Abstract
Surfactant protein A (SP-A) suppresses lymphocyte proliferation and IL-2 secretion, in part, by binding to its receptor, SP-R210. However, the mechanisms underlying this effect are not well understood. Here, we studied the effect of antibodies against the SP-A-binding (neck) domain (α-SP-R210n) or nonbinding C-terminal domain (α-SP-R210ct) of SP-R210 on human peripheral blood T cell immune responses against Mycobacterium tuberculosis. We demonstrated that both antibodies bind to more than 90% of monocytes and 5–10% of CD3+ T cells in freshly isolated PBMC. Stimulation of PBMC from healthy tuberculin reactors [purified protein derivative-positive (PPD+)] with heat-killed M. tuberculosis induced increased antibody binding to CD3+ cells. Increased antibody binding suggested enhanced expression of SP-R210, and this was confirmed by Western blotting. The antibodies (α-SP-R210n) cross-linking the SP-R210 through the SP-A-binding domain markedly inhibited cell proliferation and IFN-γ secretion by PBMC from PPD+ donors in response to heat-killed M. tuberculosis, whereas preimmune IgG and antibodies (α-SP-R210ct) cross-linking SP-R210 through the non-SP-A-binding, C-terminal domain had no effect. Anti-SP-R210n also decreased M. tuberculosis-induced production of TNF-α but increased production of IL-10. Inhibition of IFN-γ production by α-SP-R210n was abrogated by the combination of neutralizing antibodies to IL-10 and TGF-β1. Together, these findings support the hypothesis that SP-A, via SP-R210, suppresses cell-mediated immunity against M. tuberculosis via a mechanism that up-regulates secretion of IL-10 and TGF-β1.
Keywords: cytokine, tuberculosis
INTRODUCTION
The function of pulmonary surfactant was originally found to be the reduction of surface tension at the alveolar air-liquid interface, preventing alveolar collapse during respiration. However, recent studies have established that surfactant also regulates pulmonary immune defense against infections and local inflammatory responses [1]. The immunomodulatory functions of surfactant are primarily mediated through surfactant proteins A (SP-A) and SP-D [2], which belong to the mammalian collectin family of proteins that includes mannose-binding lectin and conglutinin [3, 4]. All collectins have an amino-terminal collagen-like stalk and a carboxy-terminal c-type lectin domain, the latter binding carbohydrate-containing molecules on the cell walls or membranes of infectious agents. Recognition of microorganisms by collectins triggers innate immune responses that facilitate microbial clearance.
SP-A is one of the most abundant SPs and is produced primarily by alveolar type II cells. SP-A enhances innate immunity by increasing phagocytosis of pathogenic microorganisms, including intracellular pathogens such as Mycobacterium tuberculosis [1, 5, 6]. SP-A can also up-regulate the synthesis of reactive oxygen intermediates and secretion of inflammatory cytokines [7, 8]. On the other hand, SP-A mediates resolution of inflammation [9] through enhanced clearance of apoptotic neutrophils [10, 11], suppression of cytokine production induced by Gram-negative organisms [12], and inhibition of NADPH oxidase [13]. In addition, studies in SP-A null mice suggested that SP-A regulates adaptive immunity in vivo [9, 14, 15], and corresponding in vitro studies show that SP-A influences migration and differentiation of APC [16]. Furthermore, SP-A suppresses allergen- and mitogen-induced T cell proliferation [15, 17,18,19] and IL-2 secretion [18], suggesting that SP-A regulates inflammation through inhibiting cell-mediated immunity. The inhibition of mitogen-induced lymphocyte proliferation in PBMC was associated with binding of SP-A to its receptor SP-R210 [20].
SP-R210 was originally purified from macrophage cell lines and alveolar type II cells [21] and subsequently shown to be present on human monocytes and nonadherent cells in PBMC [20]. Although several other mammalian proteins also bind SP-A [22,23,24,25], only SP-R210 has been shown to mediate inhibition of T cell proliferation and IL-2 secretion by SP-A [20]. However, the mechanisms underlying this inhibition remain unclear. We recently identified SP-R210 as unconventional myosin 18A (Myo18A) [26]. Alternative splicing of the Myo18A gene produces several long and short isoforms in a tissue- and cell-specific manner. A short isoform, SP-R210, serves as an extracellular receptor for SP-A in macrophages [26]. The antibodies generated against the neck region of the molecule, designated as α-SP-R210n, blocked SP-A binding, whereas the antibodies to the carboxy-terminal domain, α-SP-R210ct, did not, indicating that SP-R210n contains the SP-A-binding site [27]. Based on previous work indicating that SP-A inhibits lymphocyte proliferation through SP-R210 [18], the present study determined whether SP-R210 regulates lymphocyte proliferation and cytokine production during adaptive recall immune responses to M. tuberculosis antigens.
MATERIALS AND METHODS
Antibodies and SP-A
Polyclonal antibodies against SP-R210n or SP-R210ct were generated as described previously [26] by immunizing rabbits with recombinant domains of SP-R210 [28]. IgG from preimmune or immune sera was purified by affinity chromatography on a HiTrap protein G-sepharose column (GE Healthcare-Biosciences, Uppsala, Sweden). The purity of the preparation was assessed by SDS-PAGE, and the protein content was measured by the bicinchoninic acid (BCA) colorimetric assay (Pierce, Rockford, IL, USA). The endotoxin concentrations in αSP-R210n, αSP-R210ct, and preimmune IgG were in the range of 3.5–6.3 pg/μg IgG, as determined by the QCL-1000 Limulus amoebocyte lysate assay (Cambrex, Walkersville, MD, USA). Purified antibodies were lyophilized and stored at –70°C until use, and they were dissolved in sterile PBS before use. PE-conjugated antibodies to human CD3 (clone UCHT1) and CD14 (clone 61D3) were purchased from eBioscience (San Diego, CA, USA). FITC-conjugated anti-rabbit IgG was obtained from R&D Systems (Minneapolis, MN, USA) or Molecular Probes (Eugene, OR, USA). Neutralizing antibodies against IL-10 and TGF-β1 were purchased from R&D Systems. Human SP-A was isolated from spent alveolar proteinosis fluid, as described previously [26].
Stimuli for activation of PBMC
Heat-killed, whole-washed M. tuberculosis Erdman strain was kindly provided by Dr. Patrick Brennan (Colorado State University, Fort Collins, CO, USA) and used at 2.5 μg/ml as an antigen to stimulate PBMC. We also used 1 μg/ml anti-CD3 (OKT3, a generous gift from Ortho Biotechnology, Raritan, NJ, USA) and 0.5 μg/ml anti-CD28 (BD Biosciences, San Jose, CA, USA) to stimulate CD3+ T cells.
Preparation and culture of PBMC and CD3+ cells
Blood was obtained from healthy tuberculin skin test-positive [purified protein derivative-positive (PPD+)] and -negative (PPD–) donors according to protocols approved by the Institutional Review Board of the University of Texas Health Center (Tyler, TX, USA). PBMC were isolated by density gradient centrifugation over Ficoll-Paque (GE Healthcare-Biosciences). CD3+ cells were isolated from PBMC by negative selection with the Pan T Cell Isolation Kit II (Miltenyi Biotec Inc., Auburn, CA, USA), with a purity of >95%, as measured by flow cytometry with a FACSCalibur (BD Biosciences).
