Abstract
Haldane's Rule (HR), which states that ‘when in the offspring of two different animal races one sex is absent, rare, or sterile, that sex is the heterozygous (heterogametic) sex', is one of the most general patterns in speciation biology. We review the literature of the past 15 years and find that among the ∼85 new studies, many consider taxa that traditionally have not been the focus for HR investigations. The new studies increased to nine, the number of ‘phylogenetically independent' groups that comply with HR. They continue to support the dominance and faster-male theories as explanations for HR, although due to increased reliance on indirect data (from, for example, differential introgression of cytoplasmic versus chromosomal loci in natural hybrid zones) unambiguous novel results are rare. We further highlight how research on organisms with sex determination systems different from those traditionally considered may lead to more insight in the underlying causes of HR. In particular, haplodiploid organisms provide opportunities for testing specific predictions of the dominance and faster X chromosome theory, and we present new data that show that the faster-male component of HR is supported in hermaphrodites, suggesting that genes involved in male function may evolve faster than those expressed in the female function.
Keywords: speciation genetics, hermaphrodites, haplodiploids, comparative studies, reproductive isolation, hybridization
A short history
Haldane (1922) famously noticed that ‘when in the offspring of two different animal races one sex is absent, rare, or sterile, that sex is the heterozygous (heterogametic) sex'. Haldane's Rule (HR), as this observation has come to be known, has since been confirmed in many groups of animals, including ones with male heterogamety, such as Mammalia (mammals), Diptera (flies, mosquitoes and gnats), Orthoptera (grasshoppers and crickets), Teleostei (bony fish) and certain Amphibia (amphibians), and with female heterogamety, such as Aves (birds), Lepidoptera (butterflies and moths) and certain Reptilia (reptiles) (Coyne, 1992; Laurie, 1997; Orr and Presgraves, 2000; Coyne and Orr, 2004; Volff, 2005; Presgraves, 2010). As HR encompasses a generality for the evolution of reproductive isolation, at least in gonochoric animals, it holds a central position in speciation research, especially since the mid-1980s, when studies of speciation genes began to blossom (Barton and Hewitt, 1985; Wu and Palopoli, 1994; Schilthuizen, 1999; Sun et al., 2004). Some debate has, however, centered around the correct measure for gauging the generality of HR: is the relevant measure the number of species pairs obeying HR or the number of independent sex determination systems in which it is found (Read and Nee, 1991). Orr (1997) showed that, even if the latter criterion is applied, HR is generally adhered to: at least seven phylogenetically independent origins are known. Given this apparent generality, it came as a surprise when experimental studies in Drosophila revealed HR to be of a composite nature (Orr, 1993; Wu and Davis, 1993). Of the various composite modes that have been suggested for the action of HR, there is strong support for two (the dominance theory and the faster male theory), and weaker support for some other modes (Coyne and Orr, 2004 see below for details).
Aims
Since its inception, literature on HR as well as reviews of that literature have accumulated (Table 1). Whereas earlier reviews tended to aim for summaries of the taxonomic distribution and general support for the phenomenon (Craft, 1938; Laurie, 1997), later ones focused on evidence for the underlying genetic explanations for HR (Orr, 1997), and by extension of speciation, and most recently on the actual genes that cause these incompatibilities (Presgraves, 2010; Johnson, 2010). In the past 15 years, attention has shifted towards traditionally less well-studied groups, such as Amphibia, Reptilia, Teleostei and a number of invertebrate groups. These studies have not only expanded the taxonomic support for HR, but have also offered interesting new insights and opportunities for testing theories underlying HR.
Table 1. Chronological overview of Haldane's Rule literature reviews and their taxonomic foci.
| Taxonomic group | Reference | Number of pairwise comparisons |
|---|---|---|
| All taxa | Haldane, 1922 | 6 Mammalia, 9 Aves, 30 Lepidoptera, 1 Diptera (Drosophila) |
| Mammalia | Craft, 1938 | ∼30a |
| Drosophila | Coyne and Orr, 1989 (based on Bock, 1984) | 145 |
| Mammalia | Coyne and Orr, 1989 (based on Gray, 1954) | 20 |
| Aves | Coyne and Orr, 1989 (based on Gray, 1958) | 43 (for fertility); 18 (for viability) |
| All taxa | Coyne, 1992 | 25 Mammalia, 30 Aves, 36 Lepidoptera, 114 Diptera (Drosophila) |
| All taxa | Wu and Davis, 1993 | 25 Mammalia, 30 Aves, 36 Lepidopterab, 202 Diptera (Drosophila)c |
| All taxa | Laurie, 1997 | 25 Mammals, 30 Avesd, 1 Amphibia, 3 Reptilia, 196 Insecta (including 71 Lepidoptera), 1 Nematodae |
| Aves | Price and Bouvier, 2002 | 407 |
| Anatinae (ducks) | Tubaro and Lijtmaer, 2002 | 161 |
| Columbidae (pigeons and doves) | Lijtmaer et al., 2003 | 21 |
| Lepidoptera | Presgraves, 2002 | 212 |
| Teleostei (bony fish) | Russell, 2003 | 19 |
Species status of some of the comparisons unclear.
Same data as in Coyne (1992).
Counting reciprocal crosses separately.
From Wu and Davis (1993).
See Laurie (1997) for original references.
Complementing previous reviews (see Table 1), we provide an update of species pairs tested for HR including some 85 studies published over the past 15 years (Table 2). We also tabulate evidence for the major explanatory genetic theories (Tables 2 and 3). Consistent with previous reviews (Wu et al., 1996; Orr, 1997) this confirms the generality of the dominance theory, but also suggests that alternative theories are often supported. Also, a relatively large number of studies (∼20%) found patterns not predicted by HR. Such cases are chiefly explained by postulating (or demonstrating) cytonuclear incompatibilities (see below). Another goal of this review is to draw attention to new taxonomic groups for studying HR, in particular those which have both male and female heterogamety in closely related taxa (for example, Teleostei). Finally, we want to highlight the potential of hitherto neglected animal groups with alternative reproductive modes, such as hermaphrodites and haplodiploids. We argue that these groups provide unique opportunities to test specific predictions of genetic theories underlying HR.
