Abstract
Many studies are currently investigating the development of safe and effective vaccines to prevent various infectious diseases. Multiple antigen-presenting peptide vaccine systems have been developed to avoid the adverse effects associated with conventional vaccines (i.e., live-attenuated, killed or inactivated pathogens), carrier proteins and cytotoxic adjuvants. Recently, two main approaches have been used to develop multiple antigen-presenting peptide vaccine systems: (1) the addition of functional components, e.g., T-cell epitopes, cell-penetrating peptides, and lipophilic moieties; and (2) synthetic approaches using size-defined nanomaterials, e.g., self-assembling peptides, non-peptidic dendrimers, and gold nanoparticles, as antigen-displaying platforms. This review summarizes the recent experimental studies directed to the development of multiple antigen-presenting peptide vaccine systems.
Introduction
From the latter half of the 18th century onwards, vaccinations have saved millions of human lives and countless animals, and vaccinology contributes to the prevention of infectious diseases (e.g., polio, measles, and rubella) and antiserums for toxoids (e.g., snake bites, spider bites and jellyfish stings). However, there is a strong demand for the development of safer and more effective vaccines toward not only the prevention of many infectious diseases, e.g., human immunodeficiency virus (HIV) [1-3], malaria [4-6], group A streptococci (GAS) [7,8], hepatitis C virus (HCV) [9,10], and severe acute respiratory syndrome (SARS) [11,12], but also for cancer immunotherapy [13-15]. Synthetic immunogenic peptides are ideal vaccine subunit components because of the following differences to traditional vaccines composed of live-attenuated, killed or inactivated pathogens e.g., bacteria or viruses: (1) no infectious material; (2) no cross-reactivity with host tissues; (3) induction of site-specific antibodies (Abs); (4) ability to chemically define and modify products; and (5) swift large-scale manufacturing and long-term storage in the event of a pandemic. However, the biological activity of peptides is generally short due to enzymatic degradation, and small peptides that are used as antigens are not recognized by immune cells, e.g., dendritic cells (DCs) and macrophages, and do not elicit a strong immune response when administered alone. The co-administration of adjuvants (e.g., water-in-oil emulsions, oil-in-water emulsions, liposomes, bacterial lipophilic compounds, etc.) with subunit peptide antigens is one of the methods used to enhance the immune response; however, only a few adjuvants are approved for clinical use [16]. Alternatively, short antigenic peptides induce strong immune responses when co-administrated or engaged with carrier proteins (e.g., ovalbumin (OVA), bovine serum albumin, keyhole limpet hemocyanin, tetanus toxoid, etc.); however, they are also associated with undesirable effects such as the suppression of the anti-peptide Ab response and the production of Abs against the carrier proteins [17-22].
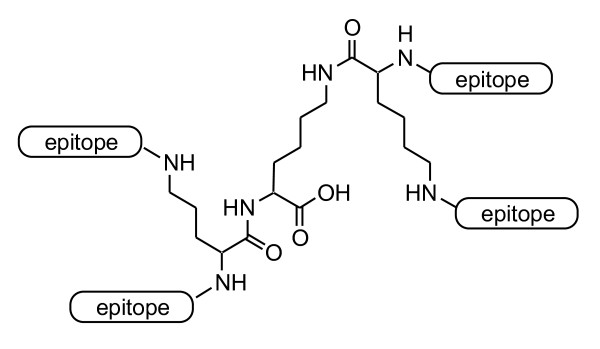
Tam developed the multiple antigenic peptide (MAP) system to improve the poor immunogenicity of subunit peptide vaccines [23]. In this MAP system, multiple copies of antigenic peptides are simultaneously bound to the α- and ε-amino groups of a non-immunogenic Lys-based dendritic scaffold (Figure 1). The protein-sized MAP molecules acquire stability from enzymatic degradation, enhanced molecular recognition by immune cells, and induction of stronger immune responses compared with small antigenic peptides. The MAP concept represented a major breakthrough for vaccine systems and further improvements in a large variety of multiple antigens-presenting peptide vaccine systems have been investigated. This review focuses on the current status of multiple antigen-presenting vaccine approaches, especially the addition of functional components and the application of a wide variety of synthetic methods and size-defined nanomaterials, e.g., self-assembling peptides, dendrimers and gold nanoparticles, as antigen-displaying platforms.
Figure 1.
Lys-based MAP vaccines.
Functional components
Tam's MAP vaccines that carried several copies of peptide antigens on a Lys-based dendrimer induced higher Ab production than single peptide monomers and carrier protein-peptide conjugates; however, additional components, such as an adjuvant, were required in many cases. Therefore, great efforts have been directed toward improvement of these MAP vaccines by the incorporation of various functions into a single vaccine molecule using helper T-cell epitopes, immune-stimulant lipid moieties, or cell-penetrating peptides have been conducted.
(1) Helper T-cell epitope
Antigens are taken up by antigen-presenting cells (APCs) and B-cells and undergo proteolysis to form peptide epitopes. Of them, T-cell epitopes are presented by class II molecules of the major histocompatibility complex (MHC) on the surface of APCs and B-cells. APCs activate helper T-cells by the interaction between the T-cell receptors on T-cells and the epitopes/MHC class II complexes on the surface of APCs. The activated helper T-cells recognize B-cells that have the same epitopes/MHC II complexes on their surfaces. This T-cell/B-cell interaction is the trigger for the differentiation of B-cells into plasma cells that secrete Abs. The use of certain T-cell epitopes induces strong immune responses, and carrier proteins are used as a source of helper T-cell epitopes. Subunit peptide vaccines containing only B-cell epitopes cannot always elicit strong immune responses due to a lack of T-cell activation.
The incorporation of helper T-cell epitopes into MAP vaccines has been investigated [22,24]. MAP vaccines containing B- and T-cell epitopes in a single construct induced strong immune responses and have been studied in clinical trials [25]. Although single vaccine molecules containing B- and T-cell epitopes induced the production of Abs against the defined T-cell epitopes, in some cases, the Ab titers of the anti-T-cell epitopes were mostly lower than those of B-cell epitopes [26,27].
(2) Lipophilic modifications
A wide variety of cells including B-cells, T-cells, DCs, and macrophages express Toll-like receptors (TLRs) on their surfaces. Eleven TLRs (named simply TLR-1 to TLR-11) in human have been identified to date [28]. TLRs are a family of pattern-recognition receptors that recognize structural components of many bacteria, viruses and fungi, and play a critical role in the early innate immune response. TLR-mediated stimulation of APCs significantly enhances the secretion of pro-inflammatory cytokines, Ab production, and immune responses and, TLR agonists may be able to be used in alternative adjuvant systems.
