Abstract
The contribution of acute phase plasma proteins to host immune responses remains poorly characterized. To better understand the role of the acute phase reactant and major hemoglobin-binding protein haptoglobin (Hp) on the function of immune cells, we generated Hp-deficient C57BL/6J mice. These mice exhibit stunted development of lymphoid organs associated with lower counts of mature T and B cells in the blood and secondary lymphoid compartments. Moreover, these mice show markedly reduced adaptive immune responses as represented by reduced accumulation of IgG antibody after immunization with adjuvant and nominal antigen, abrogation of Th1-dominated delayed-type hypersensitivity reaction, loss of mitogenic responses mounted by T cells, and reduced T cell responses conveyed by APCs. Collectively, these defects are in agreement with the observations that Hp-deficient mice are not capable of generating a recall response or deterring a Salmonella infection as well as failing to generate tumor antigen-specific responses. The administration of Hp to lymphocytes in tissue culture partially ameliorates these functional defects, lending further support to our contention that the acute phase response protein Hp has the ability to regulate immune cell responses and host immunity. The phenotype of Hp-deficient mice suggests a major regulatory activity for Hp in supporting proliferation and functional differentiation of B and T cells as part of homeostasis and in response to antigen stimulation.
Keywords: T cell, dendritic cell, humoral immune response
INTRODUCTION
Haptoglobin (Hp) is the major hemoglobin (Hb)-binding protein in the plasma of most vertebrates and all mammals [1, 2]. The primary physiological function of Hp has been described in terms of its interaction with free Hb, thereby neutralizing and restricting its oxidative damage to various organs [3]. Hp is a member of the evolutionarily conserved set of acute phase plasma proteins (APPs), whose hepatic expression is strongly induced by inflammatory mediators such as IL-6-type cytokines, increasing within 24 h the plasma concentration from as low as <50 μg/ml to ∼2.5 mg/ml [4].
The role of inflammatory mediators in directing host immune function, whether in relation to infections or a disease state such as tumorigenesis, is becoming an intensively studied process. However, the action of these mediators’ downstream targets, the APPs, in aiding this highly regulated immune response is not well understood. The tight correlation between inflammation and induction of Hp expression by cytokines, known to be critical for regulating the immune system, has led to the notion that Hp may exude a regulatory role in immune function. In line with the proposed anti-inflammatory activity of plasma Hp during the course of an acute phase reaction (APR) [4], Hp has been suggested to exert immunomodulatory effects constituent with suppression of lymphocyte function [5, 6]. Hp interferes with Langerhans cell function by preventing functional transformation [7]. Hp also binds to monocytic cells and lymphocytes through the cell surface proteins CD11b/CD18, CD163, and CD22, suggesting it may control cell functions through one or more signaling pathways [8]. The precise cellular targets and the functional manifestation of acute phase-regulated Hp are still ill-defined.
Although Hb-dependent functions of Hp in vivo were observed in a Hp-deficient strain of mice [9], the proposed anti-inflammatory and immune modulatory activities of Hp could not be adequately assessed as a result of the animals’ mixed genetic background. Therefore, in this study, we have investigated in an exploratory manner the extent by which Hp expression influences the normal development and function of the immune system in an inbred, congenic Hp−/− C57BL/6J strain. We discovered that the absence of Hp causes an impaired immune response. Furthermore, contrary to the expectation, Hp expressed by hematopoietic cells and liver appears to be required to efficiently coordinate optimal function of the immune system. This coordination occurs at multiple levels including antigen presentation and effector function. These findings emphasize a previously unappreciated, functional link between the APR and the immune response.
MATERIALS AND METHODS
Mice
A congenic strain of Hp knockout C57BL/6J (Hp−/−) mice was established through eight generations of backcrossing [9, 10] and was also used to introduce the Hp−/− genotype into C57BL/6-OT-1.PL [11, 12]. For immunizations, 0.5 ml of an emulsion of equal volumes of CFA and 2 mg/ml chicken OVA in PBS were injected s.c. Two subsequent injections containing the same amount of OVA emulsified in IFA and were given 7 and 14 days later. OVA-specific antibodies in serum were assayed by immunoblotting. Briefly, an OVA preparation was boiled in SDS sample buffer, and multiple aliquots of 1 μg were separated on one-dimensional 10% polyacrylamide gels. The protein was electrotransferred onto nitrocellulose membranes, which were cut into strips containing individual lanes of electrophoretically separated OVA. Individual strips were reacted with 1:100–1:50,000 diluted serum collected from the immunized mice at various time-points. The strips were washed extensively and then reacted with goat anti-mouse IgM (Sigma Aldrich, St. Louis, MO, USA) followed by rabbit HRP-conjugated anti-goat IgG or HRP-conjugated anti-mouse IgG After mounting the strips in order of serum collection, the Ig binding to membrane-immobilized OVA was visualized by ECL (ICN, Aurora, IL, USA).
Protection against OVA-expressing Salmonella typhimurium [13] was assessed by i.p. injection of 1 × 106 pfu bacteria at Day 56; quantification of bacterial titers in organs was done 4 days later on McConkey plates. A separate immunization used an i.v. injection of 1 × 106 bone marrow-derived dendritic cells (DC) from Hp+/+ or Hp−/− mice that had been treated with 10 μg/ml OVA for 4 h and 1 μg/ml LPS for 16 h. After 5 days, 1 × 106 syngeneic, OVA-expressing E.G7 thymoma cells [14] were injected i.p. (Day 0), and animals were monitored thereafter for morbidity.
A systemic APR was induced by i.p. injection of 10 μg LPS in 100 μl PBS; control animals received PBS alone. All mice were housed in Association for Assessment and Accreditation of Laboratory Animal Care-approved facilities under specific pathogen-free conditions and used according to the guidelines by the Institutional Animal Care and Use Committee.
Blood cell count
Aliquots of blood were transferred to EDTA microtainers (Becton Dickinson, San Diego, CA, USA) and analyzed by a Hemaret 850 Mascat blood cell counter (CDC Technologies, Oxford, CT, USA), which had been calibrated with a standard mixture of mouse leukocytes.
FACS
All antibodies were from BD Biosciences (Mountain View, CA, USA). Cells harvested from lymphoid organs and staining for surface antigen expression were performed as described previously [12] with mAb: FITC-conjugated anti-CD4, anti-Vα2, anti-IgM, anti-CD22, and anti-CD11c; PE-conjugated anti-B220, anti-Thy1.1, and anti-NK1.1; and PE/Cy5-conjugated anti-CD8, anti-CD3, anti-CD25, anti-CD11b, and anti-CD44. The cells were washed, fixed, and analyzed on a FACSCalibur/FACScan flow cytometer using CellQuest software (BD Biosciences) and WinList 5.0 software (Verity Software House, Topsham, ME, USA).
In vitro T cell stimulation assay
Cells from lymph nodes were enriched for CD4+, CD8+, or CD3+ T cells to 85–95% purity by negative selection (Cedarlane Laboratories, Ontario, Canada). Purified T cells (1×106 per well) were stimulated with 10 ng/ml PMA and 250 ng/ml ionomycin, 5 μg/ml Con A, 10 μg/ml PHA (Sigma Aldrich), or 0.5–5 μg/ml anti-CD3-coated plates. OT-1 cell preparations consisted of ≥95% Vα2+, Thy1.1+ cells and where indicated, were labeled with 5 μM CFSE (Molecular Probes, Eugene, OR, USA). BOK cells (MEC.B7. SigOVA, expressing H-2Kb, OVA, and B7.1) were used to present antigen to naïve OT-1 cells (1×105 BOK cells to 2.5×105 OT-1 cells per ml) [15]. Nonadherent cells were harvested and recultured in 96-well plates (1×105 cells per well). DNA synthesis was determined by incubation for 16 h with 1 μCi [3H]thymidine (Amersham Biosciences, Piscataway, NJ, USA). Apoptosis was determined by labeling cells with Annexin V-FITC and propidium iodide (PI; BD Biosciences) and assayed by flow cytometry.
Generation of bone marrow-derived DC
Marrow from femurs and tibias was dissociated, washed, and plated at a density of 1 × 106 cells per ml in 24-well plates in RPMI 1640 containing 10% FBS and 10 ng/ml recombinant mouse GM-CSF (eBioscience, San Diego CA, USA). Cells were fed every 2 days and harvested at Days 7–9. Cultures consisted of ∼75% CD11c+ cells, which were pulsed for 4 h with 10 μg/ml OVA prior to treatment with 1 μg/ml LPS for 16 h.
