Abstract
OBJECTIVE
The molecular mechanisms responsible for pancreatic β-cell dysfunction in type 2 diabetes remain unresolved. Increased expression of the helix-loop-helix protein Id1 has been found in islets of diabetic mice and in vitro models of β-cell dysfunction. Here, we investigated the role of Id1 in insulin secretion and glucose homeostasis.
RESEARCH DESIGN AND METHODS
Id1 knockout (Id1−/−) and wild-type mice were fed a chow or high-fat diet. Glucose tolerance, insulin tolerance, β-cell mass, insulin secretion, and islet gene expression were assessed. Small interfering RNA (siRNA) was used to silence Id1 in MIN6 cells, and responses to chronic palmitate treatment were assessed.
RESULTS
Id1−/− mice exhibited an improved response to glucose challenge and were almost completely protected against glucose intolerance induced by high-fat diet. This was associated with increased insulin levels and enhanced insulin release from isolated islets, whereas energy intake, body weight, fat pad weight, β-cell mass, and insulin action were unchanged. Islets from Id1−/− mice displayed reduced stress gene expression and were protected against high-fat diet–induced downregulation of β-cell gene expression (pancreatic duodenal homeobox-1, Beta2, Glut2, pyruvate carboxylase, and Gpr40). In MIN6 cells, siRNA-mediated inhibition of Id1 enhanced insulin secretion after chronic palmitate treatment and protected against palmitate-mediated loss of β-cell gene expression.
CONCLUSIONS
These findings implicate Id1 as a negative regulator of insulin secretion. Id1 expression plays an essential role in the etiology of glucose intolerance, insulin secretory dysfunction, and β-cell dedifferentiation under conditions of increased lipid supply.
The critical contribution of deficient insulin secretion to the pathogenesis of type 2 diabetes is accepted (1–3). Despite the presence of insulin resistance at the level of peripheral tissues and the liver, the majority of overweight and obese individuals do not develop diabetes because their pancreatic β-cells adequately respond and prevent overt hyperglycemia through increased insulin secretion. Diabetes arises when insulin secretion cannot match insulin demand (1–3). This failure of β-cell compensation is associated with a decline in insulin secretory function, which is manifested primarily as a selective loss of glucose-stimulated insulin secretion (GSIS), and a reduction in β-cell mass, which is linked with an increased rate of apoptosis (1–5).
In animal models of diabetes, β-cells have been found to lose the unique differentiation pattern that optimizes GSIS (6–10). Thus, genes that are highly expressed and thought to be involved in the function and maturation of the β-cell phenotype (insulin, Glut2, pancreatic duodenal homeobox-1 [Pdx1], and others) are decreased with diabetes. In contrast, genes that are normally suppressed and would theoretically interfere with optimal β-cell function are increased. While this altered phenotype may underlie the loss of insulin secretion in diabetes, the cellular and molecular mechanisms causing β-cell dedifferentiation have not been identified. In the db/db mouse model of type 2 diabetes, we recently found that insulin secretory dysfunction and a loss of β-cell differentiation were associated with increased islet expression of the helix-loop-helix (HLH) protein Id1 (8).
Id1 is a member of a family of proteins (Id1–4) that are capable of inhibiting differentiation (11–15). Id proteins are negative regulators of HLH transcription factors (15–17) but can also act via non-HLH proteins (14). Expression of Id1 in other cell types is associated with cell growth, enhanced proliferation, and dedifferentiation (11–15). Id1 has been extensively studied for its potential role in the cancer process, since high Id1 expression along with enhanced proliferation and dedifferentiation characterizes transformed cells (11).
Previous reports have demonstrated that Id1 expression is induced in vitro in chronically fatty acid–treated MIN6 β-cells, a model that is characterized by insulin secretory dysfunction and β-cell dedifferentiation (18). In a similar manner, Id1 expression is induced by glucose in human islets and insulin-secreting cell lines but not in liver or other non–β-cell lines (19,20). However, the role of Id1 expression in the regulation of insulin secretion and β-cell gene expression has not been examined.
Here we studied the effects of Id1 deletion on glucose tolerance, insulin secretion, and β-cell gene expression in mice. We also studied the consequences of small interfering RNA (siRNA)-mediated inhibition of Id1 in the MIN6 cell model of chronic fatty acid exposure. The studies provide novel evidence that Id1 expression inhibits insulin secretion and plays a crucial role in the development of glucose intolerance and β-cell dedifferentiation under conditions of chronic lipid oversupply.
RESEARCH DESIGN AND METHODS
Mice.
Wild-type (C57BL/6/129/Sv), Id1−/−, and Id3−/− mice (21) were bred in-house using animals provided by Professor Robert Benezra (Memorial Sloan-Kettering Cancer Center, New York, NY). Animals were kept under conventional conditions with free access to food and water. Ethical approval for mouse studies was granted by the Garvan Institute/St. Vincent’s Hospital Animal Experimentation Ethics Committee, following guidelines issued by the National Health and Medical Research Council of Australia. Mice were fed ad libitum with either a standard chow diet (8% calories from fat; Gordon’s Specialty Stockfeeds, Yanderra, Australia) or a high-fat diet containing lard/sucrose (45% calories from fat, based on rodent diet D12451; Research Diets, New Brunswick, NJ) commencing at 7–9 weeks of age. Food intake and body weight were measured for the determination of energy intake. Blood samples were taken via tail prick for measurement of glucose and insulin levels. Blood collected in EDTA via a terminal heart bleed was used for measurement of plasma glucagon, triglyceride, and nonesterified fatty acid (NEFA) levels. An insulin resistance index, homeostasis model assessment of insulin resistance (HOMA-IR), was calculated from glucose and insulin levels [glucose concentration (mmol/L) × insulin concentration (mU/L) ÷ 22.5].
Metabolic studies and assays.
Intraperitoneal glucose tolerance tests (GTTs; 2 g/kg glucose) and insulin tolerance tests (ITTs; 0.75 units/kg insulin) were performed in conscious male mice after 6 h of fasting. Blood glucose was measured using an Accu-Chek Performa glucose monitor (Roche Diagnostics, Castle Hill, Australia), and insulin was measured using an ELISA (Crystal Chem Inc., Downers Grove, IL). Plasma glucagon was measured using a radioimmunoassay (Millipore, Billerica, MA). Plasma triglyceride was measured using an enzymatic colorimetric method (glycerol-3-phosphate oxidase p-aminophenazone [GPO-PAP] reagent, Roche Diagnostics). Plasma NEFA was measured by an acyl-CoA oxidase–based colorimetric method (Wako Pure Chemical Industries, Osaka, Japan).
Measurement of β-cell mass, islet number, and apoptosis.
Sections (5 μm thick) of pancreata were stained for insulin (I2018; dilution 1:200; Sigma Aldrich, St. Louis, MO) and counterstained with hematoxylin. Whole slide digital images were captured using Aperio Scansope XT (Aperio Technologies, Vista, CA). β-Cell mass and islet number were quantified using ImageScope software (Aperio Technologies). Four sections separated by at least 100 µm were used for each mouse. β-Cell mass was calculated from relative cross-sectional β-cell area and total pancreas mass. Islet number was quantified as number of islets per mm2 of total pancreas. Apoptosis was assessed in pancreas sections using the terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling technique (In Situ Cell Death Detection Kit, POD, Roche).
