Abstract
Background
The objective of this study was to examine whether cytomegalovirus (CMV), herpes simplex virus type-1 (HSV-1), and C-reactive protein (CRP) are associated with functional impairment in older Latinos.
Methods
A cross-sectional analysis of a cohort study conducted with a community dwelling elderly population. The sample was a subset (N = 1507/1789) of participants in the Sacramento Area Latino Study on Aging (SALSA) ages 60–101 with available serum samples and functional impairment measures. Baseline serum samples were assayed for levels of immunoglobulin G antibodies to CMV and HSV-1 and for levels of CRP. Several measures were used to assess functional impairment, including activities of daily living (ADL), instrumental activities of daily living (IADL), and walking pace.
Results
CMV and CRP showed statistically significant graded associations with ADL functional impairment, even after controlling for age and gender. The relationship between CMV and ADL was slightly attenuated, and the confidence interval contained the null value when adjusted for total number of health conditions, body mass index, and household income. Only high levels of CRP were significantly related to ADL and IADL impairment even after adjusting for all other covariates.
Conclusion
Inflammation is clearly linked to physical functioning among aging Latinos. This study also suggests a role for CMV infection in relation to ADL impairment. Further research examining the influence of infection, immune response, and inflammation on longitudinal trajectories of physical functioning is warranted.
Keywords: Cytomegalovirus (CMV), CRP, Latinos, Physical function, Community
Functional impairment, a common occurrence in aging, is generally measured as any difficulty that interferes with or limits functioning in one or more major life activities, including basic and instrumental activities of daily living (ADL, IADL) (1–3). Research suggests that physiologic processes such as the production of pro-inflammatory complexes may underlie functional impairment. Several studies have described a relationship between markers such as C-reactive protein (CRP), interleukin-6 (IL-6), and tumor necrosis factor-α (TNF-α) and measures of functional impairment, physical decline, and walking speed, in older populations (4–7). Ferrucci and colleagues (8) suggest that overproduction of cytokines and maintenance of the inflammatory state over a long period may lead to physical degeneration in elderly populations.
It has also been reported that persistent infections may act as pro-inflammatory agents and are involved as stimuli that underlie and sustain the process of chronic inflammation–(9–11). For example, one study has shown that asymptomatic chronic cytomegalovirus (CMV) infection is linked to greater frailty in older women and that the relationship is enhanced by IL-6 (9). Chronic latent infections, such as CMV and herpes simplex virus-1 (HSV-1), have the potential to induce chronic inflammatory states because they undergo periodic reactivation, thereby triggering subsequent inflammatory responses (10,12). CMV and HSV-1 infections often pass undiagnosed because of their asymptomatic properties, and remain persistent in the system for life, with an increasing risk of reactivation during old age and in response to stress (13–16). More recently, immunological studies have suggested that aging populations infected with CMV experience large clonal expansion of CD8+ effector T cells resulting in a reduction in the “immunological space” and a loss of response to foreign antigens other than CMV (17). Persistent CMV has been called one of the “driving forces” behind age-related alterations among T cells in elderly persons (18–20). The immunological effects of persistent CMV and other herpesviruses, such as HSV, may influence chronic conditions associated with aging. In addition to frailty, infection with CMV has been linked to cardiovascular disease, cognitive outcomes, and Alzheimer’s disease (11,21–23).
Both CMV and HSV-1 are common worldwide and are found in >83% of individuals 60 years old and older in the United States (24,25). Factors such as age, gender, race/ethnicity, and education are associated with HSV-1 and CMV seropositivity (24,25). Ethnic differences in infection have been found among persons 60–69 years old, with 95% of Mexican Americans having antibody to HSV-1 compared to 83% in non-Latino white populations (24). Similar prevalence estimates as well as race/ethnic disparities have been reported for CMV in the United States (25). Among individuals who are seropositive for HSV-1 or CMV, factors that may lead to higher levels of antibodies and possibly clinical reaction include stress, organ transplant, and immunosuppression (10,26–28). Collectively, these data suggest important roles for persistent infection and resultant chronic inflammation in the development of older age functional impairment, especially among minority populations such as Mexican Americans.
To date, CMV has been assessed in relation to a composite measure of frailty among a group of predominantly white women (9). There are no data, to our knowledge, that have assessed the influence of CMV on measures of ADL, IADL, and walking pace. In this study, we assess whether persistent antibodies to CMV and HSV-1 and CRP levels are associated with functional impairment in older Latino adults.
Methods
Study Population
The participants were enrolled in the Sacramento Area Latino Study on Aging (SALSA) study, which is a large, ongoing prospective cohort study of predominantly Mexican Americans living in the community, who were 60–101 years old at baseline in 1998–1999. More detailed information on sampling frame, recruitment procedures, and interviews are published elsewhere (29). At the end of baseline, 1789 people were enrolled in the study. The results of blood tests for infection and inflammatory markers were available for 1559 participants of which 1507 had measurements of ADL impairment, 1261 had measurements of IADL impairment, and 1444 had measurements of walking pace. Therefore, our analyses used these subgroups with available data regarding infection, inflammation, ADL, IADL, and walking pace impairment.
Study Design
Baseline data collection began in 1998–1999. Each participant answered questions regarding sociodemographic factors, lifestyle factors, acculturation, and medical diagnoses. Blood was drawn for measurement and analyses of antibodies, inflammatory markers, lipids, glucose, and insulin on the same day as the interview. Additionally, participants completed several physical performance tests.
Laboratory Analyses
Baseline frozen serum samples were analyzed for both CMV and HSV-1 infections and CRP levels. A standard commercial, highly sensitive, enzyme-linked immunosorbent assay (ELISA) kit was used for detecting type-specific immunoglobulin G (IgG) antibody responses to CMV and HSV-1 (Wampole Laboratories, Princeton, NJ). Antibody levels were measured by optical density units. The sensitivity and specificity of the assay for HSV-1 are reported to be 97.1% and 96.8%, respectively; for CMV, the sensitivity and specificity are reported to be 99% and 94%, respectively. Serum samples were also used to analyze levels of CRP by means of the CRP Ultra Wide Range Reagent Kit latex-enhanced immunoassay (Equal Diagnostics, Exton, PA).
