Abstract
Objective
The aim of this study was to compare the distribution and immunoreactivity of cyclooxygenase (COX)-1 and COX-2 in normal uterus and breast after long-term hormone therapy in post menopausal monkeys.
Method
Female adult cynomolgus macaques were bilaterally ovariectomized 3 months before initiation of hormone treatment. The animals were treated (experiment 1) with conjugated equine estrogens (CEE), medroxyprogesterone acetate (MPA), CEE+MPA, tamoxifen (TAM) or vehicle (controls=C). In experiment 2, animals were treated with CEE, CEE+MPA, tibolone (TIB), or vehicle (C). Breast tissue and uteri were collected, fixed and paraffin embedded. Immunohistochemistry assays for COX-1 and COX-2 were performed.
Results
COX-1 immunostaining was decreased by TAM and CEE treatment in endometrial stroma and by CEE+MPA in the myometrium. COX-1 immunostaining of breast epithelia was down regulated by CEE+MPA, while other cell types in the breast seem less affected by hormone treatment.
COX-2 immunoreactivity in endometrial stroma was increased by CEE+MPA. In glandular epithelium CEE+MPA and TIB increased COX-2 immunostaining compared to C and CEE groups. No effect from hormone treatment on COX-2 immunostaining was found in the myometrium. COX-2 immunostaining in glandular epithelium of the breast was, in experiment 2, increased after CEE treatment as compared to controls. No other effects by hormone therapy on COX-2 expression were found in the breast.
Conclusions
Our results show that COX-1 and COX-2 are differently distributed and regulated by hormones in normal uterus and breast from ovariectomized macaques. COX-1 is prevailing in the uterus, while COX-2 is dominant in the mammary gland.
Keywords: COX-1, COX-2, breast, uterus, macaque
Introduction
Prostaglandins are a group of autocrine and paracrine hormones that play an important role in many physiological processes. The cyclooxygenase (COX) enzymes are key factors in the production of eicosanoids from arachidonic acid. The formation of the prostaglandins depends on an intermediate molecule, prostaglandin H2 (PGH2). The prostaglandin synthases, COX-1 and COX-2, catalyze the formation of PGH2 from arachidonic acid and are therefore important steps in prostaglandin formation. Cyclooxygenases and prostaglandins have been linked to inflammation, cell proliferation, enhanced angiogenesis and promoting invasion and metastasis of cancers (1, 2).
COX-1 is generally considered to be constitutively expressed in most tissues, whereas COX-2 is inducible (2). Recent evidence shows that COX-1 is involved in not only physiological but also pathological processes, including tumorigenesis in tissues like breast, cervix and endometrium (3-5). COX-2 is induced by mitogens, cytokines, growth factors, endotoxins, oncogenes and carcinogens (1). Its involvement in rheumatic disease, inflammation and tumorigenesis has been demonstrated (1, 4). COX-2 is indicated as a tumor promoter and a regulator of cell proliferation (6). The biosynthesis of COX-1 and/or COX-2 is also up-regulated in breast and endometrial cancers (1, 4).
Hormone therapy (HT) with estrogen and estrogen + progestogen has been associated with an increased risk of cancer in hormone sensitive tissues like the uterus and breast (7).
Breast cancer is the most common malignancy in women (8). The estrogen receptor α (ERα) is expressed in the majority of breast cancers. Estrogen is known to be a mitogen in human breast epithelium and that effect is mediated through ERα (9).
Tamoxifen (TAM) has anti-estrogenic properties in breast tissue and is commonly used as adjuvant treatment for patients with ER-positive breast cancers and as a prevention strategy for women at high risk of developing this disease (10). TAM is pharmacologically complex since it manifests agonistic or antagonistic activity in a tissue specific manner. TAM acts as an estrogen antagonist with anti proliferative effects in breast, decreasing the risk of both invasive and noninvasive breast cancer, while having agonistic effects in the endometrium (10, 11).
Tibolone (TIB) is a tissue-selective synthetic steroid, representing an alternative to conventional HT for women in Europe and Asia (12). We and others have shown that the effects of TIB on breast tissue are less pronounced than those seen with estrogen-based HT (13-17). In addition, TIB does not induce hyperplasia or carcinoma in the endometrium (18, 19).
During the last years studies on vascular endothelium have indicated regulation of COXs by estrogen and/or progestogen (20). Since uterus and breast are hormone sensitive organs, and hormone therapy is widely used in clinical practice, it is of utmost interest to study hormone regulation of COX expression in these tissues.
Knowledge of normal physiology of the human breast and uterus is limited due to the difficulty in accessing normal tissues. The cynomolgus macaque (Macaca fascicularis) is a non-human primate which has well-documented similarities to humans in terms of reproductive physiology and anatomy, mammary gland development, peripheral steroid hormone metabolism and sex steroid receptor expression (21). In general, experimental findings in macaques have been predictive of outcomes in human reproductive studies. The aim of the present investigation was to compare the expression of COX-1 and COX-2 in the normal uterus and breast after long-term hormonal therapy.
