLOGY
NEFA circulate in the plasma bound to plasma albumin. Their function was largely elucidated in the 1950s through the work of Vincent Dole (3) at the Rockefeller Institute in New York and Robert Gordon (4,5) at the National Institutes of Health. Gordon demonstrated the origin of plasma NEFA from adipose tissue and their use by tissues such as the liver and myocardium, but not the brain.
We now recognize that NEFA are the vehicle by which triacylglycerol (TG) stored in adipose tissue is transported to its sites of utilization. NEFA turnover is rapid, with a plasma half-life around 2–4 min (6). The only significant site of NEFA liberation into plasma is adipose tissue. The abdominal subcutaneous fat depot is the dominant source of NEFA; considerably less comes from leg adipose tissue and—against popular belief—only a small proportion of the systemic NEFA concentrations arises from intraabdominal adipose tissue (7). In the fasting state, plasma NEFA arise almost entirely from hydrolysis of TG within the adipocyte. This process is highly regulated over short periods via modulation of the activity of the enzymes involved and conformation of the proteins that coat the adipocyte lipid droplet. It has been reviewed recently (8), and we will not cover those aspects here. After a meal that contains fat, however, there is an additional and important route of generation of plasma NEFA. Lipoprotein lipase (LPL) in the capillaries of adipose tissue hydrolyses circulating TG, mainly the dietary fat carried in the chylomicrons. Fatty acids thus released are taken up into the adipocytes for storage, but a proportion always escapes and joins the plasma NEFA pool (9) in a process sometimes called spillover. As a result, the plasma NEFA pool changes in composition (the contributions of different fatty acids) after a meal to reflect the composition of the meal fat (10,11). Spillover fatty acids may constitute 40–50% of the total plasma NEFA pool in the postprandial period (12). Spillover can be demonstrated using isotopic tracers in a number of other tissues (13), but these do not contribute in a net sense to plasma NEFA. Spillover is also high (in percentage terms) when VLDL-TG is hydrolyzed by LPL in the fasting state, but the rate of hydrolysis is low in relation to intracellular lipolysis, so the contribution to systemic plasma NEFA is small (14,15). In obesity, when the LPL rate of action (per unit fat tissue) is normally lower than in lean individuals, the contribution of spillover fatty acids to the total NEFA delivery from adipose tissue is actually lower than in lean individuals (12). The lower LPL activity leaves more TG unlipolysed in the triglyceride-rich lipoproteins passing adipose tissue, and these remnant lipoproteins are eventually taken up by the liver. In effect, this pathway converges with the spillover pathway in that it represents fatty acids that have not been taken up/been reesterified by the tissue.
Fat mobilization is suppressed rapidly by insulin. Plasma NEFA concentrations therefore fall after any meal that contains carbohydrates, which stimulate insulin release. Spillover fatty acids somewhat reduce this effect but do not override it. Circadian profiles of plasma NEFA concentrations therefore show the highest concentrations after an overnight fast, with suppression after each meal (15–18). Typical plasma NEFA concentrations in various physiological states have been reviewed previously (2). They range from around 300–600 μmol/L in the overnight fasting state (depending upon methodology and cohort) to ∼1,300 μmol/L after a 72-h fast. Plasma NEFA concentrations are usually found to be higher in women than in men (19–22), and this difference becomes more marked with short fasting (23,24). Our own data on this will be discussed below. They are suppressed during high-carbohydrate diets (25) and increased in stress states, e.g., concentrations of 1,720 μmol/L were found in racing drivers before a race (26). During exercise, when fat is mobilized from adipose tissue to supply the working muscles, concentrations may rise somewhat (2), but often plasma NEFA concentrations remain relatively stable as removal by muscle increases to match adipose tissue lipolysis (27,28). In many studies of individuals with reasonably well controlled type 2 diabetes, plasma NEFA concentrations are not notably elevated (17,29,30), although they increase in individuals with poorly controlled type 2 diabetes (17). We summarize measurements made in obesity later in this review.
FATTY ACIDS AND PATHOPHYSIOLOGY
In 1963, understanding of the importance of NEFA changed from physiology to pathophysiology with the description of the glucose-fatty acid cycle by Randle et al. (31). The authors suggested that elevated NEFA concentrations were associated with “several abnormalities of carbohydrate metabolism, common to many endocrine and nutritional disorders.” The first of these abnormalities described was impaired sensitivity to insulin. The link between elevated NEFA concentrations and insulin resistance has remained firmly entrenched in the literature. For instance, in an authoritative review on metabolic syndrome in 2005, Eckel et al. (32) wrote that “a major contributor to the development of insulin resistance is an overabundance of circulating fatty acids.”
There have been many experimental verifications of this concept. Because of their limited solubility, it is not possible to infuse sufficient NEFA intravenously to raise plasma NEFA concentrations appreciably. Therefore an alternative technique has been developed based on the spillover concept described above. A lipid (TG) emulsion is infused intravenously, coupled with a low-dose infusion of heparin. Heparin releases LPL from its endothelial binding sites and also activates the enzyme. LPL therefore interacts with the lipid emulsion in the vasculature and releases substantial amounts of NEFA. With this approach, it is easy to show that elevated NEFA concentrations are associated acutely with insulin resistance, both with peripheral glucose uptake (33,34) and hepatic gluconeogenesis (35,36) and with impaired glucose tolerance (37). Hepatic insulin clearance is also reduced (38). Whether the excessively high NEFA concentrations (often in the range of 1,500 μmol/L or higher) usually achieved with this procedure are representative of those seen in states such as diabetes and obesity is a question we will return to later. It should also be noted that the situation is not one of pure NEFA elevation—TG concentrations are also increased, as are those of monoacylglycerols (39). In addition, lipid emulsions contain large amounts of free glycerol, and a control experiment with equivalent glycerol infusion is usually called for.
