Abstract
Background
Macrophage migration inhibitory factor (MIF) is essential for controlling parasite burden and survival in a model of systemic Toxoplasma gondii infection. Peroral T. gondii infection induces small intestine necrosis and death in susceptible hosts, and in many aspects resembles inflammatory bowel disease (IBD). Considering the critical role of MIF in the pathogenesis of IBD, we hypothesized that MIF participates in the inflammatory response induced by oral infection with T. gondii.
Methodology/Principal Findings
Mif deficient (Mif−/−) and wild-type mice in the C57Bl/6 background were orally infected with T. gondii strain ME49. Mif−/− mice had reduced lethality, ileal inflammation and tissue damage despite of an increased intestinal parasite load compared to wt mice. Lack of MIF caused a reduction of TNF-α, IL-12, IFN-γ and IL-23 and an increased expression of IL-22 in ileal mucosa. Moreover, suppressed pro-inflammatory responses at the ileal mucosa observed in Mif−/− mice was not due to upregulation of IL-4, IL-10 or TGF-β. MIF also affected the expression of matrix metalloproteinase-9 (MMP-9) but not MMP-2 in the intestine of infected mice. Signs of systemic inflammation including the increased concentrations of inflammatory cytokines in the plasma and liver damage were less pronounced in Mif−/− mice compared to wild-type mice.
Conclusion/Significance
In conclusion, our data suggested that in susceptible hosts MIF controls T. gondii infection with the cost of increasing local and systemic inflammation, tissue damage and death.
Introduction
The immune/inflammatory response to Toxoplasma gondii infection is essential to control parasite replication and tissue spread but also can cause tissue damage, being descisive to pathogenesis. Selective tissue invasion by T. gondii causes compartmental immune responses unique to each tissue such as the one present in the placenta, central nervous system and intestinal mucosa, while parasite spreading promotes a systemic response that resembles bacterial sepsis [1]–[3]. Inhibition or abrogation of TNF-α, IL-12 and/or IFN-γ increase host susceptibility to toxoplasmosis as a result of uncontrolled parasite burden [4]–[6]. In natural T. gondii infection through the oral route, exacerbated inflammatory responses at the small intestine (ileitis) resemble human inflammatory bowel disease (IBD) [1], [7].The inflammatory response coordinate by IL-23/IL-22 is involved in T. gondii-induced disruption of intestinal homeostasis and immunopathology, an effect at least in part due to increase expression of matrix metalloproteinase-2 (MMP) [7]. These pro-inflammatory mediators and MMPs are involved in the immune pathogenesis and tissue damage in T. gondii peoral infection, increasing host morbidity and mortality despite parasite control [7]–[9]. Deficient regulation of the inflammatory response observed in the absence of IL-10 results in massive leukocyte infiltration, extensive tissue damage despite control of parasite load, systemic inflammation and increase lethality [2].
MIF is a versatile molecule that comprises hormonal and enzymatic activities in addition to exert pro-inflammatory and chemotactic functions [10]–[13]. First described as a T-cell-derived factor involved in the inhibition of macrophage migration in delayed–type hypersensitivity response, MIF is also produced by other immune cells such as macrophages, neutrophils and eosinophils but also non immune cells such as endothelial, epithelial, muscle and pituitary cells [10]–[15]. MIF expression and production are regulated by adrenocorticotrophic hormone which results in counter-regulation of cortisol [11], [16]. MIF production is also regulated by TNF and LPS-Toll-like receptor 4 (TLR4) in a positive feedback cascade resulting in enhanced proinflammatory responses [15], [17], [18]. Furthermore, MIF is an essential regulator of innate and adaptive immune responses with a crucial role in host protection against different pathogens and is involved in the pathogenesis of a broad spectrum of infectious and non infectious inflammatory diseases [10]–[23].
In parasitic infections MIF is involved both in protective as well as immune pathogenic responses [12], [21], [24]–[28]. Upon systemic infection with T. gondii, IFN-γ and TNF-α mediated responses are upregulated by MIF, and Mif−/− mice are more susceptible to infection presenting increased parasite burden [27]. A recent study also demonstrated an increased susceptibility, reduced IL-12 production and dendritic cell activation on Mif−/− mice in the Balb/c background to an oral T. gondii infection [28]. Balb/c mice are naturally resistant to oral infection with T. gondii, while C57BL/6 are highly susceptible and display intestinal inflammation, especially in the ileum [29]–[31].