Functional Measures
ADL and IADL impairment was measured based on self-reported functional status (2,30). The ADL measurement consisted of seven items, and was used to measure ability to perform activities such as walking across a small room, bathing, eating, and using the toilet (1). The IADL measurement consisted of 15 items and was used to measure ability to perform activities as described earlier (3). Both the ADL and IADL measurements used a standard Likert-type scale (30). Additionally, a self-reported measure of walking pace was used in which participants reported their usual walking pace outdoors, using a multiple-category scale consisting of, “Never walks outdoors,” “Unable to walk,” “Easy, casual,” “Normal, average,” “Brisk pace,” or “Very Brisk/striding” (31).
Covariates
Age and gender of each participant were recorded. Birthplace was measured by asking participants for their country of birth. Self-reported gross household income was measured based on last month’s income, and educational attainment by asking the total number of school years completed. A modified Charlson comorbidity index was created to control for the effect of chronic health conditions on functional impairment. Points were assigned for having myocardial infarction, congestive heart failure, stroke, dementia, liver disease, diabetes, renal disease, any malignancy, and leukemia or lymphoma and then summed to generate a comorbidity score for each participant. The following conditions were not included in the modified index due to lack of data: peripheral vascular disease, cerebrovascular disease, chronic pulmonary disease, connective tissue disease, ulcer disease, hemiplegia, diabetes with end organ damage, and acquired immunodeficiency syndrome (AIDS). In addition, it was not possible to differentiate severity of liver disease and renal disease or differentiate between leukemia and malignant lymphomas. Body mass index (BMI) at baseline was calculated using measured height and weight. Smoking status was measured in pack-years.
Statistical Analyses
Statistical analyses were performed using SAS (version 9.1; SAS Institute, Cary, NC). Baseline demographic analysis was carried out using t tests for difference in means and chi-squared tests for differences in proportions or for trends among demographic groupings. All significance tests were two-tailed, and p values < .05 were considered statistically significant.
Each of the 7 ADL and 15 IADL outcome measures were transformed from the Likert-type scale into a binary variable. A response of “no difficulty” in completing an activity was defined as having no difficulty and assigned a score of 0. Responses of “some difficulty,” “a lot of difficulty,” or “only with help from a person or equipment” were defined as having difficulty and assigned a score of 1. A summary score was created as a continuous variable for ADL and IADL (range 0–7 and 0–15, respectively) and then transformed into a binary variable. The final binary ADL variable was dichotomized as: (1) difficulty in one or more activities or (2) no difficulty in activities. The final binary IADL variable was dichotomized as: (1) difficulty in three or more activities, or (2) difficulty in two or fewer activities. This method of dichotomizing ADL and IADL has been used elsewhere (32). CMV and HSV-1 antibody titer and CRP level were assessed as continuous variables and in tertiles to generate odds ratios. Because continuous CRP levels were not normally distributed, values were transformed using natural logarithm. Lastly, outdoor walking pace was transformed into a binary variable in which a response of “Easy, casual” or “Unable to walk” was defined as having a walking pace below average and a response of “Normal, average,” “Brisk pace,” or “Very Brisk/striding” was defined as having a walking pace at or above average. Participants who responded, “Never walks outdoors” were omitted (N = 51).
Binary logistic regression was conducted to assess the relationship between each predictor variable (CMV, HSV-1, and CRP) and each outcome of interest (ADL, IADL, and walking pace). First, unadjusted associations between each exposure and outcome were assessed using odds ratios and 95% confidence intervals (CI). Variables that were significantly associated with both the main predictors and outcomes of interest in bivariate analyses and were not hypothesized to be mediators were included as potential confounders in the models. Factors such as age, gender, socioeconomic status, chronic health conditions, BMI, nativity, and smoking status were examined because they have been shown to be potential confounders of either the exposures or functional impairment outcomes (4,6,8,9,25,32,33).
Results
Comparisons of demographic and clinical characteristics by ADL, IADL, and walking pace impairment are shown in Table 1. Twelve percent of participants reported having difficulty completing one or more ADL, and for IADL, 42% reported having difficulty completing three or more activities. Increased age, female gender, lower monthly income, and lower educational attainment were significantly associated with having difficulty in ADL and IADL. Twenty-seven percent of participants reported below average walking pace. Increased age, female gender, nativity, lower monthly income, and lower educational attainment were significantly associated with below average walking pace.
Table 1.
