Abstract
Background
To prepare for a possible major radiation disaster involving large numbers of potentially exposed people, it is important to be able to rapidly and accurately triage people for treatment or not, factoring in the likely conditions and available resources. To date, planners have had to create guidelines for triage based on methods for estimating dose that are clinically available and which use evidence extrapolated from unrelated conditions. Current guidelines consequently focus on measuring clinical symptoms (e.g., time-to-vomiting), which may not be subject to the same verification of standard methods and validation processes required for governmental approval processes of new and modified procedures. Biodosimeters under development have not yet been formally approved for this use. Neither set of methods has been tested in settings involving large-scale populations at risk for exposure.
Objective
To propose a framework for comparative evaluation of methods for such triage and to evaluate biodosimetric methods that are currently recommended and new methods as they are developed.
Methods
We adapt the NIH model of scientific evaluations and sciences needed for effective translational research to apply to biodosimetry for triaging very large populations following a radiation event. We detail criteria for translating basic science about dosimetry into effective multi-stage triage of large populations and illustrate it by analyzing 3 current guidelines and 3 advanced methods for biodosimetry.
Conclusions
This framework for evaluating dosimetry in large populations is a useful technique to compare the strengths and weaknesses of different dosimetry methods. It can help policy-makers and planners not only to compare the methods’ strengths and weaknesses for their intended use but also to develop an integrated approach to maximize their effectiveness. It also reveals weaknesses in methods that would benefit from further research and evaluation.
Keywords: Biodosimetry, Comparative Effectiveness, Models
1 Introduction
There is growing recognition worldwide of the need to develop integrated and effective uses of biodosimetric methods to assess unexpected exposure to radiation in the event of a large-scale event (Simon et al. 2010; Alexander et al. 2007). The context of needing to prepare for a large-scale event brings particular challenges, not only to rapidly evaluate potentially hundreds of thousands of people to determine whose exposure level would benefit from treatment for Acute Radiation Syndrome (ARS), but to do so within chaotic and compromised systems of transportation, communication, and health care delivery (Gougelet et al. 2010).
Because of the recognized importance of being prepared for such possibilities, several governments have undertaken broad programs to develop new methods of biodosimetry and medical countermeasures against radiation, with the aim of speeding up the pathway from basic science investigations to practical, deployable means to accomplish this daunting task (Grace et al. 2010). Nevertheless, at present there are very few methods available for approved use in such events, and even these have had little or no evaluation within the context of triaging hundreds of thousands of people with compromised healthcare and other systems.
The usual circumstance of conducting comparative evaluations of clinical methods to diagnose or treat ‘the same’ disease focuses only on methods currently in practice. Such evaluations assume ‘usual’ clinical care settings and, by implication, ignore any special contexts pertaining to use in a large-scale public disaster.
We argue that it is necessary and constructive to build a framework for evaluating biodosimetric methods that takes into account the traditional scientific standards and rigor applied to clinical problems, while also recognizing the complexities of the particular context of a public health disaster involving radiation. We focus on evaluating biodosimetric methods currently approved and used in guidelines for mass triage. However, because this framework can inform policy makers, we also illustrate its applicability to biodosimetric methods under development.
2 Contexts for Evaluating Biodosimetry
Briefly, four contexts distinguish effective biodosimetry in large-scale events from the context for screening a population under ordinary circumstances:
2.1 Assumptions about Impact on People and Systems in the Immediate Area
The capability of current and developing methods of biodosimetry to efficiently triage potential victims of radiation accidents or terrorism varies in proportion to the number being assessed and the complexity of transporting and treating them. In this paper, we adopt the assumptions of the US government for planning responses, i.e., a10 kiloton (KT) improvised nuclear device (IND) explodes in an urban setting (Grace et al. 2010; Waselenko et al. 2004; National Security Staff, 2010). This scenario predicts that up to a million people may seek to know if they received an exposure justifying treatment for ARS, of which 200,000 are likely to have received a significant dose. (We assume the standard 2 gray [Gy] is the threshold dose for healthy adults.) The initial task then is to determine which of the one million seeking dosimetry could potentially benefit from treatment for ARS, so they can be triaged for further consideration.
Several factors complicate effective triage in the face of such a massive surge of victims. First, victims may experience concurrent injuries and burns; consequently, they may need non-ARS treatment, and sampling or measuring them with dosimetry may be impacted. Perhaps more importantly, concomitant injury interacts with radiation to exaggerate the impact (Ledney and Elliott, 2010; Blakely et al. 2010). Therefore, injured individuals (or others such as children or people with unrelated health problems) may require assessment at levels accurate below 2 Gy. Second, the blast and associated dangers are likely to produce mass fear and confusion, mass exodus with unknown destinations for people who are mobile, difficulty in transporting victims and products out of the area or devices and emergency personnel into the area, severely compromised local health care capabilities along with destruction of medical records and credentials of medical personnel, and severely damaged infrastructures such as buildings to use for triage and treatment, power and water, and communication networks (Gougelet et al. 2010; Bell and Dallas 2007).
