Abstract
Lipoteichoic acid (LTA) is a central inducer of inflammatory responses caused by Gram-positive bacteria, such as Staphylococcus aureus, via activation of TLR2. Localization of TLR2 in relation to its coreceptors may be important for function. This study explores the signaling, uptake, and trafficking pattern of LTA in relation to expression of TLR2 and its coreceptors CD36 and CD14 in human monocytes. We found TLR2 expressed in early endosomes, late endosomes/lysosomes, and in Rab-11-positive compartments but not in the Golgi apparatus or endoplasmic reticulum (ER). Rapid internalization of fluorescently labeled LTA was observed in human monocytes, colocalizing with markers for early and late endosomes, lysosomes, ER, and Golgi network. Blocking CD14 and CD36 with antibodies inhibited LTA binding and LTA-induced TNF release from monocytes, emphasizing an important role for both molecules as coreceptors for TLR2. Importantly, blocking CD36 did not affect TNF release induced by N-palmitoyl-S-[2,3-bis(palmitoyloxy)-(2R,S)-propyl]-(R)-cysteinyl-seryl-(lysyl)3-lysine or LPS. Expression of CD14 markedly enhanced LTA binding to the plasma membrane and also enhanced NF-κB activation. LTA internalization, but not NF-κB activation, was inhibited in Dynamin-I K44A dominant-negative transfectants, suggesting that LTA is internalized by receptor-mediated endocytosis but that internalization is not required for signaling. In fact, immobilizing LTA and thereby inhibiting internalization resulted in enhanced TNF release from monocytes. Our results suggest that LTA signaling preferentially occurs at the plasma membrane, is independent of internalization, and is facilitated by CD36 and CD14 as coreceptors for TLR2.
Keywords: TLR, LTA, endocytosis
INTRODUCTION
Gram-negative and Gram-positive bacteria, as well as viruses and fungi, induce proinflammatory responses that can cause fatal sepsis syndrome [1, 2]. Staphylococcus aureus is the most commonly isolated infectious Gram-positive pathogen, and strains are rapidly becoming resistant to nearly all current antibiotics [3]. Although LPS from Gram-negative bacteria is suggested as a principal inducer of Gram-negative septic shock [4, 5], lipoteichoic acid (LTA) may be an equivalent component responsible for septic shock provoked by Gram-positive bacteria [6].
TLRs recognize a range of pathogen-associated molecular patterns (PAMPs), such as LPS and LTA. TLRs further initiate proinflammatory responses required for clearance of infection by the same mechanisms that potentially cause sepsis [7]. Thirteen mammalian TLRs (TLR1–13), which recognize different PAMPs, have been identified to date [7]. These are germ-line-encoded transmembrane proteins, consisting of an extracellular leucine-rich repeat domain and a cytoplasmic domain sharing homology with the mammalian IL-1R [8, 9]. A signaling cascade initiated by activation of the TLRs results in translocation of the transcription factor NF-κB, which subsequently induces the expression of TNF, IL-1β, IL-6, and IL-8 and maturation of APCs [10]. Although TLR4, in complex with the small, secreted glycoprotein myeloid differentiation protein 2 (MD2), recognizes LPS from Gram-negative bacteria [11, 12], TLR2 recognizes a particularly broad range of ligands, including Gram-positive bacteria and cell-wall components such as LTA as well as peptidoglycan and lipoproteins [13,14,15,16,17,18]. Additional TLR2 ligands may include zymosan, glycolipids from spirochetes,lipoarabinomannan, and porins from Neisseria, among others [19]. The ability of TLR2 to recognize such a wide repertoire of ligands is partially explained by heterodimerization of TLR2 with TLR1 and TLR6. TLR2/TLR1 heterodimerization occurs in response to triacylated lipopeptides, such as N-palmitoyl-S-[2,3-bis(palmitoyloxy)-(2R,S)-propyl]-(R)-cysteinyl-seryl-(lysyl)3-lysine (Pam3CysSK4) [20, 21], and an optimal response toward diacylated lipopeptides is attained by heterodimerization of TLR2 with TLR6 [22, 23]. LTA is recognized by the TLR2/TLR6 heterodimer, supposedly as a result of the two diacyl chains in the molecule [24].
The monocyte differentiation antigen CD14 is a GPI-linked receptor expressed by cells of the monocytic lineage [25, 26]. The receptor is shown to be highly concentrated in lipid raft microdomains of these cells [27]. Soluble CD14 and LPS-binding protein in serum transfer LPS to membrane-bound CD14, which further presents LPS to the TLR4/MD2 signaling complex [28,29,30,31]. The entire complex has further been shown to shuttle between the plasma membrane and the Golgi, independent of signaling, which is believed to occur predominantly at the plasma membrane [32, 33]. CD14 has further been shown to bind lipopeptides and LTA in a similar manner [34,35,36]. The multifunctional B class scavanger receptor CD36 has, however, also been found to be involved in immune responses to TLR2/TLR6 ligands such as LTA in a manner analogous to CD14 [37]. Whether LTA binding to the plasma membrane is sufficient to induce signaling through TLR2 or whether internalization of the ligand is required is still under debate. Although some reports support that signaling occurs in lipid rafts, independent of ligand internalization [36, 38], other reports show that reduced internalization of S. aureus and its component LTA correlates with diminished inflammatory response [39]. The relative role of CD14 and CD36 in response to LTA is furthermore in question with regard to whether the coreceptors participate in the same TLR2/TLR6 signaling complex or whether they enhance TLR2-mediated responses independent of one another.
In this study, we explored the uptake and trafficking pattern of LTA from S. aureus in relation to subcellular expression of TLR2 and its coreceptors CD36 and CD14 in human monocytes. We found TLR2 expressed in the plasma membrane, endosomes, lysosomes, and in Rab-11-positive compartments but not in the Golgi apparatus or the endoplasmic reticulum (ER). LTA rapidly accumulated in early and late endosomes, lysosomes, as well as in the ER and Golgi. CD14 and CD36 were required for optimal LTA binding/internalization and TNF release in monocytes. We further found that LTA internalization, but not NF-κB activation, was inhibited in Dynamin-I K44A dominant-negative transfectants, showing that LTA is internalized by receptor-mediated endocytosis but that internalization is not required for signaling. These results support the hypothesis that signaling in response to LTA preferentially occurs at the plasma membrane, is independent of internalization, and requires CD36 and CD14 as coreceptors for TLR2.
MATERIALS AND METHODS
Reagents
Tissue-culture medium, trypsin/EDTA, penicillin, streptomycin, and PBS were obtained from BioWhittaker (Walkersville, MD, USA). Culture medium was supplemented with 2 mM L-glutamine and 10 μg/ml ciprofloxacin (Cellgro/Mediatech, Herndon, VA, USA, or from BioWhittaker). G418 was purchased from Calbiochem (San Diego, CA, USA) and Life Technologies (Gaithersburg, MD, USA). Low endotoxin FBS was purchased from Hyclone (Logan, UT, USA) and Integro (Zaandam, The Netherlands). LTA from S. aureus was prepared by butanol extraction as described [16]. The purity of LTA was over 99%, measured by nuclear magnetic resonance and mass spectrometry [16]. Endotoxin contamination was minimal (<0.1 pg/μg), measured by negative Limulus amoebocyte lysate assay, QCL-1000 (Charles River Endosafe, Charleston, WV, USA). Fluorescein (FITC)- and rhodamine-conjugated LTA was prepared by sonifying LTA from S. aureus (3 mg) and FITC-5 or sulforhodamine Q 5 acid fluoride (4.5 mg, Fluka, Buchs, Switzerland), DMSO (2.5 ml, Wak-Chemie-Medical GmbH, Steinbach, Germany), and trimethylamine (25 μl, Acros Organics, Leicestershire, UK) for 10 min and then shaken overnight at 37°C. The mixture was further spun at 7000 g for 90 min at room temperature four times in a pyrogen-free centrifugal ultrafilter unit (cut-off 3 kDa, Microsep 3K Centricons, Pall Corp., Ann Arbor, MI, USA) and additionally filtered through a PD-10 desalting column (Amersham Biosciences, Freiburg, Germany). The yield of labeled LTA was determined by phosphate content, measured by the molybdenum blue method; LTA solution (50 μl) was mixed with ashing solution [H2SO4:HClO4:H2O (556:105:3339, v:v:v; 200 μl)] and incubated at 145°C for 2 h. Reducing solution [ascorbic acid:ammoniumheptamolybdenum sodium acetate (1:9, v:v; 1 ml)] was subseqently added prior to incubation at 50°C for 2 h. Absorption was measured at 700 nm. Labeling efficiency, calculated as fluorescence (560 nm/620 nm) per phosphate content, was ∼1 molecule rhodamine or fluorescein per LTA. The labeled LTA was negative in the Limulus test for Gram-negative endotoxin (<0.1 pg/μg). LPS was from Escherichia coli strain O111:B4 and purchased from Invivogen (San Diego, CA, USA). Synthetic Pam3CysSK4 was purchased from EMC Microcollections (Tübingen, Germany). Antibodies used were anti-TLR2 (TL2.1) [18], anti-TLR4 (HTA125), purified from hybridoma cells, kindly provided by Dr. Kensuke Miyake (Saga Medical School, Japan) [40], unconjugated and FITC-conjugated anti-CD36 (FA6-152; Immunotech, France), anti-CD14 mAb 3C10 [41] and 5C5 [42], and MEM-18 (HyCult Biotechnology, Uden, The Netherlands). Additional antibodies used include mouse IgG and a Tricolor (PE-Cy5)-conjugated goat anti-mouse secondary antibody (Invitrogen, Carlsbad, CA, USA), anti-lysosome-associated membrane protein-1 (LAMP-1; R&D Systems, Minneapolis, MN, USA), anti-Golgin-97 (CDF4; Invitrogen), anti-Rab-11A (Abcam, UK), anti-GM130, anti-early endosomal antigen-1 (Eea-1), and unconjugated and FITC-conjugated anti-calnexin and mouse IgG (BD Biosciences, Franklin Lakes, NJ, USA). Alexa-conjugated antibodies were generated by protein labeling with Alexa 488 (A488), A546, or A647, according to the manufacturer’s instructions (Invitrogen). A633-labeled transferrin was purchased from Invitrogen. The following expression vectors were used: pcDNA3 (Invitrogen), human CD14 and TLR2-yellow fluorescent protein in pcDNA3 [43], and Eea-1 tagged with GFP (Eea-1GFP) [44]. The expression vector pORF9 and human CD36 in pORF9 were purchased from Invivogen. MD-2 in the expression vector pEF-BOS was kindly provided by Dr. K. Miyake [45]. ERGFP encoding the ER-targeting sequence of calreticulin fused to cyan fluorescent protein (CFP; Clontech, Palo Alto, CA, USA). Dynamin-I wild-type and Dynamin-I K44A in pcDNA3 were kindly provided by Dr. Sandy Schmid (The Scripps Research Institute, La Jolla, CA, USA). Transient transfections were performed using GeneJuice™ transfection reagent (Novagen, Darmstadt, Germany), according to the manufacturer’s instructions if not stated otherwise.
