Abstract
OBJECTIVE
This study addressed the hypothesis that placental endothelial lipase (EL) expression is affected by pregnancies complicated by obesity and gestational diabetes mellitus (GDM).
RESEARCH DESIGN AND METHODS
EL expression in placental tissues from pregnancies complicated by obesity, GDM, or obesity combined with GDM (obese-GDM) was analyzed by quantitative RT-PCR. Moreover, primary placental cells were isolated and treated with insulin, glucose, leptin, or tumor necrosis factor (TNF)-α, and EL expression was measured. Inhibitors of nuclear factor (NF)-κB or mitogen-activated protein kinase (MAPK) signaling were used to detect potential pathways of EL regulation in primary placental endothelial cells (ECs).
RESULTS
In placentas from obese-GDM pregnancies, EL expression was upregulated by 1.9-fold (P < 0.05) compared with lean pregnancies, whereas obesity or GDM alone had no significant effect. Analyses of metabolic parameters in maternal venous and umbilical venous plasma revealed significantly increased insulin and leptin as well as slightly increased glucose and TNF-α values in the obese and obese-GDM groups. Cell culture experiments identified TNF-α and leptin, but not glucose or insulin, as regulators of EL expression in ECs. Induction of EL expression by these mediators occurred in a para/endocrine manner, since only leptin and TNF-α receptors, but not the cytokines themselves, were expressed in ECs. Inhibitor experiments suggested that TNF-α and leptin-mediated upregulation of EL may occur via two different routes. Whereas TNF-α induced EL upregulation in ECs by activation of the NF-κB pathway, leptin did not stimulate NF-κB or MAPK signaling pathways in these cells.
CONCLUSIONS
Metabolic inflammation with high leptin and locally increased TNF-α concentrations at the fetal-placental interface regulates placental EL expression.
During the last third of human gestation, not only fetal growth rate but also fetal fat accretion reaches its maximum. The rising fetal fat demand is primarily covered by maternal fat depots, which become available via enhanced lipolytic activity in maternal adipose tissue, leading to the development of maternal/gestational hyperlipidemia during the third trimester (1–3). Mobilized lipids are transported to the placenta primarily as lipoproteins. At the materno-placental interface, they are either taken up as holoparticles by their respective lipoprotein receptors (4–8) or undergo enzyme-dependent hydrolysis. Triglyceride hydrolase activities were initially detected in microvillous membrane preparations from term placental villous tissue (9,10), but these activities were, however, not attributed to specific lipases.
Subsequent studies suggested members of the triglyceride lipase gene (TLG) family as putative candidate lipases capable of extracellular hydrolysis and release of lipoprotein-associated fatty acids at the materno-placental interface. Members of the TLG family cover a broad range of substrate specificity, including triglyceride and phospholipase activity. They are secreted glycoproteins that bind to heparan sulfate proteoglycans on the surface of epithelial and endothelial cells (ECs) (11–13). Thus, close contact to passing lipoproteins enables bridging between the cell surface and the lipoproteins. By their phospholipase and triglyceride lipase activity, fatty acids are released from bridged lipoproteins and are provided for cellular uptake.
TLG family members show considerable sequence homologies and conserved domains (14). Among the TLG family, endothelial lipase (EL) and lipoprotein lipase (LPL) were described in the human placenta (14–16). Whereas LPL is a triglyceride hydrolase, EL is primarily a phospholipase with predominant phospholipase A1 activity and has a restricted ability to release sn-2–bound unsaturated fatty acids from phospholipids (17). In addition to its phospholipase activity, EL is also capable of hydrolyzing triglycerides, since the ratio of triglyceride lipase to phospholipase activity of EL was determined as 0.65 (18).
Aberrant hydrolysis of maternal lipoprotein-borne lipids at the materno-placental interface may be linked with pregnancy pathologies, such as fetal growth restriction or diabetes. Triglyceride hydrolase activity, measured in microvillous plasma membrane preparations at pH 8.0, was decreased by 27% in fetal growth restriction cases, compared with respective control preparations (19). Previous quantitative mRNA expression analyses showed that both EL and LPL expression was significantly dysregulated in fetal growth restriction placentas at term (15,20). According to that, LPL expression was significantly increased, whereas EL expression was significantly downregulated in fetal growth restriction placentas compared with control.
Dysregulated EL mRNA expression was also detected in placentas from pregnancies complicated by type 1 diabetes under suboptimal metabolic control (16). The pathophysiology of type 1 diabetes is different from obesity and gestational diabetes mellitus (GDM). Insulin resistance is one of the common features leading to maternal metabolic oversupply. This result may affect placental lipid handling, ultimately resulting in changes of fetal growth and fat accretion. Therefore, this study tested the hypothesis that GDM in obese mothers affects placental EL expression during gestation.
RESEARCH DESIGN AND METHODS
Subjects and tissues.
The protocol was approved by the hospital institutional review board, and written informed consent was obtained from each subject before study entry. Recruitment of lean (BMI <25 kg/m2) and overweight or obese (BMI >25 kg/m2) healthy women with a singleton term pregnancy took place at the time of admission for elective cesarean delivery at term (37–40 weeks), with no clinical evidence of infection. Pregnancies complicated by hypertension, preeclampsia, metabolic disease, steroid treatment, AIDS, alcohol abuse, and/or drug abuse were excluded. Smoking was recorded. GDM was defined as an abnormal glucose tolerance during the third trimester according to the criteria defined by Carpenter and Coustan (21). All GDM subjects required dietary regimen or insulin therapy for glucose control. Maternal blood was obtained on admission to labor and delivery, before placement of an intravenous line for hydration. Umbilical venous blood was drawn via syringe from the double-clamped cord immediately after delivery of the placenta. Plasma was separated by centrifugation and kept frozen at −20°C for glucose, insulin, and cytokine assays. Insulin resistance was estimated using the homeostasis model assessment–insulin resistance (HOMA-IR), i.e., fasting plasma glucose × fasting plasma insulin/22.5 (22). Neonatal body composition measurements were estimated using skinfolds and were obtained within 24 h from delivery by one examiner experienced in technique (23). From a subset of these pregnancies, placentas were collected for further studies. The subset was randomly chosen from the study populations. Placentas were weighed after trimming the umbilical cord and fetal membranes. Thereafter, pieces (1 × 1 × 1 cm) of placental tissue were excised from areas in cotyledons free of infarcts and calcifications. They were collected on ice and snap-frozen for subsequent gene expression analysis. In addition, placental RNA from lean GDM mothers was taken from archival material. Collection of placental tissue and RNA isolation followed the same protocols as for the other groups.