Table 2. Summary of studies of Haldane's Rule since 1996, as well as totals when combined with Coyne and Orr (2004), after removal of any overlap. (for a full list, see Supplementary Table 1).
| Taxon | Sex determination | This study: N (obeying Haldane′s Rule) | This study: N (not obeying HR) | Coyne and Orr, 2004 (based on Coyne, 1992) N (obeying HR) | Total obeying | |
|---|---|---|---|---|---|---|
| Mammalia | Male heterogamety | Sterility | 9 | 0 | 25 | 34 |
| Inviability | 4 | 0 | 1 | 5 | ||
| Aves (birds) | Female heterogamety | Sterility | 3 | 1 | 21 | 24 |
| Inviability | 215 | 2 | 30 | 245 | ||
| Reptilia | Female heterogamety | Sterility | 0 | 1 | 0 | |
| Inviability | 1 | 0 | 1 | |||
| Amphibia | Multiple | Sterility | 0 | 1 | 0 | |
| Inviability | 66 | 28 | 66 | |||
| Teleostei (fish) | Multiple | Sterility | 5 | 0 | 5 | |
| Inviability | 6 | 37 | 6 | |||
| Insecta | ||||||
| Diptera | Male heterogamety | Sterility | 17 | 0 | 112 | 129 |
| Inviability | 4 | 1 | 13 | 17 | ||
| Hemiptera | Male heterogamety | Sterility | 1 | 0 | 1 | |
| Inviability | 1 | 1 | 1 | |||
| Hymenoptera | Haplodiploidy | Sterility | 0 | 0 | 0 | |
| Inviability | 4 | 0 | 4 | |||
| Coleoptera | Male heterogamety | Sterility | 3 | 0 | 3 | |
| Inviability | 1a | 1 | 1 | |||
| Lepidoptera | Female heterogamety | Sterility | 6 | 1 | 11 | 17 |
| Inviability | 4 | 1 | 29 | 33 | ||
| Crustacea | Male heterogamety | Sterility | 0 | 5 | 0 | |
| Inviability | 0 | 0 | 0 | |||
| Gastropoda | Female heterogamety | Sterility | 0 | 1 | 0 | |
| Inviability | 1 | 0 | 1 | |||
| Nematoda | Male heterogamety | Sterility | 0 | 0 | 0 | |
| Inviability | 1 | 0 | 1 | |||
| Total | Sterility | N=44 | 10 (19%) | 169 | 213 | |
| Inviability | N=308 | 71 (19%) | 73 | 381 |
Abbreviation: HR, Haldane's Rule.
Deformities.
Table 3. Numbers of species pairs tested since 1996 in which explanatory theories for Haldane's Rule have been explicitly invoked.
| Dominance | Faster male | Faster heterogametic sex | Faster X (or Z) | X–Y (or W–Z) interactions | X (or Z)-autosome interactions | |
|---|---|---|---|---|---|---|
| Male heterogametic | 12 | 7 | 1 | 6 | 2 | 4 |
| Female heterogametic | 70 | 0 | 34 | 1 | 0 | 1 |
Taxonomic distribution
Haldane (1922) provided the first broad taxonomic inventory of HR, tabulating Lepidoptera, Aves, Mammalia and one species pair of Diptera. Craft (1938) expanded the list for Mammalia. After 50 years, Coyne and Orr (1989) partly using data from Bock (1984) compiled the list of Drosophila species, followed by Wu and Davis (1993) partly based on data from Gray (1954, 1958), who focused on Mammalia and Aves. Laurie (1997) provided a list of all taxa examined thus far. More recent authors have focused in more detail on taxa with female heterogamety, viz Aves (Price and Bouvier, 2002; Tubaro and Lijtmaer, 2002; Lijtmaer et al., 2003), Lepidoptera (Presgraves, 2002) and certain Teleostei (Russell, 2003). The most recent update is by Coyne and Orr (2004) who provide a summary table of the number of species that support HR. At that time, for male heterogamety, 25 species of Mammalia support HR for sterility and 1 for inviability, and 112 species of Drosophila support HR for sterility and 13 for inviability. For species with female heterogamety these numbers were 21 and 30 for Aves, and 11 and 29 for Lepidoptera, for sterility and inviability, respectively. In the past 15 years, studies of taxa that have not previously received much attention from HR researchers (for example, Reptilia, Amphibia, Teleostei and non-dipteran Insecta) found broader taxonomic support for HR, making the number of phylogenetically independent supports larger.
Supplementary Table 1 lists all known species tested for HR since Laurie (1997) and expands Coyne and Orr's (2004) table to 213 species pairs for sterility and 381 species pairs for inviability, by the addition of 9 (sterility)+4 (inviability) Mammalia, 13 Drosophila (for sterility), 3+215 Aves, 6+4 Lepidoptera, and newly, 66 Amphibia (inviability), 1 Reptilia (inviability), 5+6 Teleostei and 4+6 non-dipteran Insecta (Table 2). This increases the number of phylogenetically independent supports for HR to nine (male-heterogametic fish and male-heterogametic Gastropoda are added to the seven independently evolved sex determination systems with HR already mentioned by Orr (1997)). These new taxa also throw a somewhat different light upon Orr's (1997) contention that heterogametic infertility would develop more easily than inviability in male-heterogametic taxa whereas the reverse would be true for female-heterogametic taxa. The new studies generally support this pattern, but the male-heterogametic Teleostei tend to show the pattern usually encountered in female-heterogametic taxa.
Indirect evidence
The surge of interest in HR as part of the intensified speciation research of the past decades has led to increased familiarity with the phenomenon among students of non-model organisms. This has not only resulted in a taxonomically broader basis of case studies (see above), but also in a shift in the data type used to infer HR. As many non-model organisms cannot easily be studied under laboratory conditions, HR patterns are increasingly inferred indirectly from field data on the sex of F1 hybrids, and from differential introgression of nuclear versus mitochondrial DNA in natural hybrid zones, rather than from controlled laboratory crosses. As an example, Aguiar et al. (2008) studied species identity in groups of the howler monkeys Alouatta caraya and A. clamitans within their area of sympatry. They found that adult individuals of hybrid origin (on the basis of their mosaic coloration pattern) were almost always females. This led them to conclude that this (male-heterogametic) species adheres to HR.
