SUMMARY
Significant advances in gene therapy have been made as a result of the improvement of gene delivery systems, discovery of new therapeutic genes, better understanding of mechanisms of disease progression, exploration and improvement of tissue-specific gene regulatory sequences, and development of better prodrug/enzyme systems. We will discuss adenoviral-based and prostate-specific cancer gene therapy, emphasizing tissue-specific promoter choices to increase gene therapy safety and specificity, and the development of prostate-targeted vectors, with a focus on the two-step transactivation (TSTA) system for amplifying gene expression specifically in prostate cancer cells. Several examples will be discussed for the scientific basis and therapeutic applications. In addition, prostate cancer gene therapy clinical trials and future directions in this field also will be described briefly.
Keywords: Viral Based Gene Therapy, Prostate Cancer, TSTA system, Tissue-specific gene expression
1. Introduction
Prostate cancer has become the most frequently diagnosed male cancer next to non-melanocytic skin cancer in the Western world, and the second leading cause of cancer death in the US. In 2005, an estimated 232,090 men will be diagnosed with prostate cancer, and 30,350 will die of this disease [1], which exhibits varying degrees of aggressiveness, patterns of metastasis, and response to therapy. Current therapies such as prostatectomy, external-beam radiation therapy, brachytherapy, and cryotherapy can affect local tumor control and are potentially curative in patients with clinically localized disease. An estimated 40% of men over age 50 have slow-growing and well-differentiated prostate cancer, and the incidence increases with age. However, only 11% of the cases become clinically apparent, and 3% of them become lethal [2]. The current diagnostic methods, which are based on histology and Gleason scoring, are effective in the diagnosis and prediction of clinical outcome, however, these methods can be limited for patients with intermediate neoplastic grades, which may or may not become aggressive. An effective method for early detection has been to test for prostate-specific antigen (PSA) levels in serum, however, many cases are not diagnosed until the disease has advanced or metastasized beyond the reach of current treatments. In these more advanced cases, hormonal therapy and chemotherapy are the only systemic treatments presently available.
Unfortunately, current treatments have significant limitations and therefore, interest has shifted considerably towards the new therapy strategies, such as the development of gene therapy specific to prostate cancer, which has the promise to affect both local and systemic control of the disease. The term ‘gene therapy’ refers to the delivery of genetic material (DNA) to the particular tissue to be treated in order to slow cell growth or induce cell death as a treatment modality. The therapeutic gene generally is positioned adjacent to a regulatory sequence for RNA polymerase (promoter and/or enhancer), allowing the therapeutic gene to be expressed. Promoter sequences regulate gene expression and can modulate gene expression in a tissue-specific manner.
An ideal gene delivery vector for cancer treatment should be capable of efficient gene transfer, non-toxic or mutagenic to the patient, minimally immunogenic, and be produced at a reasonable cost and at high concentrations. Viral vectors come the closest to these challenging specifications. Of these, adenoviral (Ad) vectors are the most commonly used in clinical trials for cancer therapy. Ads enter target cells by receptor-mediated endocytosis with an epithelial cell tropism. Since infection does not depend on cell replication, these vectors can be efficiently delivered into replicating or non-replicating epithelial cells. These features make adenoviral vectors well suited for the relatively slow-growing prostate cancer. In contrast to retroviruses, genomic integration rate of adenovirus is negligible, diminishing the risk for mutagenesis. Short-term expression due to induction of neutral antibody response provided limitations for chronic gene replacement, but it may be considered advantageous for the treatment of neoplasia.
Presently, more than 160 active protocols are evaluating gene therapy in cancer treatment, with at least 13 of these protocols open for patients with prostate cancer [3]. However, the majority of these protocols do not utilize prostate cancer-specific gene therapy, and only one is prostate-specific (Phase I/II for combination CG7870 and docetaxel therapy) (Table 1). Therefore, although prostate-targeted gene therapy holds great promise for increasing safety and specificity of gene expression for therapeutic applications, achieving tissue-specific delivery and gene expression remains a main challenge, requiring further development and testing of improved specialized gene expression systems for in vivo gene therapy applications. We will discuss in this review the current and most promising prostate-specific gene expression systems tested in vitro and in vivo, focusing on viral vectors for delivery for therapeutic applications.
Table 1.
Overview of ongoing prostate cancer gene therapy clinical trials in the U.S.
| Therapeutic modality | Description | Trial Phase (reference) |
|---|---|---|
| hIL12 | • Adenovirus-mediated human interleukin-12 for recurrent non-metastatic prostate cancer following radiation therapy. | • Phase I [71] |
| • Modified vaccinia virus with genes for MUC1 and hIL12 (TG4010) to stimulate the immune system for patients with prostate adenocarcinoma. | • Phase II [72] | |
|
| ||
| Transgenic Lymphocyte Immunization | • Lymphocytes are rendered transgenic for a selected portion of the enzyme Telomerase ex vivo, then returned to the patient to produce an immune response. For patients with prostate adenocarcinoma. | • Phase I [73] |
|
| ||
| GM-CSF | • Human granulocyte-stimulating colony-factor (GM-CSF) adeno-associated (AAV) vaccine (GVAX). Allogeneic prostate cancer cell lines are gene- transduced. For treating advanced prostate cancer patients which have been rendered lymphopenic (by chemotherapy) and infused with autologous peripheral blood mononuclear cells (PBMC). | • Phase I/II [74] |
| • Combination therapy of GVAX and docetaxel versus docetaxel and prednisone. For HRPC patients with metastases. | • Phase III [75] | |
| • Priming with recombinant vaccinia containing the genes for PSA and a triad of costimulatory molecules (B7-1, ICAM1, LFA3, or TRICOM) to enhance immunogenicity, with subsequent boosts of recombinant fowlpox-PSA- TRICOM with and without recombinant GM-CSF. | • Phase I and II [76] | |
| • Sargramostim (GM-CSF) and fowlpox-PSA-TRICOM or vaccinia-PSA- TRICOM for patients with prostate cancer that have progressed following surgery and/or radiation therapy. | • Phase II [77] | |
| • Sargramostim and fowlpox-PSA-TRICOM or vaccinia-PSA-TRICOM plus anti-cytotoxic T-lymphocyte-associated antigen-4 monoclonal antibody. (MDX-010). | • Phase I [78] | |
|
| ||
| Provenge (APC8015) | • Immunotherapy designed to activate the patient’s own antigen-presenting cells (APC) from cryopreserved precursors loaded with prostate antigen PA2024 (prostatic acid phosphatase (PAP) and GM-CSF fusion), which directs the antigen to APCs, promoting antigen uptake and processing. For metastatic HRPC. | • Phase III [79] • Phase II [80] |
|
| ||
| Galactosyl transferase | • Murine Alpha(1,3)Galactosyltransferase-expressing allogeneic tumor cell therapy to induce hyperacute rejection of tumor cells. Using Moloney murine leukemia virus as a delivery vector. For patients with HRPC. | • Phase I/II [81] |
|
| ||
| CG7870 | • Combination therapy of prostate-specific oncolytic adenovirus CG7870 with docetaxel in chemotherapy-naïve patients with metastatic HRPC. | • Phase I/II [82] |
2. Biology of Prostate Cancer
Tumorigenesis is a multistep process that involves initiation, proliferation, and loss of contact inhibition, invasion, and metastasis. It is believed that the path to malignancy involves many complex genetic and epigenetic influences. Several genetic changes have been documented in prostate cancer, and include allelic loss, point mutations, and changes in DNA methylation patterns. Among these, most common allelic losses affect the short arm of chromosome 8 (containing MSR1, NKX3.1, and NKX3A), the long arm of chromosome 16 (containing Atbf1), the short arm of chromosome 12 (containing p27kip1), and the long arm of chromosome 13 (containing KLF5), suggesting the presence of candidate or novel tumor suppressor genes in these regions [4]. Other events include gene inactivation of tumor suppressor genes, with loss of p53 expression reported in 3–42% and functional inactivation or loss of Pten expression in up to 50% of cases [4–6]. Loss of expression of other genes such as glutathione-S-transferase (GSTP1) and IGFBP-3 are also common. Genes whose expression affect prostate cancer metastasis include c-met, cyclin E1, MTA1, EZH2 (expressed at higher levels in metastases than in primary tumors), E-cadherin (metastasis-suppressor gene), and Kai1 (overexpression inhibits metastasis occurrence) [5]. Finally, the genes that commonly provide prognostic information for this disease include PSA, p53, PIM1, hepsin, EZH2, and E-cadherin [5], but only PSA has been incorporated into clinical management decisions to date.
Alterations in gene function and expression also are involved in the emergence of a biological characteristic of more aggressive prostate cancer, the androgen-independent disease. Several potential mechanisms for the development of androgen-independency have been described, including activation of epidermal (EGF), fibroblast (FGF), or other growth-factor pathways; alteration of genes such as p21waf1, fibronectin, IGFBP2, and insulin receptor, and overexpression of bcl-2 (suppressor of apoptosis) in prostate cancer cell lines (reviewed in [5]). Gene amplification also plays a role in cancer progression, with higher levels of Myc and Androgen receptor (AR) expression as some of the most frequent alterations observed in prostate cancer [4, 5]. The AR signaling pathway mediates the biological effects of androgen. In androgen-dependent prostate cancer, the ligand-bound AR translocates from the cytoplasm to the nucleus, binding to the androgen-responsive elements (ARE) upstream of target genes, such as prostate specific antigen (PSA). Several mechanisms have indicated the continual involvement of AR in HRPC (reviewed in [7]), including AR gene amplification/overexpression; altered ligand specificity; and activation through crosstalks with other AI pathways. The precise role of AR in clinical situations is not fully understood. However, given the fact that AR expression is documented in the majority of HRPC cases and that PSA remains the most reliable marker for recurrent, metastatic prostate cancer, it is highly probable that the gene regulatory activity of AR is functional in the clinical setting. This is particularly relevant to prostate cancer gene therapy since most regulatory elements used to target gene expression to prostate cancer are regulated by AR.