Material and methods
Animals
Female adult cynomolgus macaques (Macaca fascicularis) were imported from Indonesia to the United States. They were housed in social groups of four to six monkeys each. All experimental procedures involving animals were approved by the Institutional Animal Care and Use Committee and compliant with relevant federal regulations. Wake Forest University School of Medicine is fully accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care. Bilateral ovariectomy was performed 3 months before initiation of hormone treatment. The compounds were administered in the diet at doses equivalent, on a caloric basis, to those given to women (22). The daily doses were thus designed to correspond to 0.625mg CEE (conjugated equine estrogens), 2.5 mg MPA (medroxyprogesteroneacetate), 20 mg TAM (tamoxifen) and 3 mg TIB (tibolone).
Experiment 1
More detailed data on animal treatment can be found in Cline et al. (23). All the macaques were 4–6 years old when the study started. The ovariectomized animals were treated orally for 35 months by CEE, MPA, CEE+MPA or TAM. Animals without hormone treatment served as controls (C).
We obtained uterine tissues from 25 animals (C=5, CEE=4, MPA=5, CEE+MPA=5, TAM=6) and breast tissue from 75 animals (n=15 in all groups).
Experiment 2
Additional data on the animals studied are presented elsewhere (15, 24). The monkeys were 6–8-year-old at entry into the study. The ovariectomized animals were treated orally for 2 years with CEE, CEE + MPA or TIB. Animals without hormone treatment served as controls (C). We obtained uterine tissues from 88 monkeys (C=20, CEE=20, CEE+MPA=20 and TIB=28) and breast tissue from 60 animals (n=15 in all groups).
Tissue collection
All monkeys were euthanized after the treatment period, and reproductive tissues were evaluated as part of a multi-system approach. Uterus and breast tissue were collected and fixed in 4% paraformaldehyde for 24 h, transferred to 70% ethanol and stored in 4°C. The tissues were trimmed to 3mm in thickness before embedded in paraffin, and then sectioned at 5μm for immunostaining.
Immunohistochemistry
All sections were deparaffinized with Bio-Clear, rehydrated in graded ethanol, and subjected to microwave treatment for antigen retrieval in 0.01M citrate buffer (pH 6.0). Non-specific endogenous peroxidase activity was blocked by treatment with 3% hydrogen peroxide. Before incubation with the primary antibody all tissue sections were exposed to 10% normal horse serum for 60 min at room temperature. Two polyclonal goat antihuman antibodies (Santa Cruz Biochnology), diluted 1:100, were used for detection of COX-1 (Sc-1752) and COX-2 (Sc-1745), respectively. The primary antibody was incubated 1 hour at 37°C. The secondary antibody, biotinylated horse anti-goat (BA-9500, Vector Laboratory, Burlingame, CA, USA) was incubated for 30 min at room temperature. Immunostaining was performed with the Vectastain Elite ABCkit (Vector Laboratories Inc., USA) and diaminobenzidine (DAB, DAKOcytomation, USA) as chromogen. Negative controls were obtained by omitting the primary antibody and replacing it with the corresponding concentration of goat IgG.
Manual scoring
The intensity and tissue distribution of COX-1 and COX-2 immunostained cells were manually and independently evaluated by two observers blinded to treatment, using a semi-quantitative manual scoring on a four point scale: (−) = negative, (+) = faint, (++) = moderate and (+++) = strong immunostaining.
Statistics
Statistical calculations were performed by ANOVA on ranks (Kruskal–Wallis test) and significances were evaluated by Dunn's test. The significance level was set at p < 0.05.
Results
Immunohistochemistry showed that COX-1 and COX-2 proteins were present in the cytoplasm of cells in both uterus and breast, in treated as well as control animals. Representative images from IHC are shown in Figure 1 (experiment 1) and Figure 2 (experiment 2), and the scoring results are presented in Tables 1 (uterus) and 2 (breast). The results from IHC that reached significant differences, as assessed by manual scoring, are presented in Figures 3-5.
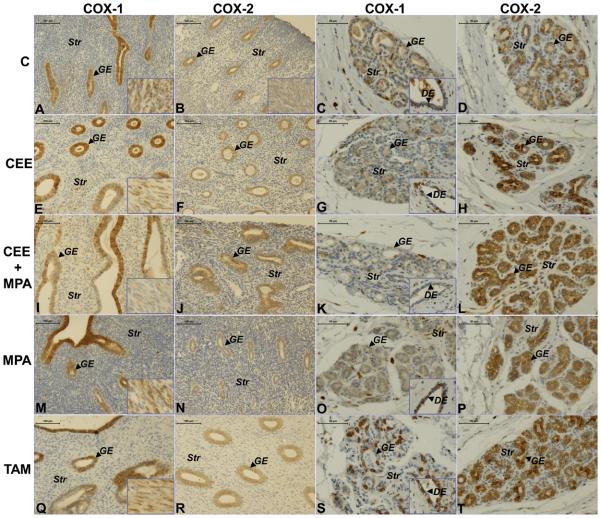
Figure 1.
