Abstract
The development of malignant tumors results from deregulated proliferation or an inability of cells to undergo apoptotic cell death. Experimental works of the past decade have highlighted the importance of calcium (Ca2+) in the regulation of apoptosis. Several studies indicate that the Ca2+ content of the endoplasmic reticulum (ER) determines the cell's sensitivity to apoptotic stress and perturbation of ER Ca2+ homeostasis appears to be a key component in the development of several pathological situations. Sensitivity to apoptosis depends on the ability of cells to transfer Ca2+ from the ER to the mitochondria. The physical platform for the interplay between the ER and mitochondria is a domain of the ER called the mitochondria-associated membranes (MAMs). The disruption of these contact sites has profound consequences for cellular function, such as imbalances of intracellular Ca2+ signaling, cellular stress, and disrupted apoptosis progression. The promyelocytic leukemia (PML) protein has been previously recognized as a critical and essential regulator of multiple apoptotic response. Nevertheless, how PML would exert such broad and fundamental role in apoptosis remained for long time a mystery. In this review, we will discuss how recent results demonstrate that the elusive mechanism whereby the PML tumor suppressor exerts its essential role in apoptosis triggered by Ca2+-dependent stimuli can be attributed to its unexpected and fundamental role at MAMs in the control of the functional cross-talk between ER and mitochondria.
Keywords: PML, mitochondria, endoplasmic reticulum (ER), apoptosis, calcium (Ca2+), mitochondria-associated membranes (MAMs)
The Promyelocytic Leukemia Protein
The promyelocytic leukemia (PML) protein is a tumor suppressor gene originally identified at the break point of the t(15;17) chromosomal translocation of acute promyelocytic leukemia (APL), a distinct subtype of acute myeloid leukemia. As a consequence of this translocation, PML fuses to the retinoic acid (RA) receptor alpha (RARα) gene. Two fusion genes are generated encoding PML-RARα and RARα-PML fusion proteins, which coexist in the leukemic cells, blocking heamatopoietic differentiation (for a review, see Pandolfi;1 Salomoni and Pandolfi.2 PML has, therefore, become the object of intense research on the basis of this premise. Since then, PML has been shown to regulate diverse cellular functions, such as transcriptional regulation, DNA-damage response, sumoylation process, cellular senescence, neoangiogenesis, and, of relevance to this review, apoptosis.3, 4
PML belongs to a large family of proteins harboring a tripartite structure that contains a zinc-finger called the RING motif (R) located N-terminally followed by two additional zinc-fingers motifs (B-boxes; B) and an α-helical coiled-coil domain (CC), collectively referred to as the RBCC domain. The RBCC domain mediates protein–protein interactions and is responsible for PML multimerization and the formation of macromolecular complexes. The C-terminal region of PML is less structured and varies between PML isoforms. Alternative splicing of C-terminal exons is responsible for the existence of at least seven PML isoforms characterized by different C-terminal regions and functional specificity.4, 5
PML is typically concentrated in subnuclear macromolecular structures termed PML-nuclear bodies (PML-NBs), of which PML is the essential component. PML-NBs have a diameter of 0.2–1 μm and the shape of a doughnut. PML-NBs are multi-protein dynamic structures that undergo significant changes in number, size, and position, particularly in response to cellular stress.4, 6 They critically depend on PML to be correctly assembled.7
PML functionally interacts with a large number of proteins within PML-NBs.4, 6 Some are in direct physical contact with PML, while others are not.4, 6 PML SUMOylation and non-covalent binding of PML to SUMOylated PML through the SUMO-binding motif constitutes the nucleation event for subsequent recruitment of SUMOylated proteins and/or proteins containing SUMO-binding motifs to the PML NBs.7
In the APL blasts, PML-RARα associates physically with PML and causes its delocalization into microspeckled nuclear structures with consequent disruption of the PML-NBs.8 Loss of PML in a mouse model of APL causes a dramatic acceleration of leukemia and increased incidence of the disease, indicating the importance of the functional disruption of PML and PML-NBs for disease progression.1, 9 Moreover, PML has been more recently shown to modulate the subcellular localization of the tumor suppressor PTEN.10 As a consequence, both in APL blasts and in PML-loss conditions, PTEN is excluded from the nucleus,11 in turn suggesting that PTEN delocalization and loss of its nuclear function may have an important role in the pathogenesis of this and possibly other forms of leukaemia. Treatment with drugs commonly used for APL,11 such as all-trans RA or arsenic trioxide, restores normal nuclear PTEN localization.10
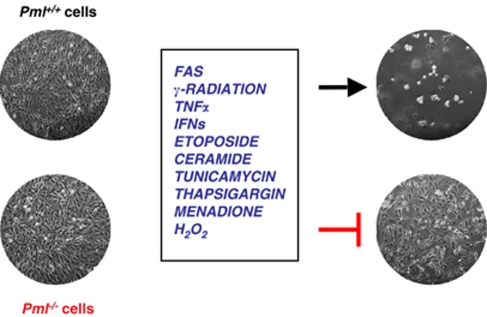
Pml null mice and cells are protected from multiple and diverse apoptotic stimuli (Figure 1).12 A possible explanation for why Pml null cells are resistant to many apoptotic stimuli can be ascribed to the fact that PML can act as a pleiotropic factor in the functional regulation of several pro- and anti-apoptotic factors.13
Figure 1.
