Abstract
The correct establishment and maintenance of cell polarity are crucial for normal cell physiology and tissue homeostasis. Conversely, loss of cell polarity, tissue disorganisation and excessive cell growth are hallmarks of cancer. In this review, we focus on identifying the stages of tumoural development that are affected by the loss or deregulation of epithelial cell polarity. Asymmetric division has recently emerged as a major regulatory mechanism that controls stem cell numbers and differentiation. Links between cell polarity and asymmetric cell division in the context of cancer will be examined. Apical–basal polarity and cell–cell adhesion are tightly interconnected. Hence, how loss of cell polarity in epithelial cells may promote epithelial mesenchymal transition and metastasis will also be discussed. Altogether, we present the argument that loss of epithelial cell polarity may have an important role in both the initiation of tumourigenesis and in later stages of tumour development, favouring the progression of tumours from benign to malignancy.
Keywords: cell polarity, cancer, asymmetric cell division, EMT, Par, scribble
Epithelial tissues are widely distributed, lining the external and internal surfaces of our bodies and playing a number of specialised roles. Each specialist function is achieved by the distinct structural organisation of epithelial cells within those tissues. Consequently, the integrity of their architecture is crucial. The majority of human cancers are derived from epithelial tissues, and display loss of cell polarity and often, as a consequence, tissue disorganisation.
Although the tumour suppressive function of polarity complexes is well established in Drosophila, it remains unclear whether a loss of cell polarity is a consequence or cause of human cancers. However, it is emerging that epithelial cell polarity may exert a tumour suppressive function in mammals through its participation in the establishment and maintenance of the three dimensional organisation of epithelial tissues as a whole. This theory is supported by the findings that polarity proteins are cellular targets of oncogenes, and an increasing list of tumour suppressors has been shown to regulate polarity pathways.
Functionally, apical–basal polarity has two fundamental roles in epithelial cells that are intimately linked to tumour suppression: (1) the regulation of asymmetric cell division and (2) the maintenance of the apical junctional complex (AJC). In epithelial stem cells, polarity proteins control asymmetric cell division by regulating the polarised localisation of cell fate determinants and the correct orientation of mitotic spindles. As a result, asymmetric cell division has a fundamental role in the control of progenitor or stem cell numbers and differentiation. This is of particular interest in the context of the cancer stem cell theory, as a shift from asymmetric division of epithelial stem cells or cancer-initiating cells towards symmetric divisions would result in dedifferentiation on one hand and an increase in cancer-initiating cells on the other. Thus, a defect in asymmetric division could contribute to the emergence of tumours. As a result, over the last few years, there has been increasing interest in the identification of core cell-polarity mechanisms that govern the asymmetric division of epithelial stem cells, and understanding how their disruption may contribute to the development of cancer.
In addition to their role in the prevention of tumour initiation, core epithelial cell polarity mechanisms may also constitute a barrier to tumour metastasis and malignancy through their close connection to the AJC. The AJC encompasses tight and adherens junction complexes, and its structure is dependent on the integrity of the apical and basolateral polarity complexes. The loss of one of the key components of adherens junctions, E-cadherin, often occurs in later stages of tumourigenesis and is thought to contribute to epithelial mesenchymal transition (EMT), which represents a crucial step in metastasis. The importance of the AJC in suppressing cancer malignancy is supported by cancer genome sequencing data, which show that a large number of AJC components are frequently mutated in human cancers. In this review, we will therefore discuss whether a loss or deregulation of epithelial cell polarity favours tumour initiation, or is responsible for later stages of tumour development and malignancy.
The Main Players in the Establishment and Maintenance of Epithelial Cell Polarity
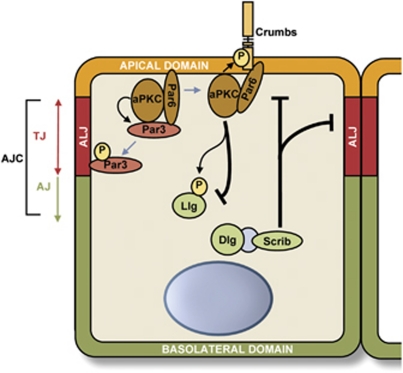
In order to understand how cells become disorganised in tumours it is vital to understand the key players and regulators that control and maintain cell polarity, which lead to epithelial tissue organisation. As expected, the molecular complexes involved in the establishment and maintenance of cell polarity are largely conserved throughout the metazoa, despite the wide range of epithelial tissue types and biological processes that require them. Three major complexes involved in the regulation of apical–basal cell polarity of epithelial cells have been described: The Crumbs-Pals1 (Stardust)-Patj-Lin-7 (Crumbs complex) and Par3 (Bazooka)-Par6-aPKC (Par complex) complexes, which are found apically, and the lethal giant larvae (Lgl)-Scribble (Scrib)-Disc large (Dlg) proteins (Scribble complex) that localise at the basolateral membrane.1, 2, 3 Both the Par and Crumbs complexes promote apical membrane identity, whereas the Scribble complex promotes basolateral membrane identity by antagonising the other two (Figure 1).
Figure 1.
Diagram representing the core polarity components involved in the maintenance of apical–basal polarity in epithelial cells and the establishment of membrane domain identity. AJ, adherens junctions; ALJ, apical/lateral junction; TJ, tight junctions
Among the three polarity complexes, the Par complex is the best studied in the context of apical–basal polarity in epithelial cells. Genetic screens in C. elegans have identified six Par genes (Par1–6). Classically, the proteins considered to be the core of the Par complex include the two Par PDZ domain-containing proteins Par3 and Par6, with the addition of atypical protein kinase C (aPKC), and the Par complex has been shown to be required for the establishment and the maintenance of apical–basal polarity and apical domain development in epithelial cells.4, 5, 6 Phosphorylation has a key role in controlling polarity, and this is reflected by the fact that Par1 and Par4, homologous in mammals to microtubule affinity regulating kinases (MARKs) and LKB1, respectively, are serine/threonine kinases and Par5 is a member of the 14-3-3 family of proteins that generally binds Ser/Thr phosphorylated proteins. Additionally, the functions of the Par complex are regulated by phosphorylation. Par3 is phosphorylated on Ser827 of its aPKC-binding region by aPKC itself, resulting in decreased affinity for aPKC.7 Rho kinase also prevents the interaction between Par3 and aPKC by phosphorylating Thr883 of Par3, thereby suppressing the activity of the Par complex.8
The activity of the Par complex is further regulated by the dynamic nature of Par3's association with the stable Par6–aPKC complex. A compelling study in Drosophila demonstrated that Par3 is in fact excluded from the apical domain by the Par6–aPKC complex.9 Instead, the Drosophila Par3 homologue Baz localised independently of aPKC and Par6 in the follicular epithelium, and below them at the level of the apical/lateral junction. This correlates with the observation that in many epithelial tissues, including in mammals, Par3 and the Par6–aPKC complex do not colocalise.10, 11, 12, 13 However, in mammals, the apical/lateral domain is formed by tight junctions, which are more apical and distinct from the adherens junctions, thus Par3 is essentially localised at the level of tight junctions where it colocalises with zonula occludens-1 (ZO-1).14, 15 This model for Par3 exclusion from the apical domain involves both the Par6–aPKC complex and the Crumbs complex, in order to prevent the interaction between Par3 and the Par6–aPKC complex. On one hand, aPKC phosphorylates Par3 on Ser827 in mammalian Par3 to decrease their affinity for each other while, on the other hand, Crumbs and Stardust compete with Par3 to interact with the same domain of Par6 (Figure 1). This exclusion mechanism is crucial to restrict the extent of the apical/lateral junction and define the border between the apical and lateral domains in Drosophila epithelial cells. Further investigation is required, but the existing evidence suggests that the observations outlined above may be generalised to epithelial tissues in mammals.9
The three members of the Scribble complex have been shown to interact genetically,16 with Dlg and Scribble physically interacting through a protein called GUK-holder in Drosophila neuronal synapses.17 However, there is little evidence for their physical interaction in mammalian epithelial cells and, as a result, the term ‘module' is sometimes used when referring to the Scribble complex. More recently, Scribble and Lgl2 have been reported to interact directly in polarised mammalian epithelial cells, although this interaction has not yet been reported in other experimental systems.18 Lgl, by competing with Par3 for Par6–aPKC, restricts the Par complex to the apical domain.5, 19 Furthermore, phosphorylation and inactivation of Lgl at the apical domain by aPKC restricts the Par and Scribble complex apically and basolaterally in epithelial cells, respectively.20 Interestingly, in Drosophila, aPKC is also able to phosphorylate Crumbs to promote the apical localisation of the Crumbs complex.21 Key phosphorylation events and protein/protein interactions therefore result in the exclusion of Scribble from the apical domain, and of the Crumbs and Par complexes from the basolateral domain. These membrane domains consequently acquire unique identities that set the basis for the establishment of apical–basal polarity in epithelial cells.