Representative images of COX-1 and COX-2 immunostaining in uterus and breast from ovariectomized animals without treatment (C) and those treated with CEE, CEE+MPA, MPA or TAM. The first column shows COX-1 immunostaining in the endometrium represented by a sample from each group ((A) C; (E) CEE; (I) CEE+MPA; (M) MPA; (Q) TAM) at magnification x200 (bar=100μm). The second column shows COX-2 immunostaining in the different groups: ((B) C; (F) CEE; (J) CEE+MPA; (N) MPA; (R) TAM) at magnification x200 (bar=100μm). Images of myometrium are shown in the bottom right corner of the pictures. The third column shows COX-1 staining in the breast sample from each group ((C) C; (G) CEE; (K) CEE+MPA; (O) MPA; (S) TAM) at magnification x400 (bar=50μm). Images of ductal epithelium (DE) are shown in the bottom right corner of the COX-1 pictures. The fourth column shows COX-2 staining in a breast sample from each group ((D) C; (H) CEE; (L) CEE+MPA; (P) MPA; (T) TAM) at magnification x400 (bar=50μm). GE=glandular epithelium; Str= stroma.
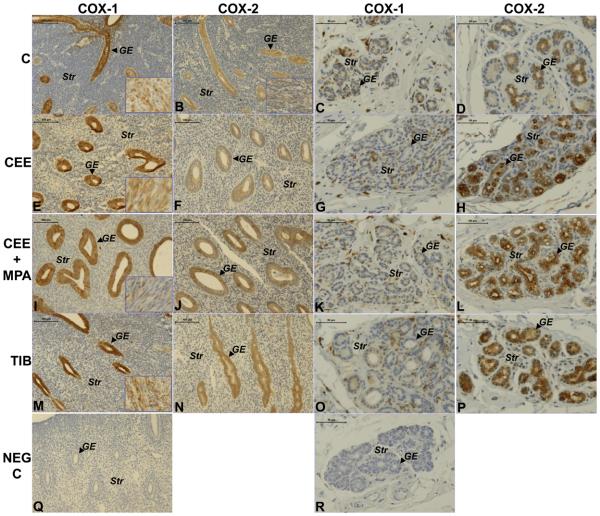
Figure 2.
Representative images of COX-1 and COX-2 immunostaining in uterus and breast from ovariectomized animals without treatment (C) and those treated with CEE, CEE+MPA or TIB. The first column shows COX-1 immunostaining in endometrium from each group ((A) C; (E) CEE; (I) CEE+MPA; (M) TIB) at magnification x200 (bar=100μm). The second column shows COX-2 immunostaining in the endometrium from each group ((B) C; (F) CEE; (J) CEE+MPA; (N) TIB) at magnification x200 (bar=100μm). Images of the myometrium are shown in the right bottom corner. The third column shows COX-1 immunostaining in a breast sample from each group ((C) C; (G) CEE; (K) CEE+MPA; (O) TIB) at magnification x400 (bar=50μm). The fourth column shows COX-2 staining in a breast sample from each group ((D) C; (H) CEE; (L) CEE+MPA; (P) TIB at magnification x400 (bar=50μm). Negative controls, where the primary antibody is replaced by goat IgG, are shown in uterus (Q) and breast (R). GE=glandular epithelium; Str= stroma.
Table 1. Uterus experiment 1 and 2.
Results from manual scoring [median (IQR)] of immunostaining in stroma, glandular epithelium (GE) and myometrium (Myo).
| Stroma 1 | OvxC | CEE | CEE+MPA | MPA | TAM | Stroma 2 | OvxC | CEE | CEE+MPA | TIB |
|---|---|---|---|---|---|---|---|---|---|---|
|
COX-1 p=0.027 |
1 (1-1.5) | 1 (0.5-1) | 1 (0.75-2) | 2 (1.75-3)b ▲ |
0 (0-0) |
COX-1 P=0.011 |
2 (2-3) | 1 (1-1.75)a ▼ |
2 (1-2) | 2 (1-2) |
|
COX-2 p=0.007 |
0 (0-1) | 2 (1-2) | 2 (1.75-2.25)b ▲ |
1 (0.75-2.25) | 0 (0-0) |
COX-2 P=0.003 |
1 (1-1) | 0.5 (0-2)c ▼ |
2 (1-2)a ▲ |
1 (1-2) |
| GE 1 | OvxC | CEE | CEE+MPA | MPA | TAM | GE 2 | OvxC | CEE | CEE+MPA | TIB |
|
COX-1 p=0.071 |
2 (1.75-2) | 2.5 (2-3) | 1 (0.75-1.25) | 1 (0.75-1.25) | 2 (1-3) |
COX-1 P<0.001 |
1 (1-2) | 2 (2-3)e ▲ |
1 (0-1) | 1 (1-1) |
|
COX-2 p=0.569 |
2 (1.75-2.25) | 2.5 (2-3) | 3 (1.75-3) | 2 (1.75-2.25) | 2.5 (2-3) |
COX-2 P<0.001 |
2 (2-2) | 2 (2-2) | 3 (2.25-3)a,d ▲ |
3 (2-3)a,d ▲ |
| Myo 1 | OvxC | CEE | CEE+MPA | MPA | TAM | Myo 2 | OvxC | CEE | CEE+MPA | TIB |
|
COX-1 p=0.009 |
3 (2.75-3) | 2.5 (1.5-3) | 1 (0-1.25)a ▼ |
3 (2.75-3)c ▲ |
2 (1-2) |
COX-1 P<0.001 |
1.5 (1-2) | 2 (1.25-2) | 1 (1-1)e ▼ |
2 (1-2.5) |
|
COX-2 p=0.866 |
2 (0-2) | 1.5 (1-2) | 2 (1.75-2) | 2 (1-2.25) | 1.5 (1-2) |
COX-2 P=0.137 |
3 (2-3) | 1.5 (1-3) | 3 (2-3) | 3 (2-3) |
= statistically significant from OvxC
= statistically significant from TAM/TIB
= statistically significant from CEE+MPA
= statistically significant from CEE
= statistically significant from all other treatments
Table 2. Breast experiment 1 and 2.