3. The Status of Current Prostate Cancer Gene Therapy
i. Immune-based gene therapy
A major focus of new treatments is to modulate the immune system, particularly by utilizing dendritic cell-based vaccines transduced with granulocyte-macrophage stimulating colony-stimulating factor (GM-CSF). There have been a number of studies and the treatment appears to be well tolerated. Among current clinical trials for immune stimulation are trials involving Ad-GM-CSF, Ad-hIL12 administration, recombinant vaccinia or fowlpox viruses containing PSA and costimulatory molecules, and dendritic cell vaccines (Table 1 and reviewed in [8]).
Of these studies, GM-CSF-transduced vaccines appear to be one of the most common current therapy trials for prostate cancer involving gene therapy (Table 1). Three previous trials employed the adenovirus as a vector [9], and the current trials involve various gene delivery vehicles (retrovirus vaccinia, fowlpox) to transduce the GM-CSF gene into the malignant tissue ex vivo. Following vaccine administration, dendritic cells are recruited to the site where antigen can be processed and presented to T lymphocytes in the local lymph nodes. Two types of GM-CSF-secreting tumor vaccines have been utilized in clinical trials for prostate cancer, autologous (manufactured from a patient’s surgically harvested tumor, then transduced by a viral vector to secrete GM-CSF) or allogeneic (produced using established tumor cell lines). The commercial form of GM-CSF therapy (GVAX) has been used in prostate cancer clinical trials (reviewed in [9]). In a Phase I trial, autologous GM-CSF-secreting irradiated tumor vaccines were prepared from ex vivo retroviral transduction of cells surgically harvested. In a Phase II trial of the allogeneic GVAX prostate vaccine, two irradiated prostate cancer cell lines, PC3, and LNCaP were transduced with GM-CSF plasmid [9]. Clinical trials with GVAX continue, and Phase II trials are ongoing in HRPC patients that have not yet received chemotherapy using allogeneic prostate cancer cell lines (Table 1). Another promising modality of immune-based therapy are dendritic cell vaccines stimulated by GMCSF plus prostatic acid phosphatase, currently in Phase III [10] (Table 1). Immune-activating therapy is a promising direction for prostate cancer treatment and may be combined with prostate-specific cytotoxic gene therapy for added efficacy in the future.
ii. Corrective gene therapy: cell cycle genes, proapoptotic genes
Besides the previously mentioned approaches taken to stimulate host anti-tumor responses, there are several approaches aimed at weakening or damaging prostate tumor cells. They include corrective therapy by tumor suppressor gene replacement or silencing of oncogenes, suicide gene therapy approaches involving transduction of tumor cells with genes producing an enzyme that converts a prodrug into a toxic agent, and oncolytic gene therapy by replication-restricted viruses.
Genetic alterations responsible for prostate oncogenesis often represent either inactivation of a tumor suppressor gene or activation and overexpression of a proto-oncogene. Therefore, tumor suppressor gene reintroduction and technology to reduce oncogene expression are strategies used for corrective gene therapy. p53 is the most commonly mutated tumor suppressor gene in cancer. Restoration of wild-type p53 in many tumor cell lines causes growth arrest or apoptosis. The recent approval of adenoviral vector expressing wildtype p53 as a cancer therapeutic drug in China greatly facilitates the use of this corrective gene therapy in patients with head and neck, colorectal and breast cancer [11], including isolated cases of prostate cancer. Also, alterations in other tumor suppressor candidate genes such as GSTP1, p27, Kai1, or Pten may represent attractive targets for corrective gene therapy research.
For down regulating oncogene overexpression, promising strategies include antisense oligonucleotides and small interfering RNA (siRNA) technology. Antisense oligonucleotides are single-stranded synthetic nucleotide sequences designed to form complementary duplex with the targeted transcript, inhibiting its transcription. Several clinical trials have used or are using antisense in prostate cancer treatment, including antisense myc RNA, and total tumor RNA (reviewed in [8]). Also currently recruiting patients are four trials, using oblimersen, an antisense oligonucleotide directed to the Bcl-2 mRNA, and OGX11, an antisense directed to clusterin, an anti-apoptotic chaperone that confers resistance to cell death triggers, including hormone, radiation, and chemotherapies. These trials include (1) a Phase I using EFGR antisense DNA for HRPC, gastric and ovarian cancers, (2) a Phase II using docetaxel in the presence or absence of oblimersen for HRPC, (3) a Phase II using docetaxel and prednisone in the presence or absence of OGX11 for HRPC, and (4) a Phase II using OGX11 prior to radical prostatectomy [10, 12–14].
Future directions in prostate cancer gene therapy will likely include a novel specific gene silencing methodology, RNA interference (RNAi). RNAi is a naturally occurring gene-silencing mechanism mediated by small double-stranded RNA molecules (small interfering RNAs, siRNAs, ~20 nucleotides in length) processed from long double-stranded RNAs or from transcripts that form stem-loops (reviewed in [15]). siRNAs silence gene expression either by targeting mRNA for degradation, by preventing mRNA translation, or by establishing regions of silenced chromatin. The mechanism for silencing gene expression occurs when one strand (the antisense) of the siRNA directs the RNA-induced silencing complex (RISC) that contains an RNA endonuclease to cleave its target mRNA bearing a complementary sequence. Although some RNAi mechanisms, especially inhibition of translation, do not require extensive base pairing over the length of the siRNA, efficient mRNA cleavage requires high complementarity to the transcript. To date, there is only one published study in patients using siRNA delivered by SV40 vectors for chronic myeloid leukemia. These siRNA molecules were demonstrated to repress, in tissue culture cells, one of the two types of the oncogenic fusion genes (BCR/ABL) present in CML patients [16]. Development of these RNAi strategies will require an expanded understanding of molecular targets for knockdown and will likely incorporate more stable, small hairpin RNA (shRNA) approaches to become fully effective. These strategies will likely expand over time and be refined further, becoming more common in the future for preclinical and clinical applications.
iii. Cytolytic strategies: Suicide and oncolytic gene therapies
Cytolytic gene therapy can involve the transfer of drug-susceptible (“suicide”) genes or oncolytic, replication-restricted viruses. In the “suicide” gene strategy, a gene is transfected into tumor cells that encodes an enzyme that converts a nontoxic prodrug into a cytotoxic form in transfected tumor cells. The two common suicide gene therapy systems are the classic Herpes simplex virus thymidine kinase (HSV-tk), and the E. coli cytosine deaminase (CD) gene. HSV-tk converts nontoxic nucleoside analogs such as ganciclovir (GCV) into phosphorylated compounds that act as chain terminators of DNA synthesis, while the prodrug for cytosine deaminase is 5-fluorocytosine. Tumor cell killing is achieved by necrosis and apoptosis.
Applying cytolytic gene therapy to treat bone metastasis of prostate cancer is a very important research focus for the field. Osteoblastic response to prostate cancer is the hallmark of progression at this metastatic site. Osteocalcin (OC), a noncollagenous bone matrix protein, is expressed at high levels by osteoblast and metastatic prostate cancer cells. A recombinant adenovirus, Ad-OC-tk, which contains the OC promoter driving expression of HSV-tk as a suicide gene resulted in gene expression in osteoblasts and advanced prostate cancer cells. Hence, this approach has potential to eradicate both the metastatic prostate cancer cells (“seed”) and osteoblastic cells (“soil”), which may be required to maintain survival of prostate cancer once it has metastasized to bone tissue [17, 18].
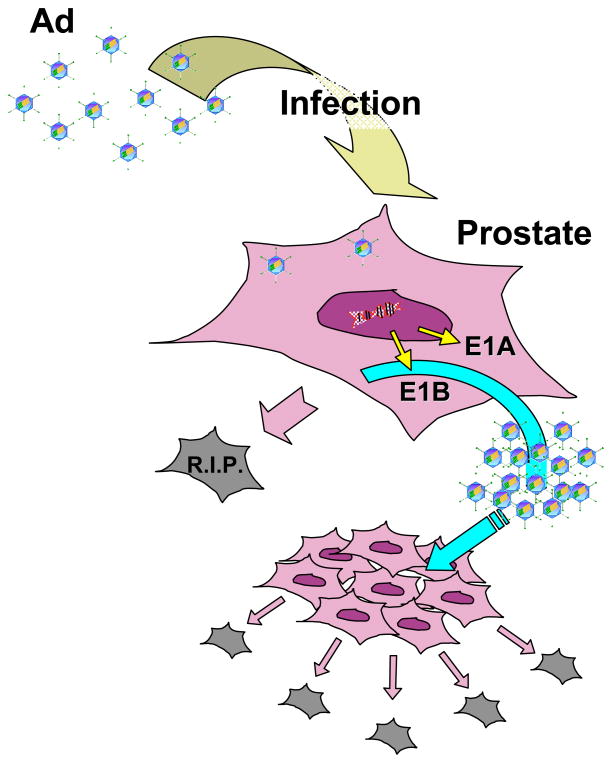
Oncolytic, replication-restricted adenovirus is actively being developed as another form of cytolytic therapy. Adenovirus is an efficient lytic virus, capable of producing up to 100,000 progeny viruses per host cell and lysing the host cell during this productive infection. To harness this potent viral replicative power towards prostate tumor destruction, a key consideration is how to turn on this process specifically in prostate tumors. The most promising approach is to restrict the expression of viral replication genes only in prostate tumor cells (Figure 1). The prototype CG7870 prostate-specific replication-selective adenovirus has the E1A gene under the control of the rat probasin promoter, E1B under control of the PSA promoter and it showed potent PSA-selective cytotoxic activity in preclinical testing [19]. The vector has been tested in a Phase I/II trial, where it showed activity as measured by reduction or stabilization of serum PSA [20].