PML is critical in multiple apoptotic pathways. The absence of PML inhibits cell death induced by various apoptotic stimuli. PML is critical for both transcription-dependent (e.g., p53-mediated responses) responses as well as transcription-independent early apoptotic responses
Indeed, PML is functioning as part of a complex tumor-suppressive network. For instance, it is well established that PML is an important factor in the regulation of both p53-dependent and -independent apoptotic pathways.13, 14 PML activates p53 by several means: by recruiting p53 to PML-NBs by promoting its acetylation and phosphorylation,15, 16, 17, 18 and by binding and inhibiting Mdm2, the main negative regulator of p53,19, 20, 21 as well as by promoting p53 de-ubiquitination by the ubiquitin protease HAUSP.22, 23
Moreover, PML can act as a suppressor of other major oncogenic pathways, such as the PI3K/Akt pathway, through its ability to interact with the protein phosphatase PP2A and inhibit the nuclear function of Akt, thus leading to suppression of its prosurvival and promitogenic functions.24 In Pten+/– animals, reduction of the Pml gene dosage results in transition to invasive carcinoma, which is accompanied by increased Akt phosphorylation,24 suggesting a genetic interaction between the two pathways. Finally, as aforementioned, PML regulates the function of the PTEN, which is the main suppressor of the PI3K pathway. This occurs through inhibition of PTEN de-ubiquitination by HAUSP and its nuclear retention.11, 25, 26 In conclusion, PML-NBs emerge as signaling coordination centers toward the regulation, availability, post-translational modification, and activation of multiple and diverse proteins implicated in apoptotic pathways.
However, no unified mechanism appeared to explain the global resistance of Pml null cells to apoptosis. Moreover, all these observations failed to clarify whether or not PML might also be involved directly in the execution of the apoptotic response and if the extra-nuclear fraction of PML could have a role in this respect. Indeed, in addition to its nuclear localization, some PML isoforms are found to accumulate into the cytosolic fraction,5 as well as adjacent to mitochondria,27 and to be critical in the regulation of TGF-β signaling and anti-viral responses.28, 29
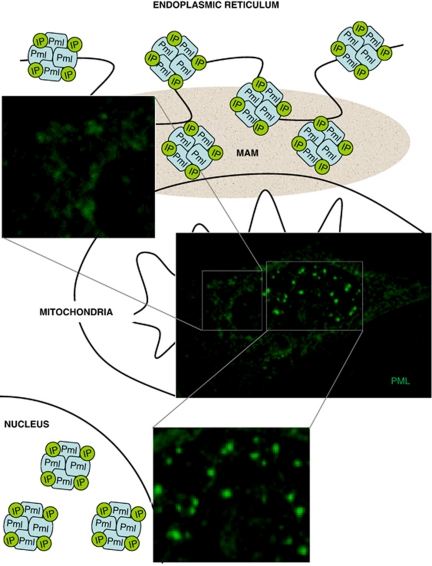
More recently, fractionation analysis by ultracentrifugation, immunogold labeling, and immunofluorescence in mouse embryonic fibroblasts (MEFs) revealed that PML associates to the surface of endoplasmic reticulum (ER) and in the proximity of the mitochondrial membrane at the ER/mitochondria contact sites (MAMs; mitochondria-associated membranes) (Figure 2), suggesting that PML might have additional as yet unidentified functions independent from the PML-NB.30
Figure 2.
Intracellular localization of PML. The PML protein accumulates in the nucleus where it forms PML-nuclear bodies (PML-NBs), as well as at endoplasmic reticulum (ER) in the contact sites with mitochondria (MAMs). In both sites, PML interacts with several proteins (indicated in figure as IP: interacting proteins) generating multi-protein complexes of large molecular size
Importantly, this unexpected intracellular localization of PML has been observed also in vivo through fractionation analysis of mouse liver cells (Giorgi, Pinton, and Pandolfi unpublished observations). Further fractionation experiments and immunohistochemical analysis that takes advantage of PML-specific antibodies will address whether the localization of PML at MAM is tissue specific or rather ubiquitous.
The ER–Mitochondria Contact Sites as a Hot Spot Signaling Unit
Mitochondrial and ER networks are fundamental for the maintenance of cellular homeostasis and for the determination of cell fate under stress conditions.31 Structural and functional studies revealed that as much as 20% of the mitochondrial surface is in direct contact with the ER, underscoring the dynamic and highly regulated communication between the ER and mitochondria.32
These specific zones of close contact between ER and mitochondria have been termed MAMs by Jean Vance who characterized for the first time, from a biochemical point of view, the intimate relationship between the two compartments.33
Experiments in living cells with ER and mitochondria differentially labeled by mutants of GFP have subsequently demonstrated the existence of a physical interaction between the two organelles and highlighted the functional importance of these contact sites.32
The physiological function of the close apposition between ER and mitochondria is related to bioenergetics and cell survival.
MAMs contain multiple phospholipid- and glycosphingolipid-synthesizing enzymes, including long-chain fatty acid-CoA ligase type 4 (FACL4) and phosphatidylserine synthase-1 (PSS-1), and support direct transfer of lipids between the ER and mitochondria.34, 35
In addition to the function in phospholipid homeostasis, MAMs have been implicated in the metabolism of cholesterol36, 37 and its metabolites and in the trafficking of sphingolipids.38
MAMs have also an important role in ER–mitochondrial calcium (Ca2+) transfer. In support of this notion, western blot analysis of MAMs fraction after subcellular fractionation showed a selective enrichment of signaling elements,39 such as the inositol 1,4,5-trisphosphate (IP3)-sensitive Ca2+ release channels (IP3Rs),40 the most important molecular component of Ca2+ handling machinery, identifying these zones as ‘hotspots' of Ca2+ transfer from the ER to the mitochondria (Figures 3 and 4).