PBMC were cultured at 2 × 106 cells/ml in RPMI 1640, supplemented with 5 mg/ml glutamine, 100 μM nonessential amino acids, 100 U/ml penicillin, 100 μg/ml streptomycin, and 10% heat-inactivated pooled human AB serum (Atlanta Biologicals, Norcross, GA, USA) in a 96-well plate and incubated at 37°C in 5% CO2 for different time-points in the presence of heat-killed M. tuberculosis or medium alone, as indicated. The cell culture supernatants were collected after 48 h incubation, aliquoted, and stored at –70°C until the cytokine concentration was measured by ELISA.
Flow cytometry
Freshly isolated or cultured PBMC were washed and resuspended in FACS buffer, composed of Dulbecco’s PBS, pH 7.4, containing 2% heat-inactivated goat serum and 0.5% BSA at a cell density of 5–10 × 106 PBMC/ml. The cells were then distributed in 100 μl aliquots in Eppendorf tubes containing 2.5 μg preimmune IgG, α-SP-R210ct, or α-SP-R210n. After 30 min on ice, the cells were washed twice in FACS buffer and incubated for an additional 30 min with predetermined dilutions of FITC-conjugated goat anti-rabbit IgG and PE-conjugated mouse anti-human CD3 or CD14 mAb or rat isotype control IgG. The cells were washed twice in FACS buffer and resuspended in PBS, and the stained cells were analyzed by flow cytometry using a FACSCalibur (BD Biosciences).
Measurement of cell proliferation
The CFSE-based cell proliferation assay was performed as described previously [29]. Briefly, PBMC were washed three times with sterile HBSS, resuspended at 2 × 107 cells/ml in HBSS, and incubated with 2 μM CFSE (Invitrogen Life Technologies, Gaithersburg, MD, USA) at 37°C for 3 min. Labeling was then stopped by adding an equal volume of human serum, and cells were washed three times with serum-free RPMI 1640. The cells were then resuspended in RPMI 1640, supplemented with 10% pooled human AB serum, and cultured under different conditions as indicated. After 96 h, the cells were collected, washed, and resuspended in FACS staining buffer, and the distribution of CFSE-labeled cells was evaluated by flow cytometry.
Measurement of cytokine concentrations
The levels of IFN-γ, TNF-α, and IL-10 were measured in culture supernatants by sandwich ELISA using commercially available capture and detection antibodies (BD Biosciences).
Quantitation of IFN-γ mRNA by real-time PCR
Total RNA of PBMC cultured under different conditions was extracted with TRIzol LS reagent (Invitrogen Life Technologies), and 250 ng total RNA was treated with DNase Ι and reverse-transcribed to cDNA, as described previously [30]. Expression of IFN-γ and 18S rRNA was measured by real-time PCR using commercial primers and probe sets (Applied Biosystems, Foster City, CA, USA). Reactions were performed with the ABI Prism 7700 sequence detection system (Applied Biosystems). The expression levels of IFN-γ mRNA were calculated using the Δ comparative threshold (Ct) method after normalization for 18S rRNA and expressed as a ratio of M. tuberculosis-stimulated cells/cells cultured in medium alone: ΔCt = CtIFN-γ – Ct18srRNA; ΔΔCt sample = ΔCtIFN-γ with M. tuberculosis (M. tb) – ΔCtIFN-γ medium; fold change = 2–ΔΔCt sample.
Preparation of total cell extracts and Western blotting
PBMC were collected after incubation with 2.5 μg/ml heat-killed M. tuberculosis for different time-points, total cell protein extracts were prepared, as described previously [31], and the protein concentration was measured by the BCA method (Pierce), aliquoted, and stored at –70°C until use. SDS-PAGE and Western blotting were performed as described previously [31], loading 35 μg cell protein extracts for each sample and blotting with α-SP-R210ct or α-SP-R210n. The blots were stripped and reblotted for β-actin (Abcam Inc., Cambridge, MA, USA) as a loading control.
Statistical analysis
The paired or unpaired Student’s t-test was performed with GraphPad InStat3 software (GraphPad Software, Inc., San Diego, CA, USA), and P < 0.05 was considered statistically significant.
RESULTS
Recognition of SP-R210 on PBMC subpopulations by anti-SPR210 antibodies
The α-SP-R210ct and α-SP-R210n antibodies were used previously to study the expression of SP-R210 on murine tissues and cells and in human THP-1 and U937 cell lines [26], but their binding activities and the expression of their target molecule SP-R210 have not been studied in human primary lymphocytes. To study the effect of these antibodies on human adaptive immune responses, we first determined if these antibodies bind specific cellular subpopulations of PBMC.
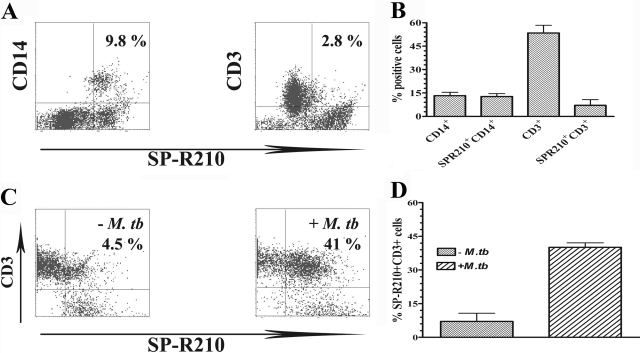
First, freshly isolated PBMC from three PPD+ donors were stained with α-SP-R210ct, in combination with PE-conjugated antibodies to CD14 or CD3, to identify monocytes and T cells that express SP-R210, respectively. Figure , 1Aand 1B, showed that α-SP-R210ct binds to virtually all CD14+ cells and a small fraction of CD3+ T cells in freshly isolated, unstimulated PBMC. However, the percentage of CD3+ cells that binds with α-SP-R210ct increased five- to eight-fold in 48 h M. tuberculosis-stimulated PBMC (Fig. , 1Cand 1D). Similar results were obtained with antibody to α-SP-R210n (data not shown).
Figure 1.
Binding of PBMC by anti-SP-R210 antibodies. After blocking in FACS staining buffer, PBMC were incubated with α-SP-R210ct for 30 min on ice. The cells were washed and further incubated with FITC-conjugated mouse anti-rabbit secondary antibody in combination with PE-CD14 or PE-CD3 antibodies for an additional 30 min. The cells were then washed and analyzed by flow cytometry. (A) Representative dot plots of freshly isolated PBMC, stained with anti-SP-R210 and anti-CD14 or anti-CD3 as indicated. The percentages of double-positive cells are shown in the upper right quadrant of each dot plot. (B) The mean ± se of cell populations and cells that express SP-R210 from four different donors. (C) Representative dot plots of SP-R210+ and CD3+ lymphocytes in PBMC, cultured in the absence (left panel) or presence (right panel) of heat-killed M. tuberculosis. (D) The mean ± se of SP-R210-expressing CD3+ cells from four donors, cultured with medium alone or with heat-killed M. tuberculosis.