Although patterns of hybridization in nature form a potentially rich source of additional information, the reliance on such patterns can be misleading because of confounding factors. First of all, sampling techniques may have a bias towards one sex or the other, leading to incorrect assessments of rarity. Also, asymmetric patterns for interspecific cytonuclear conflict (caused by, for example, coadaptation between nuclear and mitochondrially encoded components of mitochondrial enzymes; Ellison and Burton, 2006) may confuse the results. Arntzen et al. (2009), for example, carefully dissected the pattern found in hybridization between the newts Triturus marmoratus and T. cristatus in France, to show that the apparent weak HR pattern of an excess of F1 females (the species pair is male-heterogametic) was in fact composed of a female-biased class of cristatus-mothered hybrids and a much smaller class of all-male marmoratus-mothered hybrids, suggesting that HR does not apply in the crosses in the one direction, because of an incompatibility between the marmoratus cytoplasm and the cristatus X-chromosome, leading to a loss of female offspring from that cross. Thus, when the pattern of fitness values among all four classes of F1 hybrids (males and females, mothered by species 1 or by species 2) result in patterns expected under HR (not the case in, for example, Limnoporus water striders (Abe et al., 2005) and centrarchid fish (Bolnick et al., 2008), where they were found in the opposite direction), the role of cytonuclear incompatibilities is probably more often overlooked in field studies than in laboratory crosses.
Differential introgression patterns of uniparentally versus biparentally inherited markers across a hybrid zone have been used to infer HR with some success. Studies include work on taxa with male heterogamety, where maternally inherited loci are seen to introgress further than autosomal and paternally inherited loci (for example, in the Mus musculus–M. domesticus hybrid zone, mitochondrial DNA introgresses to a greater degree than autosomal loci (Raufaste et al., 2005)), as well as taxa with female heterogamety (where biparentally inherited loci are seen to introgress deeper than maternally inherited ones; Jiggins et al., 1997; Helbig et al., 2001; Cianchi et al., 2003; Kronforst et al., 2006). As a particularly comprehensive example, Carling and Brumfield (2008) studied cline width in a (female-heterogametic) avian hybrid zone and found that Z-linked and mitochondrial genes introgressed less deeply than autosomal markers, which is consistent with HR.
The tendency to detect HR indirectly from data on nuclear DNA and organelle DNA introgression should, on the one hand, be applauded, as it allows the evaluation of HR across a much broader range of taxa than is possible with laboratory crossing data only. On the other hand, caution is warranted, as sex-biased fertility and viability is only one of the possible causes for discrepancies in mitochondrial DNA and nuclear DNA cline widths. Cytonuclear conflicts (see above), male or female philopatry (Guillaume and Perrin, 2009), the relative effects of smaller effective population size (which tends to reduce mitochondrial DNA introgression) and its unlinked nature (which may increase mitochondrial DNA introgression; Ballard and Whitlock, 2004) all could (and will) interact with the effects of HR.
Genetic theories
The most important component underlying HR is dominance, as first suggested by Muller (1940). If Bateson–Dobzhansky–Muller incompatibilities (BDM) exist between genes on the autosomes and on the sex chromosomes, then homogametic F1 hybrids will be heterozygous for all these genes, and suffer fitness loss only for X-linked incompatibility genes that are dominant. Heterogametic hybrids, however, will suffer an additional fitness loss for those X-linked genes that are recessive, the effects of which are under the hemizygous sex chromosome condition, no longer shielded (Coyne, 1985; Orr, 1993). Turelli and Orr (1995) and Orr and Turelli (1996) further refined Muller's idea by showing that, as the homogametic sex inherits a double load of X-linked incompatibility genes, HR applies only when the average degree of dominance (d) is <0.5.
The other component that certainly contributes to HR is faster-male evolution. As Wu and Davis (1993) and Wu et al. (1996) have pointed out, male-expressed genes are subject to two types of sexual selection (female choice and male–male competition), whereas female-expressed genes are only subject to one type (female choice). This increased selection pressure on male genes would result in faster evolution for these loci. Genetic studies in which chromosome regions were moved between species show that, indeed, the introgressed regions much more often contain male sterility genes than female sterility genes (True et al., 1996; Hollocher and Wu, 1996; Tao and Hartl, 2003). In addition, Michalak and Noor (2003) and Hearty and Singh (2006) proved in Drosophila-microarray studies that, in hybrids, male-specific genes are the ones most likely to be misexpressed.
Turelli and Orr (1995) developed a mathematical expression in which both dominance and faster-male evolution are incorporated (as well as faster-X evolution; see below). They showed that HR occurs under certain conditions regarding the relationship among the degree of dominance (d), the proportion of X-linked BDM genes in comparison with those on the autosomes (px) and the ratio between the evolutionary rates for male and female sterility (τ), namely, when
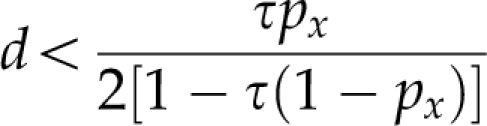 |
if τ=1, that is, if there is no faster-male evolution, HR applies when d<0.5 (see above). However, when τ>1 (faster-male evolution), the occurrence of HR is less straightforward. Under male heterogamety, faster-male evolution and dominance will amplify one another, but under female heterogamety, both effects will act in different directions, and their relative strengths will determine whether or not HR occurs.
A large number of additional theories have been proposed to explain HR (reviewed by Laurie, 1997; Kulathinal and Singh, 2008). Among these, divergence of meiotic drive suppressor systems (Frank, 1991; Hurst and Pomiankowski, 1991; Tao and Hartl, 2003 reviewed in McDermott and Noor, 2010) and other systems of genomic conflict (Johnson, 2010), inherent lability of spermatogenesis (Wu and Davis, 1993), faster X-chromosome evolution (Charlesworth et al., 1987) and Y chromosome and maternal effects (Sawamura, 1996; Turelli and Orr, 2000) have received most attention. In a recent paper, Moyle et al. (2010) revisit the old, but largely neglected argument that intragenomic rearrangements may contribute to HR, and they present support for this in mammals, but not Drosophila.
Supplementary Table 1 provides an overview of recent studies that have considered the three main underlying theories of HR and it tabulates for each species pair and taxon which theory is supported. Out of a total of some 85 studies since 1996, 15 found unambiguous support for the dominance theory, 8 for the faster male theory and 5 for the faster X theory. This broad support for the dominance theory confirms earlier conclusions of Orr (1997). Aves and Lepidoptera have been studied extensively because they have female heterogamety, which allows for testing the faster male theory. As female hybrids suffer much more frequently from sterility and inviability than male birds, these groups tend not to support the faster male theory.