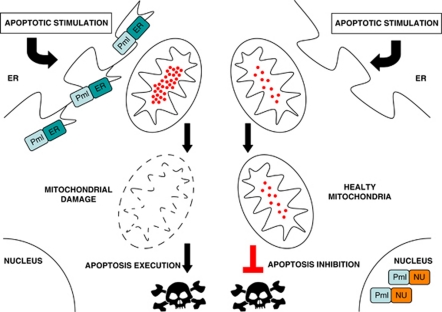
Figure 3.
PML localization at ER/MAM is critical for calcium-dependent apoptotic cell death. Re-expression of an erPML chimera does rescue the sensitivity to cell death of PML null cells. In this case, the ER Ca2+ release induced by various apoptotic stimuli causes a mitochondrial Ca2+ overload and in turn the damage of the mitochondrial structure with release of apoptotic factors. On the contrary, the re-introduction of a nuPML chimera is able to rescue the PML-NBs formation, but not the sensitivity to apoptosis. In this case, the absence of PML at the ER/MAMs sites still impairs ER-Ca2+ release and in turn mitochondrial damage and the apoptotic response
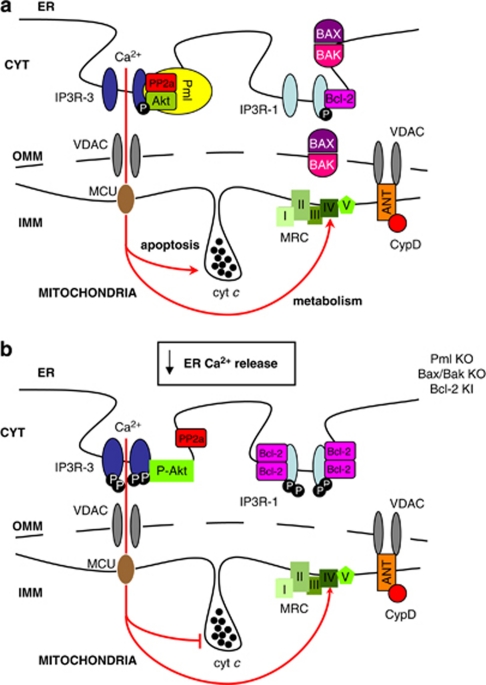
Figure 4.
Schematic representation of ER–mitochondria Ca2+ cross-talk perturbations in physiological and oncogenic states. (a) In normal cells, the PML protein is in protein complexes with the type 3 inositol trisphosphate receptor (IP3R-3), the protein kinase Akt and the phosphatase PP2a. This complex is fundamental to regulate the phosphorylation state of IP3R-3, while the Bcl-2-Bax ratio controls the phosphorylation state of the type 1 of IP3R (IP3R-1). In normal conditions, the stimulation of ER Ca2+ release through IP3 generating stimuli allows a transient mitochondrial Ca2+ uptake and in turn the stimulation of mitochondrial ATP production through the mitochondrial respiratory chain (MRC). In contrast, a Ca2+-dependent pro-apoptotic stimulus induces a much larger Ca2+ release from the ER with a dramatic effect on mitochondria, which induces the release of cytocrome c and the opening of the mitochondrial permeability transition pore. This chain of event, combined with the activation of cytoplasmic effectors, is an important trigger of the apoptotic program. (b) In cancer cells, where PML is often missing or when the Bcl-2-Bax ratio is abnormal, IP3R-3 and IPR1 are, respectively, hyper-phosphorylated as demonstrated on Pml KO, Bax/Bak KO, or Bcl-2 KI cells. Under these conditions, pro-apoptotic stimuli induce a smaller release of Ca2+ from ER, with minor repercussions on mitochondrial physiology and intracellular Ca2+-modulated processes
Interestingly, on the mitochondria side, this specialized domain is enriched in components of the permeability transition pore.41, 42 This large-conductance channel (which has attracted a large interest given its potential role in pathophysiological conditions) includes the adenine nucleotide translocator (ANT), the voltage-dependent anion channel (VDAC), cyclophilin D, and other proteins (including pro-apoptotic members of the Bcl-2 family such as BAX/BAK). Both VDAC and ANT were shown by us and other groups to be enriched in the MAMs fraction (Figure 4a).39
The continuous flow of Ca2+ between these two organelles regulates processes ranging from ER chaperone-assisted folding of newly synthesized proteins to the regulation of mitochondria-localized dehydrogenases involved in ATP-producing Krebs cycle reactions, and the activation of Ca2+-dependent enzymes that execute cell death programs.43 Hence alterations in the ER/mitochondrial coupling and in Ca2+ homeostatic mechanisms provides a powerful molecular basis for the activation of apoptosis.31
ER/Mitochondrial Coupling in Ca2+ Signal and Apoptosis
The ER is the main Ca2+ store of the mammalian cell, mitochondria are the powerhouse of the cell and they need Ca2+ signal to carry out their function. If we consider that Ca2+ ion is one of the most important mediators of life and death (and that mitochondria are main effectors of these processes), then the dynamic cross-talk between ER and mitochondria in the regulation of Ca2+ signal becomes a key step in physiology and pathology44 (Figure 4).