Considering the role of MIF on intestinal inflammation, we hypothesize that MIF participates in the pathogenesis of T. gondii-induced intestinal tissue damage. Our results indicate that MIF mediates pathogenic responses during T. gondii oral infection by exacerbating compartmental and systemic inflammatory responses. The lack of MIF caused an overall reduction of tissue damage despite of an increase in local parasite burden.
Materials and Methods
Mice
C57BL/6 (wild type, wt) and Mif−/− (backcrossed to the C57BL/6 genetic background, n = 8) were obtained from the animal facility at the Universidade Federal do Rio de Janeiro (UFRJ, Rio de Janeiro). Sex and age-matched (8–12 week-old) C57BL/6 were used as controls for Mif−/−. The animals were kept at constant temperature (25°C) with free access to chow and water in a room with a 12 h light/dark cycle. All animal experiments were performed in accordance to Brazilian Government's ethical and animal experiment regulations. The experiments were approved by the Institutional Animal Welfare Committee (approval ID: CEUA/CCS/UFRJ/IMPPG 011).
Parasites and per oral infection
The T. gondii strain ME49 was kindly provided by Dr R. Gazzinelli, (UFMG, Minas Gerais, Brazil) and Dr J. Lannes-Vieira (FIOCRUZ, Rio de Janeiro, Brazil). For peroral infection, mice were infected by gavage using 20 and 100 ME49 cysts/animal. Cysts were obtained from C57BL/6 brain homogenates as previously described [2].
Detection of T. gondii DNA
Parasites in tissues were quantified by a real-time polymerase chain reaction (RT-PCR) as previously described [7] targeting a repetitive 529-bp DNA fragment of T. gondii (GenBank accession no AF487550) [32] which are specific for a cryptic gene as described [33]. Fifty to 100 µg of ileum and liver tissues were crushed in a rotor-stator after frozen in liquid nitrogen and total DNA were isolated with DNAzol reagent (Invitogen Life Technologies) following the manufactures protocol. Five hundred ng of total DNA extracted from each sample were submitted to real-time PCR using Power SYBR® Green PCR master mix (Applied Biosystems) using each oligonucleotide primer (TOX-9, 5′-AGGAGAGATATCAGGACTGTAG-3′; TOX-10, 5′-GCGTCGTCTCGTCTAGATCG-3′). The amplification was performed using one cycle at 95°C for 10 min, 45 cycles at 95°C for 30 s, 60°C for 1 min. A standard curve was performed using 5000-5 pg T. gondii DNA to quantification of total DNA of samples.
Determination of small intestinal length, histopathology and histological scores
Small intestinal shortening was determined by measuring whole intestinal segment of wt and Mif−/− controls and T. gondii-infected mice. It was calculated by dividing the difference of the mean length of the small intestine from control mice minus the length from infected mice at day 8 after infection and then multiplied by 100 over the mean length of mice small intestines [34]. Intestines, liver and brain were fixed in 10% neutral buffered formalin, embedded in paraffin, sectioned at 5 µm, and stained with hematoxylin and eosin using standard techniques. Blinded samples were submitted for semi-quantitative histopathologic analysis. Histological scores were determined in fixed and paraffin-embedded tissue sections taken from the terminal ileum, as previously described [34]. Liver sections were analyzed for the numbers of inflammatory foci at a magnification of ×200 at day 9 after infection in each group. We analyzed the number of inflammatory foci per field (6.5×9 mm, microscope Zeiss, Germany) counting 10 fields of each section.
Determination of cytokine concentrations at the intestinal tissue and plasma
Plasma samples were obtained to measure cytokine concentrations. IFN-γ and TNF-α concentrations were measured by a commercial ELISA from Peprotech (NJ, USA). Concentrations of IL-10 was determined by ELISA using a commercial kit from R&D Systems (MN, USA).