HR in taxa without sex chromosomes
The view has been emerging that HR reveals important generalities about the genetic processes involved in the evolution of reproductive isolation and speciation (Coyne and Orr, 2004). Even if it is indeed a composite phenomenon, in which dominance, faster-male evolution and perhaps faster-X evolution and meiotic drive all have a role, it paints a picture of BDM arising through sexual selection processes (sensu lato) in diverging lineages, which could well be of general importance in speciation. If so, then HR-like patterns should also be visible in taxa with sex, but without sex chromosomes. Moreover, there is another reason why it would be welcome to extend HR to such taxa: prudent choice of study organisms with particular sex-determination mechanisms may allow diverse causes for HR to be disentangled, which is harder in taxa with heterogamety. Therefore, perhaps paradoxically, we here advocate the investigation of HR in two groups of taxa without sex chromomomes, viz haplodiploids and hermaphrodites.
Haplodiploids
Organisms with haplodiploid reproduction have haploid males (which develop from unfertilized eggs) and diploid females (developing from fertilized eggs). They are found in Rotifera, Nematoda, Arachnida and a significant fraction of the Insecta (Mable and Otto, 1998). Koevoets and Beukeboom (2009) found some support for the haploid males being more strongly negatively affected by hybridization than hybrid diploid females (although the number of studies was very limited) and suggested to rephrase HR to apply to ‘the heterogametic or hemizygous sex'. They emphasized that the adherence of haplodiploids to HR has some interesting implications and possibilities to test predictions from several HR theories. First, in interspecies crosses, truly hybrid males only appear in the F2 (as F1 males develop from unfertilized gametes of a parental species mother). Second, under the dominance theory, it would allow the evaluation of the relative importance of BDM on the X-chromosome versus those on the autosomes, as in haplodiploid hemizygous hybrids, recessive genes on all chromosomes will be revealed. Third, the faster-male theory yields similar predictions for haplodiploids as diploids, as it specifically refers to faster evolution of genes involved in spermatogenesis without a disproportional role for the sex chromosomes. Finally, if the faster-X chromosome theory applies, it would mean that genes on the hemizygous chromosomes, which are all autosomes in haploid males, are affected more when they are male-biased or unbiased in their expression. Unfortunately, sex specific gene expression studies in haplodiploids are not yet available to test this prediction. In the haplodiploid Nasonia, most genic incompatibilities appear to be cyto-nuclear rather than nuclear, but it is too early to conclude that this is a general pattern resulting from the absence of heteromorphic sex chromosomes. More comparative studies are also needed to validate the impression that hybrid male sterility in haplodiploids evolves more slowly than in diploids. If this turns out to be true, it would argue against a strong role for the faster male theory in haplodiploids, which in turn would be consistent with absence of strong sexual selection on males in most haplodiploid groups (Reeve and Pfennig, 2003). Although haploidy is confounded with maleness in haplodiploids, the independent experimental manipulation of ploidy level and sex in Nasonia provides exciting opportunities for distinguishing between the dominance and faster male theories (Koevoets, in preparation).
Hermaphrodites
Simultaneous hermaphrodites, (for example, many Angiosperms, Pulmonata and Platyhelminthes), although having both sexes united in one individual, do have separate male and female organs, functions and genes, which are under separate, and often conflicting, selection pressures (Michiels and Newman, 1998; Schilthuizen, 2002; Koene and Schulenburg, 2005).
Despite the absence of separate sexes, a Haldane's-Rule-like male-female asymmetry in BDM might be revealed in hybrid hermaphrodites by greater loss of male function than female function, as a result of faster-male evolution. In fact, Orr and Presgraves (2000) state: ‘one might expect (for example, monoecious plant hybrids) hermaphrodite hybrids to show breakdown of male reproductive structures (for example, flower parts) more often than female structures, regardless of whether these taxa possess differentiated sex chromosomes'.
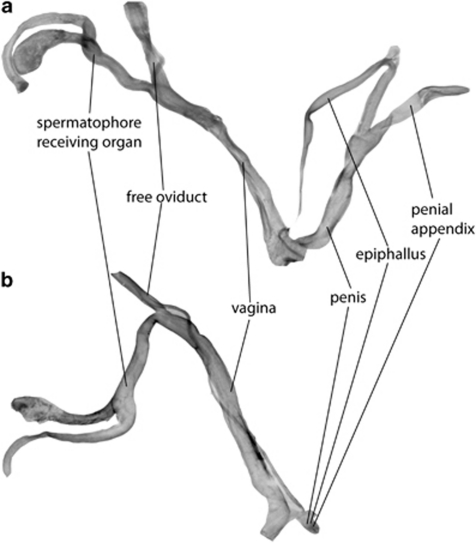
We tested Orr's and Presgraves's prediction by close examination of case studies of reproductive anatomy in hermaphrodite hybrids. An initial search of the literature on hybridization and hybrid zones in various hermaphroditic groups of organisms indicated that data on abnormalities in reproductive structures are rarely reported explicitly and that detailed, time-consuming analysis of raw data, lab journals and correspondence with researchers would be necessary. For this reason, we decided to restrict this search to the Pulmonata (Mollusca: Gastropoda), the only hermaphrodite group with which one of us (MS) had broad experience. In these simultaneous, obligately-outcrossing hermaphrodites without sex chromosomes, the genitalia are composed of structures with male functions (the intromittent penis and the spermatophore-forming complex of penial appendix and epiphallus) as well as female functions (the penis-receiving vagina, the spermatophore-receiving organ and the free oviduct; see Figure 1a). We used the following search strategies. First, peer-reviewed literature was identified by searching the Web of Science (Thompson Reuters, New York, NY, USA) with the keywords ‘hybrid' and ‘Pulmonata'. Next, in addition, non-SCI literature was detected by using the same two keywords in Google Scholar, and in two malacological databases at the Netherlands Centre for Biodiversity Naturalis, viz MS's personal database (∼5500 entries) and the digitized collection of Mollusca reprints (∼17 000 entries) by searching for the keyword ‘hybrid'. Third, we manually searched the abstracts of the triennial World Congress of Malacology for the years 1989–2007. All these searches yielded hints at the existence of useful data, which in many cases could only be confirmed by personally approaching the researchers concerned; we did so for some 25 case studies (see Acknowledgements). The results are summarized in Table 4, which contains much previously unpublished information.