On this basis, it has been argued that the switch from a life to a death signal occurs when normal Ca2+distribution between the ER and the mitochondria is distorted leading to a breakdown of mitochondrialfunctions.45 During normal signaling, there is a continuous flow of Ca2+ between these two organelles where a small bolus of Ca2+ is periodically released to the cytoplasm to be then re-sequestered with a proportion passing through the mitochondria.46
In contrast, perturbation of ER/mitochondrial coupling provide a massive and/or a prolonged mitochondrial Ca2+ overload that in turn activates apoptosis.44, 47 Indeed, a wide number of apoptotic stimuli, such as ceramide, arachinodic acid, and oxidative stress induced by H2O2 or menadione, trigger both a progressive release of Ca2+ from the ER and an activation of the capacitative Ca2+ influx. This sustained ER Ca2+ release, in turn, induced a mitochondrial Ca2+ overload with a consequent release of mitochondria proteins involved in the apoptotic process, such as cytocrome c, AIF, and Smac/Diablo.44, 48
In this respect, conditions that reduce the ER Ca2+ storage and thus Ca2+ released from ER to mitochondria decrease the probability of Ca2+-dependent apoptosis;49 on the other hand, an increase in the Ca2+ released has the opposite effect.
Interestingly, over-expression of the anti-apoptotic protein Bcl-2 decreases the steady-state Ca2+content of the ER, resulting in a reduced amount of agonist-releasable Ca2+ and in a diminution of cytosolic and mitochondrial Ca2+ response.48, 50 Thus, by diminishing ER Ca2+ levels, Bcl-2 is able to protect from Ca2+-dependent apoptotic stimuli.51 Moreover, knocking out the pro-apoptotics Bax and Bak leads to a dramatic reduction of the steady-state Ca2+ concentration in the ER, rendering the knock-out cells more resistant to apoptosis.52 On the contrary, after over-expression of Bax, the ER Ca2+ content increases and the cells are more susceptible to apoptosis treatment.53 Therefore, manipulating the level of ER Ca2+, adjusting the load of Ca2+ imposed upon mitochondria, it is possible to regulate apoptosis.
This concept was corroborated by the case of PML that was discovered to modulate Ca2+ homeostasis and apoptosis in view of its ability to localize and function at the ER and MAMs, as mentioned above30 (Figure 3). Indeed, in the absence of Pml, the ER steady-state values are lower and the decreases of [Ca2+]ER after agonist treatment are drastically smaller and lower compared with Pml+/+ MEFs. In agreement with the observed low [Ca2+]ER, the [Ca2+] increases evoked in the cytosol and mitochondria both by ATP stimulation and by oxidative apoptotic stimulation were significantly smaller in Pml−/− than in Pml+/+ MEFs and cells were unable to die. Furthermore, the expression of a PML chimera that exclusively localizes to the outer surface of the ER (erPML) in Pml−/− MEFs restores mitochondrial Ca2+ signals to values comparable to those measured in Pml+/+ MEFs re-establishing also their sensitivity to the apoptosis. On the contrary, a PML targeted exclusively to the nucleus, restores the formation of NB but is not able to rescue Ca2+ response and the sensitivity to ER-stress-dependent cell death30 (Figure 3).
Altogether, these data highlight the link between Ca2+ homeostasis and the regulation of apoptotic cell death and clearly indicate ER/mitochondria contacts as a critical, although not unique checkpoints.
IP3Rs as Target of Cell Death
The most important molecular component of the Ca2+ handling machinery of the ER is represented by the IP3Rs. IP3Rs are ligand-gated channels that serve to discharge Ca2+ from ER stores in response to agonist stimulation.40, 54 After IP3-mediated release of Ca2+ from the ER through the IP3 receptor, high-Ca2+ microdomains (estimated to be in the range of 50–100 μM) are generated at the tight ER–mitochondrial junctions, activating the low-affinity mitochondrial Ca2+ uniporter and resulting in mitochondrial Ca2+ uptake32 (Figure 4).
Being directly responsible for mitochondrial Ca2+ overload, the release of Ca2+ from ER stores by IP3Rs is linked to multiple models of apoptosis.55 Indeed, cells deficient of IP3R are resistant to apoptosis56, 57, 58 (Figure 4).
There are three isoforms of IP3Rs: IP3R-1 to 3. At present, it is not clear whether different IP3 receptor isoforms have an equivalent role in apoptosis, but types 1 and 3 have been described as important in mediating Ca2+-dependent cell death.59 Importantly, their function is regulated by post-transcriptional modifications. In particular IP3R phosphorylation appears to be a key common feature for modulation of channel function and, as consequence, apoptotic signaling.60, 61, 62
In this respect, the Korsmeyer's group found that Bcl-2 and IP3R-1 physically interact at the ER surface and proposed a model by which Bcl-2 family members regulate IP3R-1 phosphorylation to control the rate of ER Ca2+ leak from intracellular stores and, as consequence, the apoptotic response63 (Figure 4).
Recent data showed that IP3R-3, localized in the MAMs, has a selective role in the induction of apoptosis by preferentially transmitting apoptotic Ca2+ signals to mitochondria.64, 65 Accordingly, siRNA silencing of IP3R-3 blocked apoptosis,65 as KO of IP3R-3 significantly decreased agonist-induced mitochondrial Ca2+ uptake.66
IP3Rs possess consensus sequences for phosphorylation by numerous kinases, including protein kinase B (Akt/PKB).60 This is an interesting observation, because in some cancer cells in which Akt is constitutively active (e.g., prostatic carcinoma cells), IP3Rs are hyper-phosphorylated.60 In turn, the hyper-phosphorylation of IP3Rs by Akt inhibits ER Ca2+ release and reduces significantly cellular sensitivity to Ca2+-mediated pro-apoptotic stimulation.62, 67
Interestingly, similarly to Bcl-2 with IP3R-1, the tumor suppressor PML was found to physically interact with IP3R-3 modulating its phosphorylation state. Indeed in MEF Pml−/− cells, IP3R-3 is hyper-phosphorylated. This was demonstrated to be mediated by a specific multi-protein complex, localized at ER/MAMs contact sites, including PML, IP3R-3, the protein phosphatase PP2a, and Akt. In particular, PML appeared to be essential for the binding of PP2a to the IP3R-3, hence favoring IP3R-3 de-phosphorylation (Figures 3 and 4).
