Abstract
The vertebrate pituitary gland is a key endocrine control organ that contains six distinct hormone secreting cell types. In this study, we analyzed the role of direct cell-to-cell Delta-Notch signaling in zebrafish anterior pituitary cell type specification. We demonstrate that initial formation of the anterior pituitary placode is independent of Notch signaling. Later however, loss of Notch signaling in mind bomb (mib) mutant embryos or by DAPT treatment leads to increased numbers of lactotropes and loss of corticotropes in the anterior pars distalis (APD), increased number of thyrotropes and loss of somatotrope cell types in the posterior pars distalis (PPD), and fewer melanotropes in the posterior region of the adenohypophysis, the pars intermedia (PI). Conversely, Notch gain of function leads to the opposite result, loss of lactotrope and thyrotrope cell specification, and an increased number of corticotropes, melanotropes, and gonadotropes in the pituitary. Our results suggest that Notch acts on placodal cells, presumably as a permissive signal, to regulate progenitor cell specification to hormone secreting cell types. We propose that Notch mediated lateral inhibition regulates the relative numbers of specified hormone cell types in the three pituitary subdomains.
Keywords: adenohypophysis, cell differentiation, cranial, DAPT, Delta, hormone, Notch, organogenesis, patterning, placode
Introduction
Several long-range signals, including Nodals, Hedgehogs (Hh), Bone Morphogenetic Proteins (BMP), Fibroblast Growth Factors (FGF), and Wnts, regionalize and pattern the anterior pituitary (also called adenohypophysis) and instruct pituitary cells about their positions and later cell fates (Dasen and Rosenfeld, 2001; Dutta et al., 2005; Ericson et al., 1998; Herzog et al., 2003; Sbrogna et al., 2003; Treier et al., 1998). However, it remains unclear how pituitary cells acquire competence to respond to these signals or how they adopt different cell fates in the three pituitary subdomains, even though they are exposed to similar concentrations of particular long-range signals. The potential functions of short-range, cell-to-cell signals, for example by Delta ligands and Notch receptors that mediate lateral inhibition, for pituitary cell specification have not been tested.
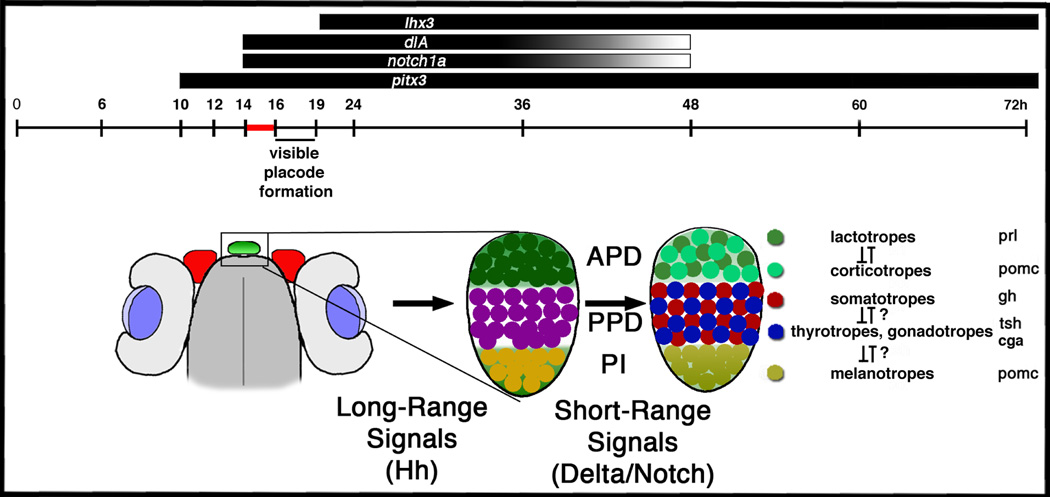
The vertebrate anterior pituitary develops from unspecified ventral ectoderm cells at the anterior midline of the neural plate border (Couly and Le Douarin, 1988; Eagleson and Harris, 1990). Mouse pituitary expresses genes encoding homeodomain transcription factors such as Pitx1 and Pitx2, and in zebrafish, pituitary precursor cells and pituitary placode are demarcated by pitx3 and dlx3b (Dutta et al., 2005). The visible placode forms by mid-somitogenesis stages (Glasgow et al., 1997). The anterior pituitary placode is later regionalized into three distinct subdomains: the anterior pars distalis (APD), the adjacent posterior pars distalis (PPD), and the posterior pars intermedia (PI) (Herzog et al., 2003). The 6 hormone secreting cell types of the pituitary are distinguished by their morphologies, by the hormones they secrete (Bentley, 1998), and by their locations in subdomains along the anteroposterior axis (Dasen and Rosenfeld, 2001; Herzog et al., 2003). Two cell types are located in the APD. One cell type expresses pomc that encodes Proopiomelanocortin (POMC). This pre-proprotein is differentially processed to form several functional peptides, such as Adrenocorticotropin (ACTH) that is secreted by corticotrope cells in the APD (Dasen and Rosenfeld, 2001). The second cell type in the APD are lactotropes that secrete Prolactin (Prl; Herzog et al., 2003). Three cell types are located in the PPD, thyrotropes that express thyroid-stimulating hormone β subunit (tsh-β), somatotropes that express growth hormone (gh; Herzog et al., 2003), and gonadotropes that secrete Follicle-Stimulating Hormone (FSH) or Luteinizing Hormone (LH). Like TSH, these hormones are glycoproteins composed of a common α-glycosylated subunit (α-GSU; encoded by cga) and a hormone specific β-subunit (Dasen and Rosenfeld, 2001). In the posterior most region of the anterior pituitary placode (PI), melanotropes express pomc and secrete the posttranslational POMC product, α-Melanocyte-Stimulating Hormone (α-MSH).
Recently, we have shown that long-range Hh signal specifies pituitary characteristics in a field of undifferentiated placodal precursor cells (Dutta et al., 2005). In addition, varying concentrations of Hh signal contribute to the regionalization of the pituitary placode into three subdomains (Herzog et al., 2003; Sbrogna et al., 2003). Presently, it is unclear how different cell fates are specified within the subdomains of the pituitary placode. During development of many organs, differentiated cell types emerge from a common field of progenitor cells by a mechanism termed "lateral inhibition.” During this process cells initially co-express Notch receptors and Delta ligands. The extracellular domain of the Delta ligand binds to the Notch receptor on adjacent cell surfaces. This interaction activates bidirectional signaling that ultimately results in unidirectional signaling between adjacent cells and adoption of distinct cell fates (Schweisguth, 2004). This direct cell-cell interaction through Delta-Notch signaling contributes to cell differentiation in a wide variety of organisms (Artavanis-Tsakonas et al., 1999).
Delta ligands are single pass transmembrane proteins present on the cell surface. Notch receptors are also transmembrane proteins with extracellular and intracellular domains. Furin cleaves the Notch extracellular domain and the two resulting peptides form a Notch heterodimer on the cell surface. Binding of Delta to Notch extracellular domain on neighboring cell surfaces triggers TACE metalloprotease mediated cleavage at an extracellular site of the Notch transmembrane domain (Brou et al., 2000). This cleavage facilitates removal of the Notch extracellular domain from the neighboring cell. Experiments in Drosophila and Xenopus indicate that ubiquitinylation of Delta and subsequent endocytosis of the Delta-Notch heterodimers play a key role in the activation of the Notch signaling cascade (Lai, 2002; Parks et al., 2000). Similarly, mind bomb (mib) mutant zebrafish embryos lack functional ubiquitin ligase, are unable to activate Notch signaling, and display a neurogenic phenotype (Itoh et al., 2003). Ubiquitinylation and sequestration of the Delta-Notch heterodimers activates cleavage of membrane bound Notch by the γ-secretase complex that releases the Notch intracellular domain (NICD). In the nucleus, NICD forms a functional transcription complex in association with Su(H), CBF1, and lag-1 proteins that subsequently regulates Notch target gene expression (De Strooper et al., 1999). Pharmacological treatment with N-[N-(3,5-difluorophenacetyl)-L-alanyl]-(S)-phenylglycine t-butyl ester (DAPT), a γ-secretase inhibitor, specifically prevents NICD formation (Dovey et al., 2001) and blocks Notch signal transduction thus leading to typical phenotypes consistent with loss of Notch signaling in zebrafish and Drosophila (Geling et al., 2002).
Prop1 controls pituitary progenitor cell differentiation and a hypomorphic mutation of Prop1 leads to pituitary hypoplasia by an unknown mechanism. A recent study in Prop1 mutant mice showed that Notch2, Delta like 3 (Dll3) expression is almost entirely absent in the pituitary, suggesting an indirect role of Notch in pituitary development (Raetzman et al., 2004). Overexpression of Notch2 delays gonadotrope development in the anterior lobe (Raetzman et al., 2006). Moreover, the transcriptional repressor Hes1 regulates survival and proliferation of Rathke’s Pouch precursor cells and is necessary for anterior lobe size. Hes1 is also necessary for melanotrope cell differentiation in the intermediate lobe (Raetzman et al., 2007). These studies suggest that downstream targets of Notch signaling regulate pituitary cell survival, proliferation, and differentiation in the anterior pituitary. However, direct functional studies of Notch signaling in pituitary cell type differentiation have not been carried out in all of the pituitary subdomains and it remains unclear which other cell types differentiate from delta or notch expressing progenitor cells. In the present study, we analyzed the role of Notch signaling in specification of zebrafish adenohypophysis cells because of their limited number, defined locations within pituitary placode subdomains, and experimental and genetic accessibility.