Induction of a delayed-type hypersensitivity (DTH) reaction
2,4-Dinitro-1-fluorobenzene (DNFB; Sigma Aldrich) was diluted in acetone/olive oil (4:1). Mice were sensitized with 25 μl 0.5% DNFB solution painted onto the shaved dorsal skin. Controls were treated with vehicle alone. Five days later, 10 μl 0.2% DNFB was applied onto both sides of the right ear and the same amount of vehicle alone onto the left ear. Ear thickness was measured at Day 5 before challenge and at 24 h after challenge using a Quick Mine thickness gauge caliper (Mitutoyo, Japan). Additional controls included in each experimental series were nonsensitized but challenged mice.
Protein analysis
Samples were solubilized in radioimmunoprecipitation assay buffer (50 mM Tris, pH 7.4, 1% Nonidet P-40, 0.25% sodium deoxycholate, 150 mM NaCl, 1 mM EDTA, 1 μl/ml aprotinin, 1 μg/ml leupeptin, 1 mM sodium vanadate, and 0.1 mM PMSF), and protein concentrations were determined (Bio-Rad, Hercules, CA, USA). Aliquots of extracts (20 μg protein) or 0.1 μl sera were separated on 8% polyacrylamide-SDS gel and immunoblotted using antibodies for mouse α1-acid glycoprotein (AGP; RPCI Springville Laboratories, Springville, NY, USA), Hp (Dako, Carpinteria, CA, USA), and ECL detection (ICN).
Purification of mouse plasma Hp
Hb isolated from erythrocytes from Hp−/− mice was covalently coupled to cyanogen bromide-activated Sepharose 4B-CL (Pharmacia, Uppsala, Sweden). Acute phase mouse serum (24–48 h post-turpentine injection [16]) was bound to Hb-Sepharose and washed extensively with PBS and high salt buffer (1 M NaCl in 50 mM Tris-HCl, pH 7.0). Hp was eluted in 5 M guanidine-HCl-25 mM sodium acetate, pH 4.5. The eluted protein was neutralized immediately, dialyzed against PBS, and subjected to a second Hb-affinity chromatography. Final Hp preparation was dialyzed against 25 mM NH4HCO3, lyophilized, and redissolved in pyrogen-free, sterile PBS to a concentration of 10 mg/ml. The absence of irritant activity was tested on human lung macrophages (lower detection level 0.1 pg/ml endotoxin equivalent).
RNA extraction and analysis
Total RNA was extracted with TRIzol (Life Technologies, Grand Island, NY, USA). Aliquots of RNA (8 ng–5 μg) were subjected to cDNA synthesis with 1 unit SuperScript™II RT and 0.5 μg oligo(dT) 15-mer (Invitrogen, Carlsbad, CA, USA). The cDNA was amplified with 0.5 unit Taq polymerase (Invitrogen) and 10 pmol each sense and antisense primers. The thermal cycle profile was as follows: denaturation for 1 min at 95°C, annealing for 1 min at 56°C, and extension for 1.5 min at 72°C for 30–35 cycles. The primer pairs were: Hp, 5′ ACGAGAAGCAATGGGTGAACACA 3′ and 5′ AGCCAGACACGTAGCCCACA 3′; GAPDH, 5′ AACGACCCCTTCATTGAC 3′ and 5′ TCCACGACATACTCAGCAC 3′. For Northern blot analysis, 20 μg RNA was separated on a 1.5% formaldehyde-agarose gel, transferred to a nylon membrane (Schleicher and Schuell BioScience, Dassel, Germany), and reacted with 32P-labeled cDNA probes. The radioactive patterns were visualized by autoradiography and by phosphorimaging (Molecular Dynamics, Sunnyvale, CA, USA).
Cytokine assay
Cytokine concentrations in tissue extracts and conditioned media of cell cultures were determined by a multiplex microsphere assay (Luminex Inc., Austin, TX, USA) and calculated using the power function generated from the trend line (R=0.98) of the linear portion of the standard curve for each cytokine. The assay of mouse IL-12 determined p70 and p40 combined.
Statistics
Significance between control and experimental groups was examined using Student’s t-test. A value of P < 0.05 was regarded as significant.
RESULTS
Hp deficiency impairs lymphoid organ development but not APR
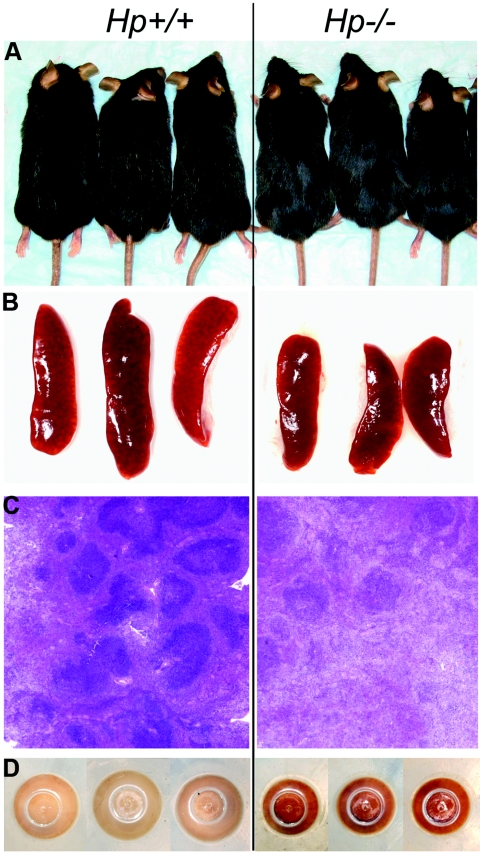
To define a bona fide physiological role for Hp that would connect the APR to immune cell regulation, we generated the congenic Hp knockout strain C57BL/6J.Hp−/−. As already observed in the mixed genetic background of 129/Sv and C57BL/6J [9, 10], the congenic, Hp-deficient C57BL/6J mice, when reared under conditions of minimized exposure to pathogens and stress, showed no significant impairment in viability, fertility, and fecundity. Nevertheless, with age, Hp–/– animals exhibited several characteristic features that distinguished them from age-matched Hp+/+ animals. An externally visible marker for both genders of Hp−/− mice was the retention of deeply black and shiny hair through at least 12 months of age; in contrast, Hp+/+ animals showed increasingly discolored ruffled and duller fur by 6 months of age (Fig. 1A). At 4 weeks postnatal and becoming more prominent with advancing age was the presence of smaller spleens in Hp−/− animals (Fig. 1B, and see Table 2). This correlated with a reduced size and number of germinal centers (Fig. 1C) and a significant, twofold reduction of splenic and circulating lymphocytes (Tables 1 and 2). Another consistently observed feature of Hp deficiency was the much darker brown color of splenocytes in Hp−/− mice relative to age-matched wild-type mice (Fig. 1D). The color may be attributed to Hb precipitates ingested by resident splenic phagocytes. Although an age-dependent accumulation of Hb precipitates was also evident in spleens of Hp+/+ animals, the amount of precipitates in age-matched Hp−/− animals (determined up to 18 months of age) was invariably higher.
Figure 1.
Phenotypic markers for Hp deficiency in C57BL/6J mice. Comparison of age-matched (8-month-old) Hp+/+ and Hp−/− mice: (A) black and shining hair; (B) smaller spleen size; (C) lower number and smaller size germinal centers in spleen (H&E staining); and (D) presence of Hb precipitates within splenocytes (bottom view of the pellet of isolated cells in centrifuge tube).
TABLE 1.
Cellular Composition of Blood from Age-Matched (10-Week) Hp−/− and Hp+/+ C57BL/6 Mice (n = 10)
| Cell Types | Hp+/+ | Hp−/− |
|---|---|---|
| Erythrocytes | 8600 ± 700 | 8600 ± 600 |
| Total leukocytes | 5.2 ± 0.7 | 3.1 ± 0.3a |
| Lymphocytes | 4.8 ± 0.7 | 2.5 ± 0.2a |
| B220+ | 2.88 ± 0.42 (60.1±1.3%) | 1.63 ± 0.13 (65.1±3.2%)a |
| CD3+ | 0.91 ± 0.14 (19.1±1.5%) | 0.35 ± 0.03 (13.7±0.8%)a |
| CD4+ | 0.46 ± 0.06 (9.5±1.1%) | 0.11 ± 0.01 (4.2±0.3%)a |
| CD8+ | 0.45 ± 0.06 (9.4±0.7%) | 0.30 ± 0.02 (11.6±0.6%)a |
| Neutrophils | 0.41 ± 0.06 | 0.63 ± 0.09 |
| Monocytes | 0.03 ± 0.01 | 0.03 ± 0.01 |
| Eosinophils | 0.04 ± 0.02 | 0.06 ± 0.03 |
| Basophils | 0.01 ± 0.01 | 0.01 ± 0.01 |
| Platelets | 1074 ± 116 | 1058 ± 127 |
The values represent means ± sem and are expressed in 106 cells/ml.