Figure 1. The concept of oncolytic gene therapy for prostate cancer treatment.
Oncolytic adenoviruses infect tumor cells, replicate their genome (important viral gene expression products are E1A and E1B, which allow the virus to take over the cellular replication machinery), assemble new viral particles and kill the host tumor cells by lysis, resulting in the release of the progeny viruses. This new virus generation spreads, and starts a new cycle of virus replication and tumor cell killing. Conditionally replicative adenoviruses are restricted in their replication to specific factors in the target cancer cells (certain cell cycle pathway defects). As a consequence, infection of normal cells by oncolytic adenoviruses and/or their replication within these cells is attenuated. Thus, the ideal oncolytic adenovirus represents an efficient and specific anti-cancer agent.
Directing cytolytic and oncolytic therapy using the prostate- or prostatic cancer-specific promoters is a promising strategy for treatment of prostate cancer. We will expand on these important gene expression-targeting concepts in the following section.
iv. Combining Therapeutic Modalitites
Due to intratumoral cell heterogeneity, combined modality therapies may achieve additive or synergistic therapeutic activity. Combined therapies can take on the form of (1) simultaneous delivery of multiple therapeutic genes, (2) combining gene and radiation therapy, and (3) treating with both gene therapy and chemotherapy. Multiple therapeutic genes that have demonstrated enhanced efficacy over single modality include dual suicide genes for enzyme/prodrug strategies (HSV-tk and CD) [21, 22], suicide plus apoptotic therapies (HSV-tk and p53) [23], and combined cytotoxic (HSV-tk) and immune-activation (Flt-3) [24]. The rationale for the combination of radiation and cytotoxic gene therapy is that prodrug activation cytotoxic treatment can sensitize cells to the effects of radiation [25, 26]. An interesting pre-clinical study has reported that an adenoviral-mediated double suicide gene therapy strategy (CD/TK) in combination with radiation resulted in prostate tumor cell killing higher than radiation alone and tumor growth delay up to 70% [21]. More significantly, no added toxicity was observed. A follow-up study in a preclinical setting provided supporting evidence for the advantages of this multimodal combination therapy. The study showed that intraprostatic directed therapy using the double fusion suicide virus (CD/HSV-tk) plus radiation exhibited no dose limiting toxicities or adverse effects [27]. In a phase I/II trial, suicide HSV-tk therapy was combined with radiotherapy in the presence or absence of hormonal therapy. The transgene was expressed up to 3 weeks following Ad injection, and therapeutic effects (all 52 patients experienced a decline in PSA levels) occurred by one week of prodrug administration. The tri-modal therapy appeared to achieve good locoregional control of prostate cancer in patients as assessed by serological and histological means. However, the systemic control was inadequate in those patients with positive pelvic lymph nodes [28].
Chemotherapy may also be combined with gene therapy for higher efficiency. Combined administration of paclitaxel and p53 gene revealed therapeutic synergy in prostate DU145 xenografts [29]. Currently, the prostate-specific oncolytic Ad CG7870 is undergoing a Phase I/II clinical trial in combination with Taxotere chemotherapy in patients with advanced-stage prostate cancer [30]. This combined therapeutic approach displayed significant synergistic antitumor activity in mouse tumor models of prostate cancer [31]. In the future, it might be important to design rational protocols that incorporate gene-based therapy in conjunction with conventional therapeutic modalities to boost the efficacy of prostate cancer treatment. Augmenting the prostate-specificity and the magnitude of expression of cytotoxic genes will be very important in providing the “safety valve” needed in escalating the potency of therapy. Some of the strategies to enhance prostate-specific therapy are discussed below.
4. Novel expression and therapeutic strategies targeting Prostate Cancer
a. Promoters to direct prostate-specific gene expression
The use of prostate-specific transcriptional activity should improve the efficacy and safety of cancer gene therapy. Transcriptional targeting refers to the use of a particular cell-specific regulatory element (promoters or promoter/enhancers) to restrict gene expression to a particular tissue or cell type. Many prostate-specific gene regulatory regions are well characterized and several of them have been assessed in pre-clinical and clinical therapeutic studies (Table 1). The best studied to date is the prostate-specific antigen (PSA or hK3) gene, which encodes a serine protease [32]. Since PSA is expressed at all stages of prostate carcinogenesis, it remains an excellent candidate for directing gene expression and therapy specifically for prostate cancer. Within the 6 kilobase (kb) native regulatory region upstream of the PSA gene, there are several essential components needed to direct prostate-specific expression. These include a proximal promoter that contains a TATA box and two functionally important binding sites for the transcription factor AR, called androgen responsive elements (AREs) [33, 34], and upstream enhancer regions, which greatly augment prostate-specific gene expression [35–37]. Putative recognition sites such as for AR, AP-1, and c-fos span the larger enhancer regions. However, a minimal 440 base pair core enhancer region was found to most critical in conferring strong activity and cell specificity. This enhancer core contains one high affinity ARE [38], and at least four non-consensus AREs with variable AR affinity [39], and these elements contribute to the synergistic activation of PSA gene expression.
Extensive studies have been performed to boost the inherent weak but specific activity of the native PSA promoter and enhancer [40, 41]. The most effective method to date is to duplicate the core enhancer and contracting the intervening sequences between the enhancer and the proximal promoter [42]. Studies from our laboratory have illustrated that the most efficacious construct, PSE-BC (duplication of core enhancer plus the PSA promoter), achieved nearly 20-fold enhancement of activity comparing to a native construct while retaining high degree of androgen inducibility and tissue-selectivity. The PSE-BC promoter was inserted in an Ad vector driving the firefly luciferase reporter gene (Ad-PSE-BC-FL). In comparison to the constitutive cytomegalovirus (CMV) promoter-driven vector, the Ad-PSE-BC-FL exhibited great prostate-selective and tissue discriminatory capability upon systemic administration in SCID mice bearing prostate tumors [42]. More significantly, the prostate-selectivity of Ad-PSE-BC-FL enables the visualization of metastatic lesions in spine and lungs of living animals by bioluminescence imaging [43].
Along with PSA (hK3), the human glandular kallikrein 2 (hK2) belongs to the same large kallikrein family and they share similar gene regulatory elements that confer prostate-restricted gene expression [44–46]. hK2 gene expression is increased in prostate carcinoma [47], suggesting that this element can be used to target gene expression to more advanced prostate cancer. Approaches taken to enhance the PSA and the rat probasin promoter, such as increasing androgen responsive elements [48], have also been effective in increasing the activity and androgen inducibility of hK2 promoter [49].
Most of the prostate-specific promoters are androgen-regulated. However, the two notable exceptions are the osteocalcin (OC) promoter [18] and the prostate-specific membrane antigen (PSMA) [50]. As mentioned previously, OC is a bone matrix protein found to be expressed in osteoblasts but interestingly also in primary and metastatic prostate cancer cells [51]. OC gene expression is controlled by multiple elements, including an osteo-specific element (OSE2) and the vitamin D-responsive element [52, 53]. Although PSMA encodes an integral membrane protein that is expressed in primary prostate cancer and lymph node metastases [54], studies have suggested that expression is not restricted to prostate [55]. Interestingly, PSMA gene expression is induced by androgen deprivation. Hence, PSMA promoter-driven cytotoxic gene therapy has been developed specifically to eradicate AI prostate cancer in the face of hormone ablation treatment [56].
Due to the genetic heterogeneity of prostate tumors, a legitimate concern is that using a cell-specific promoter might limit transgene expression to a subset of the tumor cells, thus reducing therapeutic efficacy. To address this issue, investigators have developed chimeric promoters by fusing critical transcriptional elements from distinct prostate-specific promoters. An early rendition of this concept involved the insertion of multiple synthetic AREs into the PSA enhancer, which resulted in enhanced activity and androgen responsiveness [42]. Combining hK2 and PSA enhancer elements also greatly enhanced the promoter activity in androgen receptor positive prostate cancer cells [57]. An interesting approach is to create chimeric fusions from promoters with disparate functional activities. A novel PSES chimeric promoter, composed of regulatory elements of PSA and PSMA genes, remained silent in non-prostate cell lines, but mediated high levels of expression in PSA- and PSMA-expressing prostate cancer cell lines in the presence and absence of androgen [58]. An oncolytic adenovirus that employs the PSES promoter to drive its replication was able to achieve significant inhibition of prostate tumor growth after systemic administration [59]. The most recently described chimeric promoter construct (PPT) is the most complex, combining the T cell-receptor gamma-chain alternate reading frame protein (TARP) promoter, the PSMA enhancer, and PSA enhancer [60]. It was reported to be highly active in testosterone-deprived prostate cancer cells (PC-346C tumor model). An advantage of the PSES and the PPT promoters is their ability to remain active both in the presence and in the absence of androgen [60].
Despite the high prostatic specificity of many prostate-specific regulatory regions developed to date, the magnitude of expression directed by these promoters may not be sufficient to mediate robust expression in vector-based gene therapy applications. Therefore, an approach for amplifying transcription from cell-specific promoters has been developed and is discussed in the following sections.
b. The two-step transcriptional amplification (TSTA) system for increasing prostate-specific gene expression for in vivo applications
The ubiquitously active strong viral promoters (e.g. cytomegalovirus CMV enhancer/promoter or SV40 promoter) have been the benchmark promoters used in gene therapy. Hence, a goal in augmenting the activity of tissue-specific promoters is to achieve levels comparable to the CMV promoter, while retaining the cell discriminatory activity. Recently, a useful two-step transcriptional amplification (TSTA) system has been developed to boost the activity of a weak but tissue-specific PSA promoter/enhancer (PSE-BC) [61]. This two-tiered amplification methodology has been extensive tested and validated in amplifying expression from the PSA promoter [61–63]. In the TSTA system, a potent transcriptional activator, driven by a cell-specific promoter, acts on a responsive construct, and turns on expression of the reporter/therapeutic gene (Figure 2). The potent activator is the GAL4-VP16 fusion protein, comprising the DNA binding domain from the yeast transcription activator GAL4 and the activation domain from the HSV1 viral protein 16 (VP16) transactivator. This GAL4-VP16 fusion protein binds specifically to multiple repeats of GAL4-binding sites upstream of the gene of interest. This transcriptional amplification system presents an extensive dynamic range that can be modulated by increasing the number of VP16 activator domains expressed in the fusion protein, and/or the number of GAL4 binding sites in the reporter/therapeutic construct.