Figure 1.
Haldane's Rule in hermaphrodites: a normal reproductive system in Albinaria hippolyti aphrodite (a) with parts that serve male (penis, penial appendix and epiphallus) and female (vagina, spermatophore receiving organ and free oviduct) functions, and in an A. hippolyti aphrodite × A. hippolyti harmonia hybrid individual (b) with strongly deformed male parts and unaffected female parts.
Table 4. Observed defects in genitalia of hermaphrodite hybrids (Mollusca: Gastropoda: Pulmonata).
| Parental taxon 1 | Parental taxon 2 | Type of hybrids | N (m) | N (f) | N (total) | Reference |
|---|---|---|---|---|---|---|
| Bradybaena pellucida | Bradybaena similaris | lab F1 | 0 | 0 | 30–40 | Wiwegweaw et al., 2008; Asami, pers. comm., 2 March 2010 |
| Albinaria hippolyti aphrodite | Albinaria hippolyti harmonia | hybrid zone | 3 | 0 | ∼60 | Schilthuizen and Lombaerts, 1995 |
| Albinaria hippolyti aphrodite | Albinaria hippolyti holtzi | hybrid zone | 2 | 0 | ∼50 | Schilthuizen, 1995 |
| Albinaria hippolyti hippolyti | Albinaria hippolyti harmonia | hybrid zone | 0 | 0 | ∼30 | Schilthuizen, 1995 |
| Albinaria hippolyti aphrodite | Albinaria hippolyti holtzi | hybrid zone | 0 | 0 | ∼50 | Schilthuizen, 1995 |
| Partula suturalis | Partula mooreana | lab F1 | 1 | 1 | 14 | Murray and Clarke, 1980; Murray, pers. comm., 26 February 2007 |
| Partula mirabilis | Partula mooreana | lab F1 | 1 | 1 | 19 | Murray and Clarke, 1980; Murray, pers. comm., 26 February 2007 |
| Mandarina mandarina | Mandarina chichijimana | lab F1 and hybrid zone | 7 | 0 | 34 | Chiba, pers. comm., 3 March 2010 |
| Mandarina aureola | Mandarina polita | hybrid zone | 6 | 0 | 30 | Chiba, pers. comm., 3 March 2010 |
| Mandarina aureola | Mandarina ponderosa | hybrid zone | 0 | 0 | 4 | Chiba, pers. comm., 3 March 2010 |
| Mandarina anijimana | Mandarina n. sp. | lab F1 and hybrid zone | 0 | 0 | 11 | Chiba, pers. comm., 3 March 2010 |
| Ainohelix editha subsp. 1 | Ainohelix editha keeled subsp. | lab F1 and hybrid zone | 0 | 0 | 13 | Chiba, pers. comm., 3 March 2010 |
| Albinaria lerosiensis | Albinaria brevicollis | field hybrids | 2 | 0 | 18 | Giokas, pers. comm., 29 March 2010 |
| Albinaria caerulea subsp. 1 | Albinaria caerulea subsp. 2 | lab F1 and field hybrids | 0 | 0 | 20 | Giokas, pers. comm., 29 March 2010 |
| Iberus gualtieranus | Iberus alonensis | lab F1, F2, and backcross | 0 | 0 | 34 | Gomez, pers. comm., 9 March 2010 |
| Cepaea nemoralis | Cepaea hortensis | lab F1 | 1 | 1 | 15 | Lang, 1908 |
| Luchuphaedusa ophidoon form SL | Luchuphaedusa ophidoon form Sm | field F1 and backcross | 0 | 0 | 66 | Ueshima, 1993 |
N (m) is the number of hybrid individuals with defects in male parts of the genitalia; N (f) is the number of hybrid individuals with defects in female parts of the genitalia. N (total) is the total number of hybrid individuals dissected.
Our literature survey revealed that many cases (hybridizing pairs of species, subspecies or morphs) exhibit defects in reproductive structures, but that per case, such defects are not very common. In 8 out of 17 cases of hybridization in Pulmonata with good anatomical data, defects were found, and out of those 8 cases, 3 involved defects in both male and female genitalia, whereas 5 involved defects in only the male genitalia. Overall, however, out of ∼440 hybrid individuals assayed, deformed male genitalia were found in 23 individuals and deformed female genitalia in only 3 individuals. Thus, in hybrid Pulmonata, Presgraves's and Orr's prediction appears to be borne out, as deformed male genitalia were significantly more common than female ones (Fisher exact test, P<0.001). Although these numbers appear relatively low, it must be remembered that only observable defects in gross anatomical structure were scored. These included such dramatic deformities as the complete lack of penis and epiphallus, the blind ending of the vas deferens, etcetera. Such macroscopic deformities are extremely rare under non-hybrid conditions (Hausdorf (1989) describes a single individual in Cernuella virgata). More subtle or macroanatomically cryptic deformities and compromised functioning, would have remained undetected, so the true rate of deformation may in reality be higher. What was clear was that these deformities are generally strongly associated with hybridization. For most (sub) species pairs equal or larger numbers of dissected individuals from the parental forms were available, and only in one case (Cepaea) were similar deformations found in these individuals as well.
Like in haplodiploids, the presence of this HR-like pattern in hermaphrodites provides unique opportunities for testing components of the explanatory theories for HR. First of all, given the absence of sex chromosomes in hermaphrodites, it would allow for the faster-male theory to be studied in isolation, something that is much harder to do in gonochorists (but see Presgraves and Orr (1998) on HR in gonochoric animal taxa lacking a hemizygous X). Second, comparing the rate of loss of hybrid male function in hermaphroditic taxa and gonochoric sister taxa (for example, the hermaphroditic Heterobranchia versus the gonochoric Caenogastropoda) would allow the relative importance of sexual selection (which affects both) versus dominance (which cannot affect the hermaphrodites) in generating reproductive isolation to be assessed.