2.2 Policy-Relevant and Practical Imperatives and Constraints on Biodosimetry
Initial triage imposes special constraints. Its purpose is to rapidly determine which people might need treatment or further assessment for ARS and, in order to reduce the surge on the healthcare system, which should not enter the healthcare system immediately. Thus, biodosimetry methods for initial triage need to be able to triage a million people within a few days, where samples or measurements are collected under ‘field’ conditions near the event. All field-related activities need to be operated independently of the healthcare system, without external sources of power and water, and preferably minimizing field-use of specialized facilities, expertise or devices. Field activities must also fit within the larger context of an integrated emergency response, i.e., responses are expected to be fully operational within 1 to 3 days of the event, and there are separate sites for evacuation, for first-aid/triage, and for use of healthcare facilities. Since the government plans to address the need of the population at large under emergency conditions, dosimetry should also be available to vulnerable populations such as pregnant women, children, and the elderly and disabled.
The technical requirements for initial triaging include that it be accurate enough to identify anyone with a dose above a threshold for consideration of urgent treatment for ARS (usually 2 Gy but thresholds might vary to fit special populations, injured individuals or available resources). To fit the needs for triage, samples for dosimetry need to be obtainable as soon as possible following the event and remain obtainable for a period of up to at least one week. Results should be available as quickly as possible to facilitate triaging for treatment and minimize difficulties in re-finding the person. Because the intent is to reliably keep people out of the local healthcare system who do not need care, methods should minimize misidentifying anyone as being below the threshold (‘false negative assignment’) so that very few people who could benefit are overlooked for receiving treatment. Unfortunately, this criterion concomitantly increases the likelihood of false positives, i.e., misidentifying people as being above the threshold. For this and other reasons, dosimetry for secondary triage, with more accurate dose estimation, is needed to permit better-informed triage or treatment decisions. Secondary triage, involving fewer people, can use more resource intensive methods or which require expertise and specialized facilities offsite to analyze (Grace et al. 2010; Fliedner et al. 2001). (These considerations are built in Figure B, discussed below in 3.2.)
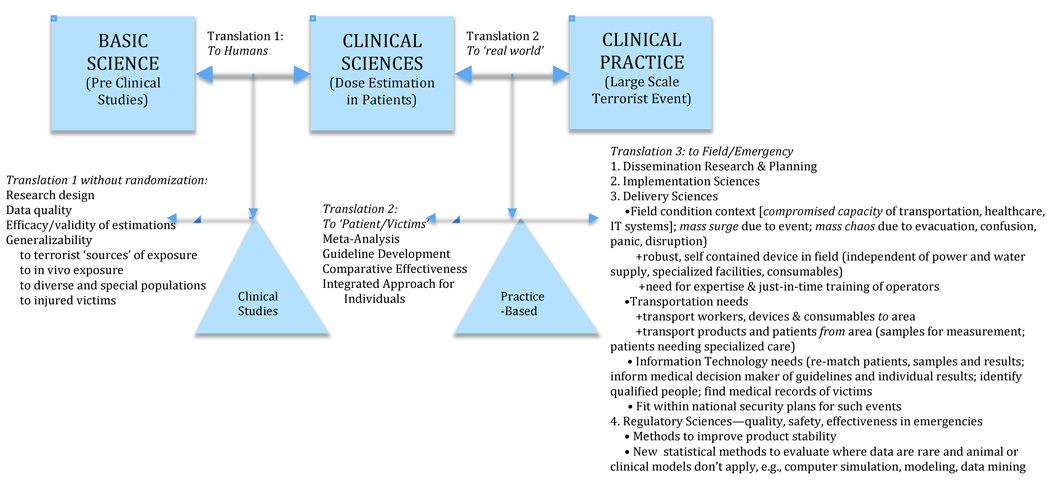
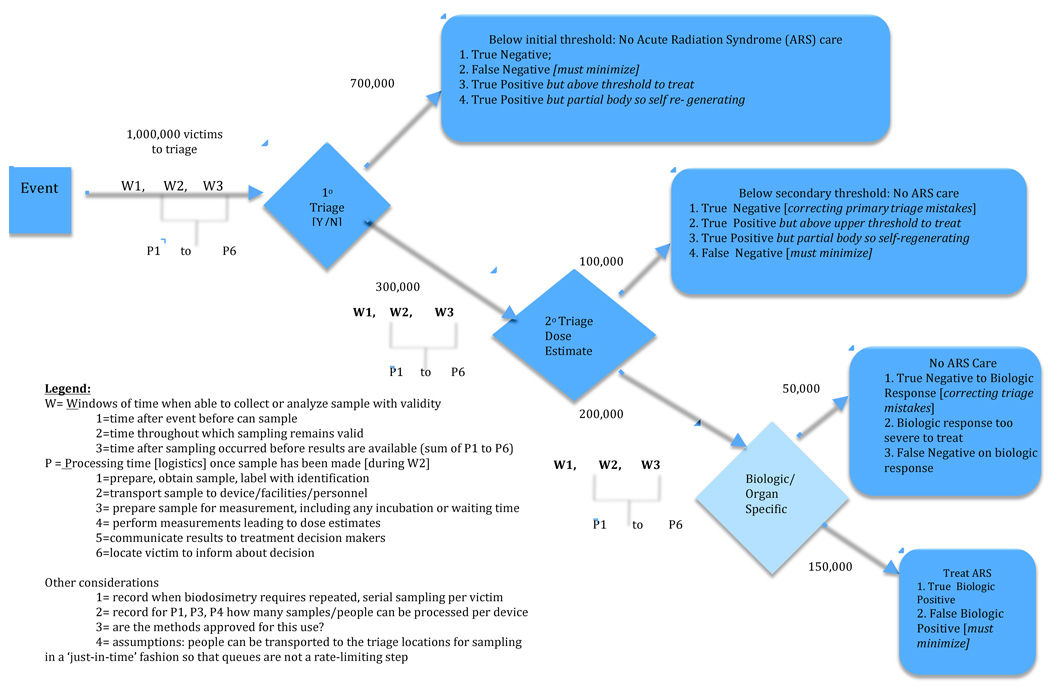
Figure.