Cells and cell lines
Human monocytes were isolated from PBMC by adherence. PBMC were seperated from A+ buffy coats (Blood Bank, St. Olav’s Hospital Trondheim, Norway) using Lymphoprep, as described by the manufacturer (Axis-Shield, Norway). Monocytes were allowed to adhere in RPMI supplemented with 5% or 10% pooled A+ serum (St. Olav’s Hospital Trondheim) for 1 h at 37°C, 5% CO2, before cells were washed three times and fresh medium added. Human epithelial kidney 293 (HEK293) cell lines expressing TLR2 or TLR2 in combination with CD14 [43] were cultured in DMEM supplemented with 10% FBS and the selection antibiotic G418 (0.5 mg/ml). Untransfected HEK293 cells were cultured in 10% FBS/DMEM. For confocal imaging, cells were seeded on 35 mm glass-bottom γ-irradiated tissue cell dishes (MatTek Corp., Ashland, MA, USA). Madine Darby canine kidney (MDCK) cells stably expressing Eea-1GFP were grown in DMEM supplemented with 9% FBS, 2 mM glutamine, 25 U/ml penicillin, and 25 μg/ml streptomycin at 37°C, 6% CO2.
Live microscopy
MDCK cells stably expressing Eea-1GFP were transiently transfected with the TLR2 overnight in Microwell dishes for microscopy (MatTek Corp.) using Lipofectamin, according to the manufacturer’s protocol (Invitrogen). Preceding imaging the cells, microscopy medium, DMEM without phenol red, and sodium carbonate supplemented with 3.5 g/L D-glucose to a final concentration of 4.5 g/L and 25 mM HEPES with 10% FBS were added. Cells were kept on ice for 45 min and then incubated with A546-conjugated anti-TLR2 mAb TL2.1 on ice for an additional 45 min. Image acquisition was performed on an Olympus Fluoview 1000 at 37°C with an Olympus PlanApo 60×/1.42 oil objective. Internalization analysis was carried out with ImageJ software, measuring the intensity of conjugated TLR2 as a function of time.
Confocal microscopy of subcellular expression of TLR2 and LTA internalization
Freshly isolated, live monocytes were added fresh RPMI medium supplemented with 0.1% A+ serum, incubated with LTArhodamine (20 μg/ml), and internalization of the ligand was followed by confocal microscopy at 37°C for 1 h. Freshly isolated monocytes were left unstimulated or stimulated with LTArhodamine for 1 h, 37°C, 8% CO2, prior to fixating with 4% paraformaldehyde, 10 min on ice, and then with 0.5 M K-PIPES/0.5 M EGTA/1 M MgCl2buffer, 10 min, at room temperature. Cells were then permeabilized with 50 mM NH4Cl/0.05% BSA/0.05% saponin, 20 min, at room temperature. Cells were subsequently stained in 50 mM NH4Cl/0.05% BSA/0.05% saponin, 20 min, at room temperature. Cells were also stained with A488- or A647-conjugated anti-TLR2 and antibodies against early endosome marker Eea-1FITC, lysosome marker anti-LAMP-1A647, trans-Golgi marker anti-Golgin-97A647, and cis-Golgi marker anti-GM130, anti-CD36FITC, anti-CD14A647 (3C10), or anti-CD14A488 (5C5). Freshly isolated monocytes were additionally stained intracellularly, as described, with anti-Rab-11A and subsequently with secondary antibody goat anti-rabbitA647, prior to staining with TL2.1A488. HEK293 cells were stained intracellularly after fixing cells with 4% paraformaldehyde for 10 min on ice and permeabilization with 20% A+/0.1% saponin/PBS for 20 min at room temperature. Cells were stained in 2% A+/0.1% saponin/PBS for 45 min at room temperature using 2–10 μg/ml antibodies before cells were washed and PBS added. Cells were observed by confocal microscopy using an Axiovert 100-M inverted microscope (Zeiss, Thornwood, NY, USA), equipped with an LSM 510 laser-scanning unit and a 63× 1.4-NA plan Apochromat oil-immersion objective (Zeiss). Appropriate filters were selected for the individual stainings.
A quantitative measure of colocalization of flourochromes was determined using the colocalization module of Imaris 5.0.2, 64-bit version (Bitplane AG, Zürick). “Percentage of material colocalized above threshold” of each subcellular marker or CD14 or CD36 that colocalized with TLR2 or LTA was calculated. This value takes into account the number of pixels that colocalizes, as well as the intensities (“material”) of the two labels in each pixel. User-defined thresholds were set conservatively in a rectangular selection mode chosen above the apparent noise level for each channel. Colocalization maps showing colocalization events of each marker or CD36 or CD14 with TLR2 or LTA were created in Imaris. In these images, white denotes colocalization events between two channels, and pixels above the threshold that failed to colocalize were set to zero (black).
LTA-binding/internalization studies
A+ buffy coat from healthy donors (St. Olav’s Hospital Trondheim) was incubated with LTARhodamine Green (10 μg/ml) for 45 min at 4°C or at 37°C, 8% CO2. Incubation at 4°C should permit LTA binding to receptors on the plasma membrane but delay LTA internalization, and binding and internalization of LTA in monocytes were expected to proceed normally at 37°C. Erythrocytes were lysed with formic acid-based lysis buffer for 1 min, neutralized, and fixed using the Coulter Immunoprep Epics leukocyte preparation system (Coulter, Miami, FL, USA). Samples of cells were stained with fluoroscein (FITC)- or PE-conjugated mAb against CD14, CD3, or CD19. Samples were analyzed by flow cytometry, and populations were gated by CD14 high expression (monocytes), CD14 low expression (granulocytes), and CD3 or CD19 expression (lymphocytes), as well as by size and granularity. Gates were subsequently applied to determine LTARhodamine Green internalization in the samples by determining median fluorescence of each population. Monocytes were also stimulated by plating cells in sterile 96-plate wells coated overnight at 4°C with titrations of LTA or PBS or stimulated by adding titrations of LTA in solution or medium. Wells were washed four times with PBS after coating and prior to addition of cells to remove excess, unbound LTA. Cells were stimulated overnight in 1% A+/RPMI at 37°C, 5% CO2, before supernatant was harvested, and TNF levels were assessed by ELISA.
Cell staining for flow cytometry
Freshly islolated monocytes were detached with 0.02% EDTA, fixed with 4% paraformaldehyde, and stained extracellularly in 1% FBS/PBS with anti-TLR2A488, CD36FITC, CD14A488 (5C5), or mouse IgGA488/FITC and analyzed by flow cytometry. Freshly isolated monocytes were furthermore left unstimulated or stimulated for 16 h with LTA (0.1, 1, 10, 100, or 1000 ng/ml) prior to staining with anti-TLR2 or mouse IgG and subsequently, with secondary antibody goat anti-mouseTricolor, CD36FITC, or mouse IgG FITC, as described.
Luciferase reporter assay
NF-κB activation was determined by a NF-κB luciferase reporter assay as described previously [43]. Briefly, HEK293 cells were transiently transfected with reporter plasmid endothelial leukocyte adhesion molecule-luciferase reporter gene (ELAM-Luc), containing a NF-κB-dependent portion of the ELAM promoter-driving luciferase. Cells were additionally transfected with control plasmids pcDNA3 and pORF9 and/or CD36, CD14, and TLR2; CD36, CD14, and TLR2 in combination; or TLR4, CD14, and MD2 for 24 h. The total amount of each vector was kept constant by filling up with the appropriate control plasmids. Cells were subsequently stimulated with LTA (5 μg/ml) or LPS (100 ng/ml) for 5 h before cells were lysed and assayed for luciferase activity as a measure for NF-κB activation using the luciferase assay system (Promega, Madison, WI, USA). Similar results were obtained upon repeating the experiment and including the pRL-TK Renilla luciferase control plasmid (Promega) as a control for transfection efficiency and cell viability in the setup. The dual luciferase assay system (Promega) was used to measure ELAM-Luc and Renilla luciferase activity, which was found to be similar in all wells (not shown).