HR in a comparative context
Johnson (2010) predicts that HR may be less prevalent in taxa without heteromorphic sex chromosomes because there is less scope for intragenomic conflict. Currently, too few non-model systems have been investigated, but this hypothesis could very well be tested with haplodiploids, and possibly with hermaphrodites and sex-reversal organisms (see below). In addition to looking for general patterns in HR across taxa, further comparative approaches involving closely related species differing in crucial reproductive traits may be helpful in elucidating aspects of HR. Turelli and Begun (1997), for example, showed that pairs of Drosophila species with relatively large X-chromosomes evolved HR faster, confirming the role for dominance. Another example are Culicidae, where Aedes lack differentiated sex chromosomes, but comply with HR for sterility, but not lethality, whereas Anopheles with heteromorphic X and Y show both male sterility and inviability in interspecific crosses (Presgraves and Orr, 1998). Other such comparative studies could be imagined. Some diecious plants, for example, have sex chromosomes and some (such as the papaya) have close relatives that are hermaphroditic (Vyskot and Hobza, 2004). Similarly, Teleostei (Volff, 2005) and Gastropoda (Avise et al., 2004) show a great diversity in sex determination systems and many groups have male heterogamety, female heterogamety and hermaphroditism in closely related taxa. Another promising approach may be to look for HR in groups that regularly or occasionally undergo sex change, such as some Teleostei (Pandian and Sheela, 1995), or that can be artificially induced to change sex (certain Amphibia; Wallace et al., 1999). Perrin (2009) realized that sex reversal may halt the differentiation of sex chromosomes following the cessation of recombination in the heterogametic sex. This could also delay the accumulation of antagonistic and sex-specifically expressed genes on the sex chromosomes and predicts smaller roles for faster-male and faster-X in causing HR in taxa undergoing sex reversal. Cases such as these would allow study of the primary causes for HR. By mapping the evolution of male and female sterility and inviability, and aberrations in male and female reproductive structures, upon phylogenetic trees in relation to the evolution of sex determination systems, a detailed dissection of the evolutionary pathways towards HR may be possible.
Note added in proof
While this paper was in press, the first case of HR in a plant with differentiated sex chromosomes (Silene) was published (Brothers & Delph, 2010; Evolution, 64: 3643–3648).
Acknowledgments
We are extremely grateful to the following colleagues, who provided us with previously unpublished data for hermaphrodite hybrids (some of them even went as far as dissecting stored voucher specimens for us), or assisted in some other way: Takahiro Asami, Bruno Baur, Satoshi Chiba, Bryan Clarke, Angus Davison, Aline Dépraz, Robert Dillon, Arantza Elejalde, Sinos Giokas, Benjamín Gómez Moliner, James Murray, Martin Haase, Bernhard Hausdorf, Joseph Heller, Jukka Jokela, Paula Lourenço, Heike Reise and David Woodruff. We thank Tosca Koevoets for discussions, Graham Wallis for reading a draft version of the manuscript and three anonymous reviewers for comments that helped improve the paper.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on Heredity website (http://www.nature.com/hdy)
Supplementary Material
References
- Abe TA, Spence JR, Sperling FAH. Mitochondrial introgression is restricted relative to nuclear markers in a water strider (Hemiptera: Gerridae) hybrid zone. Can J Zool. 2005;83:432–444. [Google Scholar]
- Aguiar LM, Pie MR, Passos FC. Wild mixed groups of howler species (Alouatta caraya and Alouatta clamitans) and new evidence for their hybridization. Primates. 2008;49:149–152. doi: 10.1007/s10329-007-0065-y. [DOI] [PubMed] [Google Scholar]
- Arntzen JW, Jehle R, Bardakci F, Burke T, Wallis GP. Asymmetric viability of reciprocal-cross hybrids between crested and marbled newts (Triturus cristatus and T. marmoratus) Evolution. 2009;63:1191–1202. doi: 10.1111/j.1558-5646.2009.00611.x. [DOI] [PubMed] [Google Scholar]
- Avise JC, Power AC, Walker D. Genetic sex determination, gender identification, and pseudohermaphroditism in the knobbed whelk, Busycon carica (Mollusca: Melongenidae) Proc R Soc Lond B. 2004;271:641–646. doi: 10.1098/rspb.2003.2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballard JWO, Whitlock MC. The incomplete natural history of mitochondria. Mol Ecol. 2004;13:729–744. doi: 10.1046/j.1365-294x.2003.02063.x. [DOI] [PubMed] [Google Scholar]
- Barton NH, Hewitt GM. Analysis of hybrid zones. Annu Rev Ecol Syst. 1985;16:113–148. [Google Scholar]
- Bock IR. Interspecific hybridization in the genus Drosophila. Evol Biol. 1984;18:41–70. [Google Scholar]
- Bolnick DI, Turelli M, López-Fernández H, Wainwright PC, Near TJ. Accelerated mitochondrial evolution and ‘Darwin's Corollary': asymmetric viability of reciprocal F1 hybrids in centrarchid fishes. Genetics. 2008;178:1037–1048. doi: 10.1534/genetics.107.081364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carling MD, Brumfield RT. Haldane's Rule in an avian system: using cline theory and divergence population genetics to test for differential introgression of mitochondrial, autosomal and sex-linked loci across the Passerina bunting hybrid zone. Evolution. 2008;62:2600–2615. doi: 10.1111/j.1558-5646.2008.00477.x. [DOI] [PubMed] [Google Scholar]
- Charlesworth B, Coyne JA, Barton NH. The relative rates of evolution of sex chromosomes and autosomes. Am Nat. 1987;130:113–146. [Google Scholar]
- Cianchi R, Ungaro A, Marini M, Bullini L. Differential patterns of hybridization and introgression between the swallowtails Papilio machaon and P. hospiton from Sardinia and Corsica islands (Lepidoptera, Papilionidae) Mol Ecol. 2003;12:1461–1471. doi: 10.1046/j.1365-294x.2003.01813.x. [DOI] [PubMed] [Google Scholar]