Demographic and Clinical Characteristics by ADL, IADL, and Walking Pace in the SALSA Study
| Characteristics | ADL (N = 1507) | IADL (N = 1261) | Walking Pace (N = 1444) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| No Difficulty | Difficulty | p Value | No Difficulty | Difficulty | p Value | No Difficulty | Difficulty | p Value | |
| N = 1332 (88.39%) | N = 175 (11.61%) | N = 736 (58.37%) | N = 525 (41.63%) | N = 1047 (72.51%) | N = 397 (27.49%) | ||||
| Age | |||||||||
| Mean ± SD | 69.79 ± 6.36 | 73.88 ± 8.57 | <.0001* | 68.75 ± 5.74 | 71.30 ± 7.19 | <.0001* | 69.38 ± 5.99 | 71.69 ± 7.41 | <.0001* |
| Category, n (%) | |||||||||
| 60–69 | 693 (91.55) | 64 (8.45) | <.0001* | 426 (64.55) | 234 (35.45) | <.0001* | 561 (76.02) | 177 (23.98) | <.0001* |
| 70–79 | 536 (88.89) | 67 (11.11) | 281 (56.09) | 220 (43.91) | 426 (73.32) | 155 (26.68) | |||
| ≥80 | 103 (70.07) | 44 (29.93) | 29 (29.00) | 71 (71.00) | 60 (48.00) | 65 (52.00) | |||
| Gender | |||||||||
| Male | 564 (90.53) | 59 (9.47) | .0293* | 364 (69.33) | 161 (30.67) | <.0001* | 456 (75.62) | 147 (24.38) | .0248* |
| Female | 768 (86.88) | 116 (13.12) | 372 (50.54) | 364 (49.46) | 591 (70.27) | 250 (29.73) | |||
| Country of Birth | |||||||||
| United States | 688 (89.12) | 84 (10.88) | .3636 | 388 (58.17) | 279 (41.83) | .8814 | 516 (69.73) | 224 (30.27) | .0154* |
| Mexico/Other Latin country | 644 (87.62) | 91 (12.38) | 348 (58.59) | 246 (41.41) | 531 (75.43) | 173 (24.57) | |||
| Monthly income | |||||||||
| <1,000 | 535 (83.99) | 102 (16.01) | <.0001* | 239 (47.14) | 268 (52.86) | <.0001* | 417 (68.81) | 189 (31.19) | .0006* |
| 1,000–1,999 | 417 (89.68) | 48 (10.32) | 232 (60.26) | 153 (39.74) | 322 (72.36) | 123 (27.64) | |||
| ≥2,000 | 361 (94.75) | 20 (5.25) | 254 (72.78) | 95 (27.22) | 292 (79.13) | 77 (20.87) | |||
| Education (y), n (%): | |||||||||
| 0–3 | 385 (83.70) | 75 (16.30) | <.0001* | 176 (50.29) | 174 (49.71) | <.0001* | 304 (70.37) | 128 (29.63) | .0143* |
| 4–11 | 513 (88.30) | 68 (11.70) | 275 (56.58) | 211 (43.42) | 392 (70.00) | 168 (30.00) | |||
| ≥12 | 434 (93.13) | 32 (6.87) | 285 (67.06) | 140 (32.94) | 351 (77.65) | 101 (22.35) | |||
| Charlson comorbidity index score category, n (%) | |||||||||
| 0–3 | 1285 (89.11) | 157 (10.89) | <.0001* | 714 (59.06) | 495 (40.94) | .0126* | 1018 (73.55) | 366 (26.45) | <.0001* |
| 4–6 | 46 (71.88) | 18 (28.13) | 22 (43.14) | 29 (56.68) | 29 (49.15) | 30 (50.85) | |||
| 7–9 | 1 (100.0) | 0 (0.00) | 0 (0.00) | 1 (100.00) | 0 (0.00) | 1 (100.00) | |||
| BMI | |||||||||
| Mean ± SD | 29.61 ± 5.60 | 29.98 ± 7.86 | .5537 | 28.98 ± 5.29 | 30.47 ± 6.25 | <.0001* | 29.38 ± 5.52 | 30.48 ± 6.35 | .0028* |
| Smoking (pack-years) | |||||||||
| Mean ± SD | 10.30 ± 20.91 | 10.10 ± 22.49 | .8824 | 10.79 ± 22.63 | 9.45 ± 17.90 | .2607 | 10.03 ± 21.96 | 11.42 ± 19.53 | .2439 |
| Category, n (%) | |||||||||
| Never smoker | 637 (88.84) | 80 (11.16) | .5995 | 334 (56.13) | 261 (43.87) | .1286 | 508 (74.82) | 171 (25.18) | .0641 |
| Ever smoker | 695 (87.97) | 95 (12.03) | 402 (60.36) | 264 (39.64) | 539 (70.46) | 226 (29.54) | |||
Notes: Difficulty in activities of daily living (ADL) defined as difficulty in ≥1 activities. Difficulty in instrumental activities of daily living (IADL) defined as ≥3 activities. Difficulty in walking pace defined as below average walking pace versus at or above average walking pace. For ADL, 24 participants were missing values on income and 12 participants were missing values on body mass index (BMI). For IADL, 20 participants were missing values on income and 7 participants were missing values on BMI. For walking pace, 24 participants were missing values on income and 11 participants were missing values on BMI. SALSA, Sacramento Area Latino Study on Aging; SD, standard deviation.
p value based on an independent samples t test or chi-square tests for trend or differences in proportions.
Clinical characteristics such as BMI, smoking status, and total chronic health conditions (modified Charlson comorbidity index) were also assessed in relation to ADL and IADL difficulties (see Table 1). There was no significant difference in BMI by ADL difficulties. In contrast, the mean BMI among participants with IADL difficulties was significantly higher than that among participants without difficulties. Participants with difficulties in ADL and IADL and below average walking pace had significantly more chronic health conditions.
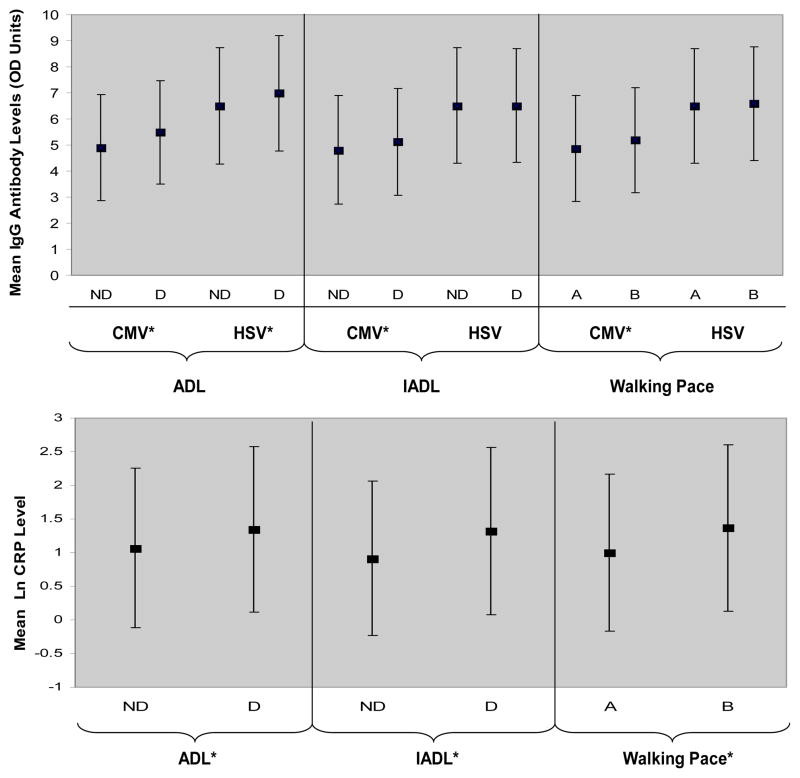
Of the 1507 participants, approximately 96% were considered CMV seropositive (i.e., having signs of prior exposure) by the ELISA testing cut points, and 98% were found seropositive for HSV-1. There were only small differences in seropositivity by ADL and IADL impairment. CMV and HSV-1 antibody titers were significantly higher among those with ADL difficulties (p = .0003) and (p = .0078), respectively (see Figure 1). CMV was significantly higher among those with IADL difficulties (p = .0083) and those with below average walking pace (p = .0067) (see Figure 1). Mean CRP levels were significantly higher among those with ADL and IADL difficulty (p = .0042 and p < .0001, respectively) (see Figure 1). Mean CRP levels were also significantly higher among those with below average walking pace versus above average walking pace (p < .0001). CMV was significantly correlated with CRP (r = 0.1156, p < .0001), but there was no significant correlation between HSV and CRP or between HSV and CMV.