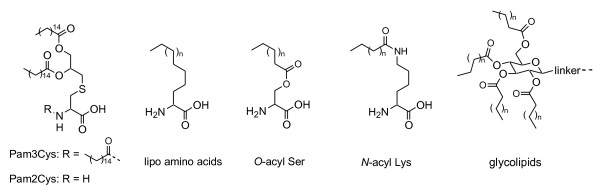
Bacterial lipid components such as Pam3Cys (the inner and outer membrane components of gram-negative bacteria) [29,30], and Pam2Cys (the lipid component of macrophage-activating lipopeptide 2 isolated from mycoplasma) [31,32] as well as synthetic α-lipo amino acids [33,34] are characterized as TLR-2 agonists and used as immune-stimulating lipophilic moieties (Figure 2) that are attached to antigenic peptides (lipopeptides). Other lipophilic compounds such as O-acyl serine, N-acyl lysine and glycolipids are also utilized for this purpose [35-37]. Lipopeptides stimulate innate immunity by their interaction with TLRs on DCs, [38-42] and elicit long-lasting systemic immune responses and T-cell proliferations [43-45], resulting in the enhanced immunogenicity of unmodified peptides by more than a few orders of magnitude. In addition, lipopeptides show none of the harmful side effects of adjuvants. Lipopeptide vaccines are currently in pre-clinical and clinical trials treating HIV and hepatitis B virus [46-48]. The incorporation of lipids with known immune-stimulating characteristics into the MAP system produces self-adjuvanting lipopeptide vaccine candidates that can induce Ab and cellular responses in the absence of additional adjuvants [33,49-52].
Figure 2.
Structure of immune-stimulating lipid moieties.
(3) Cell-penetrating peptides
Since the discovery of the cellular membrane translocating property of the human immunodeficiency virus transactivating regulatory protein (HIV TAT), several peptides with the ability to translocate to the plasma membrane have been identified and named as cell-penetrating peptides (CPPs). CPPs have been studied for their ability to deliver peptides/proteins [53,54], oligonucleotides/DNA/RNA [55-58], and even much bigger molecules such as liposomes [59,60] into the cytoplasm of cells. Fully exploiting this ability, CPPs are being utilized in vaccine studies for the delivery of antigens to APCs with the aim of antigen presentation and the induction of an immune response [61,62]. CPP-antigen conjugates enhance the cross-penetration of antigens into DCs [63,64]; however, CPPs function in all cell types, and a dual functionalized compound (CPP conjugation and DC targeting) that was designed in one study showed no synergistic enhancement of the immune response [65].
As such, numerous attempts to incorporate various functional components into single molecules have been performed. One consideration for the design of multiple antigen-presenting vaccines composed of various components is their molecular geometry, i.e., altering the linkage and spatial arrangement of each component evidently influences the conformational structure (lack of α-helicity), solubility, and molecular recognition by immune cells [27,66-71]. Zeng et al. studied the influence of diverse chemical linkages and epitope orientations on immunological activity [27]. Either N-terminus or C-terminus of both T-cell epitope and B-cell epitope were linked via disulfate, thioether, oxime, or peptide bond. The immunological evaluation of the same epitope-containing molecules with different linkages showed unique properties in terms of their stabilities to serum and the ability to induce Abs against T-cell and/or B-cell epitopes. Abdel-Aal et al. studied the relationship between immunological activity and the structural arrangement of multiple antigens-presenting vaccine molecules with 3 components (J14 as a B-cell epitope, P25 as a CD4+ T-cell epitope, and α-lipo amino acids), showing clearly that changing the position of each component significantly affected the production of IgG Abs against B-cell epitopes and, in some cases, T-cell epitopes [66].
Structural platforms and enabling chemistry
In the case of the MAP systems, the number of peptide epitopes that can be incorporated into a Lys-based dendritic scaffold is limited. Furthermore, stepwise solid phase peptide synthesis (SPPS) of branching peptides such as MAPs commonly encounters the difficulty of purification to homogeneity and characterization of the final products. Using purified short peptides as building blocks, several chemoselective conjugation techniques (e.g., native chemical ligation [52], thioether ligation [72,73], thiazolidine/oxazolidine ligation [74], oxime ligation [75], maleimide [76], and Cu(I)-catalyzed azide-alkyne cycloaddition [77]) have been applied to the synthesis of multiple antigen-presenting vaccines; however, the difficulties associated with the stoichiometrical stepwise incorporation of many of the same or different peptide epitopes into Lys-based dendritic scaffolds remain [73,78-80]. Concerning the design, preparation and biological activity of multiple antigen-presenting vaccine molecules, the stoichiometric epitope density of multivalent ligands is an important parameter [81,82]. Hence, we now introduce the alternative synthetic approaches that are used to attach multiple copies of antigenic peptides onto organic or inorganic scaffolds instead of a Lys-based dendrimer.
(1) Radical-induced polymerization
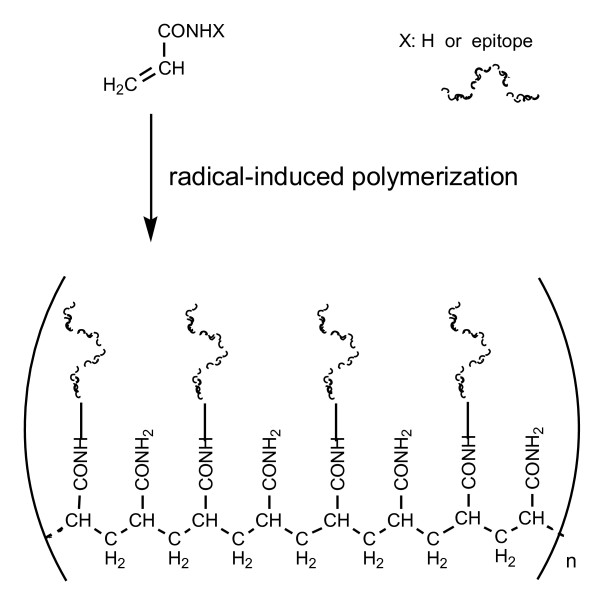
Polymer chemistry using purified antigenic peptides is suitable to prepare multiple antigen-presenting vaccine molecules. Free radical-induced polymerization of vinyl monomers is one of the most common and useful reactions for making polymers. An acryloyl (CH2 = CHCO-) group, as a key functional group for polymerization, can easily be introduced into peptides during conventional SPPS. Free radical-induced polymerization of acryloyl-peptide building blocks yields polymeric immunogens bearing hundreds of the same or different peptide epitopes attached to the alkane backbone (Figure 3) [83-86]. The size of the polymer construct and the distance between individual peptidic components can be controlled by the addition of acrylamide or its analog along with chain transfer agents (dithiothreitol or mercaptoacetic acid) during the polymerization process [85].
Figure 3.
Vaccine molecules presenting a number of epitopes on alkyl backbone formed by free radical-induced polymerization of acryloyl groups.
Jackson et al. evaluated the vaccine efficacy of polymeric immunogens containing 7 B-cell epitopes (88/30, Y504S, BSA10, NS27, NS1, NS5, and PL1; the N-terminal GAS M protein sequences) and the chimeric peptide J14 (a B-cell epitope consisting of the GAS M-protein C-region and GCN4 DNA-binding protein sequence). The polymeric GAS vaccine molecules showed outstanding broad immunogenicity and protection from GAS infection in mice. Abs against each of the individual epitopes presented in the polymers were successfully elicited [86].
