Abstract
OBJECTIVE
Diabetes is associated with vascular oxidative stress, activation of NADPH oxidase, and uncoupling of nitric oxide (NO) synthase (endothelial NO synthase [eNOS]). Pentaerithrityl tetranitrate (PETN) is an organic nitrate with potent antioxidant properties via induction of heme oxygenase-1 (HO-1). We tested whether treatment with PETN improves vascular dysfunction in the setting of experimental diabetes.
RESEARCH DESIGN AND METHODS
After induction of hyperglycemia by streptozotocin (STZ) injection (60 mg/kg i.v.), PETN (15 mg/kg/day p.o.) or isosorbide-5-mononitrate (ISMN; 75 mg/kg/day p.o.) was fed to Wistar rats for 7 weeks. Oxidative stress was assessed by optical methods and oxidative protein modifications, vascular function was determined by isometric tension recordings, protein expression was measured by Western blotting, RNA expression was assessed by quantitative RT-PCR, and HO-1 promoter activity in stable transfected cells was determined by luciferase assays.
RESULTS
PETN, but not ISMN, improved endothelial dysfunction. NADPH oxidase and serum xanthine oxidase activities were significantly reduced by PETN but not by ISMN. Both organic nitrates had minor effects on the expression of NADPH oxidase subunits, eNOS and dihydrofolate reductase (Western blotting). PETN, but not ISMN, normalized the expression of GTP cyclohydrolase-1, extracellular superoxide dismutase, and S-glutathionylation of eNOS, thereby preventing eNOS uncoupling. The expression of the antioxidant enzyme, HO-1, was increased by STZ treatment and further upregulated by PETN, but not ISMN, via activation of the transcription factor NRF2.
CONCLUSIONS
In contrast to ISMN, the organic nitrate, PETN, improves endothelial dysfunction in diabetes by preventing eNOS uncoupling and NADPH oxidase activation, thereby reducing oxidative stress. Thus, PETN therapy may be suited to treat patients with cardiovascular complications of diabetes.
Diabetes is a major risk factor for the development of cardiovascular disease (1), and endothelial dysfunction is encountered early during the development of vascular complications in this setting (2). Animal and human studies have demonstrated that increased oxidative stress largely accounts for this phenomenon. In particular, diabetes has been associated with endothelial dysfunction and vascular superoxide production attributed to the activation of the vascular NADPH oxidase and uncoupling of the endothelial nitric oxide synthase (eNOS) (3). In the setting of diabetes, 3-hydroxy-3-methylglutaryl-coenzyme A-reductase inhibition and AT1 receptor blockade have been demonstrated to prevent the activation of NADPH oxidase and to recouple the dysfunctional eNOS. These effects primarily were mediated by an increase in the expression of the BH4-synthesizing enzyme, GTP cyclohydrolase-1 (GCH-I), leading to the normalization of vascular BH4 levels and to the reduction of vascular oxidative stress and endothelial dysfunction (4,5). Alternatively, the oxidation of the zinc-sulfur complex (4,6) or phosphorylation at Thr495 (7) may contribute to eNOS uncoupling in diabetic tissue.
Organic nitrates are largely used in the therapy of coronary artery disease. These drugs, however, have important adverse effects, such as the induction of nitrate tolerance and endothelial dysfunction (8–10). Complicating these issues, nitrate resistance (i.e., a reduced responsiveness to organic nitrates mainly as a result of increased oxidative stress in vascular tissue) frequently is encountered in the setting of diabetes. Rather than improving vascular function, however, administration of exogenous NO in the form of organic nitrates would lead in this setting to its deterioration, an effect likely attributed to an increased formation of the NO/O2− reaction product, peroxynitrite, and an effect also observed in the setting of hypercholesterolemia (11).
An interesting compound with recently discovered new features is the organic nitrate pentaerithrityl tetranitrate (PETN). In contrast to all other organic nitrates, such as nitroglycerin (GTN), isosorbide-5-mononitrate (ISMN), and isosorbide dinitrate, PETN is an organic nitrate that is devoid of adverse effects, such as the induction of nitrate tolerance, vascular oxidative stress, and endothelial dysfunction (12,13). Providing an explanation for these observations at a molecular level, PETN has been shown to be a powerful inducer of heme oxygenase-1 (HO-1), which subsequently stimulates the expression of ferritin and increases vascular bilirubin and CO levels (14–16), leading to a reduction of oxidative stress in vascular tissue. In contrast, ISMN was reported to induce endothelial dysfunction, a phenomenon that was corrected by vitamin C, pointing to the reactive oxygen species (ROS) stimulatory effects of this organic nitrate (17). In a recent study, we reported that PETN, but not ISMN, improves vascular oxidative stress and dysfunction in two different animal models of arterial hypertension (18).
With the present studies, we sought to determine whether the treatment of diabetic animals with NO donors with and without antioxidant capacities may influence endothelial dysfunction and oxidative stress in a rat model of diabetes.
RESEARCH DESIGN AND METHODS
The GeneJuice transfection reagent was from Merck (Darmstadt, Germany). Zeocin and psiRNAhH1-GFPzeo were purchased from InvivoGen (San Diego, CA). The negative control (SI03650325), the validated anti-NRF2 siRNA (SI03246950), and the HiPerFect HTS reagent were purchased from Qiagen (Hilden, Germany). The passive lysis buffer and the luciferase assay system were from Promega (Mannheim, Germany). Restriction enzymes, calf intestine alkaline phosphatase, Taq DNA polymerase, and dNTPs were purchased from New England Biolabs (Frankfurt, Germany). The High-Capacity cDNA Reverse Transcription Kit was purchased from Applied Biosystems (Darmstadt, Germany). All oligonucleotides and dual-labeled probes were purchased from MWG Biotech (Ebersberg, Germany). The Bradford reagent was obtained from BioRad (Munich, Germany). The plasmid pGL3/hHO-1 was a gift of Dr. S.H. Juan (19). For isometric tension studies, GTN was used from a nitrolingual infusion solution (1 mg/mL) from G. Pohl-Boskamp (Hohenlockstedt, Germany). PETN (25% with 75% lactose) was a gift from Actavis Deutschland (Langenfeld, Germany). For induction of diabetes, we used streptozotocin (STZ) from Fluka (Steinheim, Germany). L-012 (8-amino-5-chloro-7-phenylpyrido[3,4-d]pyridazine-1,4-[2H,3H]dione sodium salt) was purchased from Wako Pure Chemical Industries (Osaka, Japan). All other chemicals were of analytical grade and were obtained from Sigma-Aldrich, Fluka, or Merck.
Animals and in vivo treatment.