Figure 1. Mean CMV and HSV-1 Antibody Levels and Log CRP By Levels of Difficulty in Activities of Daily Living (ADL), Instrumental Activities of Daily Living (IADL) and Walking Pace.
The plots show the mean and standard error bars for CMV and HSV-1 IgG antibody optical density units and (ln) natural log CRP levels by ADL and IADL difficulty status, and walking pace. ADL: Activities of Daily Living, IADL: Instrumental Activities of Daily Living. For ADL: ND= No Difficulty and D=Difficulty in ≥ 1 activities. For IADL: ND= No Difficulty in ≤ 2 activities and D= Difficulty in ≥ 3 activities. For walking pace: A= At or above average walking pace outdoors and B= Below average walking pace outdoors. OD=Optical Density Units, CMV=Cytomegalovirus, HSV= Herpes Simplex Virus 1 and CRP=C-reactive protein. *Significant at the P < .05
Association Between Infection, Inflammation, and ADL Difficulty
Table 2 shows the associations between CMV and HSV-1 infection and CRP in relation to ADL impairment in unadjusted and adjusted models. Figure 2 shows that, after adjustment for age and gender, a statistically significant association was observed between ADL difficulty and the highest tertile of CMV antibody titer. For CMV, statistical significance was lost in the fully adjusted model, and the odds ratio was reduced by 27% compared to the unadjusted model, but the graded relationship between increasing CMV levels and difficulty was still evident. A statistically significant relationship between the middle and highest level of CRP and ADL difficulty was evident before and after control for age and gender (Table 2), but the relationship was no longer significant after controlling for age, gender, education, income, and total number of chronic health conditions (Figure 3). Increasing HSV-1 levels were not associated with ADL impairment.
Table 2.
Associations Between CMV Infection, HSV-1 Infection, CRP, and ADL in the SALSA Study
| Model 1 | Model 2 | Model 3 | ||||
|---|---|---|---|---|---|---|
| Exposure in Tertiles | OR (95% CI) | p Value | OR (95% CI) | p Value | OR (95% CI) | p Value |
| CMV (N = 1507): | ||||||
| Low | 1.000 | 1.000 | 1.000 | |||
| Middle | 1.365 (0.895–2.083) | .1482 | 1.244 (0.808–1.914) | .3220 | 1.215 (0.773–1.910) | .3995 |
| High | 2.037 (1.369–3.032)* | .0005 | 1.551 (1.021–2.358)* | .0399 | 1.411 (0.911–2.185) | .1232 |
| HSV-1 (N = 1507): | ||||||
| Low | 1.000 | 1.000 | 1.000 | |||
| Middle | 1.255 (0.840–1.876) | .2667 | 1.266 (0.839–1.909) | .2606 | 1.257 (0.826–1.913) | .2859 |
| High | 1.437 (0.970–2.130) | .0706 | 1.453 (0.971–2.175) | .0691 | 1.352 (0.895–2.042) | .1525 |
| CRP (N = 1506): | ||||||
| Low | 1.000 | 1.000 | 1.000 | |||
| Middle | 1.532 (1.014–2.314)* | .0430 | 1.564 (1.024–2.388)* | .0384 | 1.443 (0.931–2.236) | .1006 |
| High | 1.842 (1.235–2.748)* | .0027 | 1.925 (1.271–2.915)* | .0020 | 1.528 (0.990–2.358) | .0556 |
Notes: Activities of daily living (ADL) outcome defined as difficulty in ≥1 activities versus no difficulty. Model 1: Unadjusted. Model 2: Adjusted for age and gender. Model 3: Model with cytomegalovirus (CMV) adjusted for age, gender, education, modified Charlson comorbidity index and income; model with herpes simplex virus type-1 (HSV-1) adjusted for age, gender, income, and education; and model with C-reactive protein (CRP) adjusted for age, gender, income, and modified Charlson comorbidity index. The total N was reduced by 24 participants for exposure to CMV, HSV-1, and CRP in Model 3 due to missing data on income and body mass index. SALSA, Sacramento Area Latino Study on Aging; OR, odds ratio; CI, confidence interval.
Significant at p < .05.
Association Between Infection, Inflammation, and IADL Difficulty
In the unadjusted analysis, there was a significant association between being in the highest tertile of CMV antibody titer and IADL difficulty (see Table 3). After full adjustment, there was no association between being in the highest tertile of CMV and IADL difficulty. HSV-1 levels were not associated with IADL difficulty. There were significantly higher odds of IADL difficulty among participants in the middle and high CRP tertiles (see Table 3). After full adjustment, only high levels of CRP remained significantly associated with difficulty completing IADL.
Table 3.