- Coyne JA. The genetic basis of Haldane's Rule. Nature. 1985;314:736–738. doi: 10.1038/314736a0. [DOI] [PubMed] [Google Scholar]
- Coyne JA. Genetics and speciation. Nature. 1992;355:511–515. doi: 10.1038/355511a0. [DOI] [PubMed] [Google Scholar]
- Coyne JA, Orr HA.1989Two rules of speciationIn: Otte D and Endler J (eds).Speciation and Its Consequences Sinauer Associates: Sunderland, Massachusetts; 189–211. [Google Scholar]
- Coyne JA, Orr HA. Speciation. Sinauer Associates: Sunderland, Massachusetts; 2004. [Google Scholar]
- Craft WA. The sex ratio in mules and other hybrid mammals. Quart Rev Biol. 1938;13:19–40. [Google Scholar]
- Ellison CK, Burton RS. Disruption of mitochondrial function in interpopulation hybrids of Tigriopus californicus. Evolution. 2006;60:1382–1391. [PubMed] [Google Scholar]
- Frank SA. Divergence of meiotic drive-suppression systems as an explanation for sex-biased hybrid sterility and inviability. Evolution. 1991;45:262–267. doi: 10.1111/j.1558-5646.1991.tb04401.x. [DOI] [PubMed] [Google Scholar]
- Guillaume F, Perrin N. Inbreeding load, bet hedging, and the evolution of sex-biased dispersal. Am Nat. 2009;173:536–541. doi: 10.1086/597218. [DOI] [PubMed] [Google Scholar]
- Gray AP. Mammalian Hybrids; A Check-List with Bibliography. Commonwealth Agricultural Bureaux, Farnham Royal; 1954. [Google Scholar]
- Gray AP. Bird Hybrids; A Check-List with Bibliography. Commonwealth Agricultural Bureaux, Farnham Royal; 1958. [Google Scholar]
- Haldane JBS. Sex ratio and unisexual sterility in hybrid animals. J Genet. 1922;12:101–109. [Google Scholar]
- Hausdorf B. Über eine Anomalie der männlichen Endwege des Genitalsystems bei Cernuella virgata (Da Costa) (Gastropoda: Hygromiidae) Heldia. 1989;1:175–176. [Google Scholar]
- Hearty W, Singh RS. Gene regulation divergence is a major contributor to the evolution of Dobzhansky-Muller incompatibilities between species of Drosophila. Mol Biol Evol. 2006;23:1707–1714. doi: 10.1093/molbev/msl033. [DOI] [PubMed] [Google Scholar]
- Helbig AJ, Salomon M, Bensch S, Seibold I. Male-biased gene flow across an avian hybrid zone: evidence from mitochondrial and microsatellite DNA. J Evol Biol. 2001;14:277–287. [Google Scholar]
- Hollocher H, Wu CI. The genetics of reproductive isolation in the Drosophila simulans clade: X vs. autosomal effects and male vs. female effects. Genetics. 1996;143:1243–1255. doi: 10.1093/genetics/143.3.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurst LD, Pomiankowski A. Causes of sex ratio bias may account for unisexual sterility in hybrids: a new explanation of Haldane's Rule and related phenomena. Genetics. 1991;128:841–858. doi: 10.1093/genetics/128.4.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiggins CD, McMillan WO, King P, Mallet J. The maintenance of species differences across a Heliconius hybrid zone. Heredity. 1997;79:495–505. [Google Scholar]
- Johnson NA. Hybrid incompatibility genes: remnants of a genomic battlefield. Trends Genet. 2010;26:317–325. doi: 10.1016/j.tig.2010.04.005. [DOI] [PubMed] [Google Scholar]
- Koene JM, Schulenburg H. Shooting darts: coevolution and counter-adaptation in hermaphroditic snails. BMC Evol Biol. 2005;5:25. doi: 10.1186/1471-2148-5-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koevoets T, Beukeboom LW. Genetics of postzygotic isolation and Haldane's Rule in haplodiploids. Heredity. 2009;102:16–23. doi: 10.1038/hdy.2008.44. [DOI] [PubMed] [Google Scholar]
- Kronforst MR, Young LG, Blume LM, Gilbert LE. Multilocus analyses of admixture and introgression among hybridizing Heliconius butterflies. Evolution. 2006;60:1254–1268. [PubMed] [Google Scholar]
- Kulathinal RJ, Singh RS. The molecular basis of speciation from patterns to processes, rules to mechanisms. J Genet. 2008;87:327–338. doi: 10.1007/s12041-008-0055-x. [DOI] [PubMed] [Google Scholar]
- Lang A. Über die Bastarde von Helix hortensis Müller und Helix nemoralis L.; eine Untersuchung zur Experimentellen Vererbungslehre. Gustav Fischer: Jena; 1908. [Google Scholar]
- Laurie CC. The weaker sex is heterogametic: 75 years of Haldane's rule. Genetics. 1997;147:937–951. doi: 10.1093/genetics/147.3.937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lijtmaer DA, Mahler B, Tubaro PL. Hybridization and postzygotic isolation patterns in pigeons and doves. Evolution. 2003;57:1411–1418. doi: 10.1111/j.0014-3820.2003.tb00348.x. [DOI] [PubMed] [Google Scholar]
- Mable BK, Otto SP. The evolution of life cycles with haploid and diploid phases. BioEssays. 1998;20:453–462. [Google Scholar]
- McDermott SR, Noor MAF. The role of meiotic drive in hybrid male sterility. Phil Trans R Soc B. 2010;365:1265–1272. doi: 10.1098/rstb.2009.0264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalak P, Noor MAF. Genome-wide patterns of expression in Drosophila pure species and hybrid males. Mol Biol Evol. 2003;20:1070–1076. doi: 10.1093/molbev/msg119. [DOI] [PubMed] [Google Scholar]
- Michiels NK, Newman LJ. Sex and violence in hermaphrodites. Nature. 1998;391:647. [Google Scholar]
- Moyle LC, Muir CD, Han MV, Hahn MW. The contribution of gene movement to the ‘two rules of speciation'. Evolution. 2010;64:1541–1557. doi: 10.1111/j.1558-5646.2010.00990.x. [DOI] [PubMed] [Google Scholar]
- Muller HJ.1940Bearing of the Drosophila work on systematicsIn: Huxley JS (ed).The New Systematics Clarendon Press: Oxford; 185–268. [Google Scholar]
- Murray J, Clarke B. The genus Partula on Moorea: speciation in progress. Proc R Soc Lond B. 1980;211:83–117. [Google Scholar]
- Orr HA. Haldane's rule has multiple genetic causes. Nature. 1993;361:532–533. doi: 10.1038/361532a0. [DOI] [PubMed] [Google Scholar]
- Orr HA. Haldane's rule. Annu Rev Ecol Syst. 1997;28:195–218. [Google Scholar]