Isolation of placental cells.
Primary term trophoblasts (TTs) and ECs were isolated from term placentas after healthy pregnancy and vaginal delivery following standard protocols. Placental ECs were isolated from term placentas after healthy pregnancy and vaginal delivery, as described previously (24,25). All primary cell isolations were scrutinized for identity and purity. TTs were used only at a purity of >98%; ECs were used at a purity of >99% and only between passages 4 and 6 to avoid phenotypic drift.
Culture of isolated TTs and ECs.
After isolation, TTs were cultured in Dulbecco’s modified Eagle’s medium (Gibco, Paisley, U.K.) with 10% FCS (vol/vol), 100 mg/mL streptomycin, and 100 IU/mL penicillin (Gibco). A representative proportion of trophoblasts was characterized by immunohistochemistry (24,26). ECs were cultured in endothelial basal medium (Clonetics; Cambrex, Walkersville, MD) supplemented with the EGM-MV BulletKit (Clonetics). They were cultured in 1% (vol/vol) gelatin-coated flasks or plates, respectively. Purity was tested by staining for the classic endothelial marker, von Willebrand factor, and for vimentin to identify cells of other origin. Primary cells were cultured at 37°C in a humidified atmosphere containing 5% CO2 in air.
Culture of placental primary cells with insulin, glucose, leptin, tumor necrosis factor-α, BAY and UO126.
TTs and ECs (50,000 cells per well) were seeded in a gelatin (1%)-coated 12-well plate and cultured for 24 h in their appropriate culture medium. Thereafter, either insulin (10 nmol/L), d-glucose (25 mmol/L), leptin (1 μg/mL), or tumor necrosis factor (TNF)-α (20 ng/mL) was supplemented to the media, and cultures were run for another 24 h. For dose-response experiments, cells were prepared the same way, but 1 day after seeding, increasing concentrations of TNF-α (2 pg/mL, 2 ng/mL, and 20 ng/mL) or leptin (50 ng/mL, 0.5 μg/mL, and 1 μg/mL) were added to the culture media.
For inhibitor experiments, ECs were serum-starved for 5 h before experiment start and preincubated for 2 h with or without inhibitors of nuclear factor (NF)-κB signaling (BAY 11–7082, 10 μmol/L; Calbiochem, an affiliate of Merck, Darmstadt, Germany) or mitogen-activated protein kinase (MAPK) signaling (UO126, 10 μmol/L; Calbiochem), respectively. Preincubation was followed by treatment of ECs with leptin (1 μg/mL; Sigma, St. Louis, MO), endotoxin-free TNF-α (20 ng/mL; Sigma), or vehicle control (DMSO) for 24 h in the presence or absence of BAY, UO126, or vehicle alone. Total RNA was isolated from cells with Tri Reagent (Molecular Research Center, Cincinnati, OH) according to the manufacturer’s instructions. All experiments were run in triplicate and repeated with five different cell preparations.
Real-time RT-PCR.
Total RNA from placental tissues and placental ECs was isolated with Tri Reagent (Molecular Research Center) as described previously (15). Thereafter, RNA was reversely transcribed to cDNA, using a High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA) according to the manufacturer’s instructions. In brief, 2 μg total RNA of each sample was mixed with the kit components in a total reaction volume of 20 μL and incubated for 10 min at 25°C, 120 min at 37°C, and 5 s at 85°C in a thermocycler. cDNA of each reaction was subsequently subjected to quantitative real-time PCR using carboxy-fluorescein dye (FAM)-labeled TaqMan Gene Expression Assays (EL: Hs00195812_m1; RPL30: Hs00265497_m1; CD34: Hs02576480_m1) and the TaqMan Universal PCR Mastermix (Applied Biosystems). Components were mixed according to the manufacturer’s instructions and amplified in 10 μL total volume/well (384 well plates; Roche, Mannheim, Germany) using a LightCycler 480 (Roche). Cycle threshold (Ct) values were automatically generated by the LightCycler 480 Software (Roche), and relative quantification of gene expression was calculated by standard ΔΔCt method using the expression of ribosomal protein L30 (RPL30) as a reference. For analysis of EL expression in placental tissue, the endothelial cell marker CD34 served as a reference. This allowed consideration of vascular changes, i.e., hypo- or hypervascularization, in pathological placentas. To rule out the possibility that infiltrated hematopoietic CD34-positive cells in pathologic placentas could have confounded the CD34 measurements, immunohistochemistry for CD34 was performed using monoclonal mouse anti-human CD34 (1:1,000, QBEnd10; DAKO, Glostrup, Denmark) and the Ultravision LP detection system (Thermo Scientific, Fremont, CA) according to the procedures previously described (15). CD34 staining was limited to the placental endothelium, i.e., neither blood cells nor any other cell within the connective tissue was labeled (not shown).
Microarray analysis of placental primary cell RNA.
Total RNA from ECs, isolated from 10 different placentas, was pooled after a quality check on a Bioanalyzer, and 5 μg RNA was prepared for hybridization as described previously (27). For expression analysis, cRNA was hybridized against Affymetrix (Santa Clara, CA) HU133A chips according to the manufacturer’s instructions. Raw data were normalized globally and processed with Microarray Suite (version 5.0) and Data Mining Tool (Affymetrix) software. Annotations were obtained from NetAffx (Affymetrix), and the data were screened for expression of leptin, TNF-α, leptin receptor, and TNF-α receptors.