Figure A: Adapting the Translational Roadmap of the National Institutes of Health
Figure B: A Comparative Framework of Biodosimetric Methods: Criteria to Assess Three Levels of Triaging Victims for Ultimate Treatment
Given the above considerations and the capability and feasibility of each method for serving as a basis for triage, researchers and policy-makers alike have called for an integrated approach where several methods are used at their most efficient and effective level (cf. Swartz et al. 2011; Grace et al. 2010).
3 Methods: Building a Comparative Conceptual Framework
Fortunately, there is no need to ‘reinvent the wheel’. Current evaluative models, although designed for usual clinical settings, can be adapted.
The US National Institutes of Health (NIH) recently called for a greater focus on the need for effective and scientifically-based translations of basic science into human applications (clinical science) and their culmination into evidence-based use in patients (practice settings) (Zerhouni 2003; Sung et al. 2003). The basic NIH model (represented by the boxes in Figure A) was seen as inadequate to the task of translating discoveries in basic science into everyday practice. To redress these problems (see triangles in Figure A), improved methods and attention were needed at two stages of translation: T1= the translation of findings from basic science to human use and T2= their subsequent translation into healthcare practice. Westfall et al. (2007) posited that this revised model still overlooked vital scientific areas, i.e., successful translation into practice also requires adding T3=the dissemination and implementation sciences.
3.1 A Modified Translational Science Model to Apply to Dosimetry (Figure A)
Further adaption of the NIH model is needed to develop, evaluate and implement dosimetry for triage in a large-scale radiological event. For example, T1 in the NIH model relies on Phase 1 and 2 Randomized Control Trials and other clinical studies to establish the efficacy for use in humans. In contrast, T1-level studies in dosimetry by necessity cannot simulate the conditions of a large-scale terrorist event. Their ‘use’ in humans rely instead on studying ex vivo irradiated samples or use cancer patients who received partial or whole body irradiation as part of their therapy. Such studies can establish proof-of-concept, but they can be poor models to evaluate efficacy of dosimetry techniques for their true intended use. For example, ex vivo irradiation may not have the same effects as in vivo irradiation. Cancer patients’ additional therapies (e.g., chemotherapy or anti-emetics) and underlying illnesses or injuries may interact with or obscure some biodosimetric markers for radiation.
Consequently, evidence gathered following small-scale nuclear accidents is often substituted (Goans et al. 2001). Such studies require a different set of statistical assumptions and design considerations, and have different limitations about how well they model large-scale radiation events or radiation associated with INDs.
While these differences in T1 are important to understand, they have been the subject of considerable focus, and we do not detail them in our analysis.
At T2, meta-analysis and structured literature reviews are conducted (cf. Cochrane Collaboration, 2010; Burgers et al. 2003; Fervers et al. 2006; Morris 2000), to build evidence-based clinical guidelines and comparative effectiveness models. This is a critical and currently missing element in evaluating dosimetry, and therefore this paper focuses especially on adapting T2 methods to compare methods for dosimetry in large-scale events.
T3 considerations become particularly important for emergency preparedness because treatment of patients in academic medical centers (the usual clinical science setting) is especially unsuited as a model for treating 100,000s of panicked people in field conditions. (Some critical T3 aspects are indicated in Figure B and Table 1 but not detailed in this paper.)
Table 1.