Statistically significant difference between the Hp−/− and Hp+/+ mice, P < 0.05.
TABLE 2.
Influence of Hp Deficiency on Cellular Composition of Primary and Secondary Lymphoid Organs
| Organ | Hp+/+ | Hp−/− |
|---|---|---|
| Spleen | ||
| Weight | 89.4 ± 11.4 mg | 58.8 ± 8.7 mga |
| Total cell number | 113.7 ± 12.8 × 106 | 41.5 ± 5.2 × 106a |
| B cells (B220+) | 69.0 ± 1.9 × 106 (60.7±1.8%) | 15.2 ± 1.1 × 106 (36.7±2.9%)a |
| T cells (CD3+) | 27.8 ± 1.4 × 106 (24.4±1.4%) | 16.7 ± 1.1 × 106 (40.2±2.0%)a |
| CD4+ CD25− | 13.9 ± 0.4 × 106 (12.2±1.1%) | 8.3 ± 1.4 × 106 (21.1±1.4%) |
| CD4+ CD25+ | 1.4 ± 0.1 × 106 (1.2±0.1%) | 1.0 ± 0.1 × 106 (2.5±0.2%) |
| CD8+ | 11.5 ± 0.6 × 106 (10.1±0.6%) | 7.1 ± 0.3 × 106 (17.0±0.8%) |
| NK cells (Nk1.1+) | 2.8 ± 0.6% | 4.4 ± 0.3% |
| Monocytes (CD11b+) | 4.3 ± 0.7% | 8.3 ± 1.2%a |
| DC (CD11c+) | 1.7 ± 0.3% | 3.8 ± 0.3%a |
| Thymus | ||
| Weight | 53.1 ± 9.2 mg | 42.9 ± 6.4 mg |
| Total cell number | 89.3 ± 14.1 × 106 | 77.4 ± 17.9 × 106 |
| CD4− CD8− | 5.9 ± 0.6% | 12.2 ± 0.9%a |
| CD4+ CD8+ | 80.5 ± 2.1% | 74.1 ± 1.7% |
| CD4+ CD8− CD25− | 8.7 ± 0.8% | 6.6 ± 0.6% |
| CD4+ CD8− CD25+ | 0.6 ± 0.1% | 0.9 ± 0.1% |
| CD4− CD8+ | 5.0 ± 0.7% | 7.0 ± 0.7% |
| CD3− CD4− CD8−b | (100%)b | (100%)b |
| CD44+ CD25− | 6.8 ± 0.3% | 3.7 ± 0.3% |
| CD44+ CD25+ | 5.1 ± 1.0% | 5.3 ± 0.5% |
| CD44− CD25+ | 42.2 ± 2.3% | 60.3 ± 0.7%a |
| CD44− CD25− | 45.9 ± 2.2% | 30.7 ± 0.6%a |
| Lymph node | ||
| Weight | 69 ± 8.7 mg | 54.7 ± 6.9 mg |
| Total cell number | 60.9 ± 12.7 × 106 | 45.8 ± 9.7 × 106 |
| B cells (B220+) | 32.2 ± 1.8% | 19.1 ± 2.0%a |
| T cells (CD3+) | 51.2 ± 1.4% | 63.2 ± 3.1% |
| CD4+ CD25− | 27.0 ± 1.4% | 24.1 ± 0.6% |
| CD4+ CD25+ | 2.6 ± 0.2% | 1.9 ± 0.3% |
| CD8+ | 19.6 ± 2.1% | 33.2 ± 2.5%a |
| Bone marrow | ||
| B220−IgM− | 49.2 ± 11.8% | 63.2 ± 8.9% |
| B220+IgM− | 32.7 ± 11.8% | 25.0 ± 8.6% |
| B220+IgM+ | 15.2 ± 3.4% | 8.7 ± 3.7%a |
Cellular composition of major lymphoid organs from age-matched (10-week) Hp−/− and Hp+/+ C57BL/6 mice (n = 7–10), as determined by well-defined markers for each cell type. The values represent means ± sem obtained by staining and flow cytometry analysis.
Statistically significant difference between Hp−/− and Hp+/+ mice.
Percentage of different stage thymocytes that are CD−CD4−CD8− have been reported as a percentage of the total CD3−CD4−CD8− population.
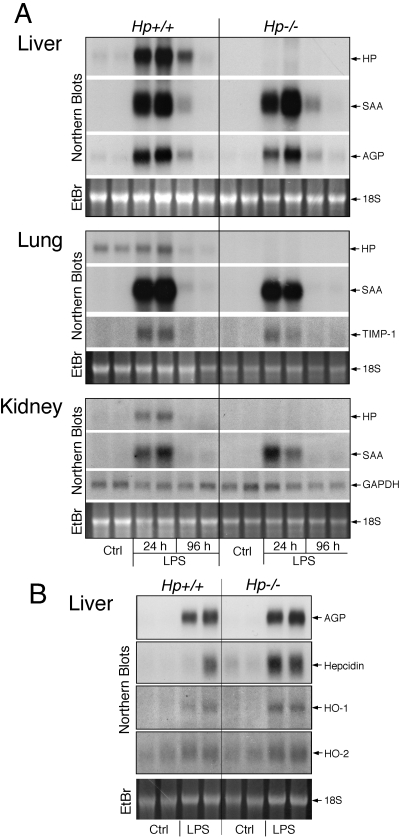
Comparison of Hp−/− and Hp+/+ animals indicated that Hp deficiency did not appreciably alter the basal expression and trauma- or endotoxin-mediated induction of APPs in various organs (Fig. 2A). Similarly, acute phase regulation of liver genes relevant for heme and iron metabolism, such as heme oxygenase-1 and -2 and hepcidin, was similar in both genotypes (Fig. 2B). Unlike hemolysis, endotoxin treatment did not generate a significantly increased and/or prolonged expression of hepatic APP in Hp−/− animals [4], although a trend of increased expression of certain acute phase proteins, such as hepcidin, was noted (Fig. 2B).
Figure 2.
LPS-mediated induction of acute phase reactants in various organs is maintained in Hp−/− mice. (A) Age-matched (10-week-old) Hp+/+ and Hp−/− C57BL/6J mice received a single i.p. injection of PBS (Ctrl) or 50 μg LPS, and after 24 h (Ctrl and LPS) or 96 h (LPS), RNAs were extracted from the indicated organs of two animals and analyzed by Northern blotting for the mRNA listed on the right. (B) A separate set of 10-week-old animals received PBS (Ctrl) or 50 μg LPS, and 24 h later, liver RNAs were isolated and analyzed by Northern blotting for the indicated mRNAs. SAA, Serum amyloid A; EtBr, ethidium bromide; TIMP-1, tissue inhibitor for metalloproteinase-1; HO, heme oxygenase.
Hp deficiency reduces the lymphocyte pool
Analysis of blood from Hp−/− animals indicated that most of the cellular constituents were in the normal range (Table 1). However, a significant, approximate twofold reduction was detected for B and T lymphocytes. Moreover, the relative composition of T cells was also shifted. In Hp+/+ mice, CD4+ and CD8+ cells were in approximately equal numbers, and in Hp−/− mice, CD8+ cells exceeded CD4+ cells. The change in blood lymphocytes counts is reflected by a reduction in the size of lymphoid organs, most notably, spleen and to a lesser extent, thymus and lymph nodes (Table 2).
The reduction of lymphocytes suggested that Hp deficiency leads to an impairment of lymphocyte development, differentiation, and/or turnover in postnatal mice. To pinpoint the potential stage of Hp-dependent action, the major hematopoietic organs of 10-week-old mice were analyzed by flow cytometry for the presence of cells representing characteristic stages of lymphoid development (Table 2). Cell analysis of bone marrow from Hp+/+ and Hp−/− animals for the three main differentiation stages of B cells (i.e., B220–IgM–, B220+IgM–, and B220+IgM+) indicated that the relative abundance of the first stage was higher, but that of the latter two stages was lower in Hp−/− animals, suggesting an attenuated differentiation to the pre-pro-B cell fraction A-D.