Acutely elevated NEFA concentrations therefore lead to insulin resistance, as described above. Plasma NEFA are the main substrate for hepatic TG production in the form of VLDL (40,41). NEFA turnover is a determinant of VLDL-TG secretion and plasma TG concentration (42). Because of these and other connections (e.g., elevated NEFA concentrations impair endothelial function [43] and raise blood pressure [44]), it seems reasonable to suppose that elevated plasma NEFA concentrations might be a marker for metabolic and cardiovascular disease (2). However, the evidence from prospective studies is far from convincing. Elevated NEFA concentrations at baseline are predictive of incident type 2 diabetes (45–47). Recently, it has been suggested that there is confounding by other metabolic variables (notably 2-h postglucose load glucose concentrations) and that with appropriate adjustments the relationship with NEFA becomes inverse (48). A similar conclusion was reached in a prospective cohort from the Ely study: fasting NEFA concentrations did not predict the development of metabolic syndrome or type 2 diabetes, but the reverse was true (49). Two studies suggest a role for plasma NEFA in incident cardiovascular disease (50,51). This was not found, however, in the large Paris Prospective Study (52); instead, in that study, elevated plasma NEFA concentrations at baseline were associated with increased risk of hypertension (53), sudden death (54), and cancer mortality (52).
Insulin resistance associated with longer-term lipid overload is now considered to involve accumulation of lipids in insulin-responsive tissues other than adipose tissue, so-called ectopic fat deposition (55). Fatty acid uptake by insulin-sensitive tissues is also likely to be modulated by altered function/expression of transporter proteins (56). The detrimental effects on insulin sensitivity and other cellular processes are known as lipotoxicity (57). In skeletal muscle, an increase in intramyocellular lipid (cellular TG), associated with insulin resistance, is seen during Intralipid/heparin infusion and clearly represents increased supply (58). Although it is as yet unresolved as to whether regulatory defects in mitochondrial fatty acid oxidation, with the apparently concomitant intramyocellular TG accumulation, are primary or secondary to insulin resistance, the phenomena appear to be strongly linked (59). Importantly, whatever the mechanism of the TG accumulation, there is evidence that interactions with insulin action are caused by a corresponding increase in active lipid moieties such as diacylglycerol, ceramide, or fatty acyl-CoAs rather than the inert TG pool. In a simple model, lipotoxicity reflects increased adipose tissue NEFA release. However, it is now recognized that fat accumulation in nonadipose tissues may reflect impaired disposal of fat as much as increased uptake (59). However, in real-life pathophysiology, the same accumulation of intramyocellular lipid may well arise more through impaired oxidative capacity. Accordingly, lipotoxicity does not necessarily imply increased NEFA delivery.
NEFA CONCENTRATIONS IN OBESITY: SYSTEMATIC LITERATURE REVIEW
As discussed above, plasma NEFA arise from adipose tissue. Adipose tissue mass is increased in obesity. It seems a natural conclusion that plasma NEFA concentrations will be increased in obese individuals and that this could lead to the insulin resistance that is usually associated with obesity.
An early and much-cited observation in small groups of individuals (60) suggested this was true, although plasma NEFA concentrations were certainly not elevated in proportion to the increase in fat mass (NEFA elevation in gross obesity vs. lean 68%; difference in fat mass 10-fold). However, over the years a number of articles have appeared in which the expected differences between lean and obese are not so clearly seen. Obvious issues in interpreting the relationship between NEFA concentrations and obesity between studies are sample size and the known within-person variability. This can be partly overcome by analyzing many studies in aggregate.
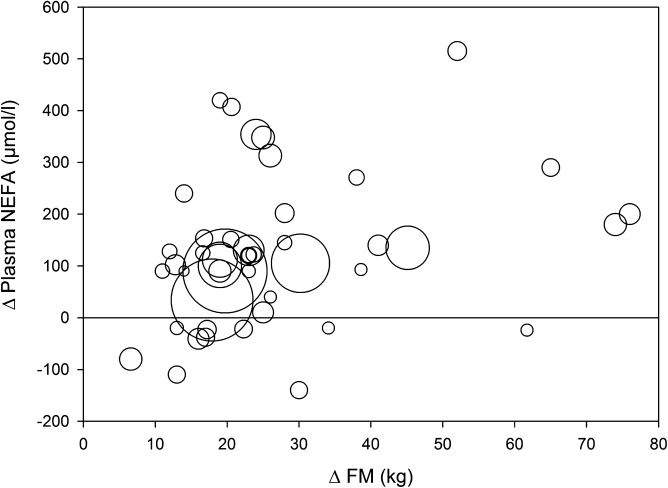
We explored this by means of a formal review of the literature. We searched the literature (PubMed, July 2009) for NEFA or FFA as well as obesity, and limited the results to original articles presenting human data. This resulted in 1,064 articles. We then required that at least two groups should be reported in each study: one overweight/obese and one control group. From each original article we then retrieved the following information: fasting plasma NEFA concentration, reported sex distribution, and BMI. We excluded studies on individuals with type 1 or type 2 diabetes. All studies published after 1990 with more than 10 subjects were included. In all, this resulted in 43 original reports including in total 953 nonobese (control) subjects and 1,410 overweight/obese subjects (Supplementary Table 1). In each study, we estimated the average body fat mass based on sex, height, and BMI according to the algorithms proposed by James and colleagues (61). The average fat mass was 16.3 kg in the control group and 41.2 kg in the overweight group. Finally, we calculated the difference in fat mass and NEFA concentration between the control group and the obese/overweight group in each respective study. The relationship between the difference in fat mass and the difference in fasting plasma NEFA concentration is depicted in Fig. 1. Most studies showed greater NEFA concentration in the obese/overweight group, but the average difference was modest (in the range of 70 μmol/L), and appeared to be unrelated to fat mass. In summary, this systematic review would support the notion that the fasting plasma NEFA concentration is largely unrelated to body fat mass.
FIG. 1.
Bubble graph representing the relationship between the difference in estimated fat mass and the difference in plasma NEFA concentration in 43 independent publications published 1990–2008 (Supplementary Table 1) between lean and overweight/obese subjects. Each bubble represents one study, and the bubble area is proportional to the number of observations (participants).