Associations Between CMV Infection, HSV-1 Infection, CRP Level, and IADL in the SALSA Study
| Model 1 | Model 2 | Model 3 | ||||
|---|---|---|---|---|---|---|
| Exposure in Tertiles | OR (95% CI) | p Value | OR (95% CI) | p Value | OR (95% CI) | p |
| CMV (N = 1261): | ||||||
| Low | 1.000 | 1.000 | 1.000 | |||
| Middle | 0.953 (0.722–1.257) | .7306 | 0.809 (0.606–1.079) | .1490 | 0.757 (0.560–1.024) | .0712 |
| High | 1.405 (1.070–1.845)* | .0144 | 1.037 (0.776–1.385) | .8071 | 0.962 (0.711–1.300) | .8000 |
| HSV-1 (N = 1261): | ||||||
| Low | 1.000 | 1.000 | 1.000 | |||
| Middle | 1.021 (0.778–1.340) | .8807 | 1.060 (0.799–1.406) | .6882 | 1.036 (0.775–1.384) | .8108 |
| High | 0.890 (0.675–1.173) | .4087 | 0.926 (0.695–1.234) | .5985 | 0.860 (0.641–1.156) | .3177 |
| CRP (N = 1260): | ||||||
| Low | 1.000 | 1.000 | 1.000 | |||
| Middle | 1.328 (1.005–1.755)* | .0464 | 1.296 (0.969–1.734) | .0802 | 1.089 (0.802–1.478) | .5863 |
| High | 2.121 (1.606–2.800)* | <.0001 | 1.981 (1.478–2.655)* | <.0001 | 1.494 (1.090–2.048)* | .0127 |
Notes: Instrumental activities of daily living (IADL) outcome defined as difficulty in ≥3 activities versus <3 activities. Model 1: Unadjusted. Model 2: Adjusted for age and gender. Model 3: Models with cytomegalovirus (CMV) adjusted for age, gender, income, education, and modified Charlson index; models with herpes simplex virus type-1 (HSV-1) adjusted for age, gender, income, and education; and model with C-reactive protein (CRP) adjusted for age, gender, income, modified Charlson comorbidity index and body mass index (BMI). The total N was reduced by 20 participants for exposure to CMV and HSV-1 and by 27 participants for exposure to CRP in Model 3 due to missing data on income and BMI. SALSA, Sacramento Area Latino Study on Aging; OR, odds ratio; CI, confidence interval.
Significant at p < .05.
Association Between Infection, Inflammation, and Walking Pace
In the unadjusted analysis, higher CMV antibody levels and higher CRP levels were significantly associated with a below average outdoor walking pace (see Table 4). After adjusting for covariates, the only significant relationship that remained was between high CRP level and below average walking pace.
Table 4.
Associations Between CMV Infection, HSV-1 Infection, CRP Level, and Walking Pace in the SALSA Study
| Model 1 | Model 2 | Model 3 | ||||
|---|---|---|---|---|---|---|
| Exposure in Tertiles | OR (95% CI) | p Value | OR (95% CI) | p Value | OR (95% CI) | p Value |
| CMV (N = 1444): | ||||||
| Low | 1.000 | 1.000 | 1.000 | |||
| Middle | 0.952 (0.712–1.273) | .7389 | 0.880 (0.654–1.184) | .3985 | 0.866 (0.637–1.178) | .3588 |
| High | 1.418 (1.072–1.876)* | .0144 | 1.190 (0.888–1.595) | .2444 | 1.140 (0.842–1.542) | .3966 |
| HSV-1 (N = 1444): | ||||||
| Low | 1.000 | 1.000 | 1.000 | |||
| Middle | 1.104 (0.834–1.461) | .4897 | 1.102 (0.829–1.467) | .5031 | 1.098 (0.821–1.469) | .5277 |
| High | 0.999 (0.750–1.332) | .9971 | 1.015 (0.758–1.358) | .9212 | 0.964 (0.715–1.300) | .8093 |
| CRP (N = 1443): | ||||||
| Low | 1.000 | 1.000 | 1.000 | |||
| Middle | 1.348 (1.000–1.815)* | .0496 | 1.324 (0.978–1.793) | .0691 | 1.222 (0.895–1.667) | .2073 |
| High | 2.009 (1.508–2.675)* | <.0001 | 2.021 (1.504–2.717)* | <.0001 | 1.710 (1.258–2.324)* | .0006 |
Notes: Walking pace outcome was defined as below average walking pace versus at or above average walking pace. Model 1: Unadjusted. Model 2: Adjusted for age and gender. Model 3: Models with cytomegalovirus (CMV) adjusted for age, gender, income, Charlson comorbidity index, and education; models with herpes simplex virus type-1 (HSV-1) adjusted for age, gender, income, and country of birth; models with C-reactive protein (CRP) adjusted for age, gender, income, nativity, and modified Charlson comorbidity index. The total N was reduced by 24 participants for exposure to CMV, HSV-1, and CRP in Model 3 due to missing data on income. SALSA, Sacramento Area Latino Study on Aging; OR, odds ratio; CI, confidence interval.
Significant at p < .05.
Discussion
This study of older Latinos suggests a consistent cross-sectional association between high CRP levels and three self-reported measures of functional impairment. There was also a significant graded association with CMV and ADL difficulties, but after adjustment for health variables the odds ratio was reduced and no longer significant. HSV-1, in contrast, showed no evidence of a relationship with ADL, IADL, or walking pace.
Only one other study at this time, by Schmaltz and colleagues (9), has looked at the association of persistent infection and a functional measure in older populations. Schmaltz and colleagues used a dichotomous measure of CMV infection status (positive or negative) and a composite measure of frailty as an outcome (9). In our study, however, continuous measures of both CMV and HSV-1 infection (antibody responses) were used because these infections were found to be highly prevalent in the study population, and it is likely that an increased antibody level may be a better reflection of the intensity of exposure and immune response to these infections over the life course (11,19). Moreover, the study by Schmaltz and colleagues focused solely on white elderly women, whereas this study analyzed data from a large population of males and females, primarily of Mexican American or Mexican descent. Given the differences in demographic and infection measures between these two studies, we cannot directly compare our results to those of Schmaltz and colleagues (9). However, our results regarding the relationship between CMV specifically and ADL impairment are further evidence supporting a potential relationship between this persistent infection and measures of physical functioning.
