Abstract
To test the hypothesis that the hexosamine biosynthesis pathway (HBP) affects cytokine production, we studied IL-2 production by Jurkat cells in response to PHA. We found that the HBP activator glucosamine (GlcN), but not glucose (Glc), dose-dependently reduced IL-2 production. Importantly, GlcN blocked trafficking of a GFP-NFAT chimeric protein to the nucleus of stimulated transfectants. Not surprisingly, changes in O-GlcNAc protein modifications were noted during cell activation with and without GlcN addition. These findings could not be explained by some non-specific change in cell metabolism because ATP concentrations did not significantly change. We speculate that HBP-active compounds may contribute to patient care in certain inflammatory and autoimmune diseases.
Keywords: Cell Activation, Inflammation, Metabolism
1. Introduction
As life began then evolved by harvesting energy from the environment, cell metabolism is tightly woven into the fabric of cell function. Nonetheless, metabolism is frequently overlooked or only seen as a power supply, not a regulatory mechanism. For example, leukocyte activation is regulated by glucose transport [1–3] and the spatial location of enzymes constituting the hexose monophosphate shunt [4, 5]. Another important metabolic regulatory pathway is the hexosamine biosynthesis pathway (HBP), although only 1–3% of the glucose entering a cell is shunted into this pathway. Traxinger and colleagues [6] discovered the HBP's cellular regulatory capacity. This pathway is now known to have multiple roles in cellular physiology: synthesis of carbohydrate starting materials, regulation of glucose transport and producing precursors for O-GlcNAc (N-acetyl-D-glucosamine) signaling [7, 8]. By attaching O-GlcNAc moieties to ser/thr residues that also undergo phosphorylation, kinase targets are blocked [7, 8]. One result of HBP activation is the inhibition of capacitative calcium signaling in leukocytes [9]. Using glucosamine (GlcN) and other reagents, the HBP has been shown to participate in several neutrophil functions such as oxidant production [10]. Recent studies have also suggested that glucosamine is immunosuppressive [11, 12]. Furthermore, the HBP also influences cytokine production in mesangial and microglial cells [11–15]. On the basis of these and other ongoing studies in this laboratory, we now hypothesize that the HBP influences cytokine production by Jurkat T cells.
2. Materials and Methods
2.1 Materials
Glucosamine (GlcN) and glucose (Glc) were obtained from Sigma-Aldrich (St. Louis, MO). GlcN stock solutions were prepared in distilled deionized water then stored at low pH at 4°C prior to use. For experimentation, GlcN solutions were neutralized and diluted in buffered solutions. Alexa-488-conjugated PHA (phytohemagglutinin) was obtained from Molecular Probes (Eugene, OR). O-(2-acetamido-2-deoxy-D-glucopyranosylidene)-amino-N-phenylcarbamate (PUGNAc) was obtained from Toronto Research Chemicals (North York, Ontario, Canada).
2.2 Cells and transient transfections
Jurkat cells (ATCC, Manassas, VA) were maintained in RPMI-1640 medium (Invitrogen, Carlsbad, CA) containing 10% FCS and 1% antibiotics. Transfection of pcDNA3-GFP-NFAT4 [16] was carried out using Lipofectamine LTX and Plus reagent following Invitrogen's protocol. Briefly, 0.5 μg of pcDNA3-GFP-NFAT4 (green fluorescent protein/nuclear factor of activated T cells) mixed with Lipofectamine LTX and Plus reagent (Invitrogen) were added to single wells containing 105 Jurkat cells suspended in RPMI-1640 medium. Mock transfections were also conducted. After 4 hours of incubation, 10% FCS was added to each well, and experiments were conducted 28 hours after transfection.
2.3 Western Blots
SDS-PAGE and Western blots were performed as described [17]. Briefly, 15 μl of a sample was loaded onto a 4–20% gradient gel for SDS-PAGE. Samples were then transferred to a PVDF membrane for O-GlcNAc modified protein detection using an anti-O-GlcNAc antibody at 1:1500 (clone CTD110.6, Covance, Berkeley, CA) and a 1:36000 dilution of HRP-conjugated anti-IgM Ab.