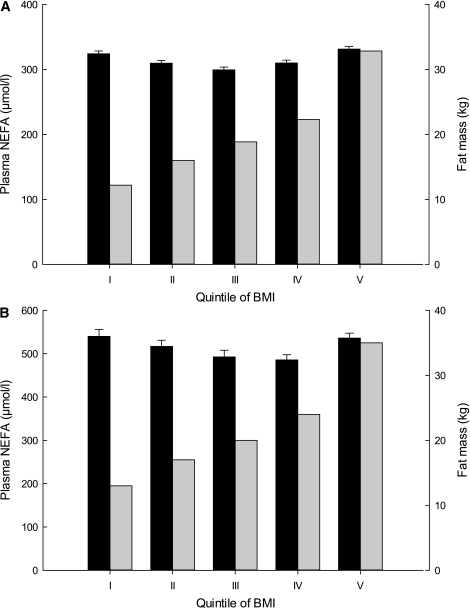
The largest study (n = 5,790) we are aware of in which the fasting NEFA concentration has been quantified along with anthropometric variables is the Paris Prospective Study (52). The results of this study were not included in the systematic review as the NEFA concentrations have never been reported in groups defined along anthropometric variables. Instead, the Paris Prospective Study reported the BMI in quintiles of NEFA concentrations, clearly showing almost no effect on BMI with rising NEFA concentration. However, we are very thankful to Beverley Balkau for providing us with the original data from the Paris Prospective Study aligned to make possible a comparison with both the Oxford Biobank (OBB; see below) and the data collected from the systematic review. We divided the data from the Paris Prospective Study participants into quintiles of BMI and calculated the mean NEFA concentration in each quintile. This clearly shows a complete absence of relationship between BMI and the fasting NEFA concentration (Fig. 2).
FIG. 2.
Relationships between plasma NEFA concentrations and BMI in two cohorts. Black bars, plasma NEFA concentrations; gray bars, fat mass. A: Data from the Paris Prospective Study (46), courtesy of Beverley Balkau, in 5,790 individuals. Fat mass has been estimated from BMI (61). B: Data from OBB for 1,591 individuals. In each case, the mean ± SE plasma NEFA concentration is shown for quintiles of BMI. The BMI quintiles were as follows: I: <22.5 kg/m2; II: 22.5–24.4 kg/m2; III: 24.4–26.4 kg/m2; IV: 26.4–29.2 kg/m2; V: >29.2 kg/m2.
NEFA CONCENTRATIONS IN GROUPS FROM A RANDOM NONDIABETIC POPULATION SAMPLE
Cross-sectional relationships.
Given the lack of association between plasma NEFA concentrations and measures of obesity in large-scale epidemiological studies, we asked whether more detailed phenotyping of fat mass would reveal the relationships that would be expected intuitively. For this purpose we analyzed data from the OBB, a population-based collection of data from healthy individuals in the Oxfordshire (U.K.) area aged 30–50 years (62). Overnight fasting plasma concentrations of NEFA and other metabolites/hormones were compared with anthropometric data.
Plasma NEFA concentrations ranged from 95 to 2,080 μmol/L (median 470). They were significantly higher in women than in men (medians 517 and 434 μmol/L, respectively, P = 2 × 10−14, Mann-Whitney test) as found in other studies (see above). Some univariate relationships for plasma NEFA concentrations are shown in Table 1. The relationships between fasting plasma NEFA and measures of adiposity (BMI, fat mass) were not strong in women and were absent in men. For illustration of the size of the effect, the relationship with fat mass in women accounts for 0.3% of the variance in fasting plasma NEFA concentration; that with insulin accounts for 0.8% of the variance in either variable. There were significant relationships with systolic blood pressure and, in women only, with diastolic blood pressure; these were only slightly weakened by adjustment for fat mass. A significant relationship with high-sensitivity C-reactive protein noted in women was lost when controlled for fat mass. The strongest relationships noted with plasma NEFA concentrations were for a metabolic product, 3-hydroxybutyrate; the relationship with NEFA explained 36% of the variance in 3-hydroxybutyrate concentrations (men and women combined).
TABLE 1.
Univariate relationships (Spearman’s rank correlation coefficient) with plasma NEFA concentrations in the Oxford BioBank
| NEFA with | BMI | Fat mass | WHR | Insulin | HOMA-IR | TG | 3-OHB | CRP | SBP | DBP |
|---|---|---|---|---|---|---|---|---|---|---|
| Males | ||||||||||
| rs | 0.057 | 0.031 | 0.034 | −0.028 | −0.025 | 0.017 | 0.570 | 0.045 | 0.157 | 0.050 |
| P | 0.094 | 0.362 | 0.323 | 0.409 | 0.466 | 0.616 | <0.001 | 0.264 | <0.001 | 0.141 |
| n | 859 | 859 | 856 | 859 | 859 | 859 | 857 | 627 | 859 | 859 |
| Females | ||||||||||
| rs | 0.091 | 0.081 | 0.043 | 0.086 | 0.094 | 0.142 | 0.654 | 0.116 | 0.132 | 0.126 |
| P | 0.007 | 0.017 | 0.209 | 0.012 | 0.006 | < 0.001 | <0.001 | 0.004 | <0.001 | <0.001 |
| n | 861 | 861 | 859 | 861 | 861 | 861 | 861 | 633 | 861 | 861 |
Samples were from 1,720 healthy individuals, median age 41 (range 2–53) years, median BMI 25 (range 15–48) kg/m2. 3-OHB, 3-hydroxybutyrate; DBP, diastolic blood pressure; HOMA-IR, homeostatic model assessment of insulin resistance; SBP, systolic blood pressure; WHR, waist-to-hip ratio.
Repeated measurements.
The lack of strong relationships between plasma NEFA concentrations and other commonly measured variables raises the question of the day-to-day reproducibility of NEFA concentrations within individuals. A carefully conducted study in which fasting samples were taken from healthy individuals on consecutive mornings suggested very high variability (within-person biological coefficient of variation 45%, compared with triglycerides 21% and glucose 5%) (63). That might imply that plasma NEFA concentrations generate almost random numbers. Magkos et al. (64) found better agreement between NEFA concentrations measured on separate occasions ≥2 weeks apart (coefficient of variation 24%). Our experience of repeated measurements of plasma NEFA concentrations has been that there is a clear within-person trend. In one study with measurements 30 days apart, the correlation coefficient between the two NEFA measurements was 0.70 (P < 0.001) (compared with triglycerides 0.87 and glucose 0.70) (65). In 81 OBB participants returning after a longer interval (1–5 years) the correlation coefficient between the two NEFA measurements was 0.35 (P = 0.002) (compared with triglycerides 0.64 and glucose 0.5, each P < 0.001).