Plasma assays.
Plasma glucose was assessed by the glucose oxidase method (Yellow Springs Instruments, Yellow Springs, OH). Plasma insulin was measured using a radioimmunoassay kit (Linco, St. Charles, MO). Leptin was measured using ELISA kits (Linco) with intra-assays coefficients of variation (CVs) of 3.0–6.2 and 6.2–8.4%. Plasma TNF-α was assayed by ELISA (Quantiglo R&D System, Minneapolis, MN) with the following CVs: 5.3–7.8 and 2.6–3.4%. All plasma samples were run in duplicate. The insulin resistance indices were calculated according to HOMA-IR: fasting plasma insulin (μU/mL) × fasting glucose (mmol/L)/22.5.
Statistical analysis.
All values are presented as mean ± SD or in the figures as mean ± SEM. Differences between dependent variables were examined with one-way or two-way repeated-measures ANOVA. Statistically significant mean differences were identified with a Fisher protected least significant difference post hoc test. The relationship between maternal and fetal insulin sensitivity was estimated using univariate correlation analysis. In multivariate linear regression analyses, the association between BMI status (lean/obese) and GDM (yes/no) with ΔCt was studied. Possible interaction between BMI and GDM was tested by entering the interaction term in the model as covariate and was considered significant if P < 0.10. The data were analyzed using the SPSS version 18 and Statview II statistical package (Abacus Concepts, Berkeley, CA). Statistical significance was set at P < 0.05.
RESULTS
Effect of obesity and GDM on EL expression in human term placenta.
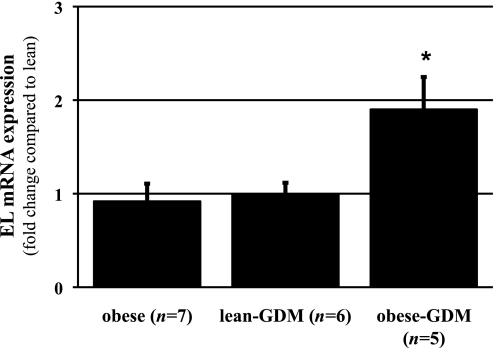
EL expression levels were analyzed in placental tissues from normal pregnancies and pregnancies complicated by either obesity or GDM (lean-GDM) or obesity combined with GDM (obese-GDM). Because previous data showed that placental EL is predominantly expressed in ECs of villous vessels at term (15), the expression of the endothelial cell marker CD34 was chosen for normalization. This way, alterations in terms of tissue composition, i.e., hypo- or hypervascularization in pathological placentas (28), were considered in the expression analysis. In obese-GDM, EL expression was 1.9-fold (± 0.35, P < 0.05) increased compared with lean, whereas obesity or lean-GDM alone had no significant effect on EL expression (Fig. 1). Correlation analysis between placental EL expression and maternal BMI, fetal weight, or placental weight, respectively, revealed no significant association.
FIG. 1.
Quantitative real-time RT-PCR analysis of EL expression in term placenta tissue from normal, obese, lean-GDM, and obese-GDM pregnancies. In obese-GDM pregnancies (n = 5), placental EL expression was 1.9-fold (± 0.35) increased compared with normal (lean, n = 8), whereas pregnancies with maternal obesity (n = 7) and placentas from lean-GDM women (n = 6) showed no difference compared with lean control subjects. Expression of placental EL was normalized to the expression of the endothelial cell marker CD34. Data are means ± SEM. *P < 0.05 vs. normal.
Analyses of metabolic parameters in maternal venous and umbilical venous plasma revealed significantly increased insulin and leptin values in the obese and obese-GDM groups compared with normal (Table 1). In addition, glucose and/or tumor necrosis factor (TNF)-α were slightly, but not significantly, increased in maternal and umbilical plasma of pregnancies complicated by obesity and obese-GDM, respectively. To test whether one of these factors could account for increased EL expression in obese-GDM placenta, placental cells facing either maternal blood (villous trophoblast) or fetal blood (placental ECs) were isolated from healthy placenta and incubated with 25 mmol/L d-glucose, 10 nmol/L insulin, 20 ng/mL TNF-α, or 1 μg/mL leptin to mimic diabetic conditions in vitro.
TABLE 1.
Anthropometrics and metabolic data of the study population
| Lean | Obese | Obese-GDM | Lean-GDM | |
|---|---|---|---|---|
| Mother (n) | 71 | 170 | 26 | 6 |
| Maternal age (years) | 28.5 ± 6.0 | 27.6 ± 5.6 | 29.2 ± 6.2 | 29.8 ± 6.2 |
| Parity (n) (0–1/>1) | 36/35 | 93/78 | 11/15 | 2/4 |
| Gestational age (weeks) | 38.7 ± 0.6 | 38.8 ± 0.6 | 38.5 ± 0.7 | 39.2 ± 1.0 |
| Maternal pregravid BMI | 22.2 ± 3.5 | 34.6 ± 7.4* | 35.8 ± 1.5* | 22.7 ± 1.6 |
| HOMA | 2.4 ± 1.4 | 4.7 ± 3.3* | 6.3 ± 5.2 * | ND |
| Maternal glucose (mg/dL) | 75 ± 8 | 80 ± 10† | 80 ± 10 | ND |
| Maternal insulin (μU/mL) | 12.5 ± 6.4 | 23.3 ± 13.1* | 30.6 ± 20.3* | ND |
| Maternal leptin (ng/mL) | 32.6 ± 20.4 | 63.6 ± 32.5* | 57.9 ± 32.4* | ND |
| TNF-α (pg/mL) | 1.4 ± 0.9 | 1.3 ± 0.5 | 1.3 ± 0.3 | ND |
| Fetus | ||||
| Placenta weight (g) | 571 ± 178 | 643 ± 176† | 689 ± 143† | 618 ± 150 |
| Birth weight (g) | 3,266 ± 508 | 3,362 ± 514 | 3,482 ± 529† | 3,428 ± 305 |
| % Neonatal body fat | 10.3 ± 4.0 | 12.1 ± 2.9* | 12.9 ± 4.4* | ND |
| Umbilical glucose (mg/dL) | 62 ± 13 | 66 ± 13 | 67 ± 13 | ND |
| Umbilical insulin (μU/mL) | 7.1 ± 3.6 | 9.1 ± 5.7† | 18.1 ± 22.9* | ND |
| Umbilical leptin (ng/mL) | 8.3 ± 4.6 | 14.0 ± 12.6† | 34.0 ± 29.3* | ND |
| Umbilical TNF-α (pg/mL) | 1.7 ± 0.6 | 1.7 ± 0.3 | 1.9 ± 0.5 | ND |
Data are means ± SD. ND, not determined.