Thus in the absence of PML, the unopposed action of Akt at ER due to an impaired PP2a activity leads to a hyper-phosphorylation of IP3R-3 and in turn a reduced Ca2+ flux from ER to mitochondria rendering cells resistant to apoptotic Ca2+-dependent stimuli30 (Figures 3 and 4).
The elucidation of the role of IP3R-3 in Ca2+ transfer from the ER to mitochondria, of its molecular mechanism and of the regulatory effect of its phosphorylation may reveal a novel unexplored pharmacological target in apoptosis.
Conclusions and Future Directions: Beyond the PML-NB
PML is a critical and essential regulator of multiple apoptotic responses. While the reported role of PML in the modulation of p53 transcription could explain some of its pro-apoptotic functions, it failed to reconcile the fundamental role played by PML in the transcription-independent early apoptotic response.
The identification of PML at the ER–MAMs regions addressed this outstanding question. Indeed, the unexpected extra-nuclear MAMs-associated PML-dependent pathway for the control of Ca2+ homeostasis and in turn Ca2+-dependent apoptosis provides a compelling explanation for such a pleiotropic role. PML exerts this role by orchestrating at ER/MAMs sites, as in the NBs, the function of different key proteins involved in cell death processes.
Further experiments will address whether this function is required also in vivo for tissue homeostasis, and in which tissues this function is critical. The generation of an inducible erPML transgenic mouse model will also allow to determine whether the PML function at the ER/MAMs sites is fundamental (and sufficient) to trigger Ca2+-dependent apoptosis in tumor models.
Strikingly, the final outcome of a PML functional loss at the cellular level is similar to the one observed in cells over-expressing Bcl-2 or lacking of Bax/Bak (albeit through a completely different molecular mechanism): a reduced mitochondrial Ca2+ overload upon pro-apoptotic stimuli that dramatically blunts the apoptotic response.
This in turn highlights a new extra-nuclear PML function critical for regulation of cell survival through the ER–mitochondria Ca2+-dependent cross-talk.
Acknowledgments
We thank members of Pinton and Pandolfi laboratories for critical discussion and suggestions. This research was supported by the Italian Association for Cancer Research (AIRC), Telethon (GGP09128), local funds from the University of Ferrara, the Italian Ministry of Education, University and Research (COFIN), the Italian Cystic Fibrosis Research Foundation, and Italian Ministry of Health to PP as well as to NIH grants to PPP.
Glossary
- AML
acute myeloid leukemia
- APL
acute promyelocytic leukemia
- ANT
adenine nucleotide translocator
- Ca2+
calcium
- cypD
cyclophilin D
- ER
endoplasmic reticulum
- IP3
inositol 1,4,5-trisphosphate
- IP3Rs
IP3-sensitive Ca2+ release channels
- MAMs
mitochondria-associated membranes
- MEFs
mouse embryonic fibroblasts
- PML
promyelocytic leukemia
- PML-NBs
PML-nuclear bodies
- nuPML
PML chimera targeted to the nucleus
- erPML
PML chimera targeted to the outer surface of the ER
- RA
retinoic acid
- RARα
retinoic acid receptor alpha
- VDAC
voltage-dependent anion channel
The authors declare no conflict of interest.
Footnotes
Edited by G Melino
References
- Pandolfi PP. Oncogenes and tumor suppressors in the molecular pathogenesis of acute promyelocytic leukemia. Hum Mol Genet. 2001;10:769–775. doi: 10.1093/hmg/10.7.769. [DOI] [PubMed] [Google Scholar]
- Salomoni P, Pandolfi PP. The role of PML in tumor suppression. Cell. 2002;108:165–170. doi: 10.1016/s0092-8674(02)00626-8. [DOI] [PubMed] [Google Scholar]
- Salomoni P, Ferguson BJ, Wyllie AH, Rich T. New insights into the role of PML in tumour suppression. Cell Res. 2008;18:622–640. doi: 10.1038/cr.2008.58. [DOI] [PubMed] [Google Scholar]
- Bernardi R, Pandolfi PP. Structure, dynamics and functions of promyelocytic leukaemia nuclear bodies. Nat Rev Mol Cell Biol. 2007;8:1006–1016. doi: 10.1038/nrm2277. [DOI] [PubMed] [Google Scholar]
- Jensen K, Shiels C, Freemont PS. PML protein isoforms and the RBCC/TRIM motif. Oncogene. 2001;20:7223–7233. doi: 10.1038/sj.onc.1204765. [DOI] [PubMed] [Google Scholar]
- Lallemand-Breitenbach V, de The H. PML nuclear bodies. Cold Spring Harb Perspect Biol. 2010;2:a000661. doi: 10.1101/cshperspect.a000661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen TH, Lin HK, Scaglioni PP, Yung TM, Pandolfi PP. The mechanisms of PML-nuclear body formation. Mol Cell. 2006;24:331–339. doi: 10.1016/j.molcel.2006.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melnick A, Licht JD. Deconstructing a disease: RARalpha, its fusion partners, and their roles in the pathogenesis of acute promyelocytic leukemia. Blood. 1999;93:3167–3215. [PubMed] [Google Scholar]
