Abstract

A practical, enantioselective synthetic route to a key precursor to the tetracycline antibiotics is reported. The route proceeds in 9 steps (21% yield) from the commercial substance methyl 3-hydroxy-5-isoxazolecarboxylate. Key steps in the route involve enantioselective addition of divinylzinc to 3-benzyloxy-5-isoxazolecarboxaldehyde and an endo-selective intramolecular furan Diels–Alder cycloaddition reaction. The route described has provided more than 40 g of chromatographically pure 1, of 93% ee.
We recently described a convergent synthetic route to the tetracyclines, broad spectrum antibiotics used widely in human and veterinary medicine.1 The key and final steps involve a Michael–Dieckmann condensation2 of the AB precursor 1 with any of a number of structurally diverse D-ring precursors, followed typically by two chemical “deprotection” reactions3 (illustrated in Scheme 1 for the specific construction of (−)-6-deoxytetracycline). Using this general route a number of novel broad-spectrum antibiotic substances have been identified, including molecules effective against tetracycline-resistant bacterial strains.1,4 Here, we describe a completely different route to the intermediate 1 than that we first reported, and show that this new route is amenable to the production of multi-gram quantities of 1 in optically active form (40 g in the largest single run to date). We believe that this innovation will fuel the discovery of large numbers of new candidate antibiotics and may provide the basis for novel tetracyclines to be synthesized on a scale sufficient for their clinical development, where hundreds of grams and, ultimately, kilograms of material will be required.5
Scheme 1.
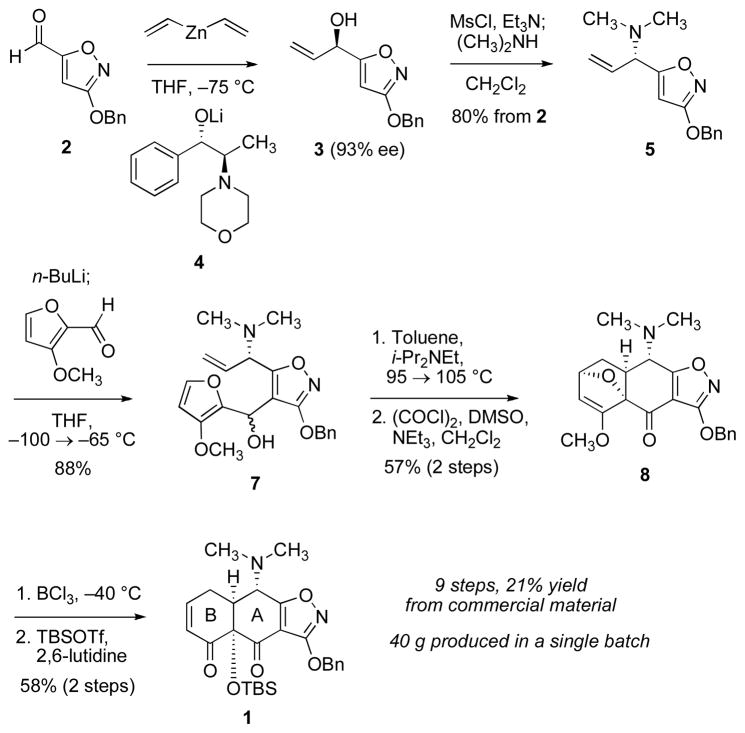
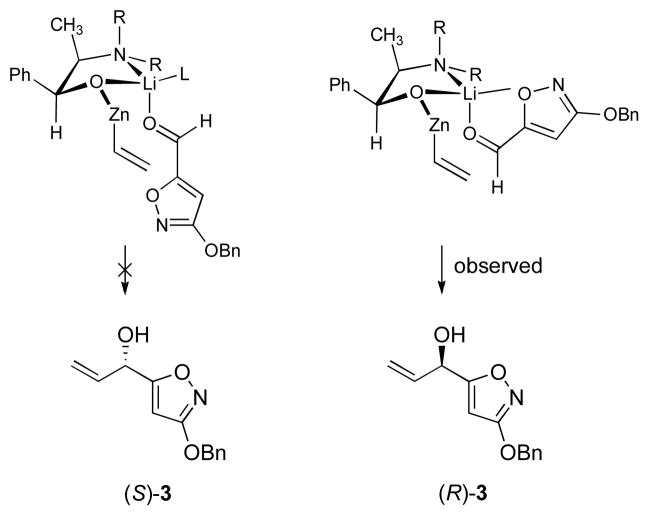
The new, convergent route to 1 begins with 3-benzyloxy-5-isoxazole carboxaldehyde (2, Scheme 2), prepared in multi-hundred gram amounts in two steps (O-benzylation, then reduction with diisobutylaluminum hydride)6 from methyl 3-hydroxy-5-isoxazolecarboxylate (a commercial product), which is available in large quantity from the reaction of N-hydroxyurea with dimethyl acetylenedicarboxylate.7 We have developed two procedures to transform 2 into the optically enriched (R)-allylic alcohol 3. The procedure currently used for large-scale preparations is derived from multiple precedents8 and involves addition at −75 °C of 1 equiv of 2 in toluene to 2 equiv of a 1:1 complex formed from the norephedrine-derived lithium alkoxide 4 and divinyl zinc. After an aqueous work-up, wherein the ligand is recovered in >95% yield, the (R)-allylic alcohol 3 is obtained with an optical purity of 93% ee (>90% yield). The configuration of the product (3) is opposite to that observed in related additions to non-heteroaromatic aldehydes,8a,9 which suggests that 2 may bind to the metal complex in bidentate fashion during the addition (thus presenting the enantiotopic π-face of the aldehyde to the nucleophile, Figure 1). The reversal in enantioselectivity we observe with a potentially bis-chelate substrate has not been documented in the Oppolzer system so far as we are aware. Dinuclear zinc catalysts apparently do not show such behavior.10 The lithium cation may prove a key