We show a direct role of the Notch signaling pathway in pituitary cell numbers and specification. We found that pituitary placode cells express delta and notch during mid-somitogenesis stages, consistent with their hypothesized role in cell differentiation or specification. To analyze Notch function, we characterized pituitary development in Notch gain and loss of function experiments. Altered Notch signaling does not affect pan-pituitary lim3 and pitx3 expression, suggesting that this short-range signal is not involved in initial formation of the pituitary preplacode or placode. However, once the placode has formed, Notch restricts specification of lactotropes and thyrotropes. Notch is also necessary and sufficient for specification of corticotropes and melanotropes, and it acts as a permissive factor for the differentiation of somatotropes. Block of Notch signaling at various developmental stages by DAPT suggests that Notch is necessary for cell type specification only during early somitogenesis stages, even though it is expressed in the pituitary until later stages.
Materials and Methods
Husbandry
Zebrafish (Danio rerio) embryos were obtained and maintained by standard procedures (Westerfield, 2007). Except for heat-shock experiments, embryos were maintained at 28.5°C. Embryos were staged by hours post fertilization (h) based on morphological staging criteria (Kimmel et al., 1995). Mutant strains used in this study were mind bomb (mibta52b; (Itoh et al., 2003) and after-eight (aeitr233; (Holley et al., 2000). We used two transgenic lines Tg(UAS:myc-Notch1a-intra)kca3 and Tg(hsp70l:Gal4)1.5kca4 (Scheer and Campos-Ortega, 1999). Embryos carrying either one or both of the transgenes were obtained either by incrossing Tg(hsp70l:Gal4)1.5kca4 carriers or by crossing Tg(UAS:myc-Notch1a-intra)kca3 with Tg(hsp70l:Gal4)1.5kca4 carriers (we abbreviate the transgenic lines as Tg(UAS:Notch1a) and Tg(hsp70:Gal4)).
Whole-mount mRNA in situ hybridization and immunohistochemistry
We analyzed gene expression by mRNA in situ hybridization with one or two mRNA probes (Hauptmann and Gerster, 2000). Antisense mRNA was in vitro synthesized using digoxygenin or fluorescein RNA labeling kits (Roche). For double in situ hybridization, the digoxygenin probe was detected using anti-digoxygenin-AP and NBT/BCIP as described earlier (Dutta et al., 2005). Fluorescein labeled probes were detected using anti-fluorescein-AP and INT/BCIP. We used probes for pitx3 and lim3 to label pituitary precursors and placode; and we used antisense mRNA probes for deltaA (dlA), deltaB (dlB), deltaC (dlC), deltaD (dlD), notch1a, notch1b, notch5, notch6 expression (Bierkamp and Campos-Ortega, 1993; Haddon et al., 1998; Westin and Lardelli, 1997). To analyze cell specification in the APD (lactotropes, corticotropes), PPD (somatotropes, thyrotropes, gonadotropes), and PI (melanotropes), we labeled pituitary hormone cell types at Prim-5 (24h) and Protruding mouth stage (72h) and used prl, gh, pomc, tsh-β and cga (previously α-gsu) mRNA probes (Herzog et al., 2003; Nica et al., 2004).
DAPT block of Notch function
We dechorionated wild-type embryos using 0.1mg/ml pronase in embryo medium at room temperature. 100 mM stock solution of the γ-secretase inhibitor (DAPT) was prepared in DMSO, stored at −20°C, and diluted to 100 µM in embryo medium. Dechorionated embryos were DAPT incubated in small Petri-dishes (3.5 cm diameter) at 28.5°C in the dark. Embryos (n=30) were incubated in 3 ml of 100 µM DAPT (Geling et al., 2002) at different developmental stages (Shield, 6h; Bud, 10h; 6-somite, 12h; 14-somoite, 16h; 18-somite, 18h; Prim-22, 36h; Long pec, 48h; Pec fin, 60h). Control embryos were incubated in embryo medium with DMSO (3 µL DMSO in 2,997 ml of embryo medium).
DAPT treatment was stopped at different stages of development (Prim-5, (24h) stage; Protruding mouth, (72h) stage) and embryos were rinsed several times in DMSO/embryo medium to remove DAPT completely. After rinsing, embryos were raised in embryo medium at 28.5°C until 72h. Embryos were fixed at Prim-5 (24h) and Protruding mouth (72h) stages in 4% paraformaldehyde and analyzed by in situ hybridization and antibody labeling.
Heat shock transactivation of Tg(UAS:myc-Notch1a-intra)
Groups of 5 to 10 transgenic embryos were heat shocked for 10 minutes at 40 °C in 0.25 ml PCR tubes using a thermocycler (PTC 200, MJ Research) heat block, which was programmed to maintain 40°C for 10 minutes and 28.5°C for 10 minutes. After the heat shock, embryos were removed from PCR tubes and maintained at 28.5°C (Scheer et al., 2001). Heat shocked, transgenic embryos Tg(UAS:myc-Notch1a-intra)kca3 × Tg(hsp70l:Gal4)1.5kca4 were identified by abnormal brain and somite morphology. We confirmed the presence of active Notch1a at different developmental stages by labeling transgenic embryos with anti-myc antibody that detects myc epitopes fused to Notch1a (Scheer et al., 2001).
mRNA injection
We injected 50 ng Xenopus dominant-negative Suppressor of Hairless XdnSu[H] (Wettstein et al., 1997), 40 ng Xenopus intracellular Notch XNIC1 (Chitnis et al., 1995) mRNA into 1-cell stage embryos. The mRNA was synthesized in-vitro using Message Machine kits (Ambion). Injected embryos were fixed in 4% paraformaldehyde at Bud, 14-somite, and Prim-5 stages.
Results
Zebrafish delta and notch genes are expressed in the pituitary placode
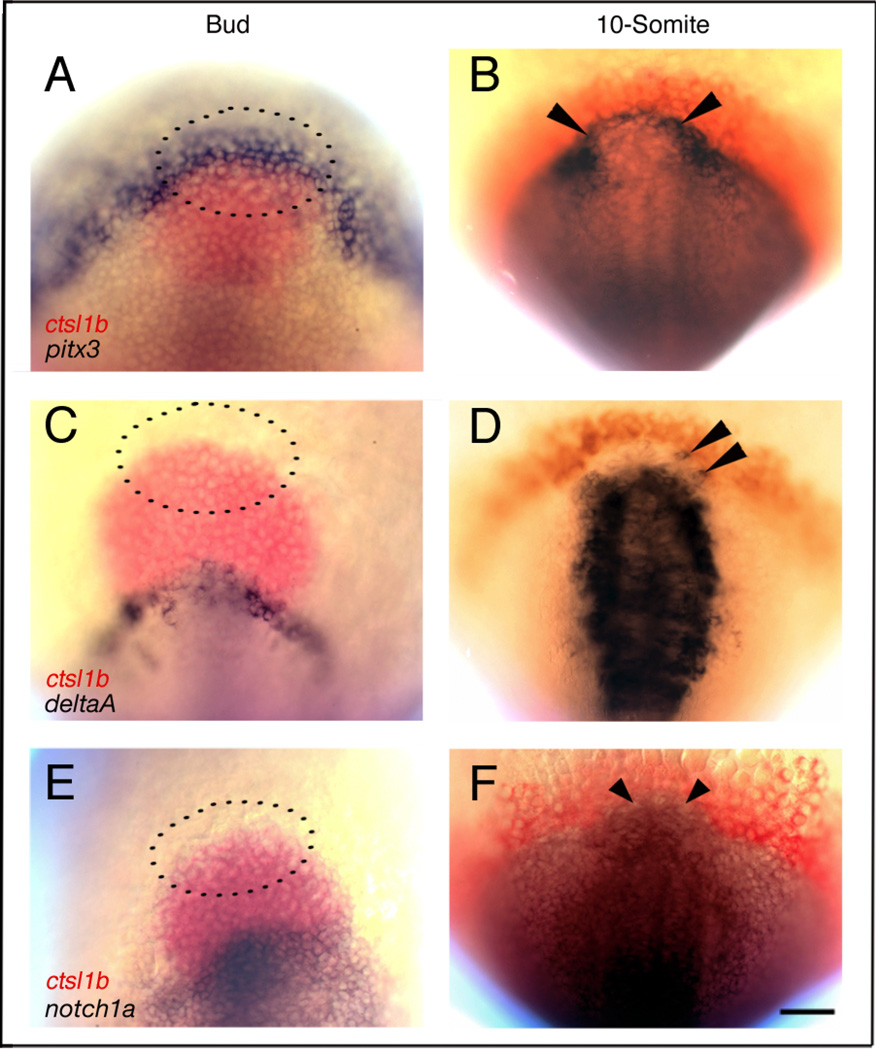
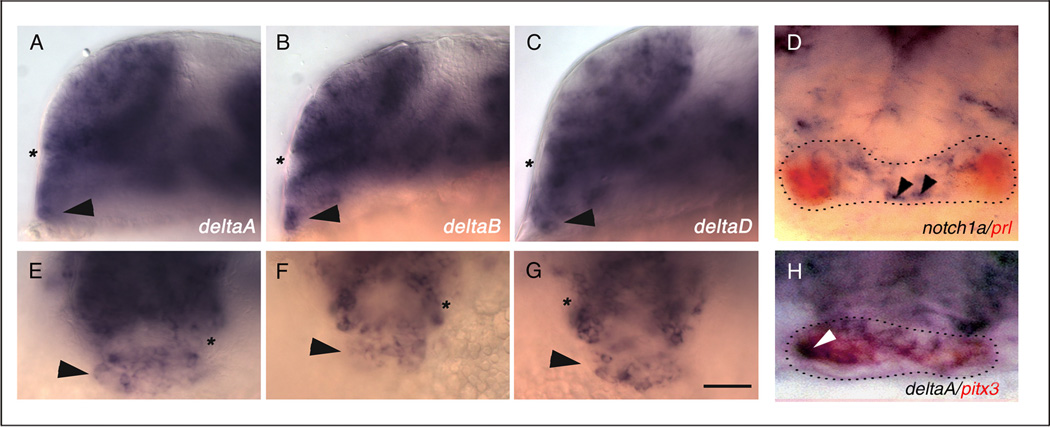
To determine when delta and notch genes are first expressed in anterior pituitary cells, we examined the expression patterns of zebrafish delta (dl) and notch genes at various developmental stages. At Bud (10h) stage, prospective pituitary precursor cells express pitx3 (Fig. 1A, area inside dotted lines; Dutta et al., 2005), however, they do not express dlA (Fig. 1C), dlB, dlC, dlD (data not shown), or notch1a (Fig. 1E), notch1b, notch5, or notch6 (data not shown). At the 10-somite (14h) stage, only a few presumptive anterior cranial placode precursors express dlA (Fig. 1D, arrowheads) and dlB (not shown). In contrast, notch1a (Fig. 1F), and notch1b (data not shown) are expressed more ubiquitously in anterior cranial placode precursors at the 10-somite stage (Dutta et al., 2005). At the 20-somite (19h) stage, after the placode has formed, pituitary cells express dlA (Fig. 2A,E,H), dlB (Fig. 2B,F), dlD (Fig. 2C,G) and the notch orthologs notch1a (Fig. 2D), notch1b, notch5 (not shown). At Prim-5 (24h) stage, some cells in the pitx3 expression domain also express dlA (Fig. 2H). However, cells expressing notch1a are excluded from the prl expression domain in the pituitary anlage (Fig. 2D). dl and notch gene expression persists in a ‘salt and pepper’ pattern throughout the pituitary anlage until Prim-22 (36h; data not shown), then decreases and is downregulated by the 48h stage (not shown). These results and our previous lineage analysis (Dutta et al., 2005) suggest that delta and notch are not expressed in pituitary precursor cells in the placodal field, but rather at and after the time the placode forms, and when the first pituitary cell types differentiate (Herzog et al., 2003).
