Abstract
Over the past 15 years, techniques for identifying visual areas using magnetic resonance imaging (MRI) in human subjects have been applied widely to multiple populations. This review will cover the basic techniques of using functional MRI and very high-resolution structural MRI to determine boundaries between different areas of the visual cortex. Recent applications of these methods to ophthalmological patient populations are discussed, and the future potential applications of very high field strength MRI are considered.
Introduction
The visual system has provided the model neural system for functional brain imaging over the past 20 years, as the presentation of flashing stimuli evokes a strong cortical response in the occipital lobe in all sighted subjects. In addition to providing a general tool, study of the visual system has also been at the forefront of experimental paradigm design. In particular, the use of mapping techniques to identify individual cortical visual areas has allowed hierarchical processing and functional specialisation to be investigated much more directly than for other brain regions.
Functional magnetic resonance imaging (fMRI) has been used extensively in neuro-ophthalmology to investigate changes in patterns of cortical activity in visual disorders, such as hemianopia,1, 2 object agnosia,3 or prosopagnosia.4 However, the ability to distinguish the primary visual cortex from surrounding visual areas also provides a tool to investigate cortical correlates of ophthalmological diseases and disorders. In this review, after discussing the basic methods for defining visual areas, a discussion of the current and potential clinical applications for fMRI is provided.
Retinotopic mapping
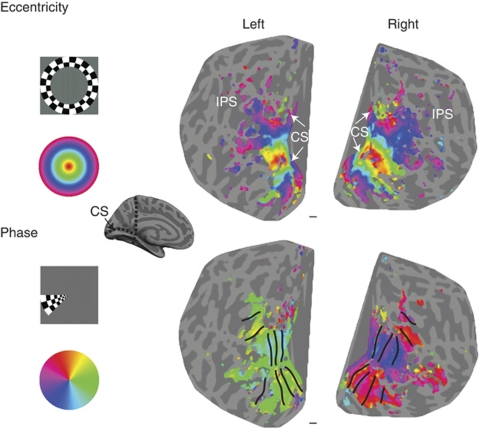
From human lesion studies,5, 6, 7 and later neurophysiology studies in non-human primates,8, 9 it had been known for nearly a century that each visual area has a retinotopic map of space, although somewhat distorted. Using this knowledge, several groups in the mid 1990s designed a paradigm, now referred to as ‘retinotopic mapping', that allows individual visual areas to be distinguished.10, 11, 12, 13 This procedure involves subjects lying in the MRI scanner fixating a central point while high contrast stimuli, usually checkerboards, are flashed at changing positions in the visual field in a cyclical manner. Figure 1 shows examples of the checkerboard stimuli used for mapping eccentricity (annulus) and visual angle (wedge). In the case of eccentricity, the radius of the annulus is increased over time, such that a wave of cortical activation spreads from the posterior occipital lobe, representing central vision, anteriorly to the regions representing more peripheral regions of the visual field. The resulting patterns of activity are displayed on a flattened section of cortex.
Figure 1.
The retinotopic mapping stimuli and resulting fMRI activations. In the eccentricity map, central regions of the visual field are shown in red, as seen in the key. Similarly, the phase stimulus (wedge) activates the left hemisphere when the stimulus is in the right visual field (green). Borders can be drawn between the visual areas at the ‘stripes' in the activation patterns, which correspond to reversals in the representation of visual angle. The brain depicted in the middle of the figure indicates the ‘cuts' made in the inflated brain to produce the flattened maps.
To produce a flattened representation, such as these, the grey matter must first be segmented from the brain image, before this folded, but continuous sheet of grey matter is computationally stretched out into a smooth surface (inflated). To ‘flatten' the surface, a restricted region of the surface is often selected and ‘cut' from the surface (here the occipital lobe). The image on the right indicates the region of cortex that has been ‘cut' from the inflated brain, a further ‘cut' was made along the calcarine sulcus to split the map into dorsal and ventral sections, as indicated by the white arrows.