Results from manual scoring [median (IQR)] of immunostaining in stroma, glandular epithelium (GE) and ductal epithelium (DE).
| Stroma 1 | OvxC | CEE | CEE+MPA | MPA | TAM | Stroma 2 | OvxC | CEE | CEE+MPA | TIB |
|---|---|---|---|---|---|---|---|---|---|---|
|
COX-1 p=0.361 |
2 (2-2.75) | 2 (2-3) | 1 (1-2.75) | 2 (1-3) | 2 (2-3) |
COX-1 p=0.845 |
2 (1.25-2) | 2 (1-2) | 2 (1-2.75) | 2 (2-2) |
|
COX-2 p=0.200 |
1 (1-1) | 1 (1-2) | 1 (1-1) | 1 (0.25-1) | 1 (0-1) |
COX-2 p=0.519 |
0 (0-0) | 0 (0-0) | 0 (0-0) | 0 (0-0) |
| GE 1 | OvxC | CEE | CEE+MPA | MPA | TAM | GE 2 | OvxC | CEE | CEE+MPA | TIB |
|
COX-1 p=0.012 |
1 (1-2) | 1 (1-2) | 1 (0-1) | 2 (1-3)c ▲ |
2 (1-2.75)c ▲ |
COX-1 p=0.075 |
1 (1-2) | 1 (1-3) | 1 (1-1) | 1 (1-2) |
|
COX-2 p=0.197 |
3 (3-3) | 3 (2-3) | 3 (2-3) | 3 (3-3) | 3 (2-3) |
COX-2 p=0.016 |
1 (1-1.75) | 2 (2-2.75)a ▲ |
2 (1-2) | 2 (1-3) |
| DE 1 | OvxC | CEE | CEE+MPA | MPA | TAM | DE 1 | OvxC | CEE | CEE+MPA | TIB |
|
COX-1 p=0.002 |
2 (1-3) | 2 (1-3) | 1 (0-1)a ▼ |
2 (2-3)c ▲ |
2 (1-2.75) |
COX-1 p=0.067 |
2 (1-2) | 2 (1-3) | 1 (1-1.75) | 1 (1-2) |
|
COX-2 p=0.265 |
3 (3-3) | 3 (3-3) | 3 (2-3) | 3 (3-3) | 3 (2-3) |
COX-2 p=0.109 |
1.5 (1-2) | 2 (1.75-2.25) | 2 (1.5-2) | 2 (2-3) |
= statistically significant from OvxC
= statistically significant from CEE+MPA.
Figure 3.
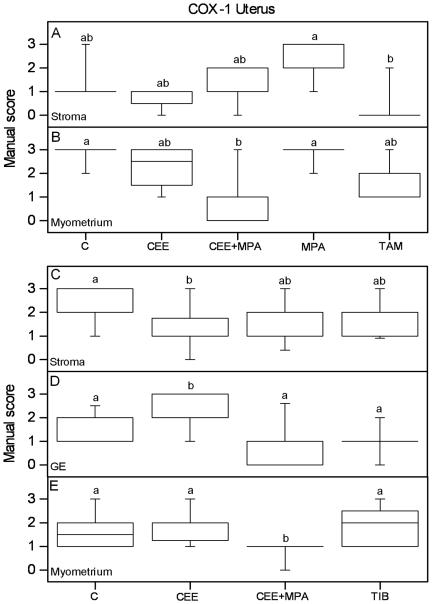
Graphs of the results from manual scoring of COX-1 immunostaining in the uterus are presented. Scores of positive COX-1 immunoreactivity in stroma (A) and myometrium (B) from experiment 1, and stroma (C), glandular epithelium (GE) (D) and myometrium (E) from experiment 2, are shown. The box and whisker plots represent the median value with 50% of all data falling within the box. The whiskers extend to the 5th and 95th percentiles. Values with different letter designations are significantly different (P<0.05). Experiment 1: n=5 in all groups but CEE=4 and TAM=6. Experiment 2: n=20 in all groups but TIB=28.
Figure 5.