Figure 1.
Prospective pituitary precursors express deltaA and notch1a after the 10-somite (14h) stage
Wild-type embryos doubly labeled with mRNA probes for cathepsin L,1b (ctsl1b), previously known as hatching gland gene 1(hgg1;A–F, red) and pitx3 (A,B), deltaA (C,D), or notch1a (E,F). (A,C,E) At Bud stage, presumptive pituitary precursor cells express pitx3 (A, n = 25; dotted lines indicate approximate location according to Dutta et al., 2005). deltaA (C, n=30) and notch1a (E, n=30) are not expressed in pituitary precursors. At 10-somite stage (14h), anterior cranial placode precursors (arrowheads) express pitx3 (B, n=25), deltaA (D, n=25), and notch1a (F, n=25). (A,C,E) Dorsal views, anterior to the top (dotted lines indicate approximate location of pituitary precursor cells according to Dutta et al., 2005); (B,D,F) dorsal views of prospective head region, anterior to the top. Scale bar: 50 µm.
Figure 2.
At the 20-somite (19h) stage, pituitary placode cells express deltaA, deltaB, and deltaD in a salt and pepper pattern. Wild-type embryos are labeled with probes for deltaA (A,E, n=19), deltaB (B,F, n=28), and deltaD (C,G, n=25). (D) Expression of notch1a (blue, n= 20) and prl (red, n=20) does not colocalize in the pituitary placode at prim-5 stage (24h). (H) Cells in the pituitary placode coexpress pitx3 (red, n=20) and deltaA (blue, n=20). (A– H) Arrowheads indicate cells in the pituitary placode, asterisk indicates optic recess. (A–C) Side views, anterior to the left, dorsal to the top; (D–H) frontal views, dorsal to the top. Scale bar: 50 µm (A–C, E–G), 20 µm (D,H).
Notch signaling specifies pituitary placode cell types
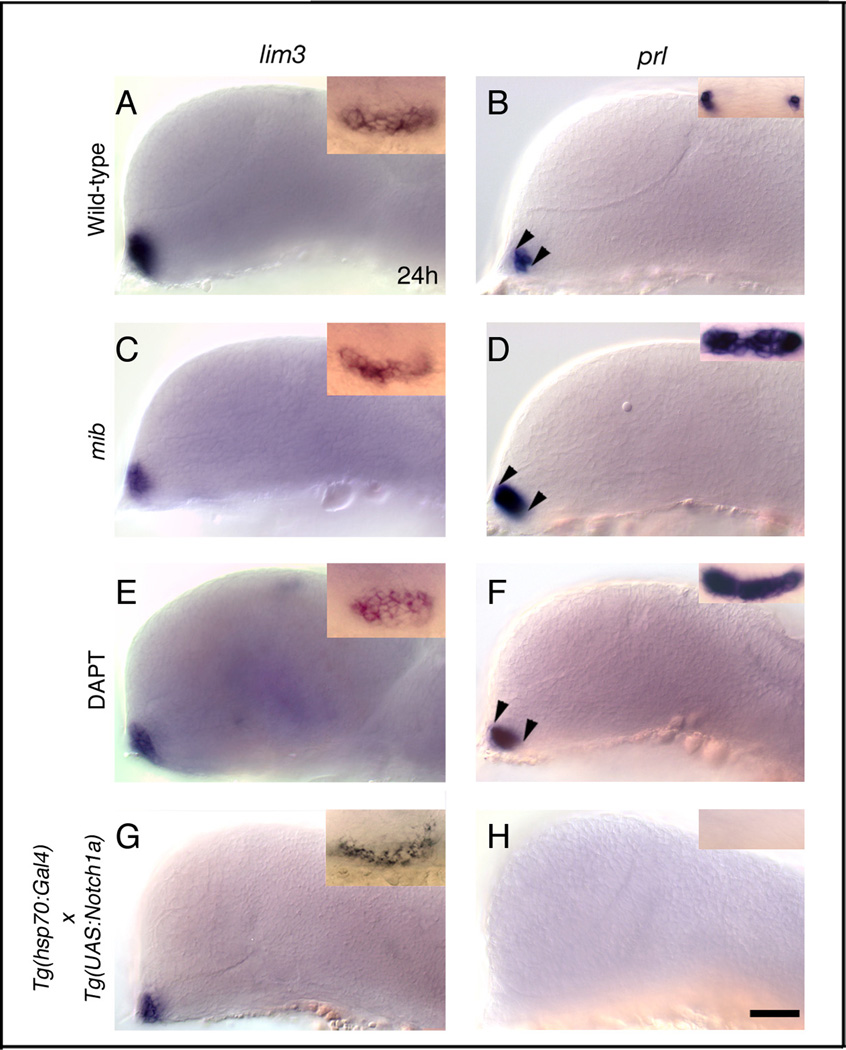
To test whether Notch signaling plays a role in pituitary placode formation, we over activated and blocked Notch signaling and analyzed expression of the pituitary placode marker genes lim3 and pitx3 by mRNA in situ hybridization. We previously showed that these two genes are expressed throughout the developing anterior pituitary (Dutta et al., 2005; Glasgow et al., 1997). In mib mutant embryos that lack functional Notch intracellular protein (NICD), expression of lim3 at Prim-5 (24h) stage (Fig. 3C) and pitx3 at Bud (10h) stage (not shown) is unaltered in pituitary placodes compared to wild-type siblings (Fig. 3A). Consistent with this result, embryos treated with DAPT between 30% epiboly and Prim-5 (24h) stage also show no significant change in lim3 (Fig. 3E) or pitx3 (not shown) expression compared to DMSO treated control embryos. These results suggest that pituitary placode formation and induction of placodal gene expression occur independently of Notch signaling.
Figure 3.
Notch is necessary and sufficient to restrict lactotrope differentiation in the anterior pituitary placode. (A,B) Wild-type embryos labeled with lim3 (A, n=85) and prl (B, n=96). (B, inset) Bilateral cells in the anterior pituitary placode express prl. Gene expression in DMSO treated (n=30), and hsp70:Gal4 heat-shocked (n=30) control embryos was similar to wild-type embryos in A and B. (A,C,E,G) Pituitary placode size and lim3 expression in mib mutant (C, n=39), in DAPT treated (E, n=45), and in heat shocked hsp70:Gal4 × UAS:notch1a (G, n=22) transgenic embryos is similar to controls (A,B). (B,D,F) Loss of functional Notch signaling in mib mutant (D, inset, n=37/37) and DAPT treated (F, inset, n=32/40) embryos leads to expansion of prl in pituitary placode (arrowheads; D,F). (H) prl expression in the anterior pituitary placode is completely lost in hsp70:Gal4 × UAS:notch1a transgenic embryos (n=48/48) following heat shock. (A–G) side views, anterior to the left, dorsal to the top; (A–G, inset) frontal views, dorsal to the top. All embryos Prim-5 (24h) stage. Scale bar: 50 µm (A–H); Insets A,C,E,G: 40 µm; Insets B,D,F,H: 30 µm.