organizational element, as suggested in Figure 1 and originally proposed by Oppolzer and Radinov. Because of the ready availability of the ligand,8c–d thus far we have not pursued efforts to reduce its levels in the addition, though this may be possible. A second route to (R)-3 proceeds by kinetic resolution. Acylation of (±)-3 (prepared in 96% yield from 2 and vinyl magnesium bromide) with vinyl acetate in hexanes using Amano Lipase AK as a catalyst afforded the (R)-allylic acetate (49% yield) and the (S)-allylic alcohol (50% yield, 93% ee), isolated separately after purification by flash column chromatography.11 Cleavage of the (R)-allylic acetate with potassium carbonate in methanol then provided (R)-3 of 93% ee (99% yield). Produced by either method, the unpurified (R)-allylic alcohol 3 was transformed into the corresponding mesylate derivative at −12 °C,12 and the latter intermediate was treated in situ with a solution of dimethylamine in THF (−30→15 °C) to afford, after extractive isolation and purification through a short column of silica gel, the (S)-allylic amine 5 as a pale yellow oil (76 g, 80% yield over 2 steps). 1H NMR analysis of the latter product in the presence of the (R)-Mosher acid13 (1.5 equiv, C6D6) established that the product was of 93% ee and thus that the transformation of 3 to 5 had proceeded with complete stereospecificity, within experimental error.
Scheme 2.
Figure 1.
A model for the reversal of enantioselectivity observed with the heterocyclic aldehyde 2. (other zinc ligands are omitted for clarity).
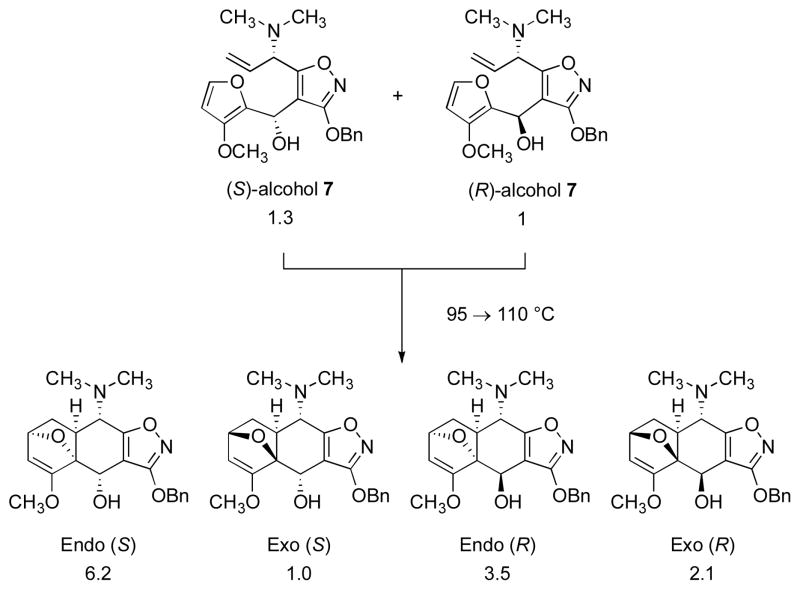
A convergent coupling was achieved by metalation of the isoxazole ring of the (S)-allylic amine 5 with n-butyllithium in tetrahydrofuran at −100 → −65 °C followed by trapping of the resulting anion at −65 °C with 3-methoxyfurfural (6),14 providing an epimeric mixture of secondary carbinol adducts 7 (1.3:1 ratio, 88%, 98 g, see Scheme 3 for assignments).15 In a key transformation, the mixture of epimeric alcohols 7 was heated in toluene with N,N-diisopropylethylamine (2 equiv) and 2,6-di-tert-butyl-4-methylphenol (0.004 equiv) first at 95 °C for 105 h, then at 110 °C for 24 h, affording a mixture of four diastereomeric intramolecular Diels–Alder cycloadducts (combined yield 78%, 77 g). Detailed analysis of the product mixture shows that cycloaddition occurs from a single π-face of the allylic amine (dienophile) and that the two major products are both endo-diastereomers, stereochemically homologous with the target 1 at the AB juncture (see Scheme 3). While both of the epimeric substrates 7 undergo endo-selective cycloaddition, the endo/exo ratios are different (6.2 : 1 for the (S)-alcohol, and 1.6 : 1 for the (R)-alcohol), as are the rates of cycloaddition, with the (S)-alcohol 7 reacting somewhat more rapidly.16 The endo selectivity of the cycloaddition reaction is notable, given that the majority of furan Diels–Alder reactions are exo-selective, reversible processes.17 Retro-cycloaddition apparently does not occur in the present case, as evidenced by the fact that each of the (purified) individual diastereomers remains unchanged after heating at 110 °C for 24 h. The lack of reversibility is likely due to an enthalpic effect of the 3-methoxyl substituent of the furan ring, as discussed in detail by Pieniazek and Houk.18