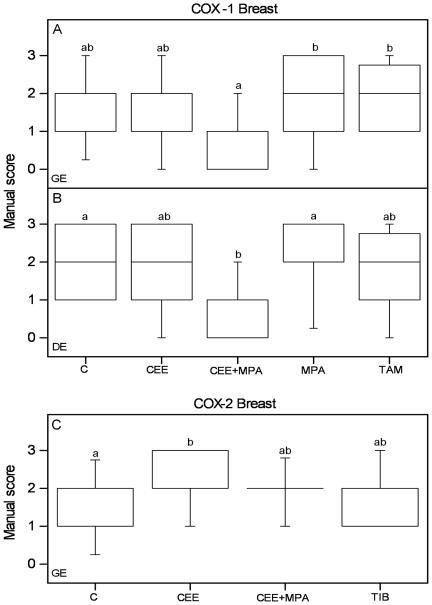
Graphs of the results from manual scoring of COX-1 and COX-2 immunostaining in the breast tissue. Scores of positive COX-1 immunoreactivity in glandular (GE) (A) and ductal (DE) (B) epithelium from experiment 1 and from COX-2 immunoreactivity in GE (C) from experiment 2 are shown. The box and whisker plots represent the median value with 50% of all data falling within the box. The whiskers extend to the 5th and 95th percentiles. Values with different letter designations are significantly different (P<0.05). Experiment 1 and 2: n=15 in all groups.
Uterus
In experiment 1 MPA treatment increased stromal COX-1 immunoreactivity as compared to TAM treatment (Figure 3A; Table 1) whereas in experiment 2 CEE treatment decreased stromal COX-1 immunoreactivity as compared to controls (Figure 3C; Table 1). In glandular epithelium (GE), experiment 2, CEE treatment increased COX-1 as compared to the other groups (Figure 3D; Table 1). Combined CEE+MPA treatment decreased COX-1 immunoreactivity compared with controls in the myometrium of both experiments (Figure 3B, E; Table 1). TAM treatment did not differ in COX-1 immunostaining as compared to controls (Figure 3A, B; Table 1). In GE of experiment 1 there was a tendency that the groups treated with estrogenic compounds only (CEE and TAM) showed increased immunostaining of COX-1 compared to the MPA and CEE+MPA groups (p=0.071; Table 1), which is in agreement with the increase found after CEE treatment in GE of experiment 2, as mentioned above. In experiment 2, TIB treatment did not affect the COX-1 immunoreactivity in any tissue type as compared to controls, but the immunostaining was lower compared to CEE treatment in GE and higher compared to CEE+MPA in myometrium (Figure 3C, D, E; Table 1).
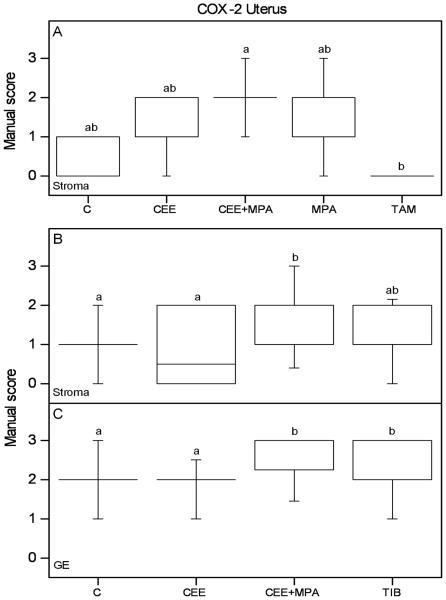
CEE or TAM treated animals did not differ in COX-2 immunostaining as compared to controls, nor did the MPA only treatment (Figure 4A; Table 1). TAM showed decreased COX-2 immunostaining as compared to CEE+MPA in the stroma (Figure 4A; Table 1). Combined treatment with CEE+MPA increased stromal COX-2 immunoreactivity compared with controls in experiment 2, with a tendency to a similar result in experiment 1 (Figure 4A, B; Table 1). In experiment 2, CEE+MPA and TIB treatments increased COX-2 immunoreactivity in GE as compared to CEE and C groups (Figure 4C; Table 1).
Figure 4.
Graphs of the results from manual scoring of COX-2 immunostaining in the uterus are presented. Scores of positive COX-2 immunoreactivity in stroma (A) from experiment 1, and in stroma (B) and glandular epithelium (GE) (C) from experiment 2, are shown. The box and whisker plots represent the median value with 50% of all data falling within the box. The whiskers extend to the 5th and 95th percentiles. Values with different letter designations are significantly different (P<0.05). Experiment 1: n=5 in all groups but CEE=4 and TAM=6. Experiment 2: n=20 in all groups but TIB=28.
Breast
In CEE, MPA, TAM and TIB groups there was no difference in COX-1 immunostaining as compared to controls (Figure 5A, B; Table 2). In experiment 1 COX-1 immunoreactivity was decreased in GE of the CEE+MPA group as compared to the TAM and MPA groups (Figure 5A; Table 2). Also in experiment 2, the CEE+MPA group showed the lowest COX-1 immunoreactivity in GE, but the difference towards the other treatment groups did not reach significance (p=0.075; Table 2).
In the ductal epithelium (DE), in experiment 1, COX-1 immunostaining was decreased in the CEE+MPA treated group as compared to the MPA and control groups (Figure 5B; Table 2). Also in experiment 2 the DE of the CEE+MPA group showed the lowest level of COX-1 immunoreactivity, but the staining was not significantly less than in any of the other groups (p=0.067; Table 2).