Cell Polarity and Asymmetric Cell Division
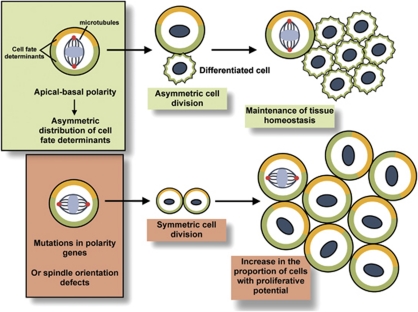
Asymmetric cell division is pivotal for the maintenance of epithelial tissue homeostasis. When a stem cell divides asymmetrically, it generates two daughter cells: one with an identical cell fate and the other with a different one. Asymmetric cell division relies on the asymmetric distribution of cell fate determinants (Numb, Pros, Brat, Pon and Mira) and, as a result, core polarity proteins and the correct orientation of mitotic spindles.2, 22 Emerging evidence, in particular from studies of neuroblasts in Drosophila, indicates that asymmetric division functions as a mechanism of tumour suppression.23 When polarity genes or cell fate determinants are deleted or mutated, neuroblasts divide symmetrically, leading to tissue overgrowth and transplantable tumours that are similar to mammalian cancers (Figure 2). For example, Dlg/Scrib/Lgl mutant neuroblasts have defects in basal protein targeting, a reduced apical cortical domain and reduced apical spindle size, which is thought to lead to symmetric divisions and, as a result, to the accumulation of cells and the development of tumours.24 In Drosophila, a number of proteins such as Pins, Mud and the mitotic kinases Aurora-A and Polo have been shown to have a major role in regulating mitotic spindle orientation and asymmetric cell division.25 Intriguingly, adenomatous polyposis coli 2 (APC2) was discovered to be part of the centrosome complex of Drosophila germline cells, where it functions in establishing the correct orientation of the mitotic spindles. Moreover, deletion of both Drosophila APC genes results in asymmetric stem cell division defects as a result of mitotic spindle misorientation.26 Interestingly, Johnston et al.27 have developed an ‘induced cortical polarity' assay in Drosophila S2 cells that should facilitate the identification of further proteins, domains and amino acids that regulate spindle orientation.
Figure 2.
Loss of cell polarity in epithelial stem cells can lead to asymmetric division defects, thereby favouring tumour initiation. Apical–basal polarity is fundamental to the asymmetric segregation of cell fate determinants. Thick yellow and green lines represent cell fate determinants. Red dots represent centrosomes. In the absence of apical–basal polarity, this segregation is defective, potentially leading to an excess in symmetric divisions and an accumulation of cells with proliferative potential
In mammals, probably as a consequence of their redundancy, the function of core polarity proteins in the regulation of asymmetric cell division has been harder to elucidate. However, Lgl1 knockout mice present some defects in the asymmetric division of neural progenitors, that may result in overproliferation and a lack of differentiation.28 Recently, in a compelling study using in vivo electroporation of mouse embryos and cortical slice cultures, Par3 has been shown to regulate the asymmetric division of neural progenitor cells via the control of the Notch signalling pathway in the developing neocortex.29 This study has also shown that Par3 is distributed and inherited asymmetrically as cells divide, suggesting that its subcellular distribution regulates the mode of progenitor cell division and daughter cell fate specification.
Polarity and EMT
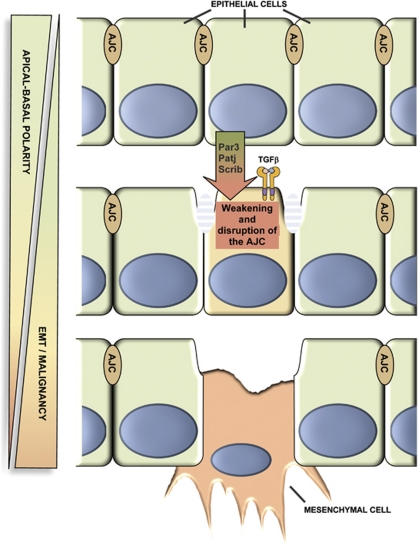
The transition from an epithelial to mesenchymal phenotype that occurs during EMT has been associated with metastatic progression; apical–basal polarity is lost during this process and cell–cell junctions are weakened and disrupted. Several lines of evidence suggest that core polarity proteins are important for the formation and maintenance of the AJC, suggesting that their loss could induce or at least contribute to EMT (Figure 3). For example, it has been shown that Par3 depletion in mammalian epithelial cells disrupts the formation of tight junctions.30 Moreover, it has been suggested that the Par6–aPKC complex, together with Cdc42, can control adherens junction remodelling through the regulation of Arp2/3-dependent endocytosis.31 Another example comes from knock-down experiments showing that PATJ is important for the proper polarisation of mammalian epithelial cells and the formation of tight junctions.32 SiRNA-mediated knockdown of Scrib in SK-CO15 cells inhibits tight junction reassembly.33
Figure 3.