FASTING NEFA RELEASE IN RELATION TO FAT MASS OR FASTING INSULIN
As noted above, the literature suggests that NEFA concentrations do not increase in proportion to fat mass, with a clear corollary that lipolysis per kilogram fat mass must be reduced in obesity. This has been repeatedly seen in studies of adipocytes from obese individuals in vitro (66,67) and is associated with downregulation of the expression of the key enzymes of fat mobilization, hormone-sensitive lipase and adipose triglyceride lipase (12,66,68,69).
This aspect of adipose tissue function in obesity has been studied with isotopic turnover techniques. Mittendorfer et al. (70) studied NEFA turnover in individuals with a range of BMIs (18–44 kg/m2). The systemic rate of appearance of NEFA, when expressed per kilogram fat mass, clearly decreased across a wide range of fat mass (correlation coefficient −0.81, P < 0.001). Although not highlighted by the authors, a similarly negative association was present (r = −0.64, P = 0.06, n = 8 [men]) when recalculating raw data presented in the very first article describing NEFA turnover in obesity (71). We have shown this also when comparing a group of abdominally obese men with lean control subjects over a 24-h period (12).
This downregulation of lipolysis per kilogram of fat mass will tend to normalize plasma NEFA concentrations in obese individuals. Some extreme examples are seen in the literature. Reeds et al. (72) studied extremely obese insulin-resistant young women compared with lean control subjects. Despite a sixfold difference in fat mass, basal NEFA concentrations were identical in the two groups. In our own studies, abdominally obese men with 2.5 times the adipose tissue mass of a lean control group had almost identical NEFA concentrations over a 24-h period (12).
However, it must be recognized that suppression of fat mobilization per kilogram adipose tissue may be counteracted by a large adipose tissue mass. In the study by Mittendorfer et al., the total rate of appearance of NEFA (whole-body) and the rate of appearance per kilogram fat-free mass were positively related to fat mass, i.e., overweight individuals may deliver more NEFA to their lean body mass. The relationship was shown over a very impressive range of fat masses (5–70 kg), but when studying the effect of NEFA delivery to lean mass within a normal fat mass range (20–40 kg), the increase in NEFA delivery was in the range of 15% for women and 25% in men in this study—albeit with a very considerable variation between individuals. Although there is an extremely close correlation between flux rate of NEFA and the systemic NEFA concentration in fasting humans (73), the former is the more important variable when it comes to evaluating fatty acid uptake by insulin sensitive tissues. It is certainly possible that the NEFA plasma concentration slightly underestimate the NEFA flux rate in obesity.
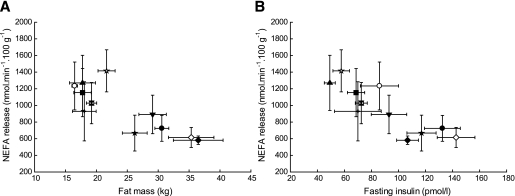
Upper-body subcutaneous fat is the largest contributor to the systemic NEFA pool (74). We therefore explored NEFA production rates further with a database of experiments in which we have directly measured fasting NEFA release from subcutaneous abdominal adipose tissue in volunteers using arteriovenous difference methodology in conjunction with measurement of adipose tissue blood flow (75). The individual studies considered are listed in Fig. 3. The subjects covered a range of metabolic phenotypes, from young, lean, healthy volunteers to middle-aged, overweight/obese, type 2 diabetic patients. When we plot the rate of NEFA release from subcutaneous abdominal adipose tissue (per 100 g tissue) against the total fat mass (kg) (Fig. 3A) we find, across the studies, a marked negative relationship—that is, as fat mass increases, so NEFA release per unit weight of adipose tissue decreases. There was a similar strong relationship when plotted against fasting insulin (Fig. 3B), suggesting that the hyperinsulinemia of insulin resistance, or long-term adaptation to hyperinsulinemia, are means by which fatty acid release is downregulated.
FIG. 3.
Relationships between NEFA release from subcutaneous abdominal adipose tissue and fat mass (A) and fasting insulin (B) in different groups of subjects. The NEFA release has been quantified with the same technique in separate group of subjects from previous studies in our laboratory using arteriovenous concentration differences across abdominal subcutaneous tissue multiplied by the tissue blood flow. Only data from men are shown. Open circle, healthy lean control subjects; solid circle, healthy obese subjects (62). Solid square, lean control subjects (80). Upward triangle, lean control subjects (81). Cross, lean control subjects (82). Square with hour-glass fill: healthy control subjects aged 31–59 years, BMI 22–28 kg/m2 (K. Manolopoulos, unpublished data). Open star, control subjects for insulin resistance study; solid star, men with insulin resistance (83). Downward triangle, healthy obese men (12). Open diamond, type 2 diabetic patients on rosiglitazone; solid diamond, same patients on placebo (84). Note that the different groups of lean control subjects had different characteristics (age, BMI) as detailed in the individual publications. For each group, mean values ± SE are shown.