Scheme 3.
Swern oxidation19 of the Diels–Alder product mixture gave two ketone products of different polarities; these were readily separated by flash-column chromatography. The more polar isomer proved to be the desired product 8, and was obtained in 73% yield (55 g, Scheme 2). Treatment of 8 with boron trichloride (3 equiv) in dichloromethane at −40 °C led to opening of the oxabicyclic ring system and demethylation of the methyl enol ether. Protection of the tertiary hydroxyl group of the product with tert-butyldimethylsilyl triflate then gave the target 1 (58% over two steps, 23% from aldehyde 2 over seven steps, 21% from the commercial precursor to 2 over nine steps, 40 g after chromatographic purification). The purified product was also recrystallized (ethyl acetate-hexanes, 27 g, crop 1, mp = 150–151 °C, 7.2 g, crop 2, mp 150–151 °C, and 1.9 g, crop 3, mp 149–150 °C). Analysis of the product (crystal crop 1) by HPLC using a chiral column showed it to be of 93% ee.20
The route we have described provides a very short sequence to (+)-1 from simple heterocyclic precursors that might readily be manufactured in multi-kilogram amounts. While further process development will undoubtedly be required to allow manufacture of 1 (and novel tetracyclines thereafter), the quantities of 1 we have prepared as described herein alone should allow for the synthesis and evaluation (antimicrobial screening panels) of literally hundreds of novel antibiotic candidates.
Supplementary Material
Acknowledgments
Financial support from the National Institutes of Health (Grant AI048825) is gratefully acknowledged. J.D.B. acknowledges an NSF predoctoral fellowship.
Footnotes
Supporting Information Available: Detailed experimental procedures, tabulated spectroscopic data (1H and 13C NMR, FT-IR, and HRMS) for all new compounds, and complete reference 8d. This material is available free of charge via the internet at http://pubs.acs.org/
References
- 1.Charest MG, Lerner CD, Brubaker JD, Siegel DR, Myers AG. Science. 2005;308:395. doi: 10.1126/science.1109755. [DOI] [PubMed] [Google Scholar]
- 2.(a) Leeper FJ, Staunton J. J Chem Soc Chem Comm. 1978:406. [Google Scholar]; (b) Hauser FM, Rhee RP. J Org Chem. 1978;43:178. [Google Scholar]; (c) Dodd JH, Weinreb SM. Tetrahedron Lett. 1979;20:3593. [Google Scholar]
- 3.Stork G, Hagedorn AA., III J Am Chem Soc. 1978;100:3609. [Google Scholar]
- 4.(a) Myers AG, Charest MG, Lerner CD, Brubaker JD, Siegel DR. Synthesis of Tetracyclines and Analogues Therof. PCT/US05/17831. May 20, 2005. [Google Scholar]; (b) Myers AG. PCT/US07/66253. Apr 6, 2007. [Google Scholar]
- 5.The Muxfeldt route to the tetracyclines (involving late-stage C- and D-ring constructions) was used to synthesize a number of analogs, and was scaled to provide (±)-6-thiatetracycline for early clinical trials (discontinued due to an apparent CNS-based toxicity). Cunha BA. Clinical Uses of the Tetracyclines. In: Hlavka JJ, Boothe JH, editors. Handbook of Experimental Pharmacology. Vol. 78. Springer-Verlag; New York: 1985. p. 393.Nelson M, Hillen W, Greenwald RA, editors. Tetracyclines in Biology, Chemistry, and Medicine. Birkhäuser Verlag; Boston, MA: 2001. p. 237.