Block of Notch signal transduction alters gene expression in the anterior pituitary anlage
The anterior pituitary contains hormone secreting cell types that express prl, pomc, gh, tsh-β, and cga (Herzog et al., 2003; Nica et al., 2004). Lactotropes express prl and differentiate first in the anterior pituitary placode at Prim-5 (24h) stage. To test whether Notch signaling plays a role in lactotrope cell differentiation, we analyzed prl expression in 24h mib mutants (Fig. 3D), DAPT treated (Fig. 3F), and wild-type sibling embryos (Fig. 3B). In mib mutants and DAPT treated embryos, we observed an expansion of the prl expression domain (Fig. 3D,F; arrowheads), although the size of the placode is largely unaffected. We also injected Xenopus dominant negative suppressor of hairless (XdnSu[H]) mRNA, which blocks the Notch pathway downstream of mib, and analyzed prl expression. At Prim-5 (24h) stage, we observed an expanded prl expression domain (data not shown) further supporting our interpretation that Notch signaling is necessary to restrict lactotrope cell differentiation.
To analyze whether Delta-Notch signaling plays a role in patterning and differentiation of other pituitary cell types, we analyzed hormone expression in aei mutant embryos that lack a functional dlD ligand (Holley et al., 2000). prl, pomc, gh, tsh-β, and cga expression in aei mutants is indistinguishable from wild-type siblings (data not shown). This result indicated that other Delta homologues might compensate for the loss of DeltaD ligand.
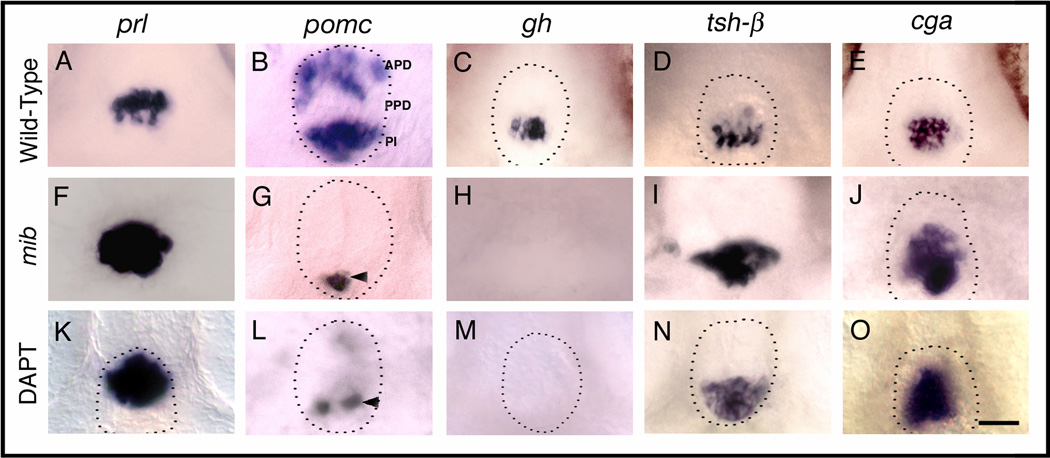
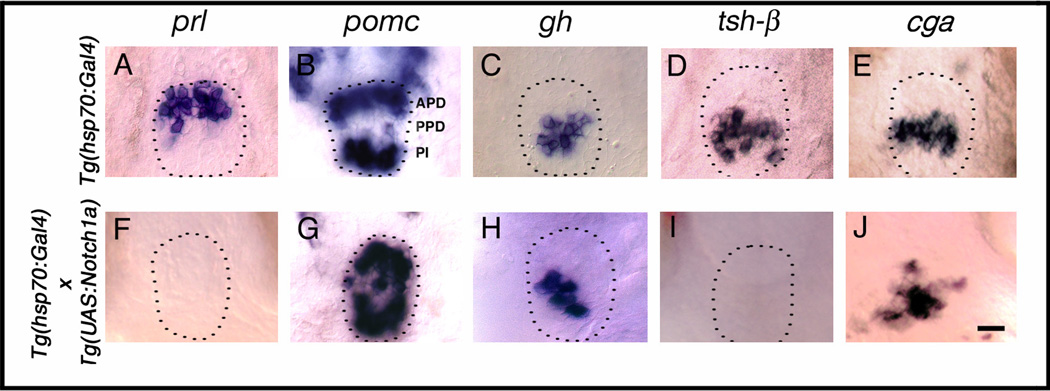
We further analyzed hormone gene expression at later stages in mib mutants when pituitary cells have differentiated. At Protruding mouth (72h) stage, we found that more cells express prl in the APD compared to wild-type siblings (Fig. 4A,F, Table 1). Conversely, pomc expression is lost in the APD cells in mib mutants compared to controls (Fig. 4B,G, Table 1), suggesting that in the APD, the number of prl and pomc expressing cells is controlled by short-range Delta- Notch signaling. In the PPD, mib mutant cells do not express gh (Fig. 4C,H, Table 1) but more cells express tsh-β (Fig. 4D,I, Table 1) and cga (Fig. 4E, Table 1) than in the wild-type siblings, indicating that Notch is necessary for differentiation of somatotropes in the PPD, whereas it restricts differentiation of thyrotropes and gonadotropes. In the PI, we found reduced pomc expression in mib mutant embryos (Fig. 4B,G) suggesting that Notch is required for differentiation of melanotropes. We also blocked Notch signaling by incubating embryos in 100 µM DAPT between shield (6h) stage and protruding mouth (72h) stage. We observed changes in the number of cells expressing prl (Fig. 4K, Table 2), pomc (Fig. 4L, Table 2), gh (Fig. 4M, Table 2), tsh-β (Fig. 4N, Table 2), and cga (Fig. 4O, Table 2) similar to the changes that occur in mib mutants.
Figure 4.
Notch signaling is necessary for corticotrope and somatotrope differentiation. (A–E) Wild-type (Gene expression in DMSO treated control embryos, not shown, was same as in wild-type embryos in A–E), mib mutant (F–J) and DAPT treated embryos (K–O) labeled with probes for anterior pituitary hormones at 72h. In mib mutant embryos, prl (F, n=25/25), tsh-β (I, n=23/23) and cga (J, n=25/25) in the anterior pituitary is expanded, pomc expression is lost in APD and reduced in PPD (G, n=26/26), and gh expression (H, n=52/52) is completely lost compared to prl (A, n=37), tsh-β (D, n=40) and cga (E, n=40), pomc (B, n=20), gh expression (C, n=40) in siblings. DAPT treatment between shield stage and 72h leads to expanded prl (K, n=26/33), tsh-β (n=50/60) and cga expression (O, n=35/42), whereas pomc expression in APD (L, n=23/25) is lost or severely reduced, pomc expression in PI (L, n=22/25) is somewhat reduced and gh expression (M, n=35/40) is entirely absent in the PPD compared to wild-type embryos (A–E). Dotted lines indicate outline of anterior pituitary tissue (omitted in panels where pituitary border was ambiguous). Arrowhead shows pomc expression in PI. (A–O) Ventral views, anterior to the top. Scale bar: 25 µm (A–O).
Table 1.
Pituitary Hormone Expressing Cell numbers are affected in mind bomb mutant and NICD overexpressing embryos.
| Hormone markers (pituitary subdomain) |
Wild-type and hsp70-gal4 controls ±S.D. |
mibP−/−P ±S.D. |
hsp70-gal4:UAS-Notch1a ±S.D. |
|
|---|---|---|---|---|
| prl | (APD) | 19±0 | 35±2 | 0±0 |
| pomc | (APD) | 18.4±0.9 | 0.6±0.9 | ≥30 |
| gh | (PPD) | 11±0.7 | 0±0 | 12.4±1.5 |
| tsh-β | (PPD) | 18±0.7 | 34±1.4 | 0±0 |
| cga | (PPD) | 19.6±0.9 | 29.2±0.5 | 20.6±1.5 |
| pomc | (PI) | 15.2±1 | 5.4±0.6 | ≥30 |
After in situ hybridization, we cryosectioned embryos and counted the number of cells expressing pituitary hormones. hsp70-gal4 control embryos and wild-type embryos had similar numbers of hormone expressing cells and the numbers were pooled. N=5 for wild-type, hsp70-gal4 controls, mibP−/−P, and NICD overexpressing embryos.
Table 2.