Applying Figure B’s Framework: Timing of 6 Biodosimetry Methods for Initial (Primary) Triaging of a Large Population
| Current Guidelines for Dosimetry | Dosimetry under Advanced Development |
|||||
|---|---|---|---|---|---|---|
| Types of Dosimetric Methods: |
Dicentric Chromosome Analysis |
Lymphocyte Depletion Rate (LDR) |
Time to Emesis |
Cytokinesis – Block Micronucleus |
γ- H2AX | EPR of Tooth Enamel |
| W= Windows of time when able to sample or measure with validity | ||||||
| W1=time after event before can sample | 0 | 12 (a) | <10 min to ≥2 hr (a) | 0 (b) | 0 (c,d) | 0 (e) |
| W2=time throughout which sampling remains valid to obtain | > 6 mo (f,g) | 48hr (a) | 12 to 48 hr (a) or longer if self-recalled | 12 mo (h) | few - 16 hr (c,d,f) | > Lifetime (e) |
| W3=time after sample is taken to triage decision [~sum of P times below] | several da, i.e., 5 – 9 da (a) | 1.5 to 2 da | < 5 min (i) | 5 da | 2 da | < 15 min (f) |
| P = Processing time once sample has been made* [occurs during W2 and W3] | ||||||
| P1=prepare, obtain sample, label with identification | 5 min | 10 min | 3 min | 3–5 min (j) | 3–5 min (j) | ~3 min (k) |
| P2=transport sample to device/facility/personnel | 2 – 96+ hr | 2 – 12 hr | 0 | 2 – 12 hr | 2 – 12 hr | 0 (l) |
| P3= prepare sample to measure, including any incubation or waiting time | 46–48 hr (c,f) | 10 min | 0 | included in P4 | included in P4 | <5 min (l) |
| P4= perform measurements leading to dose estimates | 1 hr (c,f) | 8 hr to get 3 serial results (a) | 0 | 68–76 hr (m) | 4 hr (n); 3 sec/well RABiT (a) | ~5 min (l) |
| P5=communicate results to treatment decision makers | 12 hr | 12 hr | 0 | 12 hr | 12 hr | 0 (l) |
| P6=locate victim to inform about decision | 24 hr | 24 hr | 0 | 24 hr | 24 hr | 0 (l) |
| Some additional considerations | ||||||
| FDA requires clearance | no | no | no | yes | yes | yes |
| Requires special expertise and lab facilities | labs very specialized | most clinical. labs okay | none | most clinical labs okay | none | none |
| Method validated for specific use of triage in large radiation event | no | no | no | no | no | no |
Not noted here is the time between an event and transporting the people, consumables and any devices to the field to obtain samples. We use the federal government’s assumption that all will need about the same time, i.e., 1 to 3 days, for field emergency medical sites to be operational.
Sources: (a) Berger et al. 2006; (b) Albert et al. 2009; (c) Golfier et al. 2009; (d) Turner et al. 2011; (e) Kleinerman et al. 2006; (f) Roch-Lefevre et al. 2010; (g) Roy et al. 2006; (h) Fenech 1993; (i) Demidenko et al. 2009; (k) Ivannikov et al. 2006; (j) Brenner 2010; (l) Williams et al. 2010; (m) Kirsch-Volders and Fenech 2001; (n) Avondoglio et al. 2009.
3.2 A Comparative Effectiveness Model for Biodosimetry (Figure B)
Figure B presents our framework for comparing the different biodosimetric methods for large-scale radiological events. Note: Figure B focuses only on the methods and concepts of T2. Figure B assumes T1 studies have established the efficacy of the dosimetry methods and assumes T3 studies have established appropriate application to ‘patients’ and their clinicians.
3.2.1 Staged Triage
In parallel with planned scenarios in the US (Grace et al. 2010) and UK (Fliedner et al., 2001), a staged plan for medical triage is modeled, with:
A primary level of triage (1° triage) occurring as soon as possible following the event where the full set of people present for a threshold determination of above and below 2 Gy,
A secondary level of triage (2° triage) is performed on the subset of people identified as potentially above the threshold where more refined estimates of dose are made; and
Organ-specific/biologic responses to radiation dose are monitored for the further reduced subset of people with evidence of ARS, to determine their treatment regimen.
3.2.2 Estimating Population Size
To illustrate the changes in magnitude of people at each stage of triage based on biodosimetry, we start with the US planning scenario estimates. The remaining numbers in Figure B are hypothetical, to illustrate the winnowing at each stage of triaging, the complexities of misidentification in the triage classifications, and the correction of dose estimates as people flow through the triage and medical response system.
3.3 Systematic analysis of Methods for Triage using Figure B’s Framework
Concurrently with describing our framework in greater detail in Figure B, we present an analysis of 6 types of biodosimetry in Table 1, based on this framework. The first three are the principal existing guidelines that are usually listed in official guidance and consensus documents (REMM, 2010; Rojas-Palma et al. 2009): dicentric chromosome analysis (DCA), lymphocyte depletion rate (LDR), and time to emesis. The last three are dosimetry methods in advanced stages of development; two are biologically-based biodosimetric methods using the Rapid Automated Biodosimetry Tool (RABiT) system to perform two types of cytokinesis: Block Micronucleus and γ-H2AX (Garty et al., 2010; Turner et al., 2011). The third is a physically based biodosimetric method: in vivo tooth dosimetry (Williams et al. 2011). The analysis in Table 1 compares the six methods with respect to the times needed to obtain results for use in triaging; sources where published are also noted there. Below we discuss this framework using two methods detailed in Table 1.
3.3.1 Two Dimensions for Comparing Methods in Figure B
We identify two dimensions to compare the effectiveness and feasibility of different modalities for dosimetry at different levels of triage and in the context of a large-scale event: the windows of time when sampling and measurements are valid and the logistical processes involved in order to provide the final results to a clinical decision-maker.