All animals were treated in accordance with the Guide for the Care and Use of Laboratory Animals, as adopted by the National Institutes of Health, and approval was granted by the ethics committee of the University Hospital Mainz. A total of 66 male Wistar Rats (aged 6 weeks, 250 g; Charles River Laboratories, Sulzfeld, Germany) were divided into four treatment groups: untreated controls (Ctr group); STZ-induced type 1 diabetes with placebo (STZ group); PETN (15 mg/kg/day p.o.) (+PETN group); or ISMN (75 mg/kg/d p.o.) (+ISMN group) therapy. For induction of type 1 diabetes, rats were injected with a single dose of STZ into the vena dorsalis penis (60 mg/kg i.v., in 5 mmol/L, pH 4.5, citrate buffer). Control animals were injected with the solvent. Organic nitrate treatment by diet (Ssniff Spezialdiäten, Soest, Germany) was started 1 week after STZ injection and continued for 7 weeks. After 8 weeks of total treatment duration, animals were killed under isoflurane anesthesia. Diabetes was diagnosed by measuring glucose levels in whole blood (for STZ-treated rats it was diluted 1:5 with NaCl solution) using the Accu-Chek Sensor System from Roche Diagnostics (Mannheim, Germany). STZ treatment was previously shown to be a valid type 1 diabetes model (20). We recently showed that the vascular dysfunction observed in this model of STZ-induced diabetes can be reversed after insulin administration, which demonstrates the absence of a direct vascular toxicity induced by STZ (21).
Isometric tension studies.
Vasodilator responses to ACh and GTN were assessed with endothelium-intact isolated rat aortic rings mounted for isometric tension recordings in organ chambers, preconstricted with phenylephrine, as described previously (5,22).
Detection of oxidative stress in serum, cardiac membrane fractions, and aorta.
Xanthine oxidase (XO) activity was measured in serum by tracing the reduction of cytochrome c (50 µmol/L) at 550 nm (ɛ550 = 19,500 mmol/L/cm) with hypoxanthine (1 mmol/L) versus allopurinol (1 mmol/L). Antioxidant capacity in serum was measured as previously published (23). Vascular ROS formation was determined using dihydroethidine (DHE) (1 µmol/L)-dependent fluorescence microtopography in aortic cryosections, as described (24). Membrane fractions were prepared, and NADPH oxidase activity was measured by lucigenin (5 µmol/L) enhanced chemiluminescence (ECL) in the presence of NADPH (200 µmol/L), according to a published protocol (24,25).
Western blot analysis.
Isolated aortic tissue was frozen and homogenized in liquid nitrogen. Proteins were separated by SDS-PAGE and blotted onto nitrocellulose membranes. After blocking, immunoblotting was performed with the following antibodies: monoclonal mouse α-actinin (100 kDa) or polyclonal rabbit β-actin (42 kDa) (1:2,500, Sigma-Aldrich) as controls for loading and transfer; polyclonal goat NADPH oxidase-1 (Nox1) (1:100, Santa Cruz Biotechnologies, Santa Cruz, CA) and monoclonal mouse Nox2 (gp91phox, 1:1,000, BD Biosciences, Franklin Lakes, NJ); monoclonal mouse eNOS (1:1,000, BD Biosciences); polyclonal rabbit phospho-Ser1177-eNOS (1:1,000, Upstate Biotechnology, Waltham, MA); monoclonal mouse GTP-cyclohydrolase-I (GCH-I) (1 µg/mL, Abnova, Heidelberg, Germany); monoclonal mouse dihydrofolate reductase (DHFR) (1 µg/mL, RDI Division of Fitzgerald Industries, Acton, MA); monoclonal mouse HO-1 (4 µg/mL, Stressgen, San Diego, CA); and polyclonal rabbit extracellular superoxide dismutase (ecSOD) (1:1,000, Assay Designs, Ann Arbor, MI). Detection and quantification were performed by ECL with peroxidase-conjugated anti-rabbit/mouse (1:10,000; Vector Laboratories, Burlingame, CA) and anti-goat (1:5,000; Santa Cruz Biotechnologies) secondary antibodies. Densitometric quantification of antibody-specific bands was performed with a ChemiLux Imager (CsX-1400M; Intas, Göttingen, Germany) and Gel-Pro Analyzer software (Media Cybernetics, Bethesda, MD).
Immunoprecipitation.
M-280 sheep anti-mouse IgG-coated beads from Invitrogen (Darmstadt, Germany) were used along with a monoclonal mouse eNOS (BD Biosciences) antibody. The beads were loaded with the eNOS antibody and cross-linked according to the manufacturer’s instructions. Next, the aortic homogenates were incubated with the eNOS antibody beads, precipitated with a magnet, washed and transferred to gel, and subjected to SDS-PAGE, followed by a standard Western blot procedure using a monoclonal mouse antibody against S-glutathionylated proteins from Virogen (Watertown, MA) at a dilution of 1:1,000 under nonreducing conditions.
Dot-blot analysis.
Cardiac oxidative stress was assessed by dot-blot analysis of cardiac tissues, which was modified from a previous report (26). In brief, 100 µL (0.2 µg/µL protein based on the Bradford analysis) of the homogenized tissue sample was transferred to a Protran BA85 (0.45 μm) nitrocellulose membrane (Schleicher and Schuell, Dassel, Germany) using the Minifold I vacuum dot-blot system (Schleicher and Schuell). Each slot was washed with 250 μL PBS, and the membrane was dried for 15 min at 60°C. For detection of nitrated protein, a mouse monoclonal 3-nitrotyrosine (3NT) antibody (Upstate Biotechnology, Lake Placid, NY) was used at a dilution of 1:1,000. Positive bands were detected by enhanced chemiluminescence after incubation with a peroxidase-coupled secondary antibody (GAM-POX, 1:5,000) (Vector Laboratories). All incubation and washing steps were performed according to the manufacturer’s instructions. Densitometric quantification of the dots was performed as described in the Western blot section.
Detection of oxidative stress in cardiac membrane fractions by high-performance liquid chromatography–based quantification of 2-hydroxyethidium.
Superoxide formation in heart membrane fractions also was measured by a high-performance liquid chromatography (HPLC)-based method to quantify ethidium and 2-hydroxyethidium yield from 50 µmol/L DHE, as previously described (5,27). In brief, cardiac membrane fractions were incubated with 50 µmol/L DHE for 30 min at 37°C in PBS buffer and snap frozen and stored at −80°C until they were homogenized in 50% acetonitrile/50% PBS, centrifuged, and 50 μL of the supernatant were subjected to HPLC analysis. The system consisted of a control unit, two pumps, a mixer, detectors, a column oven, a degasser, and an autosampler from Jasco (AS-2057 Plus; Groß-Umstadt, Germany) and a C18-Nucleosil 100-3 (125 × 4) column from Macherey and Nagel (Düren, Germany). A high-pressure gradient was used with acetonitrile and 25 mmol/L citrate buffer, pH 2.2, as mobile phases with the following percentages of the organic solvent: 0 min, 36%; 7 min, 40%; 8–12 min, 95%; and 13 min, 36%. The flow was 1 mL/min, and DHE was detected by its absorption at 355 nm, whereas 2-hydroxyethidium and ethidium were detected by fluorescence (excitation wavelength 480 nm/emission wavelength 580 nm). The signal was normalized on wet weight of the heart tissue.
Cell culture.
Human endothelial cells (EA.hy 926 cells) (28) were grown in Dulbecco’s modified Eagle’s medium (Sigma) with 10% FCS, 2 mmol/L l-glutamine, 1 mmol/L sodium pyruvate, 100 IU/mL penicillin, 100 μg/mL streptomycin, and 1× hypoxanthine, amethopterin/methotrexate, thymine (HAT), as previously described (29). Human epithelial colon carcinoma DLD-1 cells (American Type Culture Collection no. CCL-221) and DLD-1-HO-1-prom (see below) were grown in Dulbecco’s modified Eagle’s medium with 10% inactivated FBS, 2 mmol/L l-glutamine, penicillin, and streptomycin.