Insulin secretion assay.
Isolated islets were washed in Krebs-Ringer HEPES buffer (KRHB; containing 5 mmol/L NaHCO3, 1 mmol/L CaCl2, 2.8 mmol/L glucose, 10 mmol/L HEPES, and 10% FCS). Groups of five islets, with at least four replicates per animal, were incubated for 1 h at 37°C in KRHB containing 2.8 or 16.7 mmol/L glucose. Insulin was measured in an aliquot of the buffer by radioimmunoassay (Millipore).
Cell culture and transfection.
MIN6 cells were grown in Dulbecco’s modified Eagle’s medium (Invitrogen, Carlsbad, CA) containing 25 mmol/L glucose, 10 mmol/L HEPES, 10% FCS, 50 units/mL penicillin, and 50 µg/mL streptomycin. Cells were seeded at 2 × 105 cells per well in 24-well plates. Id1 ON-TARGETplus SMARTpool siRNA or control Non-Targeting siRNA were transfected into MIN6 cells using DharmaFECT Transfection Reagent 3 (Dharmacon, Lafayette, CO). After 24-h, cells were treated with either 0.92% BSA or 0.92% BSA coupled to 0.4 mmol/L palmitate for 48-h as previously described (18). For insulin secretion assay, cells were incubated for 1 h at 37°C in KRHB containing 2.8 or 25 mmol/L glucose in the presence of 0.4 mmol/L palmitate. Immunoblotting was performed as previously described (22) using an antibody for Id1 (Id1 [C-20] sc-488, Santa Cruz Biotechnology, Santa Cruz, CA).
RNA analysis.
Total RNA was extracted using RNeasy Mini Kit (Qiagen, Doncaster, Australia), and cDNA was synthesized using QuantiTect Reverse Transcription Kit (Qiagen). Real-time PCR was performed using oligonucleotide primers (sequences listed in Supplementary Table 1) in a LightCycler (Roche Diagnostics). The value obtained for each specific product was normalized to the control gene (cyclophilin A) and expressed as a percent of the value in control extracts. Xbp1 splicing was assessed as previously described (22).
Statistical analysis.
All results are presented as means ± SEM. Statistical analyses were performed using Student t test or ANOVA with Bonferroni post hoc tests.
RESULTS
Metabolic characteristics of wild-type and Id1−/− mice fed a chow or a high-fat diet.
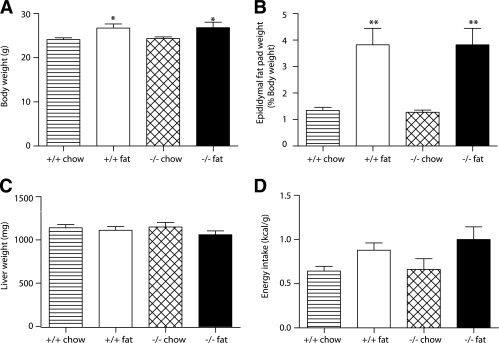
In mice fed a chow diet, body weight, epididymal fat pad weight, liver weight, and energy intake were not significantly different in wild-type and Id1−/− mice (Fig. 1A–D). In a similar manner, blood glucose, insulin, plasma glucagon, triglyceride, and NEFA levels were unchanged, although a trend toward slightly lower blood glucose levels was observed in Id1−/− mice (Table 1). Thus, consistent with previous descriptions (12), Id1−/− mice develop without obvious metabolic abnormalities. High-fat feeding of wild-type and Id1−/− mice for 6 weeks led to significant increases in body weight, fat pad weight, and energy intake, which was similar in both genotypes (Fig. 1A–D). Liver weight was not affected by high-fat feeding in either genotype (Fig. 1C). Insulin and triglyceride levels were significantly increased by high-fat feeding in both genotypes, whereas glucagon and NEFA levels were unchanged (Table 1). There was a tendency for slightly higher blood glucose levels in both wild-type and Id1−/− mice after high-fat feeding (Table 1). HOMA-IR scores (calculated from blood glucose and insulin levels) were increased by fat feeding irrespective of genotype (Table 1), suggestive of fat-induced insulin resistance in both groups of mice. Thus, Id1−/− mice displayed several characteristic features of metabolic disorder induced by high-fat diet, including increased body weight and fat accumulation, elevated circulating triglycerides, and insulin resistance.
FIG. 1.
Metabolic parameters of Id1−/− and wild-type mice fed a standard chow diet (hatched bars and striped bars, respectively) or a high-fat diet (black bars and white bars, respectively) for 6 weeks. A: Body weight for wild-type (n = 22) and Id1−/− (n = 22) mice fed a chow diet and wild-type (n = 16) and Id1−/− (n = 11) mice fed a high-fat diet. *P < 0.05 for effect of diet in wild-type and Id1−/− mice. B: Epididymal fat pad weight. Results are expressed as a percentage of body weight for wild-type (n = 5) and Id1−/− (n = 6) mice fed a chow diet and wild-type (n = 7) and Id1−/− (n = 7) mice fed a high-fat diet. **P < 0.01 for effect of diet in wild-type and Id1−/− mice. C: Liver weight for wild-type (n = 10) and Id1−/− (n = 6) mice fed a chow diet and wild-type (n = 8) and Id1−/− (n = 6) mice fed a high-fat diet. D: Energy intake of wild-type (n = 9) and Id1−/− (n = 6) mice fed a chow diet and wild-type (n = 12) and Id1−/− (n = 8) mice fed a high-fat diet. ANOVA: P < 0.05 for effect of diet.
TABLE 1.
Metabolic characteristics of wild-type and Id1−/− mice fed a chow or a high-fat diet for 6 weeks
| Wild-type |
Id1−/− |
|||
|---|---|---|---|---|
| Chow (n = 8–19) | Fat (n = 7–16) | Chow (n = 7–22) | Fat (n = 6–14) | |
| Blood glucose (mmol/L) | 8.2 ± 0.3 | 9.0 ± 0.4 | 7.5 ± 0.3 | 8.1 ± 0.3 |
| Blood insulin (ng/mL) | 0.31 ± 0.04 | 0.68 ± 0.05*** | 0.38 ± 0.04 | 0.66 ± 0.09** |
| HOMA-IR | 2.8 ± 0.4 | 6.9 ± 0.6*** | 3.3 ± 0.5 | 6.6 ± 1.0*** |
| Plasma glucagon (pg/mL) | 54.0 ± 8.5 | 50.7 ± 7.9 | 50.8 ± 9.7 | 64.4 ± 8.4 |
| Plasma triglyceride (mmol/L) | 1.37 ± 0.07 | 1.97 ± 0.15* | 1.34 ± 0.11 | 2.03 ± 0.25* |
| Plasma NEFA (mmol/L) | 0.23 ± 0.02 | 0.30 ± 0.03 | 0.25 ± 0.02 | 0.27 ± 0.01 |
Values are means ± SEM.