The composition of differentiating T cells in the thymus indicated that Hp deficiency leads to a higher relative number of CD3–CD4–CD8–CD44–CD25+ cells and a corresponding reduction of CD3–CD4–CD8–CD44–CD25– cells, suggesting an impairment of T cell development at the CD4–CD8– double-negative T cell stage (Table 2). Analysis of the spleen and lymph nodes from Hp−/− animals showed a reduction of B cells relative to T cells. Although in the spleen, no appreciable change in relative abundance of T cell subtypes was detectable, in lymph nodes, there was a shift in the relative abundance from CD4+ > CD8+ to CD4+ < CD8+. As a result of the reduced presence of lymphocytes, the relative number of splenic monocytes and DC appeared elevated with respect to wild-type mice, although the total cell number of each of these subtypes in comparison was unaltered by the absence of Hp (Table 2).
Alterations in cellular compositions of the lymphoid organs in Hp−/− mice, as determined after 10 weeks of age, were already detectable by 4 weeks of age and persisted throughout adult life (determined up to 18 months). Taken together, the absence of Hp is associated with an impairment of B and T cell differentiation in postnatal mice. This impairment may account for the reduced, steady-state level of lymphoid cells in Hp−/− animals and predicts that immune functions that depend on these cell types are also altered. This prediction has been tested in the following by subjecting Hp−/− mice to specific challenges of the immune system.
Hp deficiency impairs the immune response
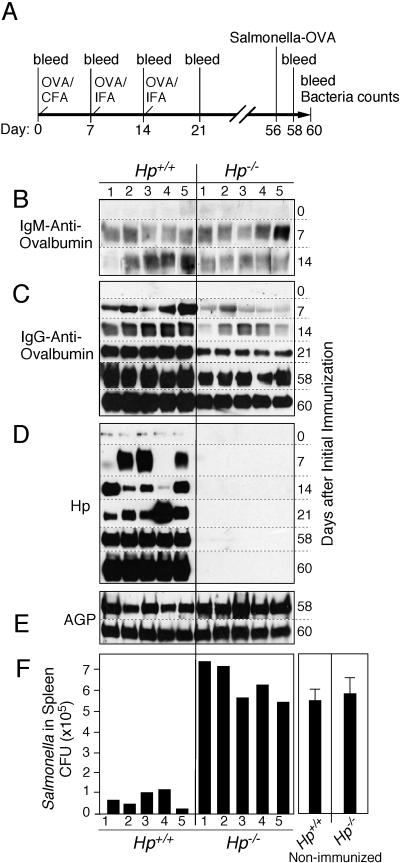
To determine the influence of Hp deficiency on the animal’s ability to mount an immune response that involves a broad range of T and B cell functions, Hp+/+ and Hp−/− animals were immunized with the nominal antigen OVA (Fig. 3A). The effectiveness of the immunization procedure was monitored by the temporal progression and relative level of anti-OVA Igs in the blood; treatment-associated induction of APPs as indicators for the systemic action of inflammatory mediators; and response to final challenge with OVA-expressing S. typhimurium [13], which indicated an immunization-dependent control of bacterial burden. The results revealed a characteristic and highly reproducible defect in the cellular immune response of Hp−/− animals. Although immunization of these animals was as effective in eliciting the production of anti-OVA IgM, comparable as in Hp+/+ animals (Fig. 3B), the class-switch to anti-OVA IgG production was significantly and persistently reduced (Fig. 3C). This immunization scheme was sufficient to increase the systemic level of APPs, including Hp in Hp+/+ animals (Fig. 3D). The final challenge with OVA-expressing Salmonella produced a comparable maximal APR, as is evident from the uniformly high plasma concentration of AGP at 48 and 96 h after bacterial challenge (Fig. 3E).
Figure 3.
Hp deficiency attenuates the immune response to OVA. (A) Schematic time-line of the experiment, indicating the sequence of bleedings and immunization with OVA in CFA or in IFA and final challenge with OVA-expressing Salmonella. (B–F) Groups of five age-matched (8-week-old) Hp+/+ and Hp−/− mice were immunized with OVA. Aliquots of the blood taken at the days indicated were analyzed by immunoblotting for the level of OVA-specific IgM (B), IgG (C), Hp (D), and AGP (E). Titer of Salmonella-OVA in the spleen extracts 4 days after challenge was determined. A significant difference was observed between Hp-deficient animals and wild-type (P=0.0002). Salmonella growth in nonimmunized animals served as reference (mean±se, n=5; F). Results are representative of two independent experiments with five mice in each group.
Despite the reduced humoral response, an immune memory was established in Hp−/− animals, which responded to challenge with OVA-expressing Salmonella by a stimulated antibody production (Fig. 3C, Days 58 and 60). However, the immune response attained in all Hp−/− animals was still insufficient to appreciably reduce proliferation of the bacteria and thus, was comparable to nonimmunized mice (Fig. 3F). These results indicate that Hp deficiency was not only associated with an impaired lymphoid cell development but also with an attenuated, Type II, Th cell-dependent, isotype-switching regulation of B cells for plasma cell function. Hp could cause these proposed regulatory functions in lymphocytes by direct action onto the various lymphocyte types and stages or through moderating the milieu within lymphoid organs by altering hematopoietic and stromal cell functions. A separate, although not mutually exclusive, mechanism of Hp-dependent action could conceivably affect the link from antigen presentation to CD4+ and CD8+ T cells and to B cells. Still to be determined is whether this regulatory process is affected by Hp, which is generated by the antigen-presenting DC and/or by liver-derived plasma Hp.
Immunization by adoptively transferred DC demonstrates the potential relative contribution of systemic Hp in directing antigen-specific immune response
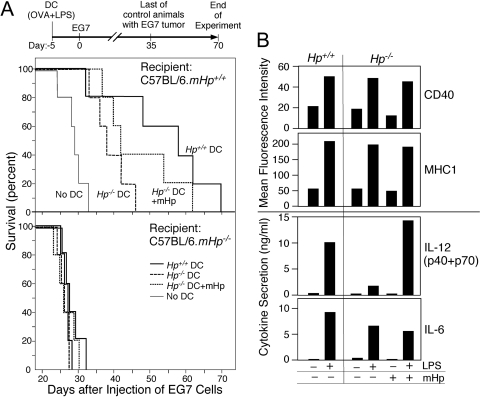
To assess the role of Hp in initiating an antigen-specific immune response in vivo, OVA-primed DC derived from Hp-deficient or wild-type mice were adoptively transferred into Hp+/+ and Hp–/– animals and tested for their capability to establish, in the recipient animals, protection against a challenge with OVA-expressing EG.7 thymoma cells (Fig. 4A). Suppression of tumor cell growth served as a measure for effective immunization. Cultures of DC were activated in vitro by treatment with OVA, LPS, and in some cases, also with purified mouse Hp. The in vitro, OVA-primed DC were adoptively transferred into Hp+/+ or Hp−/− animals; after 5 days, the animals received EG.7 tumor cells. A clear distinction between Hp−/− and Hp+/+ animals became evident by the fact that none of the DC preparations were able to provide protection against EG.7 cell-mediated lethality in Hp−/− animals, whereas the same DC preparations were effective in protecting Hp+/+ animals (Fig. 4A). A statistically significant (P=0.027) prolongation of survival was achieved with Hp+/+ DC when adoptively transferred to wild-type recipients as compared with the control group. However, Hp−/− DC were much less effective. Nevertheless, Hp−/− DC, which were maintained in medium containing the physiologically relevant concentration of 1 mg/ml mouse Hp during the in vitro antigen presentation and activation process, provided improved protection (P=0.032). The proliferation of the progenitor cells from bone marrow of Hp+/+ and Hp−/− animals did not appreciably differ between genotypes nor were there any detectable differences in the basal and LPS-induced expression of cell surface markers CD40 and MHC-I (Fig. 4B). However, although production and LPS induction of IL-6 were in comparable range between Hp+/+ and Hp−/− cells, production of IL-12 was markedly reduced in Hp−/− DC. Furthermore, when Hp was included with the treatment of LPS, this led to increased IL-12 production (Fig. 4B).
Figure 4.
Effectiveness of immunization by DC from Hp+/+ and Hp−/− mice and influence of systemic Hp. (A) Groups of 5 Hp+/+ and Hp−/− C57BL/6 mice received 1 × 106 matured bone marrow-derived DC from C57BL/6J.Hp+/+ or Hp−/− mice (Day –5). Prior to transfer, the DC had been treated for 4 h with OVA, followed by 16 h with LPS. One culture with Hp−/− DC also included 1 mg/ml mouse Hp. The recipient mice were challenged i.p. with 1 × 106 E.G7 (EL4 OVA-expressing thymoma) tumor cells (=Day 0). Mice were then monitored for survival. In Hp+/+ mice, Hp+/+ DC versus Hp−/− DC: P = 0.01; Hp−/− DC + mouse Hp (mHp) versus Hp−/− DC: P = 0.02. (B) Bone marrow-derived DC from the indicated genotypes (1×106 cells/ml) were treated for 24 h with LPS (1 μg/ml) or Hp (1 mg/ml). The expression of cell surface markers was identified by flow cytometry, and the cytokine concentrations in the conditioned medium were determined by Luminex immunobead assay. Data represent the results from one experimental series. Although secretion rate varies among independently derived cell preparations, in three series, an identical trend in Hp-dependent cytokine regulation was determined.