2.4 Bioassays
IL-2 production was measured using an ELISA kit from Bender (Burlingame, CA). Cells were stimulated with PHA (10 μg/ml) for 24 hr. ATP levels were measured using the ATPlite kit (Perkin-Elmer Life Sciences, Boston, MA) using a FlexStation II (Molecular Devices, Sunnyvale, CA).
2.5 Cell stimulation, DAPI staining
GFP-NFAT4 transfected Jurkat cells were treated with or without PHA (10 μg/ml) (Invitrogen) and/or GlcN (Sigma-Aldrich) for 10 min at 37 °C. After washing with buffer, cells were fixed with 4% paraformaldehyde at room temperature for 20 min. Subsequently, cells were treated with 4,6-diamidino-2-phenylindole (DAPI) (5 μg/ml) (Sigma-Aldrich) at room temperature for 10 min then thoroughly washed prior to microscopic imaging.
2.6 Microscopic imaging
Cells were observed using a Nikon Eclipse TE2000 Quantum inverted fluorescence microscope (Nikon Instruments, Inc., Melville, NY) with mercury illumination interfaced to a computer using Metamorph (Molecular Devices, Danville, PA) software. Images were taken with an Andor Technologies iXon model DV8 16-bit electron multiplying CCD camera cooled to −90°C (Andor Technologies, South Windsor, CT). A 96320 HYQ filter module (Nikon) was used for imaging GFP and Alexa-488-conjugated PHA. A second set containing a D355HT15 exciter, 390DCLP dichroic, and 405DF43 emitter was used for DAPI imaging.
2.7 Live cell of metabolic monitoring
NAD(P)H autofluorescence is a well-established noninvasive method to study cell and tissue metabolism [3]. An LED operating at 365nm (Rapp ElectroOptic, Wedel, Germany) was used for excitation to minimize illumination noise (both intensity fluctuations and out-of-band illumination noise). For flavoprotein fluorescence imaging, a filter set comprised of a 455DF70 nm excitation filter, a 520DF40 nm emission filter, and a 495 nm long-pass dichroic reflector was used. An iris diaphragm was adjusted to exclude light from neighboring cells. A cooled photomultiplier tube (PMT) held in a model D104 detection system (Photon Technology International, Lawrenceville, NJ) attached to a Zeiss Axiovert microscope was used. The autofluorescence intensity was recorded using Felix software (Photon Technology International). To allow the addition of GlcN, cells were attached to the surface using Cell-Tak (BD Biosciences, San Jose, CA).
3. Results
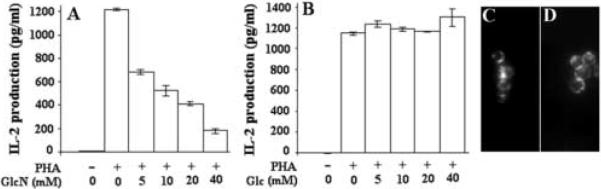
The HBP's role in cytokine production by Jurkat cells, a leading model of lymphocyte activation, was studied. Additional motivations for using Jurkat cells are the availability of mutants and their utility in gene transfection. We first studied GlcN's effect on IL-2 production by Jurkat cells. At these GlcN doses, dramatic reductions in IL-2 production were found (Fig. 1A). This was not a non-specific effect on metabolism, as glucose addition at these doses had no effect on IL-2 production (Fig. 1B). Although unlikely, it seems possible that GlcN blocked the binding of PHA to cells, thus reducing IL-2 production. To test this possibility, Jurkat cells were pre-treated with 40 mM Glc or 40 mM GlcN for 30 min. followed by the addition of 10 μg/ml Alexa-488-PHA for an additional 30 min. No differences in PHA binding were noted (Fig. 1C and D). As GlcN and the HBP promote biosynthetic activity, the IL-2 reduction is unlikely to be due to reduced biosynthetic capacity. It seems likely that this is a signaling phenomenon.
Fig. 1.