To show that increased antibody binding to M. tuberculosis-stimulated PBMC correlated with elevated expression of their target molecule SP-R210, we performed Western blotting with α-SP-R210ct on whole cell protein extracts from unstimulated and M. tuberculosis-stimulated PBMC from three healthy tuberculin reactors. Figure 2 demonstrates that SP-R210 was markedly induced in M. tuberculosis-stimulated PBMC. The antibodies to SP-R210 detected the expected 210-kDa receptor and another 150-kDa fragment, as shown previously [21, 26, 28]. The latter represents the expression of an alternatively spliced SP-R210 mRNA in murine alveolar macrophages, lymph nodes, and bone marrow cells (Z.C.C. Chroneos, unpublished data). Both SP-R210 species were maximally induced after 24 h stimulation with M. tuberculosis, and this was not a result of differences in sample loading, as evidenced by equal expression of β-actin for all samples.
Figure 2.
Elevated expression of SP-R210 by PBMC in response to M. tuberculosis stimulation. Total cell protein extracts (35 μg) of PBMC from a PPD+ donor cultured in medium alone for 48 h or stimulated with heat-killed M. tuberculosis for 24 and 48 h were resolved in 7.5% SDS-PAGE, followed by electroblotting to a nitrocellulose membrane, which was then blocked and blotted for expression of SP-R210 with α-SP-R210ct (upper panel). The membrane was subsequently reblotted for β-actin (lower panel) as a protein loading control for each sample after stripping. One representative result from three different experiments is shown.
Effect of anti-SP-R210 on proliferation by M. tuberculosis-stimulated PBMC
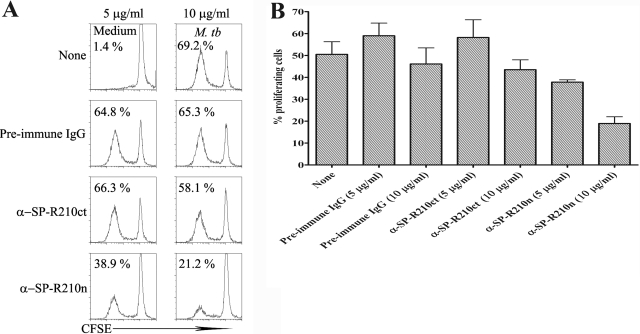
As SP-A is known to inhibit lymphocyte proliferation [18, 20], we wished to determine the effect of the anti-SP-R210 antibodies on M. tuberculosis-induced proliferation of PBMC from PPD+ persons. Using a CFSE-based assay, we found that stimulation of PBMC with heat-killed M. tuberculosis induced strong lymphocyte proliferation, which was inhibited by α-SP-R210n in a dose-dependent manner but not by α-SP-R210ct or preimmune IgG (Fig. 3A). These results were consistent in five PPD+ persons (Fig. 3B), and 10 μg/ml α-SP-R210n reduced the M. tuberculosis-induced percentage of proliferating cells to 18.9 ± 3.1% from 46.1 ± 7.3% for PBMC cultured with heat-killed M. tuberculosis and 10 μg/ml preimmune IgG (P=0.009). In contrast, α-SP-R210ct at the same concentration did not reduce proliferation (43.5±4.5% vs. 46.1±7.3% for preimmune IgG, P>0.05). These results suggest that α-SP-R210n is an agonistic antibody that mimics the T cell inhibitory effects of SP-A via binding to its receptor on PBMC.
Figure 3.
Anti-SP-R210n inhibits antigen-induced T cell proliferation. CFSE-labeled PBMC from five healthy PPD+ donors were cultured in medium alone or with 2.5 μg/ml heat-killed M. tuberculosis in the presence of preimmune IgG or anti-SP-R210 antibodies as indicated. After 96 h incubation, the cells were collected, gated on lymphocytes based on forward- and side-scatter patterns, and analyzed for CFSE content, and the percentages of proliferating cells were determined. (A) Histograms from a representative experiment are shown, indicating the proportion of proliferating cells. (B) Mean values and se shown for experiments performed with PBMC from five donors.
Effects of anti-SP-R210 on M. tuberculosis-induced cytokine secretion
Secretion of IFN-γ is essential for T cell-mediated immunity against M. tuberculosis and other intracellular pathogens. SP-A reduces production of IL-2, IL-4, and IL-5 by lymphocytes [15, 18, 20, 32], but the effects on IFN-γ production are not well-defined. Stimulation of PBMC from 12 PPD+ persons with heat-killed M. tuberculosis increased secretion of IFN-γ from 30 ± 13 pg/ml to 4291 ± 697 pg/ml, as we and others have shown previously [33]. Addition of α-SP-R210n to M. tuberculosis-stimulated PBMC markedly reduced IFN-γ concentrations by 75% to 1081 ± 375 pg/ml (P=0.0005; n=12). In contrast, preimmune IgG and α-SP-R210ct had no effect on M. tuberculosis-induced IFN-γ secretion (Fig. 4A). To evaluate the effects of α-SP-R210n in the absence of APC, purified CD3+ cells from PBMC of three PPD– donors were stimulated with α-CD3 and α-CD28 in the presence or absence of α-SP-R210n. Under these conditions, α-SP-R210n did not reduce IFN-γ production compared with results obtained with preimmune IgG at the same concentration (7318±1136 pg/ml vs. 7400±1295 pg/ml, P>0.05). This suggests that the inhibitory effect of α-SP-R210 is mediated through APC rather than a direct effect on T cells.
Figure 4.
Effects of anti-SP-R210 on M. tuberculosis-induced cytokine secretion. PBMC from 12 PPD+ donors were incubated in the presence or absence of different IgG (10 μg/ml) as indicated, together with 2.5 μg/ml heat-killed M. tuberculosis. After 48 h incubation, the culture supernatants were collected, and concentrations of IFN-γ (A), TNF-α (B), and IL-10 (C) were measured by sandwich ELISA. Mean values ± se are shown for each cytokine.
TNF-α is produced by Th1 cells and mononuclear phagocytes in response to M. tuberculosis infection and is essential for effective immunity [34]. As in the case of IFN-γ, stimulation of PBMC from PPD+ donors with M. tuberculosis increased TNF-α concentrations from 36 ± 15 pg/ml to 2739 ± 259 pg/ml. Preimmune IgG and α-SP-R210ct had no major effect on TNF-α production (2242±190 pg/ml and 2275±419 pg/ml, respectively), whereas α-SP-R210n reduced M. tuberculosis-stimulated TNF-α concentrations by almost 70% to 1039 ± 168 pg/ml (P=0.001, Fig. 4B).
As α-SP-R210n inhibited production of the proinflammatory cytokines, IFN-γ and TNF-α, we wished to determine if this was mediated by increased production of the anti-inflammatory cytokine IL-10, which inhibits IFN-γ secretion by Th1 cells [29]. Addition of heat-killed M. tuberculosis induced IL-10 secretion by PBMC from 19 ± 8 pg/ml to 366 ± 60 pg/ml. The presence of α-SP-R210n further increased M. tuberculosis-induced IL-10 nearly four-fold to 1360 ± 143 pg/ml (P<0.0001, n=15, Fig. 4C), whereas preimmune IgG and α-SP-R210ct had no effect (474±78 pg/ml and 381±58 pg/ml, respectively).