- 6.Riess R, Schön M, Laschat S, Jäger V. Eur J Org Chem. 1998:473. [Google Scholar]
- 7.Frey M, Jäger V. Synthesis. 1985:1100. [Google Scholar]
- 8.Use of lithium N-methylephedrate for the enantioselective addition of 1-alkenylzinc bromides to aldehydes: Oppolzer W, Radinov RN. Tetrahedron Lett. 1991;32:5777.Synthesis of dialkylzinc reagents by addition of Grignard reagents to zinc chloride followed by precipitation with 1,4-dioxane: von dem Bussche-Hünnefeld JL, Seebach D. Tetrahedron. 1992;48:5719.Use of (1S,2R)-2-morpholin-4-yl-1-phenylpropanol for the enantioselective addition of diisopropylzinc to aldehydes: Soai K, Hayase T, Takai K, Sugiyama T. J Org Chem. 1994;59:7908.Synthesis of (1S,2R)-2-pyrrolidiny-1-phenylpropanol by alkylation of norephedrine with 1,4-dibromobutane: Pierce ME, et al. J Org Chem. 1998;63:8536.
- 9.Layton ME, Morales CA, Shair MD. J Am Chem Soc. 2002;124:2002. doi: 10.1021/ja016585u. [DOI] [PubMed] [Google Scholar]
- 10.Furan 2-carboxaldehydes: Noyori R, Suga S, Kawai K, Okada S, Kitamura M, Oguni N, Hayashi M, Kaneko T, Matsuda Y. J Organomet Chem. 1990;382:19.Sato I, Saito T, Omiya D, Takizawa Y, Soai K. Heterocycles. 1999;51:2753.
- 11.Marshall JA, Chobanian H. Org Synth. 2005;82:43. [Google Scholar]
- 12.Crossland RK, Servis KL. J Org Chem. 1970;35:3195. [Google Scholar]
- 13.Benson SC, Cai P, Colon M, Haiza MA, Tokles M, Snyder JK. J Org Chem. 1988;53:5335. [Google Scholar]
- 14.Prepared by Villsmeier-Haack formylation (Jones G, Stanforth SP. Org React. 1997;49:1.) of 3-methoxyfurfural, synthesized in hundred-gram amounts–without chromatography–in four steps from 3-chloro-1,2-propanediol: Meister C, Scharf H-D. Synthesis. 1981:737.
-
15.Allylic metalation (removal of the proton α to the dimethylamino group) is competitive with metalation of the isoxazole ring at higher temperatures and it is the predominant reaction pathway when bases other than n-butyllithium are used for deprotonation. A preliminary experiment with the 4-iodoisoxazole 9 (see Supporting Information) reveals that magnesium-halogen exchange at −20 °C provides an efficient means of anion formation; trapping with 3-methoxyfurfural (6) gives a higher proportion of the (S)-alcohol 7.
 Magnesium-halogen exchange: Wakefield BJ. Organomagnesium Compounds in Organic Synthesis. Academic Press Inc; San Diego: 1995. Preparation of Organomagnesium Compounds; pp. 51–59.Boudier A, Bromm LO, Lotz M, Knochel P. Angew Chem, Int Ed Engl. 2000;39:4414.
Magnesium-halogen exchange: Wakefield BJ. Organomagnesium Compounds in Organic Synthesis. Academic Press Inc; San Diego: 1995. Preparation of Organomagnesium Compounds; pp. 51–59.Boudier A, Bromm LO, Lotz M, Knochel P. Angew Chem, Int Ed Engl. 2000;39:4414.
- 16.After 105 h at 95 °C cyclization of the (S)-alcohol 7 had proceeded to 89% conversion whereas cyclization of the (R)-alcohol 7 had proceeded to 77% conversion.
- 17.Kappe CO, Murphree SS, Padwa A. Tetrahedron. 1997;53:14179. [Google Scholar]
- 18.Pieniazek SN, Houk KN. Angew Chem, Int Ed Engl. 2006;45:1442. doi: 10.1002/anie.200502677. [DOI] [PubMed] [Google Scholar]
- 19.Omura K, Swern D. Tetrahedron. 1978;34:1651. [Google Scholar]
- 20.The optical rotation of synthetic 1 was [α]23D −138° (c 0.52, CHCl3); literature: [α]23D −149° (c 0.265, CHCl3).1
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.