Blocking studies
Freshly isolated monocytes were preincubated with mouse IgG, anti-TLR2, anti-CD36, anti-CD14 (MEM-18), or anti-TLR4 (10 ug/ml) in RPMI on ice (0–4°C) for 45 min and subsequently added LTARhodamine Green (2 μg/ml) for 45 min on ice. Cells were detached with 0.02% EDTA/PBS, washed, and analyzed for LTA binding and internalization by flow cytometry. Monocytes were pretreated with optimized concentrations of anti-CD36 (0.5 μg/ml), anti-CD14 (3C10; 10 μg/ml), a mixture of TL2.1 and TL2.3 (10 μg/ml), or mouse IgG (10 μg/ml) for 45 min at room temperature prior to stimulation with medium, LTA (10 μg/ml), LPS (20 ng/ml), or Pam3CysSK4 (50 ng/ml) in 1% A+/RPMI for 5 h at 37°C, 5% CO2, before the supernatant was harvested and analyzed for TNF by ELISA (R&D Systems).
Dynamin-I expression studies
HEK293-TLR2 cells were transiently transfected with wild-type Dynamin-I or the mutant Dynamin-I K44A in the presence or absence of transfected CD14 for 72 h before cells were incubated with LTArhodamine (2 μg/ml) and transferrinA633 (2 μg/ml) for 30 min at 37°C and observed by confocal microscopy. HEK-TLR2 cells were transiently transfected as described with the ELAM-Luc, combinations of CD14, Dynamin-I wild-type or the mutant Dynamin-I K44A, and/or control pcDNA3 for 72 h. Cells were subsequently stimulated with medium or LTA (5 μg/ml) for 5 h before cells were lysed and analyzed for NF-κB activation.
RESULTS
Surface TLR2 is rapidly internalized into endosomes and lysosomes
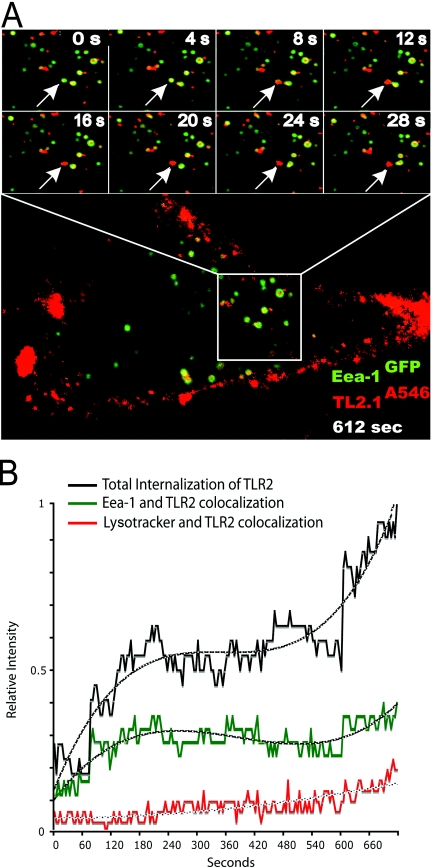
To study TLR2 trafficking, MDCK cells expressing TLR2 and Eea-1GFP were incubated with the A546-labeled TLR2 mAb TL2.1 (TL2.1A546). We observed that TL2.1A546 bound to the plasma membrane of TLR2-transfected cells (Fig. 1A). We further observed gradual internalization of the TL2.1A546 mAb into Eea-1-positive early endocytic compartments (Fig. , 1Aand 1B, and Supplementary Video 1), followed by maturation as the Eea-1 coat detached (Fig. 1A, arrows). No binding or internalization was observed upon incubation with an isotype control (not shown), demonstrating the specificity of the staining.
Figure 1.
Surface TLR2 is internalized into endosomes. (A) Confocal images of internalization of TLR2 mAb (red) in MDCK cells expressing Eea-1GFP (green) and TLR2. Images show enlargements of a portion of a representative cell at 4-s interval time-points (two upper panels). Arrows denote an EEA-1GFP (green) endosome containing TLR2 mAb (red) that matures and loses the early endosome tag. The full picture of the cell (lower picture) shows TLR2 mAb internalization after 612 s. Cells were transiently transfected with TLR2 24 h prior to the experiment using Oligofectamine transfection reagent. Cells were kept on ice for 45 min and then incubated with TLR2A546 mAb on ice for 45 min. Image-acquiring was initiated 15 min post-incubation. Image acquisition was performed on an Olympus Fluoview 1000 at 37°C with an Olympus PlanApo 60×/1.42 oil objective. (B) Plot of relative intensity of total TLR2A546 fluorescence (black) and colocalization of TLR2A546 mAb with Eea-1GFP (green) and LysotrackerGreen (red) as a function of time. Results show measurements from five representative cells. Internalization analysis was carried out with ImageJ software. Dotted lines denote polynomial trend lines.
Figure 1B shows an increase in colocalization of TL2.1A546 and Eea-1GFP within the initial 4 min of incubation, and colocalization between TL2.1A546 and LysotrackerGreen began to increase after 10 min of incubation. Calculations from images after 180 min showed that 20–30% of the TLR2 mAb colocalized with the lysosome marker LysotrackerGreen after 180 min (images not shown). These results suggest that surface TLR2 traffics along the conventional endosomal pathway.
TLR2 is expressed in the plasma membrane, endosomes, lysosomes, and Rab-11-positive compartments in monocytes
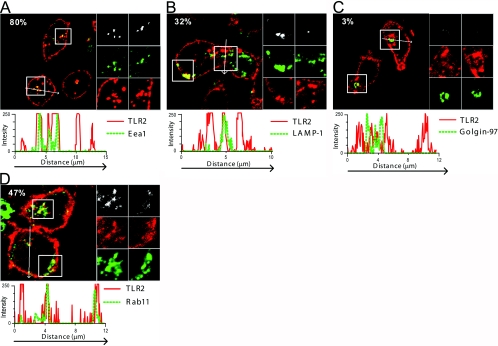
We further applied confocal microscopy to investigate the intracellular expression of TLR2 in freshly isolated monocytes to assess the subcellular compartments where LTA-induced signaling could occur. In line with previous studies [46], TLR2 was found to be highly expressed in the plasma membrane of monocytes (Fig. 2), as well as in a population of early endosomes (Fig. 2A) and lysosomes (Fig. 2B). Furthermore, we found that TLR2 was not expressed in the Golgi network, using antibodies against Golgin-97 (Fig. 2C) and GM130 (not shown), which are specific markers for the trans- and cis-Golgi, respectively [47, 48]. TLR2 expression was, however, often observed in close proximity to the trans-Golgi network, which led us to examine whether TLR2 was expressed in Rab-11A-positive compartments. The GTPase Rab-11A localizes to pericentriolar recycling endosomes and trans-Golgi and is essential for development of multivesicular body endosomal compartments [49,50,51,52]. TLR2 colocalized with Rab-11A in the perinuclear area (Fig. 2D). These results suggest that TLR2 may also be expressed in recycling endosomes and possibly in multivesicular endosomes. Minimal colocalization was observed between TLR2 and the ER marker Calnexin (not shown), showing that TLR2 is not retained in the ER, which is in contrast to other TLRs such as TLR3 and TLR9 [53, 54].
Figure 2.
TLR2 is expressed in the plasma membrane, endosomes, lysosomes, and Rab-11-positive compartments but not in the Golgi of monocytes. Confocal images of freshly isolated monocytes stained intracellularly with the TLR2 mAb TL2.1 (red) and antibodies against (A) early endosome marker Eea-1 (green), (B) lysosome marker LAMP-1 (green), (C) trans-Golgi marker Golgin-97 (green), or (D) Rab-11A (green). Areas of colocalization are shown in yellow. Percent values (A–D) denote the area percentage of each marker that colocalizes with TLR2 staining. Panels to the right of each image show enlargements of two sections, denoted by squares in each image. Colocalization maps showing colocalization events between TLR2 and respective marker stainings are shown in the top panels (white). Single tracks of the respective marker (green) and TLR2 (red) are shown in the middle and bottom panels. Profile graphs show fluorescence intensity of each color in a cross-section denoted by an arrow in each image (A–D). Images of cells shown are representative of the cells observed in each dish and are representative of three experiments.
Monocytes efficiently bind and internalize fluorescently labeled LTA and up-regulate TLR2
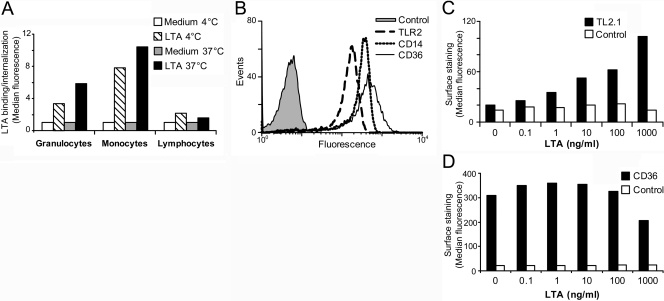
The ability of the leukocyte populations to bind and internalize LTA was assessed by incubating white blood cells from healthy human donors with fluorescently labeled LTARhodamine Green at 4°C or 37°C. We found that monocytes, as well as granulocytes, bound LTA efficiently at 4°C, and only marginal binding was observed to lymphocytes (Fig. 3A). Additional fluorescence was observed at 37°C, suggesting binding and internalization of LTA in monocytes and granulocytes. Although monocytes and granulocytes express TLR2, CD36, and CD14 [46, 55, 56], monocytes express particularly high levels of all threereceptors on the surface (Fig. 3B). We have previously shown that murine macrophages rapidly up-regulate TLR2 in response to heat-killed S. aureus [57]. Here, we show that LTA from S. aureus up-regulated surface TLR2 on human monocytes in a dose-dependent manner (Fig. 3C). The expression of surface CD36 on monocytes was, however, less affected upon stimulation with LTA and was found to be slightly down-regulated at the highest LTA concentration in contrast to TLR2 (Fig. 3D). Addition of 10 μg/ml LTA to monocytes at 0–4°C did not inhibit the surface staining with anti-CD36 (data not shown), suggesting that LTA does not interfere with the binding of the antibody to CD36.
Figure 3.