GlcN (A), but not Glc (B), reduces IL-2 production by Jurkat cells. Cells were activated with PHA in the presence of various doses of GlcN or Glc as indicated on the charts. Overnight incubations were performed. GlcN caused a dramatic reduction in IL-2 production by cells. However, the addition of GlcN or Glc did not affect the binding of Alexa-488-PHA to cells (C and D, respectively). (n=3)
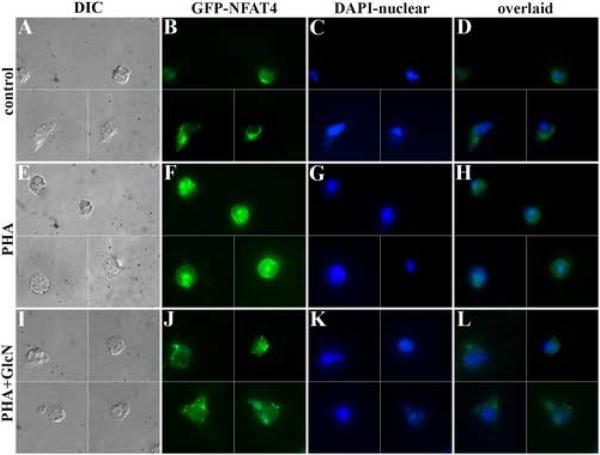
If the HBP influences cytokine production by Jurkat cells via signaling pathways, then it should be possible to detect changes upstream from IL-2 synthesis. Specifically, we tested the hypothesis that GlcN affects NFAT trafficking to the nucleus of stimulated Jurkat cells. Using an expression vector containing GFP-NFAT, transient transfectants of Jurkat cells were prepared. In this way, we can monitor the intracellular location of this transcription factor using fluorescence microscopy. Fig. 2 shows GFP-NFAT transfectants under various conditions. Cells were also stained with DAPI for nuclear localization. Under control conditions, GFP-NFAT was primarily located in the Jurkat cell cytoplasm. As others have previously shown, stimulation of cells leads to NFAT accumulation in the nucleus (Fig. 2E–H). However, inclusion of GlcN greatly diminished NFAT trafficking to the nucleus (Fig. 2I–L). Therefore, GlcN, very likely acting through the HBP, is capable of regulating NFAT trafficking and IL-2 production.
Fig. 2.
Jurkat cells transfected with NFAT-GFP were studied. Cells were DAPI-stained to label the nucleus. As the cells were relatively sparse in this transient transfection, montages are used to show several cells in each panel (data are not de-blurred). Although NFAT was cytoplasmic in unstimulated cells, it was primarily found in the nucleus in PHA-stimulated cells (E–H). When cells were treated with both PHA and GlcN (I–L), NFAT was primarily found in the cytoplasm, which accounts for the blunted cytokine responses noted above. This finding suggests that HBP activation affects Jurkat signaling pathways. (n = 3) (×1000)
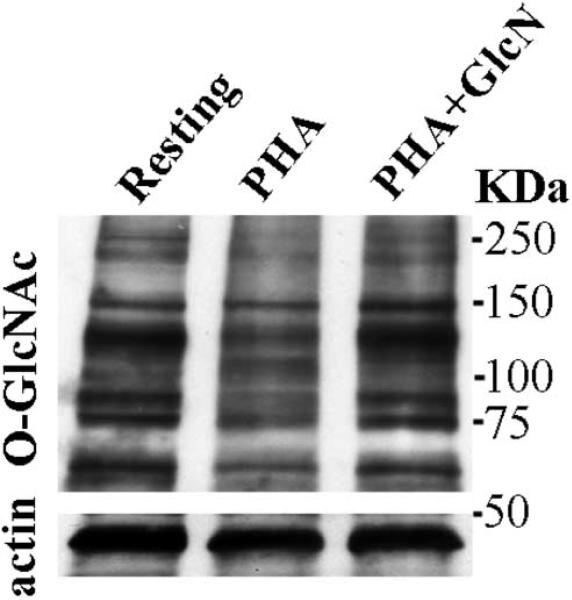
One signaling mechanism intersected by the HBP is the O-GlcNAc pathway [7]. To test the potential role of the O-GlcNAc signaling pathway, Jurkat cells were exposed to a variety of conditions then analyzed using SDS-PAGE and Western blotting. During resting conditions O-GlcNAc-modified proteins are noted in Jurkat cells (Fig. 3). PHA stimulation leads to both increases and decreases in O-GlcNAc protein modification (Fig. 3, lane 2) [18]. For example, a band at 135kDa is significantly reduced by stimulation. However, addition of GlcN with PHA stimulation normalizes O-GlcNAc staining of this band. The ability of GlcN to influence both NFAT translocation and O-GlcNAc protein modification support the hypothesis that metabolic regulatory pathways affect signal transduction and IL-2 production by Jurkat cells.
