Abstract
Purpose
To determine the pre-treatment ocular factors significantly associated with the visual outcome 24 months after intravitreal bevacizumab (IVB) for myopic choroidal neovascularization (mCNV).
Methods
A total of 23 eyes of 23 patients with mCNV were treated with IVB followed by as needed therapy. The efficacy of IVB was evaluated by the best-corrected visual acuity (BCVA) at 24 months after the initial treatment. Forward stepwise multiple linear regression analyses were performed to evaluate the influence of pre-treatment factors on the BCVA and the improvement of the BCVA at 24 months.
Results
The mean pre-IVB BCVA was 0.74±0.30 logarithm of the minimum angle of resolution (logMAR) units, and it improved to 0.43±0.31 logMAR units after 1 month (P<0.001, paired t-test). The improvement was maintained at 24 months (0.46±0.40, P<0.005). The mean number of IVB performed during the 24 months was 1.35±0.71. Forward stepwise regression analysis showed that the pre-IVB CNV size (standardized β=0.52, P<0.01) and BCVA (standardized β=−0.44, P<0.05) significantly affected the visual acuity change after 24 months. The CNV size was the only factor that significantly affected the BCVA after 24 months (standardized β=0.56, P<0.01).
Conclusions
IVB with as needed therapy for mCNV led to a rapid and sustained visual improvement. Smaller CNV size was a significant prognostic factor that predicts better visual acuity. Patients with lower pre-treatment BCVA had better visual recovery than those with better pre-treatment BCVA, however, this may be due to a ceiling/floor effect.
Keywords: myopic choroidal neovasculariation, bevacizumab, prognostic factor, intravitreal injection
Introduction
Choroidal neovascularization (CNV) is a vision-threatening complication in eyes with pathological myopia. Myopic choroidal neovascularizations (mCNVs) have been shown to develop in 5 to 10% of eyes with pathological myopia,1, 2, 3 and several studies have shown that mCNVs have a poor natural history.4, 5, 6 For example, the visual acuity at 5 years after the onset of CNV decreased to ≤20/200 in 89% of the eyes and in 96% of the eyes after 10 years.6
Because of the poor natural history of mCNVs, several procedures have been tried to treat mCNVs, for example, thermal laser photocoagulation,7 photodynamic therapy (PDT) with verteporfin (Visudyne, Novartis Pharma AG, Basel, Switzerland),8 and intravitreal bevacizumab (Avastin; Genentech, South San Francisco, CA, USA), a recombinant humanized monoclonal anti-VEGF antibody. Earlier case series have reported good visual outcomes 1 to 2 years after intravitreal bevacizumab (IVB),9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21 and at present IVB would be the first-line therapy for sub- and juxtafoveal mCNVs.22 However, there is still not enough information to predict the visual outcome of each patient with mCNV treated with IVB.
Thus, the purpose of this study was to determine the long-term visual outcome of IVB in eyes with mCNVs. We also determined which pre-IVB factors were significantly associated with the visual outcome 2-years after the IVB therapy.
Patients and methods
All of the procedures used in this study were approved by the Institutional Review Board at Kyoto University Graduate School of Medicine, and they conformed to the tenets of the Declaration of Helsinki. A written informed consent was obtained from each patient.
In this nonrandomized, non-comparative case series, we reviewed the medical records of patients with myopic CNV who were treated with IVB at the Kyoto University Hospital between 1 December 2006 and 31 December 2007. Before the IVB, all of the patients received a comprehensive ophthalmological examination, including best-corrected visual acuity (BCVA), intraocular pressure measurements, indirect ophthalmoscopy, slit-lamp biomicroscopy with a contact lens, fundus photography, optical coherence tomography (OCT), and fluorescein/indocyanine green angiography (FA/IA) using a confocal laser scanning system (HRA-2; Heidelberg Engineering, Heidelberg, Germany). The Stratus OCT3000 (Carl Zeiss, Dublin, CA, USA) or the OCT ophthalmoscope C7 (Nidek, Gamagori, Japan) examination of cross-sections (5–6 mm in length) centered on the fovea and on the mCNV were performed at the baseline examination. The size of the mCNV before treatment was measured on the FA/IA images using the embedded software programs in the HRA-2. The BCVA was measured with a Landolt chart, and the decimal values were converted to the logarithm of the minimal angle of resolution (logMAR) units.