Taken together, these results indicate that systemic Hp supports DC-dependent execution of the immune cell regulation. Moreover, the presence of Hp at the level of DC seems to be necessary to educate resident lymphocytes to combat tumor cell targets. Finally, the gain of function by addition of Hp provides evidence for a direct action of Hp on immune cells and thus, implies that the phenotype that is observed with the Hp−/− mice is a result of loss of immune cell regulatory activity of Hp.
DTH response is reduced in Hp−/− mice
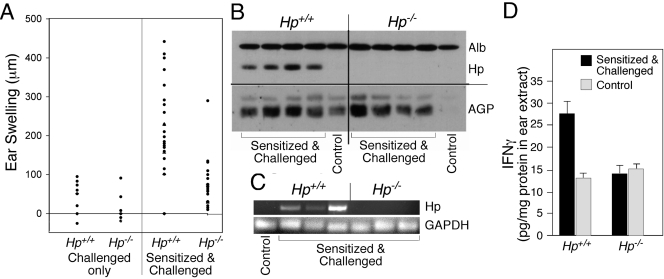
To test for the effect of Hp deficiency on DC- and Th1-dependent immune functions in vivo, a DTH assay was selected (Fig. 5). Hp−/− and Hp+/+ animals were sensitized and challenged with DNFB, and the degree of swelling of the ear tissue was used as an indicator for the impact of Hp deficiency (Fig. 5A). Hp+/+ animals presented the characteristic swelling reaction, whereas ear swelling in Hp−/− animals was marginal. Immunohistochemical analysis indicated the presence of CD4+ and CD8+ cells in challenged ear tissue from Hp−/− and Hp+/+ animals (data not shown), suggesting that the low DTH reaction in Hp−/− animals might be attributable to an insufficient, local activation of lymphocytes. Although DNFB treatment is considered to be a mild tissue irritation, it was sufficient to enhance the hepatic expression of APP (data not shown), and the presence of plasma Hp was also identified in extracts of ear tissue (Fig. 5B). Moreover, the local expression of Hp mRNA in the sensitized and challenged ear tissue could be confirmed by RNA analysis (Fig. 5C). These results indicate that the combination of Hp and inflammatory cells coexists at the challenged tissue site and that Hp could be a determining factor in the activity of effector cells, such as T cells. Indeed, extracts from ear tissue demonstrated elevated levels of IFN-γ in samples from Hp+/+ but not Hp−/− mice (Fig. 5D).
Figure 5.
Hp deficiency suppresses a DTH reaction. (A) Ear swelling was determined on Hp+/+ or Hp−/− mice 24 h after challenge with DNFB as a function of phenotype and prior sensitization. (B) Western blot analysis ear lysates from Hp+/+ and Hp−/− mice sensitized and challenged indicate the presence of Hp and AGP proteins. Albumin (Alb) is shown to demonstrate equal loading of serum proteins. Each lane represents an independent, individual mouse. Lanes labeled as “Control” represent extracts of animals with challenge only. (C) RNAs extracted from ear tissues from Hp+/+ or Hp−/− mice 24 h after a challenge were determined by RT-PCR for the expression of Hp and GAPDH mRNAs. Each lane represents an independent, individual mouse. (D) Extracts from ear tissue from Hp+/+ or Hp−/− mice 24 h after challenge were used to quantify the concentration of IFN-γ (means±sd, n=4).
T cell activation is attenuated in Hp−/− animals
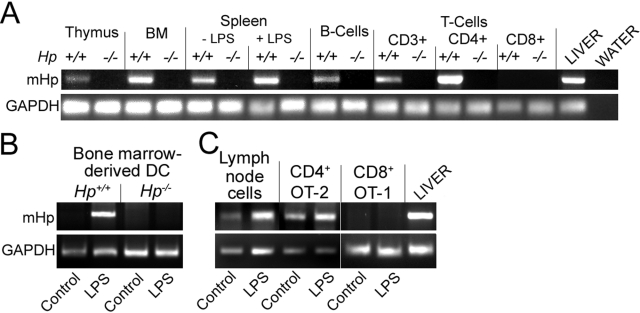
Taken together, the described in vivo analyses implied that Hp deficiency affects various aspects of adaptive immune response, but they also suggested that lymphocyte proliferation, activation, and differentiation appear to be the most prominent target of Hp action. This regulatory effect can potentially be exerted by Hp produced in the liver or at sites of immune cell regulation. Transcript analyses indicated that each lymphoid organ expressed Hp mRNA and that some of this expression was attributable to specific cell types, including DC (and monocytes) and CD4+ T cells (Fig. 6A). In contrast, CD8+ cells proved to be consistently negative for Hp expression. Moreover, Hp expression was inducible by endotoxin in monocytes and DC (Fig. 6B) but not in lymphocytes that already have an appreciable basal expression (Fig. 6C). These data suggest that local production of Hp, through autocrine or paracrine mechanisms, could serve as a possible effector and thus, in part, explain the result of the immunization with DC (Fig. 4A).
Figure 6.
Hp mRNA is expressed in lymphoid organs and in a subset of lymphoid cells. The indicated organs and hematopoietic cell types were prepared from 12-week-old mice. In selected cases, the animals received a LPS injection 24 h prior to organ collection. RNAs were extracted from intact organs after isolation of the cells (A), or from cell cultures treated in vitro for 24 h with 10 μg/ml LPS (B and C) and analyzed by RT-PCR for Hp and GAPDH. BM, Bone marrow.
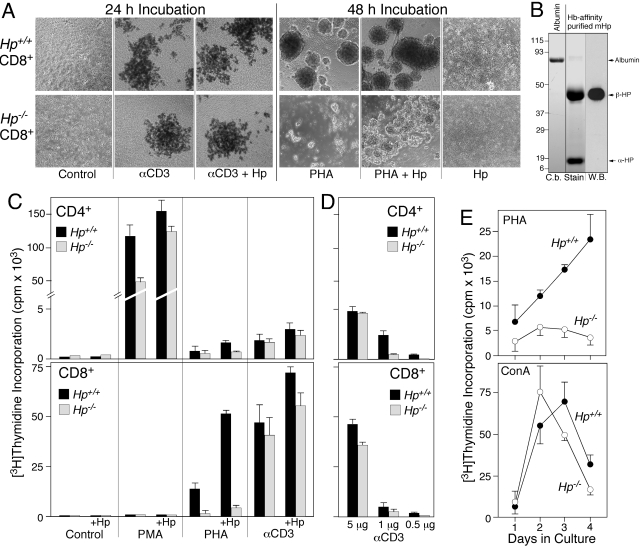
This proposal of Hp affecting lymphocyte proliferation was tested in tissue culture. Treatment of naive lymphocytes with broad-range agonists, such as lectins, PMA, αCD3, or antigen, generally stimulates proliferation. Lymphocytes derived from Hp-deficient mice consistently demonstrated a reduced, proliferative response compared with wild-type (Fig. 7). CD8+ cells from Hp−/− mice formed smaller blasts in response to PHA (Fig. 7A) and were less responsive to the mitogen in terms of proliferation as compared with wild-type (Fig. , 7Cand 7E, upper panel). When the cells were treated with anti-CD3, stimulation of proliferation was dose-dependent. At a high concentration, a similar, maximal level of proliferation was attained for the cell from Hp-deficient and wild-type mice. In contrast, at a lower dose, thymidine incorporation was much less in cells from Hp-deficient mice (Fig. 7, A, C, and D). Similarly, the strong mitogenic action of Con A was able to produce an equally high level of DNA synthesis in CD8 cells from either genotype (Fig. 7E).
Figure 7.
T cells from Hp-deficient mice show an altered response to mitogens. T cells from Hp-deficient mice show an altered response to mitogens (A), microphotographs (4×) of purified CD8 cells isolated from Hp+/+ and Hp−/− C57BL/6J mice treated for 24 h with anti-αCD3 (αCD3), or for 48 h with PHA. Selected cultures also included 1 mg/ml mouse Hp. (B) Coomassie blue-stained SDS-PAGE gel (C.b. Stain) demonstrates purity of Hp (8 μg) isolated from acute phase serum of Hp+/+ C57BL/6J. A duplicate lane was used for Western blotting (W.B.) reacted with rabbit anti-Hp (recognizes only epitopes in the β-subunit). (C and D) [3H]Thymidine incorporation was determined in cultures of purified CD4 and CD8 cells that had been maintained in the presence of the indicated mitogen for 72 h. (E) DNA synthesis was determined in nonfractionated splenocytes from Hp+/+ and Hp−/− mice as a function of duration in culture in the presence of PHA or Con A.