Epithelial cell polarity represents a barrier to the later stages of tumour development. Apical–basal polarity is involved in the formation and maintenance of the AJC. Decreased expression of core polarity proteins is linked to weakening or disruption of the AJC, thereby leading to EMT and potential malignancy. Recent evidence suggests that TGFβ, under certain conditions, can induce EMT in epithelial cells in conjunction with loss of cell polarity
TGFβ, in cooperation with the Ras pathway, is a key inducer of EMT and promotes invasion and metastasis. Several studies suggest that disrupting cell polarity may work in concert with TGFβ signalling or facilitate TGFβ-mediated EMT. For instance, it has recently been shown that disruption of the Crumbs complex predisposes to EMT in Eph4 cells, a cell line normally insensitive to TGFβ-mediated EMT.34 Another study shows that TGFβ signalling is able to downregulate the expression of Par-3 through the induction of miR-491-5p in rat proximal tubular epithelial cells, suggesting that TGFβ may induce the disruption of cell polarity.35 Probably, one of the most direct links between the disruption of cell polarity and EMT comes from the finding that TGFβ receptors directly interact with Par6 at the level of adherens junctions.36 TGFβ is able to induce phosphorylation of Par6 at Ser345, enabling the recruitment of the E3 ubiquitin ligase Smurf1, which is required for localised TGFβ-induced degradation of the actin regulator RhoA. Importantly, this phosphorylation event is required for TGFβ-dependent EMT in mammary gland epithelial cells, as mutation of this residue blocks EMT and tight junction disruption. Furthermore, in a mammary fat pad assay, over-expression of Par6/S345A in EMT-6 cells reduced their ability to induce metastasis to the lung, further demonstrating the importance of TGFβ/Par6 signalling in EMT and the development of metastasis.37 Taken together, these studies show that polarity proteins are important EMT regulators in epithelial cells.
Is Epithelial Cell Polarity a Gate Keeper Against Cancer?
Disruption of polarity by activated oncogenes
Underlying their potential role as tumour suppressors, core polarity proteins are often targeted and disrupted by oncogenic signalling. Polarity defects could collaborate with oncogenic pathways to induce tumour formation. For example, Scrib-deficient mutants cooperate with oncogenes to mediate transformation in Drosophila. Normally, Scrib-deficient mutant clones in the eye imaginal discs are eliminated by JNK-dependent apoptosis. However, in the presence of activated oncogenic pathways such as Ras or Notch, apoptosis is inhibited and neoplastic tumours occur.38 In addition, in support of these data, loss of hScrib has been shown to cooperate with H-Ras to promote cell invasion through deregulation of MAPK signalling in an organotypic culture system.39
A number of viral oncogenes have been found to directly interact with polarity proteins, suggesting that disruption of polarity is important.40 For example, the human T-cell leukemia virus type 1 tax protein binds to hScrib and alters its subcellular localisation in infected T-cells.41, 42 In addition to Scrib, Tax and the oncoprotein 9ORF1 have been shown to interact with Dlg.43, 44, 45 The interaction of Tax and hDlg was shown to affect the function of hDlg in controlling cell growth, and Tax disrupted the interaction between hDlg and APC.46 However, it remains to be shown whether disruption of the hDlg/APC complex is the critical event that leads to deregulated cell growth. E6 oncogenes in human papilloma virus (HPV) have also been found to interact with PDZ-containing polarity proteins. For example, E6 proteins from HPV-16 and HPV-18 have the ability to interact with, and induce the proteasomal degradation of, Dlg and hScrib.47 Intriguingly, E6 has also been shown to target phosphorylated forms of Dlg, and Patj of the Crumbs polarity complex, for degradation.48, 49 These findings suggest that key PDZ-containing polarity proteins are common cellular targets of viral oncoproteins. Moreover, it seems that viral proteins have evolved additional ways to target cell polarity, strongly suggesting that its disruption is necessary for malignant transformation.40
In addition to viral oncogenes, core cell polarity mechanisms are targeted by abnormally activated growth factor signalling pathways. Both the ErbB2 and TGFβ signalling pathways have been shown to be directly involved in the regulation of polarity independently of their transcriptional response.36, 50 In conclusion, many activated oncogenic pathways target and disrupt epithelial polarity in order to achieve malignant transformation, supporting the importance of cell polarity in suppressing tumour formation.
The core polarity proteins as tumour suppressors in mammals
Scrib, dlg and lgl were identified as tumour suppressors in Drosophila, in screens for mutations causing cancerous overgrowth of the larval imaginal discs and brain.51 Interestingly, the phenotypes of these mutants can be rescued by the mammalian homologues of these genes, showing that they are functionally conserved and suggesting that they may have a tumour suppressive function in human cells.52, 53, 54, 55 A growing amount of data showing mislocalisation, decreased expression or complete loss of the products of these genes in primary tumours from human patients further indicate their involvement in mammalian tumourigenesis.56 Over the past few years, there has been an increasing body of evidence that the deregulation of these core polarity complexes, both in terms of expression level and subcellular localisation, may have a causal link to disease and cancer in particular (for an exhaustive list of changes in expression and localisation of polarity proteins in carcinoma cell lines and primary tissues see Huang and Muthuswamy56).
Perhaps the best-studied polarity complex in human tumours is the Scrib/Lgl/Dlg complex. In particular, the deregulation of Scrib has been reported to promote the transformation of mammary epithelial cells in vitro and in vivo, by disrupting morphogenesis and cell polarity and by inhibiting myc-induced apoptosis, thus providing novel insight into how core polarity proteins regulate cell transformation.57 In this study, loss of Scrib was shown to cooperate with Myc to induce mammary tumours, correlating with a Drosophila study that showed that lgl requires Myc to promote clonal malignancy.58 In human keratinocytes, Scrib loss has been shown to result in ERK activation which might contribute to cancer progression.59 Scrib was found to be mislocalised or downregulated in several cancer types including cervical,60 colon adenocarcinoma61 and endometrial.62 Scrib, as well as Dlg1 and Lgl1, was also mislocalised and downregulated in a transgenic mouse model of cancer.63 In a screen of 60 tumour samples, Hugl-1 (the human homologue of Lgl) transcripts were often reduced or lost in tumour tissues of the breast (76%), prostate (53%), lung (63%), ovarian (50%) and colon carcinoma (75%).53 However, the numbers in this study are relatively small and it will be important to verify these data on a larger scale. Hugl-1 was lost in 75% of tumour samples in a cohort of 94 patients undergoing surgery for colorectal cancer, and was associated with advanced stage and lymph node metastases.64 Loss or downregulation of Hugl-1 expression was also observed in malignant melanoma and associated with an advanced stage of the disease.54 A study of 80 hepatocellular carcinomas showed that Hugl-1 mRNA is frequently mutated by aberrant splicing, and that two of the variants were able to promote hepatocellular carcinoma in nude mice.65 Dlg expression levels and localisation have also been shown to be affected in high-grade premalignant cervical neoplasia, invasive squamous cell carcinoma66 and colon adenocarcinoma.61
The deregulation of core polarity proteins in cancer is not limited to Scrib, Dlg and Lgl, however, as almost every protein involved in the core apical–basal polarity machinery of epithelial cells has been shown to be affected in some way.56 For example, the par3 gene is deleted in 15% of primary oesophageal squamous cell carcinomas and downregulated in a number of tumour tissues.67 A recent study identified homozygous intragenic microdeletions, involving genes encoding components of the core polarity complexes in a genome-wide screen of 684 cell lines.68 Interestingly, among these genes, Par3 was found to be the most commonly targeted and was disrupted in both cell lines and some primary tumours. This study will undoubtedly be complemented by data from ongoing cancer genome sequencing efforts such as the Cancer Genome Atlas. The Par6–aPKC complex has also been shown to be deregulated in cancer. For example, deregulation of Par6 was observed in ER-positive breast tumours.69 Taken together, these data strongly underline the causal link between the deregulation of core polarity proteins and human cancer.