In the eccentricity map, the regions corresponding to central, foveal vision are in red and more peripheral representations are in shades of blue. Orthogonal to the changing eccentricity is the change in phase, or visual angle. The lower panel shows the wedge stimulus that is moved around the visual field.
Creating an atlas
Although retinotopy on individual subjects is a ‘gold standard' method of defining visual areas and investigating the neural responses in different regions, obtaining sufficiently high quality data can be problematic. Specifically, retinotopic mapping requires that the subject maintain fixation on a central target while the stimulus is moved within the visual field. It can, therefore, be difficult to conduct mapping in a range of patient groups with visual dysfunction. In the extreme case, it is clearly impossible to define visual areas in blind subjects, even though an ability to subdivide the occipital lobe may be beneficial for understanding any differences in this population. Finally, to acquire sufficiently high data quality to define the areas accurately often requires a dedicated scan session in addition to the main experiment, which is time consuming in large studies.
Given the difficulty of defining visual areas in patient groups, having an approximate location for different visual areas can be beneficial. There are obstacles for comparing visual areas across subjects, notably the variability in the location of sulci and gyri, in addition to shape and size differences. One method of addressing these is to use surface-based registration, which has been shown to produce more reliable results than linear registration.14 Surface-based registration requires several steps, (i) classification of different brain tissues and segmentation of the grey matter, (ii) inflation of the grey matter to a sphere, and (iii) alignment of spheres based on the major sulci. To create an atlas using this method in Freesurfer,15, 16, 17 retinotopically mapped visual areas from 16 subjects with normal visual function were registered using the above technique to a template constructed from the average of all 16 brains. The visual areas were summed, and then thresholded at the point at which 50% of the subjects showed overlapping regions. Figure 2 shows the excellent alignment of the primary visual cortex, because of its location within the calcarine sulcus, a major landmark. The locations of extrastriate areas show considerably more variability between subjects, with very little, or sometimes no area of overlap of all subjects.
Figure 2.
Creating a retinotopy atlas to provide a probabilistic location for the early visual areas. The area with the best consensus is in V1, whereas variability increases considerably in higher areas. Note that the anterior extent of the visual areas is limited by the size of the stimuli that were used to perform the retinotopic mapping. Hence, V1 does not extend the whole length of the calcarine sulcus. The colour map shows the percentage overlap in the visual areas across all subjects. Darker regions are sulci, lighter areas are gyri.
Anatomical definition of striate cortex in vivo
In addition to the functional specialisation of the brain, it has been known since the work of Gennari, Baillarger, Elliot-Smith, and Brodmann18, 19, 20, 21 over a century ago, that there are cytoarchitectural differences between different brain regions. Exploiting this anatomical variation is an alternative route to identifying areas for individual subjects. Such a definition does not require fixation or visual function, and therefore can be performed on any subject who can remain still.
Most well characterized, and easiest to detect is the stria of Gennari,21 the thick band of myelin found within layer 4 of the primary visual cortex (V1). Detection of this structure has now been demonstrated in a number of studies, including a demonstration of the correspondence between V1, defined by the presence of the stria of Gennari, and retinotopic mapping, as described above.22 One issue to be resolved is that the studies to date have required subjective definition of the stria of Gennari. Objective, automated detection, and quantification of the myeloarchitecture would be the most useful tool for definition of V1 in patients with whom it is impossible to perform the retinotopic mapping.