The inclusion criteria were: (1) an axial length of ≥26.50 mm or spherical equivalent refractive error of ≥−6.0 diopters (D) in phakic eyes; (2) fundus changes typical of pathological myopia, such as chorioretinal atrophy, lacquer cracks, or atrophic patches; (3) FA documentation of subfoveal or juxtafoveal CNV that showed active leakage; and (4) BCVA of ≥1.3 logMAR units (0.05 decimal units, 10/200 in Snellen acuity). The exclusion criteria were: (1) history of intraocular surgery except for cataract surgery; (2) previous treatment for the mCNV; and (3) other ocular disease that can influence the BCVA, such as corneal opacity or myopic foveoschisis. In patients who had undergone IVB in both eyes, the data from the right eye was used for the statistical analyses.
All patients who had a recent visual disturbance due to active subfoveal or juxtafoveal mCNV were offered the IVB treatment with an explanation of possible complications. The intravitreal dose of bevacizumab was 1.25 mg per 0.05 ml. All injections were performed in a sterile manner, and prophylactic topical antibiotics were applied from a few days before to 1 week after the injection. After the initial IVB, the BCVA was measured, indirect ophthalmoscopy, slit-lamp biomicroscopy, and OCT examination of cross-sectional images centered on the fovea and on the mCNV was performed at each visit, and additional examinations such as angiography were performed as needed. Retreatment with IVB was performed if the evaluating clinician judged a re-injection was needed. The re-injection criteria were any of following finding with visual loss at least 1 month after the previous IVB: (1) persistence or recurrence of macular edema and/or serous retinal detachment in the OCT images; (2) persistence or recurrence of dye leakage in the FA images; and (3) new subretinal hemorrhage from the mCNV.
The efficacy of IVB for mCNV was based on the BCVA measured at 1, 3, 6, 12, 18, and 24 months after the initial IVB. In addition, the change in the BCVA, number of IVBs, and number of serious complications during the 24 months follow-up were evaluated.
Paired t-tests were used to evaluate the significance of differences in the BCVA at two time points. Pearson's correlation analyses were used to assess the influence of each pre-treatment factor, viz, age in years, duration of symptom in months, axial length in mm, pre-treatment BCVA in logMAR units, pre-treatment CNV size in μm, and pre-treatment CNV location as subfoveal or juxtafoveal, on the BCVA change, and the BCVA at 24 months after the initial IVB. Stepwise forward multivariate linear regression analyses were also performed to evaluate the contribution of each pre-treatment factor to the BCVA change and the BCVA at 24 months after the initial IVB. These statistical analyses were performed using software R (http://www.r-project.org/). The pre-IVB location of the mCNV was given a numerical value of 1 for subfoveal mCNVs and 0 for juxtafoveal CNVs for the correlation and multiple regression analyses. Stepwise forward regression analyses were performed using the software R package ‘maSigPro' (http://bioconductor.org/packages/ release/bioc/html/maSigPro.html). All continuous values are presented as means±SD. The level of statistical significance was set at P<0.05.
Results
A total of 28 eyes of 28 patients met the inclusion criteria. Of these 28 eyes, one eye showed severe inflammation with dense vitreous opacity 1 day after the fifth IVB and underwent pars plana vitrectomy.23 We excluded this eye from the statistical analyses. There were no other severe ocular or systemic adverse effects after the IVB. Of the remaining 27 eyes, four eyes of four patients were lost to follow-up 6 to 18 months after the initial treatment. Then the final number of eyes analyzed was 23 eyes of 23 patients.
The demographics of the 23 eyes of 23 patients are shown in Table 1. The mean age of the 23 patients (7 men and 16 women) at the time of the initial IVB was 65.1±10.2 years with a range of 39 to 81 years. The mean axial length was 28.94±1.70 mm with a range of 26.50 to 32.63 mm. All of the 23 CNVs were predominantly classic on FA, and the CNV was subfoveal in 14 eyes (60.9%) and juxtafoveal in 9 eyes (39.1%). The mean size of the CNV before treatment was 1803±725 μm with a range of 750 to 3750 μm. The duration of the symptoms was 3.5±2.2 months with a range of 1.0 to 8.5 months.