Thus, COX-1 immunostaining of the breast epithelia seem to be down regulated by combined CEE+MPA treatment. There were no differences in stromal COX-1 immunoreactivity between the treatment groups.
COX-2 immunostaining of GE in experiment 2 was increased in the CEE group as compared to the controls (Fig 5C; Table 2). There was no difference between any of the treatment groups in experiment 1 (data not shown). There were no differences in the COX-2 immunostaining in DE or stroma between the different treatment groups, in either experiment 1 or 2. Hence, only CEE treatment in experiment 2 showed an effect on COX-2 immunoreactivity in the breast.
Discussion
Although COX-2 has been extensively studied in the context of endometrial and breast cancers (1, 2), little is known about the functions of COX-1 and COX-2 in normal primate uterus and mammary gland.
We found that both COX-1 and COX-2 are expressed in the uterus and breast of surgically post menopausal monkeys. COX-1 seems to be the dominant cyclooxygenase in endometrium and myometrium. This is well in agreement with a previous report where COX-1 was found to be a resident enzyme which synthesizes endogenous prostaglandins and maintains basic physiological functions of prostaglandins in the normal non-pregnant porcine uterus (25). It has also been demonstrated that the expression of COX-1 is dominant in the uterus of normal non-pregnant sheep (26) and mice (27).
We found combined CEE+MPA to decrease COX-1 immunoreactivity in the myometrium whereas CEE treatment increased COX-1 in endometrial GE. CEE+MPA treatment increased COX-2 immunoreactivity in endometrial epithelium and stroma, which is in contrast to a previous study that reported no COX-2 immunoreactivity after treatment by HT (29). Taken together, the combined treatment seems to inhibit COX-1, but stimulate COX-2 expression, in endometrium. As mentioned before, COX-1 is dominant in uterus, especially in myometrium. Therefore, it is possible that the decrease in the COX-1 level could balance, or even overcome, the increase in COX-2. Progestogen is known to protect the endometrium from cancer in a combined treatment, as compared to estrogen alone therapy (30).
In contrast to the uterus COX-2 was more prevalent than COX-1 in the mammary gland. There was widespread immunostaining of COX-2 in the monkey breast epithelium. Women expressing COX-2 in normal tissue also express it in cancer tissue (28). Despite its high expression in the breast, COX-2 was less regulated than COX-1 by the treatments in our study. Only the single CEE treatment increased COX-2 immunostaining in breast glandular epithelium. Results from the WHI trial showed that single CEE use induces a significant increase in mammographic density that is persistent over at least 2 years (31). In a recent article COX-2 expression was shown to be higher in dense versus non-dense breast (32). Mammographic density relates to proliferation in local breast tissue, and is positively associated with breast cancer risk (33).
TAM is an anti-estrogen in the breast, with agonistic effects in the endometrium (10). Women on long-term TAM therapy have an increased risk of developing endometrial cancer (11). In the present study TAM treatment had no significant effect on immunostaining of COX-1 and COX-2 in uterus and breast as compared to controls. A previous study showed that the uterine mRNA expression of COX-1 is not affected by TAM treatment (34), which is in agreement with our results.
In the interest of optimizing the risk/benefit ratio of HT, TIB was developed. After oral administration it is converted into three major metabolites, two of them estrogenic and one with progestogenic and androgenic properties. The summary effect of the metabolites on the endometrium is a weak estrogenic activity (19).
TIB has low estrogenic effects on the breast due to blocking some of the local enzyme systems, which impair the active estrogen formation (19). Clinically, few women on TIB treatment complain of adverse effects in breast, and breast density is not increased (19). In our study TIB increased COX-2 immunostaining in endometrial GE, but showed no effects on the breast.
Conclusions
Our results show that COX-1 and COX-2 are differently distributed and regulated by hormone treatment in normal uterus and breast from ovariectomized macaques. COX-1 is more dominant in the uterus, and is decreased by combined estrogen and progestogen treatment whereas COX-2 immunostaining is increased by the same treatment. On the other hand, COX-2 is prevailing in the mammary gland, but less regulated in this tissue than COX-1, which is decreased by the combined treatment.
Acknowledgements
We are grateful for skilful technical assistance by Britt Masironi. We thank Dr C.S. Blesson for critical review of the manuscript.
This study received financial support from The Swedish Research Council (project 20137 (LS)) and Karolinska Institutet. Financial support was also provided through the regional agreement on medical training and clinical research (ALF) between Stockholm County Council and Karolinska Institutet. The parent studies from which tissues were collected were supported by the NIH grants HL-490852 and HL-45666 and Organon. Hua Zhang is a post doc fellow supported by the Chinese Government (grant 2008850544).