Clinical applications of visual area definition
Although the majority of work in using retinotopic mapping has concentrated on understanding basic mechanisms of early vision, the recent increase in studies investigating opthalmological disorders, predominantly glaucoma, age-related macular degeneration (AMD), and amblyopia makes the localisation of V1 in clinical populations applicable.23, 24, 25, 26, 27, 28 Retinotopic mapping can be performed in some circumstances, particularly if the disease is monocular, otherwise an anatomical definition using high-resolution imaging of the stria of Gennari may be more useful.22, 29
Glaucoma
In experimental models of glaucoma, the loss of retinal ganglion cells is associated with structural changes in the lateral geniculate nucleus and V1. However, it is more difficult to determine whether the disease process in human leads to comparable changes. A loss of grey matter in the occipital lobe that appears to correspond roughly to the mean location of visual loss has been reported using MRI.24 However, the localisation to V1 is difficult using gross anatomical landmarks unless the changes are deep in the calcarine sulcus. To investigate changes in cortical function related to human primary open-angle glaucoma, Duncan et al.23 compared the V1 responses to stimulation of the glaucomatous and fellow eye. The authors used the retinotopic map of visual space to define V1 and then looked at the relative activation in the affected and unaffected regions of the visual field. They found that activation to the fellow eye was significantly greater than that to the glaucomatous eye only in the affected region. Moreover, the magnitude of the difference in response to the eyes was correlated with multiple measures of optic disc damage. Determining the time scale of effects in the cortical visual system could provide insight into the progression of the disease.
Macular degeneration
In addition to the definition of visual areas, retinotopic mapping also provides the opportunity to determine the eccentricities of visual stimulation at which different regions of cortex respond. In the case of macular degeneration (MD), in which the central retina is damaged, the major input to the posterior section of V1 is lost. This raises the question of whether this area is able to reorganise in order to use the now redundant regions of grey matter. A conclusive answer to this question is yet to be determined. An early study by Baker et al28 found that two patients with MD activated the foveal confluence (which is difficult to subdivide into individual visual areas) when viewing stimuli at their (peripheral) preferred retinal locations (PRLs). This suggests plasticity in the visual system to use regions of cortex that no longer have input from the retina. However, an earlier case study30 and a subsequent experiment by Masuda et al.25 disagree with this level of reorganisation. The latter authors suggest that the discrepancy may depend on the task patients are given, specifically that performing a task is more likely to activate the ‘deafferented' region than passively viewing stimuli at the PRL. A task is more likely to cause feedback from extrastriate areas that could activate this region. An additional variable that could underlie the differences in activation is the age at which the MD began, so juvenile MD could be more likely to result in reorganisation than AMD. These issues have been recently reviewed in detail31, 32. It would clearly be very useful to determine whether any activation in the foveal confluence is in V1 rather than V2 or V3, an example in which the stria of Gennari may be a useful marker.
Presurgical planning
From a neuro-ophthalmological and neurosurgical approach, there are benefits of determining the location of a tumour relative to known visual areas. In particular, tumors that are neither malignant nor cause visual field deficits but may lead to visual disturbance indicative of seizure activity, are a case for consideration. The specific location of the tumor determines the likely residual deficits if surgery is performed. Using MRI, there are several possible methods of predicting post-surgical outcome. First, it is possible to use diffusion imaging to identify the optic radiation and establish whether any surgery is likely to interfere with the pathway from the lateral geniculate nucleus to V1. An alternative method is to use standard retinotopic mapping (described above) or to apply a more basic paradigm to look at the region of the visual field that would be affected by surgery. A combination of both approaches would provide the most accurate prediction.
This type of pre-surgical planning already occurs in some cases, when surgery to relieve epilepsy is planned for the medial temporal lobe, as it is important to consider the path of Meyer's loop to reduce the likelihood of visual field deficits after surgery.33, 34 Identification of visual areas, defined by retinotopic mapping used in combination with diffusion tractography to map Meyer's loop can indicate the fiber bundles that carry the critical information to V1.
Future directions of mapping
With an increase in the field strength of the human scanners, the spatial resolution at which brain imaging, both structural and functional, is performed has also increased. At 7 T, it is possible to achieve very rapid acquisition of high-resolution structural data (0.3 × 0.3 × 1.5 mm3), using T2*-weighted rather than standard T1-weighted imaging. This type of scan is believed to be sensitive to iron concentration, which has been shown to co-localize with myelin within V1.35 It is possible to visualize the stria of Gennari in extended regions of V1, as shown in Figure 3 (black arrows). These data were acquired in 4 min, and such a rapid scan could be applied to a patient population, particularly those who have abnormal visual function, or may even be blind, in order to determine the location of the primary visual cortex. Knowing the location of the striate cortex can then be used to determine the locus of any abnormal function, such as in amblyopia.