A major strength of this study is that, to our knowledge, this is the first study to examine collectively the relationships among multiple markers of persistent infection, an infection-related inflammatory marker, and three validated measures of physical functioning. In addition, our statistical models controlled for many known risk factors for functional impairment, such as age, comorbid health conditions, and BMI (33,34). In addition, all of the laboratory testing of the biological markers was conducted using validated test kits with high sensitivity and specificity and the use of positive and negative controls.
Although a graded association between CMV and ADL impairment was observed after full adjustment for covariates such as health conditions, income, and BMI, the effect estimates were no longer statistically significant. Given that control for factors such as chronic health conditions reduces the relationship between CMV and ADL impairment, it is possible that chronic health conditions associated with aging may concurrently lead to higher levels of CMV antibodies and functional impairments in aging populations. Both CMV and HSV-1 have been implicated as initiators of chronic inflammation related conditions including cardiovascular disease and Alzheimer’s disease (22,23). A longitudinal study is needed to more clearly explicate the temporal relationships among CMV antibody levels and physical decline. The reactivation of persistent CMV infection has been associated with activation of inflammatory molecules, such as increased levels of CRP and IL-6 (10). However, in this cross-sectional analysis, CRP levels were weakly correlated with CMV. It may be worthwhile, however, to further explore the relationship between CMV infection and cytokines such as IL-6, in relation to ADL and IADL impairment.
Our earlier research in the SALSA population showed a significant relationship between CMV antibody levels and cognitive decline over a 5-year period, but no relationship with HSV-1 (11). Similarly, there were no associations between HSV-1 IgG antibody levels and measures of functional impairment. It is possible that HSV-1 infection may still be related to decline in functioning over time. However, the specificity of the association with CMV in our study and the consistency with other researcher’s findings (9) suggests that CMV, in particular, may be an important indicator of physical functioning.
Several studies have observed a heightened IgG antibody response to CMV as well as other herpesviruses in older compared to younger persons (13–15). For example, a recent study by Stowe and colleagues (35) reported a 4 unit higher average serum CMV IgG antibody level among persons 66 years old or older compared to persons 25–55 years old (p < .0001) (35). Stowe and colleagues also identified detectable CMV DNA in the urine of the old individuals but did not identify CMV DNA in the blood (35). They suggest that the presence of heightened serum CMV antibody levels in combination with CMV DNA in urine only is a sign of frequent CMV reactivation that occurs at subclinical levels in old persons (35). Cell-mediated control is important for keeping persistent viral infections such as HSV-1 and CMV from reactivating (16,36,37). Changes in cell-mediated immune parameters often occurs with aging (19,38). For these reasons, it is likely that higher IgG antibody titers to CMV among infected aging populations is correlated with subclinical reactivation.
It is unclear how CMV might directly influence physical functioning, but indirect pathways may include changes in cellular components of immune function. Recently, several researchers have identified CMV as an important component of an immune risk phenotype that is associated with immunosenescence, inflammation, and several chronic health conditions observed with aging (19). CMV infection occurs early in life, and CD8+CD28− cells are generated to maintain control over persistent CMV infection over the life course (39). Wikby and colleagues (39) recently demonstrated that CMV induces changes in the CD4 T-cell compartment that are different than what usually occurs during aging and emphasized the importance of considering CMV status when examining immunosenescent changes in the elderly population. Longitudinal data assessing the interaction between CMV, specific cellular markers, and inflammation in relation to physical decline is necessary.
ADL, IADL, and walking pace impairment were found to be significantly associated with increasing levels of CRP. These results are consistent with those from previous studies that have examined various markers of inflammation and measures of physical functioning in elderly populations (4,8,40). High CRP levels have been associated with loss of muscle strength and mass in older men and women and have been found to be an important factor in cardiovascular conditions, type 2 diabetes, and metabolic syndrome (41,42). Inflammation and health conditions, in turn, impact physical function and functional impairment. Nonetheless, the relationship between CRP and physical functioning in our study was independent of comorbid conditions, such as diabetes and cardiovascular conditions, and BMI, suggesting that CRP influences physical functioning via other pathophysiological processes.
A few limitations must be considered. First, this is a cross-sectional study; therefore, caution should be taken in making temporal inferences. However, CMV infection often occurs in childhood, antibody levels to persistent herpesvirus infections are generally stable over time, and CRP is known to increase in response to infection, suggesting that CMV antibody response would biologically precede CRP increases (25,43).
Second, we used a modified version of the Charlson comorbidity index because some of the conditions that are generally applied to this index were not available for our study population. However, the variables that we did include in the modified index are known to be important factors associated with increases in CRP and possibly CMV, such as myocardial infarction and liver disease. In addition, the application of the Charlson comorbidity index in this population may be overly stringent because our population is a community-dwelling sample and the index is generally applied to patients who are already ill in the clinical setting.
It is also possible that Mexican Americans possess unique characteristics that impact their exposure and response to CMV infection (25) as well as functional decline. For example, factors such as country of birth, age at migration, and duration since migration to the United States might influence both exposure to CMV infection and physical activities. CMV was highly prevalent among our study sample, and nativity was associated with walking pace, suggesting potential cultural differences in general walking speed by place of birth. Therefore, the results of this study may be specific to individuals with characteristics similar to the SALSA study population.
In conclusion, this study provides further evidence that CRP and possibly infection with CMV may have important roles in the aging process, and more specifically, functional impairment. Further longitudinal studies examining the influence of CRP and persistent infections are needed to more fully elucidate the biological roles and underlying mechanisms associated with physical decline and the aging process. The clinical implications of this study indicate that preventive efforts targeting inflammation and possibly persistent infection may help enhance physical functioning among aging Latinos.
Acknowledgments
Funding for the SALSA study was aided by a grant from the National Institute on Aging, National Institutes of Health (AG12975). This work used the Chemistry Core of the Michigan Diabetes Research and Training Center funded by NIH5P60 DK20572 and DK60753 from the National Institute of Diabetes and Digestive and Kidney Diseases. We also acknowledge (1) the Robert Wood Johnson Health & Society Scholar grant at the University of Michigan (045823) for funding the viral testing and (2) the University of Michigan Medical School, Institute of Gerontology for fellowship support of A.E. Aiello. The only role that the sponsors had was financial support.