Figure 2. The two-step transcription amplification (TSTA) system.
(a) The TSTA system consists of a two-step transcriptional activation process. In the first step, the artificial GAL4-VP16 activator is expressed in a tissue-specific manner by virtue of regulation by a Tissue/cancer-specific promoter. In the second step, the GAL4-VP16 protein binds to five GAL4 DNA binding sites upstream of a minimal promoter, and activates expression of a reporter or therapeutic gene (FL, for example). (b) The “all-in-one” Single System TSTA Ad (AdTSTA-FL): the components of the TSTA system can be combined into a single Ad vector, where both activator (GAL4-VP16×2, or VP2) and reporter are inserted into the deleted E1 (ΔE1) region of the same Ad in a head-to-head orientation [63]. ψ denotes the packaging signal of adenovirus and open rectangles at both termini denote inverted terminal repeats of the viral genome.ΔE3, the deleted E3 region of the Ad genome.
The bipartite configuration of the TSTA system (Figure 2) offers great flexibility in modulating its activity. Enhancement of TSTA activity can come from increasing the strength of the promoter, the potency of the VP16 activator, and, most remarkably, by increasing the number of GAL4 binding sites driving the gene of interest [61]. For instance, increasing the activator binding sites from one to five amplified the activity by 200- to 400-fold [61]. Based on extensive molecular engineering refinements, a very effective adenoviral vector containing all the TSTA components in a contiguous DNA insert was generated (see Figure 2b) [63, 64]. This AdTSTA-FL maintains prostate-selectivity, androgen responsiveness and amplifies reporter gene expression nearly 1000-fold above that driven by the native PSA promoter. Importantly, these TSTA constructs display activity levels significantly higher than the CMV promoter in prostate tumors [43, 61, 63–66].
Molecular imaging is a useful technology to validate the functionality of the TSTA vectors for in vivo applications. Hence, we used the TSTA system to amplify the expression of imaging reporter genes, FL and HSV1 mutant tk (sr39tk), which can be visualized by a cooled charge-coupled device optical camera and a Positron Emission Tomography (PET) scanner, respectively. The engineered sr39tk enzyme exhibits significant higher binding affinity for GCV and 18F-labeled GCV analogue, and thus, improved sensitivity for PET imaging. Systemic administration of TSTA vectors clearly demonstrated silent expression in non-targeted organs (e.g. liver), while prostate tumor-directed administration displayed robust signals [66]. A critical consideration in applying the prostate-targeted TSTA gene therapy to patients is whether it would be functional in HRPC [64]. The TSTA-driven vectors were tested on AD and AI prostate tumors. Real-time gene expression was monitored by optical and the combined PET and computed tomography (CT) modality [64]. Our results clearly illustrated that the activity of the TSTA vectors was AR dependent and recapitulated the functional status of endogenous AR in the tumors. In several cases, the TSTA-mediated expression was more robust in the AI or HRPC tumors than AD tumors, supporting the conclusion that AR function is activated in HRPC despite of castrated levels of androgen [64].
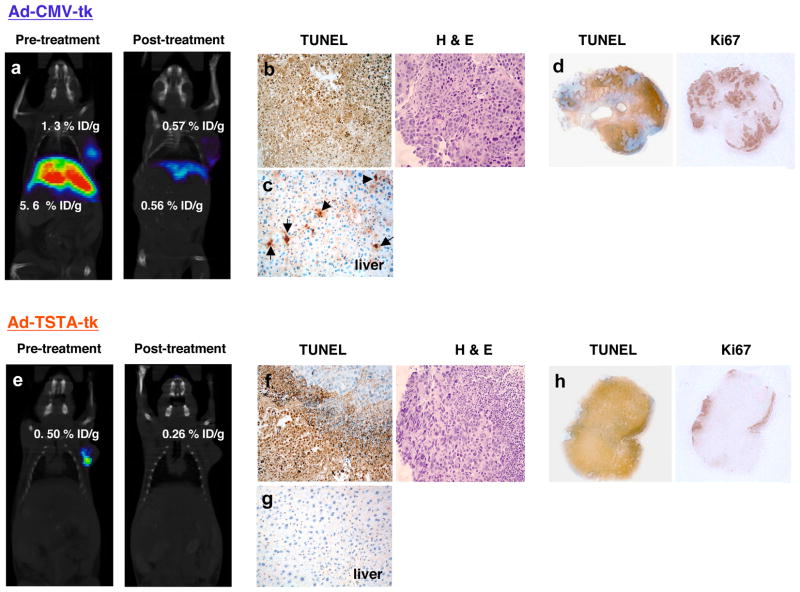
The efficacy of the two-step gene amplification mediated therapeutic interventions has been tested in several pre-clinical cancer models. Segawa et al. fused a 5 kb native PSA promoter to drive the expression of GAL4-VP16 activator [67], which was able to control the expression of the expanded polyglutamine protein at the secondary step in an androgen-dependent and PSA-selective manner. Ablation of prostate cancer cells was demonstrated using this two-step amplified expression of the toxic polyglutamine gene [67]. The two-tiered amplification strategy also has effectively enhanced a carcinoembryonic antigen (CEA) promoter driven suicide gene therapy targeting colon cancer [68]. Recently, we exploited the dual-capacity of HSV-tk as a toxic suicide and a PET reporter gene in an image-guided therapeutic study [66]. In the study, we directly compared the therapeutic effects of Ad-TSTA-to Ad-CMV-driven sr39tk. Vectors were delivered into human prostate tumors and serial optical and PET-CT imaging revealed restriction of gene expression to the tumors when the prostate-specific TSTA vector was employed. In contrast, administration of a constitutively expressed Ad-CMV-sr39tk resulted in strong tumor and liver signals. In fact, HSV-sr39tk-based PET enables the direct determination of the locations and magnitude of therapeutic gene expression prior to and post GCV prodrug treatment. The therapeutic outcome could be predicted based on the imaging findings. Overall, the augmented prostate-specific TSTA expression system was thus superior to the constitutive approach in safeguarding against systemic toxicity due to inadvertent leakage of vector outside of the tumor, while achieving effective tumor killing (results summarized in Figure 3).
Figure 3. In vivo Molecular Imaging of Prostate-specific Gene Therapy.
To illustrate the usefulness of in vivo molecular imaging for visualization of therapy effectiveness and specificity, microPET/CT imaging was performed of suicide gene therapy targeted to human prostate cancer in vivo. 109 infectious units of constitutive active AdCMV-sr39tk (CMV) or prostate-targeted AdTSTA-sr39tk (TSTA) were intratumorally injected into androgen dependent human prostate LAPC-4 xenograft tumors on day 0. MicroPET/CT imaging performed prior to ganciclovir (GCV) treatment on day 7 (Pre-treatment) showed tumor-limited expression in the TSTA-treated animals (e), but the CMV-treated animals showed strong expression in the both the tumor and the liver (a). After receiving GCV treatment, F18-FHBG PET signals at day 22 (Post-treatment) were diminished in the tumors and the liver of the CMV animals (a) and tumor of TSTA treated animals (e). Histology performed at the endpoint (day 22) revealed that both tk therapy modalities induce extensive apoptosis as measured by TUNEL positivity (brown), however, in CMV-treated animals both the tumor and the liver are affected (b, c), while in TSTA-treated only the tumor shows TUNEL positivity (f) (only low TUNEL signals are present in liver (g)). Lower magnification analyses of tumor global proliferation showed that the cell killing as evidenced by TUNEL is comparable in CMV- (d) versus TSTA-treated (h) tumors, and the proliferation index, as determined by the marker Ki-67 appear to be diminished in the areas of tumor killing and are overall comparable between both therapy strategies.
The integration of noninvasive imaging into prostate-specific cytotoxic gene therapy is an excellent example of how current treatment strategies can be improved. Several interesting and powerful applications of AdTSTA-FL take advantage of its real-time production of imaging signals in living animals. As explained in the previous section, AR function plays a critical role in modulating the growth and progression of prostate cancer and blockage of this activity is the cornerstone of therapeutic interventions for advanced stage disease. The PSA promoter activity and hence, the TSTA system, are exquisitely dependent on the AR function in vivo. For example, instillation of AdTSTA-FL into a prostate tumor under androgen ablation treatment enabled the visualization of the tumor transitioning from AD to AI state. This transition was denoted by a recovery of luciferase optical signals despite the maintenance of an androgen-deprived environment [65]. AdTSTA-FL was also applied to monitor the real-time treatment effects of anti-androgens, such as flutamide, in tumors [69].
Given the power and flexibility of the TSTA system, we are continually modifying the configuration of the TSTA viral vectors in an attempt to improve their specificity and utility. Our recent findings suggested that the orientation and placement of the two components of TSTA in the adenoviral genome can impact the mechanism of transcriptional activation [63]. Several new configurations of the two-step TSTA system in the same viral vector are illustrated in Figure 4. Preliminary findings indicated that separating the two components by great genomic distances (Figure 4a) reduce background activity, possibly interrupted the feed-forward loop described for the parental all-in-one AdTSTA-FL (Figure 2b). The advantage of the bi-directional (Figure 4b) and multiple gene (Figure 4c) configurations is that simultaneous expression of several genes can be regulated by the same stringent tissue-specific promoter [70]. These improved constructs can be applied to direct more effective multi-gene combined therapy or coupled imaging and therapeutic strategies in a single vector.