Table 1. Demographics and Ocular Characteristics of the Study Population.
| Category | Subcategory | Value |
|---|---|---|
| Number of patients (eye) | 23 (23) | |
| Age (year) | Mean±SD | 65.1±10.2 |
| Median (range) | 65 (39–81) | |
| Gender, no (%) | Men | 7 (30.4%) |
| Women | 16 (69.6%) | |
| Axial length (mm) | Mean±SD | 28.94±1.70 |
| Median (range) | 28.90 (26.50–32.63) | |
| Refraction of phakic eyes | Mean±SD | −12.62±3.47 |
| (diopter)a | Median (range) | −13.06 (−6.85 to −19.50) |
| Duration of symptoms | Mean±SD | 3.5±2.2 |
| (month) | Median (range) | 2.5 (1.0–8.5) |
| CNV size before treatment | Mean±SD | 1803±725 |
| (μm) | Median (range) | 1700 (750–3750) |
| CNV location, no (%) | Subfoveal | 14 (60.9%) |
| Juxtafoveal | 9 (39.1%) |
Abbreviation: CNV, choroidal neovascularization.
For the calculation, seven eyes (30.4%) that had undergone cataract surgery were excluded. None of the eyes had undergone corneal refractive surgery.
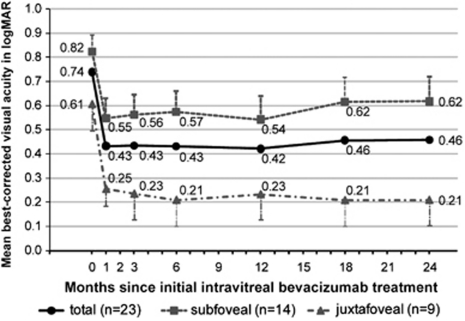
The mean BCVA before and 1, 3, 6, 12, 18, and 24 months after the initial treatment are shown in Figure 1. The mean pre-IVB BCVA was 0.74±0.30 logMAR units with a range of 0.22 to 1.30 logMAR units. At 1 month after the initial IVB, the mean BCVA improved significantly to 0.43±0.31 logMAR units (P<0.001; paired t-test). The improved BCVA was maintained at 0.46±0.40 logMAR units 24 months after the first treatment (P<0.005). The BCVA in logMAR units was inversely proportional to decimal BCVA, and thus negative values of the mean BCVA change of the 23 eyes was −0.28±0.40 logMAR units, which indicated that the mean BCVA had improved 24 months after the initial IVB. Of the 23 eyes, 14 eyes (60.9%) showed a visual improvement of >0.2 logMAR units at 24 months after the first treatment, and two eyes (8.7%) showed a visual loss of >0.2 logMAR units at 24 months after the first treatment. The mean number of IVB injections performed during the 24 months was 1.35±0.71; 17 eyes (73.9%) had IVB only once; 5 eyes (21.7 %) had IVB twice; and 1 eye (4.4%) required four IVB injections.
Figure 1.
Changes of mean best-corrected visual acuity over 24 months after initial IVB therapy for a mCNV. The squares represent the results of subfoveal mCNVs (n=14), the triangles represent the juxtafoveal mCNVs (n=9), and the circles represent all of the eyes, that is, sum of the subfoveal and juxtafoveal mCNVs (n=23). Visual acuity was converted to a logarithm of the minimal angle of resolution (logMAR) units. The error-bar represents the SEMs.
We next evaluated whether significant correlations existed between pre-treatment factors and the change in the BCVA at 24 months after the initial IVB (Table 2). Pearson's correlation analyses showed that among the pre-treatment factors, the pre-treatment CNV size (r=0.45, P<0.05) and duration of symptoms (r=0.42, P<0.05) were positively correlated with the change in the BCVA at 24 months. These results suggested that smaller CNVs and shorter durations of symptoms before the IVB were significantly associated with a greater improvement of the BCVA at 24 months after the initial IVB therapy. The pre-treatment BCVA showed marginally but not significant correlation with the change in the BCVA at 24 months (r=−0.37, P=0.087). The pre-treatment CNV location (P=0.27), age (P=0.70), and axial length (P=0.93) were not significantly correlated with the change in the BCVA.