(2) Self-assembling nanoparticles as antigen-presenting platforms
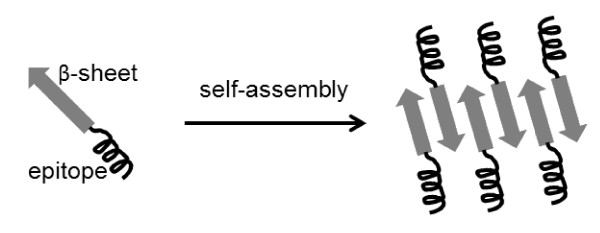
Peptides with certain helical or β-hairpin/sheet secondary structures can assemble themselves to form tubular, fibrillar, or spherical structures on the nano-scale via non-covalent interactions (e.g., van der Waals bonds, electrostatic interactions, hydrogen bonds or stacking interactions). They are called self-assembling peptides and applied as topologically defined building blocks in various fields including material sciences, molecular electronics, tissue engineering, and drug delivery [87-89]. The ability of these self-assembling peptides to form nanostructures is also of great interest for a repetitive antigen display system (Figure 4).
Figure 4.
Systematic self-assembling peptide vaccine with repetitive antigens.
Burkhard's group used self-assembling peptide nanoparticles composed of a pentameric coiled-coil oligomerization domain derived from cartilage oligomeric matrix protein [90] and a de novo trimeric coiled-coil oligomerization domain [91] as multiple antigen-display platforms [71,82]. An α-helical coiled-coil B-cell epitope HRC1, derived from the SARS S protein, was then attached on the self-assembling peptide nanoparticles. Self-assembly in phosphate-buffered saline yielded nanoparticles (~ 25 nm in diameter) that exposed multiple copies of HRC1 on their surface. Circular dichroism (CD) spectra indicated that the peptide nanoparticles maintain an α-helical conformation. Immunological evaluation of the nanoparticles containing HRC1 showed specific Ab production and moderate neutralization activity of SARS coronavirus infectivity [71]. Furthermore, immunization of mice with peptide nanoparticles bearing the malaria B-cell epitope (DPPPPNPN)2D successfully induced high Ab titers and long-lasting protection. The majority of these mice were protected against an initial challenge of parasites for up to 6 months after the last immunization or up to 15 months against a second challenge [82]. This self-assembling peptide nanoparticle vaccine against Malaria is currently undergoing pre-clinical trial in the US.
A short fibrillizing peptide Q11 self-assembles in a salt-containing aqueous solution to form networks of β-sheet-rich nanofibers with a width of 15 nm; and the resulted nanoparticles are non-cytotoxic and minimally immunogenic [92,93]. Rudra et al. used Q11 as a multiple antigen-presenting vaccine system. Self-assembling peptide nanofibers (O-Q11), in which the OVA323-339 epitope, derived from chicken egg OVA, and Q11 were linked via a spacer, were subcutaneously administrated into mice and elicited high IgG titers in the absence of an additional adjuvant. The Ab titers were remarkably higher compared with the admixture of OVA323-339 and complete Freund's adjuvant (CFA). According to this study, Ab titers against Q11 were not determined even when it was co-administrated with CFA [94].
The usage of a self-assembling non-peptidic dendritic polymer as an antigen-display system was recently reported. Multiple copies of J14 (B-cell epitope from the GAS M-protein) were attached on dendrimers via Cu(I)-catalyzed azide-alkyne cycloaddition and the subsequent self-assembly was carried out in water to form nanoparticles (20 nm in diameter). After subcutaneous immunization with the J14-bearing nanomolecules, several subclasses of IgG were successfully produced without any adjuvants [77].
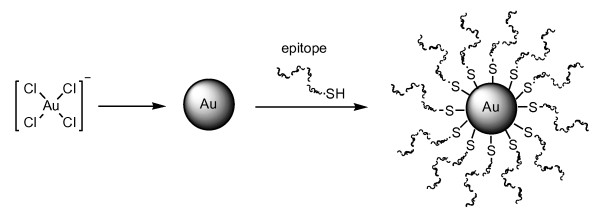
(3) Gold nanoparticles as antigen carriers
As an alternative to organic compounds (certain peptides or polymers), inorganic materials such as SiO2, Ag, Pt, and Au are also known to form nanoparticles. Colloidal gold nanoparticles (GNPs) are of interest for various biomedical applications [95,96], e.g., imaging [97-99], photo-activated therapeutics [100], tumor detection [101], and drug delivery [102-104] as well as multivalent antigen carrier scaffoldings [105-107] instead of conventional carrier proteins and synthetic dendrimers/polymers. GNPs are an ideal material primarily because of their biocompatibility and lack of immunogenicity [108,109]. GNPs can be easily prepared from gold salt (H[AuCl4]) in water and their particle sizes are controllable (ranging from 1-100 nm in diameter) [110-113]; consequently, gold surface electrodes can be readily reacted with molecules bearing a mercapto (SH) group (Figure 5). The strong attachment between the mercapto group and the gold surface is easily prepared as well as the chemoselective conjugation, e.g., native chemical ligation, click chemistry, oxime ligation, and thioether ligation [52,72-77] of the antigenic peptide and synthetic core particles (sophisticated in some cases). The size-dependent toxicity of naked GNPs can be reduced by modifying their surfaces [114]. The intracellular uptake of GNPs is mediated through endocytosis [115-117], enabling GNPs to deliver the attached molecules into the cell. GNP-peptide conjugates resistant to enzymatic and lysosomal degradation. As such, GNPs are suitable materials to use as antigen-presenting scaffolds.
Figure 5.
Multiple antigens-presenting gold nanoparticle vaccines.
Chen et al. demonstrated that the addition of extra Cys residues to the C-terminus of an epitope, derived from the VP1 protein of the foot-and-mouth disease virus, allowed its conjugation to different sized GNPs as antigen carriers. Mice that were immunized with these GNP-epitope conjugates showed significant size-dependent immunogenicity and biodistribution in the spleen [107].
Conjugation with the Fc fragment from human IgG targeting the Fcγ receptor on human DCs into GNPs successfully enhanced the cellular uptake of antigens. In addition, GNP-epitope-Fc conjugates showed better antigen-uptake activity, immunological responses and lymphocyte proliferation compared with the use of liposomes as an antigen-delivery system [118].
Conclusion
The current status of multiple antigens-presenting vaccine systems has been reviewed. Various functional components (T-helper epitopes, immune-stimulating lipids, and cell-penetrating peptides) have been attached with antigens to improve subunit peptide vaccine potentiation, such as the effective and selective activation of a particular immune system and mucosal immunity. Meanwhile, the recent use of size-defined organic and inorganic nanomaterials without either immunogenicity or cytotoxicity as antigen-display platforms surely advances the development of subunit peptide vaccines without the use of adjuvants. Each of these nanomaterials as antigen-display platforms, however, has shortcomings such as requirement of several synthetic steps and cost. Optimization of the balance between vaccine efficacy and the amounts or ratio of attached B-epitopes and additional functional components, and exploring alternative safe and economical materials for multiple antigen-presenting systems instead of Au would help reduce the cost for their practical use. As the optimization of the balance continues to investigate, antigen-display platforms would be an ideal component to develop vaccines.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
YF drafted the manuscript. HT helped to draft the manuscript. All authors read and approved the final manuscript.
Contributor Information
Yoshio Fujita, Email: yfujita@suzuka-u.ac.jp.
Hiroaki Taguchi, Email: taguchi@suzuka-u.ac.jp.
Acknowledgements
This work was supported by Grants-in-Aid for Young Scientists (B) from the Ministry of Education, Culture, Sports, Science and Technology in Japan to YF (23790144).