Notch Signal is necessary between 12h and 15h for pituitary cell specification
| Hormone expression at 72h compared to DMSO treated control embryos | |||||
|---|---|---|---|---|---|
| DAPT treatment |
Hormone | Expanded (%) |
Reduced (%) | Lost (%) |
Total Embryos |
| 6h – 72h | pomc anterior | - | - | 96 | 25 |
| prl | 78 | - | - | 45 | |
| gh | - | - | 76 | 63 | |
| tsh-β | 73 | - | - | 40 | |
| cga | 80 | - | - | 25 | |
| pomc posterior | - | 92 | - | 25 | |
| 10h – 72h | pomc anterior | - | - | 92 | 25 |
| prl | 77 | - | - | 60 | |
| gh | - | - | 75 | 60 | |
| tsh-β | 80 | - | - | 60 | |
| cga | 76 | - | - | 50 | |
| pomc posterior | - | 84 | - | 25 | |
| 12h – 72h | pomc anterior | - | - | 88 | 25 |
| prl | 84 | - | - | 44 | |
| gh | - | - | 77 | 30 | |
| tsh-β | 80 | - | - | 30 | |
| cga | 83 | - | - | 30 | |
| pomc posterior | - | 84 | - | 25 | |
| 16h – 72h | pomc anterior | - | - | - | 20 |
| prl | 2 | - | - | 55 | |
| gh | - | - | - | 32 | |
| tsh-β | 4 | - | - | 52 | |
| cga | 2 | - | - | 50 | |
| pomc posterior | - | - | - | 20 | |
| 6h – 40h | pomc anterior | - | - | 96 | 25 |
| prl | 82 | - | - | 34 | |
| gh | - | - | 76 | 29 | |
| tsh-β | 84 | - | - | 38 | |
| cga | 75 | - | - | 40 | |
| pomc posterior | - | 84 | - | 25 | |
DAPT treatment affects hormone expression in Protruding mouth (72h) stage embryos. The frequency of change in hormone gene expression domain was scored as expanded (as observed in Fig. 4K,N,O), reduced (as observed in Fig. 4L), or lost (as observed in Fig. 4M).
Notch signaling is necessary for pituitary cell specification before the placode forms
To learn when Notch signaling acts on pituitary cell differentiation, we blocked NICD formation by incubating embryos at various developmental stages in DAPT. In comparison to DMSO treated control embryos (data not shown), DAPT treatment starting at 6h, 10h, and 12h until 72h leads to expanded prl and tsh-β expression domains at Protruding mouth (72h) stage, loss of (or strongly reduced) pomc expression in the APD, complete loss of gh expression in the PPD, and reduced pomc expression in the PI (Table 2). In contrast, DAPT treatment after 16h does not affect hormone gene expression patterns (Table 2), suggesting that Notch function is necessary between 12h and 16h of development for the pituitary and that cell fates might already be determined by 16h.
dl and notch genes are expressed in the pituitary anlage until 36h and are later downregulated and completely absent from the pituitary primordium by 48h (data not shown). To investigate whether Delta and Notch proteins may also function during late stages of placode development, we blocked Notch signaling during the entire time when delta and notch genes are expressed in the placode (6h – 48h), and we blocked Notch signaling before hormone genes are expressed in the pituitary placode (6h – 22h).
First, we incubated embryos in DAPT from shield (6h) until 48h of development, removed DAPT, and allowed the embryos to recover and develop until protruding mouth (72h) stage. Control and DAPT treated embryos were fixed and analyzed for hormone gene expression. In DAPT treated embryos, prl and tsh-β expression is expanded, pomc expression is absent (in the APD) or reduced (in the PI), and gh is not expressed in PPD cells (Table 2). For the second series of tests, we incubated embryos in DAPT from shield (6h) stage until 22h, removed DAPT, and allowed the embryos to develop until Protruding mouth (72h) stage. We found that the pituitary hormone gene expression pattern in these embryos is indistinguishable from the control (data not shown). This suggests that NICD protein formed after 22h, before prl is expressed at Prim-5 (24h) stage, is sufficient to pattern the pituitary.
Our results suggest that 1) Notch is necessary only during pre-placode development (Table 2, Fig. 6), however not for placode formation per se or regionalization, but for cell specification in the prospective pituitary subdomains. 2) Block of NICD just before the placode forms and later has no effect on pituitary cell differentiation (DAPT from 15h and later, until 72h). 3) Once NICD has formed, it is sufficient to pattern the pituitary (DAPT from 6h – 22h; no DAPT from 22h until 72h).
Figure 6.
Delta-Notch Signaling acts during a critical 2-hour time-period to specify pituitary placodal cell fates. Adenohypophysis placode cells express delta and notch homologs between 15h –48h. delta and notch expression begins around 14h in the immature placode. Delta-Notch expression is downregulated by 48h when the three subdomains APD, PPD, and PI are composed of terminally differentiated pituitary cell types. Long-range signals are thought to regionalize the placode into three subdomains, whereas short-range delta notch signals specify the cell-types within each subdomain. The black and grey stripes above the timeline indicate delta and notch expression between 15h–48h post fertilization. The sketch below the timeline indicates the position of the immature pituitary placode (left, green) around 19h. The magnification of the placode indicates the regionalized placode (middle) and the regionalized adenohypophysis with specified cell types (right).
Increased Notch signaling increases the number of pomc and cga cells at the expense of prl and tsh-β cells
To test whether gain of Notch function is sufficient to regulate gene expression in pituitary cell types, we used two transgenic lines, one that contains the heat shock inducible hsp70:Gal4 and another with the Gal4 responsive UAS:notch1aICD element. Embryos were heat shocked at shield stage and maintained until Bud (10h), or Prim-5 (24h) stage at 28.5°C, fixed, and hybridized with mRNA probes for pitx3 and lim3. At Bud (10h) stage, hsp70:Gal4 control and hsp70:Gal4 × UAS:notch1a NICD overexpressing embryos display similar pitx3 expression patterns (data not shown). At Prim-5 (24h) stage, the lim3 expression pattern in the pituitary of heat-shocked hsp70:Gal4 × UAS:notch1a transgenic embryos (Fig. 3G) is similar to that of hsp70:Gal4 control embryos (Fig. 3A). We also injected mRNA encoding Xenopus Notch intracellular protein (XNIC), which constitutively activates Notch signaling, and analyzed pitx3 and lim3 expression at Prim-5 (24h) stage. We found that both pitx3 and lim3 expression patterns are similar to controls (data not shown), suggesting that excess Notch signaling is not sufficient to initiate additional placode formation or progenitor cell specification. We confirmed by myc immunolabeling that NICD was formed in embryos after heat shock at Bud stage and persisted until 5 days of development.
To analyze the role of Notch in pituitary cell differentiation, we transactivated NICD at Bud (10h) stage before the placode forms and analyzed hormone gene expression at Prim-5 (24h) stage or Protruding mouth (72h) stage. We found that in the APD of heat-shocked hsp70:Gal4 × UAS:notch1a embryos, prl expression is lost at the Prim-5 (24h) stage (Fig. 3H, Table 1, Table 3) and at the Protruding mouth (72h) stage (Fig. 5F, Table 1, Table 3). Similarly, tsh-β (Fig. 5I) expression is lost or strongly reduced in the PPD. In contrast, pomc expression is expanded in the APD, PPD, and PI (Fig. 5G, Table 3). Increased numbers of cells express cga in the PPD (Fig. 5J). However, gh expression remains largely unaffected in NICD overexpressing embryos (Fig. 5H, Table 1, Table 3). Thus, overactivation of Notch increases the numbers of pomc and cga expressing cells at the expense of prl and tsh-β.
Table 3.
Overexpression of NICD between 10h and 24h, but not after 36h, affects pituitary hormone expression
| Hormone expression in Tg(hsp70:GAL4) × Tg(UAS:Notch1a) embryos compared to Tg(hsp70:GAL4) control embryos | |||||
|---|---|---|---|---|---|
| Heat-Shock at |
Hormone | Expanded (%) |
Reduced (%) |
Lost (%) |
Total Embryos |
| 10h | pomc anterior | 95 | - | - | 25 |
| prl | - | - | 98 | 89 | |
| gh | - | - | - | 68 | |
| tsh-β | - | - | 100 | 57 | |
| cga | 90 | - | - | 30 | |
| pomc posterior | 95 | - | - | 25 | |
| 18h | pomc anterior | 93 | - | - | 30 |
| prl | - | - | 100 | 80 | |
| gh | - | - | - | 38 | |
| tsh-β | - | - | 100 | 50 | |
| cga | 100 | - | - | 40 | |
| pomc posterior | 93 | - | - | 30 | |
| 24h | pomc anterior | 90 | - | - | 40 |
| prl | - | - | 96 | 25 | |
| gh | - | - | - | 25 | |
| tsh-β | - | - | 96 | 25 | |
| cga | 90 | - | - | 20 | |
| pomc posterior | 90 | - | - | 40 | |
| 36h | pomc anterior | - | - | - | 50 |
| prl | - | - | - | 55 | |
| gh | - | - | - | 55 | |
| tsh-β | - | - | - | 50 | |
| cga | - | - | - | 50 | |
| pomc posterior | - | - | - | 55 | |
Figure 5.