A. E. Aiello conceived the aims of the study, assisted in the statistical analyses, and led the writing of the manuscript. M. N. Haan is the Principal Investigator for the SALSA study and contributed to the design, concepts, and writing of the manuscript. J. Liang contributed to the concepts and design of the study. A. M. Simanek and C.M. Pierce assisted with model building and statistical analyses of the data as well as aided in writing drafts of the manuscript.
References
- 1.Katz S, Downs TD, Cash HR, et al. Progress in development of the index of ADL. Gerontologist. 1970;10:20–30. doi: 10.1093/geront/10.1_part_1.20. [DOI] [PubMed] [Google Scholar]
- 2.Spector WD, Katz S, Murphy JB, et al. The hierarchical relationship between activities of daily living and instrumental activities of daily living. J Chronic Dis. 1987;40:481–489. doi: 10.1016/0021-9681(87)90004-x. [DOI] [PubMed] [Google Scholar]
- 3.Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9:179–186. [PubMed] [Google Scholar]
- 4.Ravaglia G, Forti P, Maioli F, et al. Peripheral blood markers of inflammation and functional impairment in elderly community-dwellers. Exp Gerontol. 2004;39:1415–1422. doi: 10.1016/j.exger.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 5.Cohen HJ, Harris T, Pieper CF. Coagulation and activation of inflammatory pathways in the development of functional decline and mortality in the elderly. Am J Med. 2003;114:180–187. doi: 10.1016/s0002-9343(02)01484-5. [DOI] [PubMed] [Google Scholar]
- 6.Taaffe DR, Harris TB, Ferrucci L, et al. Cross-sectional and prospective relationships of interleukin-6 and C-reactive protein with physical performance in elderly persons: MacArthur studies of successful aging. J Gerontol Med Sci. 2000;55A:M709–M715. doi: 10.1093/gerona/55.12.m709. [DOI] [PubMed] [Google Scholar]
- 7.Enright PL, McBurnie MA, Bittner V, et al. The 6-min walk test: a quick measure of functional status in elderly adults. Chest. 2003;123:387–398. doi: 10.1378/chest.123.2.387. [DOI] [PubMed] [Google Scholar]
- 8.Ferrucci L, Harris TB, Guralnik JM, et al. Serum IL-6 level and the development of disability in older persons. J Am Geriatr Soc. 1999;47:639–646. doi: 10.1111/j.1532-5415.1999.tb01583.x. [DOI] [PubMed] [Google Scholar]
- 9.Schmaltz HN, Fried LP, Xue QL, et al. Chronic cytomegalovirus infection and inflammation are associated with prevalent frailty in community-dwelling older women. J Am Geriatr Soc. 2005;53:747–754. doi: 10.1111/j.1532-5415.2005.53250.x. [DOI] [PubMed] [Google Scholar]
- 10.Soderberg-Naucler C. Does cytomegalovirus play a causative role in the development of various inflammatory diseases and cancer? J Intern Med. 2006;259:219–246. doi: 10.1111/j.1365-2796.2006.01618.x. [DOI] [PubMed] [Google Scholar]
- 11.Aiello A, Haan M, Blythe L, et al. The influence of latent viral infection on rate of cognitive decline over 4 years. J Am Geriatr Soc. 2006;54:1046–1054. doi: 10.1111/j.1532-5415.2006.00796.x. [DOI] [PubMed] [Google Scholar]
- 12.Padgett DA, Sheridan JF, Dorne J, et al. Social stress and the reactivation of latent herpes simplex virus type 1. Proc Natl Acad Sci U S A. 1998;95:7231–7235. doi: 10.1073/pnas.95.12.7231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Musiani M, Zerbini M, Zauli D, et al. Impairment of cytomegalovirus and host balance in elderly subjects. J Clin Pathol. 1988;41:722–725. doi: 10.1136/jcp.41.7.722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Musiani M, Zerbini ML, Muscari A, et al. Antibody patterns against cytomegalovirus and Epstein-Barr virus in human atherosclerosis. Microbiologica. 1990;13:35–41. [PubMed] [Google Scholar]
- 15.Weymouth LA, Gomolin IH, Brennan T, et al. Cytomegalovirus antibody in the elderly. Intervirology. 1990;31:223–229. doi: 10.1159/000150157. [DOI] [PubMed] [Google Scholar]
- 16.Glaser R, Kiecolt-Glaser JK. Stress-induced immune dysfunction: implications for health. Nat Rev Immunol. 2005;5:243–251. doi: 10.1038/nri1571. [DOI] [PubMed] [Google Scholar]
- 17.Almanzar G, Schwaiger S, Jenewein B, et al. Long-term cytomegalovirus infection leads to significant changes in the composition of the CD8+ T-cell repertoire, which may be the basis for an imbalance in the cytokine production profile in elderly persons. J Virol. 2005;79:3675–3683. doi: 10.1128/JVI.79.6.3675-3683.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koch S, Solana R, Dela Rosa O, et al. Human cytomegalovirus infection and T cell immunosenescence: a mini review. Mech Ageing Dev. 2006;127:538–543. doi: 10.1016/j.mad.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 19.Pawelec G, Koch S, Franceschi C, et al. Human immunosenescence: does it have an infectious component? Ann N Y Acad Sci. 2006;1067:56–65. doi: 10.1196/annals.1354.009. [DOI] [PubMed] [Google Scholar]
- 20.Mattay VS, Fera F, Tessitore A, et al. Neurophysiological correlates of age-related changes in working memory capacity. Neurosci Lett. 2006;392:32–37. doi: 10.1016/j.neulet.2005.09.025. [DOI] [PubMed] [Google Scholar]