NONFASTING NEFA CONCENTRATIONS
NEFA concentrations are normally at their highest in the fasting state, and because of the antilipolytic action of insulin released in response to ingestion of carbohydrates, the concentrations go down in the postprandial state. We will now examine the possibility that postprandial, diurnal, or nocturnal NEFA concentrations are elevated in obesity or insulin resistance despite the apparent absence of a difference with lean individuals in the fasted state as outlined above. The rationale for this supposition comes from the fact that adipose tissue could be seen as insulin resistant in obesity and therefore as not responding adequately to the antilipolytic action of insulin (29). The literature is limited in this area, and we have deliberately avoided studies in diabetes because that would inevitably involve a relative deficiency in insulin or its action. Golay et al. (76) studied diurnal NEFA concentrations in lean and obese individuals from the fasted state and over two meals. In that study the fasting NEFA concentration was 50% higher (P = 0.02) in the fasted state in obese individuals, which is more than expected from the systematic review above, and overall 30% higher (P = 0.02) in the 8-h postprandial state. However, the authors noted that “the postprandial declines in plasma FFA [NEFA] concentrations were similar, in both absolute and relative terms” in the two groups. Heptulla et al. (77) studied lean and very obese (3–4 × body fat content) teenaged girls and boys over a 24-h period under metabolic ward conditions with fixed caloric intake. NEFA concentrations over 24 h were higher in the obese children, but clear suppression of NEFA concentrations in response to feeding was seen in both lean and obese children. However, as both groups received a fixed daily 2,200 kcal diet, it could be speculated that the obese children were kept on the hypocaloric side compared with the lean children and that this could have been driving lipolysis in this group. We compared circadian NEFA profiles in lean and abdominally obese men over a 24-h period, finding no overall differences (12). A slightly reduced antilipolytic effect was seen after the breakfast meal in the obese group, but over the remaining 19 h there were no differences. The fat to carbohydrate composition of a diet could influence postprandial NEFA concentration. In a recent study by Hernandez et al. (78) comparing the metabolic effects of weight loss in groups on either a high-carbohydrate or a high-fat diet, the fasting NEFA concentration was not different between the groups whereas the postprandial and diurnal suppression of NEFA was almost completely abolished in the high-fat diet group, leading to drastically higher 24-h NEFA concentrations. Of note, in the same study conventional measures of insulin sensitivity (fasting glucose and fasting insulin concentrations) were unchanged in the high-fat diet group. Finally, Rosenthal and Woodside (79) reported on nocturnal NEFA concentrations comparing a group of young with a group of old equally nonobese men. The younger group had on average 20% higher (P < 0.05) nocturnal NEFA concentrations, while having clearly lower nocturnal insulin concentrations, the latter of which could be taken as a sign of higher insulin sensitivity. In summary, there is mixed evidence for the notion that obesity is associated raised postprandial, diurnal, or nocturnal NEFA concentrations. There is need for more studies in this area, and close attention must be given to the nutritional and methodological elements of such studies.
SUMMARY AND CONCLUSIONS
The idea that increased adipose tissue mass leads to elevated plasma NEFA concentrations, and these in turn to insulin resistance in insulin target tissues, is appealing and entrenched in the literature. However, many studies over several decades lend credence to a different picture. As adipose tissue mass expands, NEFA release per kilogram adipose tissue is downregulated, not increased. In many obese individuals, this can lead to normalization of plasma NEFA concentrations. Some elevation of NEFA concentrations is undeniable in certain groups of obese individuals and in type 2 diabetes, and tracer studies tend to show that NEFA delivery to nonfat tissues is increased even if plasma concentrations are not much raised. However, it is clear that insulin resistance, even severe insulin resistance, can exist in obesity without elevation of NEFA concentrations. It is also clear that elevated NEFA concentrations are not necessarily associated with insulin resistance. Two commonly seen examples are women versus men and younger versus older subjects. Women have very significantly raised NEFA concentrations compared with men, yet tend to be more insulin-sensitive and to have better lipid profiles.
We believe that more dynamic studies are needed to clarify relationships between obesity and fatty acid kinetics. We suggest that clamp studies may be misleading when applied to NEFA dynamics because in everyday life plasma insulin concentrations are elevated in insulin resistance, and so-called insulin resistance of adipose tissue lipolysis may simply reflect an adaptation to consistently high ambient insulin concentrations. In the absence of a convincing demonstration of excessive NEFA delivery from adipose tissue in the obese state to explain insulin resistance, more emphasis should be put on alternative explanations such as impaired adipose tissue fat storage (12) or dysfunctional regulation of adipokines or adipose-related inflammatory cytokines. We propose that it is time to reevaluate the relationships between adiposity, fatty acids, and insulin resistance.
ACKNOWLEDGMENTS
This study was supported by the Wellcome Trust.
No potential conflicts of interest relevant to this article were reported.
J.R.D. assembled and analyzed data. F.K. and K.N.F. further interpreted data and wrote the manuscript.
The authors thank Sandy Humphreys (OCDEM, University of Oxford, Oxford, U.K.) for assistance as well as their many colleagues who contributed to the data presented. They would like to thank previous and current fellows in their group who have developed protocol and conducted studies helping them to formulate the ideas contained in this review. The authors would like to thank Beverley Balkau (CESP, INSERM U1018, Paris, France) for the valuable reanalysis of the Paris Prospective Study.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db11-0425/-/DC1.