Monocytes efficiently bind and internalize LTA and up-regulate TLR2. (A) Monocytes efficiently bind and internalize LTA. A+ buffy coat from healthy donors was incubated with LTARhodamine Green for 45 min at 4°C or at 37°C, 8% CO2. RBCs were subsequently lysed, and remaining cells were analyzed by flow cytometry to determine LTA binding and uptake. Populations were gated by size and granularity and CD14 high expression (monocytes), CD14 low expression (granulocytes), and CD3 or CD19 expression (lymphocytes). (B) Monocytes were fixed and stained extracellularly with antibody against TLR2, CD14, and CD36 for 45 min on ice and analyzed by flow cytometry. Monocytes were stimulated with LTA (0, 0.1, 1, 10, 100, or 1000 ng/ml) for 16 h and were subsequently stained for surface expression of (C) TLR2 or (D) CD36 prior to determination of median fluorescence by flow cytometry. Results shown are representative of three independent experiments.
LTA is rapidly internalized in tubular endocytic structures and targeted to the trans-Golgi network and the ER
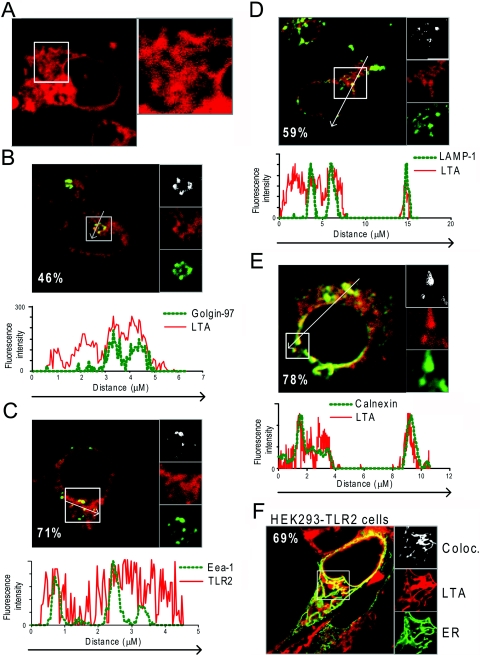
We next investigated whether LTA localized to the same compartments as TLR2 in human monocytes. Using fluorescently labeled LTA, we observed LTA internalization in live monocytes by confocal microscopy. LTA initially bound to the plasma membrane of monocytes and was rapidly internalized (not shown). Figure 4A shows LTA in extensive tubular structures and concentrated in the perinuclear area in monocytes after 20 min of incubation. Confocal images and fluorescence intensity profiles of fluorescently conjugated antibody markers and LTArhodamine showed that LTA was localized in the Golgi network (Fig. 4B), in early endosomes (Fig. 4C), as well as in lysosomes (Fig. 4D) after 1 h of incubation. LTA localization to LAMP-1-positive vesicles was more apparent at later time-points, (2–6 h; data not shown). Extensive overlay was observed between fluorescent LTA and calnexin staining, particularly in close proximity to the nucleus (Fig. 4E), suggesting that LTA localizes to the ER. These results were confirmed in HEK293-TLR2 cells transiently expressing the targeting sequence of calreticulin fused to CFP, which localizes the protein to the ER (ERCFP). Confocal images of these cells incubated with LTArhodamine revealed overlap between LTA and the ER marker (Fig. 4F), suggesting that LTA is targeted to the ER upon internalization. Although LTA clearly localized to the trans-Golgi network and the ER of monocytes, TLR2 was not expressed in these compartments (Fig. 2C, and data not shown). Colocalization between LTA and TLR2 was, however, observed in early endosomes and lysosomes (not shown). As LTA requires TLR2 for signaling, the results suggest that signaling may occur at the plasma membrane and along the endocytic pathway.
Figure 4.
LTA is rapidly internalized in tubular structures and targeted to the trans-Golgi network and the ER. (A) Internalization of LTArhodamine (20 μg/ml; red) in live monocytes after 20 min of incubation at 37°C. (B–E) Monocytes incubated with LTArhodamine (red; 20 μg/ml) for 1 h at 37°C, 8% CO2, and subsequently fixed and stained intracellularly with antibodies against (B) Golgin-97 (green), (C) Eea-1 (green), (D) LAMP-1 (green), or (E) ER marker Calnexin and secondary antibody goat anti-mouseA647 (green). (F) Confocal images of live HEK293-TLR2 cells transiently expressing CFP fused to the targeting sequence of calreticulin (ERCFP), which localizes to the ER (green), incubated with LTArhodamine (red; 20 μg/ml) for 1 h at 37°C. (B–F) Percent values denote percent area of each marker that colocalizes with LTArhodamine. Panels to the right of each image (B–F) show enlargements of sections denoted by squares in each image. Colocalization maps showing colocalization events between LTArhodamine and respective marker stainings are shown in the top panels (white). Single tracks of markers are shown in green and LTArhodamine in red. Profile graphs are included, showing fluorescence intensity of each color in a cross-section denoted by an arrow in images (A–E). Images of cells shown are representative of the cells observed in each dish and are representative of three independent experiments.
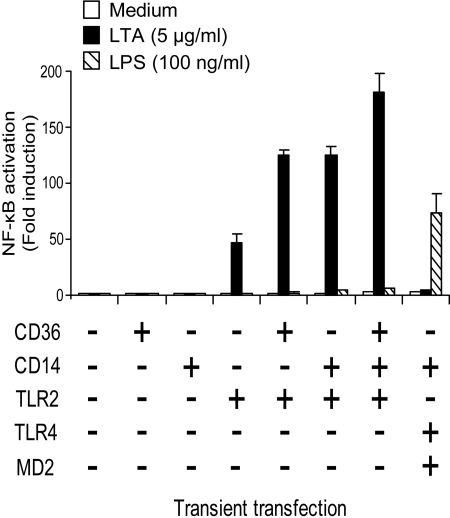
CD36 and CD14 enhance TLR2-mediated NF-κB activation in response to LTA
CD36 and CD14 are shown to act as coreceptors for TLR2 in response to LTA [34, 37]. In this experiment, the role of CD36 and CD14 in response to LTA was compared. HEK293 cells were transiently transfected with CD36, CD14, TLR2, or in combination. HEK293 cells were also transiently transfected with TLR4, MD2, and CD14 as a control. NF-κB activation in response to LTA was only observed upon expression of TLR2 (Fig. 5). Coexpression of TLR2 with CD36 or CD14 enhanced LTA-induced NF-κB activation approximately threefold compared with cells expressing TLR2 alone (Fig. 5). These results indicate that CD36 and CD14 function as coreceptors for TLR2 in response to LTA and that expression of either coreceptor enhances LTA-induced NF-κB activation markedly. A small, additive effect on NF-κB activation was observed upon coexpression of CD36 and CD14 with TLR2 in response to LTA (Fig. 5). The transfection efficiency and cell viability in all the samples were similar as determined by cotransfection with a constitutively expressed Renilla luciferase vector (not shown). NF-κB activation was not observed in HEK293 cells transfected with TLR4, CD14, and MD2 in response to LTA, although these cells responded normally to LPS (Fig. 5), illustrating the purity of the LTA.
Figure 5.
CD14 and CD36 enhance LTA-induced NF-κB activation mediated by TLR2. (A) HEK293 cells transfected with a NF-κB luciferase reporter plasmid, and TLR2 or TLR2 in combination with CD36 and/or CD14 for 24 h was stimulated with LTA (5 μg/ml) or LPS (100 ng/ml) for 5 h at 37°C, 8% CO2. Cells were subsequently lysed and assayed for NF-κB activation. Results shown are representative of three independent experiments.
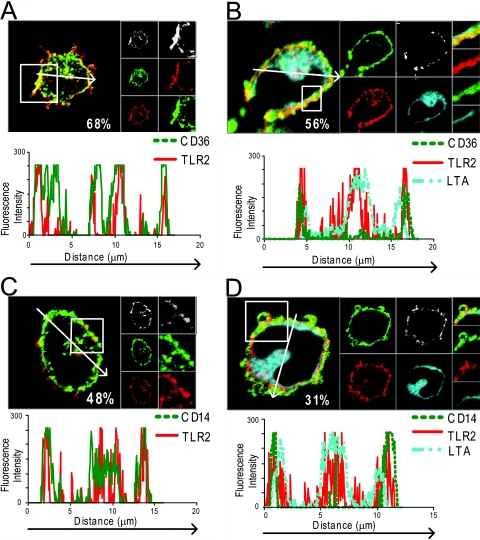
CD36 and CD14 colocalize with TLR2 at the plasma membrane in monocytes
As CD36 and CD14 function as coreceptors for TLR2 in response to LTA, we studied the localization of these receptors in unstimulated and LTA-stimulated monocytes. Freshly isolated human monocytes were left unstimulated or incubated with LTArhodamine for 1 h and then stained for TLR2, CD36, or CD14. Confocal microscopy showed that all three receptors were highly expressed in the plasma membrane, as well as in intracellular vesicles (Fig. 6). TLR2 was also found to colocalize with CD36 (Fig. 6A) and CD14 (Fig. 6C) at the plasma membrane and in internal vesicular structures, which may represent early endosomes and lysomes, where TLR2 is expressed (Fig. , 2Aand 2B). Upon stimulation of monocytes with fluorescent LTA, particularly CD36 (Fig. 6B) but also CD14 (Fig. 6D) redistributed to the plasma membrane (Fig. 6, profile graphs). LTA was predominantly localized in the perinuclear area after 1 h incubation, and surprisingly, little LTA was observed bound to the plasma membrane. However, profile graphs of fluorescence intensity in a cross-section of the cells show that LTA was often localized in close proximity to the plasma membrane (Fig. , 6Band 6D), often overlapping partially with TLR2, CD14, and CD36 at the plasma membrane. Thus, it is likely that LTA signaling occurs at the plasma membrane, as well as along the endocytic pathway.