- 21.Liu R, Moroi M, Yamamoto M, et al. Presence and severity of Chlamydia pneumoniae and cytomegalovirus infection in coronary plaques are associated with acute coronary syndromes. Int Heart J. 2006;47:511–519. doi: 10.1536/ihj.47.511. [DOI] [PubMed] [Google Scholar]
- 22.Sorlie PD, Nieto FJ, Adam E, et al. A prospective study of cytomegalovirus, herpes simplex virus 1, and coronary heart disease: the atherosclerosis risk in communities (ARIC) study. Arch Intern Med. 2000;160:2027–2032. doi: 10.1001/archinte.160.13.2027. [DOI] [PubMed] [Google Scholar]
- 23.Itzhaki RF, Dobson CB, Shipley SJ, et al. The role of viruses and of APOE in dementia. Ann N Y Acad Sci. 2004;1019:15–18. doi: 10.1196/annals.1297.003. [DOI] [PubMed] [Google Scholar]
- 24.Schillinger JA, Xu F, Sternberg MR, et al. National seroprevalence and trends in herpes simplex virus type 1 in the United States, 1976–1994. Sex Transm Dis. 2004;31:753–760. doi: 10.1097/01.olq.0000145852.43262.c3. [DOI] [PubMed] [Google Scholar]
- 25.Staras SA, Dollard SC, Radford KW, et al. Seroprevalence of cytomegalovirus infection in the United States, 1988–1994. Clin Infect Dis. 2006;43:1143–1151. doi: 10.1086/508173. [DOI] [PubMed] [Google Scholar]
- 26.Glaser R, Gotlieb-Stematsky T. Human Herpes Virus Infections: Clinical Aspects. New York: Marcel Dekker; 1982. [Google Scholar]
- 27.Hirata M, Terasaki PI, Cho YW. Cytomegalovirus antibody status and renal transplantation: 1987–1994. Transplantation. 1996;62:34–37. doi: 10.1097/00007890-199607150-00007. [DOI] [PubMed] [Google Scholar]
- 28.Sarid O, Anson O, Yaari A, et al. Human cytomegalovirus salivary antibodies as related to stress. Clin Lab. 2002;48:297–305. [PubMed] [Google Scholar]
- 29.Haan MN, Mungas DM, Gonzalez HM, et al. Prevalence of dementia in older Latinos: the influence of type 2 diabetes mellitus, stroke and genetic factors. J Am Geriatr Soc. 2003;51:169–177. doi: 10.1046/j.1532-5415.2003.51054.x. [DOI] [PubMed] [Google Scholar]
- 30.Katz S, Ford AB, Moskowitz RW, et al. Studies of illness in the aged. the index of ADL: a standardized measure of biological and psychosocial function. JAMA. 1963;185:914–919. doi: 10.1001/jama.1963.03060120024016. [DOI] [PubMed] [Google Scholar]
- 31.Wolf AM, Hunter DJ, Colditz GA, et al. Reproducibility and validity of a self-administered physical activity questionnaire. Int J Epidemiol. 1994;23:991–999. doi: 10.1093/ije/23.5.991. [DOI] [PubMed] [Google Scholar]
- 32.Wu JH, Haan MN, Liang J, et al. Diabetes as a predictor of change in functional status among older Mexican Americans: a population-based cohort study. Diabetes Care. 2003;26:314–319. doi: 10.2337/diacare.26.2.314. [DOI] [PubMed] [Google Scholar]
- 33.Jung SH, Ostbye T, Park KO. A longitudinal study of the relationship between health behavior risk factors and dependence in activities of daily living. J Prev Med Pub Health. 2006;39:221–228. [PubMed] [Google Scholar]
- 34.Walston J, McBurnie MA, Newman A, et al. Frailty and activation of the inflammation and coagulation systems with and without clinical comorbidities: results from the Cardiovascular Health Study. Arch Intern Med. 2002;162:2333–2341. doi: 10.1001/archinte.162.20.2333. [DOI] [PubMed] [Google Scholar]
- 35.Stowe RP, Kozlova EV, Yetman DL, et al. Chronic herpesvirus reactivation occurs in aging. Exp Gerontol. 2007;42:563–570. doi: 10.1016/j.exger.2007.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cytomegaloviruses: Molecular Biology and Immunology. Norfolk, U.K: Caister Academic Press; 2006. [Google Scholar]
- 37.Rawls W. Virology. New York: Raven Press; 1985. Herpes simplex virus. [Google Scholar]
- 38.De Martinis M, Franceschi C, Monti D, et al. Inflamm-ageing and lifelong antigenic load as major determinants of ageing rate and longevity. FEBS Lett. 2005;579:2035–2039. doi: 10.1016/j.febslet.2005.02.055. [DOI] [PubMed] [Google Scholar]
- 39.Wikby A, Nilsson BO, Forsey R, et al. The immune risk phenotype is associated with IL-6 in the terminal decline stage: findings from the Swedish NONA immune longitudinal study of very late life functioning. Mech Ageing Dev. 2006;127:695–704. doi: 10.1016/j.mad.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 40.Visser M, Pahor M, Taaffe DR, et al. Relationship of interleukin-6 and tumor necrosis factor-alpha with muscle mass and muscle strength in elderly men and women: the Health ABC Study. J Gerontol Med Sci. 2002;57A:M326–M332. doi: 10.1093/gerona/57.5.m326. [DOI] [PubMed] [Google Scholar]
- 41.Xu Y, Whitmer K. C-reactive protein and cardiovascular disease in people with diabetes: high-sensitivity CRP testing can help assess risk for future cardiovascular disease events in this population. Am J Nurs. 2006;106:66–72. doi: 10.1097/00000446-200608000-00027. [DOI] [PubMed] [Google Scholar]
- 42.Schaap LA, Pluijm SM, Deeg DJ, Visser M. Inflammatory markers and loss of muscle mass (sarcopenia) and strength. Am J Med. 2006;119:526.e9–526.e17. doi: 10.1016/j.amjmed.2005.10.049. [DOI] [PubMed] [Google Scholar]
- 43.Salonen EM, Vaheri A. C-reactive protein in acute viral infections. J Med Virol. 1981;8:161–167. doi: 10.1002/jmv.1890080302. [DOI] [PubMed] [Google Scholar]