Table 2. Correlation analysis and stepwise forward regression analysis to access the influence of each pre-treatment factor on LogMAR change at 24 months after initial IVB for mCNV.
| Covariate (Pre-treatment factors) |
Pearson's correlation analysis |
Stepwise forward regression analysisa |
||
|---|---|---|---|---|
| r | P-value | Standardized β | P-value | |
| CNV size | 0.45 | 0.032 | 0.52 | 0.0082 |
| BCVA (logMAR) | −0.37 | 0.087 | −0.44 | 0.020 |
| Duration of symptoms | 0.42 | 0.044 | Not included | — |
| CNV location | 0.24 | 0.27 | Not included | — |
| Age | −0.08 | 0.70 | Not included | — |
| Axial length | 0.02 | 0.93 | Not included | — |
Abbreviations: BCVA, best-corrected visual acuity; logMAR, logarithm of the minimal angle of resolution; mCNV, myopic choroidal neovascularization; r, Pearson's correlation coefficient; β, regression coefficient.
Adjusted R2 (the coefficient of multiple determination)=0.333.
Forward stepwise multiple linear regression analysis with the visual acuity change at 24 months as the dependent variable showed that pre-treatment CNV size (standardized β (multiple regression coefficient)=0.52, P<0.01) and pre-treatment BCVA (standardized β=−0.44, P<0.05) were significant contributing determinants. The duration of symptoms was not included in the stepwise selection procedure. The adjusted R2 (coefficient of multiple determination) of the final model was 0.333.
We also evaluated the possible association of the pre-treatment factors with the BCVA at 24 months after the initial IVB treatment (Table 3). Pearson's correlation analyses showed that pre-treatment CNV size and pre-treatment CNV location were significantly associated with BCVA at 24 months (r=0.56, P<0.01 and r=0.50, P<0.05, respectively). The duration of the symptoms was also significantly correlated with the BCVA at 24 months (r=0.49, P<0.05). The pre-treatment BCVA showed marginally but not significant correlation (r=0.39, P=0.065) with BCVA at 24 months after initial treatment. Forward stepwise regression analysis showed that only the pre-treatment CNV size (standardized β=0.56, P<0.01) was included in the final model. The adjusted R2 of the final model was 0.279.
Table 3. Correlation analysis and stepwise forward regression analysis to access the influence of each pre-treatment factor on BCVA in LogMAR at 24 Months after Initial IVB for mCNV.
| Covariate (Pre-treatment factors) |
Pearson's correlation analysis |
Stepwise forward regression analysisa |
||
|---|---|---|---|---|
| r | P-value | Standardized β | P-value | |
| CNV size | 0.56 | 0.0056 | 0.56 | 0.0056 |
| CNV location | 0.50 | 0.014 | Not included | — |
| Duration of symptoms | 0.49 | 0.019 | Not included | — |
| BCVA in logMAR | 0.39 | 0.065 | Not included | — |
| Age | 0.25 | 0.24 | Not included | — |
| Axial length | −0.22 | 0.32 | Not included | — |
Abbreviations: BCVA, best-corrected visual acuity; logMAR, logarithm of the minimal angle of resolution; mCNV, myopic choroidal neovascularization; r, Pearson's correlation coefficient; β, regression coefficient.
Adjusted R2 (the coefficient of multiple determination)=0.279.