*P < 0.001.
†P < 0.01 vs. control subjects.
Effects of glucose, insulin, TNF-α, or leptin on EL expression in placental primary cells.
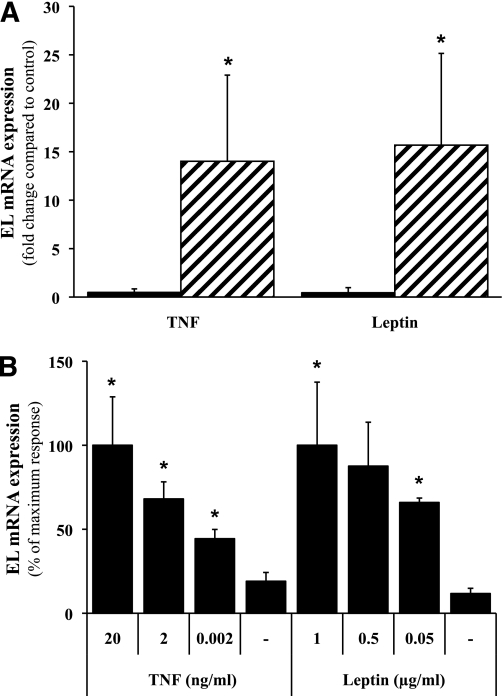
Although treatment with glucose or insulin did not show any effects on EL expression in both cell types (data not shown), incubation with TNF-α or leptin altered EL expression after 24 h. In primary TTs, EL expression was slightly, but not significantly, downregulated by 52% (± 36.8%) and 56.6% (± 53.6%) in the presence of TNF-α and leptin, respectively. However, in primary placental ECs, both TNF-α (14.0 ± 8.9-fold increase; P = 0.035) and leptin (15.7 ± 9.5-fold increase; P = 0.026) increased EL expression after a 24-h incubation (Fig. 2A). The TNF-α and leptin concentrations chosen here have been frequently used for in vitro experiments (29–32), but are higher than systemic levels in the fetal circulation. However, both cytokines may originate either from the umbilical circulation (endocrine effect) or locally from the trophoblast and/or placental macrophages (paracrine effect). Depending on the mechanism of action, i.e., endocrine or paracrine, the concentrations of both cytokines may vary, with higher paracrine and lower endocrine concentrations. Thus, EL expression in response to various concentrations of TNF-α and leptin, respectively, was determined in ECs. Incubation of ECs with increasing concentrations of TNF-α or leptin showed that both cytokines induced EL expression in a dose-dependent manner (Fig. 2B).
FIG. 2.
Effects of TNF-α or leptin on EL mRNA expression in primary TTs and placental ECs. A: Primary TTs (■) and placental ECs (▨) were incubated with TNF-α (20 ng/mL) or leptin (1 μg/mL) for 24 h, and EL expression was analyzed with quantitative real-time RT-PCR. In TTs, incubation with TNF-α or leptin decreased EL expression by 52% (± 36%) and 56% (± 53%), respectively, which was not statistically different compared with controls. In ECs, TNF-α led to a 14.0-fold (± 8.9) upregulation and leptin to a 15.7-fold (± 9.5) upregulation of EL. Experiments were performed in triplicate with five different cell preparations. B: Dose-response experiments with indicated concentrations of TNF-α or leptin showed a concentration-dependent induction of EL expression in ECs after 24 h. Data are presented as mean ± SEM. *P < 0.05 vs. untreated control.
Expression of TNF-α, leptin, and their receptors in ECs.
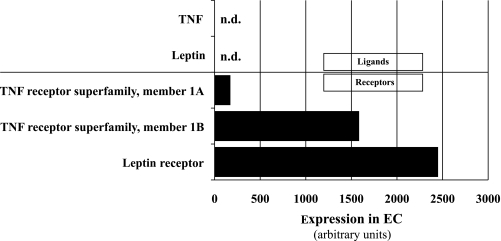
As mentioned above, both TNF-α and leptin can act either in an autocrine/paracrine manner, when produced and secreted by placental cells, or in an endocrine manner because they circulate in fetal blood. In an attempt to distinguish between both principle mechanisms, the EC transcriptome was screened for expression of TNF-α, leptin, and their respective receptors. Whereas TNF-α and leptin were not expressed in ECs, the leptin receptor and TNF receptor superfamily member 1B were abundantly expressed and the TNF receptor superfamily member 1A was moderately expressed in ECs (Fig. 3). This observation makes an autocrine mechanism of TNF-α− or leptin-mediated upregulation of EL in ECs rather unlikely and suggests that endocrine/paracrine-secreted TNF-α or leptin (or both) accounts for the changes.
FIG. 3.
Expression of TNF-α, leptin, and their receptors in primary placental ECs. Microarray analysis of primary placental ECs isolated from 10 different placentas revealed abundant expression of leptin receptor and TNF receptor superfamily member 1B. While TNF receptor superfamily member 1A was moderately expressed, TNF-α and leptin expression itself was not detectable (n.d.) in ECs.