Figure 6.
CD36 and CD14 are expressed at the plasma membrane, where they colocalize with TLR2. Freshly isolated monocytes incubated with medium (A and C) or LTArhodamine for 1 h (B and D) at 37°C, 5% CO2, and subsequently fixed and stained intracellularly with TLR2 mAb TL2.1A647 (red) and anti-CD36FITC (green; A and B) or TL2.1A647 (red) and anti-CD14A488 (green; C and D). Staining was observed by confocal microscopy. Percent values shown in images denote the percentage area of CD36 or CD14 that colocalizes with TLR2 staining. Panels to the right of each image show enlargements of a section, denoted by a square in each image. Areas of colocalization between CD36 (A) or CD14 (C) and TLR2 stainings are shown in the top panels (white). Single tracks of CD36 (A) or CD14 (C) are shown in green and TLR2 in red in separate panels. (B and D) Separate tracks are shown of CD36 (B) or CD14 (D) in green, TLR2 (red), and LTA (blue). (A–D) Profile graphs show fluorescence intensity of cross-sections denoted by an arrow.
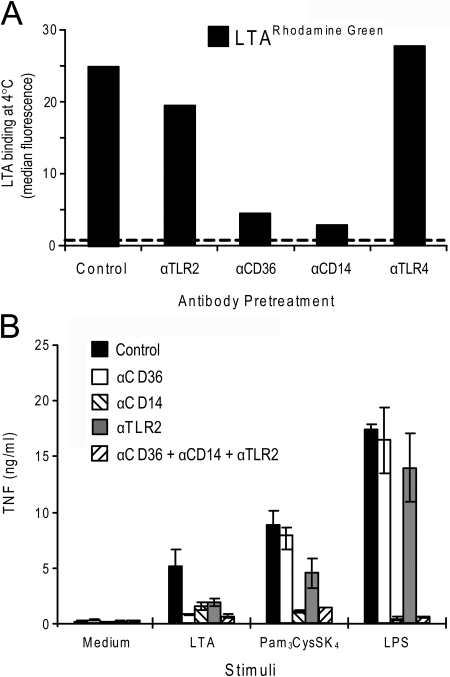
Blocking CD14 or CD36 impairs LTA binding and subsequent TNF release in monocytes
We further investigated the contribution of TLR2 and its coreceptors CD36 and CD14 in binding LTA to freshly isolated human monocytes, which were pretreated with antibodies against TLR2, CD36, and CD14 prior to addition of fluorescently labeled LTA. All treatments were done on ice (0–4°C). As can be seen from Figure 7A, blocking CD36 or CD14 inhibited the binding of LTA to monocytes, suggesting that CD36 and CD14 are involved in binding LTA to monocytes. LTA binding was minimally affected by pretreatment with antibodies against TLR2. Pretreatment of monocytes with antibodies against TLR4 did not affect LTA binding (Fig. 7A). Thus, CD14 and CD36 play an important role in the binding of LTA in human monocytes.
Figure 7.
Blocking CD14 or CD36 impairs LTA cell association and subsequent TNF release in monocytes. (A) Monocytes were pretreated with mAb against TLR2, CD36, CD14, or control antibody (10 μg/ml) on ice (0–4°C) for 45 min before addition of LTARhodamine Green (2 μg/ml) for 45 min on ice. Cells were subsequently washed and analyzed by flow cytometry to assess LTA binding. Dotted line denotes background fluorescence of cells incubated with medium only. (B) Monocytes were pretreated with control antibody or mAb against CD36, CD14, or TLR2 or CD36, CD14, and TLR2 in combination for 45 min at room temperature before cells were stimulated with medium, LTA (10 μg/ml), Pam3CysSK4 (50 ng/ml), or LPS (20 ng/ml) for 5 h at 37°C, 5% CO2. Supernatant was harvested and analyzed for TNF by ELISA. Results shown are representative of three independent experiments.
Given the reduction in LTA binding upon blocking CD36 and CD14, we next determined whether inhibiting CD36 and CD14 with blocking mAb also had an effect on LTA-induced signaling in monocytes. Indeed, inhibition of CD36 was found to markedly reduce TNF release in monocytes in response to LTA but had no effect on the response to the TLR4 ligand LPS or the TLR2/TLR1 ligand Pam3CysSK4(Fig. 7B). Although inhibition of TLR2 had minimal effect on LTA binding, inhibition of TLR2 significantly reduced TNF release from monocytes in response to LTA, as well as in response to Pam3CysSK4, but not in response to LPS (Fig. 7B). Pretreatment of monocytes with anti-CD14 inhibited TNF release in response to LTA, as well as LPS and Pam3CysSK4(Fig. 7B). These results suggest that although TLR2 is required for signaling in response to LTA, CD14 and CD36 play a prominent role in LTA binding and in enhancing LTA-induced signaling in human monocytes.
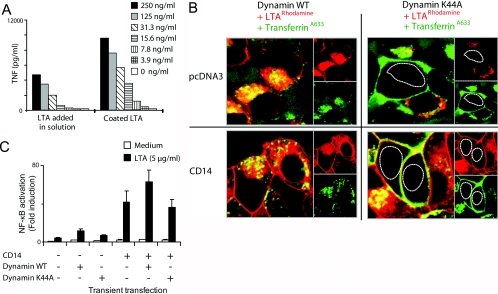
TLR2 signaling in response to LTA occurs mainly at the plasma membrane, independently of receptor-mediated endocytosis via Dynamin-I
As LTA colocalized with TLR2 at the plasma membrane and along the endocytic pathway, we further examined whether internalization of LTA was necessary for signaling. Monocytes were incubated on LTA-coated wells or with LTA added in solution. Immobilization of LTA on a plastic surface should allow binding but restrict internalization of the ligand. Interestingly, we found that immobilizing LTA greatly enhanced TNF release from monocytes, compared with cells that received LTA in solution (Fig. 8A). We argue that the prominent effect on TNF release from monocytes observed upon immobilizing LTA shows that signaling predominantly occurs at the plasma membrane and does not require internalization of LTA.
Figure 8.
TLR2 signaling in response to LTA occurs at the plasma membrane and is not dependent on Dynamin-I. (A) Immobilizing LTA on a plastic surface enhances TNF release in monocytes, which were stimulated by plating cells in wells coated with LTA or PBS or stimulated by adding medium or LTA in solution. Supernatant was harvested after overnight incubation, and TNF levels were analyzed by ELISA. Results show average TNF release of duplets and are representative of three independent experiments. (B) LTA is internalized by a receptor-mediated mechanism. Confocal images of HEK-TLR2 cells transiently expressing wild-type (WT) Dynamin-I or the mutant Dynamin-I K44A and control pcDNA3 or CD14 incubated with LTArhodamine (red) or transferrinA633 (green) for 30 min at 37°C, 8% CO2, prior to imaging. The nucleuses of cells are outlined in Dynamin-I K44A-expressing cells. (C) LTA-induced NF-κB activation occurs at the plasma membrane, independent of LTA uptake. HEK293-TLR2 cells were transfected with a NF-κB luciferase reporter plasmid and wild-type Dynamin-I or the mutant Dynamin-I K44A in the presence of control pcDNA3 or CD14. Cells were subsequently stimulated with LTA (5 μg/ml) or medium for 6 h, 37°C, 8% CO2, before cells were lysed and assayed for NF-κB activation. Results shown are representative of three experiments.
The significance of LTA internalization with regard to signaling was further studied by inhibiting LTA internalization. A dominant-negative mutant of Dynamin-I (Dynamin-I K44A) inhibits receptor-mediated endocytosis by interfering with the function of endogenous Dynamin-I by blocking vesicle internalization before membrane scission occurs [58]. The internalization route of LTA was studied in HEK-TLR2 cells, transiently expressing wild-type Dynamin-I or the mutant Dynamin-I K44A in the presence or absence of CD14 expression. Transfected cells were incubated with LTArhodamine as well as with transferrinA633 as a control for receptor-mediated endocytosis prior to imaging by confocal microscopy (Fig. 8B). Confocal images show that the internalization of LTA and transferrin occurred in cells expressing wild-type Dynamin-I but not in cells expressing the mutant Dynamin-I K44A (Fig. 8B). As expected, transferrin bound to the plasma membrane but was not internalized in the presence of Dynamin-I K44A (Fig. 8B). LTA internalization was inhibited in cells expressing the mutant Dynamin-I K44A in the presence and absence of CD14. Although LTA binding to the plasma membrane was observed upon expression of CD14, in cells expressing wild-type and mutant Dynamin-I, LTA internalization was restricted upon expression of Dynamin-I K44A (Fig. 8B). These results suggest that LTA is internalized by a receptor-mediated mechanism.
To assess whether internalization of LTA was necessary for signaling, NF-κB activation was assessed in HEK-TLR2 cells in the absence or presence of CD14 and in the presence of wild-type Dynamin-I or the mutant K44A Dynamin-I. Figure 8C shows that introduction of CD14 strongly enhanced LTA-induced NF-κB activation but that the Dynamin-I K44A mutant did not affect NF-κB activation, neither in the presence nor absence of CD14. These results suggest that CD14 enhances signaling by binding LTA to the plasma membrane and that LTA internalization is not required for signaling.
DISCUSSION
In this study, we have investigated the mechanisms of LTA internalization in monocytes. It has been suggested that LTA uptake is required for signaling [39]; however, this is in contrast with previous reports suggesting that TLR2 activation by LTA occurs in lipid rafts in the plasma membrane, independent of LTA internalization [36, 38]. In this study, we examined the intracellular trafficking of LTA in human monocytes using directly labeled LTA. We found that monocytes, which express high levels of surface TLR2 and its coreceptors CD14 and CD36, bind and internalize LTA efficiently.