Figure 4. The new configurations for the TSTA system for in vivo applications.
The engineering of higher efficiency and safer vectors for TSTA delivery and expression has promoted the design of alternative configurations for potential in vivo applications. Here we illustrate this idea by using the adenovirus vector genome as a prototype. The Single System TSTA Ads incorporate the components of the TSTA system into a single Ad vector. (a) New Generation AdTSTA-FL: the components are separated, with the activator inserted in the E3 region, and the reporter inserted into the E1 region; (b) Bidirectional New Generation AdTSTA-FL-TK: the activator is inserted in the E3 region, and the reporter/therapeutic gene is inserted in a head-to-head orientation in the E1 region to achieve simultaneous expression of two genes; (c) Multiple Genes New Generation AdTSTA-FL-TK-ires-EGFP: the activator is inserted in the E3 region, and a combination of genes can be expressed in a head-to-head fashion. The number of genes expressed can be increased by virtue of internal ribosome entry site (IRES) sequences placed in between them. ψ denotes the packaging signal of adenovirus and open rectangles at both termini denote inverted terminal repeats of the viral genome.
Collectively, the TSTA approach has been demonstrated to be an effective mechanism to boost many weak but cell-specific transcriptional regulatory elements. Besides the PSA promoter, many other promoters (e.g. PSMA, ARR2PB, hK2) could be augmented to target primary tumor and its metastases (e.g. OC, PSA). The robust and specific expression nature of TSTA imaging vectors, in particular PET imaging vector, hold promise that they can be developed to detect and treat metastatic lesions in pre-clinical and clinical settings. Based on the current data, we anticipate that the incorporation of TSTA system in place of constitutive (CMV) driven gene therapy strategies might improve the safety of current status of prostate cancer clinical trials.
5. Conclusion
Achieving cell-specific targeting remains a great challenge in the future of cancer therapy. Transcriptional targeting is a promising and feasible alternative with which to improve both the specificity and efficacy of gene therapy. However, significant obstacles remain in seeking out and destroying the hidden metastatic cancer cells in the whole organism. To further enhance tumor selectivity, approaches that target cell surface antigens or biochemical pathways unique to tumors can augment the transcriptional targeting of TSTA system. Combinations of multiple targeting strategies into tissue- or cancer-specific viral vectors should achieve synergistic selectivity and efficacy of therapy. Non-invasive imaging will be a useful tool to assess the performance of transcriptionally targeted vectors in vivo. As these prostate-specific regulatory systems (highly boosted by TSTA utilization) and safer and more efficient viral vector configurations continue to evolve, it will remain critical to design and stringently test cancer-targeted gene therapies in preclinical settings, and promote translation to the clinic of the most promising strategies.
6. Future Perspectives
Gene therapy offers extraordinary potential for the management and correction of human disease, including cancer. While the expectations and the promise of gene therapy are great, major difficulties include shortcomings in current gene transfer vectors and an inadequate understanding of the biological interaction of these vectors with the host, and several research groups currently focus on examining these issues in depth. Basic disease pathophysiology studies (often carried out in animal models) will likely contribute to the eventual success of gene therapy, and can lead to better definition of the important target cell(s) and therefore to more effective design of therapeutic approaches. In order to confront the major outstanding obstacles to successful gene therapy, greater focus is necessary on basic aspects of gene transfer and gene expression. Such efforts need to be applied to improving vectors for gene delivery, enhancing and maintaining high level expression of genes transferred to somatic cells, achieving tissue-specific and regulated expression of transferred genes, and directing gene transfer to specific cell types. Increased emphasis on research dealing with the mechanisms of disease pathogenesis, further development of animal models of disease, enhanced use of preclinical gene therapy approaches in these models, and greater study of stem cell biology in diverse organ systems will improve our understanding of prostate cancer and thus, the outlook of gene therapy. Development of PSA-based strategies is a promising avenue for targeting prostate cancer and detecting metastases. The refining of this and other prostate-specific transcriptionally based strategies will definitely involve extensive testing in vitro and in vivo (animal models) prior to translation to the clinic and many approaches are likely to be tested. The addition of PSA-based TSTA strategies to these combined treatment strategies to specifically suppress growth and/or induce cell killing in the prostate and prostatic metastases will likely become a trend to achieve more effective therapeutic outcomes.
7. Executive Summary
Prostate Cancer Facts
The second most frequently diagnosed male cancer in the Western world, and the second leading cause of cancer death in the US (232,090 new cases and 30,350 deaths in 2005).
Prostate cancer is a heterogeneous disease. The current diagnostic methods (e.g. PSA, Gleason scoring) are useful in the prediction of clinical outcome, but these methods can be limited for patients with intermediate grades or aggressive cancer Prostatectomy, external-beam radiation therapy, brachytherapy, and cryotherapy can control and cure clinically localized disease with high probability.
Serum PSA testing is very helpful towards early detection. However, many cases present at advanced or metastasized stages.
In advanced disease, hormonal therapy and chemotherapy are palliative and offer no curative potential. New therapy strategies that can potentially control both local and systemic disease are sorely needed. Gene therapy specific to prostate cancer holds significant promise.
Biology of Prostate Cancer
Genetic alterations contributing to prostate oncogenesis include allelic loss, point mutations, changes in DNA methylation patterns, and gene inactivation of tumor suppressor genes (TSG).
Amplification or mutation of the androgen receptor (AR) gene or alteration of AR function is likely involved in the emergence of aggressive HRPC.
Current Status of Prostate Cancer Gene Therapy
Immune Modulating Gene Therapy comprises a major effort of new treatments. Dendritic cell-based vaccines transduced with GM-CSF, Ad-hIL12 administration, and viruses expressing PSA and/or costimulatory molecules are a few examples of therapeutic strategies.
Corrective Gene Therapy
Reintroduction of TSG is a common approach. Due to the high prevalence of p53 gene mutation in cancer, it is a favorite target of correction. Many TSG candidates are good targets for prostate cancer.
Methods to downregulate oncogene expression include the use of antisense oligonucleotides, several of which are being evaluated in clinical trials. Future directions will likely include a novel specific gene silencing methodology, RNA interference (RNAi).
Cytolytic Gene Therapy
The Suicide approach involves transduction of tumor cells with genes producing an enzyme that converts a prodrug into a toxic agent, The herpes simplex virus thymidine kinase (HSV-tk), and the E. coli cytosine deaminase (CD) are two such suicide genes.
The Oncolytic approach involves restricted expression of viral replication genes in prostate tumor cells. The CG7870 is such a prostate-specific replication-selective adenovirus, which holds promise in achieving prostate-selective cytotoxicity in preclinical testing, and a Phase I/II trial is underway.
Combination therapies
Combination therapies in the form of (1) simultaneously expressing multiple therapeutic genes, (2) combining gene and radiation therapy, and (3) treating with both gene therapy and chemotherapy are being developed with the goal of achieving more effective, synergistic therapy to treat the typically heterogeneous prostate tumors.
Novel strategies to target prostate cancer
Transcriptional targeting involves the use of a particular cell-specific regulatory element to restrict gene expression to a particular tissue or cell type.
Many well-characterized prostate-specific promoters have been applied in targeted gene therapy. They include the prostate-specific antigen (PSA), and kallikrein 2, osteocalcin, and probasin promoters.
Improving promoter/enhancer activity while retaining proper regulation and prostate-selectivity can take on the form of duplicating key enhancer elements and removal of inert DNA sequences. In the case of the PSE-BC construct, a nearly 20-fold gain in activity was achieved.
Chimeric fusion between disparate prostate-specific promoters is an innovative approach to achieve broader expression in a heterogeneous prostate tumor.
The two-step transcriptional amplification system and its applications
The TSTA system is an excellent method to boost the activity of weak but specific promoters.
The prostate-specific TSTA system comprises a potent transcriptional activator, driven by the PSE-BC prostate-specific promoter, which then turns on expression of the reporter and/or therapeutic gene. The potent activator is the GAL4-VP16 fusion protein, which binds specifically to multiple repeats of GAL4-binding sites upstream of the gene of interest.
The TSTA system achieves nearly 1000-fold enhancement of activity over native PSA promoter. It maintains androgen responsiveness, prostate-selectivity and is able to drive robust gene expression in both AD and AI prostate tumors.
A TSTA adenoviral vector expressing a variant HSV-tk gene (AdTSTA-sr39tk) achieved tissue-restricted expression in vivo, as supported by PET imaging and cytotoxic activity mediated by the vector. The prostate-specific TSTA expression system is superior to the constitutive (CMV) approach in safeguarding against systemic toxicity, while achieving effective tumor killing.
The expression of imaging reporter gene in TSTA vector (AdTSTA-FL) is completely reliant on AR function in the cell. This fact was exploited to develop methods which enable the visualization of cancer transition from AD to AI state or therapeutic efficacy of anti-androgen in living animals.
Coupled imaging and therapeutic capabilities of TSTA vector will provide great benefits in monitoring in vivo therapeutic activity in prostate cancer clinical trials in the future.
Table 2.