Footnotes
No conflicts of interests.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Harris RE. Cyclooxygenase-2 (cox-2) and the inflammogenesis of cancer. Subcell Biochem. 2007;42:93–126. doi: 10.1007/1-4020-5688-5_4. [DOI] [PubMed] [Google Scholar]
- 2.Sales KJ, Jabbour HN. Cyclooxygenase enzymes and prostaglandins in pathology of the endometrium. Reproduction. 2003 Nov;126(5):559–67. doi: 10.1530/rep.0.1260559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Richards JA, Petrel TA, Brueggemeier RW. Signaling pathways regulating aromatase and cyclooxygenases in normal and malignant breast cells. J Steroid Biochem Mol Biol. 2002 Feb;80(2):203–12. doi: 10.1016/s0960-0760(01)00187-x. [DOI] [PubMed] [Google Scholar]
- 4.Jabbour HN, Sales KJ. Prostaglandin receptor signalling and function in human endometrial pathology. Trends Endocrinol Metab. 2004 Oct;15(8):398–404. doi: 10.1016/j.tem.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 5.Sales KJ, Katz AA, Howard B, Soeters RP, Millar RP, Jabbour HN. Cyclooxygenase-1 is up-regulated in cervical carcinomas: autocrine/paracrine regulation of cyclooxygenase-2, prostaglandin e receptors, and angiogenic factors by cyclooxygenase-1. Cancer Res. 2002 Jan 15;62(2):424–32. [PMC free article] [PubMed] [Google Scholar]
- 6.Sobolewski C, Cerella C, Dicato M, Ghibelli L, Diederich M. The role of cyclooxygenase-2 in cell proliferation and cell death in human malignancies. Int J Cell Biol. 2010:215158. doi: 10.1155/2010/215158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Santen RJ, Allred DC, Ardoin SP, Archer DF, Boyd N, Braunstein GD, et al. Postmenopausal hormone therapy: an Endocrine Society scientific statement. J Clin Endocrinol Metab. Jul;95(7 Suppl 1):s1–s66. doi: 10.1210/jc.2009-2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008 Mar-Apr;58(2):71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 9.Nilsson S, Makela S, Treuter E, Tujague M, Thomsen J, Andersson G, et al. Mechanisms of estrogen action. Physiol Rev. 2001 Oct;81(4):1535–65. doi: 10.1152/physrev.2001.81.4.1535. [DOI] [PubMed] [Google Scholar]
- 10.Cuzick J, Powles T, Veronesi U, Forbes J, Edwards R, Ashley S, et al. Overview of the main outcomes in breast-cancer prevention trials. Lancet. 2003 Jan 25;361(9354):296–300. doi: 10.1016/S0140-6736(03)12342-2. [DOI] [PubMed] [Google Scholar]
- 11.Polin SA, Ascher SM. The effect of tamoxifen on the genital tract. Cancer Imaging. 2008;8:135–45. doi: 10.1102/1470-7330.2008.0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kloosterboer HJ. Tibolone: a steroid with a tissue-specific mode of action. J Steroid Biochem Mol Biol. 2001 Jan-Mar;76(1-5):231–8. doi: 10.1016/s0960-0760(01)00044-9. [DOI] [PubMed] [Google Scholar]
- 13.Hofling M, Ma L, Sahlin L, Haglund C, Nordling S, von Schoultz B, et al. Expression of the androgen receptor and syndecan-1 in breast tissue during different hormonal treatments in cynomolgus monkeys. Climacteric. 2009 Feb;12(1):72–9. doi: 10.1080/13697130802448387. [DOI] [PubMed] [Google Scholar]
- 14.Ma L, Hofling M, Masironi B, von Schoultz B, Cline JM, Sahlin L. Effects of tibolone and conventional HRT on the expression of estrogen and progesterone receptors in the breast. Maturitas. 2008 Dec 20;61(4):345–9. doi: 10.1016/j.maturitas.2008.09.018. [DOI] [PubMed] [Google Scholar]
- 15.Cline JM, Register TC, Clarkson TB. Comparative effects of tibolone and conjugated equine estrogens with and without medroxyprogesterone acetate on the reproductive tract of female cynomolgus monkeys. Menopause. 2002 Jul-Aug;9(4):242–52. doi: 10.1097/00042192-200207000-00005. [DOI] [PubMed] [Google Scholar]
- 16.Lundstrom E, Christow A, Kersemaekers W, Svane G, Azavedo E, Soderqvist G, et al. Effects of tibolone and continuous combined hormone replacement therapy on mammographic breast density. Am J Obstet Gynecol. 2002 Apr;186(4):717–22. doi: 10.1067/mob.2002.121896. [DOI] [PubMed] [Google Scholar]
- 17.Cummings SR, Ettinger B, Delmas PD, Kenemans P, Stathopoulos V, Verweij P, et al. The effects of tibolone in older postmenopausal women. N Engl J Med. 2008 Aug 14;359(7):697–708. doi: 10.1056/NEJMoa0800743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Archer DF, Hendrix S, Gallagher JC, Rymer J, Skouby S, Ferenczy A, et al. Endometrial effects of tibolone. J Clin Endocrinol Metab. 2007 Mar;92(3):911–8. doi: 10.1210/jc.2006-2207. [DOI] [PubMed] [Google Scholar]