In fact, umbilical plasma leptin levels were 4.1- and 2.4-fold higher in obese-GDM cases compared with normal and obese cases, respectively (Table 1). Umbilical plasma TNF-α, in contrast, was only increased by 14% in the obese-GDM group compared with obese and normal cases.
NF-κB pathway is involved in TNF-α−mediated upregulation of EL in ECs.
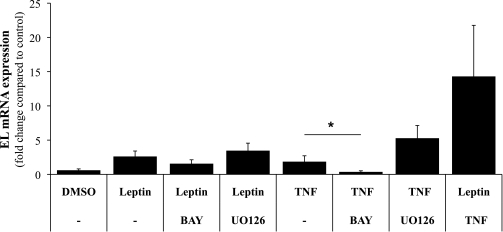
Induction of EL expression by TNF-α was previously demonstrated to be regulated by the NF-κB pathway in human umbilical vein ECs (33) and human microvascular endothelial cell line-1 (34). So far, no pathways were described responsible for upregulation of EL expression by leptin. To further define potential pathways involved in TNF-α− and leptin-mediated upregulation of EL in our placental primary ECs, BAY, an inhibitor of NF-κB signaling, and UO126, an inhibitor of MAPK signaling, were added to TNF-α− or leptin-treated cells. Administration of BAY decreased EL expression in leptin-treated cells by 29.5% compared with expression in cells treated with leptin alone. When UO126 was present, EL expression in leptin-treated ECs increased by 32.4% after 24 h (Fig. 4). However, the observed effects of both inhibitors did not reach statistical significance. In TNF-α−treated ECs, the inhibitors had more pronounced effects on EL expression, which followed the same trend as in leptin experiments. BAY decreased TNF-α−induced upregulation of EL by 84.1% (P < 0.001), and UO126 further increased EL expression (2.0-fold, P = 0.073) compared with cells treated with TNF-α alone.
FIG. 4.
Effect of inhibitors of NF-κB or MAPK signaling on TNF-α and leptin-induced upregulation of EL in primary placental ECs. Primary placental ECs were incubated with TNF-α (20 ng/mL), leptin (1 μg/mL), or both for 24 h in the presence or absence of NF-κB (BAY, 10 μmol/L) or MAPK (UO126, 10 μmol/L) signaling inhibitors. Neither BAY nor UO126 showed significant effects on leptin-induced upregulation of EL. In ECs treated with TNF-α, BAY significantly reduced expression of EL to 15.9% (*P < 0.001) compared with expression level in cells treated with TNF-α alone. DMSO was used as a solvent control for signaling inhibitors and showed no significant effects compared with untreated cells. Data are presented as mean ± SEM from experiments with five different cell preparations, each performed in triplicate.
Interestingly, combined application of TNF-α and leptin significantly increased EL expression (23.4-fold, P < 0.001), which by far exceeded expression in cells separately treated with TNF-α or leptin (Fig. 4). This observation suggests an additive effect of TNF-α and leptin on EL expression in ECs. Unfortunately, no data were available for experiments with combined application of both inhibitors, since these conditions were lethal for our primary cells.
DISCUSSION
Dysregulation of placental EL expression was previously demonstrated in pregnancies complicated either by fetal growth restriction or type 1 diabetes. Although EL was significantly downregulated in fetal growth restriction placentas (15), its expression was increased in placentas from women with type 1 diabetes (16). In the current study, placental EL expression was compared in healthy pregnancies and pregnancies complicated by obesity, lean-GDM, or obesity combined with GDM (obese-GDM). EL expression was significantly increased in placentas from obese-GDM cases, but not in placentas from obese women without GDM or lean women with GDM. These data suggest that neither obesity nor GDM alone accounts for the dysregulation of placental EL, and only the combination of both conditions alters placental EL. This interpretation is further substantiated by the in vitro experiments showing that combined administration of TNF-α and leptin resulted in an additive effect on EL upregulation.
The human placenta represents a barrier between maternal and fetal blood circulation. At the materno-placental interface, the villous trophoblast is in contact with maternal blood, while at the feto-placental interface, ECs of stromal vessels are in contact with fetal blood. Both cell types express EL, although EL expression in ECs prevails at term (15).
Thus, primary ECs and trophoblasts were treated with glucose, insulin, leptin, and TNF-α to test their putative effect on EL expression. Only primary ECs, but not trophoblasts, responded to leptin and TNF-α, whereas glucose and insulin had no effect on EL expression in both compartments. These cell culture experiments confirmed that increased fetal plasma leptin and/or TNF-α are able to upregulate EL expression in the placental endothelium, i.e., the ECs. This result may also explain EL upregulation in total placental tissue in type 1 diabetes pregnancies, in which fetal leptin (35) and TNF-α (36) concentrations are elevated.
The source of leptin and TNF-α effecting these changes may be different because only leptin concentration was elevated in the cord blood. Fetal leptin levels correlate with fetal insulin in fetuses with excess weight (37), strongly suggesting fetal fat as the source. TNF-α and leptin levels in placental tissue are elevated in conditions associated with fetal adiposity (32), which, in the absence of their production in ECs, may be accounted for by trophoblast or macrophage synthesis and release. Dose-response experiments suggest that EL expression is upregulated by TNF-α or leptin in a concentration-dependent manner. Leptin and TNF-α are not transferred from the maternal to the fetal circulation, hence originating from fetal tissue release (38,39). This finding is substantiated by the absence of higher umbilical plasma levels in preeclampsia despite elevated maternal levels (40). Indeed, neither leptin nor TNF-α expression could be detected in primary ECs, suggesting either an endocrine or paracrine effect of these factors. Because placental expression of leptin and TNF-α is increased in GDM and obesity (41,42), a paracrine contribution of leptin and/or TNF-α derived from placental macrophages (Hofbauer cells) or the villous trophoblast is conceivable. Interestingly, both TNF-α and leptin also induced overexpression of two other placental phospholipases (32). Both molecules should be able to bind to placental ECs, since expression of receptors for leptin and TNF-α was detected. The presence of leptin receptors in placental ECs found here is in conflict with previous studies that localized leptin receptors only at the syncytiotrophoblast (43,44). However, leptin receptors were found in ECs lining umbilical veins and arteries, a vascular system closely linked to that of the placental chorionic tissue proper (45).