Figure 3.
The stria of Gennari imaged in a sighted control subject in a T2*-weighted scan at 7 T. The resolution of the scan is 0.3 × 0.3 × 1.5 mm3 and took 4 min. White matter is dark in these images and the dark line in the middle of the cortex indicates the stria of Gennari (black arrows).
At 7 T, fMRI scanning at a resolution of 0.75 mm3, or even higher, provides the opportunity to investigate the functional architecture of visual areas. In V1, wherein this architecture is best understood, it is possible to image both the ocular dominance columns and the orientation structure.36, 37 The ocular dominance columns in V1 have now been imaged several times, including a paper showing the reproducibility over scanning sessions days apart.37 In ophthalmology, such an approach could be used in the future to determine whether and how this functional architecture is disrupted in conditions, such as amblyopia and congenital cataract.
Beyond V1, there are reports in the macaque, using optical imaging, that the ‘stripes' of V2 are selective for disparity, and show and have an ordered representation of depth.38, 39 The scale of these stripes on the cortical surface is of the order 1 mm2 in the macaque, and is likely to be slightly greater in the human, because of the different size of the cortex. For binocular disorders, a window on the representation of depth in the cortex would provide some insight into the mechanisms of disrupted depth perception.
Summary
The methods to distinguish cortical visual areas used as standard by vision scientists working on subjects with normal vision may assist in studies of ophthalmological conditions. Furthermore, the increase in the strength of the MRI scanners available means that in the future it will be possible to investigate changes in the functional architecture at a macroscopic level.
Acknowledgments
This work was funded by a Royal Society University Research Fellowship and the Medical Research Council. I would like to thank Andrew Parker for comments on the article.
The author declares no conflict of interest.
References
- Goebel R, Muckli L, Zanella FE, Singer W, Stoerig P. Sustained extrastriate cortical activation without visual awareness revealed by fMRI studies of hemianopic patients. Vision Res. 2001;41:1459–1474. doi: 10.1016/s0042-6989(01)00069-4. [DOI] [PubMed] [Google Scholar]
- Baseler HA, Morland AB, Wandell BA. Topographic organization of human visual areas in the absence of input from primary cortex. J Neurosci. 1999;19:2619–2627. doi: 10.1523/JNEUROSCI.19-07-02619.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James TW, Culham J, Humphrey GK, Milner AD, Goodale MA. Ventral occipital lesions impair object recognition but not object-directed grasping: an fMRI study. Brain. 2003;126:2463–2475. doi: 10.1093/brain/awg248. [DOI] [PubMed] [Google Scholar]
- Hadjikhani N, de Gelder B. Neural basis of prosopagnosia: an fMRI study. Hum Brain Mapp. 2002;16:176–182. doi: 10.1002/hbm.10043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes G. Disturbances of vision by cerebral lesions. Br J Ophthalmol. 1918;2:353–384. doi: 10.1136/bjo.2.7.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton JC, Hoyt WF. The representation of the visual field in human striate cortex. A revision of the classic Holmes map. Arch Ophthalmol. 1991;109:816–824. doi: 10.1001/archopht.1991.01080060080030. [DOI] [PubMed] [Google Scholar]
- Horton JC, Hoyt WF. Quadrantic visual field defects. A hallmark of lesions in extrastriate (V2/V3) cortex. Brain. 1991;114 (Part 4:1703–1718. doi: 10.1093/brain/114.4.1703. [DOI] [PubMed] [Google Scholar]