RT-PCR analysis of matrix metalloproteinases and cytokines
Approximately 50–100 µg of ileum tissue were crushed in a rotor–stator after frozen in liquid nitrogen and total RNA were isolated with TRIzol reagent (Invitogen Life Technologies) following the manufactures protocol. Four micrograms of total RNA extracted were reverse transcribed using a High capacity cDNA reverse transcription kit (Applied Biosystems, Foster City, CA). Each sample was submitted to real-time PCR using Power SYBR® Green PCR master mix (Applied Biosystems). The reactions were carried out using specific primers for the following genes: MMP-2 (forward, 5′-CAATCTTTTCTGGGAGCTC-3′; reverse, 5′-GCTGATACTGACACTGGTACTG-3′), MMP-9 (forward, 5′-CCTGGAACTCACACGACATCTTC-3′; reverse, 5′-TGGAAACTCACACGCCAGAA-3′), TNF-α (forward, 5′-CCTCACACTCAGATCATCTTCTCA-3′; reverse, 5′- TGGTTGTCTTTGAGATCCATGC-3′), IL-6 (forward, 5′- TCATATCTTCAACCAAGAGGTA-3′; reverse, 5′- CAGTGAGGAATGTCCACAAACTG-3′), IFN-γ (forward, 5′-AGCAACAGCAAGGCGAAAA-3′; reverse, 5′- CTGGACCTGTGGGTTGTTGA -3′), IL-17A (forward, 5′-TCCCTCTGTGATCTGGGAAG-3′; reverse, 5′-CTCGACCCTGAAAGTGAAGG-3′), TGF-β (forward, 5′-GACCGCAACAACGCCATCTA-3′; reverse, 5′-AGCCCTGTATTCCGTCTCCTT-3′), IL-12p35 (forward, 5′-CCACCCTTGCCCTCCTAAAC-3′; reverse, 5′-GGCAGCTCCCTCTTGTTGTG-3′), IL-23 (forward, 5′-TGGCATCGAGAAACTGTGAGA-3′; reverse, 5′-TCAGTTCGTATTGGTAGTCCTGTTA-3′), IL-22 (forward, 5′-GACAGGTTCCAGCCCTACAT-3′; reverse, 5′-GTCGTCACCGCTGATGTG-3′), IL-10 (forward, 5′-TAAGGGTTACTTGGGTTGCCAAG-3′; reverse, 5′-CAAATGCTCCTTGATTTCTGGGC-3′). The samples were subjected to 45 amplification cycles consisting in 95°C for 30 s, 60°C for 1 min. The expression of the hypoxanthine guanine phosphoribosyl transferase 1 (Hprt1) gene (forward, 5′-GCTGGTGAAAAGGACCTCT-3′; reverse 5′-CAC AGG ACT AGA ACA CCT GC-3′) was used to normalize the results, which were presented as fold induction of mRNA expression relative to control samples. Moreover, mouse TaqMan pre-developed assay reagents (Applied Biosystems) were used for IL-4 and Hprt amplification. The analyses of relative gene expression data were performed by 2−ΔΔC T method [35].
Statistical analysis
Data are expressed as means ± SEM. The statistical significance of differences in mean values was determined by using Student's t test or ANOVA. Survival data are presented as a Kaplan-Meier survival curve and analyzed with Log-rank test by software Prism 5 (USA). Differences of at least p≤0.05 are considered significant.
Results
Reduced mortality and morbidity of Mif−/− mice after T. gondii oral infection
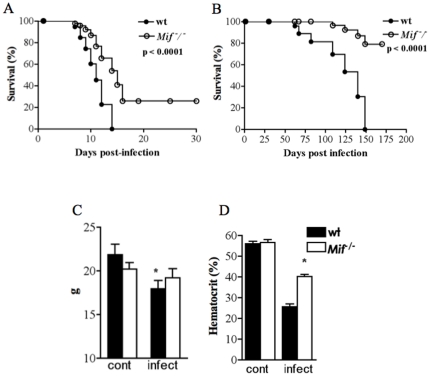
It has been recently demonstrated that MIF determines host susceptibility to T. gondii infection by peritoneal route [27]. Mif−/− mice presente increased parasite load and systemic inflammatory response when compared to wt mice. However, T. gondii infection through the oral route might determine a different outcome depending on the inflammatory response within the intestinal mucosa [36], . To define the role of MIF during natural T. gondii infection in susceptible hosts, wt and Mif−/− mice were orally infected with 100 or 20 cysts per animal. Wt mice infected with 100 cysts were all dead after 10–12 days post infection while Mif−/− mice had a significant increase of survival (Fig. 1A). The increase survival of Mif−/− compared to wt mice was even more pronounced upon infection with 20 cysts/animal (Fig. 1B). To assess morbidity during acute T. gondii infection, we used weight loss and hematocrit measurement, considered good markers of morbidity in acute acquired toxoplasmosis [38]. Although both wt and Mif−/− mice presented weight loss, it was more severe in wt than on Mif−/− mice (Fig. 1C). Similarly, hematocrit was significantly reduced in infected wt when compared to Mif−/− mice (Fig. 1D). Thus, Mif−/− mice displayed increased resistance to acute infection and reduced morbidity following per oral T. gondii infection.
Figure 1. MIF increases morbidity and mortality during peroral T. gondii infection.