Fig. 3.
O-GlcNAcylation of Jurkat cell proteins at 24hrs. (A) Western blot of O-GlcNAc-labeled proteins was prepared at 3 × 106 cells/lane for all lanes. Lane 1: control. Lane 2: PHA stimulated. Lane 3: PHA + 40 mM GlcN. (B) Actin loading controls are shown in the bottom panel for this same gel. Jurkat stimulation alters O-GlcNAcylation patterns, which are offset in part by GlcN. This finding suggests that GlcN affects signaling pathways. (n=3)
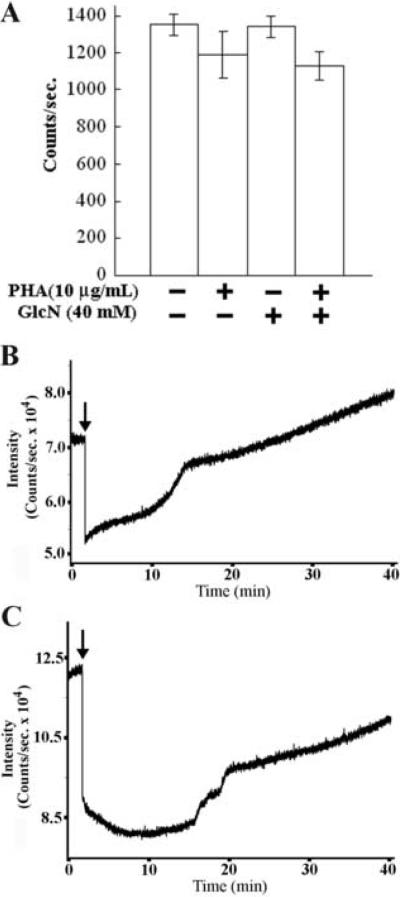
Although unlikely, it is possible that GlcN influenced IL-2 production and NFAT trafficking by reducing ATP levels. For example, GlcN might compete for one of the several leukocyte glucose transporters; this could affect ATP production when HBP-to-glycolytic coupling via glucosamine-6-phosphate deaminase is extremely weak (tight coupling would form higher amounts of ATP powered by F6P formed from GlcN6P). This seems very unlikely because we have found that Jurkat cells express significant amounts of glucosamine-6-phosphate deaminase by using immunofluorescence microscopy (unpublished data). Nonetheless, to control for this potential confounding factor, we assessed ATP levels. ATP concentrations were measured using the ATPlite kit (Perkin-Elmer), which employs luciferase-based ATP detection in conjunction with a pH protocol to inhibit ATPase activity. Cells were stimulated for 10 min. then extracted using the manufacturer's protocol. Analysis of PHA stimulated and GlcN treated cells showed that total ATP levels are not affected significantly by GlcN (Fig. 4A).
Fig. 4.