- Daniel PM, Whitteridge D. The representation of the visual field on the cerebral cortex in monkeys. J Physiol. 1961;159:203–221. doi: 10.1113/jphysiol.1961.sp006803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Essen DC, Newsome WT, Maunsell JH. The visual field representation in striate cortex of the macaque monkey: asymmetries, anisotropies, and individual variability. Vision Res. 1984;24:429–448. doi: 10.1016/0042-6989(84)90041-5. [DOI] [PubMed] [Google Scholar]
- DeYoe EA, Carman GJ, Bandettini P, Glickman S, Wieser J, Cox R, et al. Mapping striate and extrastriate visual areas in human cerebral cortex. Proc Natl Acad Sci USA. 1996;93:2382–2386. doi: 10.1073/pnas.93.6.2382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel SA, Glover GH, Wandell BA. Retinotopic organization in human visual cortex and the spatial precision of functional MRI. Cereb Cortex. 1997;7:181–192. doi: 10.1093/cercor/7.2.181. [DOI] [PubMed] [Google Scholar]
- Engel SA, Rumelhart DE, Wandell BA, Lee AT, Glover GH, Chichilnisky EJ, et al. fMRI of human visual cortex. Nature. 1994;369:525. doi: 10.1038/369525a0. [DOI] [PubMed] [Google Scholar]
- Sereno MI, Sereno MI, Dale AM, Reppas JB, Kwong KK, Belliveau JW, et al. Borders of multiple visual areas in humans revealed by functional magnetic resonance imaging. Science. 1995;268:889–893. doi: 10.1126/science.7754376. [DOI] [PubMed] [Google Scholar]
- Van Essen DC. A population-average, landmark- and surface-based (PALS) atlas of human cerebral cortex. Neuroimage. 2005;28:635–662. doi: 10.1016/j.neuroimage.2005.06.058. [DOI] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Tootell RB, Dale AM. High-resolution intersubject averaging and a coordinate system for the cortical surface. Hum Brain Mapp. 1999;8:272–284. doi: 10.1002/(SICI)1097-0193(1999)8:4<272::AID-HBM10>3.0.CO;2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis. II: inflation, flattening, and a surface-based coordinate system. Neuroimage. 1999;9:195–207. doi: 10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 1999;9:179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- Baillarger JGF. Recherches sur la structure de la couche corticale des circonvolutions du cerveau. Mém Acad R Méd. 1840;8:149–183. [Google Scholar]
- Brodmann K. Vergleichende Lokalisationslehre der Großhirnrinde in ihren Prinzipeien dargestellt auf Grund des Zellenbaues. Leipzig: Barth; 1909. [Google Scholar]
- Elliott Smith G. A new topographical survey of the human cerebral cortex being an account of the distribution of the anatomically distinct cortical areas and their relationship to the cerebral sulci. J Anat Physiol. 1907;41:237–254. [PMC free article] [PubMed] [Google Scholar]
- Gennari F. Francisci Gennari Parmensis Medicinae Doctoris Collegiati de Peculiari Structura Cerebri Nonnullisque Eius Morbis-Paucae Aliae Anatom. Observat. Accedunt. Parma: Regio Typographeo; 1782. [Google Scholar]
- Bridge H, Clare S, Jenkinson M, Jezzard P, Parker AJ, Matthews PM, et al. Independent anatomical and functional measures of the V1/V2 boundary in human visual cortex. J Vis. 2005;5:93–102. doi: 10.1167/5.2.1. [DOI] [PubMed] [Google Scholar]
- Duncan RO, Sample PA, Weinreb RN, Bowd C, Zangwill LM. Retinotopic organization of primary visual cortex in glaucoma: a method for comparing cortical function with damage to the optic disk. Invest Ophthalmol Vis Sci. 2007;48:733–744. doi: 10.1167/iovs.06-0773. [DOI] [PubMed] [Google Scholar]