REFERENCES
- 1.Frayn KN, Coppack SW. Insulin resistance, adipose tissue and coronary heart disease. Clin Sci (Lond) 1992;82:1–8 [DOI] [PubMed] [Google Scholar]
- 2.Frayn KN, Williams CM, Arner P. Are increased plasma non-esterified fatty acid concentrations a risk marker for coronary heart disease and other chronic diseases? Clin Sci (Lond) 1996;90:243–253 [DOI] [PubMed] [Google Scholar]
- 3.Dole VP. A relation between non-esterified fatty acids in plasma and the metabolism of glucose. J Clin Invest 1956;35:150–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gordon RS., Jr Unesterified fatty acid in human blood plasma. II. The transport function of unesterified fatty acid. J Clin Invest 1957;36:810–815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gordon RS, Jr, Cherkes A. Unesterified fatty acid in human blood plasma. J Clin Invest 1956;35:206–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eaton RP, Berman M, Steinberg D. Kinetic studies of plasma free fatty acid and triglyceride metabolism in man. J Clin Invest 1969;48:1560–1579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nielsen S, Guo Z, Johnson CM, Hensrud DD, Jensen MD. Splanchnic lipolysis in human obesity. J Clin Invest 2004;113:1582–1588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lafontan M, Langin D. Lipolysis and lipid mobilization in human adipose tissue. Prog Lipid Res 2009;48:275–297 [DOI] [PubMed] [Google Scholar]
- 9.Evans K, Burdge GC, Wootton SA, Clark ML, Frayn KN. Regulation of dietary fatty acid entrapment in subcutaneous adipose tissue and skeletal muscle. Diabetes 2002;51:2684–2690 [DOI] [PubMed] [Google Scholar]
- 10.Heimberg M, Dunn GD, Wilcox G. The derivation of plasma-free fatty acids from dietary neutral fat in man. J Lab Clin Med 1974;83:393–402 [PubMed] [Google Scholar]
- 11.Fielding BA, Callow J, Owen RM, Samra JS, Matthews DR, Frayn KN. Postprandial lipemia: the origin of an early peak studied by specific dietary fatty acid intake during sequential meals. Am J Clin Nutr 1996;63:36–41 [DOI] [PubMed] [Google Scholar]
- 12.McQuaid SE, Hodson L, Neville MJ, et al. Downregulation of adipose tissue fatty acid trafficking in obesity: a driver for ectopic fat deposition? Diabetes 2011;60:47–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miles JM, Nelson RH. Contribution of triglyceride-rich lipoproteins to plasma free fatty acids. Horm Metab Res 2007;39:726–729 [DOI] [PubMed] [Google Scholar]
- 14.Samra JS, Clark ML, Humphreys SM, Macdonald IA, Frayn KN. Regulation of lipid metabolism in adipose tissue during early starvation. Am J Physiol 1996;271:E541–E546 [DOI] [PubMed] [Google Scholar]
- 15.Ruge T, Hodson L, Cheeseman J, et al. Fasted to fed trafficking of Fatty acids in human adipose tissue reveals a novel regulatory step for enhanced fat storage. J Clin Endocrinol Metab 2009;94:1781–1788 [DOI] [PubMed] [Google Scholar]
- 16.Singer P, Gödicke W, Voigt S, Hajdu I, Weiss M. Postprandial hyperinsulinemia in patients with mild essential hypertension. Hypertension 1985;7:182–186 [DOI] [PubMed] [Google Scholar]
- 17.Reaven GM, Hollenbeck C, Jeng C-Y, Wu MS, Chen Y-DI. Measurement of plasma glucose, free fatty acid, lactate, and insulin for 24 h in patients with NIDDM. Diabetes 1988;37:1020–1024 [DOI] [PubMed] [Google Scholar]
- 18.Kruszynska YT, Munro J, Home PD, McIntyre N. Twenty-four hour C-peptide and insulin secretion rates and diurnal profiles of glucose, lipids and intermediary metabolites in cirrhosis. Clin Sci (Lond) 1992;83:597–605 [DOI] [PubMed] [Google Scholar]
- 19.Bakewell L, Burdge GC, Calder PC. Polyunsaturated fatty acid concentrations in young men and women consuming their habitual diets. Br J Nutr 2006;96:93–99 [DOI] [PubMed] [Google Scholar]
- 20.Shadid S, Kanaley JA, Sheehan MT, Jensen MD. Basal and insulin-regulated free fatty acid and glucose metabolism in humans. Am J Physiol Endocrinol Metab 2007;292:E1770–E1774 [DOI] [PubMed] [Google Scholar]
- 21.Magkos F, Patterson BW, Mohammed BS, Klein S, Mittendorfer B. Women produce fewer but triglyceride-richer very low-density lipoproteins than men. J Clin Endocrinol Metab 2007;92:1311–1318 [DOI] [PubMed] [Google Scholar]
- 22.Stefan N, Kantartzis K, Celebi N, et al. Circulating palmitoleate strongly and independently predicts insulin sensitivity in humans. Diabetes Care 2010;33:405–407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Soeters MR, Sauerwein HP, Groener JE, et al. Sex-related differences in the metabolic response to fasting. J Clin Endocrinol Metab 2007;92:3646–3652 [DOI] [PubMed] [Google Scholar]
- 24.Marinou K, Adiels M, Hodson L, Frayn KN, Karpe F, Fielding BA. Young women partition fatty acids towards ketone body production rather than VLDL-TAG synthesis, compared with young men. Br J Nutr 2011;105:857–865 [DOI] [PubMed] [Google Scholar]
- 25.Schlierf G, Dorow E. Diurnal patterns of triglycerides, free fatty acids, blood sugar, and insulin during carbohydrate-induction in man and their modification by nocturnal suppression of lipolysis. J Clin Invest 1973;52:732–740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Taggart P, Carruthers M. Endogenous hyperlipidaemia induced by emotional stress of racing driving. Lancet 1971;1:363–366 [DOI] [PubMed] [Google Scholar]
- 27.Hodgetts V, Coppack SW, Frayn KN, Hockaday TDR. Factors controlling fat mobilization from human subcutaneous adipose tissue during exercise. J Appl Physiol 1991;71:445–451 [DOI] [PubMed] [Google Scholar]
- 28.Romijn JA, Coyle EF, Sidossis LS, et al. Regulation of endogenous fat and carbohydrate metabolism in relation to exercise intensity and duration. Am J Physiol 1993;265:E380–E391 [DOI] [PubMed] [Google Scholar]