*P < 0.05,
**P < 0.01, and
***P < 0.001 for effect of diet in wild-type and Id1−/− mice.
Id1−/− mice exhibit improved glucose tolerance.
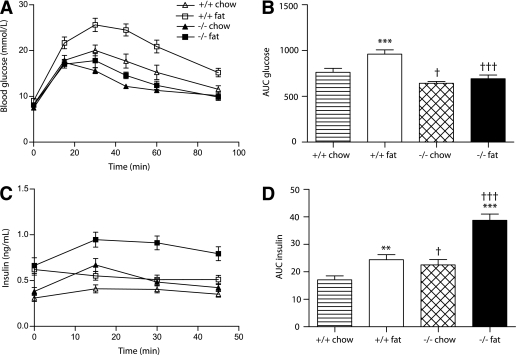
To determine whether Id1 plays a role in the regulation of glucose tolerance, we performed intraperitoneal GTT in wild-type and Id1−/− mice fed a chow or a high-fat diet for 6 weeks. After 6 h of fasting, blood glucose levels were similar among the diets and genotypes (indicated at 0 min) (Fig. 2A). After intraperitoneal glucose administration in mice fed a chow diet, blood glucose levels (Fig. 2A) and the resultant area under the curve (AUC) for glucose values from 0–90 min (Fig. 2B) were significantly reduced in Id1−/− mice compared with wild-type mice. This indicates that Id1 deletion leads to improved glucose tolerance in chow-fed mice (Fig. 2A and B). Compared with chow-fed mice, high-fat feeding of wild-type mice led to significantly increased blood glucose levels after the intraperitoneal bolus (Fig. 2A and B), indicating that as expected, a 6-week exposure to a high-fat diet results in marked glucose intolerance. It is striking that Id1−/− mice were almost completely protected from glucose intolerance induced by high-fat diet (Fig. 2A and B). After the glucose challenge, the blood glucose levels of fat-fed Id1−/− mice were only slightly elevated compared with levels in chow-fed Id1−/− mice, and they remained below the range observed in chow-fed wild-type mice (Fig. 2A and B). These data provide the first evidence that deletion of Id1 confers protection against diet-induced glucose intolerance. This protective effect in Id1−/− mice was also observed after a prolonged 18-week period of high-fat feeding (Supplementary Fig. 1).
FIG. 2.
Effect of Id1 deletion on glucose tolerance and insulin levels in wild-type and Id1−/− mice fed a standard chow diet (white triangles/striped bars and black triangles/hatched bars, respectively) or a high-fat diet (white squares/white bars and black squares/black bars, respectively) for 6 weeks. A: Blood glucose levels during an intraperitoneal GTT of wild-type (n = 19) and Id1−/− (n = 22) mice fed a chow diet and wild-type (n = 12) and Id1−/− (n = 6) mice fed a high-fat diet. ANOVA: P < 0.0001 for effect of diet in wild-type mice, P < 0.05 for effect of diet in Id1−/− mice, P < 0.0001 for effect of Id1 deletion in chow-fed mice, and P < 0.0001 for effect of Id1 deletion in fat-fed mice. B: AUC of blood glucose levels during the intraperitoneal GTT. ***P < 0.001 for effect of fat diet in wild-type mice, †P < 0.05 for effect of Id1 deletion in chow-fed mice, and †††P < 0.001 for effect of Id1 deletion in fat-fed mice. C: Insulin levels during intraperitoneal GTT of wild-type (n = 14) and Id1−/− (n = 17) mice fed a chow diet and wild-type (n = 11) and Id1−/− (n = 6) mice fed a high-fat diet. ANOVA: P < 0.01 for effect of fat diet in wild-type mice, P < 0.0001 for effect of fat diet in Id1−/− mice, P < 0.05 for effect of Id1 deletion in chow-fed mice, and P < 0.001 for effect of Id1 deletion in fat-fed mice. D: AUC of insulin levels during intraperitoneal GTT. **P < 0.01 for effect of fat diet in wild-type mice, †P < 0.05 for effect of Id1 deletion in chow-fed mice, ***P < 0.001 for effect of fat diet in Id1−/− mice, and †††P < 0.001 for effect of Id1 deletion in fat-fed mice.
In contrast, deletion of Id3 did not affect glucose tolerance in chow- or fat-fed mice (Supplementary Fig. 2). Taken together, the data suggest a specific role of Id1, and not its closely related Id3 family member (12,23), in the regulation of glucose tolerance in mice.
Improved glucose tolerance in Id1−/− mice is associated with increased insulin levels.
During the intraperitoneal GTT, insulin levels (Fig. 2C) and the resultant AUC for insulin (Fig. 2D) were significantly increased in Id1−/− mice compared with wild-type controls. This was particularly evident after high-fat feeding. Compared with chow-fed controls, fat-fed wild-type mice exhibited higher fasting insulin levels (indicated at 0 min) (Fig. 2C), but these did not increase further after intraperitoneal glucose administration (Fig. 2C), despite the presence of marked hyperglycemia (Fig. 2A). In contrast, in fat-fed Id1−/− mice, insulin levels were significantly increased after intraperitoneal glucose administration (Fig. 2C and D). These findings indicate that the ability of Id1−/− mice to improve glucose tolerance is associated with enhanced circulating insulin levels, especially in the face of insulin resistance induced by high-fat diet.
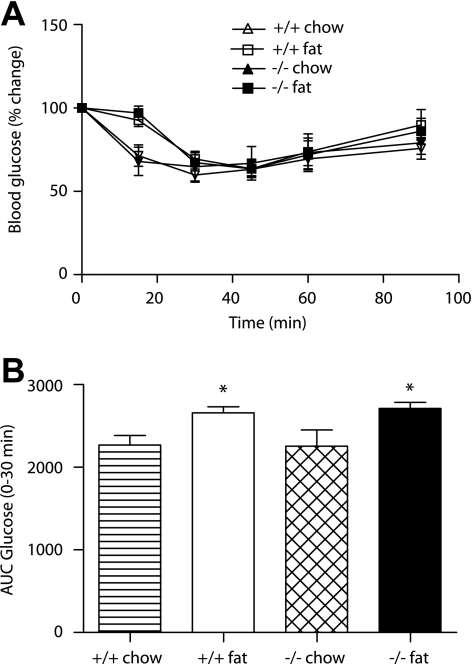
Id1 deletion does not affect insulin action.
To investigate whether changes in insulin action contribute to the improved glucose tolerance in Id1−/− mice, we performed intraperitoneal ITT in wild-type and Id1−/− mice fed a chow or a high-fat diet. Chow-fed wild-type and Id1−/− mice exhibited similar time-course changes in blood glucose levels after insulin injection (Fig. 3A). Accordingly, the AUC of glucose values from 0–30 min after insulin injection were similar in both genotypes (Fig. 3B). After high-fat feeding, the blood glucose response to insulin was delayed in both wild-type and Id1−/− mice, indicating that diet-induced insulin resistance was not affected by the deletion of Id1. This suggests that the improved glucose tolerance in Id1−/− mice is a consequence of increased insulin levels, rather than changes in insulin action. Id1 might therefore inhibit insulin secretion, particularly under conditions of increased lipid supply.