Prostate-specific regulatory promoter/enhancers applied in adenoviral vector mediated interventions in vivo.
| Regulatory Element Configuration | Gene Therapy; Summary of Results | References |
|---|---|---|
| (a) Native promoters: | ||
| PSA (prostate-specific antigen) | • HSV-tk (therapeutic); cell killing in vitro and growth inhibition in tumor model. | • Huang, 1999 [39] |
| hK2 (Kallikrein-2, PSA-related) | • E1 protein (oncolytic); expression in PSA+, prostate tumor selective replication. | • Yu, 1999 [45] |
| OC (osteocalcin; prostatic metastasis and bone cell-specific) | • HSV-tk (therapeutic); Phase I clinical trials; | • Koeneman, 2000[17]; Kubo, 2003 [83] |
| • E1a and E1b (oncolytic); conditional replication competence in OC-expressing cells, growth inhibition in tumor model; | • Hsieh, 2002 [52] | |
|
| ||
| (b) Improved promoters: | ||
| PSE (PSA promoter/enhancer) | • Luciferase (detection of metastasis); expression (PSE-BC) higher in AI than in AD tumors and excluded from liver. Non-invasive bioluminescence imaging showed that the Ad could locate and illuminate lung and spine metastases. Systemic injection of low Ad doses also illuminated lung metastasis; | • Adams, 2002 [66] |
| • Nitroreductase (therapeutic); expression (PSE).in PSA+ cells inducible by androgen and comparable to that of CMV vector. | • Latham, 2000 [49] | |
| Rat Probasin (ARRPB2) | • E1a (oncolytic); selective replication in PSA+ cells | • Yu, 1999 [30] |
| • Bad (apoptotic); cell-specific expression and cell death in vitro; tumor size reduction in tumor models; | • Zhang, 2002 [84] | |
| • HSV-tk (therapeutic); retinoid inducible; growth suppression of AI tumor model. | • Furuhata, 2003 [85] | |
|
| ||
| (c) Chimeric elements: | ||
| PSMA/PSES (prostate- specific membrane antigen/ artificial chimeric enhancer) | • E1a (oncolytic); replication competent virus, exclusively in prostatic AI cells, efficient killing of PSMA+ cells in vitro and tumor model; | • Lee, 2004 [58] |
| • E1a and E4 (oncolytic); controlled viral vector replication and specific cell killing in prostate cancer cell lines and tumor model. | • Li, 2005 [59] | |
| PPT (PSMA enhancer/PSA enhancer/ T cell receptor gamma- chain alternate reading frame protein) | • Luciferase (reporter gene); high prostate-specificity and expression levels in the presence and absence of testosterone in vitro and in vivo; higher activity and selectivity than the CMV promoter following intravenous virus administration. | • Cheng, 2004 [60] |
Acknowledgments
This work is supported by the California Cancer Research Program 3NI0226 (to LW), DAMD17-03-1-0095 (to LW), and NIH R01 CA101904 (to LW). MF is supported by NCI Cancer Education Grant R25 CA098010 (Scholars in Oncologic Molecular Imaging Program).
References
- 1.ACS. American Cancer Society(issue) Washington, DC: [Accessed October 31, 2005]. 2005. American Cancer Society: Cancer Facts and Figures. [Google Scholar]
- 2.Scardino PT, Weaver R, Hudson MA. Early detection of prostate cancer. Hum Pathol. 1992;23(3):211–222. doi: 10.1016/0046-8177(92)90102-9. [DOI] [PubMed] [Google Scholar]
- 3.NIH: US National Institutes of Health (NIH) ClinicalTrials.gov. US National Library of Medicine; 2005. p. 2005. (issue) [Google Scholar]
- 4.Dong JT. Prevalent mutations in prostate cancer. J Cell Biochem. 2005 doi: 10.1002/jcb.20696. [DOI] [PubMed] [Google Scholar]
- 5.Foley R, Hollywood D, Lawler M. Molecular pathology of prostate cancer: the key to identifying new biomarkers of disease. Endocr Relat Cancer. 2004;11(3):477–488. doi: 10.1677/erc.1.00699. [DOI] [PubMed] [Google Scholar]
- 6.Bertram J, Peacock JW, Fazli L, et al. Loss of PTEN is associated with progression to androgen independence. Prostate. 2006 doi: 10.1002/pros.20411. [DOI] [PubMed] [Google Scholar]
- 7.Feldman BJ, Feldman D. The development of androgen-independent prostate cancer. Nat Rev Cancer. 2001;1(1):34–45. doi: 10.1038/35094009. [DOI] [PubMed] [Google Scholar]
- 8.Mabjeesh NJ, Zhong H, Simons JW. Gene therapy of prostate cancer: current and future directions. Endocr Relat Cancer. 2002;9(2):115–139. doi: 10.1677/erc.0.0090115. [DOI] [PubMed] [Google Scholar]
- 9.Eager R, Nemunaitis J. GM-CSF gene-transduced tumor vaccines. Mol Ther. 2005;12(1):18–27. doi: 10.1016/j.ymthe.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 10.Dendreon. ClinicalTrials.gov [Internet] Bethesda (MD): National Library of Medicine; 2000. Provenge® Immunotherapy Vaccine for the Treatment of Metastatic Prostate Cancer After Failing Hormone Therapy. Available from: http://clinicaltrials.gov/show/NCT00065442. [cited 2006 Apr 24] [Google Scholar]
- 11.Peng Z. Current status of gendicine in China: recombinant human Ad-p53 agent for treatment of cancers. Hum Gene Ther. 2005;16(9):1016–1027. doi: 10.1089/hum.2005.16.1016. [DOI] [PubMed] [Google Scholar]
- 12.University of British Columbia/ Department of Defense. ClinicalTrials.gov [Internet] Bethesda (MD): National Library of Medicine; 2000. A Phase II Study of OGX-011 Given Prior to Radical Prostatectomy in Patients With Localized Prostate Cancer. Available from: http://clinicaltrials.gov/show/NCT00138918. [cited 2006 Apr 24] [Google Scholar]
- 13.Southwest Oncology Group/ National Cancer Institute. ClinicalTrials.gov. Bethesda (MD): National Library of Medicine; 2000. Phase I Study of EGFRvIII Peptide Vaccine With Sargramostim (GM-CSF) Versus Keyhole Limpet Hemocyanin as Adjuvant in Patients With EGFRvIII-Expressing Cancer. [Internet] Available from: http://clinicaltrials.gov/show/NCT00023634. [cited 2006 Apr 24] [Google Scholar]
- 14.National Cancer Institute of Canada. ClinicalTrials.gov [Internet] Bethesda (MD): National Library of Medicine; 2000. Docetaxel and Prednisone With or Without OGX-011 in Treating Patients With Recurrent or Metastatic Prostate Cancer That Did Not Respond to Previous Hormone Therapy. Available from: http://clinicaltrials.gov/show/NCT00258388. [cited 2006 Apr 24] [Google Scholar]
- 15.Dykxhoorn DM, Palliser D, Lieberman J. The silent treatment: siRNAs as small molecule drugs. [DOI] [PubMed] [Google Scholar]
- 16.Aichberger KJ, Mayerhofer M, Krauth MT, et al. Low-level expression of proapoptotic Bcl-2-interacting mediator in leukemic cells in patients with chronic myeloid leukemia: role of BCR/ABL, characterization of underlying signaling pathways, and reexpression by novel pharmacologic compounds. Cancer Res. 2005;65(20):9436–9444. doi: 10.1158/0008-5472.CAN-05-0972. [DOI] [PubMed] [Google Scholar]
- 17.Koeneman KS, Kao C, Ko SC, et al. Osteocalcin-directed gene therapy for prostate-cancer bone metastasis. World J Urol. 2000;18(2):102–110. doi: 10.1007/s003450050181. [DOI] [PubMed] [Google Scholar]
- 18.Yeung F, Law WK, Yeh CH, et al. Regulation of human osteocalcin promoter in hormone-independent human prostate cancer cells. J Biol Chem. 2002;277(4):2468–2476. doi: 10.1074/jbc.M105947200. [DOI] [PubMed] [Google Scholar]
- 19.Rodriguez R, Schuur ER, Lim HY, Henderson GA, Simons JW, Henderson DR. Prostate attenuated replication competent adenovirus (ARCA) CN706: a selective cytotoxic for prostate-specific antigen-positive prostate cancer cells. Cancer Res. 1997;57(13):2559–2563. [PubMed] [Google Scholar]
- 20.Cancer Gene Ther; Abstracts of the 11th International Conference on Gene Therapy of Cancer; December 12–14, 2002; San Diego, California, USA. 2003. pp. S1–44. [PubMed] [Google Scholar]
- 21.Freytag SO, Paielli D, Wing M, et al. Efficacy and toxicity of replication-competent adenovirus-mediated double suicide gene therapy in combination with radiation therapy in an orthotopic mouse prostate cancer model. Int J Radiat Oncol Biol Phys. 2002;54(3):873–885. doi: 10.1016/s0360-3016(02)03005-5. [DOI] [PubMed] [Google Scholar]
- 22.Uckert W, Kammertons T, Haack K, et al. Double suicide gene (cytosine deaminase and herpes simplex virus thymidine kinase) but not single gene transfer allows reliable elimination of tumor cells in vivo. Hum Gene Ther. 1998;9(6):855–865. doi: 10.1089/hum.1998.9.6-855. [DOI] [PubMed] [Google Scholar]
- 23.Xie Y, Gilbert JD, Kim JH, Freytag SO. Efficacy of adenovirus-mediated CD/5-FC and HSV-1 thymidine kinase/ganciclovir suicide gene therapies concomitant with p53 gene therapy. Clin Cancer Res. 1999;5(12):4224–4232. [PubMed] [Google Scholar]