- 29.Groop LC, Bonadonna RC, DelPrato S, et al. Glucose and free fatty acid metabolism in non-insulin-dependent diabetes mellitus. Evidence for multiple sites of insulin resistance. J Clin Invest 1989;84:205–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sørensen LP, Andersen IR, Søndergaard E, et al. Basal and insulin mediated VLDL-triglyceride kinetics in type 2 diabetic men. Diabetes 2011;60:88–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Randle PJ, Garland PB, Hales CN, Newsholme EA. The glucose fatty-acid cycle. Its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet 1963;1:785–789 [DOI] [PubMed] [Google Scholar]
- 32.Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet 2005;365:1415–1428 [DOI] [PubMed] [Google Scholar]
- 33.Boden G, Chen X, Ruiz J, White JV, Rossetti L. Mechanisms of fatty acid-induced inhibition of glucose uptake. J Clin Invest 1994;93:2438–2446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shah PV, Vella A, Basu A, et al. Effects of free fatty acids and glycerol on splanchnic glucose metabolism and insulin extraction in nondiabetic humans. Diabetes 2002;51:301–310 [DOI] [PubMed] [Google Scholar]
- 35.Roden M, Stingl H, Chandramouli V, et al. Effects of free fatty acid elevation on postabsorptive endogenous glucose production and gluconeogenesis in humans. Diabetes 2000;49:701–707 [DOI] [PubMed] [Google Scholar]
- 36.Staehr P, Hother-Nielsen O, Landau BR, Chandramouli V, Holst JJ, Beck-Nielsen H. Effects of free fatty acids per se on glucose production, gluconeogenesis, and glycogenolysis. Diabetes 2003;52:260–267 [DOI] [PubMed] [Google Scholar]
- 37.Kruszynska YT, Mulford MI, Yu JG, Armstrong DA, Olefsky JM. Effects of nonesterified fatty acids on glucose metabolism after glucose ingestion. Diabetes 1997;46:1586–1593 [DOI] [PubMed] [Google Scholar]
- 38.Wiesenthal SR, Sandhu H, McCall RH, et al. Free fatty acids impair hepatic insulin extraction in vivo. Diabetes 1999;48:766–774 [DOI] [PubMed] [Google Scholar]
- 39.Fielding BA, Humphreys SM, Allman RFC, Frayn KN. Mono-, di- and triacylglycerol concentrations in human plasma: effects of heparin injection and of a high-fat meal. Clin Chim Acta 1993;216:167–173 [DOI] [PubMed] [Google Scholar]
- 40.Friedberg SJ, Klein RF, Trout DL, Bogdonoff MD, Estes EHJ., Jr The incorporation of plasma free fatty acids into plasma triglycerides in man. J Clin Invest 1961;40:1846–1855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hodson L, Bickerton AS, McQuaid SE, et al. The contribution of splanchnic fat to VLDL triglyceride is greater in insulin-resistant than insulin-sensitive men and women: studies in the postprandial state. Diabetes 2007;56:2433–2441 [DOI] [PubMed] [Google Scholar]
- 42.Nestel PJ. Relationship between FFA flux and TGFA influx in plasma before and during the infusion of insulin. Metabolism 1967;16:1123–1132 [DOI] [PubMed] [Google Scholar]
- 43.Steinberg HO, Paradisi G, Hook G, Crowder K, Cronin J, Baron AD. Free fatty acid elevation impairs insulin-mediated vasodilation and nitric oxide production. Diabetes 2000;49:1231–1238 [DOI] [PubMed] [Google Scholar]
- 44.Florian JP, Pawelczyk JA. Non-esterified fatty acids increase arterial pressure via central sympathetic activation in humans. Clin Sci (Lond) 2010;118:61–69 [DOI] [PubMed] [Google Scholar]
- 45.Paolisso G, Tataranni PA, Foley JE, Bogardus C, Howard BV, Ravussin E. A high concentration of fasting plasma non-esterified fatty acids is a risk factor for the development of NIDDM. Diabetologia 1995;38:1213–1217 [DOI] [PubMed] [Google Scholar]
- 46.Charles MA, Eschwège E, Thibult N, et al. The role of non-esterified fatty acids in the deterioration of glucose tolerance in Caucasian subjects: results of the Paris Prospective Study. Diabetologia 1997;40:1101–1106 [DOI] [PubMed] [Google Scholar]
- 47.Pankow JS, Duncan BB, Schmidt MI, et al. ; Atherosclerosis Risk in Communities Study Fasting plasma free fatty acids and risk of type 2 diabetes: the atherosclerosis risk in communities study. Diabetes Care 2004;27:77–82 [DOI] [PubMed] [Google Scholar]
- 48.Il’yasova D, Wang F, D’Agostino RB, Jr, Hanley A, Wagenknecht LE. Prospective association between fasting NEFA and type 2 diabetes: impact of post-load glucose. Diabetologia 2010;53:866–874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Byrne CD, Maison P, Halsall D, Martensz N, Hales CN, Wareham NJ. Cross-sectional but not longitudinal associations between non-esterified fatty acid levels and glucose intolerance and other features of the metabolic syndrome. Diabet Med 1999;16:1007–1015 [DOI] [PubMed] [Google Scholar]
- 50.Pirro M, Mauriège P, Tchernof A, et al. Plasma free fatty acid levels and the risk of ischemic heart disease in men: prospective results from the Québec Cardiovascular Study. Atherosclerosis 2002;160:377–384 [DOI] [PubMed] [Google Scholar]
- 51.Pilz S, Scharnagl H, Tiran B, et al. Free fatty acids are independently associated with all-cause and cardiovascular mortality in subjects with coronary artery disease. J Clin Endocrinol Metab 2006;91:2542–2547 [DOI] [PubMed] [Google Scholar]
- 52.Charles MA, Fontbonne A, Thibult N, et al. High plasma nonesterified fatty acids are predictive of cancer mortality but not of coronary heart disease mortality: results from the Paris Prospective Study. Am J Epidemiol 2001;153:292–298 [DOI] [PubMed] [Google Scholar]
- 53.Fagot-Campagna A, Balkau B, Simon D, et al. High free fatty acid concentration: an independent risk factor for hypertension in the Paris Prospective Study. Int J Epidemiol 1998;27:808–813 [DOI] [PubMed] [Google Scholar]
- 54.Jouven X, Charles M-A, Desnos M, Ducimetière P. Circulating nonesterified fatty acid level as a predictive risk factor for sudden death in the population. Circulation 2001;104:756–761 [DOI] [PubMed] [Google Scholar]
- 55.Yki-Järvinen H. Ectopic fat accumulation: an important cause of insulin resistance in humans. J R Soc Med 2002;95(Suppl. 42):39–45 [PMC free article] [PubMed] [Google Scholar]