FIG. 3.
Effect of Id1 deletion on insulin action in wild-type and Id1−/− mice fed a chow diet (white triangle/striped bar and black triangle/hatched bar, respectively) or a high-fat diet (white square/white bar and black square/black bar, respectively). A: Blood glucose levels during an intraperitoneal ITT of wild-type (n = 5) and Id1−/− (n = 4) mice fed a chow diet and wild-type (n = 8) and Id1−/− (n = 6) mice fed a high-fat diet. ANOVA: P < 0.05 for effect of fat diet in wild-type and Id1−/− mice. B: AUC 0–30 min of blood glucose levels during intraperitoneal ITT. *P < 0.05 for effect of diet in wild-type and Id1−/− mice.
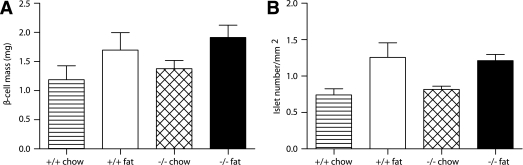
Id1 deletion does not affect β-cell mass or islet number.
We next investigated potential mechanisms responsible for the increased insulin levels observed after a glucose challenge in Id1−/− mice. To determine whether Id1 plays a role in the regulation of β-cell mass, we performed morphometric analyses of pancreas sections from wild-type and Id1−/− mice fed a chow or a high-fat diet. There were no differences in β-cell mass or in the number of islets between wild-type and Id1−/− mice fed a chow diet (Fig. 4A and B). As observed previously (24), β-cell mass was increased in fat-fed mice, but this occurred irrespective of the genotype (Fig. 4A). In a similar manner, high-fat feeding increased the number of islets in both genotypes (Fig. 4B). We found no evidence of β-cell apoptosis irrespective of genotype or diet. These data suggest that changes in β-cell capacity do not contribute to the increased insulin levels in Id1−/− mice.
FIG. 4.
Comparison of β-cell mass and islet number in wild-type and Id1−/− mice fed either a chow (striped bars and hatched bars, respectively) or a high-fat (white bars and black bars, respectively) diet. A: β-Cell mass of wild-type (n = 3) and Id1−/− (n = 4) mice fed a chow diet and wild-type (n = 4) and Id1−/− (n = 5) mice fed a high-fat diet. ANOVA: P < 0.05 for effect of fat diet in wild-type and Id1−/− mice. B: Number of islets per area of pancreas. ANOVA: P < 0.01 for effect of fat diet in wild-type and Id1−/− mice.
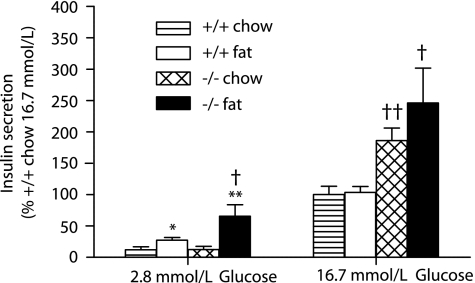
Islets from Id1−/− mice display enhanced insulin secretion.
To investigate the role of Id1 in insulin secretion, we assessed GSIS in islets isolated from wild-type and Id1−/− mice fed either a chow or a high-fat diet. Compared with chow-fed mice, insulin secretion at a low stimulatory level of glucose (2.8 mmol/L) was significantly increased in islets isolated from fat-fed mice (Fig. 5). This fat diet–induced enhancement of insulin secretion at low glucose was greater in islets from Id1−/− mice compared with wild-type controls, suggesting that Id1 expression inhibits basal secretion under conditions of lipid oversupply. At a high stimulatory level of glucose (16.7 mmol/L), insulin secretion in islets isolated from Id1−/− mice was significantly increased compared with wild-type controls (Fig. 5), especially after high-fat feeding. Note, islet insulin content (Supplementary Fig. 3) and total protein levels (Supplementary Fig. 4) were similar between the genotypes, and no differences were detected in KCl-stimulated insulin secretion (Supplementary Fig. 5). These results suggest that Id1 expression inhibits GSIS in mouse islets, particularly under conditions of lipid oversupply and/or insulin resistance. The increased insulin levels in mice with Id1 deletion are therefore likely the result of enhanced insulin release from islets.
FIG. 5.
Effects of Id1 deletion on GSIS ex vivo in isolated islets. Batches of islets isolated from wild-type mice fed a chow (striped bar, n = 6) or a high-fat (white bar, n = 7) diet and Id1−/− mice fed a chow (hatched bar, n = 7) or a high-fat (black bar, n = 5) diet were incubated at low (2.8 mmol/L) or high glucose (16.7 mmol/L) for 1 h. Insulin was measured in an aliquot of the media by radioimmunoassay. *P < 0.05 for effect of fat diet in wild-type mouse islets at low glucose, **P < 0.01 for effect of fat diet in Id1−/− mouse islets at low glucose, †P < 0.05 for effect of genotype in fat-fed mouse islets at low and high glucose, and ††P < 0.01 for effect of genotype in chow-fed mouse islets at high glucose.
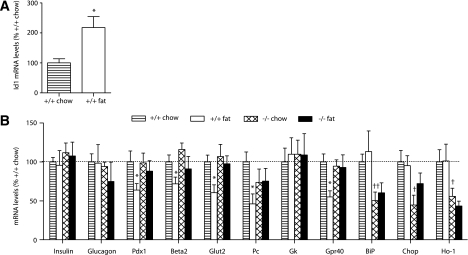
Id1−/− mice are protected against diet-induced loss of β-cell gene expression.
We next examined expression of several genes involved in the maintenance and specialized function of the β-cell phenotype. mRNA levels were assessed in islets isolated from wild-type and Id1−/− mice fed a chow or a high-fat diet. Id1 mRNA levels were increased by twofold in islets from fat-fed mice compared with chow-fed controls (Fig. 6A). Id1 mRNA levels were undetectable in islets from Id1−/− mice (not shown). Expression of the islet hormones, insulin and glucagon, were not affected by either diet or genotype (Fig. 6B). Pdx1 and Beta2 are transcription factors that are important for the maintenance of β-cell differentiation (25). mRNA levels of Pdx1 and Beta2 were significantly reduced in islets of fat-fed wild-type mice; Pdx1 was downregulated by ∼40% and Beta2 by ∼30% (Fig. 6B). It is striking that in islets from fat-fed Id1−/− mice, expression of Pdx1 and Beta2 were maintained at levels observed in chow-fed mice (Fig. 6B). We next evaluated several genes involved in β-cell glucose metabolism. The glucose transporter Glut2 and the anaplerotic enzyme pyruvate carboxylase (Pc) were downregulated in islets from fat-fed wild-type mice, whereas these metabolic genes were not affected by high-fat feeding in Id1−/− islets (Fig. 6B). Not all genes involved in glucose metabolism were altered; glucokinase mRNA levels were unchanged by diet or genotype. The G-protein–coupled receptor Gpr40 may play a role in both fatty acid and glucose stimulation of insulin secretion (26–28). Consistent with previous findings (26), we found that Gpr40 expression was downregulated by ∼50% in fat-fed wild-type mice (Fig. 6B). In contrast, Gpr40 expression was unchanged after fat feeding in Id1−/− mice (Fig. 6B). These data demonstrate that islets from Id1−/− mice are protected against high-fat diet–induced loss of β-cell gene expression, suggesting a role for Id1 in β-cell dedifferentiation under conditions of increased lipid supply.