Collectively, the dysregulation of EL in obese-GDM tissue can be attributed at least in part to TNF-α− or leptin-mediated upregulation of EL in vascular ECs of placental villi. A contribution of other dysregulated factors, such as partial oxygen pressure, cannot be excluded.
TNF-α induces EL upregulation in placental primary ECs by activating the NF-κB pathway, as demonstrated by inhibitor experiments in vitro. According to these experiments, leptin may not activate NF-κB or MAPK signaling pathways in these cells (Fig. 5). Activation of leptin signaling pathways may be cell type-dependent, since leptin activates MAPK in the trophoblast-derived cell line BeWo (46). Interestingly, there seems to be an additive effect of both leptin and TNF-α leading to augmented EL expression in primary placental ECs, suggesting a cross-talk between both signaling pathways.
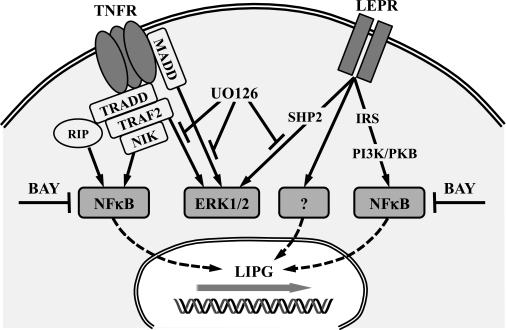
FIG. 5.
Proposed signaling of TNF-α− and leptin-induced EL upregulation in primary placental ECs. TNF receptor (TNFR) can induce NF-κB–related gene expression via TNF receptor type 1–associated death domain protein (TRADD) and receptor-interacting serine/threonine-protein kinase (RIP), as well as via TNF receptor–associated factor 2 (TRAF2)/NF-κB–inducing kinase (NIK) (47). Extracellular signal–related kinase (ERK)-1/2 can be activated by TNF receptor via MAP kinase activating death domain (MADD) (48) or via TNF receptor-associated factor 2 (49). The leptin receptor (LEPR) can induce ERK1/2 activation via protein tyrosine phosphatase, non-receptor type 11 (SHP2)/growth factor receptor-bound protein 2 (GRB2) and/or NF-κB–related gene expression by insulin receptor substrate (IRS)/phosphatidylinositol 3-kinase (PI3K)/protein kinase B (PKB) signaling (50). Inhibition of ERK1/2 signaling by UO126 had no effect on TNF-α− and leptin-induced upregulation of the EL gene (LIPG) expression, whereas the NF-κB inhibitor BAY completely abolished TNF-α−induced EL upregulation. BAY only in part (not significantly) block leptin-induced EL upregulation in primary placental ECs, suggesting that another pathway is involved.
The pathophysiological consequence of the EL upregulation in obese-GDM is unclear, because the role of EL on placental ECs for regulating lipid homeostasis or materno-fetal lipid transfer has not yet been fully defined. The current study did not measure placental EL activity, since the relative contribution of individual lipases to total placental lipase activity would be difficult to separate. As a speculation, higher EL activity in placental ECs exposed to the fetal circulation may facilitate release of free fatty acids from triglycerides and predominantly phospholipids for subsequent uptake by the placental endothelium. Whether EL upregulation may contribute to the synthesis of inflammatory mediators or vasoregulators is unknown. We propose that metabolic inflammation with elevated TNF-α and leptin concentrations at the fetal-placental endothelium determines EL regulation. Absence of correlation between placental EL expression and fetal growth measures makes a direct interaction of placental EL and fetal growth rather unlikely.
ACKNOWLEDGMENTS
M.G. was supported by the START funding program of the Medical University of Graz, Austria. U.H. and G.D. were supported by the Jubilee Fund, Austrian National Bank, Vienna (grants 10896, 12601, and 13307).
No potential conflicts of interest relevant to this article were reported.
M.G. generated data and wrote the manuscript. U.H. and S.H-d.M. generated data and reviewed the manuscript. S.F. and C.W. reviewed the manuscript. M.v.P. carried out statistical analyses and reviewed the manuscript. G.D. wrote and edited the manuscript. All authors contributed to discussion of data.
The authors are indebted to R. Michelmayr, H. Miedl, and S. Kopp (Department of Obstetrics and Gynecology, Medical University of Graz, Austria) for their cell isolation and cell culture work. The archived placental tissues used for immunohistochemistry were provided by the Biobank of the Medical University of Graz.