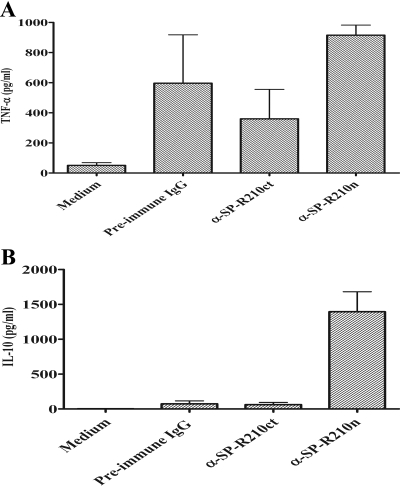
Effects of anti-SP-R210 alone on cytokine secretion
To determine if the antibodies used in this study directly induced cytokine secretion by PBMC in the absence of M. tuberculosis stimulation, PBMC from five PPD– donors were cultured with medium alone or with 10 μg/ml preimmune IgG, α-SP-R210n, or α-SP-R210ct for 48 h, and cytokine concentrations were measured in culture supernatants. Mean IFN-γ concentrations were extremely low, ranging from 5 to 18 pg/ml, and did not differ between groups (data not shown). The two antibodies and preimmune IgG elicited production of modest concentrations of TNF-α, and mean values ranged from 300–900 pg/ml (Fig. 5A). This may be a result of binding of rabbit IgG to the FcRI on monocytes, as reported previously [35]. In contrast to the findings with IFN-γ and TNF-α, α-SP-R210n induced production of 1394 ± 287 pg/ml IL-10 compared with 74 ± 42 pg/ml for cells treated with preimmune IgG and 62 ± 32 pg/ml for those treated with α-SP-R210ct (Fig. 5B, P=0.002, compared with α-SP-R210n). These findings suggest that cross-linking of SP-R210 through the SP-A-binding domain with α-SP-R210n directly stimulates PBMC to produce IL-10, which in turn inhibits secretion of M. tuberculosis-stimulated IFN-γ and TNF-α.
Figure 5.
Direct effect of anti-SP-R210 antibodies on cytokine secretion. PBMC from five PPD– donors were cultured in the absence or presence of different IgGs (10 μg/ml) as indicated. After 48 h incubation, concentrations of TNF-α (A) and IL-10 (B) were measured in culture supernatants by sandwich ELISA. Mean values ± se are shown.
Effects of SP-A on M. tuberculosis-induced cytokine secretion
To determine if the effects we observed with α-SP-R210n paralleled those of SP-A, we added 5–20 μg/ml purified human SP-A to M. tuberculosis-stimulated PBMC from four PPD+ persons. SP-A inhibited IFN-γ production in a dose-dependent manner, and 20 μg/ml reduced IFN-γ production by 60% (1168±487 pg/ml vs. 2978±2131 pg/ml). This concentration of SP-A also inhibited M. tuberculosis-induced TNF-α secretion by PBMC by 30% (579±111 pg/ml vs. 813±148 pg/ml) and increased M. tuberculosis-stimulated IL-10 secretion by 43% (420±66 pg/ml vs. 241±48 pg/ml). The effects of SP-A on production of IFN-γ, TNF-α, and IL-10 paralleled those of α-SP-R210n, although they were generally more modest. Thus, we concluded that α-SP-R210n behaved as an agonistic antibody.
Effects of anti-IL-10 and anti-TGF-β1 on inhibition of IFN-γ production by α-SP-R210n
The results above indicate that α-SP-R210n inhibits production of IFN-γ and stimulates secretion of IL-10. To determine if the reduction in IFN-γ was mediated through IL-10, we added IL-10 neutralizing antibodies to PBMC from five PPD+ persons that were cultured with M. tuberculosis in the presence of α-SP-R210n. Anti-IL-10 partially reversed the inhibitory effect of α-SP-R210n, and IFN-γ concentrations increased from 1323 ± 351 pg/ml to 1963 ± 613 pg/ml (Fig. 6A). As TGF-β1 also inhibits the Th1 response, and SP-A induces alveolar macrophages to produce TGF-β1 [11], we also tested the effect of neutralizing TGF-β1. Anti-TGF-β1 had effects similar to those of anti-IL-10, increasing IFN-γ levels to 2537 ± 844 pg/ml, and the combination of both antibodies markedly increased IFN-γ concentrations to M. tuberculosis-stimulated levels of 4519 ± 951 pg/ml (P=0.01, n=5, compared with 1323±351 pg/ml with M. tuberculosis and α-SP-R210n only, Fig. 6A).
Figure 6.
Neutralization of IL-10 and TGF-β1 abrogates the inhibitory effect of α-SP-R210n on M. tuberculosis-induced production of IFN-γ. PBMC were cultured in medium alone or with 2.5 μg/ml heat-killed M. tuberculosis in the absence or presence of different IgG (10 μg/ml) as indicated. In some cases, 1 μg/ml neutralizing antibodies to IL-10 and TGF-β1 were also added. After 48 h incubation, IFN-γ levels in the culture supernatants were measured by ELISA (A), cells were collected, and IFN-γ mRNA expression was measured by real-time PCR, normalized to 18S rRNA content (B). The mean ± se of five (A) or six (B) donors is shown.
As IFN-γ expression is regulated primarily at the transcriptional level, we also studied the effect of α-SP-R210n on M. tuberculosis-induced expression of IFN-γ mRNA by PBMC from six PPD+ subjects, using real-time PCR. Addition of α-SP-R210n to M. tuberculosis-stimulated PBMC inhibited IFN-γ mRNA expression by more than 90% (39±17-fold, compared with 534±119-fold, P<0.001, Fig. 6B). In contrast, preimmune IgG and α-SP-R120ct had no effect. IFN-γ mRNA levels were increased significantly by anti-IL-10 (244±65-fold vs. 39±17-fold, P=0.004) and α-TGF-β1 (328±77-fold vs. 39±17-fold, P=0.001). When both cytokines were neutralized, IFN-γ mRNA levels reached baseline values of 606 ± 12-fold, similar to those of M. tuberculosis-stimulated PBMC. These findings suggest that α-SP-R210n inhibited M. tuberculosis-induced IFN-γ secretion by PBMC through induction of IL-10 and TGF-β1.
DISCUSSION
The data presented in the current report demonstrate that anti-SP-R210 antibodies, α-SP-R210n and α-SP-R210ct, bind to more than 90% of CD14+ monocytes and less than 10% of CD3+ cells in freshly isolated PBMC. Stimulation of PBMC from PPD+ donors with heat-killed M. tuberculosis markedly increased the binding of antibodies to CD3+ cells, and this was correlated with increased expression of SP-R210 in PBMC after stimulation. The antibody, α-SP-R210n, inhibited proliferation of lymphocytes and secretion of IFN-γ by PBMC from healthy PPD+ donors in response to heat-killed M. tuberculosis, whereas preimmune IgG and the antibody α-SP-R210ct had no effect. Inhibition of IFN-γ production depended on the presence of APC. The antibodies against SP-R210n also inhibited M. tuberculosis-induced production of TNF-α and enhanced production of IL-10, and inhibition of IFN-γ production by α-SP-R210n was abrogated by neutralization of IL-10 and TGF-β1 simultaneously. As our studies were performed with polyclonal rather than mAb to SP-R210, we cannot exclude with certainty the possibility that some of the effects observed may be a result of binding of these polyclonal antibodies to unintended targets. Future studies with monoclonal reagents would be helpful to resolve this issue. Nevertheless, our current findings indicate that SP-A, via SP-R210, suppresses cell-mediated, immune responses to M. tuberculosis by enhanced production of IL-10 and TGF-β1 by APC.