- 56.Holloway GP, Luiken JJ, Glatz JF, Spriet LL, Bonen A. Contribution of FAT/CD36 to the regulation of skeletal muscle fatty acid oxidation: an overview. Acta Physiol (Oxf) 2008;194:293–309 [DOI] [PubMed] [Google Scholar]
- 57.Unger RH. Lipotoxicity in the pathogenesis of obesity-dependent NIDDM. Genetic and clinical implications. Diabetes 1995;44:863–870 [DOI] [PubMed] [Google Scholar]
- 58.Bachmann OP, Dahl DB, Brechtel K, et al. Effects of intravenous and dietary lipid challenge on intramyocellular lipid content and the relation with insulin sensitivity in humans. Diabetes 2001;50:2579–2584 [DOI] [PubMed] [Google Scholar]
- 59.Kelley DE. Skeletal muscle fat oxidation: timing and flexibility are everything. J Clin Invest 2005;115:1699–1702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Opie LH, Walfish PG. Plasma free fatty acid concentrations in obesity. N Engl J Med 1963;268:757–760 [DOI] [PubMed] [Google Scholar]
- 61.James WPT. Research on Obesity: a report of the DHSS/MRC Group. London, Her Majesty’s Stationery Office, 1976 [Google Scholar]
- 62.Tan GD, Neville MJ, Liverani E, et al. The in vivo effects of the Pro12Ala PPARgamma2 polymorphism on adipose tissue NEFA metabolism: the first use of the Oxford Biobank. Diabetologia 2006;49:158–168 [DOI] [PubMed] [Google Scholar]
- 63.Widjaja A, Morris RJ, Levy JC, Frayn KN, Manley SE, Turner RC. Within- and between-subject variation in commonly measured anthropometric and biochemical variables. Clin Chem 1999;45:561–566 [PubMed] [Google Scholar]
- 64.Magkos F, Patterson BW, Mittendorfer B. Reproducibility of stable isotope-labeled tracer measures of VLDL-triglyceride and VLDL-apolipoprotein B-100 kinetics. J Lipid Res 2007;48:1204–1211 [DOI] [PubMed] [Google Scholar]
- 65.Hodson L, Harnden KE, Roberts R, Dennis AL, Frayn KN. Does the DASH diet lower blood pressure by altering peripheral vascular function? J Hum Hypertens 2010;24:312–319 [DOI] [PubMed] [Google Scholar]
- 66.Large V, Reynisdottir S, Langin D, et al. Decreased expression and function of adipocyte hormone-sensitive lipase in subcutaneous fat cells of obese subjects. J Lipid Res 1999;40:2059–2066 [PubMed] [Google Scholar]
- 67.Hellström L, Reynisdottir S. Influence of heredity for obesity on adipocyte lipolysis in lean and obese subjects. Int J Obes Relat Metab Disord 2000;24:340–344 [DOI] [PubMed] [Google Scholar]
- 68.Langin D, Dicker A, Tavernier G, et al. Adipocyte lipases and defect of lipolysis in human obesity. Diabetes 2005;54:3190–3197 [DOI] [PubMed] [Google Scholar]
- 69.Jocken JW, Langin D, Smit E, et al. Adipose triglyceride lipase and hormone-sensitive lipase protein expression is decreased in the obese insulin-resistant state. J Clin Endocrinol Metab 2007;92:2292–2299 [DOI] [PubMed] [Google Scholar]
- 70.Mittendorfer B, Magkos F, Fabbrini E, Mohammed BS, Klein S. Relationship between body fat mass and free fatty acid kinetics in men and women. Obesity (Silver Spring) 2009;17:1872–1877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nestel PJ, Whyte HM. Plasma free fatty acid and triglyceride turnover in obesity. Metabolism 1968;17:1122–1128 [DOI] [PubMed] [Google Scholar]
- 72.Reeds DN, Stuart CA, Perez O, Klein S. Adipose tissue, hepatic, and skeletal muscle insulin sensitivity in extremely obese subjects with acanthosis nigricans. Metabolism 2006;55:1658–1663 [DOI] [PubMed] [Google Scholar]
- 73.Hagenfeldt L. Turnover of individual free fatty acids in man. Fed Proc 1975;34:2246–2249 [PubMed] [Google Scholar]
- 74.Jensen MD. Adipose tissue and fatty acid metabolism in humans. J R Soc Med 2002;95(Suppl. 42):3–7 [PMC free article] [PubMed] [Google Scholar]
- 75.Bickerton AS, Roberts R, Fielding BA, et al. Preferential uptake of dietary fatty acids in adipose tissue and muscle in the postprandial period. Diabetes 2007;56:168–176 [DOI] [PubMed] [Google Scholar]
- 76.Golay A, Swislocki AL, Chen Y-DI, Jaspan JB, Reaven GM. Effect of obesity on ambient plasma glucose, free fatty acid, insulin, growth hormone, and glucagon concentrations. J Clin Endocrinol Metab 1986;63:481–484 [DOI] [PubMed] [Google Scholar]
- 77.Heptulla R, Smitten A, Teague B, Tamborlane WV, Ma Y-Z, Caprio S. Temporal patterns of circulating leptin levels in lean and obese adolescents: relationships to insulin, growth hormone, and free fatty acids rhythmicity. J Clin Endocrinol Metab 2001;86:90–96 [DOI] [PubMed] [Google Scholar]
- 78.Hernandez TL, Sutherland JP, Wolfe P, et al. Lack of suppression of circulating free fatty acids and hypercholesterolemia during weight loss on a high-fat, low-carbohydrate diet. Am J Clin Nutr 2010;91:578–585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rosenthal MJ, Woodside WF. Nocturnal regulation of free fatty acids in healthy young and elderly men. Metabolism 1988;37:645–648 [DOI] [PubMed] [Google Scholar]
- 80.Tan GD, Savage DB, Fielding BA, et al. Fatty acid metabolism in patients with PPARgamma mutations. J Clin Endocrinol Metab 2008;93:4462–4470 [DOI] [PubMed] [Google Scholar]
- 81.McQuaid SE, Humphreys SM, Hodson L, Fielding BA, Karpe F, Frayn KN. Femoral adipose tissue may accumulate the fat that has been recycled as VLDL and nonesterified fatty acids. Diabetes 2010;59:2465–2473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Robertson MD, Bickerton AST, Dennis AL, Vidal H, Frayn KN. Insulin-sensitizing effects of dietary resistant starch and effects on skeletal muscle and adipose tissue metabolism. Am J Clin Nutr 2005;82:559–567 [DOI] [PubMed] [Google Scholar]
- 83.Bickerton AST, Roberts R, Fielding BA, et al. Adipose tissue fatty acid metabolism in insulin-resistant men. Diabetologia 2008;51:1466–1474 [DOI] [PubMed] [Google Scholar]
- 84.Tan GD, Fielding BA, Currie JM, et al. The effects of rosiglitazone on fatty acid and triglyceride metabolism in type 2 diabetes. Diabetologia 2005;48:83–95 [DOI] [PubMed] [Google Scholar]