REFERENCES
- 1.Desoye G, Schweditsch MO, Pfeiffer KP, Zechner R, Kostner GM. Correlation of hormones with lipid and lipoprotein levels during normal pregnancy and postpartum. J Clin Endocrinol Metab 1987;64:704–712 [DOI] [PubMed] [Google Scholar]
- 2.Herrera E. Lipid metabolism in pregnancy and its consequences in the fetus and newborn. Endocrine 2002;19:43–55 [DOI] [PubMed] [Google Scholar]
- 3.Knopp RH, Warth MR, Charles D, et al. Lipoprotein metabolism in pregnancy, fat transport to the fetus, and the effects of diabetes. Biol Neonate 1986;50:297–317 [DOI] [PubMed] [Google Scholar]
- 4.Alsat E, Bouali Y, Goldstein S, et al. Low-density lipoprotein binding sites in the microvillous membranes of human placenta at different stages of gestation. Mol Cell Endocrinol 1984;38:197–203 [DOI] [PubMed] [Google Scholar]
- 5.Malassiné A, Besse C, Roche A, et al. Ultrastructural visualization of the internalization of low density lipoprotein by human placental cells. Histochemistry 1987;87:457–464 [DOI] [PubMed] [Google Scholar]
- 6.Quinn KA, Pye VJ, Dai YP, Chesterman CN, Owensby DA. Characterization of the soluble form of the low density lipoprotein receptor-related protein (LRP). Exp Cell Res 1999;251:433–441 [DOI] [PubMed] [Google Scholar]
- 7.Wadsack C, Hammer A, Levak-Frank S, et al. Selective cholesteryl ester uptake from high density lipoprotein by human first trimester and term villous trophoblast cells. Placenta 2003;24:131–143 [DOI] [PubMed] [Google Scholar]
- 8.Wittmaack FM, Gåfvels ME, Bronner M, et al. Localization and regulation of the human very low density lipoprotein/apolipoprotein-E receptor: trophoblast expression predicts a role for the receptor in placental lipid transport. Endocrinology 1995;136:340–348 [DOI] [PubMed] [Google Scholar]
- 9.Waterman IJ, Emmison N, Dutta-Roy AK. Characterisation of triacylglycerol hydrolase activities in human placenta. Biochim Biophys Acta 1998;1394:169–176 [DOI] [PubMed] [Google Scholar]
- 10.Waterman IJ, Emmison N, Sattar N, Dutta-Roy AK. Further characterization of a novel triacylglycerol hydrolase activity (pH 6.0 optimum) from microvillous membranes from human term placenta. Placenta 2000;21:813–823 [DOI] [PubMed] [Google Scholar]
- 11.Fuki IV, Blanchard N, Jin W, et al. Endogenously produced endothelial lipase enhances binding and cellular processing of plasma lipoproteins via heparan sulfate proteoglycan-mediated pathway. J Biol Chem 2003;278:34331–34338 [DOI] [PubMed] [Google Scholar]
- 12.Ji ZS, Lauer SJ, Fazio S, Bensadoun A, Taylor JM, Mahley RW. Enhanced binding and uptake of remnant lipoproteins by hepatic lipase-secreting hepatoma cells in culture. J Biol Chem 1994;269:13429–13436 [PubMed] [Google Scholar]
- 13.Strauss JG, Zimmermann R, Hrzenjak A, et al. Endothelial cell-derived lipase mediates uptake and binding of high-density lipoprotein (HDL) particles and the selective uptake of HDL-associated cholesterol esters independent of its enzymic activity. Biochem J 2002;368:69–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jaye M, Lynch KJ, Krawiec J, et al. A novel endothelial-derived lipase that modulates HDL metabolism. Nat Genet 1999;21:424–428 [DOI] [PubMed] [Google Scholar]
- 15.Gauster M, Hiden U, Blaschitz A, et al. Dysregulation of placental endothelial lipase and lipoprotein lipase in intrauterine growth-restricted pregnancies. J Clin Endocrinol Metab 2007;92:2256–2263 [DOI] [PubMed] [Google Scholar]
- 16.Lindegaard ML, Damm P, Mathiesen ER, Nielsen LB. Placental triglyceride accumulation in maternal type 1 diabetes is associated with increased lipase gene expression. J Lipid Res 2006;47:2581–2588 [DOI] [PubMed] [Google Scholar]
- 17.Gauster M, Rechberger G, Sovic A, et al. Endothelial lipase releases saturated and unsaturated fatty acids of high density lipoprotein phosphatidylcholine. J Lipid Res 2005;46:1517–1525 [DOI] [PubMed] [Google Scholar]
- 18.McCoy MG, Sun GS, Marchadier D, Maugeais C, Glick JM, Rader DJ. Characterization of the lipolytic activity of endothelial lipase. J Lipid Res 2002;43:921–929 [PubMed] [Google Scholar]
- 19.Magnusson AL, Waterman IJ, Wennergren M, Jansson T, Powell TL. Triglyceride hydrolase activities and expression of fatty acid binding proteins in the human placenta in pregnancies complicated by intrauterine growth restriction and diabetes. J Clin Endocrinol Metab 2004;89:4607–4614 [DOI] [PubMed] [Google Scholar]
- 20.Tabano S, Alvino G, Antonazzo P, Grati FR, Miozzo M, Cetin I. Placental LPL gene expression is increased in severe intrauterine growth-restricted pregnancies. Pediatr Res 2006;59:250–253 [DOI] [PubMed] [Google Scholar]
- 21.Carpenter MW, Coustan DR. Criteria for screening tests for gestational diabetes. Am J Obstet Gynecol 1982;144:768–773 [DOI] [PubMed] [Google Scholar]
- 22.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985;28:412–419 [DOI] [PubMed] [Google Scholar]
- 23.Catalano PM, Thomas AJ, Avallone DA, Amini SB. Anthropometric estimation of neonatal body composition. Am J Obstet Gynecol 1995;173:1176–1181 [DOI] [PubMed] [Google Scholar]
- 24.Lang I, Pabst MA, Hiden U, et al. Heterogeneity of microvascular endothelial cells isolated from human term placenta and macrovascular umbilical vein endothelial cells. Eur J Cell Biol 2003;82:163–173 [DOI] [PubMed] [Google Scholar]
- 25.Lang I, Schweizer A, Hiden U, et al. Human fetal placental endothelial cells have a mature arterial and a juvenile venous phenotype with adipogenic and osteogenic differentiation potential. Differentiation 2008;76:1031–1043 [DOI] [PubMed] [Google Scholar]