Establishment of DLD-1-HO-1-prom cells.
To generate a PCR fragment containing an 11-kb promoter fragment of the human HO-1 gene, chromosomal DNA from DLD-1 cells and the oligonucleotides huHO-1prom5P1 CCGATATCAAGGCTGATCCCAGGCTAAC and huHO-1prom3P1 TGGGCAACATCAGGAACTTAG were used for the amplification reaction. The PCR fragment was digested with Eco RV and Eco R1 and cloned into pGL3/hHO-1 (19) to generate pGL3-huHO-1-prom-11kb. To generate DLD-1 cells stably transfected with pGL3-huHO-1-prom-11kb, cells were cotransfected with 2.5 µg pGL3-huHO-1-prom-11kb and 2.5 µg psiRNAhH1-GFPzeo (expressing a zeocin–green fluorescent protein fusion protein [GFP]) with GeneJuice, according to the manufacturer’s recommendations. Pools of stable transfectants from one plate were selected with zeocin (0.2 mg/mL) and for GFP expression by fluorescence-activated cell sorting, resulting in the DLD-1-HO-1-prom cell line.
Transient transfection of cells with siRNAs.
Cells were transiently transfected with a validated anti-NRF2 siRNA (SI03246950) or a negative control siRNA (SI03650325) by lipofection with the HiPerFect HTS Reagent, according to the manufacturer’s recommendations.
Statistical analysis.
Results are expressed as means ± SEM. t Tests or one-way ANOVA (with Bonferroni or Dunn correction for comparison of multiple means) were used for comparisons of vasodilator potency and efficacy, ROS detection by chemiluminescence, cytochrome c reduction or fluorescence, as well as protein expression, RNA expression, luciferase activity, and antioxidant capacity. The EC50 value for each experiment was obtained by log transformation. P values < 0.05 were considered significant.
RESULTS
Blood glucose and weight gain.
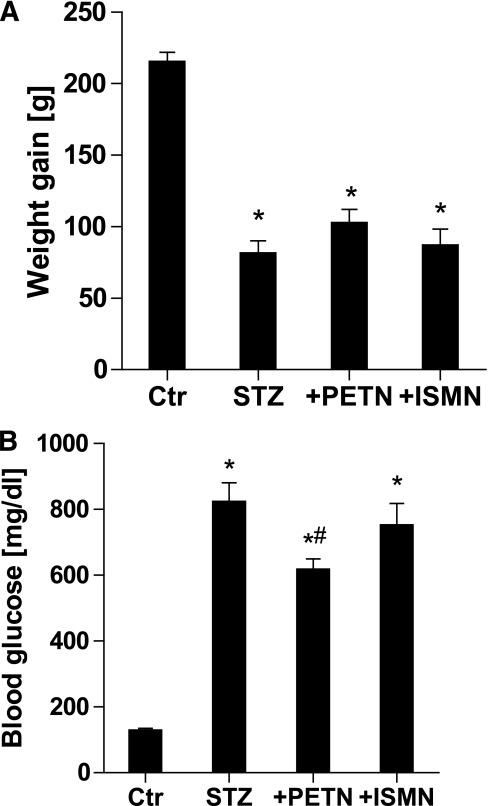
STZ-induced diabetes in Wistar rats caused a four- to fivefold increase in blood glucose and a 60% decrease in body weight gain within the therapy interval (Fig. 1). The organic nitrates did not affect weight gain in diabetic rats, but PETN, in contrast to ISMN, caused a minor but significant decrease in blood glucose in diabetic animals (Fig. 1).
FIG. 1.
Weight gain and blood glucose levels in control and diabetic rats 8 weeks after STZ injection and 7 weeks of organic nitrate treatment. A: Weight gain was calculated from the difference of values before and after STZ injection. B: Blood glucose was determined on the day the animals were killed (8 weeks after STZ injection). Data are the means ± SEM of 10–11 (+PETN/+ISMN groups) or 20–22 (Ctr/STZ groups) animals per group. *P < 0.05 vs. control; #P < 0.05 vs. STZ-injected group.
Vascular function.
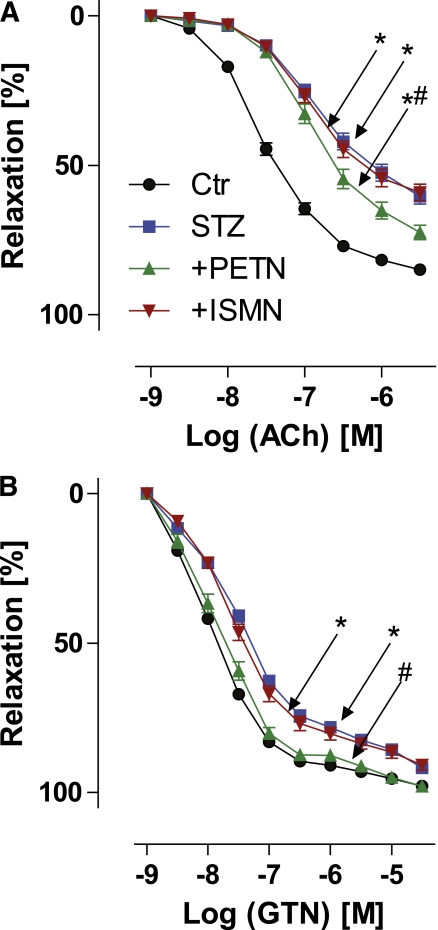
Diabetes-induced endothelial dysfunction in diabetic vessels was manifest as a significant right shift of the acetylcholine concentration-relaxation curve, as observed by isometric tension studies (Fig. 2A). Therapy with PETN, but not ISMN, partially normalized diabetes-induced endothelial dysfunction (Fig. 2A). Also, endothelium-independent, GTN-mediated relaxation was impaired in the setting of diabetes and was completely normalized by PETN cotreatment but not improved by ISMN (Fig. 2B).
FIG. 2.
Effects of organic nitrate treatment on endothelium-dependent and -independent vasodilation in diabetic rats. Vascular function was determined by isometric tension studies and relaxation in response to endothelium-dependent (ACh) (A) and endothelium-independent with requirement of bioactivation (GTN) (B) vasodilators. Data are shown for control (Ctr) (circles), diabetic (STZ) (squares), STZ+PETN (triangles), and STZ+ISMN (inverted triangles) animals. Data are the means ± SEM of 37–77 (ACh) and 40–88 (GTN) aortic rings from 10–22 animals per group. *P < 0.05 vs. control; #P < 0.05 vs. STZ.
XO- and Nox-derived ROS.