For CD4+ cells, the prominent growth response to PMA/ionomycin was twofold lower in cells from Hp−/− mice as compared with Hp+/+ mice (Fig. 7C). Although treatments of CD4+ cells with PHA and αCD3 were less effective than PMA in stimulating proliferation, the response of Hp−/− cells was consistently lower (Fig. , 7Cand 7D). These results suggested that T lymphocytes activated in the absence of Hp have a reduced ability to sustain elevated levels of proliferation and that this deficiency may have some specificity in regards to the strength and mode of activation.
To determine whether Hp would rectify the abnormal cellular phenotype of T cells from Hp−/− mice, purified Hp (Fig. 7B) was added at a mid-acute phase concentration of 1 mg/ml to cultures of CD4+ or CD8+ cells (Fig. , 7Aand 7C). Incubation with Hp alone did not detectably affect the cultures. In combination with PHA, however, Hp enhanced growth of Hp−/− CD8+ cells by threefold. Yet, this level of activation was still a fraction of that seen for Hp+/+ cells (Fig. , 7Aand 7C). Hp added to CD4+ cells treated with PMA enhanced the proliferation up to twofold and restored proliferation to close to normal levels (Fig. 7C). These data support the notion that Hp can assume a costimulatory function on T cells. Furthermore, differentiation of CD8+ T cells in Hp-deficient mice led to an altered response pattern that in the case of PHA-mediated activation, could not be simply restored to normal levels by exogenous Hp. Important is that none of the treatments led to a preferential cell death of Hp−/− cells (data not shown).
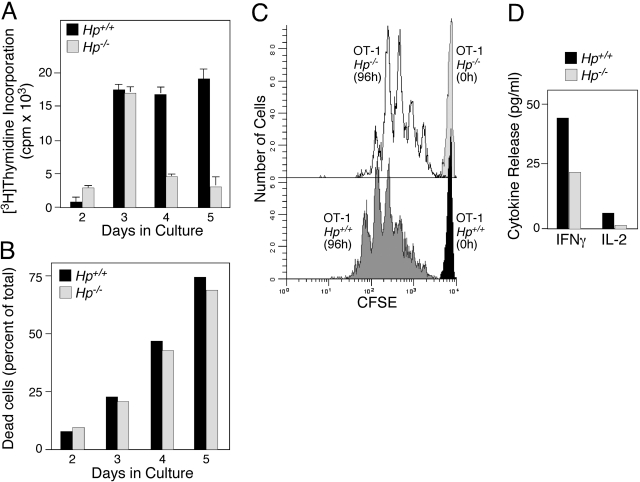
To evaluate whether activation of CD8+ cells through TCR-specific signals was also the target for alteration in Hp−/− mice, we generated TCR transgenic (OT-1) Hp−/− mice. OT-1 CD8+ cells were isolated from Hp−/− and Hp+/+ animals, labeled with CFSE, and activated by coculture in the presence of adherent BOK fibroblasts presenting H-2KB-SIINFEKL and B7.1 (Fig. 8). Thymidine incorporation was stimulated to the same extent within the first 3 days in Hp-deficient and wild-type OT-1 cells. DNA synthesis was maintained at elevated levels in Hp+/+ cells, whereas it abruptly declined in Hp−/− cells during the subsequent 2 days (Fig. 8A). As the same cell cultures from both genotypes did not show a detectable difference in the apoptotic rate (Fig. 8B), the dilution of a CFSE label as a function of cell division (Fig. 8C) indicated that Hp−/− OT-1 cells underwent fewer cell divisions, yielded smaller cultures, and released proportionally less cytokines such as IFN-γ and IL-2 (Fig. 8D). These data support the conclusion that CD8+ T cells obtained from Hp−/− mice have a reduced proliferative capacity in vitro, not only in response to broadly acting mitogens as determined in Figure 7 but also to antigen-specific TCR ligands, which may be a consequence of reduced autocrine IL-2 production. These in vitro studies support the notion that Hp at the level of T cells is necessary for proper activation and proliferation and may partially account for the phenotypes observed in vivo.
Figure 8.
Attenuated response of CD8+ T cells from Hp−/− mice. (A) CD8+ OT-1 cells were isolated from spleens of Hp+/+ or Hp−/− C57BL/6J background and cultured in the presence of irradiated BOK cells presenting H-2Kb-SIINFEKL and B7.1. DNA synthesis ([3H]Thymidine Incorporation) at Days 2–5 was determined. (B) The relative presence of apoptotic cells in the cultures at the times indicated was determined by flow cytometric analysis using labeling with Annexin V-FITC and PI. (C) CFSE-labeled, naive OT-1 T cells from Hp+/+ or Hp−/− at the onset and after 96 h stimulation by coculture with BOK cells were analyzed by flow cytometry. (D) OT-1 T cells from Hp+/+ or Hp−/− were stimulated by coculture with BOK cells for 72 h, and conditioned medium was analyzed for concentration of IFN-γ and IL-2.
DISCUSSION
Hp in inflammation and the immune response
APPs are generally viewed as being relevant in providing protective functions at sites of tissue injury such as containment of tissue degradation, facilitation of wound healing, and restoration of homeostasis. Hp fulfills this role by restricting oxidation via sequestration of free Hb, acting as a powerful antioxidant [17], inhibiting cathepsin, serving as a chaperone, and assisting in iron recycling [18]. Acute phase-induced Hp reaches maximal systemic levels between 1 and 2 days following initiation of the inflammatory insult (Fig. 2), a time-point considered to be relevant for replenishing the depleted systemic Hp pool, supporting the resolution of inflammation [19,20,21], and activation of immune cells (Fig. 3) [22].
Acute phase-induced expression of Hp represents a substantial change in the extracellular milieu, one that is required for tissue repair and immune cell regulation. Hence, it seems reasonable to propose that Hp acts directly on innate and adaptive immune cells at local and systemic levels. Studies performed in vivo and in tissue culture [23, 24] indicated that purified Hp exerts a moderate inhibitory activity on T cells [8, 25, 26] but a stimulatory activity on myeloid cells [27]. These effects combined have been interpreted to be anti-inflammatory and immune-suppressive in nature [28,29,30]. As a result of the variability in purity and presence of contaminating irritants in commercially available human Hp, confirmation of the suggested activities of Hp activity under a correct physiological setting in vivo has been elusive (Y. Wang, unpublished).
Hp-deficient mice as reporters of Hp function
In the current study and unconstrained by a preset working model, the in vivo role of Hp in linking APR and immune response was assessed by defining the effects of Hp deficiency in Hp−/− mice. The data demonstrate that the absence of Hp is associated with the development of smaller lymphoid organs and immature B cells in the bone marrow and T cells in the thymus (Table 2). Although the differences may appear modest, they are associated with a significant reduction of the steady-state pool of mature lymphocytes (Tables 1and 2). The most striking consequence of Hp deficiency is the reduced T and B cell-mediated immune responses. These data suggest a less-effective communication from the APCs to the CD4 and CD8 compartments. T cells that have undergone differentiation in the absence of Hp fail to proceed to complete maturation and to fully carry out their effector functions. These defects can be corrected in part by treatment of isolated cells with the plasma form of mouse Hp in vitro. Yet, Hp expression by hematopoietic cells in vivo appears to be required for an optimal immune response (Figs. 345).
These data support the interpretation that Hp has regulatory effects at various levels of immune compartment development and function of the cells that inhabit these compartments. The findings also permit the conclusion that the most prominent action rests in the growth control of lymphocytes as part of homeostasis and following antigen stimulation. This leads to the current proposed model that Hp is one of the principle intermediaries between APR and immune responses by acting as a coactivator of T cells. This activity is perceived to be particularly relevant in conjunction with the moderate level of signaling elicited by antigen presentation and TCR action (Figs. 7and 8), resulting in graded expansion of effector cells and communication to B cells. We also hypothesize that the temporal profile of Hp action on the immune cells is governed by its tissue-specific and inflammation-induced expression, its contextual presentation to the target cells, and the ability of the target cells to recognize and to respond to it. The aim of future work will be delineation of the Hp-directed regulatory mechanisms.