- Rego EM, Wang ZG, Peruzzi D, He LZ, Cordon-Cardo C, Pandolfi PP. Role of promyelocytic leukemia (PML) protein in tumor suppression. J Exp Med. 2001;193:521–529. doi: 10.1084/jem.193.4.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song MS, Salmena L, Carracedo A, Egia A, Lo-Coco F, Teruya-Feldstein J, et al. The deubiquitinylation and localization of PTEN are regulated by a HAUSP-PML network. Nature. 2008;455:813–817. doi: 10.1038/nature07290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de The H, Chen Z. Acute promyelocytic leukaemia: novel insights into the mechanisms of cure. Nat Rev Cancer. 2010;10:775–783. doi: 10.1038/nrc2943. [DOI] [PubMed] [Google Scholar]
- Wang ZG, Ruggero D, Ronchetti S, Zhong S, Gaboli M, Rivi R, et al. PML is essential for multiple apoptotic pathways. Nat Genet. 1998;20:266–272. doi: 10.1038/3073. [DOI] [PubMed] [Google Scholar]
- Bernardi R, Papa A, Pandolfi PP. Regulation of apoptosis by PML and the PML-NBs. Oncogene. 2008;27:6299–6312. doi: 10.1038/onc.2008.305. [DOI] [PubMed] [Google Scholar]
- Takahashi Y, Lallemand-Breitenbach V, Zhu J, de The H. PML nuclear bodies and apoptosis. Oncogene. 2004;23:2819–2824. doi: 10.1038/sj.onc.1207533. [DOI] [PubMed] [Google Scholar]
- Louria-Hayon I, Grossman T, Sionov RV, Alsheich O, Pandolfi PP, Haupt Y. The promyelocytic leukemia protein protects p53 from Mdm2-mediated inhibition and degradation. J Biol Chem. 2003;278:33134–33141. doi: 10.1074/jbc.M301264200. [DOI] [PubMed] [Google Scholar]
- Alsheich-Bartok O, Haupt S, Alkalay-Snir I, Saito S, Appella E, Haupt Y. PML enhances the regulation of p53 by CK1 in response to DNA damage. Oncogene. 2008;27:3653–3661. doi: 10.1038/sj.onc.1211036. [DOI] [PubMed] [Google Scholar]
- Yang S, Jeong JH, Brown AL, Lee CH, Pandolfi PP, Chung JH, et al. Promyelocytic leukemia activates Chk2 by mediating Chk2 autophosphorylation. J Biol Chem. 2006;281:26645–26654. doi: 10.1074/jbc.M604391200. [DOI] [PubMed] [Google Scholar]
- Moller A, Sirma H, Hofmann TG, Rueffer S, Klimczak E, Droge W, et al. PML is required for homeodomain-interacting protein kinase 2 (HIPK2)-mediated p53 phosphorylation and cell cycle arrest but is dispensable for the formation of HIPK domains. Cancer Res. 2003;63:4310–4314. [PubMed] [Google Scholar]
- Bernardi R, Pandolfi PP. Role of PML and the PML-nuclear body in the control of programmed cell death. Oncogene. 2003;22:9048–9057. doi: 10.1038/sj.onc.1207106. [DOI] [PubMed] [Google Scholar]
- Dellaire G, Bazett-Jones DP. PML nuclear bodies: dynamic sensors of DNA damage and cellular stress. Bioessays. 2004;26:963–977. doi: 10.1002/bies.20089. [DOI] [PubMed] [Google Scholar]
- Kurki S, Latonen L, Laiho M. Cellular stress and DNA damage invoke temporally distinct Mdm2, p53 and PML complexes and damage-specific nuclear relocalization. J Cell Sci. 2003;116 (Part 19:3917–3925. doi: 10.1242/jcs.00714. [DOI] [PubMed] [Google Scholar]
- Everett RD, Meredith M, Orr A, Cross A, Kathoria M, Parkinson J. A novel ubiquitin-specific protease is dynamically associated with the PML nuclear domain and binds to a herpesvirus regulatory protein. EMBO J. 1997;16:1519–1530. doi: 10.1093/emboj/16.7.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Chen D, Shiloh A, Luo J, Nikolaev AY, Qin J, et al. Deubiquitination of p53 by HAUSP is an important pathway for p53 stabilization. Nature. 2002;416:648–653. doi: 10.1038/nature737. [DOI] [PubMed] [Google Scholar]
- Trotman LC, Alimonti A, Scaglioni PP, Koutcher JA, Cordon-Cardo C, Pandolfi PP. Identification of a tumour suppressor network opposing nuclear Akt function. Nature. 2006;441:523–527. doi: 10.1038/nature04809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trotman LC, Wang X, Alimonti A, Chen Z, Teruya-Feldstein J, Yang H, et al. Ubiquitination regulates PTEN nuclear import and tumor suppression. Cell. 2007;128:141–156. doi: 10.1016/j.cell.2006.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Trotman LC, Koppie T, Alimonti A, Chen Z, Gao Z, et al. NEDD4-1 is a proto-oncogenic ubiquitin ligase for PTEN. Cell. 2007;128:129–139. doi: 10.1016/j.cell.2006.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condemine W, Takahashi Y, Zhu J, Puvion-Dutilleul F, Guegan S, Janin A, et al. Characterization of endogenous human promyelocytic leukemia isoforms. Cancer Res. 2006;66:6192–6198. doi: 10.1158/0008-5472.CAN-05-3792. [DOI] [PubMed] [Google Scholar]
- Lin HK, Bergmann S, Pandolfi PP. Cytoplasmic PML function in TGF-beta signalling. Nature. 2004;431:205–211. doi: 10.1038/nature02783. [DOI] [PubMed] [Google Scholar]