3.3.1.1 The Windows of time when sampling and measurements are valid
W1 refers to the time after the event before sampling is valid. For example, for LDR, W1=12 hours because it takes time for the lymphocytes to undergo apoptosis. For tooth dosimetry with EPR, W1=0 because the physical changes measured occur instantly.
W2 is the entire window during which a sample is valid to obtain. Continuing these examples, W2=48 hours for LDR because the subsequent changes after this period no longer predict dose. For tooth EPR, W2=the foreseeable lifetime of the person because the changes are stable in tooth enamel for thousands of years.
W3 is the total amount of time after a sample is taken until a triage decision can be made, i.e., the total logistical considerations detailed below.. W3=1.5 to 2 days for LDR, because it takes that long to process once sampled. In EPR tooth dosimetry, W3=15 minutes, i.e., the time from when a person sits in the device until a result is available to the medical triage physician and person. Note that W3 can overlap with W2 if the sample is taken early within the W2 window, or W3 can be much shorter than W2 if W2 extends weeks or years. (Although not noted in this framework, a sample taken during the appropriate W2 window could be processed so as to be measurable later.)
3.3.1.2 Process Logistics: from obtaining samples to using the results
The Process timeframes (P1 to P6) measure the average time for each logistical step to be completed, beginning after sampling has occurred and ending when the results are available for the clinician to triage the patient.
Typically, only sample preparation (P3) and measurement times (P4) are reported in the literature. Continuing our example, both LDR and tooth EPR do not require an incubation period or complex measurements and so both require only a few minutes for sample preparation and measurement.
However, a complete evaluation in the context of the methods’ intended use requires looking at the entire set of logistics involved, including transportation and communication. For biodosimeters analyzing blood—including LDR—a minimally trained person can take a sample in under 5 minutes. However, LDR requires serial (at least 3) collections of blood over a period of 12–24 hours. For tooth dosimetry, no ‘sample’ is needed; instead the measurements require proper placement of the device on the tooth; less than 5 minutes is needed now and automation is projected to reduce placement time to less than a minute.
If transportation of samples to remote, highly specialized facilities for processing (P2) and long-distance communication of individual results are needed (P5 and P6), a number of hours to days may be required. For example, LDR samples need to be transported to a clinical laboratory for analysis and results conveyed later to the clinician who may in turn need to relocate the victim. For tooth EPR, where measurements are performed in the field with immediate output of results, these processing times are near negligible.
3.3.2 Other Dimensions in T1 and T3 (not detailed in this paper)
Though not detailed in this paper and Table 1, other factors are key to comparing the different methods and to deciding which are most appropriate for primary or secondary triage or in assessing treatment response. For example, in T1 the relative sensitivity and specificity of the dose estimate, especially when assessing special populations, is important. In T3, the feasibility of handling the volume of cases and the suitability for its intended use need to be considered (and none of the current or developing modalities has been well-evaluated for this intended use). Subtleties abound too, such as whether or not the dose estimate is cumulative or dose-rate dependent, site specific (irrespective of whether the actual dose was whole or partial body), or applicable to special populations such as children without further adaptation (e.g., do they require different technical details or calibration curves?)
4. Conclusions, Discussion and Next Steps
Starting with the overall NIH Translational Science Roadmap, we have begun an analysis of triage under an assumption of a 10 KT IND. Our framework modified the existing evaluative scientific methods and adapted the criteria to fit the context of a being applied to a large-scale public disaster. We applied these criteria to 3 current guidelines for triaging victims and 3 advanced biodosimetric methods under development. This framework provides a useful way to compare and contrast the feasibility of these methods to meet the needs for triaging at the primary level (where potentially millions of people present for potential treatment for ARS) or at subsequent stages of triaging (where only people with substantial evidence of exposure are further evaluated).
Rather than comparing methods to choose the best option, our framework supports what others have called for: an integrated approach to triage large numbers of people for effective care for ARS. While some have focused on integrating triage by building an effective network of facilities to handle the surge needs for one method of biodosimetry (Christie, Chu and Carr, 2010), others have urged using the information from several methods for estimating an individual’s dose, e.g., by using a formula or multiparametric approaches (AFRRI 2011; Rjecke, Ruf and Meineke 2010). Here we espouse the approach others have advocated (Grace et al. 2010, Swartz et al. 2011) that seeks to maximize effective triaging by creating tiers of assessment. The first tier focuses on techniques that can quickly manage large numbers in the field with immediate feedback based on whether the exposure exceeded a threshold. Higher tiers have fewer people to evaluate and can therefore use more specialized facilities and expertise to estimate the dose with greater precision and perhaps with more sensitivity to the victim’s biologic responses.
Arguably all three definitions of an integrated approach are needed. Our framework and approach emphasizes the needs to make comparisons across methods, paying particular attention to the context of their intended use. More information needs to be gathered, based on rigorous definitions of the logistical assumptions, to make these comparisons. In addition to those criteria identified in this paper, comparisons need to include important criteria such as the basic sensitivity and specificity of the estimates made by each method under the conditions that mirror their intended use.