Until now, a potential role of Hp in regulating the immune response during the inflammatory reaction has not been recognized. This is largely a result of the fact that Hp is invariably present and often represents a major constituent of the plasma, lymph, and other extravascular fluids. In humans, only few cases of Hp null patients have been described, which do not present overt immune deficiencies [31, 32]. Based on evolutionary considerations, the essential function of Hp in vertebrates is underscored by the strict conservation of its structure and function. Among mammals, no species is known to lack the Hp gene or its acute phase regulation. Our results propose that Hp has two modes of action on the immune system: Hp expression per se contributes to the development and differentiation of immune organs, and inflammation-induced Hp assists in executing an optimal immune response. The application of Hp knockout mice has provided compelling evidence in support of both aspects.
Although the direct action of Hp onto immune cells could be demonstrated by a gain-of-function approach in vitro (Fig. 7), the corresponding approach in vivo has not yet been technically feasible. The alternative approach by transplantation of immune cells identified as being able to express Hp (Fig. 6) suggested a contribution of Hp derived from hematopoietic and nonhematopoietic (primarily liver-derived) Hp (Fig. 4). At present, we cannot formally rule out that a low level of free Hb, through oxidative action at the site of lymphocyte differentiation and regulation, also contributes to the immune response observed in Hp−/− animals. In part, as a result of maintenance of the animals in a pathogen- and stress-free environment, the probability of hemolytic reactions during immunization reactions used in Figures 345 is kept at a minimum. Moreover, oxidative reactions associated with T cell activation had been considered to add to the activation process [33, 34]. Yet, in our system, if free Hb were effective as an oxidant on immune cells, it was unable to elicit a measurable immune response.
Cellular action of Hp
In the future, two major points will need to be addressed: the identification of the sources of Hp that is effective in directing immune cell development and immune response and the mode of action of Hp in targeting lymphocytes. Although it is evident that the bulk of Hp present in animals undergoing experimental inflammation is derived from the liver, the origin of Hp in mice that are not undergoing an inflammation appears to be diverse with contribution from phagocytes, CD4+ T cells, and epithelial/stromal cells [35,36,37] (Figs. 1, 5, and 6). Biochemical analysis of Hp synthesized and secreted by phagocytes and epithelial cells has shown structural differences from that processed by hepatocytes, including distinct glycosylation patterns present on the β-subunit (K. M. Huntoon, unpublished). Currently, however, it is not known whether the structural differences correlate with distinct functions. Nevertheless, structural differences present with the two allelic forms of human Hp, Hp1 and Hp2 [38, 39], demonstrate functional consequences on progression of chronic disease. Prominent expression of Hp in DC and CD4+ cells (Fig. 6) and enhanced immune response via these cell types (Fig. 4A) suggest a functional contribution of nonhepatic Hp to the overall outcome of immunization. Nonhepatic Hp forms have not been detected in the circulation, suggesting that Hp made by leukocytes is restricted to the site of protein production, where it may act as autocrine and/or paracrine component and/or is subject to rapid clearance. Potential paracrine targets could be naïve CD8+ T cells that do not express Hp.
Membrane proteins on numerous cell types can serve as binding proteins for Hp; those described thus far are the CD11b/CD18 integrin on monocytic cells, CD163 scavenger receptor on macrophages or as a soluble component shed from these, and CD22 lectin on B cells [18]. Interactions between these cell surface molecules and Hp have been associated with changes in cell proliferation, expression of stimulated cytokine genes, and cell motility [8, 40]. A specific Hp receptor for T cells has not yet been described. However, for Hp to be effective, particularly at a peak acute phase level, a low-affinity binding to various cell surface glycoconjugates may suffice for triggering a signaling pathway and/or a cellular response. As evident from the stimulation experiments (Figs. 7and 8), T cells derived from Hp−/− animals are already programmed to respond differently to Hp. At present, it is not known whether this is a result of altered cell surface components or to altered downstream signaling activity.
The action of Hp in immune responsiveness was defined by the effects of Hp deficiency as compared with wild-type C57BL/6 mice. For plasma Hp to be effective in wild-type animals, in particular, those kept in a pathogen- and stress-free environment, its expression needs to be increased from the very low (<50 μg/ml) basal level (Fig. 2). Normally, Hp expression is induced by an IL-6-mediated process, which inevitably occurs as part of inflammatory reactions or also part of immunization (Fig. 3) or DTH reactions (Fig. 5). As Hp-inducing cytokines are themselves effective modulators of immune cells, a dual effect of cytokines may be in operation: a direct effect on target cells, coupled with an indirect effect via Hp induction. Considering the timeframe of events that leads to an increased presence of Hp in vivo, Hp will act on target cells that have been exposed to the same inflammatory cytokines that induced Hp expression.
In all, the current study suggests a functionally relevant link between an APP and immune regulation. The data derived from the immunization model suggest an immune-supporting action of Hp. In view of this immune regulatory function, Hp may also prove to be relevant in directing disease progression such as chronic degenerative diseases and cancer.
Acknowledgments
This work was supported by the National Institutes of Health Grants DK033886 and P30CA16056 (Roswell Park Cancer Institute’s Cancer Center Support Grant). We thank Dr. James Clements for critically reading this manuscript and histology core for H&E stain organ sections.
References
- Jayle M. F., Boussier G., Badin J. Electrophoresis of haptoglobin and of its hemoglobin complex. Bull Soc Chim Biol (Paris) 1952;34:1063–1069. [PubMed] [Google Scholar]
- Wicher K. B., Fries E. Haptoglobin, a hemoglobin-binding plasma protein, is present in bony fish and mammals but not in frog and chicken. Proc Natl Acad Sci USA. 2006;103:4168–4173. doi: 10.1073/pnas.0508723103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobryszycka W. Biological functions of haptoglobin—new pieces to an old puzzle. Eur J Clin Chem Clin Biochem. 1997;35:647–654. [PubMed] [Google Scholar]
- Wang Y., Kinzie E., Berger F. G., Lim S. K., Baumann H. Haptoglobin, an inflammation-inducible plasma protein. Redox Rep. 2001;6:379–385. doi: 10.1179/135100001101536580. [DOI] [PubMed] [Google Scholar]
- Oh S. K., Ross S., Walker J., Zeisel S. Role of a SER immune suppressor in immune surveillance. Immunology. 1988;64:73–79. [PMC free article] [PubMed] [Google Scholar]
- Israel L., Samak R., Edelstein R., Bogucki D., Breau J. L. Immunosuppressive effects of acute phase reactant proteins. Physiopathological role in cancer patients Ann. Med. Interne (Paris) 1981;132:26–29. [PubMed] [Google Scholar]
- Xie Y., Li Y., Zhang Q., Stiller M. J., Wang C. L., Streilein J. W. Haptoglobin is a natural regulator of Langerhans cell function in the skin. J Dermatol Sci. 2000;24:25–37. doi: 10.1016/s0923-1811(00)00078-5. [DOI] [PubMed] [Google Scholar]
- Arredouani M., Matthijs P., Van Hoeyveld E., Kasran A., Baumann H., Ceuppens J. L., Stevens E. Haptoglobin directly affects T cells and suppresses T helper cell type 2 cytokine release. Immunology. 2003;108:144–151. doi: 10.1046/j.1365-2567.2003.01569.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim S. K., Kim H., Lim S. K., bin Ali A., Lim Y. K., Wang Y., Chong S. M., Costantini F., Baumman H. Increased susceptibility in Hp knockout mice during acute hemolysis. Blood. 1998;92:1870–1877. [PubMed] [Google Scholar]