Tumour suppressors that regulate epithelial cell polarity
Over the past decade, a number of tumour suppressor pathways have been directly linked to epithelial cell polarity, suggesting that the integrity of apical–basal polarity is crucial for the prevention of tumour development. Importantly, one such tumour suppressor is Par4/LKB1. Although LKB1 deficiency does not cause a gross defect in cell polarity in the intestine, for example,70 it may regulate cell polarity through its ability to phosphorylate members of the AMPK-related kinase (ARK) family in other tissues.71, 72 Hence, Par4/LKB1 is able to activate Par1/MARK in the Drosophila oocyte and, in epithelial cells, may have its role through Par1/MARK activation.73 Alternatively, Par4/LKB1 may regulate polarity through the activation of AMPK (AMP-dependent activated protein kinase), which in turn activates Myosin II.73 Among all of the Par genes identified in model organisms, Par4/LKB1 is the most well-established tumour suppressor. Germline mutations in the LKB1 gene cause Peutz–Jeghers syndrome and predispose patients to develop colon cancer.74, 75 All of these factors suggest that regulators of cell polarity may have an important role in suppressing tumours. In agreement with this, a growing list of tumour suppressors has been identified that regulate cell polarity. For example, the tumour suppressor von Hippel-Lindau exerts its regulation on polarity at several levels: it is able to directly interact with aPKC and mediate its ubiquitination and subsequent degradation, and its interaction with the Par complex is involved in the regulation of polarised microtubule growth and formation of primary cilia.76, 77 Another tumour suppressor that regulates cell polarity is phosphatase and tensin homolog (PTEN), which is likely to be involved in different aspects of epithelial cell polarity. However, as apical accumulation of phosphatidylinositol 4,5 biphosphate is dependent on apical targeting of PTEN, and as membrane targeting of Par3 is mediated by direct binding to phosphoinositide lipids, PTEN may be instrumental in the apical localisation of Par3.78, 79, 80 Interestingly, APC has been shown to interact with several core polarity proteins. For example, hDlg can interact with the APC tumour suppressor, and their interaction negatively regulates cell cycle progression.81 APC was also found to interact with hScrib and it has been suggested that Scrib controls its localisation at the level of the adherens junctions in epithelial cells.82
Recently, apical–basal polarity in epithelial cells has been linked to another tumour suppressor, with the discovery that ASPP2 is a new binding partner of Par3.83, 84 ASPP2 is critical for the localisation of Par3 at the level of tight junctions in epithelial cells and, therefore, has a crucial role in the establishment and maintenance of apical–basal polarity in epithelial cells in culture. In vivo, ASPP2 deficiency results in defects arising during the development of the central nervous system, characterised by a loss of apical–basal polarity and an expansion of neural progenitor cells.84 ASPP2 colocalises with Par3 at the level of tight junctions in a variety of epithelial cells and tissues, suggesting that its role in controlling apical–basal polarity is common to other epithelia.83, 84 ASPP2 is a transcriptional target of E2F185 and was first identified as a p53 regulator that specifically promotes its apoptotic function.86 Tumour studies in mice have identified ASPP2 as a haploinsufficient tumour suppressor gene,87, 88 and ASPP2 levels have been shown to be deregulated in human tumours and tumour cell lines, suggesting that its tumour suppressive role is conserved in humans.89, 90, 91 Studies in Drosophila have shown that dASPP, the unique ASPP protein in Drosophila, localises at adherens junctions. These studies suggest that dASPP regulates retinal morphogenesis by acting in concert with dRASSF8 to promote dCSK activity, thus, the function of ASPP2 in the establishment and maintenance of apical–basal polarity in epithelial cells appears to have been conserved from Drosophila to humans.92, 93 However, it remains unclear whether the function of ASPP2 in regulating apical–basal polarity is linked to its ability to regulate p53 and its family members, p63 and p73. One possibility is that ASPP2, upon external stimuli, may be able to shuttle from tight junctions to the nucleus to have its transcriptional role, in a similar way to other junctional proteins such as β-catenin or ZO-1. Therefore, it will be of great interest to investigate the extent to which ASPP2's regulation of the localisation of Par3 and the apical–basal polarity of epithelial cells contributes to its tumour suppressive function, and how this interplays with its role in regulating p53, the most mutated tumour suppressor in human cancers, and p63, a key regulator of epithelial stratification.
There are a number of studies that also suggest that mitotic spindle orientation is crucial for asymmetric cell division in mammals. For instance, p63 has been shown to be important for mitotic spindle orientation during asymmetric cell division of epidermal stem cells.94 Finally, emerging evidence suggests that there is also a direct relationship between loss of cell polarity of stem cells and asymmetric division and tumour initiation in mammals. For example, the tumour suppressor p53 has recently been linked with the regulation of asymmetric divisions of mammary stem cells.95 In the future, due to their redundancy in mammals, the real challenge will be to investigate whether core polarity proteins regulate asymmetric stem cell division in mammalian epithelia, and whether their deregulation consequently drives tumour initiation as a result of asymmetric stem cell division defects.
Taken together, these studies emphasise the role and importance of known tumour suppressors in the control of epithelial cell polarity. Many of those tumour suppressors regulate the functions of core polarity proteins through direct interactions suggesting that, in addition to their better-known roles in the control of cellular proliferation, these roles are crucial for the prevention of tumour development.
Conclusion
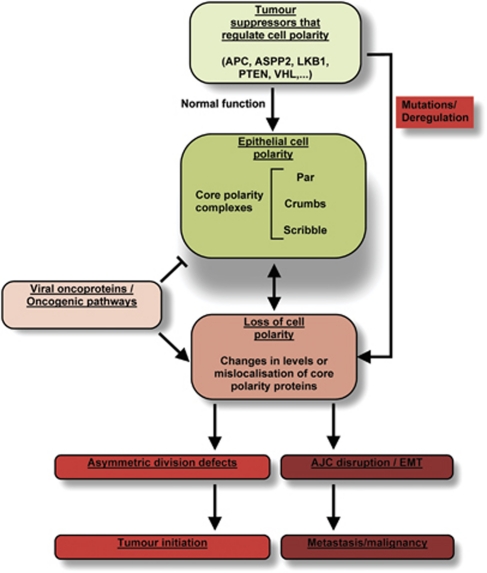
In this review, we have highlighted how epithelial cell polarity may contribute to tumour suppression (Figure 4), through its role in controlling asymmetric cell division and the integrity of the AJC. Loss of cell polarity is a hallmark of cancer, however, studies in transgenic mouse models have so far been unable to clearly answer the question of whether core polarity proteins are tumour suppressors or not. Future studies using multiple and/or conditional knockout mouse models will be essential to finally demonstrate their direct role in tumour suppression. Due to the vital importance of core polarity proteins in maintaining tissue homeostasis, redundancy mechanisms have evolved in mammals that may make this issue too complex to address. Nonetheless, it is emerging that an increasing number of well-known tumour suppressors have a pivotal role in regulating cell polarity. Hence, regulators of polarity may themselves represent a new class of tumour suppressors.
Figure 4.
A summary of epithelial cell polarity's role in suppressing tumour initiation and malignancy
Glossary
- EMT
epithelial mesenchymal transition
- AJC
apical junctional complex
- aPKC
atypical protein kinase C
- Lgl
lethal giant larvae
- Dlg
Disc large
- APC
adenomatous polyposis coli
- ZO-1
zonula occludens-1 protein
- PDZ
post synaptic density protein 95 (PSD95), Dlg and ZO-1
The authors declare no conflict of interest.