- McNally BA, Trgovcich J, Maul GG, Liu Y, Zheng P. A role for cytoplasmic PML in cellular resistance to viral infection. PLoS One. 2008;3:e2277. doi: 10.1371/journal.pone.0002277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giorgi C, Ito K, Lin HK, Santangelo C, Wieckowski MR, Lebiedzinska M, et al. PML regulates apoptosis at endoplasmic reticulum by modulating calcium release. Science. 2010;330:1247–1251. doi: 10.1126/science.1189157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giorgi C, De Stefani D, Bononi A, Rizzuto R, Pinton P. Structural and functional link between the mitochondrial network and the endoplasmic reticulum. Int J Biochem Cell Biol. 2009;41:1817–1827. doi: 10.1016/j.biocel.2009.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzuto R, Pinton P, Carrington W, Fay FS, Fogarty KE, Lifshitz LM, et al. Close contacts with the endoplasmic reticulum as determinants of mitochondrial Ca2+ responses. Science. 1998;280:1763–1766. doi: 10.1126/science.280.5370.1763. [DOI] [PubMed] [Google Scholar]
- Vance JE. Phospholipid synthesis in a membrane fraction associated with mitochondria. J Biol Chem. 1990;265:7248–7256. [PubMed] [Google Scholar]
- Piccini M, Vitelli F, Bruttini M, Pober BR, Jonsson JJ, Villanova M, et al. FACL4, a new gene encoding long-chain acyl-CoA synthetase 4, is deleted in a family with Alport syndrome, elliptocytosis, and mental retardation. Genomics. 1998;47:350–358. doi: 10.1006/geno.1997.5104. [DOI] [PubMed] [Google Scholar]
- Stone SJ, Vance JE. Phosphatidylserine synthase-1 and -2 are localized to mitochondria-associated membranes. J Biol Chem. 2000;275:34534–34540. doi: 10.1074/jbc.M002865200. [DOI] [PubMed] [Google Scholar]
- Rusinol AE, Cui Z, Chen MH, Vance JE. A unique mitochondria-associated membrane fraction from rat liver has a high capacity for lipid synthesis and contains pre-Golgi secretory proteins including nascent lipoproteins. J Biol Chem. 1994;269:27494–27502. [PubMed] [Google Scholar]
- Thomson M. Does cholesterol use the mitochondrial contact site as a conduit to the steroidogenic pathway. Bioessays. 2003;25:252–258. doi: 10.1002/bies.10243. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Rizzuto R, Hajnoczky G, Su TP. MAM: more than just a housekeeper. Trends Cell Biol. 2009;19:81–88. doi: 10.1016/j.tcb.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieckowski MR, Giorgi C, Lebiedzinska M, Duszynski J, Pinton P. Isolation of mitochondria-associated membranes and mitochondria from animal tissues and cells. Nat Protoc. 2009;4:1582–1590. doi: 10.1038/nprot.2009.151. [DOI] [PubMed] [Google Scholar]
- Mikoshiba K. IP3 receptor/Ca2+ channel: from discovery to new signaling concepts. J Neurochem. 2007;102:1426–1446. doi: 10.1111/j.1471-4159.2007.04825.x. [DOI] [PubMed] [Google Scholar]
- Azzolin L, von Stockum S, Basso E, Petronilli V, Forte MA, Bernardi P. The mitochondrial permeability transition from yeast to mammals. FEBS Lett. 2010;584:2504–2509. doi: 10.1016/j.febslet.2010.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halestrap AP. What is the mitochondrial permeability transition pore. J Mol Cell Cardiol. 2009;46:821–831. doi: 10.1016/j.yjmcc.2009.02.021. [DOI] [PubMed] [Google Scholar]
- Berridge MJ. The endoplasmic reticulum: a multifunctional signaling organelle. Cell Calcium. 2002;32:235–249. doi: 10.1016/s0143416002001823. [DOI] [PubMed] [Google Scholar]
- Pinton P, Giorgi C, Siviero R, Zecchini E, Rizzuto R. Calcium and apoptosis: ER-mitochondria Ca2+ transfer in the control of apoptosis. Oncogene. 2008;27:6407–6418. doi: 10.1038/onc.2008.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzuto R, Marchi S, Bonora M, Aguiari P, Bononi A, De Stefani D, et al. Ca(2+) transfer from the ER to mitochondria: when, how and why. Biochim Biophys Acta. 2009;1787:1342–1351. doi: 10.1016/j.bbabio.2009.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapham DE. Calcium signaling. Cell. 2007;131:1047–1058. doi: 10.1016/j.cell.2007.11.028. [DOI] [PubMed] [Google Scholar]
- Ferri KF, Kroemer G. Organelle-specific initiation of cell death pathways. Nat Cell Biol. 2001;3:E255–E263. doi: 10.1038/ncb1101-e255. [DOI] [PubMed] [Google Scholar]
- Pinton P, Ferrari D, Rapizzi E, Di Virgilio FD, Pozzan T, Rizzuto R. The Ca2+ concentration of the endoplasmic reticulum is a key determinant of ceramide-induced apoptosis: significance for the molecular mechanism of Bcl-2 action. EMBO J. 2001;20:2690–2701. doi: 10.1093/emboj/20.11.2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinton P, Ferrari D, Rapizzi E, Di Virgilio F, Pozzan T, Rizzuto R. The Ca2+ concentration of the endoplasmic reticulum is a key determinant of ceramide-induced apoptosis: significance for the molecular mechanism of Bcl-2 action. EMBO J. 2001;20:2690–2701. doi: 10.1093/emboj/20.11.2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinton P, Ferrari D, Magalhaes P, Schulze-Osthoff K, Di Virgilio F, Pozzan T, et al. Reduced loading of intracellular Ca(2+) stores and downregulation of capacitative Ca(2+) influx in Bcl-2-overexpressing cells. J Cell Biol. 2000;148:857–862. doi: 10.1083/jcb.148.5.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinton P, Ferrari D, Rapizzi E, Di Virgilio F, Pozzan T, Rizzuto R. A role for calcium in Bcl-2 action. Biochimie. 2002;84:195–201. doi: 10.1016/s0300-9084(02)01373-1. [DOI] [PubMed] [Google Scholar]