Using a TLR2-specific mAb, we found that TLR2 rapidly traffics from the plasma membrane to early endosomes in live cells that overexpressed the receptor. Early endosomes containing TLR2 subsequently matured (Fig. 1A). We further observed TLR2 in lysosomes. Although only 20–30% of the TLR2 antibody was observed colocalizing with LysotrackerGreen after 3 h, this observation could be a result of several factors. Incubation for longer time periods or during stimulation may have shown more TLR2 in the lysosomes. The fluorochrome bound to the TL2.1 antibodies may lose fluorescence in the acidic environment of lysosomes of live cells, or the antibodies may be degraded or uncoupled from TLR2 in these compartments, resulting in the low percentage of TLR2 mAb observed in lysosomes. Although TLR2 has been reported not to be ubiquitinylated by the ubiquitin-protein ligase TRIAD3A, in contrast to TLR4 and TLR9 [59], other ligases, for instance, TRIAD3B, may ubiquitinylate TLR2 and target it for degradation. The TL2.1 mAb used in this study has been shown to be presented on MHC class II and induce proliferation of a mouse C-specific human CD4+ T cell clone [60], supporting that TLR2 traffics along the classical MHC II pathway.
TLR2 was further found to be highly expressed in a population of Eea-1-positive early endosomes and LAMP-1-positive lysosomes in human monocytes. In contrast to previous reports [36], we did not observe TLR2 in the Golgi network in human monocytes; however, we did observe TLR2 in the Golgi network of HEK293 cells overexpressing the receptor (data not shown). We interpret these results as characteristic of epithelial cells or an effect of overexpression of TLR2. In monocytes, TLR2 colocalized with Rab-11A-positive structures localized in close proximity to the trans-Golgi network, suggesting that TLR2 is expressed in endosomal recycling compartments. Whether Rab-11A is required for LTA internalization and signaling remains to be investigated.
TLR2 is essential for inflammatory responses toward highly purified LTA [34]. The high amount of TLR2 at the plasma membrane and up-regulation of the receptor in response to LTA support the notion that TLR2 signaling occurs at the plasma membrane. Localization of TLR2 in endosomes and lysosomes, however, suggests that signaling may occur in these compartments as well, as shown for TLR4 [61]. Signaling from endosomes/lysosomes also occurs for TLR3 [53] and TLR9 [54], although these receptors are recruited to endosomes from the ER. Using directly labeled, functional LTA, we found that LTA was rapidly internalized in characteristic tubular structures, which colocalized with markers for ER and Golgi network, showing that LTA has an uptake and trafficking pattern in phagocytic cells, which is different from other TLR ligands such as LPS, CpG, and polyinosinic:polycytidylic acid [32, 53, 54, 61]. The colocalization of LTA with markers of the ER and the Golgi suggests that it follows a retrograde pathway, possibly resembling the trafficking pattern of the plant toxin ricin and the bacterial toxin shiga toxin [62], which follow a retrograde transport to the ER [63,64,65,66]. Although shiga toxin is shown to be internalized in clathrin-coated pits, ricin is internalized by dynamin-, clathrin-, and caveolea-independent mechanisms [62]. In light of the finding that LTA appears to be targeted to the Golgi and ER, it would be interesting to compare LTA internalization with ricin and shiga toxin.
Our results demonstrate that LTA was endocytosed in a Dynamin-I-dependent manner. Previous reports have suggested that LTA is internalized by a lipid raft-dependent pathway [67,68,69]. Colocalization between LTA and transferrin was observed during the initial minutes of endocytosis in HEK293 cells expressing TLR2 (N. J. Nilsen, unpublished data). In addition, some colocalization between LTA and cholera toxin B was seen, predominantly in the Golgi network (N. J. Nilsen, unpublished data). These results suggest that LTA may be internalized by clathrin- and caveolea-dependent pathways. Colocalization between LTA and transferrin and cholera toxin B was, however, only partial, and the internalization pattern of LTA did not mimic the uptake of LPS, fibroblast-stimulating lipopeptide-1, or Pam3CysSK4. These ligands are internalized slower than LTA and are seen in endocytic vesicles that clearly colocalize with transferrin [35, 61] (N. J. Nilsen, unpublished data). Our findings suggest that LTA may use several endocytic pathways, which also have been described for CD14-mediated LPS uptake [61, 70].
The role of CD36 as a coreceptor for TLR2 in response to LTA has predominantly been studied in the presence of CD14 [37, 39], and the contribution of each of these coreceptors has not been compared previously. In accordance with previous reports [34, 39], we found that CD36 and CD14 enhanced LTA-induced, TLR2-mediated NF-κB activation in transfected cells. Only a minor additive effect was observed upon coexpression of both receptors in HEK-TLR2 cells. In monocytes, CD36 and CD14 mAb inhibited cell association of LTA and TNF release to a similar extent (Fig. 7). The results suggest that inhibiting CD14 or CD36 on monocytes prevents binding of LTA, which in turn reduces signaling and the induction of TNF. Consequently, CD36 and CD14 appear to be important in enhancing TLR2 signaling in response to LTA. CD36, however, appears to be specifically involved in TLR2/TLR6-mediated responses but not in TLR2/TLR1-mediated responses, in line with previous observations [37]. CD14, in contrast, enhances signaling in response to LPS and Pam3CysSK4, as well as in response to LTA.
Expression of CD14 in HEK-TLR2 cells profoundly enhanced binding of LTA to the plasma membrane and NF-κB activation, suggesting that this coreceptor may up-regulate signaling by accumulating LTA at the plasma membrane. Furthermore, immobilizing LTA on a plastic surface induced a high level of TNF release in monocytes, independent of internalization of the ligand. This appears to be a specific property of LTA but not LPS or Pam3CysSK4 (S. Deininger, unpublished data). Expression of Dynamin-I K44A inhibited LTA internalization in the absence and presence of CD14, suggesting that LTA is internalized by a receptor-mediated mechanism. Expression of Dynamin-I K44A had, however, no significant effect on LTA-induced NF-κB activation, showing that binding of LTA to the plasma membrane is sufficient to induce signaling. This finding is in contrast to previous reports describing that CD36-mediated internalization of LTA is required for signaling [39]. The presence of CD36 may, however, also be required for formation of the correct receptor clustering in response to LTA or for recruitment of LTA to lipid rafts, as suggested in recent reports [38]. In summary, our results show that signaling in response to LTA occurs independent of internalization of the ligand and provide further insight into the mechanisms of LTA internalization, trafficking, and signaling through TLR2 and its coreceptors CD14 and CD36.
Supplementary Material
Acknowledgments
This work was funded by The Research Council of Norway, the Norwegian Cancer Society, and the Commission of the European Communities, LSMH-CT-2004-512093, AMIS, and National Institutes of Health, National Institute of Allergy and Infectious Diseases (Grant AI057588 to E. L.).
References
- Janeway C. A., Jr The immune system evolved to discriminate infectious nonself from noninfectious self. Immunol Today. 1992;13:11–16. doi: 10.1016/0167-5699(92)90198-G. [DOI] [PubMed] [Google Scholar]
- Medzhitov R., Janeway C. A., Jr Innate immunity: impact on the adaptive immune response. Curr Opin Immunol. 1997;9:4–9. doi: 10.1016/s0952-7915(97)80152-5. [DOI] [PubMed] [Google Scholar]
- Marshall C., Kossmann T., Wesselingh S., Spelman D. Methicillin-resistant Staphylococcus aureus and beyond: what’s new in the world of the golden staph? ANZ J Surg. 2004;74:465–469. doi: 10.1111/j.1445-2197.2004.03034.x. [DOI] [PubMed] [Google Scholar]
- Raetz C. R., Brozek K. A., Clementz T., Coleman J. D., Galloway S. M., Golenbock D. T., Hampton R. Y. Gram-negative endotoxin: a biologically active lipid. Cold Spring Harb Symp Quant Biol. 1988;53:973–982. doi: 10.1101/sqb.1988.053.01.112. [DOI] [PubMed] [Google Scholar]
- Raetz C. R., Ulevitch R. J., Wright S. D., Sibley C. H., Ding A., Nathan C. F. Gram-negative endotoxin: an extraordinary lipid with profound effects on eukaryotic signal transduction. FASEB J. 1991;5:2652–2660. doi: 10.1096/fasebj.5.12.1916089. [DOI] [PubMed] [Google Scholar]
- Ginsburg I. Role of lipoteichoic acid in infection and inflammation. Lancet Infect Dis. 2002;2:171–179. doi: 10.1016/s1473-3099(02)00226-8. [DOI] [PubMed] [Google Scholar]
- Lien E., Ingalls R. R. Toll-like receptors. Crit Care Med. 2002;30:S1–S11. [PubMed] [Google Scholar]