FIG. 6.
Relative gene expression levels in islets. Islets were isolated from wild-type and Id1−/− mice fed a chow (striped bars and hatched bars, respectively) or a high-fat (white bars and black bars, respectively) diet. Total RNA was extracted, reverse transcribed, and analyzed by real-time PCR. A: Expression of Id1 in islets of wild-type mice fed a chow (n = 5) or a high-fat (n = 6) diet. *P < 0.05 for effect of fat diet. B: Expression of the genes indicated in wild-type mice fed a chow (n = 5–6) or a high-fat (n = 5–7) diet and Id1−/− mice fed a chow (n = 5–7) or a high-fat (n = 4–7) diet. *P < 0.05 for effect of fat diet in wild-type mice, †P < 0.05, and ††P < 0.01 for effect of genotype in chow-fed mice.
mRNA levels of stress genes are reduced in islets of Id1−/− mice.
The role of cellular stress and stress response mediators in the failure of β-cells in diabetes has been the subject of much recent attention (29,30). BiP is an endoplasmic reticulum (ER) chaperone and key regulator of the ER stress response (31), and XBP1 and Chop are ER stress-inducible transcription factors (30,32). It is interesting that both BiP and Chop mRNA levels were significantly reduced in islets from Id1−/− mice compared with wild-type controls (Fig. 6B). Also reduced in Id1−/− islets were levels of the spliced (activated) form of Xbp1 mRNA (fold change, wild-type chow: 1.00 ± 0.02; wild-type fat: 1.04 ± 0.04; Id1−/− chow: 0.94 ± 0.04; Id1−/− fat: 0.92 ± 0.03; P < 0.05 for genotype effect). The antioxidant heme oxygenease-1 (Ho-1) is induced by oxidative stress (33). Ho-1 mRNA levels were significantly reduced in islets of Id1−/− mice compared with wild-type controls (Fig. 6B). These data indicate that the augmentation of insulin secretion is accompanied by reduced stress gene expression in islets from Id1−/− mice.
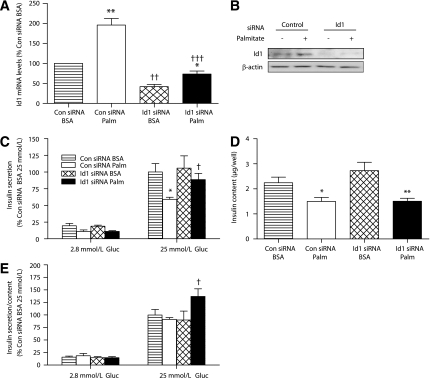
Id1 plays a role in regulating insulin secretory changes that accompany chronic palmitate exposure in MIN6 cells.
To examine the role of Id1 in insulin secretion in β-cells, we used the highly differentiated and glucose responsive mouse insulinoma β-cell line, MIN6 (34). Chronic exposure of MIN6 cells to elevated fatty acids has previously been shown to induce mild insulin secretory dysfunction and changes in gene expression consistent with a loss of β-cell differentiation (18). Exposure of MIN6 cells to the saturated fatty acid palmitate (0.4 mmol/L palmitate coupled to 0.92% BSA) for 48 h led to a twofold increase in Id1 mRNA levels (Fig. 7A). MIN6 cells were transfected with Id1 or control siRNA. Id1 siRNA transfection led to reduced Id1 mRNA (Fig. 7A) and protein (Fig. 7B) levels in MIN6 cells exposed to palmitate or BSA. We next assessed insulin secretion under these experimental conditions. In control siRNA-treated cells, chronic palmitate exposure significantly reduced the subsequent insulin secretory response to high glucose stimulation (Fig. 7C). In contrast, the insulin secretory response to high glucose stimulation was maintained in palmitate-treated MIN6 cells after knockdown of Id1 with siRNA (Fig. 7C), suggesting a requirement of Id1 expression in the fatty acid–mediated decrease in insulin secretion. We also measured cellular insulin content in these treatment groups. Consistent with previous studies (18,35,36), cellular insulin content was significantly reduced in fatty acid–treated cells (Fig. 7D). However, this was not affected by Id1 knockdown; chronic palmitate treatment led to a similar depletion of cellular insulin content in control siRNA- and Id1 siRNA-transfected cells (Fig. 7D). The recalculation of insulin secretion as a function of total insulin content negated the change in insulin secretion as a result of palmitate exposure in control siRNA-transfected cells (Fig. 7E). However, the data emphasize the significantly increased insulin secretory response to high glucose stimulation in palmitate-pretreated MIN6 after knockdown of Id1 (Fig. 7E). In a similar manner, increased insulin secretion was found in palmitate-pretreated islets with Id1 deletion (Supplementary Fig. 6). Taken together, the data suggest that Id1 expression inhibits secretory function without affecting insulin content under conditions of increased lipid supply.
FIG. 7.
siRNA-mediated silencing of Id1 in MIN6 cells increases insulin secretion after chronic palmitate exposure. MIN6 cells transfected with Id1 ON-TARGETplus SMARTpool siRNA or control Non-Targeting siRNA were pretreated with either 0.92% BSA alone or 0.92% BSA coupled to 0.4 mmol/L palmitate for 48 h. A: Expression of Id1. Total RNA was extracted, reverse transcribed, and analyzed by real-time PCR in control (Con) siRNA-transfected cells pretreated with BSA (striped bars) or BSA-coupled palmitate (Palm) (white bars) and in Id1 siRNA-transfected cells pretreated with BSA (hatched bars) or BSA-coupled palmitate (black bars) (n = 5 separate experiments in each group). **P < 0.01 for palmitate effect in control siRNA-transfected cells, *P < 0.05 for palmitate effect in Id1 siRNA-transfected cells, ††P < 0.01 for effect of Id1 siRNA in BSA-treated cells, and †††P < 0.001 for effect of Id1 siRNA in palmitate-treated cells. B: Protein extracts were immunoblotted for Id1 or β-actin. Representative images are shown (n = 3 separate experiments in each group). C–E: Control siRNA-transfected cells pretreated with BSA (striped bars) or BSA-coupled palmitate (white bars) and Id1 siRNA-transfected cells pretreated with BSA (hatched bars) or BSA-coupled palmitate (black bars). After pretreatment, cells were incubated in medium containing 2.8 or 25 mmol/L glucose (Gluc) for 1 h. Medium was taken to determine levels of insulin secretion (C), and results are expressed as a percentage of insulin secretion in control siRNA-transfected BSA-pretreated cells incubated with 25 mmol/L glucose (n = 4 separate experiments in each group; *P < 0.05 for effect of palmitate pretreatment in control siRNA-transfected cells at 25 mmol/L glucose and †P < 0.05 for effect of Id1 siRNA in palmitate-pretreated cells at 25 mmol/L glucose). Total insulin content (D) was determined in cell lysates (*P < 0.05 for effect of palmitate pretreatment in control siRNA-transfected cells and **P < 0.01 for effect of palmitate pretreatment in Id1 siRNA-transfected cells). Ratio of insulin secretion to total insulin content (E) expressed as a percentage of ratios in control siRNA-transfected BSA-pretreated cells incubated with 25 mmol/L glucose (†P < 0.05 for effect of Id1 siRNA in palmitate-pretreated cells at 25 mmol/L glucose).