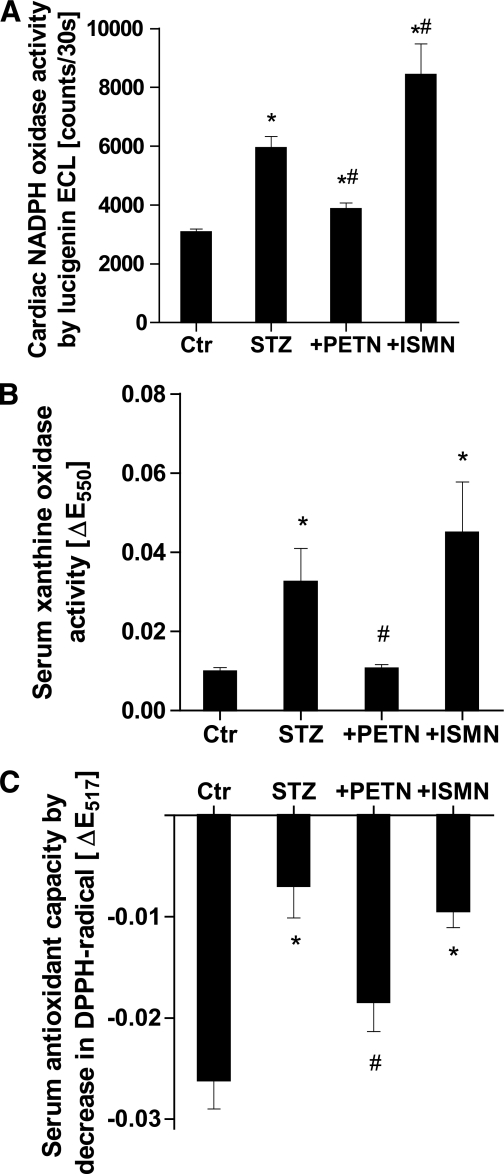
As a measure of NADPH oxidase activity, we used cardiac membrane fractions, which were stimulated with NADPH. Diabetes almost doubled the activity of membrane-bound NADPH oxidases. PETN therapy significantly decreased the signal in the hyperglycemic group (Fig. 3A). In contrast, ISMN significantly increased the NADPH oxidase activity in membranous fractions. Membranous superoxide formation also was determined by HPLC-based quantification of 2-hydroxyethidium, which was increased in samples from diabetic rats and normalized by PETN therapy (Supplementary Fig. S1).
FIG. 3.
Effects of organic nitrate treatment on cardiac and serum oxidative stress in diabetic rats. A: Quantification of cardiac NADPH oxidase activity in membranous preparations by ECL using the superoxide-specific dye, lucigenin (5 µmol/L). B: Quantification of serum XO activity by superoxide-dependent cytochrome c (50 µmol/L) reduction traced at 550 nm. C: Detection of serum antioxidant capacity by low-molecular weight antioxidant-dependent 2,2-diphenyl-1-picrylhydrazyl radical (DPPH•) (50 µmol/L) reduction traced at 517 nm. Serum was deproteinized by the addition of 50% acetonitrile. Data are the means ± SEM of heart tissues and serum samples from 10–20 (A), 6–10 (B), and 6–12 (C) animals per group. *P < 0.05 vs. control; #P < 0.05 vs. STZ.
Finally, STZ-induced diabetes activated XO and led to increased serum ROS formation in the presence of hypoxanthine, which was normalized by PETN, but not ISMN, therapy (Fig. 3B). The latter rather increased serum XO activity. As a related parameter, serum antioxidant capacity was dramatically decreased in the setting of diabetes, improved by PETN, and not altered by ISMN (Fig. 3C). These data indicate that hyperglycemic conditions cause the depletion of serum low-molecular weight antioxidants, which may contribute to ROS- and reactive nitrogen species–triggered endothelial dysfunction.
Vascular and cardiac oxidative stress.
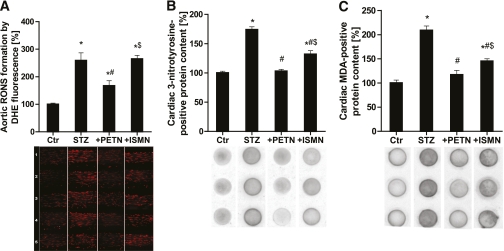
Aortic ROS formation was detected in cryosections using DHE-dependent fluorescence microtopography and quantified by densitometry (Fig. 4A). DHE fluorescence revealed a significant increase in ROS formation in the whole vessel wall (endothelium, media, and adventitia) of vessels from hyperglycemic rats. PETN, but not ISMN, therapy normalized the abnormal vascular ROS formation. As a measure for cardiac oxidative stress, the content of 3-nitrotyrosine– and malondialdehyde-positive proteins in heart tissue was determined by dot-blot analysis (Fig. 4B and C). Both markers of oxidative stress were significantly increased in the diabetic group, completely normalized by PETN therapy, and partially improved by ISMN.
FIG. 4.
Effects of organic nitrate treatment on vascular and cardiac production of RONS in diabetic rats. A: DHE (1 µmol/L) fluorescence microtopography was used to assess vascular RONS formation in aortic cryosections. Five representative microscope images are shown below. B: Dot-blot analysis with a 3NT-specific antibody was used to assess 3-nitrotyrosine content in cardiac proteins. Three representative dot-blot results are shown below. C: Dot-blot analysis with a malondialdehyde (MDA)-specific antibody was used to assess malondialdehyde content in cardiac proteins. Three representative dot-blot results are shown below. Data are the means ± SEM of samples from 10–20 (A) and 6–15 (B and C) animals per group. *P < 0.05 vs. control; #P < 0.05 vs. STZ; $P < 0.05 vs. PETN-treated group. (A high-quality digital representation of this figure is available in the online issue.)
Activation of vascular NADPH oxidases in the setting of diabetes and antioxidant, counterregulatory pathways.
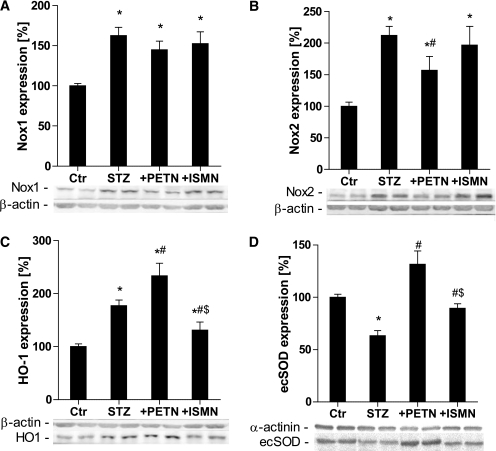
The expression of the membrane-located NADPH oxidase subunits Nox1 and Nox2 in aorta was increased by STZ-induced diabetes. Nox2 expression was partially suppressed by PETN, but not ISMN, therapy (Fig. 5A and B). As a compensatory mechanism, the expression of the antioxidant HO-1 system was increased in the setting of diabetes, which was further amplified by PETN supplementation (one of the known pleiotropic antioxidant properties of PETN) but decreased in the ISMN treatment group (Fig. 5C). Expression of the important antioxidant enzyme ecSOD was decreased by hyperglycemia but dramatically increased by PETN therapy and to a minor extent in the ISMN-treated animals (Fig. 5D).
FIG. 5.
Effects of organic nitrate treatment on the expression of vascular Nox1 and Nox2 as well as antioxidant proteins in diabetic rats. Nox1 (A), Nox2 (B), HO-1 (C), and ecSOD (D) were assessed using the Western blotting technique and specific antibodies. Representative blots are shown at the bottom of each densitometric quantification. Data are the means ± SEM of aortic protein preparations from 6–15 (A–C) and 5 (D) animals per group. *P < 0.05 vs. control; #P < 0.05 vs. STZ; $P < 0.05 vs. PETN-treated group.