- Orr HA, Presgraves DC. Speciation by postzygotic isolation: forces, genes and molecules. BioEssays. 2000;22:1085–1094. doi: 10.1002/1521-1878(200012)22:12<1085::AID-BIES6>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Orr HA, Turelli M. Dominance and Haldane's Rule. Genetics. 1996;143:613–616. doi: 10.1093/genetics/143.1.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandian TJ, Sheela SG. Hormonal induction of sex reversal in fish. Aquaculture. 1995;138:1–22. [Google Scholar]
- Perrin N. Sex reversal: a fountain of youth for sex chromosomes. Evolution. 2009;63:3043–3049. doi: 10.1111/j.1558-5646.2009.00837.x. [DOI] [PubMed] [Google Scholar]
- Presgraves DC. Patterns of postzygotic isolation in Lepidoptera. Evolution. 2002;56:1168–1183. doi: 10.1111/j.0014-3820.2002.tb01430.x. [DOI] [PubMed] [Google Scholar]
- Presgraves DC. The molecular evolutionary basis of species formation. Nature Rev Genet. 2010;11:175–180. doi: 10.1038/nrg2718. [DOI] [PubMed] [Google Scholar]
- Presgraves DC, Orr HA. Haldane's Rule in taxa lacking a hemizygous X. Science. 1998;282:952–954. doi: 10.1126/science.282.5390.952. [DOI] [PubMed] [Google Scholar]
- Price TD, Bouvier MM. The evolution of F1 postzygotic incompatibilities in birds. Evolution. 2002;56:2083–2089. [PubMed] [Google Scholar]
- Raufaste N, Orth A, Belkhir K, Senet D, Smadja C, Baird SJE, et al. Inferences of selection and migration in the Danish house mouse hybrid zone. Biol J Linn Soc. 2005;84:593–616. [Google Scholar]
- Read A, Nee S. Is Haldane's Rule significant. Evolution. 1991;45:1707–1709. doi: 10.1111/j.1558-5646.1991.tb02676.x. [DOI] [PubMed] [Google Scholar]
- Reeve HK, Pfennig DW. Genetic biases for showy males. Are some genetic systems especially conducive to sexual selection. Proc Natl Acad Sci USA. 2003;100:1089–1094. doi: 10.1073/pnas.0337427100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell ST. Evolution of intrinsic post-zygotic reproductive isolation in fish. Ann Zool Fennici. 2003;40:321–329. [Google Scholar]
- Sawamura K. Maternal effect as a cause of exceptions for Haldane's Rule. Genetics. 1996;143:609–611. doi: 10.1093/genetics/143.1.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilthuizen M. A comparative study of hybrid zones in the polytypic land snail Albinaria hippolyti (Pulmonata: Clausiliidae) Neth J Zool. 1995;45:261–290. [Google Scholar]
- Schilthuizen M. Cloning Odysseus and the seed of speciation. Trends Ecol Evol. 1999;14:90–91. doi: 10.1016/s0169-5347(98)01565-1. [DOI] [PubMed] [Google Scholar]
- Schilthuizen M. Mollusca: an evolutionary cornucopia. Trends Ecol Evol. 2002;17:8–9. [Google Scholar]
- Schilthuizen M, Lombaerts M. Life on the edge: a hybrid zone in Albinaria hippolyti from Crete. Biol J Linn Soc. 1995;54:111–138. [Google Scholar]
- Sun S, Ting CT, Wu CI. The normal function of a speciation gene, Odysseus, and its hybrid sterility effect. Science. 2004;305:81–83. doi: 10.1126/science.1093904. [DOI] [PubMed] [Google Scholar]
- Tao Y, Hartl DL. Genetic dissection of hybrid incompatibilities between Drosophila simulans and D. mauritiana. III. Heterogeneous accumulation of hybrid incompatibilities, degree of dominance, and implications for Haldane's Rule. Evolution. 2003;57:2580–2598. doi: 10.1111/j.0014-3820.2003.tb01501.x. [DOI] [PubMed] [Google Scholar]
- True JR, Weir BS, Laurie CC. A genome-wide survey of hybrid incompatibility factors by the introgression of marked segments of Drosophila mauritiana chromosomes into Drosophila simulans. Genetics. 1996;142:819–837. doi: 10.1093/genetics/142.3.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tubaro PL, Lijtmaer DA. Hybridization patterns and the evolution of reproductive isolation in ducks. Biol J Linn Soc. 2002;77:193–200. [Google Scholar]
- Turelli M, Begun DJ. Haldane's Rule and X-chromosome size in Drosophila. Genetics. 1997;147:1799–1815. doi: 10.1093/genetics/147.4.1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turelli M, Orr HA. The dominance theory of Haldane's Rule. Genetics. 1995;140:389–402. doi: 10.1093/genetics/140.1.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turelli M, Orr HA. Dominance, epistasis and the genetics of postzygotic isolation. Genetics. 2000;154:1663–1679. doi: 10.1093/genetics/154.4.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueshima R. Morphological divergence and speciation in the clausiliid snails of the Luchuphaedusa (Oophaedusa) ophidoon species complex, with special reference to the hybrid-zone. Venus. 1993;52:259–281. [Google Scholar]
- Volff JN. Genome evolution and biodiversity in teleost fish. Heredity. 2005;94:280–294. doi: 10.1038/sj.hdy.6800635. [DOI] [PubMed] [Google Scholar]
- Vyskot B, Hobza R. Gender in plants: sex chromosomes are emerging from the fog. Trends Genet. 2004;20:432–438. doi: 10.1016/j.tig.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Wallace H, Badawy GMI, Wallace GMN. Amphibian sex determination and sex reversal. Cell Mol Life Sci. 1999;55:901–909. doi: 10.1007/s000180050343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiwegweaw A, Seki K, Mori H, Asami T. Asymmetric reproductive isolation during simultaneous reciprocal mating in pulmonates. Biol Lett. 2008;5:240–243. doi: 10.1098/rsbl.2008.0714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu CI, Davis AW. Evolution of post-mating reproductive isolation—the composite nature of Haldane's Rule and its genetic bases. Am Nat. 1993;142:187–212. doi: 10.1086/285534. [DOI] [PubMed] [Google Scholar]
- Wu CI, Palopoli MF. Genetics of postmating isolation in animals. Annu Rev Genet. 1994;27:283–308. doi: 10.1146/annurev.ge.28.120194.001435. [DOI] [PubMed] [Google Scholar]
- Wu CI, Johnson NA, Palopoli MF. Haldane's Rule and its legacy: why are there so many sterile males. Trends Ecol Evol. 1996;11:281–284. doi: 10.1016/0169-5347(96)10033-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.