Discussion
Although the dose of bevacizumab and follow-up strategy were different among the studies, earlier studies have reported that IVB for mCNV leads to a significant improvement in the BCVA with only a few injections. Thus IVB may be considered as first-line therapy for mCNV.9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22 However, the follow-up periods were up to 1-year in most of the earlier studies. There have been a few studies that showed 2-years visual outcomes of IVB for mCNV, and the results have been conflicting.9, 18, 20 Our results showed that IVB for 23 eyes with mCNV significantly improved the BCVA at 1 month (from 0.74±0.30 logMAR units to 0.43±0.31 logMAR units), and following an as needed strategy, the BCVA improvement was maintained over 24 months with 1.35±0.71 times IVB. Baba et al9 reported that 12 eyes with mCNV treated by 1.25 mg IVB had significant improvement of the BCVA from 0.75±0.25 logMAR units at baseline to 0.50±0.38 logMAR unit at 24 months after IVB, and mean number of injections was 1.6±0.8 times. Ikuno et al18 reported that 11 eyes with mCNV treated by 1.0 mg IVB showed significant improvement of the BCVA from 0.68±0.29 logMAR units to 0.56±0.31 logMAR unit at 1 month, and the improvement was maintained for 12 months. However, the significance of the improvement was not present at 18 and 24 months after the initial treatment, and the mean number of injections was 2.9±2.4 times. Voykov et al20 reported that 11 eyes treated by 1.25 mg IVB monotherapy showed gradually improvements of the BCVA from 0.7 logMAR units to 0.5 logMAR unit with 2.2 times injections at 24 months after IVB, however, the improvement was marginally not significant.
There are several reasons for the differences of the results of these studies; for example, all four studies were retrospective, the sample sizes were relatively small, and there were differences of the baseline characteristics of the patients. Accumulation of the results of more studies, as well as prospective studies with a larger number of cohorts will be necessary to understand the long-term visual prognosis of IVB for mCNV. Several earlier studies also showed that IVB was more effective than photodynamic therapy for treating mCNV.9, 12, 18, 19, 21 We could not compare the efficacy of IVB with the other treatments because our study was a non-comparative design.
The prognostic factor analyses showed that the pre-treatment CNV size was significantly associated with both the BCVA and the change in the BCVA at 24 months after the initial IVB. These results indicated that eyes with smaller mCNV had both better BCVA itself and better improvement of BCVA at 24 months after the initial IVB than those with larger mCNV. Our results showed that the mCNV size could be used as a prognostic factor for the BCVA after IVB for mCNV. Similar findings were reported for age-related macular degeneration (AMD) where the size of the CNV before PDT or anti-VEGF therapy was a predictive factor for the post-treatment BCVA.8, 24, 25, 26, 27, 28 However, the mechanism of how the CNV lesion size influences the visual outcome after these treatments has not been determined.
The pre-treatment BCVA was also significantly associated with the change in the post-IVB BCVA at 24 months, but it was not significantly associated with the BCVA itself at 24 months. Thus, patients with poorer BCVA acuity before treatment had greater recovery of the BCVA than those with better pre-treatment BVCA. Similar results were reported in subgroup analyses in the MARINA25 and ANCHOR studies,26 both of which were prospective, randomized, double-masked studies that evaluated effectiveness of another anti-VEGF drug, ranibizumab, for the treatment of AMD. In both subgroup analyses, better baseline BCVA, increasing age and larger CNV lesion size were associated with less improvement of the BCVA after the ranibizumab treatment. The authors suggested that the association between the baseline BCVA and the visual improvement after treatment was because patients with higher pre-treatment BCVAs had a smaller chance for improvement (ceiling effect), whereas patients with a greater impairment of the pre-treatment BCVA had a greater chance for improvement (floor effect).25, 26 We suggest that our results might also be due to the similar ceiling/floor effect, and we should not consider that IVB was less effective for the mCNV patients with better pre-treatment BCVA.
An earlier natural history study showed that eyes with juxtafoveal mCNV had better final BCVA than those with subfoveal mCNV.5 The correlation analysis in our study showed that the pre-treatment location (subfoveal or juxtafoveal) of the mCNV was significantly correlated with BCVA at 24 months. However, the forward stepwise regression analysis did not show that the CNV location was a significant contributing determinant for the BCVA at 24 months. This might be partially because of the pre-treatment CNV location was correlated with the pre-treatment CNV size (r=0.45, P<0.05), that is, subfoveal mCNVs were larger than juxtafoveal mCNVs in this study. The duration of the symptoms was also correlated with pre-treatment CNV size (r=0.42, P<0.05). This might explain why the forward stepwise regression analysis did not include this covariant into the final models.