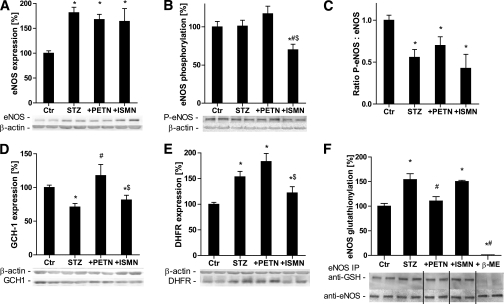
Mechanisms of eNOS uncoupling in the setting of diabetes.
eNOS expression was increased in diabetic rats and not altered by organic nitrates (Fig. 6A). Although there was a tendency for PETN-dependent improvement of the activating (Ser1177) phosphorylation state of eNOS, this difference was not significant, and this activating phosphorylation was suppressed in diabetic animals (Fig. 6B and C). Compatible with the uncoupling of eNOS, the tetrahydrobiopterin (BH4) synthase, GCH-I, was downregulated in the setting of diabetes and was normalized by PETN, but not ISMN, treatment (Fig. 6D). In contrast, the expression of the BH4 recycling DHFR was increased in the setting of diabetes and further augmented by PETN but inhibited by ISMN therapy (Fig. 6E). According to a recent publication by the group of Zweier, eNOS is glutathionylated under oxidative stress conditions and uncoupled (converted to a superoxide source) by this mechanism (30). We observed increased S-glutathionylation of eNOS in diabetic rats, which was normalized by PETN therapy but not by ISMN treatment (Fig. 6F).
FIG. 6.
Effects of organic nitrate treatment on the expression of vascular eNOS, GCH-I, and DHFR and phosphorylation, as well as the S-glutathionylation state of eNOS in diabetic rats. eNOS (A), Ser1177-phospho-eNOS (B), the ratio of Ser1177-phospho-eNOS to eNOS (C), GCH-I (D), and DHFR (E) were assessed using the Western blotting technique and specific antibodies. F: S-glutathionylation of eNOS was determined by eNOS immunoprecipitation, followed by anti-glutathione staining. After stripping the membrane, the bands were stained for eNOS to allow normalization of the signals. As a control for the specificity of the antibody for GSH-positive proteins, diabetic samples were treated with β-mercaptoethanol. Representative blots are shown at the bottom of each densitometric quantification. Data are the means ± SEM of aortic rings from 6–15 (A, D, and E) and 5–8 (B, C, and F) animals per group. *P < 0.05 vs. control; #P < 0.05 vs. STZ; $P < 0.05 vs. PETN.
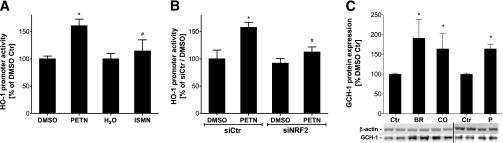
Mechanistic insights in the induction of HO-1 and GCH-I by PETN in cell culture.
The activity of the HO-1 promoter was increased by PETN but not by ISMN (Fig. 7A) or other organic nitrates, such as GTN and isosorbide dinitrate (not shown). The PETN-mediated increase in the HO-1 promoter activity was suppressed by siRNA-mediated knockdown of NFR2 mRNA (Fig. 7B). The successful downregulation of the transcription factor, NFR2, and downstream inhibition of the PETN-mediated HO-1 induction was determined at the mRNA level (Supplementary Fig. S2). Because PETN is a potent inducer of HO-1, we determined the effects of the products from HO-1–catalyzed breakdown of metal porphyrins, bilirubin, and carbon monoxide on the expression level of GCH-I. Both HO-1–derived products increased the expression of GCH-I (Fig. 7C).
FIG. 7.
Effect of organic nitrates on the activity of the 11-kb human HO-1 promoter in the dependence of NRF2 and induction of GCH-I by HO-1 products. A: DLD-1-HO-1-prom cells were washed with PBS and incubated with Dulbecco’s modified Eagle’s medium containing 2 mmol/L l-glutamine in the absence of serum and phenol red. After 16 h, cells were incubated with 50 μmol/L PETN (or DMSO as a solvent control) or 50 μmol/L ISMN (or water as a solvent control) for 8 h, lyzed in 1× passive lysis buffer, and protein concentrations were measured using the Bradford reagent. Luciferase activity in the extracts was determined using the luciferase assay system. The light units of the firefly luciferase were normalized to the protein concentration of the cell extracts. The relative luciferase activity level of cells treated with the solvent control was set to 100%. B: DLD-1-HO-1-prom cells were transiently transfected with an anti-NRF2 siRNA (siNRF2), which is shown to downregulate NRF2 expression, or a negative control siRNA (siCtr) by lipofection with HiPerFect HTS Reagent according to the manufacturer’s recommendations. After 48 h, cells were treated as described above to analyze PETN-induced human HO-1-11kb promoter activity. The relative luciferase activity level of cells treated with DMSO and siCtr was set to 100%. C: Confluent human endothelial cells (EA.hy 926) were treated in six-well plates with solvent (0.1% DMSO = Ctr), bilirubin (BR; 10 μmol/L), the carbon monoxide–releasing compound (CORM = CO; 50 μmol/L), or PETN (P; 50 μmol/L) for 15 h in the incubator. Two wells were pooled for GCH-I protein analysis (1:2,500; Dr. E. Werner, Innsbruck, Austria). Data are the means ± SEM of 6–10 (A and B) and 3–9 (C) independent experiments. *P < 0.05 vs. control; #P < 0.05 vs. PETN (siCtr).
DISCUSSION
With the present studies, we show for the first time that the significant impairment of vascular function (ACh and GTN responses) in diabetic animals is partially improved by PETN, but not ISMN, therapy. Cardiac and vascular oxidative stress was increased in the STZ-induced diabetic group and almost completely normalized by PETN treatment, whereas ISMN, in most cases, did not alter reactive oxygen and nitrogen species (RONS) formation (Nox and XO activity, serum antioxidant capacity, and vascular oxidative stress) or had less pronounced effects compared with PETN (3NT and malondialdehyde content). These observations were mirrored by the protein expression profiles of Nox isoforms, eNOS, the BH4-synthesizing GCH-I, the BH4-recycling DHFR, and the antioxidant enzymes ecSOD or HO-1.