Survival curves of Mif −/− (C57BL/6) mice (6–8 weeks old) and age and sex-matched wild type controls orally infected with 100 (A) and 20 cysts (B) of ME-49 strain of T. gondii. Morbidity was evaluated by determining animal (C) relative weight loss and (D) hematocrit. One representative experiment out of three performed. Data from 8 to 10 animals per group are given as mean ± SEM, and p values were determined by t-test (* p≤0.05) or by Log-rank test (A, B). Cont, control not infected.
Reduced ileal damage despite of increased parasite loads in the absence of MIF
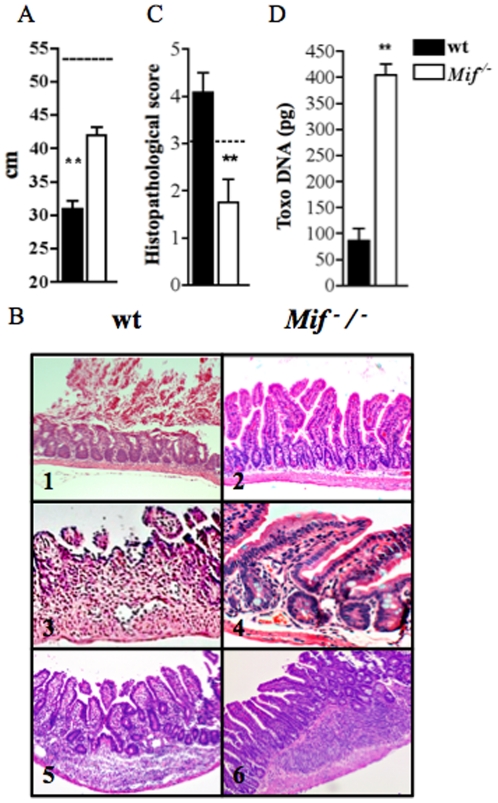
To determine the cause of reduced mortality of Mif−/− mice, we initially performed macroscopical and histopathological analysis of the small intestine of control and infected animals. At day 9 post infection, wt mice presented significant intestinal shortening compared to Mif−/− mice (Fig. 2A). This intestinal shrinkage observed in wt mice was associated with histological changes at the terminal ileum, including blunting of the villi, extensive necrosis and full thickness inflammatory infiltration of the lamina propria (Fig. 2B). Infected Mif−/− mice presented reduced necrosis and decreased loss of mucosal integrity, lamina propria and intestinal wall and more preserved areas of intestine. Tissue damage was determined by histopathological scores of the terminal ileum of infected mice, and most samples from wt mice presented higher scores when compared to Mif−/− mice (Fig. 2C). In fact, Mif−/− mice displayed less tissue damage in contrast to a significant increase in tissue parasitism (Fig. 2D). These findings indicate that MIF-mediated inflammatory response promotes tissue damage, contributing to morbidity and mortality, but also participates on T. gondii control at the intestinal mucosa.
Figure 2. T. gondii-induced ileitis is mediated by MIF that induces severe tissue damage despite efficient parasite control.
(A) Shortening of small intestine of wt and Mif −/− mice (n = 6) at 9 days post-infection (dpi). (B) Histopathologycal analysis by hematoxilin and eosin (HE) of terminal ileum was performed at 9 dpi in wt and Mif −/−. Magnification are ×200 (B1, 2, 5 and 6) and ×400 (B3 and 4). In (C) Histologycal scores of ileal biopsies of wt and Mif −/− mice at 9 dpi. The horizontal line limits absence of inflammatory response (0 to 3) and necrosis (above 3). (D) Toxo DNA concentration was determined in ileal biopsies of wt and Mif −/− mice. One representative experiment out of three independent experiments. Data from 3 to 6 mice per group is represented by means ± SEM and p values determined by t-test (* p≤0.05, ** p≤0.01). Length of intestinal segments of control not infected wt and Mif −/− mice are represented by a dotted line in A. Cont, control not infected.