Although the patients' age has been shown to have influence on natural history and visual outcome after treatment of mCNVs,24, 29, 30, 31, 32 it was not significantly associated with visual outcome after IVB treatment in our eyes. The reason might be that most of the patients in this study were older with a mean age of 65.1±10.2 years, and 20 of the 23 patients (87.0%) were over 60 years of age. We could not assess the effectiveness of IVB for young patients with mCNV and additional study are needed to determine this.
The limitations of this study are its retrospective design and small sample size. The adjusted R2 of the final regression models was 0.333 and 0.279, indicating that the revealed predictors leave a notable amount of variation of the dependent variables, that is, the BCVA and the change in the BCVA at 24 months. However, the results of this study showed that IVB followed by as needed strategy led to a rapid and significant visual recovery in patients with mCNV, and the visual recovery was maintained for 24 months after initial treatment. The pre-treatment CNV size was an important prognostic factor that was significantly associated with both the change in the BCVA and the BCVA at 24 months after initial treatment. The results indicated that patients with smaller pre-treatment mCNVs would have better visual recovery and better BCVA at 24 months after initial IVB. The patients with more impaired pre-treatment BCVA had better visual recovery than those with better pre-treatment BVCA, however, these results may be due to ceiling/floor effects.
Acknowledgments
We thank Dr Christopher Seunkyu Lee, Yonsei University College of Medicine, Seoul, Korea, for his support in data analysis and interpretation. The study was supported in part by grants-in-aid for scientific research (No. 21249084) from the Japan Society for the Promotion of Science, Tokyo, Japan, and by the Japanese National Society for the Prevention of Blindness.
The authors declare no conflict of interest.
Footnotes
The study was supported in part by grants-in-aid for scientific research (No. 21249084) from the Japan Society for the Promotion of Science, Tokyo, Japan, and by the Japanese National Society for the Prevention of Blindness.
References
- Curtin BJ, Karlin DB. Axial length measurements and fundus changes of the myopic eye. Am J Ophthalmol. 1971;71:42–53. doi: 10.1016/0002-9394(71)91092-0. [DOI] [PubMed] [Google Scholar]
- Grossniklaus HE, Green WR. Pathologic findings in pathologic myopia. Retina. 1992;12:127–133. doi: 10.1097/00006982-199212020-00009. [DOI] [PubMed] [Google Scholar]
- Ohno-Matsui K, Yoshida T, Futagami S, Yasuzumi K, Shimada N, Kojima A, et al. Patchy atrophy and lacquer cracks predispose to the development of choroidal neovascularisation in pathological myopia. Br J Ophthalmol. 2003;87:570–573. doi: 10.1136/bjo.87.5.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotchkiss ML, Fine SL. Pathologic myopia and choroidal neovascularization. Am J Ophthalmol. 1981;91:177–183. doi: 10.1016/0002-9394(81)90170-7. [DOI] [PubMed] [Google Scholar]
- Bottoni F, Tilanus M. The natural history of juxtafoveal and subfoveal choroidal neovascularization in high myopia. Int Ophthalmol. 2001;24:249–255. doi: 10.1023/a:1025488429802. [DOI] [PubMed] [Google Scholar]
- Yoshida T, Ohno-Matsui K, Yasuzumi K, Kojima A, Shimada N, Futagami S, et al. Myopic choroidal neovascularization: a 10-year follow-up. Ophthalmology. 2003;110:1297–1305. doi: 10.1016/S0161-6420(03)00461-5. [DOI] [PubMed] [Google Scholar]