Studies in animals and humans, using in vitro and in vivo models, have demonstrated that SPs, especially SP-A, exhibit potent regulatory effects on immunity and inflammatory reactions in the lung [1, 2, 14, 36]. Although the effect of SP-A on innate immunity has been studied extensively [1, 2], its effect on adaptive immunity in humans has been largely limited to studies of cell proliferation and IL-2 secretion in response to mitogens and anti-CD3 mAb [18,19,20, 32, 37]. In the current report, we evaluated the effects of SP-A in a more physiologically relevant recall immune response to microbial antigen by human primary T cells from healthy persons infected with M. tuberculosis. We found that the antibodies targeted against the SP-A-binding domain (α-SP-R210n) markedly inhibited M. tuberculosis-induced proliferation of lymphocytes and secretion of IFN-γ and TNF-α in a dose-dependent manner (Figs. 3and 4). Anti-SP-R210n also elicited production of high concentrations of the anti-inflammatory cytokine IL-10, and the combination of neutralizing antibodies to IL-10 and TGF-β1 abrogated the inhibitory effects of α-SP-R210n (Fig. 6). Previous studies showed that SP-A induced TGF-β1 production by alveolar macrophages [11], whereas the effect of SP-A on IL-10 production is more controversial. Chignard and colleagues [38, 39] showed that SP-A inhibited IL-10 production by LPS-stimulated murine alveolar macrophages, and the same group also reported that SP-A stimulated IL-10 production by bone marrow macrophages and U937 cells [40]. In another study, SP-A-mediated uptake of respiratory syncytial virus suppressed virus-induced IL-10 secretion by primary monocytes and U937 monocytic cells [41]. Importantly, SP-A-deficient mice had reduced the Th2 response and IL-10 secretion following pulmonary infection with influenza A virus, compared with wild-type animals [14], indicating that SP-A regulates secretion of IL-10 in vivo. Underlying these results is the ability of SP-A to interact with pathogens and host cells, acting to block or stimulate pathogen-induced responses on one hand and modulating the inflammatory response of immune cells on the other [1, 2]. Here, direct probing of the functional domain of the SP-A receptor SP-R210 with an antibody revealed that this receptor inhibits Th1 immune responses. The current findings suggest that SP-A binds to SP-R210n, which is present on the vast majority of monocytes, eliciting production of IL-10 and TGF-β1, known to inhibit production of IFN-γ by reducing the capacity of APC to produce IL-12 and to express costimulatory molecules [42, 43]. Our results extend the findings of previous reports that SP-A inhibits expression of costimulatory molecules on murine bone marrow-derived dendritic cells and reduces their capacity to support T cell proliferation in response to antigen [16].
We found that the inhibitory effects of α-SP-R210n on production of M. tuberculosis-induced IFN-γ depended on the presence of monocytes. Although SP-A is primarily produced by airway epithelial cells, many reports document that SP-A is present in multiple tissues, including the gastrointestinal and sinus mucosa, peritoneum, pericardium, and skin [44,45,46,47]. SP-A is also present in plasma at low levels, and these levels increase in patients with lung diseases such as pulmonary fibrosis and acute lung injury [48, 49]. We speculate that SP-A plasma levels may be elevated in chronic lung infections such as tuberculosis and that this may contribute to the reduced production of IFN-γ by peripheral blood T cells in response to mycobacterial antigens in tuberculosis patients [33, 50].
The lung is continually exposed to foreign antigens, and uncontrolled inflammatory responses to these antigens could cause significant tissue damage and reduced gas exchange. Alveolar macrophages have potent phagocytic activity but low immunostimulatory capacity and inhibit the response of alveolar T lymphocytes to mitogens, in part, through SP-A [51, 52], which also inhibits the Th2 immune response in vivo in a mouse model of Aspergillus fumigatus-induced allergic disease [15]. We found that expression of SP-R210 was markedly increased in T lymphocytes after stimulation by M. tuberculosis(Fig. 1) and therefore, hypothesize that this may provide a back-up control mechanism to dampen T cell activation, in addition to the immunosuppressive effects of SP-A mediated through alveolar macrophages. Activated T cells that express SP-R210 may become susceptible to the effects of SP-A, which can directly inhibit cell-cycle progression of T cells and attenuate intracellular calcium release in the absence of APC [19]. Further studies are needed to clarify the direct effects of SP-A on T cells.
High levels of TGF-β1 are associated with active pulmonary tuberculosis [50, 53], but its precise role in the pathogenesis of tuberculosis remains unclear. TGF-β1 inhibits expression of SP-A and other SPs by alveolar type II epithelial cells [54, 55], and we recently found that SP-A levels were reduced during the first 15 days of pulmonary Mycobacterium bovis BCG infection, preceding development of adaptive immunity [27]. We speculate that SP-R210 mediates secretion of TGF-β1 by alveolar macrophages, which in turn inhibits SP-A production, facilitating initiation of protective Th1 immune response against tuberculosis. In later stages of infection, SP-R210-mediated TGF-β1 secretion may help control the inflammatory response and reduce tissue damage.
IL-10 is produced during the local immune response to M. tuberculosis [53, 56], but its role in tuberculosis pathogenesis is uncertain. Mice overexpressing IL-10 from T cells had increased susceptibility to reactivation tuberculosis at later stages of infection [57]. On the other hand, IL-10-deficient mice did not have enhanced resistance to tuberculosis [58]. The primary role of IL-10 in the lung is believed to be to reduce excessive inflammation and injury [59, 60]. Alveolar type I and type II epithelial cells, recruited monocytes, and alveolar macrophages are important sources of IL-10 in the lung [60,61,62], and the latter three cell types are known to express SP-R210 and respond to SP-A [26, 63]. In combination with previous studies, our findings support the hypothesis that SP-R210 is involved in balancing immune responses to mycobacterial infection through secretion of IL-10 and TGF-β1 in vivo.
In conclusion, we found that in a physiologically relevant system, α-SP-R210n antibodies inhibited production of TNF-α and IFN-γ as well as proliferation of antigen-specific T lymphocytes through eliciting enhanced production of IL-10 and TGF-β1. These results provide insight into the receptor-mediated mechanisms that underlie the anti-inflammatory roles of SP-A in innate and adaptive immunity in multiple previous studies. The present experimental approach obviates technical limitations arising from using native or recombinant SP-A molecules that may be contaminated with different amounts of LPS [64] or TGF-β1 [65]. The agonistic α-SP-R210n antibodies represent a new tool to understand the physiological functions of the SP-A and its receptor SP-R210 in modulating innate and adaptive immune responses.
Acknowledgments
This work was supported in part by grants from National Institutes of Health (A1063514 to P. F. B. and B. S.; HL068127 to Z. C. C.), the Margaret E. Byers Cain Chair for Tuberculosis Research (P. F. B.), and the Juvenile Diabetes Research Foundation International (5-2007-813 to Z. C. C. and B. S.).