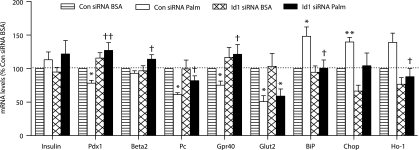
Id1 plays a role in regulating gene expression changes that accompany chronic palmitate exposure in MIN6 cells.
We next investigated the role of Id1 in the regulation of gene expression in MIN6 cells. Chronic (48-h) palmitate treatment of control MIN6 cells led to reduced expression of Pdx1, Pc, Gpr40, and Glut2, whereas insulin mRNA levels were not affected (Fig. 8). It is striking that the knockdown of Id1 in MIN6 cells prevented the palmitate-mediated downregulation of Pdx1, Pc, and Gpr40 (Fig. 8). On the other hand, Glut2 was not affected by Id1 siRNA. Knockdown of Id1 in palmitate-treated cells significantly increased Pdx1 by ∼50%, Pc by ∼20%, and Gpr40 by ∼40% compared with control palmitate-treated cells. Although not reduced by palmitate treatment in control MIN6 cells, Beta2 mRNA levels were significantly increased by ∼20% in palmitate-treated cells after knockdown of Id1 (Fig. 8). These results suggest that Id1 expression is necessary for the downregulation of several important β-cell genes under conditions of chronic lipid oversupply. In addition, the knockdown of Id1 prevented the palmitate-mediated upregulation of stress genes BiP, Chop, Ho-1 (Fig. 8), and spliced Xbp1; palmitate treatment induced the levels of spliced Xbp1 mRNA by 1.07 ± 0.01 fold in control cells and by 1.03 ± 0.003 fold in cells with Id1 knockdown (P < 0.05 for effect of Id1 siRNA).
FIG. 8.
Relative gene expression levels in MIN6 cells. MIN6 cells transfected with Id1 ON-TARGETplus SMARTpool siRNA or control Non-Targeting siRNA were treated with either 0.92% BSA alone or 0.92% BSA coupled to 0.4 mmol/L palmitate (Palm) for 48 h. Total RNA was extracted, reverse transcribed, and relative expression of the genes indicated determined by PCR for control siRNA-transfected cells treated with BSA (striped bars) or BSA-coupled palmitate (white bars) and Id1 siRNA-transfected cells treated with BSA (hatched bars) or BSA-coupled palmitate (black bars). Results are expressed as a percentage of mRNA levels in control (Con) siRNA-transfected cells treated with BSA (n = 4–7 in each group). *P < 0.05, **P < 0.01 for effect of palmitate treatment in control siRNA- and Id1 siRNA-transfected cells, †P < 0.05, and ††P < 0.01 for effect of Id1 siRNA in palmitate-treated cells.
DISCUSSION
The molecular mechanisms by which factors such as lipids and glucose contribute to β-cell failure in type 2 diabetes have been the subject of much attention. We have shown that ablation of Id1 improves whole body glucose disposal by augmenting insulin secretion. This enhanced insulin release compensates for insulin resistance and is associated with reduced stress gene expression within islets and protection from lipid-mediated β-cell dedifferentiaton. These findings suggest a novel role of Id1 in the development of β-cell dysfunction and glucose intolerance.
The improved glucose tolerance with ablation of Id1 is likely the result of direct effects in β-cells. Energy intake, body weight, fat accumulation, glucagon levels, and insulin action were similar in the absence of Id1 expression, and an equivalent degree of insulin resistance was evident after high-fat feeding. Furthermore, the phenotype of enhanced insulin secretion and protection against β-cell dedifferentiaton in Id1−/− mice is broadly recapitulated in MIN6 cells after knockdown of Id1 using siRNA. It is interesting that enhanced GSIS was dependent on prior lipid exposure in MIN6 cells and was also most apparent in fat-fed mice. These striking outcomes likely represent the effects of chronically elevated Id1 expression under conditions of prolonged lipid oversupply compared with the more subtle effects of low-level Id1 expression in normal β-cells. While conditions of chronic lipid oversupply were examined in the current study, we do not rule out the possibility that Id1 expression is also involved in the β-cell secretory dysfunction induced by other factors, including chronically increased glucose levels (8).
Although additional mechanisms may contribute, reduced levels of cellular stress and protection against loss of β-cell gene expression may underlie the improvement in insulin secretion after Id1 ablation. The loss of β-cell gene expression has been linked to secretory dysfunction in several animal models of diabetes (6–10). The current study demonstrates that Id1 expression is required for the dysregulation of several β-cell genes under conditions of chronic lipid oversupply. This includes the downregulation of important β-cell transcription factors Pdx1 and Beta2, which could contribute to the altered expression of genes essential for GSIS (25). Furthermore, we demonstrate that Id1 expression is required for the downregulation of Gpr40 expression, which has potentially important implications for GSIS and its potentiation by fatty acids (26–28). Cellular stress can play a major role in the severity of β-cell dedifferentiation and dysfunction (29,30,33,37). That Id1 could be involved in oxidative stress is supported by a study in cardiac myocytes (38). Islets from Id1−/− mice may be less susceptible to stress and, consequently, better able to adapt to demanding conditions such as occur with chronic lipid oversupply and insulin resistance. It is noteworthy that forced expression of Id1 in insulinoma cells has been shown to inhibit insulin promoter activity (39). However, the increased levels of Id1 expression in fat-fed mouse islets and lipid-treated MIN6 cells (approximately twofold in both models) are associated with unchanged insulin mRNA levels. Furthermore, the beneficial effects of Id1 inhibition on insulin secretion occur without changes in insulin transcription or insulin content; that is, cellular insulin depletion was not affected by knockdown of Id1 in lipid-treated MIN6 cells (Fig. 7D), and the intensity of insulin staining was similar in pancreas sections of wild-type and Id1−/− mice fed a high-fat diet (not shown). Thus, under the conditions of the current study, abnormalities in the glucose-sensing machinery accompany Id1 expression, whereas insulin expression is better maintained. A similar phenomenon was observed in several animal models of diabetes in which glucose-sensing genes and islet transcription factors were reduced in the early/mild phases of the disease, whereas insulin levels were affected only after progression to more severe stages (6,8,9).
In other cell types, Id proteins are implicated in a number of cellular processes, including cell cycle regulation, growth, and proliferation (11–15,40). It is noteworthy that Id2 expression may play a role in the expansion of pancreatic ductal progenitor cells and β-cells (41,42). Moreover, several cell cycle and early growth response genes that could interact with Id1 are important for the regulation of β-cell growth (43–48). Our studies show that Id1 expression is not required for the establishment of normal β-cell mass in adult mice or for its expansion after high-fat feeding. This suggests that the effects of Id1 expression on β-cell differentiation are direct and independent of proliferation. It is interesting that a role for Id1 in promoting the β-cell phenotype has been proposed (20). Thus, Id1 regulation of the β-cell phenotype may be well correlated with the effects of glucose and fatty acids after both acute (stimulatory) and chronic (inhibitory) exposure.