Effect of cell stimulation and GlcN addition on cell metabolism. (A) ATP levels of Jurkat cells were measured using the ATPlite kit as directed by the manufacturer. Luminescence was measured using a Molecular Devices FlexStation II. No statistically significant differences were found. (Raw data in counts are shown.) (n=3) Effect of GlcN on Jurkat NAD(P)H (B) and flavoprotein (C) autofluorescence. Experiments were conducted at 37°C. Jurkat cells were placed on Cell-Tak-coated coverslips, to allow addition of GlcN without disturbing the cells. GlcN (40 mM) was added at the arrowheads in both panels. A decrease in NADH was observed, followed by slow recovery over time. Similar changes in flavoprotein autofluorescence were found. A PTI photometer using a low noise LED at 365nm for excitation was used for NADH and a conventional Hg lamp at 460nm for the flavoprotein excitation. (n=3) (Unprocessed data are shown.)
Fig. 1 shows that GlcN's effect on IL-2 production cannot be duplicated by Glc. Fig. 4A demonstrates the fact that ATP levels are not affected by GlcN. These data indicate strongly that GlcN's effects are not mediated by some “non-specific” effect on cell metabolism. To further rule out this unlikely possibility, cellular NADH levels were monitored using autofluorescence (Fig. 4B). A transient decrease in NAD(P)H level was observed followed by a slow recovery. In addition, we measured the autofluorescence of mitochondrial flavoproteins as a second indicator of metabolic activity. Changes in endogenous flavoprotein autofluorescence (Fig. 4C) paralleled those noted for NAD(P)H fluorescence. These metabolic perturbations may be due in part to glucosamine-6-phosphate deaminase metabolism of GlcN6P to F6P. These findings indicate that ATP production (Fig. 4A), NAD(P)H production (Fig. 4B) and flavoprotein activity (Fig. 4C) are intact. Although these manipulations may cause some changes in how carbon is routed through metabolism, no significant losses of metabolic energy exist. Indeed, energy availability from all sources may be slightly elevated in the presence of GlcN. As Glc is unable to inhibit IL-2 production (Fig. 1B), these approaches all indicate that the effect is specific for the HBP.
To provide another approach to test the HBP's role in cytokine production, we evaluated the effects of several HBP inhibitors on IL-2 production by Jurkat cells. At 500 μM, PUGNAc, an inhibitor of O-GlcNAc-selective N-acetyl-β-D-glucosaminidase (O-GlcNAcase), reduced IL-2 production by PHA-stimulated Jurkat cells by about one-half in the absence of GlcN; levels dropped from 1170±20 pg/ml in controls to 550±40 pg/ml in the presence of PUGNAc (n=3, p<0.001). This reagent did not change ATP levels as judged by the ATPlite kit (data not shown). This suggests that the O-GlcNAc signaling pathway may participate in regulating IL-2 production. These results further support a role for the HBP in regulating IL-2 production.
4. Discussion
Today's most pressing medical challenge is the control of inflammation. Inflammatory reactions participate in clinical settings such as: autoimmune diseases (multiple sclerosis, arthritis, type 1 diabetes, uveitis, etc.), transplant rejection, heart attack, stroke, cerebral palsy, sepsis, and many others. Despite intense research, the options available to physicians are limited. In contrast to conventional approaches relying upon rational structure-based drug design or proteomic screening methods, our approach has been to analyze sites of intersection between metabolic and signaling circuitry to identify control or “choke” points that influence immune cell physiology. For example, we have identified epigenetic metabolic regulatory mechanisms such as the translocation of cytosolic enzymes and the inhibition of glucose transport that influence leukocyte activation [3–5]. In the present work, we have extended this strategic approach to lymphocyte activation. Our studies suggest that the HBP is capable of inhibiting Jurkat cell production of IL-2 by suppressing the translocation of NFAT to the nucleus.
Our study suggests that Jurkat T cell IL-2 production is regulated by the HBP via downstream elements of the signaling apparatus in a fashion that does not significantly alter several measures of energy production. This newly proposed role of the HBP as a metabolic anti-inflammatory regulatory pathway is consistent with previous studies using other cell types. For example, capacitative calcium entry, which required for normal lymphocyte activation, is inhibited by GlcN in macrophages [9]. TGF-β is generally considered to have anti-autoimmune properties [19]; its production by mesangial cells is enhanced by GlcN and other HBP activators [11–14]. Furthermore, in microglial cells, which resemble macrophages, GlcN and the HBP have been shown to inhibit production of the inflammatory cytokine TNF-α [15]. We speculate that the HBP broadly influences inflammatory events.