Footnotes
Edited by G Melino
References
- Humbert PO, Grzeschik NA, Brumby AM, Galea R, Elsum I, Richardson HE. Control of tumourigenesis by the Scribble/Dlg/Lgl polarity module. Oncogene. 2008;27:6888–6907. doi: 10.1038/onc.2008.341. [DOI] [PubMed] [Google Scholar]
- Lee M, Vasioukhin V. Cell polarity and cancer--cell and tissue polarity as a non-canonical tumor suppressor. J Cell Sci. 2008;121 (Part 8:1141–1150. doi: 10.1242/jcs.016634. [DOI] [PubMed] [Google Scholar]
- Medina E, Lemmers C, Lane-Guermonprez L, Le Bivic A. Role of the Crumbs complex in the regulation of junction formation in Drosophila and mammalian epithelial cells. Biol Cell/Under Aus Eu Cell Biol Organization. 2002;94:305–313. doi: 10.1016/s0248-4900(02)00004-7. [DOI] [PubMed] [Google Scholar]
- Hirose T, Izumi Y, Nagashima Y, Tamai-Nagai Y, Kurihara H, Sakai T, et al. Involvement of ASIP/PAR-3 in the promotion of epithelial tight junction formation. J Cell Sci. 2002;115 (Part 12:2485–2495. doi: 10.1242/jcs.115.12.2485. [DOI] [PubMed] [Google Scholar]
- Hutterer A, Betschinger J, Petronczki M, Knoblich JA. Sequential roles of Cdc42, Par-6, aPKC, and Lgl in the establishment of epithelial polarity during drosophila embryogenesis. Dev Cell. 2004;6:845–854. doi: 10.1016/j.devcel.2004.05.003. [DOI] [PubMed] [Google Scholar]
- Horikoshi Y, Suzuki A, Yamanaka T, Sasaki K, Mizuno K, Sawada H, et al. Interaction between PAR-3 and the aPKC-PAR-6 complex is indispensable for apical domain development of epithelial cells. J Cell Sci. 2009;122 (Part 10:1595–1606. doi: 10.1242/jcs.043174. [DOI] [PubMed] [Google Scholar]
- Nagai-Tamai Y, Mizuno K, Hirose T, Suzuki A, Ohno S. Regulated protein-protein interaction between aPKC and PAR-3 plays an essential role in the polarization of epithelial cells. Genes Cells. 2002;7:1161–1171. doi: 10.1046/j.1365-2443.2002.00590.x. [DOI] [PubMed] [Google Scholar]
- Nakayama M, Goto TM, Sugimoto M, Nishimura T, Shinagawa T, Ohno S, et al. Rho-kinase phosphorylates PAR-3 and disrupts PAR complex formation. Dev Cell. 2008;14:205–215. doi: 10.1016/j.devcel.2007.11.021. [DOI] [PubMed] [Google Scholar]
- Morais-de-Sa E, Mirouse V, St Johnston D. aPKC phosphorylation of Bazooka defines the apical/lateral border in drosophila epithelial cells. Cell. 2010;141:509–523. doi: 10.1016/j.cell.2010.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris TJ, Peifer M. The positioning and segregation of apical cues during epithelial polarity establishment in Drosophila. J Cell Biol. 2005;170:813–823. doi: 10.1083/jcb.200505127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Belmonte F, Gassama A, Datta A, Yu W, Rescher U, Gerke V, et al. PTEN-mediated apical segregation of phosphoinositides controls epithelial morphogenesis through Cdc42. Cell. 2007;128:383–397. doi: 10.1016/j.cell.2006.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nam SC, Choi KW. Interaction of Par-6 and crumbs complexes is essential for photoreceptor morphogenesis in drosophila. Development. 2003;130:4363–4372. doi: 10.1242/dev.00648. [DOI] [PubMed] [Google Scholar]
- Vogelmann R, Nelson WJ. Fractionation of the epithelial apical junctional complex: reassessment of protein distributions in different substructures. Mol Biol Cell. 2005;16:701–716. doi: 10.1091/mbc.E04-09-0827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izumi Y, Hirose T, Tamai Y, Hirai S, Nagashima Y, Fujimoto T, et al. An atypical PKC directly associates and colocalizes at the epithelial tight junction with ASIP, a mammalian homologue of Caenorhabditis elegans polarity protein PAR-3. J Cell Biol. 1998;143:95–106. doi: 10.1083/jcb.143.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joberty G, Petersen C, Gao L, Macara IG. The cell-polarity protein Par6 links Par3 and atypical protein kinase C to Cdc42. Nat Cell Biol. 2000;2:531–539. doi: 10.1038/35019573. [DOI] [PubMed] [Google Scholar]
- Bilder D, Li M, Perrimon N. Cooperative regulation of cell polarity and growth by Drosophila tumor suppressors. Science. 2000;289:113–116. doi: 10.1126/science.289.5476.113. [DOI] [PubMed] [Google Scholar]
- Mathew D, Gramates LS, Packard M, Thomas U, Bilder D, Perrimon N, et al. Recruitment of scribble to the synaptic scaffolding complex requires GUK-holder, a novel DLG binding protein. Curr Biol. 2002;12:531–539. doi: 10.1016/s0960-9822(02)00758-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallay LM, McNickle A, Brennwald PJ, Hubbard AL, Braiterman LT. Scribble associates with two polarity proteins, Lgl2 and Vangl2, via distinct molecular domains. J Cell Biochem. 2006;99:647–664. doi: 10.1002/jcb.20992. [DOI] [PubMed] [Google Scholar]
- Yamanaka T, Horikoshi Y, Izumi N, Suzuki A, Mizuno K, Ohno S. Lgl mediates apical domain disassembly by suppressing the PAR-3-aPKC-PAR-6 complex to orient apical membrane polarity. J Cell Sci. 2006;119 (Part 10:2107–2118. doi: 10.1242/jcs.02938. [DOI] [PubMed] [Google Scholar]
- Plant PJ, Fawcett JP, Lin DC, Holdorf AD, Binns K, Kulkarni S, et al. A polarity complex of mPar-6 and atypical PKC binds, phosphorylates and regulates mammalian Lgl. Nat Cell Biol. 2003;5:301–308. doi: 10.1038/ncb948. [DOI] [PubMed] [Google Scholar]
- Sotillos S, Diaz-Meco MT, Caminero E, Moscat J, Campuzano S. DaPKC-dependent phosphorylation of crumbs is required for epithelial cell polarity in drosophila. J Cell Biol. 2004;166:549–557. doi: 10.1083/jcb.200311031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoblich JA. Asymmetric cell division: recent developments and their implications for tumour biology. Nat Rev Mol Cell Biol. 2010;11:849–860. doi: 10.1038/nrm3010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Januschke J, Gonzalez C. Drosophila asymmetric division, polarity and cancer. Oncogene. 2008;27:6994–7002. doi: 10.1038/onc.2008.349. [DOI] [PubMed] [Google Scholar]
- Albertson R, Doe CQ. Dlg, Scrib and Lgl regulate neuroblast cell size and mitotic spindle asymmetry. Nat Cell Biol. 2003;5:166–170. doi: 10.1038/ncb922. [DOI] [PubMed] [Google Scholar]