As complex as this model may appear, it needs to take into account additional considerations about how well each method can handle special populations such as people injured during the event, those with chronic conditions that may confound the estimates, children, and pregnant women. These and other subpopulations may not be able to be assessed at a given tier by one method, so that having multiple approaches as each tier may be needed.
A final note: integration of biodosimetric methods, by any definition, requires being able to trace the patient and his/her samples and results across multiple settings and under chaotic communication and transportation infrastructures. The effectiveness of integration will be greatly reduced in those countries (like the US) that do not use a universal identifier for healthcare services.
For all these reasons, we feel that it is vital to develop a thorough and well-validated framework for evaluating the strengths and weaknesses of biodosimetry as applied to a variety of likely scenarios using dosimetry for triage. Comparative evaluation is needed for all methods, including the current guidelines, since none has been fully evaluated for this intended use.
Acknowledgements
This work was supported by a grant from the National Institute of Allergy and Infectious Diseases: NIH-U19AI091173.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclaimer: The opinions or assertions contained herein are the private views of the authors and are not necessarily those of the Food and Drug Administration or the Oak Ridge Institute for Science and Education.
Contributor Information
Ann Barry Flood, Email: Ann.B.Flood@Dartmouth.Edu.
Roberto J. Nicolalde, Email: Roberto.J.Nicolalde@Dartmouth.Edu.
Eugene Demidenko, Email: Eugene.Demidenko@Dartmouth.Edu.
Benjamin B. Williams, Email: Benjamin.B.Williams@Dartmouth.Edu.
Alla Shapiro, Email: Alla.Shapiro@fda.hhs.gov.
Albert L. Wiley, Jr., Email: Albert.Wiley@orise.orau.gov.
Harold M. Swartz, Email: Harold.M.Swartz@Dartmouth.Edu.
References
- AFRRI (Armed Forces Radiobiology Research Institute. [Accessed February 15, 2011];BAT and FRAT: Biodose Tools. 2011 http://www.afrri.usuhs.mil/outreach/biodostools.htm.
- Albert GC, McNamee JP, Marro L, Bellier PV, Prato FS, Thomas AW. Assessment of genetic damage in peripheral blood of human volunteers exposed (whole-body) to a 200 muT, 60 hz magnetic field. Int J Radiat Biol. 2009;85(2):144–152. doi: 10.1080/09553000802641169. [DOI] [PubMed] [Google Scholar]
- Alexander GA, Swartz HM, Amundson SA, Blakely WF, Buddemeier B, Gallez B, Dainiak N, Goans RE, Hayes RB, Lowry PC. BiodosEPR-2006 meeting: Acute dosimetry consensus committee recommendations on biodosimetry applications in events involving uses of radiation by terrorists and radiation accidents. Radiat. Measur. 2007;42(6–7):972–996. [Google Scholar]
- Avondoglio D, Scott T, Kil WJ, Sproull M, Tofilon PJ, Camphausen K. High throughput evaluation of γ-H2AX. Radiat. Oncol. 2009;24:4–31. doi: 10.1186/1748-717X-4-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell W, Dallas C. Vulnerability of populations and the urban health care systems to nuclear weapon attack: Examples from four American cities. Int J Health Geogr. 2007;6(1):5, 1–33. doi: 10.1186/1476-072X-6-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger ME, Christensen DM, Lowry PC, Jones OW, Wiley AL. Medical management of radiation injuries: Current approaches. Occup Med. 2006;56(3):162–172. doi: 10.1093/occmed/kql011. [DOI] [PubMed] [Google Scholar]
- Brenner D. Ultra-high throughput radiation biodosimetry: The RABiT. International Conference on Radiation Biology: Nanotechnology, Imaging and Stem Cells in Radiation Oncology; Chennai, India. 2010. [Google Scholar]
- Blakely WF, Ossetrova NI, Whitnall MH, Sandgren DJ, Krivokrysenko VI, Shakhov A, Feinstein E. Multiple parameter radiation injury assessment using a nonhuman primate radiation model: Biodosimetry applications. Health Phys. 2010;98(2):153–159. doi: 10.1097/HP.0b013e3181b0306d. [DOI] [PubMed] [Google Scholar]
- Buddemeier BR, Dillon MD. Key response planning factors for the aftermath of nuclear terrorism. Livermore, CA: Lawrence Livermore National Laboratory (LLNL); 2009. LLNL-TR-410067. [Google Scholar]
- Burgers JS, Grol R, Klazinga NS, Makela M, Zaat J. Towards evidence-based clinical practice: An international survey of 18 clinical guideline programs. Int. J. Qual. Health Care. 2003;15(1):31–45. doi: 10.1093/intqhc/15.1.31. [DOI] [PubMed] [Google Scholar]
- Christie DH, Chu MC, Carr Z. Global networking for biodosimetry laboratory capacity surge in radiation emergencies. Health Phys. 2010;98(2):168–171. doi: 10.1097/HP.0b013e3181abaad4. [DOI] [PubMed] [Google Scholar]
- Cochrane Collaboration. [accessed 11/18 2010];Cochrane effective practice and organisation of care group. http://www.epoc.cochrane.org/en/index.html.