- 24.Ali S, King GD, Curtin JF, et al. Combined immunostimulation and conditional cytotoxic gene therapy provide long-term survival in a large glioma model. Cancer Res. 2005;65(16):7194–7204. doi: 10.1158/0008-5472.CAN-04-3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nishihara E, Nagayama Y, Mawatari F, et al. Retrovirus-mediated herpes simplex virus thymidine kinase gene transduction renders human thyroid carcinoma cell lines sensitive to ganciclovir and radiation in vitro and in vivo. Endocrinology. 1997;138(11):4577–4583. doi: 10.1210/endo.138.11.5509. [DOI] [PubMed] [Google Scholar]
- 26.Hanna NN, Mauceri HJ, Wayne JD, Hallahan DE, Kufe DW, Weichselbaum RR. Virally directed cytosine deaminase/5-fluorocytosine gene therapy enhances radiation response in human cancer xenografts. Cancer Res. 1997;57(19):4205–4209. [PubMed] [Google Scholar]
- 27.Chhikara M, Huang H, Vlachaki MT, et al. Enhanced therapeutic effect of HSV-tk+GCV gene therapy and ionizing radiation for prostate cancer. Mol Ther. 2001;3(4):536–542. doi: 10.1006/mthe.2001.0298. [DOI] [PubMed] [Google Scholar]
- 28.Teh BS, Ayala G, Aguilar L, et al. Phase I-II trial evaluating combined intensity-modulated radiotherapy and in situ gene therapy with or without hormonal therapy in treatment of prostate cancer-interim report on PSA response and biopsy data. Int J Radiat Oncol Biol Phys. 2004;58(5):1520–1529. doi: 10.1016/j.ijrobp.2003.09.083. [DOI] [PubMed] [Google Scholar]
- 29.Freytag SO, Rogulski KR, Paielli DL, Gilbert JD, Kim JH. A novel three-pronged approach to kill cancer cells selectively: concomitant viral, double suicide gene, and radiotherapy. Hum Gene Ther. 1998;9(9):1323–1333. doi: 10.1089/hum.1998.9.9-1323. [DOI] [PubMed] [Google Scholar]
- 30.Yu DC, Chen Y, Seng M, Dilley J, Henderson DR. The addition of adenovirus type 5 region E3 enables calydon virus 787 to eliminate distant prostate tumor xenografts. Cancer Res. 1999;59(17):4200–4203. [PubMed] [Google Scholar]
- 31.Dilley J, Reddy S, Ko D, et al. Oncolytic adenovirus CG7870 in combination with radiation demonstrates synergistic enhancements of antitumor efficacy without loss of specificity. Cancer Gene Ther. 2005;12(8):715–722. doi: 10.1038/sj.cgt.7700835. [DOI] [PubMed] [Google Scholar]
- 32.Aumuller G, Seitz J, Lilja H, Abrahamsson PA, von der Kammer H, Scheit KH. Species- and organ-specificity of secretory proteins derived from human prostate and seminal vesicles. Prostate. 1990;17(1):31–40. doi: 10.1002/pros.2990170105. [DOI] [PubMed] [Google Scholar]
- 33.Schaffner DL, Barrios R, Shaker MR, et al. Transgenic mice carrying a PSArasT24 hybrid gene develop salivary gland and gastrointestinal tract neoplasms. Lab Invest. 1995;72(3):283–290. [PubMed] [Google Scholar]
- 34.Cleutjens KB, van der Korput HA, van Eekelen CC, van Rooij HC, Faber PW, Trapman J. An androgen response element in a far upstream enhancer region is essential for high, androgen-regulated activity of the prostate-specific antigen promoter. Mol Endocrinol. 1997;11(2):148–161. doi: 10.1210/mend.11.2.9883. [DOI] [PubMed] [Google Scholar]
- 35.Cleutjens KB, van der Korput HA, Ehren-van Eekelen CC, et al. A 6-kb promoter fragment mimics in transgenic mice the prostate-specific and androgen-regulated expression of the endogenous prostate-specific antigen gene in humans. Mol Endocrinol. 1997;11(9):1256–1265. doi: 10.1210/mend.11.9.9974. [DOI] [PubMed] [Google Scholar]
- 36.Pang S, Dannull J, Kaboo R, et al. Identification of a positive regulatory element responsible for tissue-specific expression of prostate-specific antigen. Cancer Res. 1997;57(3):495–499. [PubMed] [Google Scholar]
- 37.Schuur ER, Henderson GA, Kmetec LA, Miller JD, Lamparski HG, Henderson DR. Prostate-specific antigen expression is regulated by an upstream enhancer. J Biol Chem. 1996;271(12):7043–7051. doi: 10.1074/jbc.271.12.7043. [DOI] [PubMed] [Google Scholar]
- 38.Cleutjens KB, van Eekelen CC, van der Korput HA, Brinkmann AO, Trapman J. Two androgen response regions cooperate in steroid hormone regulated activity of the prostate-specific antigen promoter. J Biol Chem. 1996;271(11):6379–6388. doi: 10.1074/jbc.271.11.6379. [DOI] [PubMed] [Google Scholar]
- 39.Huang W, Shostak Y, Tarr P, Sawyers C, Carey M. Cooperative assembly of androgen receptor into a nucleoprotein complex that regulates the prostate-specific antigen enhancer. J Biol Chem. 1999;274(36):25756–25768. doi: 10.1074/jbc.274.36.25756. [DOI] [PubMed] [Google Scholar]
- 40.Lee SE, Jin RJ, Lee SG, et al. Development of a new plasmid vector with PSA-promoter and enhancer expressing tissue-specificity in prostate carcinoma cell lines. Anticancer Res. 2000;20(1A):417–422. [PubMed] [Google Scholar]
- 41.Spitzweg C, Zhang S, Bergert ER, et al. Prostate-specific antigen (PSA) promoter-driven androgen-inducible expression of sodium iodide symporter in prostate cancer cell lines. Cancer Res. 1999;59(9):2136–2141. [PubMed] [Google Scholar]
- 42.Wu L, Matherly J, Smallwood A, et al. Chimeric PSA enhancers exhibit augmented activity in prostate cancer gene therapy vectors. Gene Ther. 2001;8(18):1416–1426. doi: 10.1038/sj.gt.3301549. [DOI] [PubMed] [Google Scholar]
- 43.Adams JY, Johnson M, Sato M, et al. Visualization of advanced human prostate cancer lesions in living mice by a targeted gene transfer vector and optical imaging. Nat Med. 2002;8(8):891–897. doi: 10.1038/nm743. [DOI] [PubMed] [Google Scholar]
- 44.Mitchell SH, Murtha PE, Zhang S, Zhu W, Young CY. An androgen response element mediates LNCaP cell dependent androgen induction of the hK2 gene. Mol Cell Endocrinol. 2000;168(1–2):89–99. doi: 10.1016/s0303-7207(00)00319-1. [DOI] [PubMed] [Google Scholar]
- 45.Yu DC, Sakamoto GT, Henderson DR. Identification of the transcriptional regulatory sequences of human kallikrein 2 and their use in the construction of calydon virus 764, an attenuated replication competent adenovirus for prostate cancer therapy. Cancer Res. 1999;59(7):1498–1504. [PubMed] [Google Scholar]
- 46.Xie X, Zhao X, Liu Y, et al. Robust prostate-specific expression for targeted gene therapy based on the human kallikrein 2 promoter. Hum Gene Ther. 2001;12(5):549–561. doi: 10.1089/104303401300042483. [DOI] [PubMed] [Google Scholar]
- 47.Yousef GM, Diamandis EP. Expanded human tissue kallikrein family--a novel panel of cancer biomarkers. Tumour Biol. 2002;23(3):185–192. doi: 10.1159/000064027. [DOI] [PubMed] [Google Scholar]
- 48.Zhang J, Thomas TZ, Kasper S, Matusik RJ. A small composite probasin promoter confers high levels of prostate-specific gene expression through regulation by androgens and glucocorticoids in vitro and in vivo. Endocrinology. 2000;141(12):4698–4710. doi: 10.1210/endo.141.12.7837. [DOI] [PubMed] [Google Scholar]
- 49.Latham JP, Searle PF, Mautner V, James ND. Prostate-specific antigen promoter/enhancer driven gene therapy for prostate cancer: construction and testing of a tissue-specific adenovirus vector. Cancer Res. 2000;60(2):334–341. [PubMed] [Google Scholar]
- 50.Fair WR, Israeli RS, Heston WD. Prostate-specific membrane antigen. Prostate. 1997;32(2):140–148. doi: 10.1002/(sici)1097-0045(19970701)32:2<140::aid-pros9>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 51.Matsubara S, Wada Y, Gardner TA, et al. A conditional replication-competent adenoviral vector, Ad-OC-E1a, to cotarget prostate cancer and bone stroma in an experimental model of androgen-independent prostate cancer bone metastasis. Cancer Res. 2001;61(16):6012–6019. [PubMed] [Google Scholar]
- 52.Hsieh CL, Yang L, Miao L, et al. A novel targeting modality to enhance adenoviral replication by vitamin D(3) in androgen-independent human prostate cancer cells and tumors. Cancer Res. 2002;62(11):3084–3092. [PubMed] [Google Scholar]
- 53.Lian JB, Stein GS, Stein JL, van Wijnen AJ. Regulated expression of the bone-specific osteocalcin gene by vitamins and hormones. Vitam Horm. 1999;(55):443–509. doi: 10.1016/s0083-6729(08)60941-3. [DOI] [PubMed] [Google Scholar]
- 54.Horoszewicz JS, Kawinski E, Murphy GP. Monoclonal antibodies to a new antigenic marker in epithelial prostatic cells and serum of prostatic cancer patients. Anticancer Res. 1987;7(5B):927–935. [PubMed] [Google Scholar]
- 55.Silver DA, Pellicer I, Fair WR, Heston WD, Cordon-Cardo C. Prostate-specific membrane antigen expression in normal and malignant human tissues. Clin Cancer Res. 1997;3(1):81–85. [PubMed] [Google Scholar]