- Virgili G, Menchini F. Laser photocoagulation for choroidal neovascularisation in pathologic myopia. Cochrane Database Syst Rev. 2005. p. CD004765. [DOI] [PubMed]
- Blinder KJ, Blumenkranz MS, Bressler NM, Bressler SB, Donato G, Lewis H, et al. Verteporfin therapy of subfoveal choroidal neovascularization in pathologic myopia: 2-year results of a randomized clinical trial—VIP report no. 3. Ophthalmology. 2003;110:667–673. doi: 10.1016/s0161-6420(02)01998-x. [DOI] [PubMed] [Google Scholar]
- Baba T, Kubota-Taniai M, Kitahashi M, Okada K, Mitamura Y, Yamamoto S. Two-year Comparison of Photodynamic Therapy and Intravitreal Bevacizumab for Treatment of Myopic Choroidal Neovascularization. Br J Ophthalmol. 2010;94:864–870. doi: 10.1136/bjo.2009.166025. [DOI] [PubMed] [Google Scholar]
- Chan WM, Lai TY, Liu DT, Lam DS. Intravitreal bevacizumab (Avastin) for myopic choroidal neovascularisation: 1-year results of a prospective pilot study. Br J Ophthalmol. 2009;93:150–154. doi: 10.1136/bjo.2008.145797. [DOI] [PubMed] [Google Scholar]
- Gharbiya M, Allievi F, Mazzeo L, Gabrieli CB.Intravitreal bevacizumab treatment for choroidal neovascularization in pathologic myopia: 12-month results Am J Ophthalmol 200914784–93.e1. [DOI] [PubMed] [Google Scholar]
- Hayashi K, Ohno-Matsui K, Teramukai S, et al. Comparison of visual outcome and regression pattern of myopic choroidal neovascularization after intravitreal bevacizumab or after photodynamic therapy. Am J Ophthalmol. 2009;148:396–408. doi: 10.1016/j.ajo.2009.03.026. [DOI] [PubMed] [Google Scholar]
- Ikuno Y, Sayanagi K, Soga K, et al. Intravitreal bevacizumab for choroidal neovascularization attributable to pathological myopia: one-year results Am J Ophthalmol 200914794–100.e1. [DOI] [PubMed] [Google Scholar]
- Ruiz-Moreno JM, Montero JA, Gomez-Ulla F, Ares S. Intravitreal bevacizumab to treat subfoveal choroidal neovascularisation in highly myopic eyes: 1-year outcome. Br J Ophthalmol. 2009;93:448–451. doi: 10.1136/bjo.2008.145391. [DOI] [PubMed] [Google Scholar]
- Scupola A, Tiberti AC, Sasso P, et al. Macular functional changes evaluated with MP-1 microperimetry after intravitreal bevacizumab for subfoveal myopic choroidal neovascularization: one year results. Retina. 2010;30:739–747. doi: 10.1097/IAE.0b013e3181c59725. [DOI] [PubMed] [Google Scholar]
- Spielberg L, Leys A. Intravitreal bevacizumab for myopic choroidal neovascularization: short-term and 1-year results. Bull Soc Belge Ophtalmol. 2009;312:17–27. [PubMed] [Google Scholar]
- Wu PC, Chen YJ. Intravitreal injection of bevacizumab for myopic choroidal neovascularization: 1-year follow-up. Eye (London) 2009;23:2042–2045. doi: 10.1038/eye.2008.404. [DOI] [PubMed] [Google Scholar]
- Ikuno Y, Nagai Y, Matsuda S, Arisawa A, Sho K, Oshita T, et al. Two-year visual results for older Asian women treated with photodynamic therapy or bevacizumab for myopic choroidal neovascularization. Am J Ophthalmol. 2010;149:140–146. doi: 10.1016/j.ajo.2009.08.008. [DOI] [PubMed] [Google Scholar]
- Parodi MB, Iacono P, Papayannis A, Sheth S, Bandello F. Laser Photocoagulation, Photodynamic Therapy, and Intravitreal Bevacizumab for the Treatment of Juxtafoveal Choroidal Neovascularization Secondary to Pathologic Myopia. Arch Ophthalmol. 2010;128:437–442. doi: 10.1001/archophthalmol.2009.408. [DOI] [PubMed] [Google Scholar]