Whether Id1-mediated inhibition of β-cell differentiation is the result of the ability of Id proteins to sequester HLH transcription factors and inhibit E-box activation of transcription is not known. It is worth noting that HLH transcription factors, such as Beta2, are important for pancreas development and β-cell differentiation (25,49,50). However, an interaction with Beta2 would be expected to also affect insulin gene transcription (51). Possible mechanisms by which Id1 expression mediates its effects include the creation of an imbalance between E proteins and binding partners or interactions with Pax proteins or members of the transforming growth factor-β family (11–15).
In conclusion, we have identified a novel role of Id1 in the negative regulation of insulin secretion and β-cell differentiation. Studies from Id1−/− mice and MIN6 cells demonstrate that the beneficial effects of Id1 inhibition include augmentation of insulin secretion, reduced islet stress, and protection from lipid-induced glucose intolerance and β-cell dedifferentiation. The findings suggest that Id1 expression may provide a molecular link between chronic lipid oversupply and β-cell dedifferentiation and dysfunction. Id1 may therefore represent a new target for interventions aimed at improving disordered glucose homeostasis and β-cell dysfunction.
ACKNOWLEDGMENTS
This work was supported by a grant from the National Health and Medical Research Council (NHMRC) of Australia and a Juvenile Diabetes Research Foundation/NHMRC Program grant.
No potential conflicts of interest relevant to this article were reported.
M.C.Å. designed and performed the experiments and wrote the manuscript. D.R.L. conceived and designed the studies, performed the animal work, and wrote the manuscript.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db11-0083/-/DC1.
See accompanying commentary, p. 2455.
REFERENCES
- 1.Kahn SE, Zraika S, Utzschneider KM, Hull RL. The beta cell lesion in type 2 diabetes: there has to be a primary functional abnormality. Diabetologia 2009;52:1003–1012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Prentki M, Nolan CJ. Islet beta cell failure in type 2 diabetes. J Clin Invest 2006;116:1802–1812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weir GC, Marselli L, Marchetti P, Katsuta H, Jung MH, Bonner-Weir S. Towards better understanding of the contributions of overwork and glucotoxicity to the beta-cell inadequacy of type 2 diabetes. Diabetes Obes Metab 2009;11(Suppl. 4):82–90 [DOI] [PubMed] [Google Scholar]
- 4.Butler AE, Janson J, Bonner-Weir S, Ritzel R, Rizza RA, Butler PC. Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes 2003;52:102–110 [DOI] [PubMed] [Google Scholar]
- 5.Rahier J, Guiot Y, Goebbels RM, Sempoux C, Henquin JC. Pancreatic beta-cell mass in European subjects with type 2 diabetes. Diabetes Obes Metab 2008;10(Suppl. 4):32–42 [DOI] [PubMed] [Google Scholar]
- 6.Jonas JC, Sharma A, Hasenkamp W, et al. Chronic hyperglycemia triggers loss of pancreatic beta cell differentiation in an animal model of diabetes. J Biol Chem 1999;274:14112–14121 [DOI] [PubMed] [Google Scholar]
- 7.Tokuyama Y, Sturis J, DePaoli AM, et al. Evolution of beta-cell dysfunction in the male Zucker diabetic fatty rat. Diabetes 1995;44:1447–1457 [DOI] [PubMed] [Google Scholar]
- 8.Kjørholt C, Åkerfeldt MC, Biden TJ, Laybutt DR. Chronic hyperglycemia, independent of plasma lipid levels, is sufficient for the loss of beta-cell differentiation and secretory function in the db/db mouse model of diabetes. Diabetes 2005;54:2755–2763 [DOI] [PubMed] [Google Scholar]
- 9.Laybutt DR, Glandt M, Xu G, et al. Critical reduction in beta-cell mass results in two distinct outcomes over time. Adaptation with impaired glucose tolerance or decompensated diabetes. J Biol Chem 2003;278:2997–3005 [DOI] [PubMed] [Google Scholar]
- 10.Laybutt DR, Hawkins YC, Lock J, et al. Influence of diabetes on the loss of beta cell differentiation after islet transplantation in rats. Diabetologia 2007;50:2117–2125 [DOI] [PubMed] [Google Scholar]
- 11.Perk J, Iavarone A, Benezra R. Id family of helix-loop-helix proteins in cancer. Nat Rev Cancer 2005;5:603–614 [DOI] [PubMed] [Google Scholar]
- 12.Ruzinova MB, Benezra R. Id proteins in development, cell cycle and cancer. Trends Cell Biol 2003;13:410–418 [DOI] [PubMed] [Google Scholar]
- 13.Yokota Y, Mori S. Role of Id family proteins in growth control. J Cell Physiol 2002;190:21–28 [DOI] [PubMed] [Google Scholar]
- 14.Norton JD. ID helix-loop-helix proteins in cell growth, differentiation and tumorigenesis. J Cell Sci 2000;113:3897–3905 [DOI] [PubMed] [Google Scholar]
- 15.Sikder HA, Devlin MK, Dunlap S, Ryu B, Alani RM. Id proteins in cell growth and tumorigenesis. Cancer Cell 2003;3:525–530 [DOI] [PubMed] [Google Scholar]
- 16.Benezra R, Davis RL, Lockshon D, Turner DL, Weintraub H. The protein Id: a negative regulator of helix-loop-helix DNA binding proteins. Cell 1990;61:49–59 [DOI] [PubMed] [Google Scholar]
- 17.Yan W, Young AZ, Soares VC, Kelley R, Benezra R, Zhuang Y. High incidence of T-cell tumors in E2A-null mice and E2A/Id1 double-knockout mice. Mol Cell Biol 1997;17:7317–7327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Busch AK, Cordery D, Denyer GS, Biden TJ. Expression profiling of palmitate- and oleate-regulated genes provides novel insights into the effects of chronic lipid exposure on pancreatic beta-cell function. Diabetes 2002;51:977–987 [DOI] [PubMed] [Google Scholar]
- 19.Webb GC, Akbar MS, Zhao C, Steiner DF. Expression profiling of pancreatic beta cells: glucose regulation of secretory and metabolic pathway genes. Proc Natl Acad Sci USA 2000;97:5773–5778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wice BM, Bernal-Mizrachi E, Permutt MA. Glucose and other insulin secretagogues induce, rather than inhibit, expression of Id-1 and Id-3 in pancreatic islet beta cells. Diabetologia 2001;44:453–463 [DOI] [PubMed] [Google Scholar]
- 21.Lyden D, Young AZ, Zagzag D, et al. Id1 and Id3 are required for neurogenesis, angiogenesis and vascularization of tumour xenografts. Nature 1999;401:670–677 [DOI] [PubMed] [Google Scholar]