- 19.Hammar ML, van de Weijer P, Franke HR, Pornel B, von Mauw EM, Nijland EA. Tibolone and low-dose continuous combined hormone treatment: vaginal bleeding pattern, efficacy and tolerability. BJOG. 2007 Dec;114(12):1522–9. doi: 10.1111/j.1471-0528.2007.01537.x. [DOI] [PubMed] [Google Scholar]
- 20.Hermenegildo C, Oviedo PJ, Cano A. Cyclooxygenases regulation by estradiol on endothelium. Curr Pharm Des. 2006;12(2):205–15. doi: 10.2174/138161206775193136. [DOI] [PubMed] [Google Scholar]
- 21.Cline JM, Soderqvist G, Register TC, Williams JK, Adams MR, Von Schoultz B. Assessment of hormonally active agents in the reproductive tract of female nonhuman primates. Toxicol Pathol. 2001 Jan-Feb;29(1):84–90. doi: 10.1080/019262301301418883. [DOI] [PubMed] [Google Scholar]
- 22.Adams MR, Register TC, Golden DL, Wagner JD, Williams JK. Medroxyprogesterone acetate antagonizes inhibitory effects of conjugated equine estrogens on coronary artery atherosclerosis. Arterioscler Thromb Vasc Biol. 1997 Jan;17(1):217–21. doi: 10.1161/01.atv.17.1.217. [DOI] [PubMed] [Google Scholar]
- 23.Cline JM, Soderqvist G, von Schoultz E, Skoog L, von Schoultz B. Effects of conjugated estrogens, medroxyprogesterone acetate, and tamoxifen on the mammary glands of macaques. Breast Cancer Res Treat. 1998 Apr;48(3):221–9. doi: 10.1023/a:1005984932268. [DOI] [PubMed] [Google Scholar]
- 24.Cline JM, Register TC, Clarkson TB. Effects of tibolone and hormone replacement therapy on the breast of cynomolgus monkeys. Menopause. 2002 Nov-Dec;9(6):422–9. doi: 10.1097/00042192-200211000-00007. [DOI] [PubMed] [Google Scholar]
- 25.Cao J, Kitazawa T, Takehana K, Taneike T. Endogenous prostaglandins regulate spontaneous contractile activity of uterine strips isolated from non-pregnant pigs. Prostaglandins Other Lipid Mediat. 2006 Dec;81(3-4):93–105. doi: 10.1016/j.prostaglandins.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 26.Wu WX, Ma XH, Zhang Q, Buchwalder L, Nathanielsz PW. Regulation of prostaglandin endoperoxide H synthase 1 and 2 by estradiol and progesterone in nonpregnant ovine myometrium and endometrium in vivo. Endocrinology. 1997 Sep;138(9):4005–12. doi: 10.1210/endo.138.9.5394. [DOI] [PubMed] [Google Scholar]
- 27.Lee DS, Yanagimoto Ueta Y, Xuan X, Igarashi I, Fujisaki K, Sugimoto C, et al. Expression patterns of the implantation-associated genes in the uterus during the estrous cycle in mice. J Reprod Dev. 2005 Dec;51(6):787–98. doi: 10.1262/jrd.17039. [DOI] [PubMed] [Google Scholar]
- 28.Leo C, Faber S, Hentschel B, Hockel M, Horn LC. The status of cyclooxygenase-2 expression in ductal carcinoma in situ lesions and invasive breast cancer correlates to cyclooxygenase-2 expression in normal breast tissue. Ann Diagn Pathol. 2006 Dec;10(6):327–32. doi: 10.1016/j.anndiagpath.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 29.Hsu SC, Long CY, Yang CH, Wu CH, Chen CH, Liu FI. Cyclooxygenase-2 expression in the endometrium at the end of 2 years' continuous combined hormone replacement therapy. Maturitas. 2003 Dec 10;46(4):295–9. doi: 10.1016/s0378-5122(03)00218-4. [DOI] [PubMed] [Google Scholar]
- 30.Gambrell RD., Jr Prevention of endometrial cancer with progestogens. Maturitas. 1986 Jul;8(2):159–68. doi: 10.1016/0378-5122(86)90022-8. [DOI] [PubMed] [Google Scholar]
- 31.McTiernan A, Chlebowski RT, Martin C, Peck JD, Aragaki A, Pisano ED, et al. Conjugated equine estrogen influence on mammographic density in postmenopausal women in a substudy of the women's health initiative randomized trial. J Clin Oncol. 2009 Dec 20;27(36):6135–43. doi: 10.1200/JCO.2008.21.7166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang WT, Lewis MT, Hess K, Wong H, Tsimelzon A, Karadag N, et al. Decreased TGFbeta signaling and increased COX2 expression in high risk women with increased mammographic breast density. Breast Cancer Res Treat. Jan;119(2):305–14. doi: 10.1007/s10549-009-0350-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ziv E, Tice J, Smith-Bindman R, Shepherd J, Cummings S, Kerlikowske K. Mammographic density and estrogen receptor status of breast cancer. Cancer Epidemiol Biomarkers Prev. 2004 Dec;13(12):2090–5. [PubMed] [Google Scholar]
- 34.Wu Y, Niwa K, Onogi K, Tang L, Mori H, Tamaya T. Effects of selective estrogen receptor modulators and genistein on the expression of ERalpha/beta and COX-1/2 in ovarectomized mouse uteri. Eur J Gynaecol Oncol. 2007;28(2):89–94. [PubMed] [Google Scholar]