Gain of Notch function increases the number of pomc expressing corticotropes and melanotropes at the expense of lactotropes and thyrotropes. Embryos carrying either the hsp70:Gal4 or the hsp70:Gal4 × UAS:notch1a transgenes were heat shocked at Bud (10h) stage and analyzed for hormone expression at Protruding mouth (72h) stage (A–J). In heat-shocked hsp70:Gal4 × UAS:notch1a transgenic embryos, both prl (F, n= 36/36) and tsh-β (I, n=42/42) are completely lost, pomc expression is expanded in APD, PPD and PI (G, n=32/32), and cga is expanded (J, n=15/17). gh expression is unaffected (H, n=40/40) compared to prl (A, n=34) and tsh-β (D, n=32), pomc (B, n=37), gh (C, n=30) and cga (E, n=20) in hsp70:Gal4 embryos following heat shock. Dotted lines indicate outline of anterior pituitary tissue (omitted in panels where pituitary border was ambiguous). (A–J) Ventral views anterior to the top. Scale bar: 15 µm (A–J)
To determine the time when Notch1a protein modulates cell type specification in the adenohypophysis, we heat shocked hsp70:Gal4 × UAS:notch1aICD embryos at various developmental stages and analyzed the pattern of pituitary hormone gene expression at 72h. We found that transactivation of NICD in Bud (10h), 18-somite (18h), and Prim-5 (24h) stage leads to complete loss of prl and tsh-β and expansion of pomc and cga. gh expression in the PPD, however, remains unaffected (Table 2). We observed no changes in hormone gene expression in TG(hsp70:Gal4) control embryos (data not shown) or in TG(hsp70:Gal4 × UAS:notch1aICD) embryos that are heat shocked after 36h of development. These results further support our conclusion that Notch overexpression increases the numbers of pomc and cga expressing cells in the pituitary placode at the expense of prl and tsh-β expressing cells. Our previous observations, based on blocking Notch signaling, suggested that cell fates are already determined by 16h. Overactivation of Notch signaling, on the other hand, suggests that some cell fates are not determined until after 24h.
Discussion
The zebrafish Adenohypophysis consists of three subdomains (Fig. 6) that are characterized by a) prl (lactotrope) and pomc (corticotrope) expression (APD); b) gh (somatotrope), tsh-β (thyrotrope), and cga (gonadotrope, thyrotrope) expression (PPD); and c) pomc (melanotrope) expression (PI; Herzog et al., 2003). These subdomains develop from a field of pituitary precursor cells that initially have a uniform pattern of gene expression and developmental potential (Dutta et al., 2005). In the present study, we analyzed how different pituitary cell types are specified within the anterior pituitary subdomains and whether short-range cell-to-cell interactions mediated by Delta and Notch play a role in the process. Our results show that Delta and Notch signaling is not required for pituitary precursor cell specification or placode formation. However, at the time the placode forms, Notch is necessary for pituitary cell differentiation of somatotropes, corticotropes, and melanotropes, while restricting differentiation of lactotropes, thyrotropes, and gonadotropes. Further, we found that gain of Notch function is sufficient to induce specification of additional corticotrope and melanotrope cells.
Long-range and short-range signals regionalize and pattern the adenohypophysis
Previous studies have implicated several signaling pathways in patterning and cell type specification of the anterior pituitary gland (Dutta et al., 2005; Ericson et al., 1998; Herzog et al., 2003; Rones et al., 2000; Sbrogna et al., 2003). Hh signaling is important for ventral cell type (gonadotropes, thyrotropes) specification in Rathke’s pouch (Treier et al., 2001), and dorsoventral FGF and ventrodorsal BMP2 gradients regionalize Rathke’s pouch leading to formation of specified cell types in the subdomains of the mouse anterior pituitary (Dasen and Rosenfeld, 2001). In zebrafish, Hedgehog (Hh) plays a key role in specifying cells in the preplacode (Dutta et al., 2005; Karlstrom et al., 1999; Sbrogna et al., 2003). Hh also regionalizes the placode into subdomains (Herzog et al., 2003).
Recently, several studies reported that Notch signaling might play a role in mouse pituitary development (Raetzman et al., 2004; Raetzman et al., 2007; Raetzman et al., 2006). These studies indicate that Notch2 regulates cell proliferation, survival, and differentiation of two or more cell types in the anterior lobe. Based on our analysis in zebrafish, we suggest that Delta-Notch signaling plays a general role in regulating cell numbers and specifying cell fates within the three pituitary subdomains, in which cells are presumably exposed to the same concentrations of long-range signaling molecules. Delta ligands and Notch receptors (Fig. 1, Fig. 2) are initially expressed in presumptive pituitary precursors, at the 10-somite stage, before the placode forms and expression persists until 36h in the pituitary anlage. In Prim-5 (24h) stage mib mutant and DAPT treated embryos, we did not observe any significant changes in lim3 expression patterns or placode size, suggesting that the placode forms independently of Delta-Notch signaling, and that in contrast to mice (Raetzman et al., 2007), Delta-Notch signaling does not regulate overall placode cell proliferation in zebrafish (Fig. 3). We suggest that Notch signaling acts on cell specification at or after the time the pituitary placode has formed. This observation is consistent with the role of Notch signaling in cell type specification in the Xenopus heart field (Rones et al., 2000).
Because long-range signaling mechanisms also contribute to pituitary regionalization and cell specification (Dasen and Rosenfeld, 2001; Dutta et al., 2005; Herzog et al., 2003; Sbrogna et al., 2003; Treier et al., 2001), we suggest that Notch signaling is a permissive factor for pituitary patterning and cell specification.
Notch regulates cell specification in anterior pituitary subdomains
In mouse, the pituitary gland develops from Rathke’s pouch, whereas the zebrafish pituitary derives from a placode that forms from the anterior ventral ectoderm (Glasgow et al., 1997; Dasen and Rosenfeld, 2001). In zebrafish, lactotrope cells that express prl are the first hormone producing cells to differentiate in the APD subdomain of the anterior pituitary, pomc expressing corticotropes differentiate shortly later at the Prim-5 (24h) stage (Herzog et al., 2003). At Prim-5 (24h) and at Protruding mouth (72h) stages, we observed expansion of the prl expression domain at the expense of pomc when Notch function is reduced (Fig. 3, Fig. 4). This suggests that Notch is necessary for the specification of pomc expressing corticotrope cells in the APD. Consistent with this idea, Notch gain of function experiments show a reverse phenotype; in the APD, NICD overexpression leads to an expansion of pomc expression at the expense of prl. Based on these observations, and additionally because deltaA is expressed in many cells throughout the placode whereas notch1a is not expressed in prl expressing cells (Fig. 2), we propose that in the APD, Delta expressing cells adopt the fate of lactotrope cells, whereas Notch positive cells differentiate as corticotropes.
In the PPD, block of NICD similarly leads to expansion of tsh-β and cga, and loss of gh expression. However, overexpression of NICD also expands the number of cga cells at the expense of tsh-β cells, whereas gh expression remains largely unaffected. Thus, in the PPD, Delta-Notch function may be insufficient to explain how these three cell types are specified. Both thyrotrope and gonadotrope cell types express cga (Nica et al., 2004). Thus, in the absence of more specific cell markers, we are unable to distinguish whether expansion of cga in embryos with loss of functional Notch protein (Fig. 4J,O) is due to increased numbers of thyrotrope and/or gonadotrope cell types. In the posterior subdomain (PI), pomc is reduced in the absence of Notch function but expanded when NICD is overexpressed. Our results suggest that Delta and Notch regulate choices between different cell fates in subdomains of the pituitary, presumably by lateral inhibition as described in other sites of Delta and Notch signaling (Artavanis-Tsakonas et al., 1999).
Based on our observations in zebrafish, we suggest that lactotropes (prl) and thyrotropes (tsh-β) constitute primary cell fates whereas corticotropes/melanotropes (pomc) are secondary cell fates. This is consistent with previous results in mouse (Raetzmann et al., 2007), indicating that Notch signaling leads to Hes1 expression, which in turn blocks Pit1 that is necessary for GH, PRL, and TSH expression. In zebrafish however, gh and cga expression is not entirely consistent with the Notch signaling hypothesis when Notch signaling is blocked or upregulated (Fig. 4 and 5) as predicted by similar experiments in mouse. This indicates either that these cell types might be regulated by other, additional signals, or that Notch signal strength or timing needs to be more finely adjusted in zebrafish.
Our study does not address conclusively, whether or not particular delta or notch genes play roles in the differentiation of a particular pituitary cell type. Our analysis of delta expression in the pituitary placode shows that none of these genes is restricted to any particular subdomain (Fig. 2). Whereas notch1a is not expressed in prl positive cells, other notch paralogs are ubiquitously expressed in the placode and surrounding tissues. Therefore, it may be less likely that any particular delta or notch gene (except for notch1a) regulates the switch between cell fates in a subdomain. Nevertheless, our analyses show that Notch in general is necessary for the differentiation of some pituitary cells and that delta genes can compensate for loss of a single delta ortholog, such as deltaD. Thus, our study demonstrates that Delta-Notch interaction plays a direct role in pituitary cell patterning and differentiation.
Delta-Notch signaling acts on pituitary cell specification during a critical developmental time-period
Our expression analysis indicates that delta and notch are not expressed in preplacodal pituitary precursors. Inhibition of Notch signaling at various developmental stages supports the idea that Notch is not necessary for placode formation per se, but functions in hormone cell specification. DAPT treatment at early stages, shortly before the placode forms, leads to significant changes in anterior pituitary hormone gene expression, similar to mib mutants. However, later block of Notch signal, after the onset of delta and notch gene expression in the pituitary (at 15h as analyzed by in situ hybridization), results in a hormone expression pattern similar to wild-type embryos (Fig. 6). Thus, our results suggest that once functional Notch protein has been synthesized, NICD is sufficient to specify cells in the adenohypophysis without additional NICD at later stages. This suggests that Notch signaling specifies cell types in the anterior pituitary that cell fates are already determined by 16h, and that continued Notch expression is not necessary for the later differentiation of additional cells after the placode has formed. Presumably, NICD is not immediately degraded and enough protein might initially be produced to achieve normal pituitary patterning until all the various hormone producing cell types are formed. This idea is supported by our observation that after heat shock at Bud stage, myc labeling persists until 5 days of development.