- 26.Blaschitz A, Weiss U, Dohr G, Desoye G. Antibody reaction patterns in first trimester placenta: implications for trophoblast isolation and purity screening. Placenta 2000;21:733–741 [DOI] [PubMed] [Google Scholar]
- 27.Hiden U, Maier A, Bilban M, et al. Insulin control of placental gene expression shifts from mother to foetus over the course of pregnancy. Diabetologia 2006;49:123–131 [DOI] [PubMed] [Google Scholar]
- 28.Mayhew TM, Charnock-Jones DS, Kaufmann P. Aspects of human fetoplacental vasculogenesis and angiogenesis. III. Changes in complicated pregnancies. Placenta 2004;25:127–139 [DOI] [PubMed] [Google Scholar]
- 29.Badellino KO, Wolfe ML, Reilly MP, Rader DJ. Endothelial lipase is increased in vivo by inflammation in humans. Circulation 2008;117:678–685 [DOI] [PubMed] [Google Scholar]
- 30.Hirata K, Ishida T, Matsushita H, Tsao PS, Quertermous T. Regulated expression of endothelial cell-derived lipase. Biochem Biophys Res Commun 2000;272:90–93 [DOI] [PubMed] [Google Scholar]
- 31.Kivelä AM, Mäkinen PI, Jyrkkänen HK, et al. Sulforaphane inhibits endothelial lipase expression through NF-κB in endothelial cells. Atherosclerosis 2010;213:122–128 [DOI] [PubMed] [Google Scholar]
- 32.Varastehpour A, Radaelli T, Minium J, et al. Activation of phospholipase A2 is associated with generation of placental lipid signals and fetal obesity. J Clin Endocrinol Metab 2006;91:248–255 [DOI] [PubMed] [Google Scholar]
- 33.Jin W, Sun GS, Marchadier D, Octtaviani E, Glick JM, Rader DJ. Endothelial cells secrete triglyceride lipase and phospholipase activities in response to cytokines as a result of endothelial lipase. Circ Res 2003;92:644–650 [DOI] [PubMed] [Google Scholar]
- 34.Kempe S, Kestler H, Lasar A, Wirth T. NF-kappaB controls the global pro-inflammatory response in endothelial cells: evidence for the regulation of a pro-atherogenic program. Nucleic Acids Res 2005;33:5308–5319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nelson SM, Coan PM, Burton GJ, Lindsay RS. Placental structure in type 1 diabetes: relation to fetal insulin, leptin, and IGF-I. Diabetes 2009;58:2634–2641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Holm BC, Svensson J, Akesson C, et al. Evidence for immunological priming and increased frequency of CD4+ CD25+ cord blood T cells in children born to mothers with type 1 diabetes. Clin Exp Immunol 2006;146:493–502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wolf HJ, Ebenbichler CF, Huter O, et al. Fetal leptin and insulin levels only correlate in large-for-gestational age infants. Eur J Endocrinol 2000;142:623–629 [DOI] [PubMed] [Google Scholar]
- 38.Aaltonen R, Heikkinen T, Hakala K, Laine K, Alanen A. Transfer of proinflammatory cytokines across term placenta. Obstet Gynecol 2005;106:802–807 [DOI] [PubMed] [Google Scholar]
- 39.Radaelli T, Uvena-Celebrezze J, Minium J, Huston-Presley L, Catalano P, Hauguel-de Mouzon S. Maternal interleukin-6: marker of fetal growth and adiposity. J Soc Gynecol Investig 2006;13:53–57 [DOI] [PubMed] [Google Scholar]
- 40.Schiff E, Friedman SA, Baumann P, Sibai BM, Romero R. Tumor necrosis factor-alpha in pregnancies associated with preeclampsia or small-for-gestational-age newborns. Am J Obstet Gynecol 1994;170:1224–1229 [DOI] [PubMed] [Google Scholar]
- 41.Challier JC, Basu S, Bintein T, et al. Obesity in pregnancy stimulates macrophage accumulation and inflammation in the placenta. Placenta 2008;29:274–281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lepercq J, Cauzac M, Lahlou N, et al. Overexpression of placental leptin in diabetic pregnancy: a critical role for insulin. Diabetes 1998;47:847–850 [DOI] [PubMed] [Google Scholar]
- 43.Bodner J, Ebenbichler CF, Wolf HJ, et al. Leptin receptor in human term placenta: in situ hybridization and immunohistochemical localization. Placenta 1999;20:677–682 [DOI] [PubMed] [Google Scholar]
- 44.Challier J, Galtier M, Bintein T, Cortez A, Lepercq J, Hauguel-de Mouzon S. Placental leptin receptor isoforms in normal and pathological pregnancies. Placenta 2003;24:92–99 [DOI] [PubMed] [Google Scholar]
- 45.Akerman F, Lei ZM, Rao CV. Human umbilical cord and fetal membranes co-express leptin and its receptor genes. Gynecol Endocrinol 2002;16:299–306 [PubMed] [Google Scholar]
- 46.Caüzac M, Czuba D, Girard J, Hauguel-de Mouzon S. Transduction of leptin growth signals in placental cells is independent of JAK-STAT activation. Placenta 2003;24:378–384 [DOI] [PubMed] [Google Scholar]
- 47.Hayden MS, Ghosh S. Shared principles in NF-kappaB signaling. Cell 2008;132:344–362 [DOI] [PubMed] [Google Scholar]
- 48.Schievella AR, Chen JH, Graham JR, Lin LL. MADD, a novel death domain protein that interacts with the type 1 tumor necrosis factor receptor and activates mitogen-activated protein kinase. J Biol Chem 1997;272:12069–12075 [DOI] [PubMed] [Google Scholar]
- 49.Yuasa T, Ohno S, Kehrl JH, Kyriakis JM. Tumor necrosis factor signaling to stress-activated protein kinase (SAPK)/Jun NH2-terminal kinase (JNK) and p38: germinal center kinase couples TRAF2 to mitogen-activated protein kinase/ERK kinase kinase 1 and SAPK while receptor interacting protein associates with a mitogen-activated protein kinase kinase kinase upstream of MKK6 and p38. J Biol Chem 1998;273:22681–22692 [DOI] [PubMed] [Google Scholar]
- 50.Gonzalez-Perez RR, Xu Y, Guo S, Watters A, Zhou W, Leibovich SJ. Leptin upregulates VEGF in breast cancer via canonic and non-canonical signalling pathways and NFkappaB/HIF-1alpha activation. Cell Signal 2010;22:1350–1362 [DOI] [PMC free article] [PubMed] [Google Scholar]