Although much recent research concerning lymphocyte activation has focused upon early signaling events, these early events are not the only or best sites for therapeutic intervention. For example, our findings are not inconsistent with studies showing the GlcN promotes a Th2-biased phenotype in mice [20]. However, as GlcN is not likely to reach serum concentrations as high as those used in this study after oral uptake, its touted ability to affect human osteoarthritis cannot be explained by our studies. Nonetheless, use of the HBP to regulate the extent and, perhaps, the nature of lymphocyte activation is more elegant than existing anti-inflammatory strategies that broadly depress lymphocyte metabolic pathways [21]. This study will stimulate the search for new HBP-active drugs that inhibit inflammation by selectively activating this branch of metabolism.
Acknowledgments
We thank Dr. Masamitsu Iino of the University of Tokyo for kindly providing the GFP-NFAT construct. We also thank the UM Vector Core Facility for assistance. This work was supported by NIAID grant 51789.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Kiyotaki C, Peisach J, Bloom BR. Oxygen metabolism in cloned macrophage cell lines: Glucose dependence of superoxide production, metabolic and spectral analysis. J. Immunol. 1984;132:857–66. [PubMed] [Google Scholar]
- [2].Tan AS, Ahmed N, Berridge MV. Acute regulation of glucose transport after activaton of human peripheral blood neutrophils by phorbol myristate acetate, fMLP, and granulocyte-macrophage colony-stimulation factor. Blood. 1998;91:649–55. [PubMed] [Google Scholar]
- [3].Petty HR, Kindzelskii AL, Espinoza J, Romero R. Trophoblast contact de-activates human neutrophils. J. Immunol. 2006;176:3205–14. doi: 10.4049/jimmunol.176.5.3205. [DOI] [PubMed] [Google Scholar]
- [4].Kindzelskii AL, Huang JB, Chaiworapongsa T, Kim YM, Romero R, Petty HR. Pregnancy alters glucose-6-phosphate dehydrogenase trafficking, cell metabolism and oxidant release of maternal neutrophils. J. Clin. Invest. 2002;110:1801–11. doi: 10.1172/JCI200215973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Huang JB, Romero R, Petty HR. Human neutrophil transaldolase undergoes retrograde trafficking during pregnancy, but anterograde trafficking in cells from non-pregnant women. Metabolism. 2005;54:1027–33. doi: 10.1016/j.metabol.2005.03.005. [DOI] [PubMed] [Google Scholar]
- [6].Marshall S, Bacote V, Traxinger RR. Discovery of a metabolic pathway mediating glucose-induced desensitization of the glucose transport system. J. Biol. Chem. 1991;266:4706–12. [PubMed] [Google Scholar]
- [7].Love DC, Hanover JA. The hexosamine signaling pathway: deciphering the “OGlcNAc Code”. Science's STKE. 2005;312:1–14. doi: 10.1126/stke.3122005re13. [DOI] [PubMed] [Google Scholar]
- [8].Filippis A, Clark S, Proietto J. Increased flux through the hexosamine biosynthesis pathway inhibits glucose transport acutely by activation of protein kinase C. Biochem. J. 1997;324:981–5. doi: 10.1042/bj3240981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Vemuri S, Marchase RB. The inhibition of capacitative calcium entry due to ATP depletion but not due to glucosamine is reversed by staurosporine. J. Biol. Chem. 1999;274:20165–70. doi: 10.1074/jbc.274.29.20165. [DOI] [PubMed] [Google Scholar]
- [10].Hua J, Sakamoto K, Nagoaka I. Inhibitory actions of glucosamine, a therapeutic agent for osteoarthritis, on the functions of neutrophils. J. Leuk. Biol. 2002;71:632–40. [PubMed] [Google Scholar]