However, a transient block of Notch signaling between 6h and 22h does not affect the ratio of pituitary cell types, indicating a delay in the timing of cell determination under these conditions. In addition, overexpression of NICD (Table 3), even after 24h, leads to increased numbers of pomc and gh expressing cells at the expense of prl and tsh-β expression, suggesting that these cell types are not determined at this stage. A possible explanation for these observations is that once cells have been directed to express pomc or gh by high Notch activity, these fates are stable against loss of Notch activity after 16h. However, cells permitted by block of Notch signaling to express prl and tsh-β may remain plastic and may be induced to express pomc and gh as late as 24h by increased Notch activity.
The mechanisms that regulate later development of the pituitary are unclear. Expression of delta and notch expression is down regulated by 48h even though cells continue to proliferate after this stage; adult pituitaries are considerably larger in size and cell number (unpublished observations S. Toro and Z.M. Varga). Apparently, later born cells are specified in the absence of Delta-Notch signaling. It is possible that terminally differentiated, hormone producing pituitary cell types, or pools of specified stem cells generated before 48h, maintain their mitogenic potential and continue to produce additional daughter cells of their own kind. An alternative explanation is that other signals govern pituitary cell specification after 48h.
Additional signaling and morphogenetic mechanisms may help pattern the anterior pituitary
Our study does not address whether changes in anterior pituitary patterning occur directly due to increased or decreased Notch in pituitary cells alone or due to secondary changes in long-range signals from neighboring tissues that also express delta and notch. The primordia of adenohypophysis and hypothalamus associate by morphogenetic movements during development to form the hypothalamic-pituitary axis. Cell lineage analyses in birds and zebrafish support the view that the precursors of adenohypophysis and hypothalamus originate from distinct ectodermal positions (Couly and Le Douarin, 1985; Dutta et al., 2005; Varga et al., 1999). Several studies also indicated that development of the hypothalamus and pituitary are interdependent (Kawamura and Kikuyama, 1995; Kawamura and Kikuyama, 1998; Watanabe, 1996). These studies suggested that contact between adenohypophyseal primordium and ventral diencephalon is indispensable for the proliferation and differentiation of adenohypophyseal hormone-producing cells. This interdependence is presumably dependent on long-range signaling events necessary for proper regionalization, patterning, and cell-type specification in these tissues. Consistent with this idea, Nodal and Hh mutants that have defects in long-range signals lack hypothalamus and pituitary (Sbrogna et al., 2003; Varga et al., 2001; Zilinski et al., 2005) or have reduced pituitary cell types (Treier et al., 2001).
delta and notch genes are expressed in many tissues close to the pituitary anlage including the hypothalamus (Fig. 2). In our study, DAPT and heat-shock presumably affected all tissues including those near the pituitary. Thus, at present, we can not distinguish, whether anterior pituitary patterning changes might occur secondarily due to Delta or Notch induced changes in long-range signals such as Bmp, Fgf, or Shh from neighboring tissues. However, the analysis of bmp, fgf, and hh expression does not indicate any obvious changes in the expression pattern of these genes (unpublished observations J.E.D., S.D., and Z.V.).
At least two distinct morphogenetic movements could also pattern the pituitary. The first is the invagination of the oral ectoderm and the concomitant movement of pituitary placode ventral to the hypothalamus (Herzog et al., 2003). A second morphogenetic movement is plausible, because pomc expressing cells initially form a single domain, and later pomc positive cells populate the APD and the PI (Herzog et al., 2003). In the absence of tracing cell lineages and movements, it remains unclear whether or not Delta and Notch directly or indirectly (via other long range signals) affect this hypothetical second movement of pomc expressing cells within the pituitary placode. However, lim3 expression suggests that the first movement, the invagination of oral ectoderm, and the ventral-posterior movement of the anterior pituitary placode occur independently of Delta-Notch signaling.
Our study indicates that the three pituitary subdomains are analogous to “proneural fields” and that Delta-Notch interactions regulate specification of cell fates within them. We showed by gain and loss of Notch function that pituitary patterning is regulated by Notch, consistent with the idea that cell differentiation within the subdomains is regulated by “lateral inhibition.” Notch acts on early placodal cells, promotes differentiation of somatotropes, corticotropes, and melanotropes, and restricts differentiation of lactotropes, thyrotropes, and gonadotropes.
Acknowledgements
We thank Igor Dawid, Stefan Hans, Dong Liu, and Sabrina Toro for discussion and critical comments on the manuscript. We thank Judith Eisen, Alexandra Tallafuss, Haruki Ochi, Julian Lewis, Michael Lardelli, Wolfgang Driever, Alida Filippi, Soojin Ryu, Brant Weinstein, Matthias Hammerschmidt and the Zebrafish International Resource Center for plasmids, antibodies, and transgenic fish lines. Supported by NIH HD22486 and DC04186, S.D. supported by NIH DC04186 and DFG SFB 592/A5; Z.V. supported by NIH RR12546 and DFG SFB 592/A5.
References
- Artavanis-Tsakonas S, Rand MD, Lake RJ. Notch signaling: cell fate control and signal integration in development. Science. 1999;284:770–776. doi: 10.1126/science.284.5415.770. [DOI] [PubMed] [Google Scholar]
- Bentley PJ. "Comparative Vertebrate Endocrinology.". Cambridge: Cambridge University Press; 1998. [Google Scholar]
- Bierkamp C, Campos-Ortega JA. A zebrafish homologue of the Drosophila neurogenic gene Notch and its pattern of transcription during early embryogenesis. Mechanisms of Development. 1993;43:87–100. doi: 10.1016/0925-4773(93)90027-u. [DOI] [PubMed] [Google Scholar]
- Brou C, Logeat F, Gupta N, Bessia C, LeBail O, Doedens JR, Cumano A, Roux P, Black RA, Israel A. A novel proteolytic cleavage involved in Notch signaling: the role of the disintegrin-metalloprotease TACE. Mol Cell. 2000;5:207–216. doi: 10.1016/s1097-2765(00)80417-7. [DOI] [PubMed] [Google Scholar]
- Chitnis A, Henrique D, Lewis J, Ish-Horowicz D, Kintner C. Primary neurogenesis in Xenopus embryos regulated by a homologue of the Drosophila neurogenic gene Delta. Nature. 1995;375:761–766. doi: 10.1038/375761a0. [DOI] [PubMed] [Google Scholar]
- Couly G, Le Douarin NM. The fate map of the cephalic neural primordium at the presomitic to the 3-Somite stage in the avaian embryo. Development. 1988;103:101–113. doi: 10.1242/dev.103.Supplement.101. [DOI] [PubMed] [Google Scholar]
- Couly GF, Le Douarin NM. Mapping of the early neural primordium in quail-chick chimeras. I. Developmental relationships between placodes, facial ectoderm, and prosencephalon. Dev Biol. 1985;110:422–439. doi: 10.1016/0012-1606(85)90101-0. [DOI] [PubMed] [Google Scholar]
- Dasen JS, Rosenfeld MG. Signaling and transcriptional mechanisms in pituitary development. Annu Rev Neurosci. 2001;24:327–355. doi: 10.1146/annurev.neuro.24.1.327. [DOI] [PubMed] [Google Scholar]
- De Strooper B, Annaert W, Cupers P, Saftig P, Craessaerts K, Mumm JS, Schroeter EH, Schrijvers V, Wolfe MS, Ray WJ, Goate A, Kopan R. A presenilin-1-dependent gamma-secretase-like protease mediates release of Notch intracellular domain. Nature. 1999;398:518–522. doi: 10.1038/19083. [DOI] [PubMed] [Google Scholar]
- Dovey HF, John V, Anderson JP, Chen LZ, de Saint Andrieu P, Fang LY, Freedman SB, Folmer B, Goldbach E, Holsztynska EJ, Hu KL, Johnson-Wood KL, Kennedy SL, Kholodenko D, Knops JE, Latimer LH, Lee M, Liao Z, Lieberburg IM, Motter RN, Mutter LC, Nietz J, Quinn KP, Sacchi KL, Seubert PA, Shopp GM, Thorsett ED, Tung JS, Wu J, Yang S, Yin CT, Schenk DB, May PC, Altstiel LD, Bender MH, Boggs LN, Britton TC, Clemens JC, Czilli DL, Dieckman-McGinty DK, Droste JJ, Fuson KS, Gitter BD, Hyslop PA, Johnstone EM, Li WY, Little SP, Mabry TE, Miller FD, Audia JE. Functional gamma-secretase inhibitors reduce beta-amyloid peptide levels in brain. J Neurochem. 2001;76:173–181. doi: 10.1046/j.1471-4159.2001.00012.x. [DOI] [PubMed] [Google Scholar]