- Barbour K. W., Davis T., White A., Baumann H., Berger F. G. Haptoglobin, inflammation, and tumorigenesis in the MIN mouse. Redox Rep. 2001;6:366–368. doi: 10.1179/135100001101536553. [DOI] [PubMed] [Google Scholar]
- Hogquist K. A., Jameson S. C., Heath W. R., Howard J. L., Bevan M. J., Carbone F. R. T cell receptor antagonist peptides induce positive selection. Cell. 1994;76:17–27. doi: 10.1016/0092-8674(94)90169-4. [DOI] [PubMed] [Google Scholar]
- Shrikant P., Mescher M. F. Control of syngeneic tumor growth by activation of CD8+ T cells: efficacy is limited by migration away from the site and induction of nonresponsiveness. J Immunol. 1999;162:2858–2866. [PubMed] [Google Scholar]
- Chen Z. M., Jenkins M. K. Clonal expansion of antigen-specific CD4 T cells following infection with Salmonella typhimurium is similar in susceptible (Itys) and resistant (Ityr) BALB/c mice. Infect Immun. 1999;67:2025–2029. doi: 10.1128/iai.67.4.2025-2029.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore M. W., Carbone F. R., Bevan M. J. Introduction of soluble protein into the class I pathway of antigen processing and presentation. Cell. 1988;54:777–785. doi: 10.1016/s0092-8674(88)91043-4. [DOI] [PubMed] [Google Scholar]
- Van Stipdonk M. J., Lemmens E. E., Schoenberger S. P. Naive CTLs require a single brief period of antigenic stimulation for clonal expansion and differentiation. Nat Immunol. 2001;2:423–429. doi: 10.1038/87730. [DOI] [PubMed] [Google Scholar]
- Miale J. B., Kent J. W. Serum haptoglobin in rabbits after subcutaneous injection of Freund’s adjuvant or turpentine. Proc Soc Exp Biol Med. 1962;111:589–590. doi: 10.3181/00379727-111-27863. [DOI] [PubMed] [Google Scholar]
- Tseng C. F., Lin C. C., Huang H. Y., Liu H. C., Mao S. J. Antioxidant role of human haptoglobin. Proteomics. 2004;4:2221–2228. doi: 10.1002/pmic.200300787. [DOI] [PubMed] [Google Scholar]
- Wassell J. Haptoglobin: function and polymorphism. Clin Lab. 2000;46:547–552. [PubMed] [Google Scholar]
- Tilg H., Dinarello C. A., Mier J. W. IL-6 and APPs: anti-inflammatory and immunosuppressive mediators. Immunol Today. 1997;18:428–432. doi: 10.1016/s0167-5699(97)01103-1. [DOI] [PubMed] [Google Scholar]
- Tilg H., Vannier E., Vachino G., Dinarello C. A., Mier J. W. Antiinflammatory properties of hepatic acute phase proteins: preferential induction of interleukin 1 (IL-1) receptor antagonist over IL-1 β synthesis by human peripheral blood mononuclear cells. J Exp Med. 1993;178:1629–1636. doi: 10.1084/jem.178.5.1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabay C., Kushner I. Acute-phase proteins and other systemic responses to inflammation. N Engl J Med. 1999;340:448–454. doi: 10.1056/NEJM199902113400607. [DOI] [PubMed] [Google Scholar]
- Carroll M. C. The complement system in regulation of adaptive immunity. Nat Immunol. 2004;5:981–986. doi: 10.1038/ni1113. [DOI] [PubMed] [Google Scholar]
- Asleh R., Levy A. P. In vivo and in vitro studies establishing haptoglobin as a major susceptibility gene for diabetic vascular disease. Vasc Health Risk Manag. 2005;1:19–28. doi: 10.2147/vhrm.1.1.19.58930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guetta J., Strauss M., Levy N. S., Fahoum L., Levy A. P. Haptoglobin genotype modulates the balance of Th1/Th2 cytokines produced by macrophages exposed to free hemoglobin. Atherosclerosis. 2007;191:48–53. doi: 10.1016/j.atherosclerosis.2006.04.032. [DOI] [PubMed] [Google Scholar]
- Arredouani M., Matthys P., Kasran A., Baumann H., Ceuppen J. L. Haptoglobin and the Th1/Th2 balance: hints from in vitro and in vivo studies. Redox Rep. 2001;6:369–371. doi: 10.1179/135100001101536481. [DOI] [PubMed] [Google Scholar]
- Arredouani M. S., Kasran A., Vanoirbeek J. A., Berger F. G., Baumann H., Ceuppens J. L. Haptoglobin dampens endotoxin-induced inflammatory effects both in vitro and in vivo. Immunology. 2005;114:263–271. doi: 10.1111/j.1365-2567.2004.02071.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theilgaard-Monch K., Jacobsen L. C., Nielsen M. J., Rasmussen T., Udby L., Gharib M., Arkwright P. D., Gombart A. F., Calafat J., Moestrup S. K., Porse B. T., Borregaard N. Haptoglobin is synthesized during granulocyte differentiation, stored in specific granules, and released by neutrophils in response to activation. Blood. 2006;108:353–361. doi: 10.1182/blood-2005-09-3890. [DOI] [PubMed] [Google Scholar]
- Hellings P. W., Kasran A., Bullens D., Overbergh L., Mathieu C., Heremans H., Matthys P., Boon L., Jorissen M., Ceuppens J. L. IL-10- and IL-12-independent down-regulation of allergic sensitization by stimulation of CD40 signaling. J Immunol. 2006;177:5138–5144. doi: 10.4049/jimmunol.177.8.5138. [DOI] [PubMed] [Google Scholar]
- Hawiger J. Innate immunity and inflammation: a transcriptional paradigm. Immunol Res. 2001;23:99–109. doi: 10.1385/IR:23:2-3:099. [DOI] [PubMed] [Google Scholar]
- Meyts I., Hellings P. W., Hens G., Vanaudenaerde B. M., Verbinnen B., Heremans H., Matthys P., Bullens D. M., Overbergh L., Mathieu C., De Boeck K., Ceuppens J. L. IL-12 contributes to allergen-induced airway inflammation in experimental asthma. J Immunol. 2006;177:6460–6470. doi: 10.4049/jimmunol.177.9.6460. [DOI] [PubMed] [Google Scholar]
- Hayashi A., Wada Y., Suzuki T., Shimizu A. Studies on familial hypotransferrinemia: unique clinical course and molecular pathology. Am J Hum Genet. 1993;53:201–213. [PMC free article] [PubMed] [Google Scholar]
- Koda Y., Watanabe Y., Soejima M., Shimada E., Nishimura M., Morishita K., Moriya S., Mitsunaga S., Tadokoro K., Kimura H. Simple PCR detection of haptoglobin gene deletion in anhaptoglobinemic patients with antihaptoglobin antibody that causes anaphylactic transfusion reactions. Blood. 2000;95:1138–1143. [PubMed] [Google Scholar]
- Buttari B., Profumo E., Petrone L., Pietraforte D., Siracusano A., Margutti P., Delunardo F., Ortona E., Minetti M., Salvati B., Rigano R. Free hemoglobin: a dangerous signal for the immune system in patients with carotid atherosclerosis? Ann N Y Acad Sci. 2007;1107:42–50. doi: 10.1196/annals.1381.005. [DOI] [PubMed] [Google Scholar]
- Tse H. M., Milton M. J., Schreiner S., Profozich J. L., Trucco M., Piganelli J. D. Disruption of innate-mediated proinflammatory cytokine and reactive oxygen species third signal leads to antigen-specific hyporesponsiveness. J Immunol. 2007;178:908–917. doi: 10.4049/jimmunol.178.2.908. [DOI] [PubMed] [Google Scholar]
- Smeets M. B., Fontijn J., Kavelaars A., Pasterkamp G., De Kleijn D. P. The acute phase protein haptoglobin is locally expressed in arthritic and oncological tissues. Int J Exp Pathol. 2003;84:69–74. doi: 10.1046/j.1365-2613.2003.00336.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim I. S., Lee I. H., Lee J. H., Lee S. Y. Induction of haptoglobin by all-trans retinoic acid in THP-1 human monocytic cell line. Biochem Biophys Res Commun. 2001;284:738–742. doi: 10.1006/bbrc.2001.5041. [DOI] [PubMed] [Google Scholar]
- Yang F., Ghio A. J., Herbert D. C., Weaker F. J., Walter C. A., Coalson J. J. Pulmonary expression of the human haptoglobin gene. Am J Respir Cell Mol Biol. 2000;23:277–282. doi: 10.1165/ajrcmb.23.3.4069. [DOI] [PubMed] [Google Scholar]
- Blum S., Asaf R., Guetta J., Miller-Lotan R., Asleh R., Kremer R., Levy N. S., Berger F. G., Aronson D., Fu X., Zhang R., Hazen S. L., Levy A. P. Haptoglobin genotype determines myocardial infarct size in diabetic mice. J Am Coll Cardiol. 2007;49:82–87. doi: 10.1016/j.jacc.2006.08.044. [DOI] [PubMed] [Google Scholar]
- Nakhoul F. M., Miller-Lotan R., Awaad H., Asleh R., Levy A. P. Hypothesis–haptoglobin genotype and diabetic nephropathy. Nat Clin Pract Nephrol. 2007;3:339–344. doi: 10.1038/ncpneph0467. [DOI] [PubMed] [Google Scholar]
- De Kleijn D. P., Smeets M. B., Kemmeren P. P., Lim S. K., Van Middelaar B. J., Velema E., Schoneveld A., Pasterkamp G., Borst C. Acute-phase protein haptoglobin is a cell migration factor involved in arterial restructuring. FASEB J. 2002;16:1123–1125. doi: 10.1096/fj.02-0019fje. [DOI] [PubMed] [Google Scholar]