References
- Kim JH, Rerks-Ngarm S, Excler JL, Michael NL. HIV vaccines: lessons learned and the way forward. Curr Opin HIV AIDS. 2010;5(5):428–434. doi: 10.1097/COH.0b013e32833d17ac. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voronin Y, Manrique A, Bernstein A. The future of HIV vaccine research and the role of the Global HIV Vaccine Enterprise. Curr Opin HIV AIDS. 2010;5(5):414–420. doi: 10.1097/COH.0b013e32833cfe32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaccari M, Poonam P, Franchini G. Phase III HIV vaccine trial in Thailand: a step toward a protective vaccine for HIV. Expert Rev Vaccines. 2010;9(9):997–1005. doi: 10.1586/erv.10.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genton B. Malaria vaccines: a toy for travelers or a tool for eradication? Expert Rev Vaccines. 2008;7(5):597–611. doi: 10.1586/14760584.7.5.597. [DOI] [PubMed] [Google Scholar]
- Garcia LS. Malaria. Clin Lab Med. 2010;30(1):93–129. doi: 10.1016/j.cll.2009.10.001. [DOI] [PubMed] [Google Scholar]
- Kappe SH, Vaughan AM, Boddey JA, Cowman AF. That was then but this is now: malaria research in the time of an eradication agenda. Science. 2010;328(5980):862–866. doi: 10.1126/science.1184785. [DOI] [PubMed] [Google Scholar]
- Dale JB. Current status of group A streptococcal vaccine development. Adv Exp Med Biol. 2008;609:53–63. doi: 10.1007/978-0-387-73960-1_5. [DOI] [PubMed] [Google Scholar]
- Steer AC, Batzloff MR, Mulholland K, Carapetis JR. Group A streptococcal vaccines: facts versus fantasy. Curr Opin Infect Dis. 2009;22(6):544–552. doi: 10.1097/QCO.0b013e328332bbfe. [DOI] [PubMed] [Google Scholar]
- Yu CI, Chiang BL. A new insight into hepatitis C vaccine development. J Biomed Biotechnol. 2010;2010:548280. doi: 10.1155/2010/548280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoll-Keller F, Barth H, Fafi-Kremer S, Zeisel MB, Baumert TF. Development of hepatitis C virus vaccines: challenges and progress. Expert Rev Vaccines. 2009;8(3):333–345. doi: 10.1586/14760584.8.3.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du L, He Y, Zhou Y, Liu S, Zheng BJ, Jiang S. The spike protein of SARS-CoV--a target for vaccine and therapeutic development. Nat Rev Microbiol. 2009;7(3):226–236. doi: 10.1038/nrmicro2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roper RL, Rehm KE. SARS vaccines: where are we? Expert Rev Vaccines. 2009;8(7):887–898. doi: 10.1586/erv.09.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundaram R, Dakappagari NK, Kaumaya PT. Synthetic peptides as cancer vaccines. Biopolymers. 2002;66(3):200–216. doi: 10.1002/bip.10258. [DOI] [PubMed] [Google Scholar]
- Oka Y, Tsuboi A, Fujiki F, Li Z, Nakajima H, Hosen N, Shirakata T, Nishida S, Oji Y, Kawase I, Sugiyama H. WT1 peptide vaccine as a paradigm for "cancer antigen-derived peptide"-based immunotherapy for malignancies: successful induction of anti-cancer effect by vaccination with a single kind of WT1 peptide. Anticancer Agents Med Chem. 2009;9(7):787–797. doi: 10.2174/187152009789056958. [DOI] [PubMed] [Google Scholar]
- Carballido E, Fishman M. Sipuleucel-T: prototype for development of anti-tumor vaccines. Curr Oncol Rep. 2011;13(2):112–9. doi: 10.1007/s11912-011-0152-5. [DOI] [PubMed] [Google Scholar]
- Heegaard PMH, Dedieu L, Johnson N, Le Potier M-F, Mockey M, Mutinelli F, Vahlenkamp T, Vascellari M, Sørensen NS. Adjuvants and delivery systems in veterinary vaccinology: current state and future developments. Arch Virol. 2010;156(2):183–202. doi: 10.1007/s00705-010-0863-1. [DOI] [PubMed] [Google Scholar]
- Herzenberg LA, Tokuhisa T, Herzenberg A. Carrier-priming leads to hepten-specific suppression. Nature. 1980;285(5767):664–667. doi: 10.1038/285664a0. [DOI] [PubMed] [Google Scholar]
- Landsteiner K. The Specificity of Serological Reactions. 3. Harvard Univ Press, Cambridge, MA; 1962. [Google Scholar]
- Avery OT, Goebel WF. Chemo-immunological studies on conjugated carbohydrate protein: V. the immunological specificity of an antigen prepared by combining the capsular polysaccharide of type III pneumococcus with foreign protein. J Exp Med. 1931;54(3):437–447. doi: 10.1084/jem.54.3.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schutze MP, Leclerc C, Jolivet M, Audibert F, Chedid L. Carrier-induced epitopic suppression, a major issue for future synthetic vaccines. J Immunol. 1985;135(4):2319–2322. [PubMed] [Google Scholar]
- DiJohn D, Wasserman SS, Torres JR, Cortesia MJ, Murillo J, Losonsky GA, Herrington DA, Stürchler D, Levine MM. Effect of priming with carrier on response to conjugate vaccine. Lancet. 1989;2(8677):1415–1418. doi: 10.1016/s0140-6736(89)92033-3. [DOI] [PubMed] [Google Scholar]
- Kumar A, Arora R, Kaur P, Chauhan VS, Sharma P. "Universal" T helper cell determinants enhance immunogenicity of a Plasmodium falciparum merozoite surface antigen peptide. J Immunol. 1992;148(5):1499–1505. [PubMed] [Google Scholar]
- Tam JP. Synthetic peptide vaccine design: synthesis and properties of a high-density multiple antigenic peptide system. Proc Natl Acad Sci USA. 1988;85(15):5409–5413. doi: 10.1073/pnas.85.15.5409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etlinger HM, Knorr R. Model using a peptide with carrier function for vaccination against different pathogens. Vaccine. 1991;9(7):512–514. doi: 10.1016/0264-410X(91)90038-8. [DOI] [PubMed] [Google Scholar]
- Nardin EH, Oliveira GA, Calvo-Calle JM, Castro ZR, Nussenzweig RS, Schmeckpeper B, Hall BF, Diggs C, Bodison S, Edelman R. Synthetic malaria peptide vaccine elicits high levels of antibodies in vaccinees of defined HLA genotypes. J Infect Dis. 2000;182(5):1486–1496. doi: 10.1086/315871. [DOI] [PubMed] [Google Scholar]
- Ghosh S, Jackson DC. Antigenic and immunogenic properties of totally synthetic peptide-based anti-fertility vaccines. Int Immunol. 1999;11(7):1103–1110. doi: 10.1093/intimm/11.7.1103. [DOI] [PubMed] [Google Scholar]