- Rock F. L., Hardiman G., Timans J. C., Kastelein R. A., Bazan J. F. A family of human receptors structurally related to Drosophila Toll. Proc Natl Acad Sci USA. 1998;95:588–593. doi: 10.1073/pnas.95.2.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akira S., Hemmi H. Recognition of pathogen-associated molecular patterns by TLR family. Immunol Lett. 2003;85:85–95. doi: 10.1016/s0165-2478(02)00228-6. [DOI] [PubMed] [Google Scholar]
- Barton G. M., Medzhitov R. Toll-like receptor signaling pathways. Science. 2003;300:1524–1525. doi: 10.1126/science.1085536. [DOI] [PubMed] [Google Scholar]
- Poltorak A., He X., Smirnova I., Liu M. Y., Van Huffel C., Du X., Birdwell D., Alejos E., Silva M., Galanos C., Freudenberg M., Ricciardi-Castagnoli P., Layton B., Beutler B. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 1998;282:2085–2088. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- Qureshi S. T., Medzhitov R. Toll-like receptors and their role in experimental models of microbial infection. Genes Immun. 2003;4:87–94. doi: 10.1038/sj.gene.6363937. [DOI] [PubMed] [Google Scholar]
- Yoshimura A., Lien E., Ingalls R. R., Tuomanen E., Dziarski R., Golenbock D. Cutting edge: recognition of Gram-positive bacterial cell wall components by the innate immune system occurs via Toll-like receptor 2. J Immunol. 1999;163:1–5. [PubMed] [Google Scholar]
- Flo T. H., Ryan L., Latz E., Takeuchi O., Monks B. G., Lien E., Halaas O., Akira S., Skjak-Braek G., Golenbock D. T., Espevik T. Involvement of Toll-like receptor (TLR) 2 and TLR4 in cell activation by mannuronic acid polymers. J Biol Chem. 2002;277:35489–35495. doi: 10.1074/jbc.M201366200. [DOI] [PubMed] [Google Scholar]
- Schwandner R., Dziarski R., Wesche H., Rothe M., Kirschning C. J. Peptidoglycan- and lipoteichoic acid-induced cell activation is mediated by Toll-like receptor 2. J Biol Chem. 1999;274:17406–17409. doi: 10.1074/jbc.274.25.17406. [DOI] [PubMed] [Google Scholar]
- Morath S., Geyer A., Hartung T. Structure-function relationship of cytokine induction by lipoteichoic acid from Staphylococcus aureus. J Exp Med. 2001;193:393–397. doi: 10.1084/jem.193.3.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brightbill H. D., Libraty D. H., Krutzik S. R., Yang R. B., Belisle J. T., Bleharski J. R., Maitland M., Norgard M. V., Plevy S. E., Smale S. T., Brennan P. J., Bloom B. R., Godowski P. J., Modlin R. L. Host defense mechanisms triggered by microbial lipoproteins through Toll-like receptors. Science. 1999;285:732–736. doi: 10.1126/science.285.5428.732. [DOI] [PubMed] [Google Scholar]
- Flo T. H., Halaas O., Lien E., Ryan L., Teti G., Golenbock D. T., Sundan A., Espevik T. Human Toll-like receptor 2 mediates monocyte activation by Listeria monocytogenes, but not by group B streptococci or lipopolysaccharide. J Immunol. 2000;164:2064–2069. doi: 10.4049/jimmunol.164.4.2064. [DOI] [PubMed] [Google Scholar]
- Takeda K., Akira S. Roles of Toll-like receptors in innate immune responses. Genes Cells. 2001;6:733–742. doi: 10.1046/j.1365-2443.2001.00458.x. [DOI] [PubMed] [Google Scholar]
- Takeuchi O., Sato S., Horiuchi T., Hoshino K., Takeda K., Dong Z., Modlin R. L., Akira S. Cutting edge: role of Toll-like receptor 1 in mediating immune response to microbial lipoproteins. J Immunol. 2002;169:10–14. doi: 10.4049/jimmunol.169.1.10. [DOI] [PubMed] [Google Scholar]
- Sandor F., Latz E., Re F., Mandell L., Repik G., Golenbock D. T., Espevik T., Kurt-Jones E. A., Finberg R. W. Importance of extra- and intracellular domains of TLR1 and TLR2 in NFκ B signaling. J Cell Biol. 2003;162:1099–1110. doi: 10.1083/jcb.200304093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi O., Kawai T., Muhlradt P. F., Morr M., Radolf J. D., Zychlinsky A., Takeda K., Akira S. Discrimination of bacterial lipoproteins by Toll-like receptor 6. Int Immunol. 2001;13:933–940. doi: 10.1093/intimm/13.7.933. [DOI] [PubMed] [Google Scholar]
- Ozinsky A., Underhill D. M., Fontenot J. D., Hajjar A. M., Smith K. D., Wilson C. B., Schroeder L., Aderem A. The repertoire for pattern recognition of pathogens by the innate immune system is defined by cooperation between Toll-like receptors. Proc Natl Acad Sci USA. 2000;97:13766–13771. doi: 10.1073/pnas.250476497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henneke P., Morath S., Uematsu S., Weichert S., Pfitzenmaier M., Takeuchi O., Muller A., Poyart C., Akira S., Berner R., Teti G., Geyer A., Hartung T., Trieu-Cuot P., Kasper D. L., Golenbock D. T. Role of lipoteichoic acid in the phagocyte response to group B streptococcus. J Immunol. 2005;174:6449–6455. doi: 10.4049/jimmunol.174.10.6449. [DOI] [PubMed] [Google Scholar]
- Goyert S. M., Ferrero E., Rettig W. J., Yenamandra A. K., Obata F., Le Beau M. M. The CD14 monocyte differentiation antigen maps to a region encoding growth factors and receptors. Science. 1988;239:497–500. doi: 10.1126/science.2448876. [DOI] [PubMed] [Google Scholar]
- Haziot A., Chen S., Ferrero E., Low M. G., Silber R., Goyert S. M. The monocyte differentiation antigen, CD14, is anchored to the cell membrane by a phosphatidylinositol linkage. J Immunol. 1988;141:547–552. [PubMed] [Google Scholar]
- Pfeiffer A., Bottcher A., Orso E., Kapinsky M., Nagy P., Bodnar A., Spreitzer I., Liebisch G., Drobnik W., Gempel K., Horn M., Holmer S., Hartung T., Multhoff G., Schutz G., Schindler H., Ulmer A. J., Heine H., Stelter F., Schutt C., Rothe G., Szolosi J., Damjanovich S., Schmitz G. Lipopolysaccharide and ceramide docking to CD14 provokes ligand-specific receptor clustering in rafts. Eur J Immunol. 2001;31:3153–3164. doi: 10.1002/1521-4141(200111)31:11<3153::aid-immu3153>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Schumann R. R., Leong S. R., Flaggs G. W., Gray P. W., Wright S. D., Mathison J. C., Tobias P. S., Ulevitch R. J. Structure and function of lipopolysaccharide binding protein. Science. 1990;249:1429–1431. doi: 10.1126/science.2402637. [DOI] [PubMed] [Google Scholar]
- Schromm A. B., Brandenburg K., Rietschel E. T., Flad H. D., Carroll S. F., Seydel U. Lipopolysaccharide-binding protein mediates CD14-independent intercalation of lipopolysaccharide into phospholipid membranes. FEBS Lett. 1996;399:267–271. doi: 10.1016/s0014-5793(96)01338-5. [DOI] [PubMed] [Google Scholar]
- Blondin C., Le Dur A., Cholley B., Caroff M., Haeffner-Cavaillon N. Lipopolysaccharide complexed with soluble CD14 binds to normal human monocytes. Eur J Immunol. 1997;27:3303–3309. doi: 10.1002/eji.1830271229. [DOI] [PubMed] [Google Scholar]
- Wright S. D., Ramos R. A., Tobias P. S., Ulevitch R. J., Mathison J. C. CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science. 1990;249:1431–1433. doi: 10.1126/science.1698311. [DOI] [PubMed] [Google Scholar]
- Thieblemont N., Wright S. D. Transport of bacterial lipopolysaccharide to the Golgi apparatus. J Exp Med. 1999;190:523–534. doi: 10.1084/jem.190.4.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latz E., Visintin A., Lien E., Fitzgerald K. A., Espevik T., Golenbock D. T. The LPS receptor generates inflammatory signals from the cell surface. J Endotoxin Res. 2003;9:375–380. doi: 10.1179/096805103225003303. [DOI] [PubMed] [Google Scholar]
- Schroder N. W., Morath S., Alexander C., Hamann L., Hartung T., Zahringer U., Gobel U. B., Weber J. R., Schumann R. R. Lipoteichoic acid (LTA) of Streptococcus pneumoniae and Staphylococcus aureus activates immune cells via Toll-like receptor (TLR)-2, lipopolysaccharide-binding protein (LBP), and CD14, whereas TLR-4 and MD-2 are not involved. J Biol Chem. 2003;278:15587–15594. doi: 10.1074/jbc.M212829200. [DOI] [PubMed] [Google Scholar]
- Manukyan M., Triantafilou K., Triantafilou M., Mackie A., Nilsen N., Espevik T., Wiesmuller K. H., Ulmer A. J., Heine H. Binding of lipopeptide to CD14 induces physical proximity of CD14, TLR2 and TLR1. Eur J Immunol. 2005;35:911–921. doi: 10.1002/eji.200425336. [DOI] [PubMed] [Google Scholar]
- Triantafilou M., Manukyan M., Mackie A., Morath S., Hartung T., Heine H., Triantafilou K. Lipoteichoic acid and Toll-like receptor 2 internalization and targeting to the Golgi are lipid raft-dependent. J Biol Chem. 2004;279:40882–40889. doi: 10.1074/jbc.M400466200. [DOI] [PubMed] [Google Scholar]
- Hoebe K., Georgel P., Rutschmann S., Du X., Mudd S., Crozat K., Sovath S., Shamel L., Hartung T., Zahringer U., Beutler B. CD36 is a sensor of diacylglycerides. Nature. 2005;433:523–527. doi: 10.1038/nature03253. [DOI] [PubMed] [Google Scholar]
- Triantafilou M., Gamper F. G., Haston R. M., Mouratis M. A., Morath S., Hartung T., Triantafilou K. Membrane sorting of Toll-like receptor (TLR)-2/6 and TLR2/1 heterodimers at the cell surface determines heterotypic associations with CD36 and intracellular targeting. J Biol Chem. 2006;281:31002–31011. doi: 10.1074/jbc.M602794200. [DOI] [PubMed] [Google Scholar]