- 56.O’Keefe DS, Uchida A, Bacich DJ, et al. Prostate-specific suicide gene therapy using the prostate-specific membrane antigen promoter and enhancer. Prostate. 2000;45(2):149–157. doi: 10.1002/1097-0045(20001001)45:2<149::aid-pros9>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 57.Tsui KH, Wu L, Chang PL, Hsieh ML, Juang HH. Identifying the combination of the transcriptional regulatory sequences on prostate specific antigen and human glandular kallikrein genes. J Urol. 2004;172(5 Pt 1):2029–2034. doi: 10.1097/01.ju.0000141147.96640.76. [DOI] [PubMed] [Google Scholar]
- 58.Lee SJ, Zhang Y, Lee SD, et al. Targeting prostate cancer with conditionally replicative adenovirus using PSMA enhancer. Mol Ther. 2004;10(6):1051–1058. doi: 10.1016/j.ymthe.2004.08.028. [DOI] [PubMed] [Google Scholar]
- 59.Li X, Zhang YP, Kim HS, et al. Gene therapy for prostate cancer by controlling adenovirus E1a and E4 gene expression with PSES enhancer. Cancer Res. 2005;65(5):1941–1951. doi: 10.1158/0008-5472.CAN-04-3666. [DOI] [PubMed] [Google Scholar]
- 60.Cheng WS, Kraaij R, Nilsson B, et al. A novel TARP-promoter-based adenovirus against hormone-dependent and hormone-refractory prostate cancer. Mol Ther. 2004;10(2):355–364. doi: 10.1016/j.ymthe.2004.05.022. [DOI] [PubMed] [Google Scholar]
- 61.Zhang L, Adams JY, Billick E, et al. Molecular engineering of a two-step transcription amplification (TSTA) system for transgene delivery in prostate cancer. Mol Ther. 2002;5(3):223–232. doi: 10.1006/mthe.2002.0551. [DOI] [PubMed] [Google Scholar]
- 62.Iyer M, Wu L, Carey M, Wang Y, Smallwood A, Gambhir SS. Two-step transcriptional amplification as a method for imaging reporter gene expression using weak promoters. Proc Natl Acad Sci U S A. 2001;98(25):14595–14600. doi: 10.1073/pnas.251551098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sato M, Johnson M, Zhang L, et al. Optimization of adenoviral vectors to direct highly amplified prostate-specific expression for imaging and gene therapy. Mol Ther. 2003;8(5):726–737. doi: 10.1016/j.ymthe.2003.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sato M, Johnson M, Zhang L, Gambhir SS, Carey M, Wu L. Functionality of androgen receptor-based gene expression imaging in hormone refractory prostate cancer. Clin Cancer Res. 2005;11(10):3743–3749. doi: 10.1158/1078-0432.CCR-04-1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhang L, Johnson M, Le KH, et al. Interrogating androgen receptor function in recurrent prostate cancer. Cancer Res. 2003;63(15):4552–4560. [PubMed] [Google Scholar]
- 66.Johnson M, Sato M, Burton J, Gambhir SS, Carey M, Wu L. Micro-PET/CT Monitoring of Herpes Thymidine Kinase Suicide Gene Therapy in a Prostate Cancer Xenograft: The Advantage of a Cell-specific Transcriptional Targeting Approach. Mol Imaging. 2005;4(4):463–472. doi: 10.2310/7290.2005.05154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Segawa T, Takebayashi H, Kakehi Y, Yoshida O, Narumiya S, Kakizuka A. Prostate-specific amplification of expanded polyglutamine expression: a novel approach for cancer gene therapy. Cancer Res. 1998;58(11):2282–2287. [PubMed] [Google Scholar]
- 68.Qiao J, Doubrovin M, Sauter BV, et al. Tumor-specific transcriptional targeting of suicide gene therapy. Gene Ther. 2002;9(3):168–175. doi: 10.1038/sj.gt.3301618. [DOI] [PubMed] [Google Scholar]
- 69.Ilagan R, Zhang LJ, Pottratz J, et al. Imaging androgen receptor function during flutamide treatment in the LAPC9 xenograft model. Mol Cancer Ther. 2005;4(11):1662–1669. doi: 10.1158/1535-7163.MCT-05-0197. [DOI] [PubMed] [Google Scholar]
- 70.Ray S, Paulmurugan R, Hildebrandt I, et al. Novel bidirectional vector strategy for amplification of therapeutic and reporter gene expression. Hum Gene Ther. 2004;15(7):681–690. doi: 10.1089/1043034041361271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mount Sinai School of Medicine/U.S. Army Medical Research and Material Command. ClinicalTrials.gov [Internet] Bethesda (MD): National Library of Medicine; 2000. Gene therapy for prostate cancer that returns after radiation therapy. Available from: http://clinicaltrials.gov/show/NCT00085228. [cited 2006 Apr 24] [Google Scholar]
- 72.Transgene/ University of California Los Angeles/ The Cleveland Clinic/ University of Arizona/ University of Chicago Mount Sinai School of Medicine/U.S. Army Medical Research and Material Command. ClinicalTrials.gov [Internet] Bethesda (MD): National Library of Medicine; 2000. Vaccine Study of MVA-MUC1-IL2 in Patients With Prostate Cancer. Available from: http://clinicaltrials.gov/show/NCT00040170. [cited 2006 Apr 24] [Google Scholar]
- 73.Cosmo bioscience. ClinicalTrials.gov [Internet] Bethesda (MD): National Library of Medicine; 2000. Evaluation of Transgenic Lymphocyte Immunization Vaccine in Subjects With Prostate Adenocarcinoma. Available from: http://clinicaltrials.gov/show/NCT00061035. [cited 2006 Apr 24] [Google Scholar]
- 74.Providence Health Care/ Cell Genesys. ClinicalTrials.gov [Internet] Bethesda (MD): National Library of Medicine; 2000. GVAX in advanced prostate cancer patients made lymphopenic. Available from: http://clinicaltrials.gov/show/NCT00122005. [cited 2006 Apr 24] [Google Scholar]
- 75.Cell Genesys. ClinicalTrials.gov [Internet] Bethesda (MD): National Library of Medicine; 2000. GVAX® Vaccine for Prostate Cancer Vs Docetaxel & Prednisone in Patients With Metastatic Hormone-Refractory Prostate Cancer. Available from: http://clinicaltrials.gov/show/NCT00089856. [cited 2006 Apr 24] [Google Scholar]
- 76.NCI. ClinicalTrials.gov [Internet] Bethesda (MD): National Library of Medicine; 2000. Vaccine and Antibody Treatment of Prostate Cancer. Available from: http://clinicaltrials.gov/show/NCT00113984. [cited 2006 Apr 24] [Google Scholar]
- 77.NCI, ECOG. ClinicalTrials.gov [Internet] Bethesda (MD): National Library of Medicine; 2000. Vaccine Therapy and GM-CSF in Treating Patients With Prostate Cancer That Progressed After Surgery and/or Radiation Therapy. Available from: http://clinicaltrials.gov/show/NCT00108732. [cited 2006 Apr 24] [Google Scholar]
- 78.National Cancer Institute. ClinicalTrials.gov [Internet] Bethesda (MD): National Library of Medicine; 2000. Vaccine Therapy, MDX-010, and GM-CSF in Treating Patients With Metastatic Prostate Cancer. Available from: http://clinicaltrials.gov/show/NCT00124670. [cited 2006 Apr 24] [Google Scholar]
- 79.Dendreon/ NCI. ClinicalTrials.gov [Internet] Bethesda (MD): National Library of Medicine; 2000. Vaccine Therapy in Treating Patients with Metastatic Prostate Cancer That has not Responded to Hormone Therapy. Available from: http://clinicaltrials.gov/show/NCT00005947. [cited 2006 Apr 24] [Google Scholar]
- 80.Mayo Clinic/ Dendreon. ClinicalTrials.gov [Internet] Bethesda (MD): National Library of Medicine; 2000. Study of APC8015F Immunotherapy in Prostate Cancer Patients Who Participated in Study D9902B and Have Experienced Disease Progression and Disease-Related Pain. Available from: http://clinicaltrials.gov/show/NCT00170066. [cited 2006 Apr 24] [Google Scholar]
- 81.NewLink Genetics Corporation. ClinicalTrials.gov [Internet] Bethesda (MD): National Library of Medicine; 2000. Vaccine Treatment for Hormone Refractory Prostate Cancer. Available from: http://clinicaltrials.gov/show/NCT00105053. [cited 2006 Apr 24] [Google Scholar]
- 82.Cell Genesys. ClinicalTrials.gov [Internet] Bethesda (MD): National Library of Medicine; 2000. Intravenous CG7870 in Combination With Docetaxel in Patients With Metastatic Hormone-Refractory Prostate Cancer. Available from: http://clinicaltrials.gov/show/NCT00103428. [cited 2006 Apr 24] [Google Scholar]
- 83.Kubo H, Gardner TA, Wada Y, et al. Phase I dose escalation clinical trial of adenovirus vector carrying osteocalcin promoter-driven herpes simplex virus thymidine kinase in localized and metastatic hormone-refractory prostate cancer. Hum Gene Ther. 2003;14(3):227–241. doi: 10.1089/10430340360535788. [DOI] [PubMed] [Google Scholar]
- 84.Zhang Y, Yu J, Unni E, et al. Monogene and polygene therapy for the treatment of experimental prostate cancers by use of apoptotic genes bax and bad driven by the prostate-specific promoter ARR(2)PB. Hum Gene Ther. 2002;13(17):2051–2064. doi: 10.1089/10430340260395901. [DOI] [PubMed] [Google Scholar]
- 85.Furuhata S, Ide H, Miura Y, Yoshida T, Aoki K. Development of a prostate-specific promoter for gene therapy against androgen-independent prostate cancer. Mol Ther. 2003;7(3):366–374. doi: 10.1016/s1525-0016(02)00059-x. [DOI] [PubMed] [Google Scholar]