- Voykov B, Gelisken F, Inhoffen W, Voelker M, Ulrich Bartz-Schmidt K, Ziemssen F. Bevacizumab for choroidal neovascularization secondary to pathologic myopia: Is there a decline of the treatment efficacy after 2 years. Graefes Arch Clin Exp Ophthalmol. 2010;248:543–550. doi: 10.1007/s00417-009-1285-1. [DOI] [PubMed] [Google Scholar]
- Yoon JU, Byun YJ, Koh HJ. Intravitreal anti-VEGF versus photodynamic therapy with verteporfin for treatment of myopic choroidal neovascularization. Retina. 2010;30:418–424. doi: 10.1097/IAE.0b013e3181bd2fe4. [DOI] [PubMed] [Google Scholar]
- Cohen SY. Anti-VEGF drugs as the 2009 first-line therapy for choroidal neovascularization in pathologic myopia. Retina. 2009;29:1062–1066. doi: 10.1097/IAE.0b013e3181b1bb1a. [DOI] [PubMed] [Google Scholar]
- Yamashiro K, Tsujikawa A, Miyamoto K, Oh H, Otani A, Tamuara H, et al. Sterile endophthalmitis after intravitreal injection of bevacizumab obtained from a single batch. Retina. 2010;30:485–490. doi: 10.1097/IAE.0b013e3181bd2d51. [DOI] [PubMed] [Google Scholar]
- Ergun E, Heinzl H, Stur M. Prognostic factors influencing visual outcome of photodynamic therapy for subfoveal choroidal neovascularization in pathologic myopia. Am J Ophthalmol. 2004;138:434–438. doi: 10.1016/j.ajo.2004.04.055. [DOI] [PubMed] [Google Scholar]
- Boyer DS, Antoszyk AN, Awh CC, Bhisitkul RB, Shapiro H, Acharya NR. Subgroup analysis of the MARINA study of ranibizumab in neovascular age-related macular degeneration. Ophthalmology. 2007;114:246–252. doi: 10.1016/j.ophtha.2006.10.045. [DOI] [PubMed] [Google Scholar]
- Kaiser PK, Brown DM, Zhang K, Hudson HL, Holz FG, Shapiro H, et al. Ranibizumab for predominantly classic neovascular age-related macular degeneration: subgroup analysis of first-year ANCHOR results. Am J Ophthalmol. 2007;144:850–857. doi: 10.1016/j.ajo.2007.08.012. [DOI] [PubMed] [Google Scholar]
- Lux A, Llacer H, Heussen FM, Joussen AM. Non-responders to bevacizumab (Avastin) therapy of choroidal neovascular lesions. Br J Ophthalmol. 2007;91:1318–1322. doi: 10.1136/bjo.2006.113902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang S, Roh YJ. One-year results of intravitreal ranibizumab for neovascular age-related macular degeneration and clinical responses of various subgroups. Jpn J Ophthalmol. 2009;53:389–395. doi: 10.1007/s10384-009-0670-y. [DOI] [PubMed] [Google Scholar]
- Yoshida T, Ohno-Matsui K, Ohtake Y, Takashima T, Futagami S, Baba T, et al. Long-term visual prognosis of choroidal neovascularization in high myopia: a comparison between age groups. Ophthalmology. 2002;109:712–719. doi: 10.1016/s0161-6420(01)01007-7. [DOI] [PubMed] [Google Scholar]
- Axer-Siegel R, Ehrlich R, Weinberger D, Rosenblatt I, Shani L, Yassur Y, et al. Photodynamic therapy of subfoveal choroidal neovascularization in high myopia in a clinical setting: visual outcome in relation to age at treatment. Am J Ophthalmol. 2004;138:602–607. doi: 10.1016/j.ajo.2004.05.074. [DOI] [PubMed] [Google Scholar]
- Hayashi K, Ohno-Matsui K, Yoshida T, Kobayashi K, Kojima A, Shimada N, et al. Characteristics of patients with a favorable natural course of myopic choroidal neovascularization. Graefes Arch Clin Exp Ophthalmol. 2005;243:13–19. doi: 10.1007/s00417-004-0960-5. [DOI] [PubMed] [Google Scholar]
- Kojima A, Ohno-Matsui K, Teramukai S, Ishihara Y, Shimada N, Yoshida T, et al. Estimation of visual outcome without treatment in patients with subfoveal choroidal neovascularization in pathologic myopia. Graefes Arch Clin Exp Ophthalmol. 2006;244:1474–1479. doi: 10.1007/s00417-006-0324-4. [DOI] [PubMed] [Google Scholar]