- Boucard CC, Hernowo AT, Maguire RP, Jansonius NM, Roerdink JB, Hooymans JM, et al. Changes in cortical grey matter density associated with long-standing retinal visual field defects. Brain. 2009;132:1898–1906. doi: 10.1093/brain/awp119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda Y, Dumoulin SO, Nakadomari S, Wandell BA. V1 projection zone signals in human macular degeneration depend on task, not stimulus. Cereb Cortex. 2008;18:2483–2493. doi: 10.1093/cercor/bhm256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dilks DD, Baker CI, Peli E, Kanwisher N. Reorganization of visual processing in macular degeneration is not specific to the ‘preferred retinal locus'. J Neurosci. 2009;29:2768–2773. doi: 10.1523/JNEUROSCI.5258-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumacher EH, Jacko JA, Primo SA, Main KL, Moloney KP, Kinzel EN, et al. Reorganization of visual processing is related to eccentric viewing in patients with macular degeneration. Restor Neurol Neurosci. 2008;26:391–402. [PubMed] [Google Scholar]
- Baker CI, Peli E, Knouf N, Kanwisher NG. Reorganization of visual processing in macular degeneration. J Neurosci. 2005;25:614–618. doi: 10.1523/JNEUROSCI.3476-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbier EL, Marrett S, Danek A, Vortmeyer A, van Gelderen P, Duyn J, et al. Imaging cortical anatomy by high-resolution MR at 3.0T: detection of the stripe of Gennari in visual area 17. Magn Reson Med. 2002;48:735–738. doi: 10.1002/mrm.10255. [DOI] [PubMed] [Google Scholar]
- Sunness JS, Liu T, Yantis S. Retinotopic mapping of the visual cortex using functional magnetic resonance imaging in a patient with central scotomas from atrophic macular degeneration. Ophthalmology. 2004;111:1595–1598. doi: 10.1016/j.ophtha.2003.12.050. [DOI] [PubMed] [Google Scholar]
- Baseler HA, Gouws A, Morland AB. The organization of the visual cortex in patients with scotomata resulting from lesions of the central retina. Neuro-Ophthalmology. 2009;33:149–157. [Google Scholar]
- Wandell BA, Smirnakis SM. Plasticity and stability of visual field maps in adult primary visual cortex. Nat Rev Neurosci. 2009;10:873–884. doi: 10.1038/nrn2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell HW, Parker GJ, Alexander DC, Symms MR, Boulby PA, Wheeler-Kingshott CA, et al. MR tractography predicts visual field defects following temporal lobe resection. Neurology. 2005;65:596–599. doi: 10.1212/01.wnl.0000172858.20354.73. [DOI] [PubMed] [Google Scholar]
- Yogarajah M, Focke NK, Bonelli S, Cercignani M, Acheson J, Parker GJ, Alexander DC, McEvoy AW, et al. Defining Meyer's loop-temporal lobe resections, visual field deficits and diffusion tensor tractography. Brain. 2009;132 (Part 6:1656–1668. doi: 10.1093/brain/awp114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukunaga M, Li TQ, van Gelderen P, de Zwart JA, Shmueli K, Yao B, et al. Layer-specific variation of iron content in cerebral cortex as a source of MRI contrast. Proc Natl Acad Sci USA. 2010;107:3834–3839. doi: 10.1073/pnas.0911177107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yacoub E, Harel N, Ugurbil K. High-field fMRI unveils orientation columns in humans. Proc Natl Acad Sci USA. 2008;105:10607–10612. doi: 10.1073/pnas.0804110105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yacoub E, Shmuel A, Logothetis N, Ugurbil K. Robust detection of ocular dominance columns in humans using Hahn spin echo bold functional MRI at 7 Tesla. Neuroimage. 2007;37:1161–1177. doi: 10.1016/j.neuroimage.2007.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roe AW, Parker AJ, Born RT, DeAngelis GC. Disparity channels in early vision. J Neurosci. 2007;27:11820–11831. doi: 10.1523/JNEUROSCI.4164-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G, Lu HD, Roe AW. A map for horizontal disparity in monkey V2. Neuron. 2008;58:442–450. doi: 10.1016/j.neuron.2008.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]