- Scorrano L, Oakes SA, Opferman JT, Cheng EH, Sorcinelli MD, Pozzan T, et al. BAX and BAK regulation of endoplasmic reticulum Ca2+: a control point for apoptosis. Science. 2003;300:135–139. doi: 10.1126/science.1081208. [DOI] [PubMed] [Google Scholar]
- Chami M, Prandini A, Campanella M, Pinton P, Szabadkai G, Reed JC, et al. Bcl-2 and Bax exert opposing effects on Ca2+ signalling, which do not depend on their putative pore-forming region. J Biol Chem. 2004;279:54581–54589. doi: 10.1074/jbc.M409663200. [DOI] [PubMed] [Google Scholar]
- Joseph SK, Hajnoczky G. IP3 receptors in cell survival and apoptosis: Ca2+ release and beyond. Apoptosis. 2007;12:951–968. doi: 10.1007/s10495-007-0719-7. [DOI] [PubMed] [Google Scholar]
- Mikoshiba K. The IP3 receptor/Ca2+ channel and its cellular function. Biochem Soc Symp. 2007;74:9–22. doi: 10.1042/BSS0740009. [DOI] [PubMed] [Google Scholar]
- Jayaraman T, Marks AR. T cells deficient in inositol 1,4,5-trisphosphate receptor are resistant to apoptosis. Mol Cell Biol. 1997;17:3005–3012. doi: 10.1128/mcb.17.6.3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan AA, Soloski MJ, Sharp AH, Schilling G, Sabatini DM, Li SH, et al. Lymphocyte apoptosis: mediation by increased type 3 inositol 1,4,5-trisphosphate receptor. Science. 1996;273:503–507. doi: 10.1126/science.273.5274.503. [DOI] [PubMed] [Google Scholar]
- Sugawara H, Kurosaki M, Takata M, Kurosaki T. Genetic evidence for involvement of type 1, type 2 and type 3 inositol 1,4,5-trisphosphate receptors in signal transduction through the B-cell antigen receptor. EMBO J. 1997;16:3078–3088. doi: 10.1093/emboj/16.11.3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehning D, Patterson RL, Sedaghat L, Glebova NO, Kurosaki T, Snyder SH. Cytochrome c binds to inositol (1,4,5) trisphosphate receptors, amplifying calcium-dependent apoptosis. Nat Cell Biol. 2003;5:1051–1061. doi: 10.1038/ncb1063. [DOI] [PubMed] [Google Scholar]
- Khan MT, Wagner L, Yule DI, Bhanumathy C, Joseph SK. Akt kinase phosphorylation of inositol 1,4,5-trisphosphate receptors. J Biol Chem. 2006;281:3731–3737. doi: 10.1074/jbc.M509262200. [DOI] [PubMed] [Google Scholar]
- Vanderheyden V, Devogelaere B, Missiaen L, De Smedt H, Bultynck G, Parys JB. Regulation of inositol 1,4,5-trisphosphate-induced Ca2+ release by reversible phosphorylation and dephosphorylation. Biochim Biophys Acta. 2009;1793:959–970. doi: 10.1016/j.bbamcr.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szado T, Vanderheyden V, Parys JB, De Smedt H, Rietdorf K, Kotelevets L, et al. Phosphorylation of inositol 1,4,5-trisphosphate receptors by protein kinase B/Akt inhibits Ca2+ release and apoptosis. Proc Natl Acad Sci USA. 2008;105:2427–2432. doi: 10.1073/pnas.0711324105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakes SA, Scorrano L, Opferman JT, Bassik MC, Nishino M, Pozzan T, et al. Proapoptotic BAX and BAK regulate the type 1 inositol trisphosphate receptor and calcium leak from the endoplasmic reticulum. Proc Natl Acad Sci USA. 2005;102:105–110. doi: 10.1073/pnas.0408352102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendes CC, Gomes DA, Thompson M, Souto NC, Goes TS, Goes AM, et al. The type III inositol 1,4,5-trisphosphate receptor preferentially transmits apoptotic Ca2+ signals into mitochondria. J Biol Chem. 2005;280:40892–40900. doi: 10.1074/jbc.M506623200. [DOI] [PubMed] [Google Scholar]
- Blackshaw S, Sawa A, Sharp AH, Ross CA, Snyder SH, Khan AA. Type 3 inositol 1,4,5-trisphosphate receptor modulates cell death. FASEB J. 2000;14:1375–1379. doi: 10.1096/fj.14.10.1375. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Su TP. Sigma-1 receptor chaperones at the ER-mitochondrion interface regulate Ca(2+) signaling and cell survival. Cell. 2007;131:596–610. doi: 10.1016/j.cell.2007.08.036. [DOI] [PubMed] [Google Scholar]
- Marchi S, Rimessi A, Giorgi C, Baldini C, Ferroni L, Rizzuto R, et al. Akt kinase reducing endoplasmic reticulum Ca2+ release protects cells from Ca2+-dependent apoptotic stimuli. Biochem Biophys Res Commun. 2008;375:501–505. doi: 10.1016/j.bbrc.2008.07.153. [DOI] [PMC free article] [PubMed] [Google Scholar]