- Dutta S, Dietrich JE, Aspock G, Burdine RD, Schier A, Westerfield M, Varga ZM. pitx3 defines an equivalence domain for lens and anterior pituitary placode. Development. 2005;132:1579–1590. doi: 10.1242/dev.01723. Epub 2005 Feb 23. [DOI] [PubMed] [Google Scholar]
- Eagleson GW, Harris WA. Mapping of the presumptive brain regions in the neural plate of Xenopus laevis. J Neurobiol. 1990;21:427–440. doi: 10.1002/neu.480210305. [DOI] [PubMed] [Google Scholar]
- Ericson J, Norlin S, Jessell T, Edlund T. Integrated FGF and BMP signaling controls the progression of progenitor cell differentiation and the emergence of pattern in the embryonic anterior pituitary. "Development". 1998;Vol. 125:1005–1015. doi: 10.1242/dev.125.6.1005. [DOI] [PubMed] [Google Scholar]
- Geling A, Steiner H, Willem M, Bally-Cuif L, Haass C. A gamma-secretase inhibitor blocks Notch signaling in vivo and causes a severe neurogenic phenotype in zebrafish. EMBO Rep. 2002;3:688–694. doi: 10.1093/embo-reports/kvf124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasgow E, Karavanov AA, Dawid IB. Neuronal and Neuroendocrine Expression of Lim3, a Lim Class Homeobox Gene, Is Altered in Mutant Zebrafish With Axial Signaling Defects. Developmental Biology. 1997;192:405–419. doi: 10.1006/dbio.1997.8761. [DOI] [PubMed] [Google Scholar]
- Haddon C, Smithers L, Schneider-Maunoury S, Coche T, Henrique D, Lewis J. Multiple delta genes and lateral inhibition in zebrafish primary neurogenesis. Development. 1998;125:359–370. doi: 10.1242/dev.125.3.359. [DOI] [PubMed] [Google Scholar]
- Hauptmann G, Gerster T. Regulatory gene expression patterns reveal transverse and longitudinal subdivisions of the embryonic zebrafish forebrain. Mech Dev. 2000;91:105–118. doi: 10.1016/s0925-4773(99)00277-4. [DOI] [PubMed] [Google Scholar]
- Herzog W, Zeng X, Lele Z, Sonntag C, Ting JW, Chang CY, Hammerschmidt M. Adenohypophysis formation in the zebrafish and its dependence on sonic hedgehog. Dev Biol. 2003;254:36–49. doi: 10.1016/s0012-1606(02)00124-0. [DOI] [PubMed] [Google Scholar]
- Holley SA, Geisler R, Nusslein-Volhard C. Control of her1 expression during zebrafish somitogenesis by a delta-dependent oscillator and an independent wave-front activity. Genes Dev. 2000;14:1678–1690. [PMC free article] [PubMed] [Google Scholar]
- Itoh M, Kim CH, Palardy G, Oda T, Jiang YJ, Maust D, Yeo SY, Lorick K, Wright GJ, Ariza-McNaughton L, Weissman AM, Lewis J, Chandrasekharappa SC, Chitnis AB. Mind bomb is a ubiquitin ligase that is essential for efficient activation of Notch signaling by Delta. Dev Cell. 2003;4:67–82. doi: 10.1016/s1534-5807(02)00409-4. [DOI] [PubMed] [Google Scholar]
- Karlstrom RO, Talbot WS, Schier AF. Comparative synteny cloning of zebrafish you-too: mutations in the Hedgehog target gli2 affect ventral forebrain patterning. Genes Dev. 1999;13:388–393. doi: 10.1101/gad.13.4.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamura K, Kikuyama S. Induction from posterior hypothalamus is essential for the development of the pituitary proopiomelacortin (POMC) cells of the toad (Bufo japonicus) Cell Tissue Res. 1995;279:233–239. doi: 10.1007/BF00318479. [DOI] [PubMed] [Google Scholar]
- Kawamura K, Kikuyama S. Morphogenesis of the hypothalamus and hypophysis: their association, dissociation and reassociation before and after "Rathke". Arch Histol Cytol. 1998;61:189–198. doi: 10.1679/aohc.61.189. [DOI] [PubMed] [Google Scholar]
- Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF. Stages of embryonic development of the zebrafish. Develop Dynam. 1995;203:253–310. doi: 10.1002/aja.1002030302. [DOI] [PubMed] [Google Scholar]
- Lai EC. Protein degradation: four E3s for the notch pathway. Curr Biol. 2002;12:R74–R78. doi: 10.1016/s0960-9822(01)00679-0. [DOI] [PubMed] [Google Scholar]
- Nica G, Herzog W, Sonntag C, Hammerschmidt M. Zebrafish pit1 mutants lack three pituitary cell types and develop severe dwarfism. Mol Endocrinol. 2004;18:1196–1209. doi: 10.1210/me.2003-0377. Epub 2004 Feb 26. [DOI] [PubMed] [Google Scholar]
- Parks AL, Klueg KM, Stout JR, Muskavitch MA. Ligand endocytosis drives receptor dissociation and activation in the Notch pathway. Development. 2000;127:1373–1385. doi: 10.1242/dev.127.7.1373. [DOI] [PubMed] [Google Scholar]
- Raetzman LT, Cai JX, Camper SA. Hes1 is required for pituitary growth and melanotrope specification. Dev Biol. 2007;304:455–466. doi: 10.1016/j.ydbio.2006.11.010. Epub 2006 Nov 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raetzman LT, Ross SA, Cook S, Dunwoodie SL, Camper SA, Thomas PQ. Developmental regulation of Notch signaling genes in the embryonic pituitary: Prop1 deficiency affects Notch2 expression. Dev Biol. 2004;265:329–340. doi: 10.1016/j.ydbio.2003.09.033. [DOI] [PubMed] [Google Scholar]
- Raetzman LT, Wheeler BS, Ross SA, Thomas PQ, Camper SA. Persistent expression of Notch2 delays gonadotrope differentiation. Mol Endocrinol. 2006;20:2898–2908. doi: 10.1210/me.2005-0394. Epub 2006 Jul 13. [DOI] [PubMed] [Google Scholar]
- Rones MS, McLaughlin KA, Raffin M, Mercola M. Serrate and Notch specify cell fates in the heart field by suppressing cardiomyogenesis. Development. 2000;127:3865–3876. doi: 10.1242/dev.127.17.3865. [DOI] [PubMed] [Google Scholar]
- Sbrogna JL, Barresi MJ, Karlstrom RO. Multiple roles for Hedgehog signaling in zebrafish pituitary development. Dev Biol. 2003;254:19–35. doi: 10.1016/s0012-1606(02)00027-1. [DOI] [PubMed] [Google Scholar]
- Scheer N, Campos-Ortega JA. Use of the Gal4-UAS technique for targeted gene expression in the zebrafish. Mech Dev. 1999;80:153–158. doi: 10.1016/s0925-4773(98)00209-3. [DOI] [PubMed] [Google Scholar]
- Scheer N, Groth A, Hans S, Campos-Ortega JA. An instructive function for Notch in promoting gliogenesis in the zebrafish retina. Development. 2001;128:1099–1107. doi: 10.1242/dev.128.7.1099. [DOI] [PubMed] [Google Scholar]
- Schweisguth F. Notch signaling activity. Curr Biol. 2004;14:R129–R138. [PubMed] [Google Scholar]
- Treier M, Gleiberman AS, O'Connell SM, Szeto DP, McMahon JA, McMahon AP, Rosenfeld MG. Multistep signaling requirements for pituitary organogenesis in vivo. Genes Dev. 1998;12:1691–1704. doi: 10.1101/gad.12.11.1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treier M, O'Connell S, Gleiberman A, Price J, Szeto DP, Burgess R, Chuang PT, McMahon AP, Rosenfeld MG. Hedgehog signaling is required for pituitary gland development. Development. 2001;128:377–386. doi: 10.1242/dev.128.3.377. [DOI] [PubMed] [Google Scholar]
- Varga ZM, Amores A, Lewis KE, Yan YL, Postlethwait JH, Eisen JS, Westerfield M. Zebrafish smoothened functions in ventral neural tube specification and axon tract formation. Development. 2001;128:3497–3509. doi: 10.1242/dev.128.18.3497. [DOI] [PubMed] [Google Scholar]
- Varga ZM, Wegner J, Westerfield M. Anterior movement of ventral diencephalic precursors separates the primordial eye field in the neural plate and requires cyclops. Development. 1999;126:5533–5546. doi: 10.1242/dev.126.24.5533. [DOI] [PubMed] [Google Scholar]
- Watanabe YG. An immunohistochemical study of an anomaly of the fetal rat: adenohypophysis developing without contact with the brain. Arch Histol Cytol. 1996;59:381–387. doi: 10.1679/aohc.59.381. [DOI] [PubMed] [Google Scholar]
- Westerfield M. "The Zebrafish Book; A Guide for the Laboratory Use of Zebrafish (Danio rerio).". Eugene: University of Oregon Press; 2007. [Google Scholar]
- Westin J, Lardelli M. Three novel Notch genes in zebrafish: Implications for vertebrate Notch gene evolution and function. Dev Genes Evol. 1997;207:51–63. doi: 10.1007/s004270050091. [DOI] [PubMed] [Google Scholar]
- Wettstein DA, Turner DL, Kintner C. The Xenopus homolog of Drosophila Suppressor of Hairless mediates Notch signaling during primary neurogenesis. Development. 1997;124:693–702. doi: 10.1242/dev.124.3.693. [DOI] [PubMed] [Google Scholar]
- Zilinski CA, Shah R, Lane ME, Jamrich M. Modulation of zebrafish pitx3 expression in the primordia of the pituitary, lens, olfactory epithelium and cranial ganglia by hedgehog and nodal signaling. Genesis. 2005;41:33–40. doi: 10.1002/gene.20094. [DOI] [PubMed] [Google Scholar]