- [11].Ma L, Rudert WA, Harnaha J, Wright M, Machen J, Lakomy R, Qian S, Lu L, Robbins PD, Trucco M, Giannoukakis N. Immunosuppressive effects of glucosamine. J. Biol. Chem. 2002;277:39343–9. doi: 10.1074/jbc.M204924200. [DOI] [PubMed] [Google Scholar]
- [12].Forchhammer L, Thorn M, Met O, Gad M, Weidner MS, Claesson MH. Immunobiological effects of glucosamine in vitro. Scand. J. Immunol. 2003;58:404–11. doi: 10.1046/j.1365-3083.2003.01313.x. [DOI] [PubMed] [Google Scholar]
- [11].James LR, Tang D, Ingram A, Ly H, Thai K, Cai L, Scholey JW. Flux through the hexosamine pathway is a determinant of nuclear factor κB-dependent promoter activation. Diabetes. 2002;51:1146–56. doi: 10.2337/diabetes.51.4.1146. [DOI] [PubMed] [Google Scholar]
- [12].Kolm-Litty V, Sauer U, Nerlich A, Lehmann R, Schleicher ED. High glucose-induced transforming growth factor beta1 production is mediated by the hexosamine pathway in porcine glomerular mesangial cells. J. Clin. Invest. 1998;101:160–9. doi: 10.1172/JCI119875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Daniels MC, Kansal P, Smith TM, Paterson AJ, Kudlow JE, McClain DA. Glucose regulation of transforming growth factor-alpha expression is mediated by products of the hexosamine biosynthesis pathway. Mol. Endocrinol. 1993;7:1041–8. doi: 10.1210/mend.7.8.8232303. [DOI] [PubMed] [Google Scholar]
- [14].Weigert C, Friess U, Brodbeck K, Haring HU, Schliecher ED. Glutamine:fructose-6-phosphate aminotransferase enzyme activity is necessary for the induction of TGF-β1 and fibronectin expression in mesangial cells. Diabetologia. 2003;46:852–5. doi: 10.1007/s00125-003-1122-8. [DOI] [PubMed] [Google Scholar]
- [15].Yi HA, Yi SD, Jang BC, Song DK, Shin DH, Mun KC, Kim SP, Suh SI, Bae JH. Inhibitory effects of glucosamine on lipopolysaccharide-induced activation in microglial cells. Clin. Exp. Pharmacol. Physiol. 2005;32:1097–103. doi: 10.1111/j.1440-1681.2005.04305.x. [DOI] [PubMed] [Google Scholar]
- [16].Tomida T, Hirose K, Takizawa A, Shibasaki F, Iino M. NFAT functions as a working memory of Ca2+ signals in decoding Ca2+ oscillation. EMBO J. 2003;22:3825–32. doi: 10.1093/emboj/cdg381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Kneass ZT, Marchase RB. Neutrophils exhibit rapid agonist-induced increases in protein-associated O-GlcNAc. J. Biol. Chem. 2004;279:45759–65. doi: 10.1074/jbc.M407911200. [DOI] [PubMed] [Google Scholar]
- [18].Kearse KP, Hart GW. Lymphocyte activation induces rapid changes in nuclear and cytoplasmic glycoproteins. Proc. Natl. Acad. Sci. USA. 1991;88:1701–5. doi: 10.1073/pnas.88.5.1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Prud'homme GJ, Piccirillo CA. The inhibitory effects of transforming growth factor-beta-1 (TGF-beta1) in autoimmune diseases. J. Autoimmunol. 2000;14:23–42. doi: 10.1006/jaut.1999.0339. [DOI] [PubMed] [Google Scholar]
- [20].Zhang GX, Yu S, Gran B, Rostami A. Glucosamine abrogates the acute phase of experimental autoimmune encephalomyelitis by induction of Th2 response. J. Immunol. 2005;175:7202–8. doi: 10.4049/jimmunol.175.11.7202. [DOI] [PubMed] [Google Scholar]
- [21].Buttgereit F, Burmester GR, Brand MD. Bioenergetics of immune functions: fundamental and therapeutic aspects. Immunol. Today. 2000;21:192–9. doi: 10.1016/s0167-5699(00)01593-0. [DOI] [PubMed] [Google Scholar]