- Zeng W, Ghosh S, Macris M, Pagnon J, Jackson DC. Assembly of synthetic peptide vaccines by chemoselective ligation of epitopes: influence of different chemical linkages and epitope orientations on biological activity. Vaccine. 2001;19(28-29):3843–3852. doi: 10.1016/S0264-410X(01)00152-9. [DOI] [PubMed] [Google Scholar]
- Bhattacharjee RN, Akira S. Toll-like receptor signaling: emerging opportunities in human diseases and medicine. Curr Immunol Rev. 2005;1(1):81–90. doi: 10.2174/1573395052952897. [DOI] [Google Scholar]
- Huang W, Nardelli B, Tam JP. Lipophilic multiple antigen peptide system for peptide immunogen and synthetic vaccine. Mol Immunol. 1994;31(15):1191–1199. doi: 10.1016/0161-5890(94)90033-7. [DOI] [PubMed] [Google Scholar]
- Zeng W, Jackson DC, Murray J, Rose K, Brown LE. Totally synthetic lipid-containing polyoxime peptide constructs are potent immunogens. Vaccine. 2000;18(11-12):1031–1039. doi: 10.1016/S0264-410X(99)00346-1. [DOI] [PubMed] [Google Scholar]
- Lau YF, Deliyannis G, Zeng W, Mansell A, Jackson DC, Brown LE. Lipid-containing mimetics of natural triggers of innate immunity as CTL-inducing influenza vaccines. Int Immunol. 2006;18(12):1801–1813. doi: 10.1093/intimm/dxl114. [DOI] [PubMed] [Google Scholar]
- Mühlradt PF, Kiess M, Meyer H, Süssmuth R, Jung G. Isolation, structure elucidation, and synthesis of a macrophage stimulatory lipopeptide from Mycoplasma fermentans acting at picomolar concentration. J Exp Med. 1997;185(11):1951–1958. doi: 10.1084/jem.185.11.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horváth A, Olive C, Wong A, Clair T, Yarwood P, Good M, Toth I. Lipoamino acid-based adjuvant carrier system: enhanced immunogenicity of group A streptococcal peptide epitopes. J Med Chem. 2002;45(6):1387–1390. doi: 10.1021/jm0110441. [DOI] [PubMed] [Google Scholar]
- Zaman M, Abdel-Aal A-BM, Phillipps KSM, Fujita Y, Good MF, Toth I. Structure-activity relationships of lipopeptide group A streptococcus (GAS) vaccine candidates on toll-like receptor 2. Vaccine. 2010;28(10):2243–2248. doi: 10.1016/j.vaccine.2009.12.046. [DOI] [PubMed] [Google Scholar]
- Fujita Y, Abdel-Aal AB, Wimmer N, Batzloff MR, Good MF, Toth I. Synthesis and immunological evaluation of self-adjuvanting glycolipopeptide vaccine candidates. Bioorg Med Chem. 2008;16(19):8907–8913. doi: 10.1016/j.bmc.2008.08.064. [DOI] [PubMed] [Google Scholar]
- Hopp TP. Immunogenicity of a synthetic HBSAG peptide - enhancement by conjugation to a fatty-acid carrier. Mol Immunol. 1984;21(1):13–16. doi: 10.1016/0161-5890(84)90084-1. [DOI] [PubMed] [Google Scholar]
- Deres K, Schild H, Wiesmüller KH, Jung G, Rammensee HG. In vivo priming of virus-specific cyto-toxic lymphocytes-T with synthetic lipopeptide vaccine. Nature. 1989;342(6249):561–564. doi: 10.1038/342561a0. [DOI] [PubMed] [Google Scholar]
- Loing E, Andrieu M, Thiam K, Schörner D, Wiesmüller KH, Hosmalin A, Jung G, Gras-Masse H. Extension of HLA-A*0201-restricted minimal epitope by Nε-palmitoyl-lysine increases the life span of functional presentation to cytotoxic T cells. J Immunol. 2000;164(2):900–907. doi: 10.4049/jimmunol.164.2.900. [DOI] [PubMed] [Google Scholar]
- Zeng W, Ghosh S, Lau YF, Brown LE, Jackson DC. Highly immunogenic and totally synthetic lipopeptides as self-adjuvanting immunocontraceptive vaccines. J Immunol. 2002;169(9):4905–4912. doi: 10.4049/jimmunol.169.9.4905. [DOI] [PubMed] [Google Scholar]
- Jackson DC, Lau TF, Le T, Suhrbier A, Deliyannis G, Cheers C, Smith C, Zeng W, . Brown LE. A totally synthetic vaccine of generic structure that targets Toll-like receptor 2 on dendritic cells and promotes antibody or cytotoxic T cell responses. Proc Natl Am Soc. 2004;101(43):15440–15445. doi: 10.1073/pnas.0406740101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua BY, Healy A, Cameron PU, Stock O, Rizkalla M, Zeng W, Torresi J, Brown LE, Fowler NL, Gowans EJ, Jackson DC. Maturation of dendritic cells with lipopeptides that represent vaccine candidates for hepatitis C virus. Immunol Cell Biol. 2003;81(1):67–72. doi: 10.1046/j.1440-1711.2003.01133.x. [DOI] [PubMed] [Google Scholar]
- Zhu X, Ramos TV, Gras-Masse H, Kaplan BE, BenMohamed L. Lipopeptide epitopes extended by an Nε-palmitoyl-lysine moiety increase uptake and maturation of dendritic cells through a Toll-like receptor-2 pathway and trigger a Th1-dependent protective immunity. Eur J Immunol. 2004;34(11):3102–3114. doi: 10.1002/eji.200425166. [DOI] [PubMed] [Google Scholar]
- Langhan B, Braunschweiger I, Schweitzer S, Jung G, Inchauspé G, Sauerbruch T, Spengler U. Lipidation of T helper sequences from hepatits C virus core significantly enhances T-cell activity in vitro. Immunol. 2001;102(4):460–465. doi: 10.1046/j.1365-2567.2001.01209.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouaix F, Gras-Masse H, Mazingue C, Diesis E, Ridel PR, Estaquier J, Capron A, Tartar A, Auriault C. Effect of a lipopeptidic formulation on macrophage activation and peptide presentation to T cells. Vaccine. 1994;12(13):1209–1214. doi: 10.1016/0264-410X(94)90245-3. [DOI] [PubMed] [Google Scholar]
- BenMohamed L, Gras-Masse H, Tartar A, Daubersies P, Brahimi K, Bossus M, Thomas A, Druilhe P. Lipopeptide immunization without adjuvant induces potent and long-lasting B, T helper, and cytotoxic T lymphocyte responses against a malaria liver stage antigen in mice and chimpanzees. Eur J Immunol. 1997;27(5):1242–1253. doi: 10.1002/eji.1830270528. [DOI] [PubMed] [Google Scholar]
- Pialoux G, Gahéry-Ségard H, Sermet S, Poncelet H, Fournier S, Gérard L, Tartar A, Gras-Masse H, Levy JP, Guillet JG. ANRS VAC 04 Study Team. Lipopeptides induce cell-mediated anti-HIV immune responses in seronegative volunteers. AIDS. 2001;15(10):1239–1249. doi: 10.1097/00002030-200107060-00005. [DOI] [PubMed] [Google Scholar]
- Livingston BD, Crimi C, Grey H, Ishioka G, Chisari FV, Fikes J, Grey H, Chesnut RW, Sette A. The hepatitis B virus-specific CTL responses induced in humans by lipopeptide vaccination are comparable to those elicited by acute viral infection. J Immunol. 1997;159(3):1383–1392. [PubMed] [Google Scholar]