References
- Kuroki Y., Takahashi M., Nishitani C. Pulmonary collectins in innate immunity of the lung. Cell Microbiol. 2007;9:1871–1879. doi: 10.1111/j.1462-5822.2007.00953.x. [DOI] [PubMed] [Google Scholar]
- Wright J. R. Immunoregulatory functions of surfactant proteins. Nat Rev Immunol. 2005;5:58–68. doi: 10.1038/nri1528. [DOI] [PubMed] [Google Scholar]
- Sastry K., Ezekowitz R. A. Collectins: pattern recognition molecules involved in first line host defense. Curr Opin Immunol. 1993;5:59–66. doi: 10.1016/0952-7915(93)90082-4. [DOI] [PubMed] [Google Scholar]
- Thiel S., Reid K. B. Structures and functions associated with the group of mammalian lectins containing collagen-like sequences. FEBS Lett. 1989;250:78–84. doi: 10.1016/0014-5793(89)80689-1. [DOI] [PubMed] [Google Scholar]
- Tenner A. J., Robinson S. L., Borchelt J., Wright J. R. Human pulmonary surfactant protein (SP-A), a protein structurally homologous to C1q, can enhance FcR- and CR1-mediated phagocytosis. J Biol Chem. 1989;264:13923–13928. [PubMed] [Google Scholar]
- Tino M. J., Wright J. R. Surfactant protein A stimulates phagocytosis of specific pulmonary pathogens by alveolar macrophages. Am J Physiol. 1996;270:L677–L688. doi: 10.1152/ajplung.1996.270.4.L677. [DOI] [PubMed] [Google Scholar]
- Weikert L. F., Lopez J. P., Abdolrasulnia R., Chroneos Z. C., Shepherd V. L. Surfactant protein A enhances mycobacterial killing by rat macrophages through a nitric oxide-dependent pathway. Am J Physiol Lung Cell Mol Physiol. 2000;279:L216–L223. doi: 10.1152/ajplung.2000.279.2.L216. [DOI] [PubMed] [Google Scholar]
- Weissbach S., Neuendank A., Pettersson M., Schaberg T., Pison U. Surfactant protein A modulates release of reactive oxygen species from alveolar macrophages. Am J Physiol. 1994;267:L660–L666. doi: 10.1152/ajplung.1994.267.6.L660. [DOI] [PubMed] [Google Scholar]
- Hohlfeld J. M., Erpenbeck V. J., Krug N. Surfactant proteins SP-A and SP-D as modulators of the allergic inflammation in asthma. Pathobiology. 2002;70:287–292. doi: 10.1159/000070744. [DOI] [PubMed] [Google Scholar]
- LeVine A. M., Kurak K. E., Bruno M. D., Stark J. M., Whitsett J. A., Korfhagen T. R. Surfactant protein-A-deficient mice are susceptible to Pseudomonas aeruginosa infection. Am J Respir Cell Mol Biol. 1998;19:700–708. doi: 10.1165/ajrcmb.19.4.3254. [DOI] [PubMed] [Google Scholar]
- Reidy M. F., Wright J. R. Surfactant protein A enhances apoptotic cell uptake and TGF-β1 release by inflammatory alveolar macrophages. Am J Physiol Lung Cell Mol Physiol. 2003;285:L854–L861. doi: 10.1152/ajplung.00439.2002. [DOI] [PubMed] [Google Scholar]
- McIntosh J. C., Mervin-Blake S., Conner E., Wright J. R. Surfactant protein A protects growing cells and reduces TNF-α activity from LPS-stimulated macrophages. Am J Physiol. 1996;271:L310–L319. doi: 10.1152/ajplung.1996.271.2.L310. [DOI] [PubMed] [Google Scholar]
- Crowther J. E., Kutala V. K., Kuppusamy P., Ferguson J. S., Beharka A. A., Zweier J. L., McCormack F. X., Schlesinger L. S. Pulmonary surfactant protein A inhibits macrophage reactive oxygen intermediate production in response to stimuli by reducing NADPH oxidase activity. J Immunol. 2004;172:6866–6874. doi: 10.4049/jimmunol.172.11.6866. [DOI] [PubMed] [Google Scholar]
- LeVine A. M., Hartshorn K., Elliott J., Whitsett J., Korfhagen T. Absence of SP-A modulates innate and adaptive defense responses to pulmonary influenza infection. Am J Physiol Lung Cell Mol Physiol. 2002;282:L563–L572. doi: 10.1152/ajplung.00280.2001. [DOI] [PubMed] [Google Scholar]
- Scanlon S. T., Milovanova T., Kierstein S., Cao Y., Atochina E. N., Tomer Y., Russo S. J., Beers M. F., Haczku A. Surfactant protein-A inhibits Aspergillus fumigatus-induced allergic T-cell responses. Respir Res. 2005;6:97. doi: 10.1186/1465-9921-6-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinker K. G., Garner H., Wright J. R. Surfactant protein A modulates the differentiation of murine bone marrow-derived dendritic cells. Am J Physiol Lung Cell Mol Physiol. 2003;284:L232–L241. doi: 10.1152/ajplung.00187.2002. [DOI] [PubMed] [Google Scholar]
- Wang H. X., Liu W. K., Ng T. B., Ooi V. E., Chang S. T. Immunomodulatory and antitumor activities of a polysaccharide-peptide complex from a mycelial culture of Tricholoma sp., a local edible mushroom. Life Sci. 1995;57:269–281. doi: 10.1016/0024-3205(95)00270-g. [DOI] [PubMed] [Google Scholar]
- Borron P., Veldhuizen R. A., Lewis J. F., Possmayer F., Caveney A., Inchley K., McFadden R. G., Fraher L. J. Surfactant associated protein-A inhibits human lymphocyte proliferation and IL-2 production. Am J Respir Cell Mol Biol. 1996;15:115–121. doi: 10.1165/ajrcmb.15.1.8679215. [DOI] [PubMed] [Google Scholar]
- Borron P. J., Mostaghel E. A., Doyle C., Walsh E. S., McHeyzer-Williams M. G., Wright J. R. Pulmonary surfactant proteins A and D directly suppress CD3+/CD4+ cell function: evidence for two shared mechanisms. J Immunol. 2002;169:5844–5850. doi: 10.4049/jimmunol.169.10.5844. [DOI] [PubMed] [Google Scholar]
- Borron P., McCormack F. X., Elhalwagi B. M., Chroneos Z. C., Lewis J. F., Zhu S., Wright J. R., Shepherd V. L., Possmayer F., Inchley K., Fraher L. J. Surfactant protein A inhibits T cell proliferation via its collagen-like tail and a 210-kDa receptor. Am J Physiol. 1998;275:L679–L686. doi: 10.1152/ajplung.1998.275.4.L679. [DOI] [PubMed] [Google Scholar]
- Chroneos Z. C., Abdolrasulnia R., Whitsett J. A., Rice W. R., Shepherd V. L. Purification of a cell-surface receptor for surfactant protein A. J Biol Chem. 1996;271:16375–16383. doi: 10.1074/jbc.271.27.16375. [DOI] [PubMed] [Google Scholar]
- Tino M. J., Wright J. R. Glycoprotein-340 binds surfactant protein-A (SP-A) and stimulates alveolar macrophage migration in an SP-A-independent manner. Am J Respir Cell Mol Biol. 1999;20:759–768. doi: 10.1165/ajrcmb.20.4.3439. [DOI] [PubMed] [Google Scholar]
- Gupta N., Manevich Y., Kazi A. S., Tao J. Q., Fisher A. B., Bates S. R. Identification and characterization of p63 (CKAP4/ERGIC-63/CLIMP-63), a surfactant protein A binding protein, on type II pneumocytes. Am J Physiol Lung Cell Mol Physiol. 2006;291:L436–L446. doi: 10.1152/ajplung.00415.2005. [DOI] [PubMed] [Google Scholar]