Oxidative stress is a major cause of reduced vascular NO bioavailability in diabetes (3). Besides the direct inactivation of NO by reaction with superoxide (31), the resulting peroxynitrite is known to potently oxidize and thereby deplete the eNOS cofactor BH4 (32), which in turn causes eNOS uncoupling. This concept is supported by the present findings and our previous observations that hyperglycemia causes endothelial and smooth-muscle dysfunction, with a major role for oxidative stress (3–5,33). More importantly, there was a clear correlation between NADPH oxidase activity, endothelial dysfunction, and nitrate resistance (Supplementary Figs. S3 and S4). The present data demonstrate an activation of known sources of RONS, the NADPH oxidase, the XO, and the uncoupled eNOS, which is in accordance with our previous observations (4,5). According to the concept of “kindling radicals,” initial ROS formation (e.g., from NADPH oxidases) triggers further damage, such as eNOS uncoupling by oxidative depletion of BH4 or oxidation of the zinc-sulfur complex, both compromising the integrity of the eNOS dimer (4,6,34,35). Alternatively, protein kinase C–dependent phosphorylation of eNOS at Thr495 has been shown to impair NO synthesis and may even lead to uncoupling with superoxide formation (7). Here, we also show for the first time that eNOS S-glutathionylation may contribute to eNOS dysfunction/uncoupling in the setting of diabetes (Fig. 6F), as recently published for spontaneously hypertensive rats (30). In agreement with this hypothesis, we found an increased expression of Nox1 and Nox2 isoforms in aorta from diabetic rats, and the increased superoxide formation from Nox isoforms was further amplified by a significant decrease in ecSOD, as previously reported (36).
The inducible antioxidant enzyme HO-1, a typical stress gene, was induced by STZ treatment, as previously shown for chronic hyperglycemia (37,38). PETN therapy further amplified HO-1 expression, which was previously reported as an antioxidant feature of this organic nitrate contributing to its beneficial profile during chronic treatment (14,16,39). According to the present findings, HO-1 induction by PETN is likely determined by the activation of the transcription factor NRF2 because this beneficial effect of PETN was lost upon silencing of NRF2 in cell culture, supporting the reported role of NO in this process (40). The observed induction of the BH4 synthase, GCH-I, by bilirubin and carbon monoxide, which are HO-1–derived products, provides an attractive explanation for the PETN-mediated upregulation of GCH-I (Fig. 7). Whether this is a result of the direct and indirect antioxidant effects of bilirubin and CO interfering with the published “oxidative proteasomal” degradation of GCH-I (41,42) or to mechanisms at the transcriptional level remains to be determined. These data are in accordance with previous observations that pharmacological (16) and genetic (18) suppression of HO-1 activity results in the loss of the beneficial pleiotropic effects of PETN.
As shown previously (3), the expression of eNOS was increased in diabetic rats, suggesting that this may be a compensatory “rescue” mechanism of the organism to respond to endothelial dysfunction (Fig. 6). However, because eNOS is in its uncoupled state in diabetic animals (3–5,33), an additional increase in eNOS-produced ROS rather results in a vicious cycle. The most reasonable explanation for the uncoupling of eNOS is the downregulation of GCH-I that results in decreased BH4 levels. We observed an increase in the expression of GCH-I in the PETN-treated group (Fig. 6D), compatible with our previous finding in angiotensin II–induced hypertension that PETN increases GCH-I expression via activation of the HO-1 pathway (18). In addition, the content of eNOS that was phosphorylated at Ser1177 (Akt dependent), another known Ca2+-independent route to activate the enzyme, was significantly decreased in the setting of diabetes, which could contribute to the low NO-producing activity of eNOS in diabetic tissue (43).
Finally, the expression of the BH4-recycling DHFR, the so-called “salvage” pathway, was increased in the setting of diabetes, compatible with the attempt of the organism to compensate for the loss of BH4 de novo synthesis. This compensatory effect was supported by the administration of PETN but was abrogated by ISMN.
All of these cardiovascular complications and adverse side effects of STZ-induced diabetes were, in most cases, almost completely normalized, or at least significantly improved, by PETN therapy. In contrast, ISMN supplementation had only minor effects on these parameters and only in some cases partially improved them. Thus, our data suggest that the interaction of organic nitrates with the vascular abnormalities induced by diabetes should not be extended to all drugs in this class. Induction of additional oxidative stress evoked by organic nitrates may play an important role in the setting of nitrate-induced and diabetes-induced endothelial dysfunction and might potentially represent a major drawback for the use of nitrates in diabetic patients. In this scenario, one opportunity may be supplementation with antioxidants, such as hydralazine (44,45) or lipoic acid (46,47), which are known to suppress nitrate tolerance and improve endothelial dysfunction, or at least the use of an organic nitrate that activates intrinsic antioxidant pathways such as PETN (48,49), which displays potent antioxidant properties that are mediated by induction of HO-1 (14,16,39), ecSOD (50), and many other cardioprotective genes (51). Previous data show that the ecSOD is induced by HO-1 activity (or HO-1–derived products) (36). In addition, we recently have reported that PETN improves angiotensin II–triggered endothelial dysfunction and vascular oxidative stress by recoupling of eNOS and suppression of NADPH oxidase activity (18), in accordance with previous reports that PETN improves established atherosclerosis (52). All of these observations may explain the beneficial effects of PETN in the present model of experimental diabetes.
Conclusions and clinical implications.
The results of the present studies support previous findings that vascular dysfunction is associated with increased vascular oxidative stress in this in vivo model of diabetes. Treatment with the organic nitrate, PETN, but not with ISMN, improves endothelium-dependent and -independent relaxation, reduces oxidative stress, prevents activation of NADPH oxidase, and inhibits XO-mediated superoxide production. The important role of NADPH oxidase in this process is reflected by the correlation between vascular function and the activity of this enzyme complex (Supplementary Figs. S3 and S4). We propose that the powerful pleiotropic antioxidant effects of PETN account for the improvement of vascular function. According to preliminary data, PETN also seems to modify the metabolism of glucose in diabetic rats (Fig. 1), possibly by regulation of metabolic pathways, such as AMP-activated protein kinase (not shown). These experimental data that an organic nitrate improves vascular dysfunction in STZ-induced diabetes are new and further corroborate the concept that the organic nitrate, PETN, with intrinsic antioxidant properties, may be useful for the treatment of diabetic patients requiring anti-ischemic therapy. However, it should be noted that STZ treatment results in type 1 diabetes and that the present findings cannot necessarily be translated to type 2 diabetes, a clear limitation of the current study regarding the clinical importance of the latter form. The clinical importance of the present findings is based on the fact that cardiovascular complications (including angina pectoris) are a hallmark of diabetes, and diabetic patients with a two- to fourfold increased risk for infarction, especially, would benefit greatly from organic nitrate treatment devoid of classical adverse effects, such as nitrate-induced vascular oxidative stress, nitrate tolerance, and endothelial dysfunction (cross-tolerance).
Supplementary Material
ACKNOWLEDGMENTS
The present work was supported by generous financial support from the Johannes Gutenberg University and Medical Center Mainz (Forschungsfonds and Mainzer Forschungsförderungsprogramm [MAIFOR] grants to A.D.), a vascular biology grant from Actavis Deutschland (to A.D. and T.M.) and partially by funds from the Federal Ministry of Education and Research (BMBF 01EO1003) (to P.W. and A.D.). A.D. received honoraria and research grant support from Actavis Deutschland, Langenfeld, Germany. T.M. received research grant support from Actavis Deutschland, Langenfeld, Germany. This article contains results that are part of the doctoral thesis of C.O. No other potential conflicts of interest relevant to this article were reported.
S.Sc. and M.O. researched data and reviewed and edited the manuscript. F.B. researched data. H.K. researched data and wrote, reviewed, and edited the manuscript. C.O., T.H., S.St., M.H., M.K., and A.P. researched data. K.R. contributed to discussion. E.S. contributed to discussion and reviewed and edited the manuscript. T.G. reviewed and edited the manuscript. P.W. contributed to discussion and reviewed and edited the manuscript. T.M. contributed to discussion and wrote the manuscript. A.D. researched data, contributed to discussion, and wrote, reviewed, and edited the manuscript.