- Demidenko E, Williams BB, Swartz HM. Radiation dose prediction using data on time to emesis in the case of nuclear terrorism. Radiat Res. 2009;171(3):817–826. doi: 10.1667/RR1552.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenech M. The cytokinesis-block micronucleus technique and its application to genotoxicity studies in human populations. Environ Health Perspect. 1993;101 Suppl 3:101–107. doi: 10.1289/ehp.93101s3101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fervers B, Burgers JS, Haugh MC, Latreille J, Mlika-Cabanne N, Paquet L, Coulombe M, Poirier M, Burnand B. Adaptation of clinical guidelines: Literature review and proposition for a framework and procedure. Int. J. Qual. Health Care. 2006;18(3):167–176. doi: 10.1093/intqhc/mzi108. [DOI] [PubMed] [Google Scholar]
- Fliedner TM, Friesecke I, Beyrer K, editors. Medical management of radiation accidents: Manual on the acute radiation syndrome. London, UK: British Institute of Radiology; 2001. [DOI] [PubMed] [Google Scholar]
- Garty G, Chen Y, Salerno A, Turner H, Zhang J, Lyulko O, Bertucci A, Xu Y, Wang H, Simaan N. The RABIT: A rapid automated biodosimetry tool for radiological triage. Health Phys. 2010;98(2):209–217. doi: 10.1097/HP.0b013e3181ab3cb6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goans RE, Holloway EC, Berger ME, Ricks RC. Early dose assessment in criticality accidents. Health Phys. 2001;81(4):446–449. doi: 10.1097/00004032-200110000-00009. [DOI] [PubMed] [Google Scholar]
- Golfier S, Jost G, Pietsch H, Lengsfeld P, Eckardt-Schupp F, Schmid E, Voth M. Dicentric chromosomes and γ-H2AX foci formation in lymphocytes of human blood samples exposed to a CT scanner: A direct comparison of dose response relationships. Radiat. Prot. Dosim. 2009;134(1):55–61. doi: 10.1093/rpd/ncp061. [DOI] [PubMed] [Google Scholar]
- Gougelet RM, Rea ME, Nicolalde RJ, Geiling JA, Swartz HM. The view from the trenches: Part 1 Emergency medical response plans and the need for EPR screening. Health Phys. 2010;98(2):118–127. doi: 10.1097/HP.0b013e3181a6de7d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace MB, Moyer BR, Prasher J, Cliffer KD, Ramakrishnan N, Kaminski J, Coleman CN, Manning RG, Maidment BW, Hatchett R. Rapid radiation dose assessment for radiological public health emergencies: Roles of NIAID and BARDA. Health Phys. 2010;98(2):172–178. doi: 10.1097/01.HP.0000348001.60905.c0. [DOI] [PubMed] [Google Scholar]
- Ivannikov A, Zhumadilov K, Tieliewuhan E, Jiao L, Zharlyganova D, Apsalikov KN, Berekenova G, Zhumadilov Z, Toyoda S, Miyazawa C, Skvortsov V, Stepanenko V, Endo S, Tanaka K, Hoshi M. Results of EPR dosimetry for population in the vicinity of the most contaminating radioactive fallout trace after the first nuclear test in the Semipalatinsk test site. J of Radiat. Res. 2006;47 Suppl A:A39–A46. doi: 10.1269/jrr.47.a39. [DOI] [PubMed] [Google Scholar]
- Kirsch-Volders M, Fenech M. Inclusion of micronuclei in non-divided mononuclear lymphocytes and necrosis/apoptosis may provide a more comprehensive cytokinesis block micronucleus assay for biomonitoring purposes. Mutagenesis. 2001;16(1):51–58. doi: 10.1093/mutage/16.1.51. [DOI] [PubMed] [Google Scholar]
- Kleinerman RA, Romanyukha AA, Schauer DA, Tucker JD. Retrospective assessment of radiation exposure using biological dosimetry: Chromosome painting, electron paramagnetic resonance and the glycophorin a mutation assay. Radiat. Res. 2006;166(1,Pt 2):287–302. doi: 10.1667/RR3273.1. [DOI] [PubMed] [Google Scholar]
- Ledney GD, Elliott TB. Combined injury: Factors with potential to impact radiation dose assessments. Health Phys. 2010;98(2):145–152. doi: 10.1097/01.HP.0000348466.09978.77. [DOI] [PubMed] [Google Scholar]
- Morris AH. Developing and implementing computerized protocols for standardization of clinical decisions. Ann. Intern. Med. 2000;132(5):373–383. doi: 10.7326/0003-4819-132-5-200003070-00007. [DOI] [PubMed] [Google Scholar]
- National Security Staff. Interagency Policy Coordination Subcommittee for Preparedness and Response to Radiological and Nuclear Events. [accessed Nov. 19, 2010];US Executive Office of the President. 2010. Planning Guidance for Reaction to a Nuclear Detonation. (2nd edition). 2010 hps.org/.../Planning_Guidance_for_Response_to_a_Nuclear_Detonation-2nd_Edition_FINAL.pdf.