- Yamada C., Sano H., Shimizu T., Mitsuzawa H., Nishitani C., Himi T., Kuroki Y. Surfactant protein A directly interacts with TLR4 and MD-2 and regulates inflammatory cellular response Importance of supratrimeric oligomerization. J Biol Chem. 2006;281:21771–21780. doi: 10.1074/jbc.M513041200. [DOI] [PubMed] [Google Scholar]
- Gardai S. J., Xiao Y. Q., Dickinson M., Nick J. A., Voelker D. R., Greene K. E., Henson P. M. By binding SIRPα or calreticulin/CD91, lung collectins act as dual function surveillance molecules to suppress or enhance inflammation. Cell. 2003;115:13–23. doi: 10.1016/s0092-8674(03)00758-x. [DOI] [PubMed] [Google Scholar]
- Yang C. H., Szeliga J., Jordan J., Faske S., Sever-Chroneos Z., Dorsett B., Christian R. E., Settlage R. E., Shabanowitz J., Hunt D. F., Whitsett J. A., Chroneos Z. C. Identification of the surfactant protein A receptor 210 as the unconventional myosin 18A. J Biol Chem. 2005;280:34447–34457. doi: 10.1074/jbc.M505229200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szeliga J., Daniel D. S., Yang C. H., Sever-Chroneos Z., Jagannath C., Chroneos Z. C. Granulocyte-macrophage colony stimulating factor-mediated innate responses in tuberculosis. Tuberculosis (Edinb) 2008;88:7–20. doi: 10.1016/j.tube.2007.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szeliga J., Jordan J., Yang C. H., Sever-Chroneos Z., Chroneos Z. C. Bacterial expression of recombinant MyoXVIIIA domains. Anal Biochem. 2005;346:179–181. doi: 10.1016/j.ab.2005.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons A. B., Parish C. R. Determination of lymphocyte division by flow cytometry. J Immunol Methods. 1994;171:131–137. doi: 10.1016/0022-1759(94)90236-4. [DOI] [PubMed] [Google Scholar]
- Samten B., Ghosh P., Yi A. K., Weis S. E., Lakey D. L., Gonsky R., Pendurthi U., Wizel B., Zhang Y., Zhang M., Gong J., Fernandez M., Safi H., Vankayalapati R., Young H. A., Barnes P. F. Reduced expression of nuclear cyclic adenosine 5′-monophosphate response element-binding proteins and IFN-γ promoter function in disease due to an intracellular pathogen. J Immunol. 2002;168:3520–3526. doi: 10.4049/jimmunol.168.7.3520. [DOI] [PubMed] [Google Scholar]
- Samten B., Wizel B., Shams H., Weis S. E., Klucar P., Wu S., Vankayalapati R., Thomas E. K., Okada S., Krensky A. M., Barnes P. F. CD40 ligand trimer enhances the response of CD8+ T cells to Mycobacterium tuberculosis. J Immunol. 2003;170:3180–3186. doi: 10.4049/jimmunol.170.6.3180. [DOI] [PubMed] [Google Scholar]
- Borron P., McIntosh J. C., Korfhagen T. R., Whitsett J. A., Taylor J., Wright J. R. Surfactant-associated protein A inhibits LPS-induced cytokine and nitric oxide production in vivo. Am J Physiol Lung Cell Mol Physiol. 2000;278:L840–L847. doi: 10.1152/ajplung.2000.278.4.L840. [DOI] [PubMed] [Google Scholar]
- Zhang M., Lin Y., Iyer D. V., Gong J., Abrams J. S., Barnes P. F. T-cell cytokine responses in human infection with Mycobacterium tuberculosis. Infect Immun. 1995;63:3231–3234. doi: 10.1128/iai.63.8.3231-3234.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keane J., Gershon S., Wise R. P., Mirabile-Levens E., Kasznica J., Schwieterman W. D., Siegel J. N., Braun M. M. Tuberculosis associated with infliximab, a tumor necrosis factor α-neutralizing agent. N Engl J Med. 2001;345:1098–1104. doi: 10.1056/NEJMoa011110. [DOI] [PubMed] [Google Scholar]
- Hoffman T., Tripathi A. K., Lee Y. L., Bonvini E., Golding B. Inflammatory mediator release from human monocytes via immobilized Fc receptors. Its potential role in adverse reactions to systemic monoclonal antibody therapy Transplantation. 1992;54:343–346. doi: 10.1097/00007890-199208000-00027. [DOI] [PubMed] [Google Scholar]
- LeVine A. M., Whitsett J. A., Gwozdz J. A., Richardson T. R., Fisher J. H., Burhans M. S., Korfhagen T. R. Distinct effects of surfactant protein A or D deficiency during bacterial infection on the lung. J Immunol. 2000;165:3934–3940. doi: 10.4049/jimmunol.165.7.3934. [DOI] [PubMed] [Google Scholar]
- Wang J. Y., Shieh C. C., You P. F., Lei H. Y., Reid K. B. Inhibitory effect of pulmonary surfactant proteins A and D on allergen-induced lymphocyte proliferation and histamine release in children with asthma. Am J Respir Crit Care Med. 1998;158:510–518. doi: 10.1164/ajrccm.158.2.9709111. [DOI] [PubMed] [Google Scholar]
- Salez L., Balloy V., van Rooijen N., Lebastard M., Touqui L., McCormack F. X., Chignard M. Surfactant protein A suppresses lipopolysaccharide-induced IL-10 production by murine macrophages. J Immunol. 2001;166:6376–6382. doi: 10.4049/jimmunol.166.10.6376. [DOI] [PubMed] [Google Scholar]
- Chabot S., Salez L., McCormack F. X., Touqui L., Chignard M. Surfactant protein A inhibits lipopolysaccharide-induced in vivo production of interleukin-10 by mononuclear phagocytes during lung inflammation. Am J Respir Cell Mol Biol. 2003;28:347–353. doi: 10.1165/rcmb.4883. [DOI] [PubMed] [Google Scholar]
- Golioto A., Wright J. R. Effects of surfactant lipids and surfactant protein A on host defense functions of rat alveolar macrophages. Pediatr Res. 2002;51:220–227. doi: 10.1203/00006450-200202000-00016. [DOI] [PubMed] [Google Scholar]
- Barr F. E., Pedigo H., Johnson T. R., Shepherd V. L. Surfactant protein-A enhances uptake of respiratory syncytial virus by monocytes and U937 macrophages. Am J Respir Cell Mol Biol. 2000;23:586–592. doi: 10.1165/ajrcmb.23.5.3771. [DOI] [PubMed] [Google Scholar]
- Moore K. W., de Waal Malefyt R., Coffman R. L., O'Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- Letterio J. J., Roberts A. B. Regulation of immune responses by TGF-β. Annu Rev Immunol. 1998;16:137–161. doi: 10.1146/annurev.immunol.16.1.137. [DOI] [PubMed] [Google Scholar]
- Kim J. K., Kim S. S., Rha K. W., Kim C. H., Cho J. H., Lee C. H., Lee J. G., Yoon J. H. Expression and localization of surfactant proteins in human nasal epithelium. Am J Physiol Lung Cell Mol Physiol. 2007;292:L879–L884. doi: 10.1152/ajplung.00156.2006. [DOI] [PubMed] [Google Scholar]
- Bourbon J. R., Chailley-Heu B. Surfactant proteins in the digestive tract, mesentery, and other organs: evolutionary significance. Comp Biochem Physiol A Mol Integr Physiol. 2001;129:151–161. doi: 10.1016/s1095-6433(01)00312-9. [DOI] [PubMed] [Google Scholar]