- Salmon-Céron D, Durier C, Desaint C, Cuzin L, Surenaud M, Hamouda NB, Lelièvre JD, Bonnet B, Pialoux G, Poizot-Martin I, Aboulker JP, Lévy Y, Launay O. ANRS VAC18 trial group. Immunogenicity and safety of an HIV-1 lipopeptide vaccine in healthy adults: a phase 2 placebo-controlled ANRS trial. AIDS. 2010;24(14):2211–2223. doi: 10.1097/QAD.0b013e32833ce566. [DOI] [PubMed] [Google Scholar]
- Defoort JP, Nardelli B, Huang W, Ho DD, Tam JP. Macromolecular assemblage in the design of a synthetic AIDS vaccine. Proc Natl Acad Soc USA. 1992;89(9):3879–3883. doi: 10.1073/pnas.89.9.3879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Defoort JP, Nardelli B, Huang W, Tam JP. A rational design of synthetic peptide vaccine with a built-in adjuvant: a modular approach for unambiguity. Int J Pept Protein Res. 1992;40(3-4):214–221. doi: 10.1111/j.1399-3011.1992.tb00294.x. [DOI] [PubMed] [Google Scholar]
- Nardelli B, Haser PB, Tam JP. Oral administration of an antigenic synthetic lipopeptide (MAP-P3C) evokes salivary antibodies and systemic humoral and cellular responses. Vaccine. 1994;12(14):1335–1339. doi: 10.1016/S0264-410X(94)80062-5. [DOI] [PubMed] [Google Scholar]
- Moyle PM, Hari Y, Huang N, Olive C, Good MF, Toth I. A technique for the synthesis of highly-pure, mono-epitopic, multi-valent lipid core peptide vaccines. Tetrahedron Lett. 2007;48(29):4965–4967. doi: 10.1016/j.tetlet.2007.05.129. [DOI] [Google Scholar]
- Edenhofer F. Protein transduction revisited: novel insights into the mechanism underlying intracellular delivery of proteins. Curr Pharm Des. 2008;14(34):3628–3636. doi: 10.2174/138161208786898833. [DOI] [PubMed] [Google Scholar]
- Wagstaff KM, Jans DA. Protein transduction: cell penetrating peptides and their therapeutic applications. Curr Med Chem. 2006;13(12):1371–1387. doi: 10.2174/092986706776872871. [DOI] [PubMed] [Google Scholar]
- Crombez L, Aldrian-Herrada G, Konate K, Nguyen QN, McMaster GK, Brasseur R, Heitz F, Divita G. A new potent secondary amphipathic cellpenetrating peptide for siRNA delivery into mammalian cells. Mol Ther. 2009;17(1):95–103. doi: 10.1038/mt.2008.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner JJ, Jones S, Fabani MM, Ivanova G, Arzumanov AA, Gait MJ. RNA targeting with peptide conjugates of oligonucleotides, siRNA and PNA. Blood Cells Mol Dis. 2007;38(1):1–7. doi: 10.1016/j.bcmd.2006.10.003. [DOI] [PubMed] [Google Scholar]
- Moschos SA, Jones SW, Perry MM, Williams AE, Erjefalt JS, Turner JJ, Barnes PJ, Sproat BS, Gait MJ, Lindsay MA. Lung delivery studies using siRNA conjugated to TAT(48-60) and penetratin reveal peptide induced reduction in gene expression and induction of innate immunity. Bioconjug Chem. 2007;18(5):1450–1459. doi: 10.1021/bc070077d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meade BR, Dowdy SF. Exogenous siRNA delivery using peptide transduction domains/cell penetrating peptides. Adv Drug Deliv Rev. 2007;59(2-3):134–140. doi: 10.1016/j.addr.2007.03.004. [DOI] [PubMed] [Google Scholar]
- Kale AA, Torchillin VP. "Smart" drug carriers: PEGylated TATp-modified pH-sensitive liposomes. J Liposome Res. 2007;17(3-4):197–203. doi: 10.1080/08982100701525035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pappalardo JS, Quattrocchi V, Langellotti C, Di Giacomo S, Gnazzo V, Olivcra V, Calamante G, Zamorano PI, Levchenko TS, Torchilin VP. Improved transfection of spleen-derived antigen presenting cells in culture using TATp-liposomes. J Control Release. 2009;134(4):41–46. doi: 10.1016/j.jconrel.2008.11.006. [DOI] [PubMed] [Google Scholar]
- Brooks NA, Pouniotis DS, Sheng KC, Apostolopoulos V, Pietersz GA. A membrane penetrating multiple antigen peptide (MAP) incorporating ovalbumin CD8 epitope induces potent immune responses in mice. Biochimica Biophysica Acta. 2010;1798(12):2286–2295. doi: 10.1016/j.bbamem.2010.05.007. [DOI] [PubMed] [Google Scholar]
- Brooks NA, Pouniotis DS, Tang C-T, Apostolopoulos V, Pietersz GA. Cell-penetrating peptides: application in vaccine delivery. Biochimica Biophysica Acta. 2010;1805(1):25–34. doi: 10.1016/j.bbcan.2009.09.004. [DOI] [PubMed] [Google Scholar]
- Kim DT, Mitchell DJ, Brockstedt DG, Fong L, Nolan GP, Fathman CG, Engleman EG, Rothbard JB. Introduction of soluble proteins into the MHC class I pathway by conjugation to an HIV tat peptide. J Immunol. 1997;159(4):1666–1668. [PubMed] [Google Scholar]
- Tacken PJ, Joosten B, Reddy A, Wu D, Eek A, Laverman P, Kretz-Rommel A, Adema GJ, Torensma R, Figdor CG. No advantage of cell-penetrating peptides over receptor-specific antibodies in targeting antigen to human dendritic cells for cross-presentation. J Immunol. 2008;180(11):7687–7696. doi: 10.4049/jimmunol.180.11.7687. [DOI] [PubMed] [Google Scholar]
- Shibagaki N, Udey MC. Dendritic cells transduced with protein antigens induce cytotoxic lymphocytes and elicit antitumor immunity. J Immunol. 2002;168(5):2393–2401. doi: 10.4049/jimmunol.168.5.2393. [DOI] [PubMed] [Google Scholar]
- Abdel-Aal A-BM, Zaman M, Fujita Y, Batzloff MR, Good MF, Toth I. Design of three-component vaccines against group A streptococcal infections: importance of spatial arrangement of vaccine components. J Med Chem. 2010;53(22):8041–8046. doi: 10.1021/jm1007787. [DOI] [PubMed] [Google Scholar]
- Abdel-Aal A-BM, Batzloff MR, Fujita Y, Barozzi N, Faria A, Simerska P, Moyle PM, Good MF, Toth I. Structure-activity relationships of a series of synthetic lipopeptide self-adjuvanting group A streptococcal vaccine candidates. J Med Chem. 2008;51(1):167–172. doi: 10.1021/jm701091d. [DOI] [PubMed] [Google Scholar]
- Ni J, Powell R, Baskakov IV, DeVico A, Lewis GK, Wang L-X. Synthesis, conformation, and immunogenicity of monosaccharide-centered multivalent HIV-1 gp41 peptides containing the sequence of DP178. Bioorg Med Chem. 2004;12(12):3141–3148. doi: 10.1016/j.bmc.2004.04.008. [DOI] [PubMed] [Google Scholar]
- Lundberg P, Langel U. A brief introduction to cell-penetrating peptides. J Mol Recogn JMR. 2003;16(5):227–233. doi: 10.1002/jmr.630. [DOI] [PubMed] [Google Scholar]