- 22.Åkerfeldt MC, Howes J, Chan JY, et al. Cytokine-induced beta-cell death is independent of endoplasmic reticulum stress signaling. Diabetes 2008;57:3034–3044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pan L, Sato S, Frederick JP, Sun XH, Zhuang Y. Impaired immune responses and B-cell proliferation in mice lacking the Id3 gene. Mol Cell Biol 1999;19:5969–5980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schmitz-Peiffer C, Laybutt DR, Burchfield JG, et al. Inhibition of PKCepsilon improves glucose-stimulated insulin secretion and reduces insulin clearance. Cell Metab 2007;6:320–328 [DOI] [PubMed] [Google Scholar]
- 25.Bernardo AS, Hay CW, Docherty K. Pancreatic transcription factors and their role in the birth, life and survival of the pancreatic beta cell. Mol Cell Endocrinol 2008;294:1–9 [DOI] [PubMed] [Google Scholar]
- 26.Kebede M, Alquier T, Latour MG, Semache M, Tremblay C, Poitout V. The fatty acid receptor GPR40 plays a role in insulin secretion in vivo after high-fat feeding. Diabetes 2008;57:2432–2437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kebede MA, Alquier T, Latour MG, Poitout V. Lipid receptors and islet function: therapeutic implications? Diabetes Obes Metab 2009;11(Suppl. 4):10–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alquier T, Peyot ML, Latour MG, et al. Deletion of GPR40 impairs glucose-induced insulin secretion in vivo in mice without affecting intracellular fuel metabolism in islets. Diabetes 2009;58:2607–2615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Poitout V, Robertson RP. Glucolipotoxicity: fuel excess and beta-cell dysfunction. Endocr Rev 2008;29:351–366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eizirik DL, Cardozo AK, Cnop M. The role for endoplasmic reticulum stress in diabetes mellitus. Endocr Rev 2008;29:42–61 [DOI] [PubMed] [Google Scholar]
- 31.Bertolotti A, Zhang Y, Hendershot LM, Harding HP, Ron D. Dynamic interaction of BiP and ER stress transducers in the unfolded-protein response. Nat Cell Biol 2000;2:326–332 [DOI] [PubMed] [Google Scholar]
- 32.Wang XZ, Lawson B, Brewer JW, et al. Signals from the stressed endoplasmic reticulum induce C/EBP-homologous protein (CHOP/GADD153). Mol Cell Biol 1996;16:4273–4280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Laybutt DR, Kaneto H, Hasenkamp W, et al. Increased expression of antioxidant and antiapoptotic genes in islets that may contribute to beta-cell survival during chronic hyperglycemia. Diabetes 2002;51:413–423 [DOI] [PubMed] [Google Scholar]
- 34.Miyazaki J, Araki K, Yamato E, et al. Establishment of a pancreatic beta cell line that retains glucose-inducible insulin secretion: special reference to expression of glucose transporter isoforms. Endocrinology 1990;127:126–132 [DOI] [PubMed] [Google Scholar]
- 35.Bollheimer LC, Skelly RH, Chester MW, McGarry JD, Rhodes CJ. Chronic exposure to free fatty acid reduces pancreatic beta cell insulin content by increasing basal insulin secretion that is not compensated for by a corresponding increase in proinsulin biosynthesis translation. J Clin Invest 1998;101:1094–1101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhou YP, Grill VE. Long-term exposure of rat pancreatic islets to fatty acids inhibits glucose-induced insulin secretion and biosynthesis through a glucose fatty acid cycle. J Clin Invest 1994;93:870–876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mathews CE, Leiter EH. Constitutive differences in antioxidant defense status distinguish alloxan-resistant and alloxan-susceptible mice. Free Radic Biol Med 1999;27:449–455 [DOI] [PubMed] [Google Scholar]
- 38.Tanaka K, Pracyk JB, Takeda K, et al. Expression of Id1 results in apoptosis of cardiac myocytes through a redox-dependent mechanism. J Biol Chem 1998;273:25922–25928 [DOI] [PubMed] [Google Scholar]
- 39.Cordle SR, Henderson E, Masuoka H, Weil PA, Stein R. Pancreatic beta-cell-type-specific transcription of the insulin gene is mediated by basic helix-loop-helix DNA-binding proteins. Mol Cell Biol 1991;11:1734–1738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Swarbrick A, Åkerfeldt MC, Lee CS, et al. Regulation of cyclin expression and cell cycle progression in breast epithelial cells by the helix-loop-helix protein Id1. Oncogene 2005;24:381–389 [DOI] [PubMed] [Google Scholar]
- 41.Hua H, Sarvetnick N. ID2 promotes the expansion and survival of growth-arrested pancreatic beta cells. Endocrine 2007;32:329–337 [DOI] [PubMed] [Google Scholar]
- 42.Hua H, Zhang YQ, Dabernat S, et al. BMP4 regulates pancreatic progenitor cell expansion through Id2. J Biol Chem 2006;281:13574–13580 [DOI] [PubMed] [Google Scholar]
- 43.Garnett KE, Chapman P, Chambers JA, Waddell ID, Boam DS. Differential gene expression between Zucker fatty rats and Zucker diabetic fatty rats: a potential role for the immediate-early gene Egr-1 in regulation of beta cell proliferation. J Mol Endocrinol 2005;35:13–25 [DOI] [PubMed] [Google Scholar]
- 44.Georgia S, Hinault C, Kawamori D, et al. Cyclin D2 is essential for the compensatory beta-cell hyperplastic response to insulin resistance in rodents. Diabetes 2010;59:987–996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kushner JA, Ciemerych MA, Sicinska E, et al. Cyclins D2 and D1 are essential for postnatal pancreatic beta-cell growth. Mol Cell Biol 2005;25:3752–3762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cozar-Castellano I, Harb G, Selk K, et al. Lessons from the first comprehensive molecular characterization of cell cycle control in rodent insulinoma cell lines. Diabetes 2008;57:3056–3068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pelengaris S, Khan M, Evan GI. Suppression of Myc-induced apoptosis in beta cells exposes multiple oncogenic properties of Myc and triggers carcinogenic progression. Cell 2002;109:321–334 [DOI] [PubMed] [Google Scholar]
- 48.Laybutt DR, Weir GC, Kaneto H, et al. Overexpression of c-Myc in beta-cells of transgenic mice causes proliferation and apoptosis, downregulation of insulin gene expression, and diabetes. Diabetes 2002;51:1793–1804 [DOI] [PubMed] [Google Scholar]
- 49.Gu C, Stein GH, Pan N, et al. Pancreatic beta cells require NeuroD to achieve and maintain functional maturity. Cell Metab 2010;11:298–310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Naya FJ, Huang HP, Qiu Y, et al. Diabetes, defective pancreatic morphogenesis, and abnormal enteroendocrine differentiation in BETA2/neuroD-deficient mice. Genes Dev 1997;11:2323–2334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kaneto H, Sharma A, Suzuma K, et al. Induction of c-Myc expression suppresses insulin gene transcription by inhibiting NeuroD/BETA2-mediated transcriptional activation. J Biol Chem 2002;277:12998–13006 [DOI] [PubMed] [Google Scholar]