- Chia W, Somers WG, Wang H. Drosophila neuroblast asymmetric divisions: cell cycle regulators, asymmetric protein localization, and tumorigenesis. J Cell Biol. 2008;180:267–272. doi: 10.1083/jcb.200708159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita YM, Jones DL, Fuller MT. Orientation of asymmetric stem cell division by the APC tumor suppressor and centrosome. Science. 2003;301:1547–1550. doi: 10.1126/science.1087795. [DOI] [PubMed] [Google Scholar]
- Johnston CA, Hirono K, Prehoda KE, Doe CQ. Identification of an Aurora-A/PinsLINKER/Dlg spindle orientation pathway using induced cell polarity in S2 cells. Cell. 2009;138:1150–1163. doi: 10.1016/j.cell.2009.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klezovitch O, Fernandez TE, Tapscott SJ, Vasioukhin V. Loss of cell polarity causes severe brain dysplasia in Lgl1 knockout mice. Genes Dev. 2004;18:559–571. doi: 10.1101/gad.1178004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bultje RS, Castaneda-Castellanos DR, Jan LY, Jan YN, Kriegstein AR, Shi SH. Mammalian Par3 regulates progenitor cell asymmetric division via notch signaling in the developing neocortex. Neuron. 2009;63:189–202. doi: 10.1016/j.neuron.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Macara IG. Par-3 controls tight junction assembly through the Rac exchange factor Tiam1. Nat Cell Biol. 2005;7:262–269. doi: 10.1038/ncb1226. [DOI] [PubMed] [Google Scholar]
- Georgiou M, Marinari E, Burden J, Baum B. Cdc42, Par6, and aPKC regulate Arp2/3-mediated endocytosis to control local adherens junction stability. Curr Biol. 2008;18:1631–1638. doi: 10.1016/j.cub.2008.09.029. [DOI] [PubMed] [Google Scholar]
- Shin K, Straight S, Margolis B. PATJ regulates tight junction formation and polarity in mammalian epithelial cells. J Cell Biol. 2005;168:705–711. doi: 10.1083/jcb.200408064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov AI, Young C, Den Beste K, Capaldo CT, Humbert PO, Brennwald P, et al. Tumor suppressor scribble regulates assembly of tight junctions in the intestinal epithelium. Am J Pathol. 2010;176:134–145. doi: 10.2353/ajpath.2010.090220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varelas X, Samavarchi-Tehrani P, Narimatsu M, Weiss A, Cockburn K, Larsen BG, et al. The Crumbs complex couples cell density sensing to Hippo-dependent control of the TGF-beta-SMAD pathway. Dev Cell. 2010;19:831–844. doi: 10.1016/j.devcel.2010.11.012. [DOI] [PubMed] [Google Scholar]
- Zhou Q, Fan J, Ding X, Peng W, Yu X, Chen Y, et al. TGF-{beta}-induced MiR-491-5p expression promotes Par-3 degradation in rat proximal tubular epithelial cells. J Biol Chem. 2010;285:40019–40027. doi: 10.1074/jbc.M110.141341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozdamar B, Bose R, Barrios-Rodiles M, Wang HR, Zhang Y, Wrana JL. Regulation of the polarity protein Par6 by TGFbeta receptors controls epithelial cell plasticity. Science. 2005;307:1603–1609. doi: 10.1126/science.1105718. [DOI] [PubMed] [Google Scholar]
- Viloria-Petit AM, David L, Jia JY, Erdemir T, Bane AL, Pinnaduwage D, et al. A role for the TGFbeta-Par6 polarity pathway in breast cancer progression. Proc Natl Acad Sci USA. 2009;106:14028–14033. doi: 10.1073/pnas.0906796106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brumby AM, Richardson HE. Scribble mutants cooperate with oncogenic Ras or Notch to cause neoplastic overgrowth in drosophila. EMBO J. 2003;22:5769–5779. doi: 10.1093/emboj/cdg548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dow LE, Elsum IA, King CL, Kinross KM, Richardson HE, Humbert PO. Loss of human scribble cooperates with H-Ras to promote cell invasion through deregulation of MAPK signalling. Oncogene. 2008;27:5988–6001. doi: 10.1038/onc.2008.219. [DOI] [PubMed] [Google Scholar]
- Javier RT. Cell polarity proteins: common targets for tumorigenic human viruses. Oncogene. 2008;27:7031–7046. doi: 10.1038/onc.2008.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okajima M, Takahashi M, Higuchi M, Ohsawa T, Yoshida S, Yoshida Y, et al. Human T-cell leukemia virus type 1 Tax induces an aberrant clustering of the tumor suppressor scribble through the PDZ domain-binding motif dependent and independent interaction. Virus Genes. 2008;37:231–240. doi: 10.1007/s11262-008-0259-4. [DOI] [PubMed] [Google Scholar]
- Arpin-Andre C, Mesnard JM. The PDZ domain-binding motif of the human T cell leukemia virus type 1 tax protein induces mislocalization of the tumor suppressor hScrib in T cells. J Biol Chem. 2007;282:33132–33141. doi: 10.1074/jbc.M702279200. [DOI] [PubMed] [Google Scholar]
- Lee SS, Weiss RS, Javier RT. Binding of human virus oncoproteins to hDlg/SAP97, a mammalian homolog of the drosophila discs large tumor suppressor protein. Proc Natl Acad Sci USA. 1997;94:6670–6675. doi: 10.1073/pnas.94.13.6670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanamori M, Sandy P, Marzinotto S, Benetti R, Kai C, Hayashizaki Y, et al. The PDZ protein tax-interacting protein-1 inhibits beta-catenin transcriptional activity and growth of colorectal cancer cells. J Biol Chem. 2003;278:38758–38764. doi: 10.1074/jbc.M306324200. [DOI] [PubMed] [Google Scholar]
- Hirata A, Higuchi M, Niinuma A, Ohashi M, Fukushi M, Oie M, et al. PDZ domain-binding motif of human T-cell leukemia virus type 1 Tax oncoprotein augments the transforming activity in a rat fibroblast cell line. Virology. 2004;318:327–336. doi: 10.1016/j.virol.2003.10.006. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Ohsugi Y, Uchida-Toita M, Akiyama T, Yoshida M. Tax oncoprotein of HTLV-1 binds to the human homologue of drosophila discs large tumor suppressor protein, hDLG, and perturbs its function in cell growth control. Oncogene. 1999;18:5967–5972. doi: 10.1038/sj.onc.1203008. [DOI] [PubMed] [Google Scholar]
- Thomas M, Massimi P, Navarro C, Borg JP, Banks L. The hScrib/Dlg apico-basal control complex is differentially targeted by HPV-16 and HPV-18 E6 proteins. Oncogene. 2005;24:6222–6230. doi: 10.1038/sj.onc.1208757. [DOI] [PubMed] [Google Scholar]