- Joliot A, Prochiantz A. Transduction peptides: from technology to physiology. Nat Cell Biol. 2004;6(3):189–196. doi: 10.1038/ncb0304-189. [DOI] [PubMed] [Google Scholar]
- Pimentel TAPF, Yan Z, Jeffers SA, Holmes KV, Hodges RS, Burkhard P. Peptide nanoparticles as novel immunogens: design and analysis of a prototypic severe acute respiratory syndrome vaccine. Chem Biol Drug Des. 2009;73(1):53–61. doi: 10.1111/j.1747-0285.2008.00746.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathieu MN, Wade JD, Tan Y-Y, Summers RJ, Tregear GW. Novel Strategy for the synthesis of template-assembled analogues of rat relaxin. J Pept Sci. 2000;6(5):235–242. doi: 10.1002/(SICI)1099-1387(200005)6:5<235::AID-PSC247>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Boykins RA, Joshi M, Syin C, Dhawan S, Nakhasi H. Synthesis and construction of a novel multiple peptide conjugate system: strategy for a subunit vaccine design. Peptides. 2000;21(1):9–17. doi: 10.1016/S0196-9781(99)00172-2. [DOI] [PubMed] [Google Scholar]
- Zhang L, Tam JP. Thiazolidine formation as a general and site-specific conjugation method for synthetic peptides and proteins. Anal Biochem. 1996;233(1):87–93. doi: 10.1006/abio.1996.0011. [DOI] [PubMed] [Google Scholar]
- Zeng W, Jackson DJ, Rose K. Synthesis of a new template with a built-in adjuvant and its use in contructing peptide vaccine candidates through polyoxime chemistry. J Pept Sci. 1996;2(1):66–72. doi: 10.1002/psc.51. [DOI] [PubMed] [Google Scholar]
- Wang L-X, Ni J, Singh S. Carbohydrate-centered maleimide cluster as a new type of templates for multivalent peptide assembling: synthesis of multivalent HIV-1 gp41 peptides. Bioorg Med Chem. 2003;11(1):159–166. doi: 10.1016/S0968-0896(02)00339-5. [DOI] [PubMed] [Google Scholar]
- Skwarczynski M, Zaman M, Urbani CN, Lin I-C, Jia Z, Batzloff MR, Good MF, Monterio MJ, Toth I. Polyacrylate dendrimer nanoparticles: A self-adjuvanting vaccine delivery system. Angew Chem Int Ed. 2010;49(33):5742–5745. doi: 10.1002/anie.201002221. [DOI] [PubMed] [Google Scholar]
- Horváth A, Olive C, Karpati L, Sun HK, Good M, Toth I. Toward the development of a synthetic group A streptococcal vaccine of high purity and broad protective coverage. J Med Chem. 2004;47(16):4100–4104. doi: 10.1021/jm040041w. [DOI] [PubMed] [Google Scholar]
- Moyle PM, Olive C, Ho M-F, Good MF, Toth I. Synthesis of a highly pure lipid core peptide based self-adjuvanting priepitopic group A streptococcal vaccine, and subsequent immunological evaluation. J Med Chem. 2006;49(21):6364–6370. doi: 10.1021/jm060475m. [DOI] [PubMed] [Google Scholar]
- Fujita Y, Moyle PM, Hieu S, Simerska S, Toth I. Investigation towards multi-epitope vaccine candidates using native chemical ligation. Biopolymers (Pept Sci) 2008;90(5):624–632. doi: 10.1002/bip.21002. [DOI] [PubMed] [Google Scholar]
- Cairo CW, Gestwicki JE, Kanai M, Kiessling LL. Control of multivalent interactions by binding epitope density. J Am Chem Soc. 2002;124(8):1615–1619. doi: 10.1021/ja016727k. [DOI] [PubMed] [Google Scholar]
- Kaba SA, Brando C, Guo Q, Mitterlholzer C, Raman S, Tropel D, Aebi U, Burkhand P, Lanar DE. A nonadjuvanted polypeptide nanoparticle vaccine confers long-lasting protection against rodent malaria. J Immunol. 2009;183(11):7268–7277. doi: 10.4049/jimmunol.0901957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien-Simpson NM, Ede NJ, Brown LE, Swan J, Jackson DC. Polymerization of unprotected synthetic peptides: A view toward synthetic peptide vaccines. J Am Chem Soc. 1997;119(6):1183–1188. doi: 10.1021/ja962707k. [DOI] [PubMed] [Google Scholar]
- Jackson DC, O'Brien-Simpson NM, Ede NJ, Brown LE. Free radical induced polymerization of synthetic peptides into polymeric immunogens. Vaccine. 1997;15(15):1697–1705. doi: 10.1016/S0264-410X(97)00085-6. [DOI] [PubMed] [Google Scholar]
- Sadler K, Zeng W, Jackson DC. Synthesis of peptide epitope-based polymers: Controlling size and determining the efficiency of epitope incorporation. J Pept Res. 2002;60(3):150–158. doi: 10.1034/j.1399-3011.2002.21009.x. [DOI] [PubMed] [Google Scholar]
- Brandt ER, Sriprakash KS, Hobb RI, Hayman WA, Zeng W, Batzloff MR, Jackson DC, Good MF. New multi-determinant strategy for a group A streptococcal vaccine designed for the Australian Aboriginal population. Nat Med. 2000;6(4):455–459. doi: 10.1038/74719. [DOI] [PubMed] [Google Scholar]
- Rajagopal K, Schneider JP. Self-assembling peptides and proteins for nanotechnological applications. Curr Opin Struct Biol. 2004;14(4):480–486. doi: 10.1016/j.sbi.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Hartgerink JD, Granja JR, Milligan RA, Ghadiri MR. Self-assembling peptide nanotubes. J Am Chem Soc. 1996;118(1):43–50. doi: 10.1021/ja953070s. [DOI] [Google Scholar]
- Reches M, Gazit E. Molecular self-assembly of peptide nanostructures: Mechanism of association and potential uses. Curr Nanosci. 2006;2(2):105–111. doi: 10.2174/157341306776875802. [DOI] [Google Scholar]
- Malashkevich VN, Kammerer RA, Efimov VP, Schulthess T, Engel J. The crystal structure of a five-stranded coiled coil in COMP: a prototype ion channel? Science. 1996;274(5288):761–765. doi: 10.1126/science.274.5288.761. [DOI] [PubMed] [Google Scholar]
- Burkhard P, Meier M, Lustig A. Design of a minimal protein oligomerization domain by a structural approach. Protein Sci. 2000;9(12):2294–2301. doi: 10.1110/ps.9.12.2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung JP, Jones JL, Cronier SA, Collier JH. Modulating the mechanical properties of self-assembled peptide hydrogels via native chemical ligation. Biomterials. 2008;29(13):2143–2151. doi: 10.1016/j.biomaterials.2008.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung JP, Nagaraj AK, Fox EK, Rudra JS, Devgun JM, Collier JH. Co-assembling peptides as defined matrices for endothelial cells. Biomterials. 2009;30(12):2400–2410. doi: 10.1016/j.biomaterials.2009.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudra JS, Tian YF, Jung JP, Collier JH. A self-assembling peptide acting as an immune adjuvant. Proc Natl Acad Sci USA. 2010;107(2):622–627. doi: 10.1073/pnas.0912124107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling RA, Gil PR, Zhang F, Zanella M, Parak WJ. Biological applications of gold nanoparticles. Chem Soc Rev. 2008;37(9):1896–1908. doi: 10.1039/b712170a. [DOI] [PubMed] [Google Scholar]