- Riecke A, Ruf CG, Meineke V. Assessment of radiation damage: The need for a multiparametric and integrative approach with the help of both clinical and biological dosimetry. Health Phys. 2010;98(2):160–167. doi: 10.1097/HP.0b013e3181b97306. [DOI] [PubMed] [Google Scholar]
- Roch-Lefevre S, Mandina T, Voisin P, Gaetan G, Mesa JE, Valente M, Bonnesoeur P, García O, Voisin P, Roy L. Quantification of γ-H2AX foci in human lymphocytes: A method for biological dosimetry after ionizing radiation exposure. Radiat. Res. 2010;174(2):185–194. doi: 10.1667/RR1775.1. [DOI] [PubMed] [Google Scholar]
- Rojas-Palma C, Liland A, Jerstad AN, Etherington G, Pérez MR, Rahola T, Smith K, editors. TMT Handbook: Triage, monitoring and treatment of people exposed to ionising radiation following a malevolent act. Osteras, Norway: Norweigan Radiation Protection Authority; 2009. [accessed November 19, 2010]. ISBN (PDF version): 978-82-90362-28-2 tmthandbook.org. [Google Scholar]
- Roy L, Gregoire E, Durand V, Buard V, Delbos M, Paillole N, Sorokine-Durm I, Gourmelon P, Voisin P. Study of the tools available in biological dosimetry to estimate the dose in cases of accidental complex overexposure to ionizing radiation: The Lilo accident. Int. J. Radiat. Biol. 2006;82(1):39–48. doi: 10.1080/09553000600579207. [DOI] [PubMed] [Google Scholar]
- Simon SL, Skinner AR, Swartz HM. Guest editorial: Editors’ remarks. Health Phys. 2010;98(2):93–94. [Google Scholar]
- Sung NS, Crowley WF, Genel M, Salber P, Sandy L, Sherwood LM, Johnson SB, Catanese V, Tilson H, Getz K. Central challenges facing the national clinical research enterprise. JAMA. 2003;289(10):1278–1287. doi: 10.1001/jama.289.10.1278. [DOI] [PubMed] [Google Scholar]
- Swartz HM, Flood AB, Gougelet RM, Rea ME, Nicolalde RJ, Williams BB. A critical assessment of biodosimetry methods for large-scale incidents. Health Phys. 2010;98(2):95–108. doi: 10.1097/HP.0b013e3181b8cffd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swartz HM, Williams BB, Nicolalde RJ, Demidenko E, Flood AB. Overview of biodosimetry for management of unplanned exposures to ionizing radiation. Radiat. Measur. 2011;175 [Google Scholar]
- Turner HC, Brenner DJ, Chen Y, Bertucci A, Zhang J, Wang H, Lyulko OV, Xu Y, Shuryak I, Schaefer J, Simaan N, Randers-Pehrson G, Yao YL, Amundson SA, Garty G. Adapting the γ-H2AX assay for automated processing in human lymphocytes. 1. Technological aspects. Rad. Res. 2011 doi: 10.1667/RR2125.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REMM (Radiation Emergency Medical Management) [accessed Nov. 17, 2010];Dose estimator for exposure: 3 biodosimetry tools. 2010 http://www.remm.nlm.gov/ars_wbd.htm.
- Waselenko JK, MacVittie TJ, Blakely WF, Pesik N, Wiley AL, Dickerson WE, Tsu H, Confer DL, Coleman CN, Seed T. Medical management of the acute radiation syndrome: Recommendations of the strategic national stockpile radiation working group. Ann. Intern. Med. 2004;140(12):1037–1051. doi: 10.7326/0003-4819-140-12-200406150-00015. [DOI] [PubMed] [Google Scholar]
- Westfall JM, Mold J, Fagnan L. Practice-based research: "Blue Highways" on the NIH Roadmap. JAMA. 2007;297(4):403–406. doi: 10.1001/jama.297.4.403. [DOI] [PubMed] [Google Scholar]
- Williams BB, Dong R, Kmiec M, Burke G, Demidenko E, Gladstone D, Nicolalde RJ, Sucheta A, Lesniewski P, Swartz HM. Development of vivo tooth EPR for individual radiation dose estimation and screening. Health Phys. 2010;98(2):327–338. doi: 10.1097/HP.0b013e3181a6de5d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams BB, Dong R, Flood AB, Grinberg O, Kmiec M, Lesniewski PN, Matthews TP, Nicolalde RJ, Raynolds T, Salikhov IKZ, Swartz HM. In Vivo EPR tooth dosimetry for triage after a radiation event involving large populations. Radiat Measur. 2011;175 doi: 10.1016/j.radmeas.2011.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerhouni E. The NIH Roadmap. Science. 2003;302(5642):63–72. doi: 10.1126/science.1091867. [DOI] [PubMed] [Google Scholar]