Parts of this study were presented in abstract form at the Arteriosclerosis, Thrombosis, and Vascular Biology 2011 Scientific Sessions, Chicago, Illinois, 28–30 April 2011, and at the 17th Annual Meeting of the Society for Free Radical Biology and Medicine, Orlando, Florida, 17–21 November 2010.
The authors thank Jörg Schreiner (University Medical Center Mainz, Mainz, Germany), Angelica Karpi (University Medical Center Mainz, Mainz, Germany), and Anja Conrad (University Medical Center Mainz, Mainz, Germany) for expert technical assistance.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db10-1395/-/DC1.
REFERENCES
- 1.Nathan DM, Cleary PA, Backlund JY, et al. ; Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study Research Group Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med 2005;353:2643–2653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jay D, Hitomi H, Griendling KK. Oxidative stress and diabetic cardiovascular complications. Free Radic Biol Med 2006;40:183–192 [DOI] [PubMed] [Google Scholar]
- 3.Hink U, Li H, Mollnau H, et al. Mechanisms underlying endothelial dysfunction in diabetes mellitus. Circ Res 2001;88:E14–E22 [DOI] [PubMed] [Google Scholar]
- 4.Wenzel P, Daiber A, Oelze M, et al. Mechanisms underlying recoupling of eNOS by HMG-CoA reductase inhibition in a rat model of streptozotocin-induced diabetes mellitus. Atherosclerosis 2008;198:65–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wenzel P, Schulz E, Oelze M, et al. AT1-receptor blockade by telmisartan upregulates GTP-cyclohydrolase I and protects eNOS in diabetic rats. Free Radic Biol Med 2008;45:619–626 [DOI] [PubMed] [Google Scholar]
- 6.Zou MH, Shi C, Cohen RA. Oxidation of the zinc-thiolate complex and uncoupling of endothelial nitric oxide synthase by peroxynitrite. J Clin Invest 2002;109:817–826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lin MI, Fulton D, Babbitt R, et al. Phosphorylation of threonine 497 in endothelial nitric-oxide synthase coordinates the coupling of L-arginine metabolism to efficient nitric oxide production. J Biol Chem 2003;278:44719–44726 [DOI] [PubMed] [Google Scholar]
- 8.Münzel T, Daiber A, Mülsch A. Explaining the phenomenon of nitrate tolerance. Circ Res 2005;97:618–628 [DOI] [PubMed] [Google Scholar]
- 9.Fayers KE, Cummings MH, Shaw KM, Laight DW. Nitrate tolerance and the links with endothelial dysfunction and oxidative stress. Br J Clin Pharmacol 2003;56:620–628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johnston GD. Ulster says ‘NO’; explosion, resistance and tolerance: nitric oxide and the actions of organic nitrates. Ulster Med J 1998;67:79–90 [PMC free article] [PubMed] [Google Scholar]
- 11.Warnholtz A, Mollnau H, Heitzer T, et al. Adverse effects of nitroglycerin treatment on endothelial function, vascular nitrotyrosine levels and cGMP-dependent protein kinase activity in hyperlipidemic Watanabe rabbits. J Am Coll Cardiol 2002;40:1356–1363 [DOI] [PubMed] [Google Scholar]
- 12.Gori T, Al-Hesayen A, Jolliffe C, Parker JD. Comparison of the effects of pentaerythritol tetranitrate and nitroglycerin on endothelium-dependent vasorelaxation in male volunteers. Am J Cardiol 2003;91:1392–1394 [DOI] [PubMed] [Google Scholar]
- 13.Jurt U, Gori T, Ravandi A, Babaei S, Zeman P, Parker JD. Differential effects of pentaerythritol tetranitrate and nitroglycerin on the development of tolerance and evidence of lipid peroxidation: a human in vivo study. J Am Coll Cardiol 2001;38:854–859 [DOI] [PubMed] [Google Scholar]
- 14.Oberle S, Abate A, Grosser N, et al. Endothelial protection by pentaerithrityl trinitrate: bilirubin and carbon monoxide as possible mediators. Exp Biol Med (Maywood) 2003;228:529–534 [DOI] [PubMed] [Google Scholar]
- 15.Oberle S, Schwartz P, Abate A, Schröder H. The antioxidant defense protein ferritin is a novel and specific target for pentaerithrityl tetranitrate in endothelial cells. Biochem Biophys Res Commun 1999;261:28–34 [DOI] [PubMed] [Google Scholar]
- 16.Wenzel P, Oelze M, Coldewey M, et al. Heme oxygenase-1: a novel key player in the development of tolerance in response to organic nitrates. Arterioscler Thromb Vasc Biol 2007;27:1729–1735 [DOI] [PubMed] [Google Scholar]
- 17.Thomas GR, DiFabio JM, Gori T, Parker JD. Once daily therapy with isosorbide-5-mononitrate causes endothelial dysfunction in humans: evidence of a free-radical-mediated mechanism. J Am Coll Cardiol 2007;49:1289–1295 [DOI] [PubMed] [Google Scholar]
- 18.Schuhmacher S, Wenzel P, Schulz E, et al. Pentaerythritol tetranitrate improves angiotensin II-induced vascular dysfunction via induction of heme oxygenase-1. Hypertension 2010;55:897–904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Juan SH, Cheng TH, Lin HC, Chu YL, Lee WS. Mechanism of concentration-dependent induction of heme oxygenase-1 by resveratrol in human aortic smooth muscle cells. Biochem Pharmacol 2005;69:41–48 [DOI] [PubMed] [Google Scholar]
- 20.Like AA, Rossini AA. Streptozotocin-induced pancreatic insulitis: new model of diabetes mellitus. Science 1976;193:415–417 [DOI] [PubMed] [Google Scholar]
- 21.Oelze M, Knorr M, Schuhmacher S, et al. Vascular dysfunction in streptozotocin-induced experimental diabetes strictly depends on insulin deficiency. J Vasc Res 2011;48:275–284 [DOI] [PubMed] [Google Scholar]
- 22.Daiber A, Oelze M, Coldewey M, et al. Oxidative stress and mitochondrial aldehyde dehydrogenase activity: a comparison of pentaerythritol tetranitrate with other organic nitrates. Mol Pharmacol 2004;66:1372–1382 [DOI] [PubMed] [Google Scholar]
- 23.Cos P, Rajan P, Vedernikova I, et al. In vitro antioxidant profile of phenolic acid derivatives. Free Radic Res 2002;36:711–716 [DOI] [PubMed] [Google Scholar]
- 24.Oelze M, Daiber A, Brandes RP, et al. Nebivolol inhibits superoxide formation by NADPH oxidase and endothelial dysfunction in angiotensin II-treated rats. Hypertension 2006;48:677–684 [DOI] [PubMed] [Google Scholar]
- 25.Rajagopalan S, Kurz S, Münzel T, et al. Angiotensin II-mediated hypertension in the rat increases vascular superoxide production via membrane NADH/NADPH oxidase activation: contribution to alterations of vasomotor tone. J Clin Invest 1996;97:1916–1923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schuhmacher S, Schulz E, Oelze M, et al. A new class of organic nitrates: investigations on bioactivation, tolerance and cross-tolerance phenomena. Br J Pharmacol 2009;158:510–520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wenzel P, Mollnau H, Oelze M, et al. First evidence for a crosstalk between mitochondrial and NADPH oxidase-derived reactive oxygen species in nitroglycerin-triggered vascular dysfunction. Antioxid Redox Signal 2008;10:1435–1447 [DOI] [PubMed] [Google Scholar]