- Arvizo R, Bhattacharya R, Mukherjee P. Gold nanoparticles: opportunities and challenges in nanomedicine. Expert Opin Drug Deliv. 2010;7(6):753–763. doi: 10.1517/17425241003777010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenzie F, Faulds K, Graham D. DNA functionalized gold nanoparticles as probes for double stranded DNA through triplex formation. Chem Commun. 2008;28(20):2367–2369. doi: 10.1039/b802163e. [DOI] [PubMed] [Google Scholar]
- Eck W, Craig G, Sigdel A, Gerd R, Old LJ, Tang L, Brennan MF, Allen PJ, Mason MD. PEGylated gold nanoparticles conjugated to monoclonal F19 antibodies as targeted labeling agents for human pancreatic carcinoma tissue. ACS Nano. 2008;2(11):2263–2272. doi: 10.1021/nn800429d. [DOI] [PubMed] [Google Scholar]
- Loo C, Lin A Hirsch L, Lee MH, Barton J, Halas N, West J, Drezek R. Nanoshell-enable photonics-based imaging and therapy of cancer. Technol Cancer Res Treat. 2004;3(1):33–40. doi: 10.1177/153303460400300104. [DOI] [PubMed] [Google Scholar]
- Pissuwan D, Valenzuela SM, Cortie MB. Therapeutic possibilities of plasmonically heated gold nanoparticles. Trends Biotechnol. 2006;24(2):62–67. doi: 10.1016/j.tibtech.2005.12.004. [DOI] [PubMed] [Google Scholar]
- Qian X, Peng X-H, Ansari DO, Yin-Goen Q, Chen GZ, Shin DM, Yang L, Young AN, Wang MD, Nie S. In vivo tumor targeting and spectroscopic detection with surface-enhanced Raman nanoparticle tags. Nat Biotechnol. 2008;26(1):83–90. doi: 10.1038/nbt1377. [DOI] [PubMed] [Google Scholar]
- Paciotti GF, Myer L, Weinreich D, Goia D, Pavel N, McLaughlin RE, Tamarkin L. Colloidal gold: a novel nanoparticle vector for tumor directed drug delivery. Drug Deliv. 2004;11(3):169–183. doi: 10.1080/10717540490433895. [DOI] [PubMed] [Google Scholar]
- Gibson JD, Khanal BP, Zubarev ER. Paclitaxel-functionalized gold nanoparticles. J Am Chem Soc. 2007;129(37):11653–11661. doi: 10.1021/ja075181k. [DOI] [PubMed] [Google Scholar]
- Brown SD, Nativo P, Smith J-A, Stirling D, Edwards PR, Venuopal B, Flint DJ, Plumb JA, Graham D, Wheate NJ. Gold nanoparticles for the improved anticancer drug delivery of the active component of oxaliplatin. J Am Chem Soc. 2010;132(13):4678–4684. doi: 10.1021/ja908117a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomii A, Masugi F. Production of anti-platelet-activating factor antibodies by the use of colloidal gold as carrier. Jpn J Med Sci Biol. 1991;44(2):75–80. doi: 10.7883/yoken1952.44.75. [DOI] [PubMed] [Google Scholar]
- Zhou X, Zhang X, Yu X, Zha X, Fu Q, Liu B, Wang X, Chen Y, Chen Y, Shan Y, Jin Y, Wu Y, Liu J, Kong W, Shen J. The effect of conjugation to gold nanoparticles on the ability of low molecular weight chitosan to transfer DNA vaccine. Biomaterials. 2008;29(1):111–117. doi: 10.1016/j.biomaterials.2007.09.007. [DOI] [PubMed] [Google Scholar]
- Chen Y-S, Hung Y-C, Lin W-H, Huang GS. Assessment of gold nanoparticles as a size-dependent vaccine carrier for enhancing the antibody response against synthetic food-and-mouth disease virus peptide. Nanotechnol. 2010;21:195101. doi: 10.1088/0957-4484/21/19/195101. [DOI] [PubMed] [Google Scholar]
- Connor EE, Mwamuka J, Gole A, Murphy CJ, Wyatt MD. Gold nanoparticles are taken by human cells but do not cause acute cytotoxicity. Small. 2005;1(3):325–327. doi: 10.1002/smll.200400093. [DOI] [PubMed] [Google Scholar]
- Male KB, Lachance B, Hrapovic S, Sunahara G, Luong JH. Assessment of cytotoxicity of quantum dots and gold nanoparticles using cell-based impedance spectroscopy. Anal Chem. 2008;80(14):5487–5493. doi: 10.1021/ac8004555. [DOI] [PubMed] [Google Scholar]
- Frens G. Controlled nucleation for the regulation of the particle size in monodisperse gold suspensions. Nature Phys Sci. 1973;214:20–22. [Google Scholar]
- Turkevich J. Colloidal gold Part I. Historical and preparative aspects, morphology and structure. Gold Bull. 1985;18(3):86–91. doi: 10.1007/BF03214690. [DOI] [Google Scholar]
- Perrault SD, Chan WC. Synthesis and surface modification of highly monodispersed, spherical gold nanoparticles of 50-200 nm. J Am Chem Soc. 2009;131(47):17042–17043. doi: 10.1021/ja907069u. [DOI] [PubMed] [Google Scholar]
- Martin MN, Basham JI, Chando P, Eah S-K. Charged gold nanoparticles in non-polar solvents: 10-min synthesis and 2D self-assembly. Langmuir. 2010;26(10):7410–7417. doi: 10.1021/la100591h. [DOI] [PubMed] [Google Scholar]
- Chen Y-S, Hung Y-C, Liau I, Huang GS. Assessment of the in vivo toxicity of gold nanoparticles. Nanoscale Res Lett. 2009;4(8):858–864. doi: 10.1007/s11671-009-9334-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malugin A, Ghandehari H. Cellular uptake and toxicity of gold nanoparticles in prostate cancer cells: a comparative study of rods and spheres. J Appl Toxicol. 2010;30(3):212–217. doi: 10.1002/jat.1486. [DOI] [PubMed] [Google Scholar]
- Verma A, Uzun O, Hu Y, Han H-S, Watson N, Chen S, Irvine DJ, Stellacci F. Surface-structure-regulated cell-membrane penetration by monolayer-protected nanoparticles. Nat Mater. 2008;7(7):588–595. doi: 10.1038/nmat2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokes RJ, McKenzie F, McFarlane E, Ricketts A, Tetley L, Faulds K, Alexander J, Graham D. Rapid cell mapping using nanoparticles and SERRS. Analyst. 2009;134(1):170–175. doi: 10.1039/b815117b. [DOI] [PubMed] [Google Scholar]
- Cruz LJ, Rueda F, Cordobilla B, Simón L, Hosta L, Albericio F, Domingo JC. Targeting nanosystems to human DCs via Fc receptor as an effective strategy to deliver antigen for immunotherapy. Mol Pharm. 2011;8(1):104–116. doi: 10.1021/mp100178k. [DOI] [PubMed] [Google Scholar]