- Stuart L. M., Deng J., Silver J. M., Takahashi K., Tseng A. A., Hennessy E. J., Ezekowitz R. A., Moore K. J. Response to Staphylococcus aureus requires CD36-mediated phagocytosis triggered by the COOH-terminal cytoplasmic domain. J Cell Biol. 2005;170:477–485. doi: 10.1083/jcb.200501113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimazu R., Akashi S., Ogata H., Nagai Y., Fukudome K., Miyake K., Kimoto M. MD-2, a molecule that confers lipopolysaccharide responsiveness on Toll-like receptor 4. J Exp Med. 1999;189:1777–1782. doi: 10.1084/jem.189.11.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Voorhis W. C., Steinman R. M., Hair L. S., Luban J., Witmer M. D., Koide S., Cohn Z. A. Specific antimononuclear phagocyte monoclonal antibodies Application to the purification of dendritic cells and the tissue localization of macrophages. J Exp Med. 1983;158:126–145. doi: 10.1084/jem.158.1.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lien E., Sellati T. J., Yoshimura A., Flo T. H., Rawadi G., Finberg R. W., Carroll J. D., Espevik T., Ingalls R. R., Radolf J. D., Golenbock D. T. Toll-like receptor 2 functions as a pattern recognition receptor for diverse bacterial products. J Biol Chem. 1999;274:33419–33425. doi: 10.1074/jbc.274.47.33419. [DOI] [PubMed] [Google Scholar]
- Latz E., Visintin A., Lien E., Fitzgerald K. A., Monks B. G., Kurt-Jones E. A., Golenbock D. T., Espevik T. Lipopolysaccharide rapidly traffics to and from the Golgi apparatus with the Toll-like receptor 4-MD-2-CD14 complex in a process that is distinct from the initiation of signal transduction. J Biol Chem. 2002;277:47834–47843. doi: 10.1074/jbc.M207873200. [DOI] [PubMed] [Google Scholar]
- McBride H. M., Rybin V., Murphy C., Giner A., Teasdale R., Zerial M. Oligomeric complexes link Rab5 effectors with NSF and drive membrane fusion via interactions between EEA1 and syntaxin 13. Cell. 1999;98:377–386. doi: 10.1016/s0092-8674(00)81966-2. [DOI] [PubMed] [Google Scholar]
- Yang H., Young D. W., Gusovsky F., Chow J. C. Cellular events mediated by lipopolysaccharide-stimulated Toll-like receptor 4 MD-2 is required for activation of mitogen-activated protein kinases and Elk-1. J Biol Chem. 2000;275:20861–20866. doi: 10.1074/jbc.M002896200. [DOI] [PubMed] [Google Scholar]
- Flo T. H., Halaas O., Torp S., Ryan L., Lien E., Dybdahl B., Sundan A., Espevik T. Differential expression of Toll-like receptor 2 in human cells. J Leukoc Biol. 2001;69:474–481. [PubMed] [Google Scholar]
- Griffith K. J., Chan E. K., Lung C. C., Hamel J. C., Guo X., Miyachi K., Fritzler M. J. Molecular cloning of a novel 97-kd Golgi complex autoantigen associated with Sjogren’s syndrome. Arthritis Rheum. 1997;40:1693–1702. doi: 10.1002/art.1780400920. [DOI] [PubMed] [Google Scholar]
- Nakamura N., Rabouille C., Watson R., Nilsson T., Hui N., Slusarewicz P., Kreis T. E., Warren G. Characterization of a cis-Golgi matrix protein, GM130. J Cell Biol. 1995;131:1715–1726. doi: 10.1083/jcb.131.6.1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W., Feng Y., Chen D., Wandinger-Ness A. Rab11 is required for trans-Golgi network-to-plasma membrane transport and a preferential target for GDP dissociation inhibitor. Mol Biol Cell. 1998;9:3241–3257. doi: 10.1091/mbc.9.11.3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deretic D. Rab proteins and post-Golgi trafficking of rhodopsin in photoreceptor cells. Electrophoresis. 1997;18:2537–2541. doi: 10.1002/elps.1150181408. [DOI] [PubMed] [Google Scholar]
- Ullrich O., Reinsch S., Urbe S., Zerial M., Parton R. G. Rab11 regulates recycling through the pericentriolar recycling endosome. J Cell Biol. 1996;135:913–924. doi: 10.1083/jcb.135.4.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh A. K., O'Tousa J. E., Ozaki K., Ready D. F. Rab11 mediates post-Golgi trafficking of rhodopsin to the photosensitive apical membrane of Drosophila photoreceptors. Development. 2005;132:1487–1497. doi: 10.1242/dev.01704. [DOI] [PubMed] [Google Scholar]
- Johnsen I. B., Nguyen T. T., Ringdal M., Tryggestad A. M., Bakke O., Lien E., Espevik T., Anthonsen M. W. Toll-like receptor 3 associates with c-Src tyrosine kinase on endosomes to initiate antiviral signaling. EMBO J. 2006;25:3335–3346. doi: 10.1038/sj.emboj.7601222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latz E., Schoenemeyer A., Visintin A., Fitzgerald K. A., Monks B. G., Knetter C. F., Lien E., Nilsen N. J., Espevik T., Golenbock D. T. TLR9 signals after translocating from the ER to CpG DNA in the lysosome. Nat Immunol. 2004;5:190–198. doi: 10.1038/ni1028. [DOI] [PubMed] [Google Scholar]
- Talle M. A., Rao P. E., Westberg E., Allegar N., Makowski M., Mittler R. S., Goldstein G. Patterns of antigenic expression on human monocytes as defined by monoclonal antibodies. Cell Immunol. 1983;78:83–99. doi: 10.1016/0008-8749(83)90262-9. [DOI] [PubMed] [Google Scholar]
- Bazil V., Baudys M., Hilgert I., Stefanova I., Low M. G., Zbrozek J., Horejsi V. Structural relationship between the soluble and membrane-bound forms of human monocyte surface glycoprotein CD14. Mol Immunol. 1989;26:657–662. doi: 10.1016/0161-5890(89)90048-5. [DOI] [PubMed] [Google Scholar]
- Nilsen N., Nonstad U., Khan N., Knetter C. F., Akira S., Sundan A., Espevik T., Lien E. Lipopolysaccharide and double-stranded RNA up-regulate Toll-like receptor 2 independently of myeloid differentiation factor 88. J Biol Chem. 2004;279:39727–39735. doi: 10.1074/jbc.M405027200. [DOI] [PubMed] [Google Scholar]
- Damke H., Baba T., Warnock D. E., Schmid S. L. Induction of mutant dynamin specifically blocks endocytic coated vesicle formation. J Cell Biol. 1994;127:915–934. doi: 10.1083/jcb.127.4.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang T. H., Ulevitch R. J. Triad3A, an E3 ubiquitin-protein ligase regulating Toll-like receptors. Nat Immunol. 2004;5:495–502. doi: 10.1038/ni1066. [DOI] [PubMed] [Google Scholar]
- Schjetne K. W., Thompson K. M., Nilsen N., Flo T. H., Fleckenstein B., Iversen J. G., Espevik T., Bogen B. Cutting edge: link between innate and adaptive immunity: Toll-like receptor 2 internalizes antigen for presentation to CD4+ T cells and could be an efficient vaccine target. J Immunol. 2003;171:32–36. doi: 10.4049/jimmunol.171.1.32. [DOI] [PubMed] [Google Scholar]
- Husebye H., Halaas O., Stenmark H., Tunheim G., Sandanger O., Bogen B., Brech A., Latz E., Espevik T. Endocytic pathways regulate Toll-like receptor 4 signaling and link innate and adaptive immunity. EMBO J. 2006;25:683–692. doi: 10.1038/sj.emboj.7600991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandvig K., Grimmer S., Lauvrak S. U., Torgersen M. L., Skretting G., van Deurs B., Iversen T. G. Pathways followed by ricin and Shiga toxin into cells. Histochem Cell Biol. 2002;117:131–141. doi: 10.1007/s00418-001-0346-2. [DOI] [PubMed] [Google Scholar]
- Munro S., Pelham H. R. A C-terminal signal prevents secretion of luminal ER proteins. Cell. 1987;48:899–907. doi: 10.1016/0092-8674(87)90086-9. [DOI] [PubMed] [Google Scholar]
- Griffiths G., Ericsson M., Krijnse-Locker J., Nilsson T., Goud B., Soling H. D., Tang B. L., Wong S. H., Hong W. Localization of the Lys, Asp, Glu, Leu tetrapeptide receptor to the Golgi complex and the intermediate compartment in mammalian cells. J Cell Biol. 1994;127:1557–1574. doi: 10.1083/jcb.127.6.1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miesenbock G., Rothman J. E. The capacity to retrieve escaped ER proteins extends to the trans-most cisterna of the Golgi stack. J Cell Biol. 1995;129:309–319. doi: 10.1083/jcb.129.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandvig K., van Deurs B. Entry of ricin and shiga toxin into cells: molecular mechanisms and medical perspectives. EMBO J. 2000;19:5943–5950. doi: 10.1093/emboj/19.22.5943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torgersen M. L., Skretting G., van Deurs B., Sandvig K. Internalization of cholera toxin by different endocytic mechanisms. J Cell Sci. 2001;114:3737–3747. doi: 10.1242/jcs.114.20.3737. [DOI] [PubMed] [Google Scholar]
- Kirkham M., Fujita A., Chadda R., Nixon S. J., Kurzchalia T. V., Sharma D. K., Pagano R. E., Hancock J. F., Mayor S., Parton R. G. Ultrastructural identification of uncoated caveolin-independent early endocytic vehicles. J Cell Biol. 2005;168:465–476. doi: 10.1083/jcb.200407078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkham M., Parton R. G. Clathrin-independent endocytosis: new insights into caveolae and non-caveolar lipid raft carriers. Biochim Biophys Acta. 2005;1745:273–286. doi: 10.1016/j.bbamcr.2005.06.002. [DOI] [PubMed] [Google Scholar]
- Kitchens R. L., Wang P., Munford R. S. Bacterial lipopolysaccharide can enter monocytes via two CD14-dependent pathways. J Immunol. 1998;161:5534–5545. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.