- Storrs CH, Silverstein SJ. PATJ, a tight junction-associated PDZ protein, is a novel degradation target of high-risk human papillomavirus E6 and the alternatively spliced isoform 18 E6. J Virol. 2007;81:4080–4090. doi: 10.1128/JVI.02545-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayan N, Subbaiah VK, Banks L. The high-risk HPV E6 oncoprotein preferentially targets phosphorylated nuclear forms of hDlg. Virology. 2009;387:1–4. doi: 10.1016/j.virol.2009.02.030. [DOI] [PubMed] [Google Scholar]
- Aranda V, Haire T, Nolan ME, Calarco JP, Rosenberg AZ, Fawcett JP, et al. Par6-aPKC uncouples ErbB2 induced disruption of polarized epithelial organization from proliferation control. Nat Cell Biol. 2006;8:1235–1245. doi: 10.1038/ncb1485. [DOI] [PubMed] [Google Scholar]
- Gateff E. Malignant neoplasms of genetic origin in Drosophila melanogaster. Science. 1978;200:1448–1459. doi: 10.1126/science.96525. [DOI] [PubMed] [Google Scholar]
- Dow LE, Brumby AM, Muratore R, Coombe ML, Sedelies KA, Trapani JA, et al. hScrib is a functional homologue of the Drosophila tumour suppressor Scribble. Oncogene. 2003;22:9225–9230. doi: 10.1038/sj.onc.1207154. [DOI] [PubMed] [Google Scholar]
- Grifoni D, Garoia F, Schimanski CC, Schmitz G, Laurenti E, Galle PR, et al. The human protein Hugl-1 substitutes for drosophila lethal giant larvae tumour suppressor function in vivo. Oncogene. 2004;23:8688–8694. doi: 10.1038/sj.onc.1208023. [DOI] [PubMed] [Google Scholar]
- Kuphal S, Wallner S, Schimanski CC, Bataille F, Hofer P, Strand S, et al. Expression of Hugl-1 is strongly reduced in malignant melanoma. Oncogene. 2006;25:103–110. doi: 10.1038/sj.onc.1209008. [DOI] [PubMed] [Google Scholar]
- Thomas U, Phannavong B, Muller B, Garner CC, Gundelfinger ED. Functional expression of rat synapse-associated proteins SAP97 and SAP102 in Drosophila dlg-1 mutants: effects on tumor suppression and synaptic bouton structure. Mech Dev. 1997;62:161–174. doi: 10.1016/s0925-4773(97)00658-8. [DOI] [PubMed] [Google Scholar]
- Huang L, Muthuswamy SK. Polarity protein alterations in carcinoma: a focus on emerging roles for polarity regulators. Curr Opin Genet Dev. 2010;20:41–50. doi: 10.1016/j.gde.2009.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan L, Rosenberg A, Bergami KC, Yu M, Xuan Z, Jaffe AB, et al. Deregulation of scribble promotes mammary tumorigenesis and reveals a role for cell polarity in carcinoma. Cell. 2008;135:865–878. doi: 10.1016/j.cell.2008.09.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froldi F, Ziosi M, Garoia F, Pession A, Grzeschik NA, Bellosta P, et al. The lethal giant larvae tumour suppressor mutation requires dMyc oncoprotein to promote clonal malignancy. BMC Biol. 2010;8:33. doi: 10.1186/1741-7007-8-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagasaka K, Pim D, Massimi P, Thomas M, Tomaic V, Subbaiah VK, et al. The cell polarity regulator hScrib controls ERK activation through a KIM site-dependent interaction. Oncogene. 2010;29:5311–5321. doi: 10.1038/onc.2010.265. [DOI] [PubMed] [Google Scholar]
- Nakagawa S, Yano T, Nakagawa K, Takizawa S, Suzuki Y, Yasugi T, et al. Analysis of the expression and localisation of a LAP protein, human scribble, in the normal and neoplastic epithelium of uterine cervix. Br J Cancer. 2004;90:194–199. doi: 10.1038/sj.bjc.6601465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardiol D, Zacchi A, Petrera F, Stanta G, Banks L. Human discs large and scrib are localized at the same regions in colon mucosa and changes in their expression patterns are correlated with loss of tissue architecture during malignant progression. Int J Cancer. 2006;119:1285–1290. doi: 10.1002/ijc.21982. [DOI] [PubMed] [Google Scholar]
- Ouyang Z, Zhan W, Dan L. hScrib, a human homolog of Drosophila neoplastic tumor suppressor, is involved in the progress of endometrial cancer. Oncol Res. 2010;18:593–599. doi: 10.3727/096504010x12767359114045. [DOI] [PubMed] [Google Scholar]
- Vieira V, de la Houssaye G, Lacassagne E, Dufier JL, Jais JP, Beermann F, et al. Differential regulation of Dlg1, Scrib, and Lgl1 expression in a transgenic mouse model of ocular cancer. Mol Vis. 2008;14:2390–2403. [PMC free article] [PubMed] [Google Scholar]
- Schimanski CC, Schmitz G, Kashyap A, Bosserhoff AK, Bataille F, Schafer SC, et al. Reduced expression of Hugl-1, the human homologue of Drosophila tumour suppressor gene lgl, contributes to progression of colorectal cancer. Oncogene. 2005;24:3100–3109. doi: 10.1038/sj.onc.1208520. [DOI] [PubMed] [Google Scholar]
- Lu X, Feng X, Man X, Yang G, Tang L, Du D, et al. Aberrant splicing of Hugl-1 is associated with hepatocellular carcinoma progression. Clin Cancer Res. 2009;15:3287–3296. doi: 10.1158/1078-0432.CCR-08-2078. [DOI] [PubMed] [Google Scholar]
- Lin HT, Steller MA, Aish L, Hanada T, Chishti AH. Differential expression of human Dlg in cervical intraepithelial neoplasias. Gynecol Oncol. 2004;93:422–428. doi: 10.1016/j.ygyno.2004.01.025. [DOI] [PubMed] [Google Scholar]
- Zen K, Yasui K, Gen Y, Dohi O, Wakabayashi N, Mitsufuji S, et al. Defective expression of polarity protein PAR-3 gene (PARD3) in esophageal squamous cell carcinoma. Oncogene. 2009;28:2910–2918. doi: 10.1038/onc.2009.148. [DOI] [PubMed] [Google Scholar]
- Rothenberg SM, Mohapatra G, Rivera MN, Winokur D, Greninger P, Nitta M, et al. A genome-wide screen for microdeletions reveals disruption of polarity complex genes in diverse human cancers. Cancer Res. 2010;70:2158–2164. doi: 10.1158/0008-5472.CAN-09-3458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolan ME, Aranda V, Lee S, Lakshmi B, Basu S, Allred DC, et al. The polarity protein Par6 induces cell proliferation and is overexpressed in breast cancer. Cancer Res. 2008;68:8201–8209. doi: 10.1158/0008-5472.CAN-07-6567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shorning BY, Zabkiewicz J, McCarthy A, Pearson HB, Winton DJ, Sansom OJ, et al. Lkb1 deficiency alters goblet and paneth cell differentiation in the small intestine. PloS One. 2009;4:e4264. doi: 10.1371/journal.pone.0004264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson HB, McCarthy A, Collins CM, Ashworth A, Clarke AR. Lkb1 deficiency causes prostate neoplasia in the mouse. Cancer Res. 2008;68:2223–2232. doi: 10.1158/0008-5472.CAN-07-5169. [DOI] [PubMed] [Google Scholar]