- 28.Edgell CJ, McDonald CC, Graham JB. Permanent cell line expressing human factor VIII-related antigen established by hybridization. Proc Natl Acad Sci USA 1983;80:3734–3737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jansen T, Hortmann M, Oelze M, et al. Conversion of biliverdin to bilirubin by biliverdin reductase contributes to endothelial cell protection by heme oxygenase-1-evidence for direct and indirect antioxidant actions of bilirubin. J Mol Cell Cardiol 2010;49:186–195 [DOI] [PubMed] [Google Scholar]
- 30.Chen CA, Wang TY, Varadharaj S, et al. S-glutathionylation uncouples eNOS and regulates its cellular and vascular function. Nature 2010;468:1115–1118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Beckman JS, Koppenol WH. Nitric oxide, superoxide, and peroxynitrite: the good, the bad, and ugly. Am J Physiol 1996;271:C1424–C1437 [DOI] [PubMed] [Google Scholar]
- 32.Kuzkaya N, Weissmann N, Harrison DG, Dikalov S. Interactions of peroxynitrite, tetrahydrobiopterin, ascorbic acid, and thiols: implications for uncoupling endothelial nitric-oxide synthase. J Biol Chem 2003;278:22546–22554 [DOI] [PubMed] [Google Scholar]
- 33.Wendt MC, Daiber A, Kleschyov AL, et al. Differential effects of diabetes on the expression of the gp91phox homologues nox1 and nox4. Free Radic Biol Med 2005;39:381–391 [DOI] [PubMed] [Google Scholar]
- 34.Dikalova AE, Gongora MC, Harrison DG, Lambeth JD, Dikalov S, Griendling KK. Upregulation of Nox1 in vascular smooth muscle leads to impaired endothelium-dependent relaxation via eNOS uncoupling. Am J Physiol Heart Circ Physiol 2010;299:H673–H679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Landmesser U, Dikalov S, Price SR, et al. Oxidation of tetrahydrobiopterin leads to uncoupling of endothelial cell nitric oxide synthase in hypertension. J Clin Invest 2003;111:1201–1209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kruger AL, Peterson S, Turkseven S, et al. D-4F induces heme oxygenase-1 and extracellular superoxide dismutase, decreases endothelial cell sloughing, and improves vascular reactivity in rat model of diabetes. Circulation 2005;111:3126–3134 [DOI] [PubMed] [Google Scholar]
- 37.Laybutt DR, Kaneto H, Hasenkamp W, et al. Increased expression of antioxidant and antiapoptotic genes in islets that may contribute to β-cell survival during chronic hyperglycemia. Diabetes 2002;51:413–423 [DOI] [PubMed] [Google Scholar]
- 38.Oksala NK, Lappalainen J, Laaksonen DE, et al. Alpha-lipoic acid modulates heat shock factor-1 expression in streptozotocin-induced diabetic rat kidney. Antioxid Redox Signal 2007;9:497–506 [DOI] [PubMed] [Google Scholar]
- 39.Oberle S, Abate A, Grosser N, et al. Heme oxygenase-1 induction may explain the antioxidant profile of pentaerythrityl trinitrate. Biochem Biophys Res Commun 2002;290:1539–1544 [DOI] [PubMed] [Google Scholar]
- 40.Naughton P, Hoque M, Green CJ, Foresti R, Motterlini R. Interaction of heme with nitroxyl or nitric oxide amplifies heme oxygenase-1 induction: involvement of the transcription factor Nrf2. Cell Mol Biol (Noisy-le-grand) 2002;48:885–894 [PubMed] [Google Scholar]
- 41.Xu J, Wang S, Wu Y, Song P, Zou MH. Tyrosine nitration of PA700 activates the 26S proteasome to induce endothelial dysfunction in mice with angiotensin II-induced hypertension. Hypertension 2009;54:625–632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xu J, Wu Y, Song P, Zhang M, Wang S, Zou MH. Proteasome-dependent degradation of guanosine 5′-triphosphate cyclohydrolase I causes tetrahydrobiopterin deficiency in diabetes mellitus. Circulation 2007;116:944–953 [DOI] [PubMed] [Google Scholar]
- 43.Dimmeler S, Fleming I, Fisslthaler B, Hermann C, Busse R, Zeiher AM. Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature 1999;399:601–605 [DOI] [PubMed] [Google Scholar]
- 44.Daiber A, Oelze M, Coldewey M, et al. Hydralazine is a powerful inhibitor of peroxynitrite formation as a possible explanation for its beneficial effects on prognosis in patients with congestive heart failure. Biochem Biophys Res Commun 2005;338:1865–1874 [DOI] [PubMed] [Google Scholar]
- 45.Münzel T, Kurz S, Rajagopalan S, et al. Hydralazine prevents nitroglycerin tolerance by inhibiting activation of a membrane-bound NADH oxidase: a new action for an old drug. J Clin Invest 1996;98:1465–1470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dudek M, Bednarski M, Bilska A, et al. The role of lipoic acid in prevention of nitroglycerin tolerance. Eur J Pharmacol 2008;591:203–210 [DOI] [PubMed] [Google Scholar]
- 47.Wenzel P, Hink U, Oelze M, et al. Role of reduced lipoic acid in the redox regulation of mitochondrial aldehyde dehydrogenase (ALDH-2) activity: implications for mitochondrial oxidative stress and nitrate tolerance. J Biol Chem 2007;282:792–799 [DOI] [PubMed] [Google Scholar]
- 48.Daiber A, Münzel T. Characterization of the antioxidant properties of pentaerithrityl tetranitrate (PETN)-induction of the intrinsic antioxidative system heme oxygenase-1 (HO-1). Methods Mol Biol 2010;594:311–326 [DOI] [PubMed] [Google Scholar]
- 49.Gori T, Daiber A. Non-hemodynamic effects of organic nitrates and the distinctive characteristics of pentaerithrityl tetranitrate. Am J Cardiovasc Drugs 2009;9:7–15 [DOI] [PubMed] [Google Scholar]
- 50.Oppermann M, Balz V, Adams V, et al. Pharmacological induction of vascular extracellular superoxide dismutase expression in vivo. J Cell Mol Med 2009;13:1271–1278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pautz A, Rauschkolb P, Schmidt N, et al. Effects of nitroglycerin or pentaerithrityl tetranitrate treatment on the gene expression in rat hearts: evidence for cardiotoxic and cardioprotective effects. Physiol Genomics 2009;38:176–185 [DOI] [PubMed] [Google Scholar]
- 52.Hacker A, Müller S, Meyer W, Kojda G. The nitric oxide donor pentaerythritol tetranitrate can preserve endothelial function in established atherosclerosis. Br J Pharmacol 2001;132:1707–1714 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.