- Madsen J., Kliem A., Tornoe I., Skjodt K., Koch C., Holmskov U. Localization of lung surfactant protein D on mucosal surfaces in human tissues. J Immunol. 2000;164:5866–5870. doi: 10.4049/jimmunol.164.11.5866. [DOI] [PubMed] [Google Scholar]
- Mo Y. K., Kankavi O., Masci P. P., Mellick G. D., Whitehouse M. W., Boyle G. M., Parsons P. G., Roberts M. S., Cross S. E. Surfactant protein expression in human skin: evidence and implications. J Invest Dermatol. 2007;127:381–386. doi: 10.1038/sj.jid.5700561. [DOI] [PubMed] [Google Scholar]
- Greene K. E., King T. E., Jr, Kuroki Y., Bucher-Bartelson B., Hunninghake G. W., Newman L. S., Nagae H., Mason R. J. Serum surfactant proteins-A and -D as biomarkers in idiopathic pulmonary fibrosis. Eur Respir J. 2002;19:439–446. doi: 10.1183/09031936.02.00081102. [DOI] [PubMed] [Google Scholar]
- Doyle I. R., Bersten A. D., Nicholas T. E. Surfactant proteins-A and -B are elevated in plasma of patients with acute respiratory failure. Am J Respir Crit Care Med. 1997;156:1217–1229. doi: 10.1164/ajrccm.156.4.9603061. [DOI] [PubMed] [Google Scholar]
- Hirsch C. S., Toossi Z., Othieno C., Johnson J. L., Schwander S. K., Robertson S., Wallis R. S., Edmonds K., Okwera A., Mugerwa R., Peters P., Ellner J. J. Depressed T-cell interferon-γ responses in pulmonary tuberculosis: analysis of underlying mechanisms and modulation with therapy. J Infect Dis. 1999;180:2069–2073. doi: 10.1086/315114. [DOI] [PubMed] [Google Scholar]
- Ansfield M. J., Kaltreider H. B., Benson B. J., Caldwell J. L. Immunosuppressive activity of canine pulmonary surface active material. J Immunol. 1979;122:1062–1066. [PubMed] [Google Scholar]
- Ansfield M. J., Kaltreider H. B., Caldwell J. L., Herskowitz F. N. Hyporesponsiveness of canine bronchoalveolar lymphocytes to mitogens: inhibition of lymphocyte proliferation by alveolar macrophages. J Immunol. 1979;122:542–548. [PubMed] [Google Scholar]
- Bonecini-Almeida M. G., Ho J. L., Boechat N., Huard R. C., Chitale S., Doo H., Geng J., Rego L., Lazzarini L. C., Kritski A. L., Johnson W. D., Jr, McCaffrey T. A., Silva J. R. Down-modulation of lung immune responses by interleukin-10 and transforming growth factor β (TGF-β) and analysis of TGF-β receptors I and II in active tuberculosis. Infect Immun. 2004;72:2628–2634. doi: 10.1128/IAI.72.5.2628-2634.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beers M. F., Solarin K. O., Guttentag S. H., Rosenbloom J., Kormilli A., Gonzales L. W., Ballard P. L. TGF-β1 inhibits surfactant component expression and epithelial cell maturation in cultured human fetal lung. Am J Physiol. 1998;275:L950–L960. doi: 10.1152/ajplung.1998.275.5.L950. [DOI] [PubMed] [Google Scholar]
- Koth L. L., Alex B., Hawgood S., Nead M. A., Sheppard D., Erle D. J., Morris D. G. Integrin β6 mediates phospholipid and collectin homeostasis by activation of latent TGF-β1. Am J Respir Cell Mol Biol. 2007;37:651–659. doi: 10.1165/rcmb.2006-0428OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balkhi M. Y., Sinha A., Natarajan K. Dominance of CD86, transforming growth factor-β 1, and interleukin-10 in Mycobacterium tuberculosis secretory antigen-activated dendritic cells regulates T helper 1 responses to mycobacterial antigens. J Infect Dis. 2004;189:1598–1609. doi: 10.1086/383328. [DOI] [PubMed] [Google Scholar]
- Turner J., Gonzalez-Juarrero M., Ellis D. L., Basaraba R. J., Kipnis A., Orme I. M., Cooper A. M. In vivo IL-10 production reactivates chronic pulmonary tuberculosis in C57BL/6 mice. J Immunol. 2002;169:6343–6351. doi: 10.4049/jimmunol.169.11.6343. [DOI] [PubMed] [Google Scholar]
- Jung Y. J., Ryan L., LaCourse R., North R. J. Increased interleukin-10 expression is not responsible for failure of T helper 1 immunity to resolve airborne Mycobacterium tuberculosis infection in mice. Immunology. 2003;109:295–299. doi: 10.1046/j.1365-2567.2003.01645.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson M. S., Elnekave E., Mentink-Kane M. M., Hodges M. G., Pesce J. T., Ramalingam T. R., Thompson R. W., Kamanaka M., Flavell R. A., Keane-Myers A., Cheever A. W., Wynn T. A. IL-13Rα2 and IL-10 coordinately suppress airway inflammation, airway-hyperreactivity, and fibrosis in mice. J Clin Invest. 2007;117:2941–2951. doi: 10.1172/JCI31546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garantziotis S., Brass D. M., Savov J., Hollingsworth J. W., McElvania-TeKippe E., Berman K., Walker J. K., Schwartz D. A. Leukocyte-derived IL-10 reduces subepithelial fibrosis associated with chronically inhaled endotoxin. Am J Respir Cell Mol Biol. 2006;35:662–667. doi: 10.1165/rcmb.2006-0055OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim S., Caramori G., Tomita K., Jazrawi E., Oates T., Chung K. F., Barnes P. J., Adcock I. M. Differential expression of IL-10 receptor by epithelial cells and alveolar macrophages. Allergy. 2004;59:505–514. doi: 10.1111/j.1398-9995.2004.00455.x. [DOI] [PubMed] [Google Scholar]
- Haase M. G., Klawitter A., Geyer P., Baretton G. B. Expression of the immunomodulator IL-10 in type I pneumocytes of the rat: alterations of IL-10 expression in radiation-induced lung damage. J Histochem Cytochem. 2007;55:1167–1172. doi: 10.1369/jhc.7A7173.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chroneos Z., Shepherd V. L. Differential regulation of the mannose and SP-A receptors on macrophages. Am J Physiol. 1995;269:L721–L726. doi: 10.1152/ajplung.1995.269.6.L721. [DOI] [PubMed] [Google Scholar]
- Wright J. R., Zlogar D. F., Taylor J. C., Zlogar T. M., Restrepo C. I. Effects of endotoxin on surfactant protein A and D stimulation of NO production by alveolar macrophages. Am J Physiol. 1999;276:L650–L658. doi: 10.1152/ajplung.1999.276.4.L650. [DOI] [PubMed] [Google Scholar]
- Kunzmann S., Wright J. R., Steinhilber W., Kramer B. W., Blaser K., Speer C. P., Schmidt-Weber C. TGF-β1 in SP-A preparations influence immune suppressive properties of SP-A on human CD4+ T lymphocytes. Am J Physiol Lung Cell Mol Physiol. 2006;291:L747–L756. doi: 10.1152/ajplung.00401.2005. [DOI] [PubMed] [Google Scholar]