- Hezel AF, Gurumurthy S, Granot Z, Swisa A, Chu GC, Bailey G, et al. Pancreatic LKB1 deletion leads to acinar polarity defects and cystic neoplasms. Mol Cell Biol. 2008;28:2414–2425. doi: 10.1128/MCB.01621-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirouse V, Billaud M. The LKB1/AMPK polarity pathway. FEBS Lett. 2010;585:981–986. doi: 10.1016/j.febslet.2010.12.025. [DOI] [PubMed] [Google Scholar]
- Hemminki A, Markie D, Tomlinson I, Avizienyte E, Roth S, Loukola A, et al. A serine/threonine kinase gene defective in Peutz-Jeghers syndrome. Nature. 1998;391:184–187. doi: 10.1038/34432. [DOI] [PubMed] [Google Scholar]
- Jenne DE, Reimann H, Nezu J, Friedel W, Loff S, Jeschke R, et al. Peutz-Jeghers syndrome is caused by mutations in a novel serine threonine kinase. Nat Genet. 1998;18:38–43. doi: 10.1038/ng0198-38. [DOI] [PubMed] [Google Scholar]
- Okuda H, Saitoh K, Hirai S, Iwai K, Takaki Y, Baba M, et al. The von Hippel-Lindau tumor suppressor protein mediates ubiquitination of activated atypical protein kinase C. J Biol Chem. 2001;276:43611–43617. doi: 10.1074/jbc.M107880200. [DOI] [PubMed] [Google Scholar]
- Schermer B, Ghenoiu C, Bartram M, Muller RU, Kotsis F, Hohne M, et al. The von Hippel-Lindau tumor suppressor protein controls ciliogenesis by orienting microtubule growth. J Cell Biol. 2006;175:547–554. doi: 10.1083/jcb.200605092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng W, Wu H, Chan LN, Zhang M. Par-3-mediated junctional localization of the lipid phosphatase PTEN is required for cell polarity establishment. J Biol Chem. 2008;283:23440–23449. doi: 10.1074/jbc.M802482200. [DOI] [PubMed] [Google Scholar]
- Krahn MP, Klopfenstein DR, Fischer N, Wodarz A. Membrane targeting of Bazooka/PAR-3 is mediated by direct binding to phosphoinositide lipids. Curr Biol. 2010;20:636–642. doi: 10.1016/j.cub.2010.01.065. [DOI] [PubMed] [Google Scholar]
- Wu H, Feng W, Chen J, Chan LN, Huang S, Zhang M. PDZ domains of Par-3 as potential phosphoinositide signaling integrators. Mol Cell. 2007;28:886–898. doi: 10.1016/j.molcel.2007.10.028. [DOI] [PubMed] [Google Scholar]
- Ishidate T, Matsumine A, Toyoshima K, Akiyama T. The APC-hDLG complex negatively regulates cell cycle progression from the G0/G1 to S phase. Oncogene. 2000;19:365–372. doi: 10.1038/sj.onc.1203309. [DOI] [PubMed] [Google Scholar]
- Takizawa S, Nagasaka K, Nakagawa S, Yano T, Nakagawa K, Yasugi T, et al. Human scribble, a novel tumor suppressor identified as a target of high-risk HPV E6 for ubiquitin-mediated degradation, interacts with adenomatous polyposis coli. Genes Cells. 2006;11:453–464. doi: 10.1111/j.1365-2443.2006.00954.x. [DOI] [PubMed] [Google Scholar]
- Cong W, Hirose T, Harita Y, Yamashita A, Mizuno K, Hirano H, et al. ASPP2 regulates epithelial cell polarity through the PAR complex. Curr Biol. 2010;20:1408–1414. doi: 10.1016/j.cub.2010.06.024. [DOI] [PubMed] [Google Scholar]
- Sottocornola R, Royer C, Vives V, Tordella L, Zhong S, Wang Y, et al. ASPP2 binds Par-3 and controls the polarity and proliferation of neural progenitors during CNS development. Dev Cell. 2010;19:126–137. doi: 10.1016/j.devcel.2010.06.003. [DOI] [PubMed] [Google Scholar]
- Fogal V, Kartasheva NN, Trigiante G, Llanos S, Yap D, Vousden KH, et al. ASPP1 and ASPP2 are new transcriptional targets of E2F. Cell Death Differ. 2005;12:369–376. doi: 10.1038/sj.cdd.4401562. [DOI] [PubMed] [Google Scholar]
- Samuels-Lev Y, O'Connor DJ, Bergamaschi D, Trigiante G, Hsieh JK, Zhong S, et al. ASPP proteins specifically stimulate the apoptotic function of p53. Mol Cell. 2001;8:781–794. doi: 10.1016/s1097-2765(01)00367-7. [DOI] [PubMed] [Google Scholar]
- Kampa KM, Acoba JD, Chen D, Gay J, Lee H, Beemer K, et al. Apoptosis-stimulating protein of p53 (ASPP2) heterozygous mice are tumor-prone and have attenuated cellular damage-response thresholds. Proc Natl Acad Sci USA. 2009;106:4390–4395. doi: 10.1073/pnas.0809080106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vives V, Su J, Zhong S, Ratnayaka I, Slee E, Goldin R, et al. ASPP2 is a haploinsufficient tumor suppressor that cooperates with p53 to suppress tumor growth. Genes Dev. 2006;20:1262–1267. doi: 10.1101/gad.374006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu WK, Jiang XY, Ren JK, Zhang ZX. Expression pattern of the ASPP family members in endometrial endometrioid adenocarcinoma. Onkologie. 2010;33:500–503. doi: 10.1159/000319692. [DOI] [PubMed] [Google Scholar]
- Liu ZJ, Lu X, Zhang Y, Zhong S, Gu SZ, Zhang XB, et al. Downregulated mRNA expression of ASPP and the hypermethylation of the 5′-untranslated region in cancer cell lines retaining wild-type p53. FEBS Lett. 2005;579:1587–1590. doi: 10.1016/j.febslet.2005.01.069. [DOI] [PubMed] [Google Scholar]
- Park SW, An CH, Kim SS, Yoo NJ, Lee SH. Mutational analysis of ASPP1 and ASPP2 genes, a p53-related gene, in gastric and cololorectal cancers with microsatellite instability. Gut Liver. 2010;4:292–293. doi: 10.5009/gnl.2010.4.2.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langton PF, Colombani J, Aerne BL, Tapon N. Drosophila ASPP regulates C-terminal Src kinase activity. Dev Cell. 2007;13:773–782. doi: 10.1016/j.devcel.2007.11.005. [DOI] [PubMed] [Google Scholar]
- Langton PF, Colombani J, Chan EH, Wepf A, Gstaiger M, Tapon N. The dASPP-dRASSF8 complex regulates cell-cell adhesion during drosophila retinal morphogenesis. Curr Biol. 2009;19:1969–1978. doi: 10.1016/j.cub.2009.10.027. [DOI] [PubMed] [Google Scholar]
- Lechler T, Fuchs E. Asymmetric cell divisions promote stratification and differentiation of mammalian skin. Nature. 2005;437:275–280. doi: 10.1038/nature03922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicalese A, Bonizzi G, Pasi CE, Faretta M, Ronzoni S, Giulini B, et al. The tumor suppressor p53 regulates polarity of self-renewing divisions in mammary stem cells. Cell. 2009;138:1083–1095. doi: 10.1016/j.cell.2009.06.048. [DOI] [PubMed] [Google Scholar]