MIF regulates inflammatory cytokines in T. gondii-induced ileitis
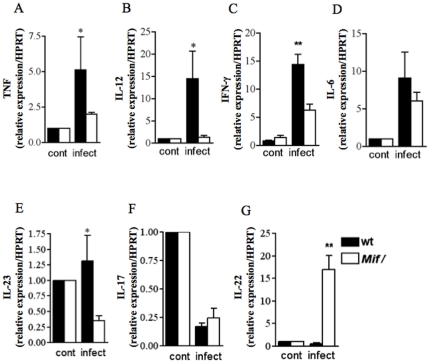
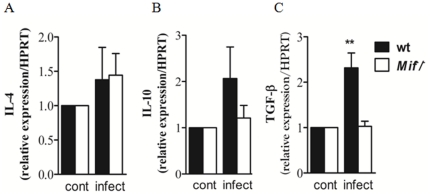
To investigate the mechanism by which MIF regulates the inflammatory response at the intestinal mucosa, we examined mRNA expression and the concentrations of inflammatory cytokines in the supernatants of ileal mucosal explants from wt and Mif−/− infected mice. TNF-α, IL-12, IFN-γ and IL-23 expression were decreased in infected Mif−/− mice when compared to wt mice (Fig. 3A–C, E). The mRNA expression of IL-6 and IL-17A were also similar in wt and Mif−/− mice (Fig. 3D, F). Surprisingly, IL-22 expression was significantly increased in infected Mif−/− compared to wt mice (Fig. 3G). To determine if IL-4, IL-10 and TGF-β could be implicated in the suppression of immunopathology and increased parasite burdens of Mif−/−, we examined the mRNA expression of these regulatory cytokines in the ileal mucosa explants of control and infected wt and Mif−/− mice. While IL-4 mRNA expression was similar in wt and Mif−/− mice at 9 days post infection (Fig. 4A), IL-10 mRNA expression and protein in ileal mucosa were reduced in Mif−/− compared to wt mice (Fig. 4B, data not shown). Similarly, T. gondii infected Mif−/− mice presented significant decrease of TGF-β expression when compared to infected wt mice (Fig. 4C). These results indicate that the reduced inflammatory response in the ileun of Mif−/− compared to wt mice is not due to up regulation of IL-4, IL-10 or TGF-β.
Figure 3. MIF upregulates proinflammatory responses in toxoplasmic ileitis.
Real-time PCR of (A) TNF-α, (B) IL-12, (C) IFN-γ, (D) IL-6, (E) IL-23, (F) IL-17 and (G) IL-22 expression in ileal explants from control and infected wt and Mif −/− mice. Results are expressed as fold changes relative to HPRT mRNA expression. Data from 3 to 6 mice per group is represented by means ± SEM and p values determined by t-test (* p≤0.05, ** p≤0.01). Cont, control not infected.
Figure 4. Reduced intestinal expression of IL-10 and TGF-β in Mif−/− mice.
Quantitative RT-PCR of of (A) IL-4, (B) IL-10 and (C) TGF-β mRNA expression in ileal explants of control and infected wt and Mif −/− mice. Results are expressed as fold changes relative to HPRT mRNA expression. Data from 3 to 6 mice per group is represented by means ± SEM and p values determined by t-test (*p≤0.05). Cont, control not infected.
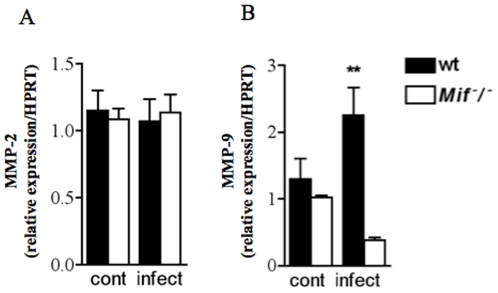
T. gondii peroral infection induces decreased expression of MMP-9, but not MMP2, in the intestinal mucosa of Mif −/− mice
Matrix metalloproteinases have been implicated in the pathogenesis of IBD and most recently in T. gondii-induced ileitis [7]. Given that MIF regulates the expression of MMPs during pathological inflammatory responses such as arthritis [39], [40], we investigated the possible involvement of MMP-2 and MMP-9 in T. gondii-induced MIF-mediated ileitis. We determined the mRNA expression of MMP-2 and MMP-9 in the ileum of control and infected wt and Mif−/− mice. At day 9 post-infection, both control and infected wt and Mif−/− mice presented similar MMP-2 expression (Fig. 5A). However, MMP-9 was significantly reduced in the ileum of Mif−/− infected mice (Fig. 5B). These results suggest that MIF might contribute to pathological responses to T. gondii infection at the intestinal compartment via MMP-9 pathway.
Figure 5. MIF upregulates MMP-9 but not MMP-2 in the terminal ileum of T. gondii infected mice.
Quantitative real-time PCR of (A) MMP-2 and (B) MMP-9 mRNAs in ileal biopsies of control and infected (9 dpi) wt and Mif −/− mice. Results are expressed as fold changes to HPRT mRNA expression. One representative experiment out of two experiments is shown. Data of three to five mice per group are given as means ± SEM and p values were determined by Mann-Whitney (**p≤0.01). Cont, control not infected.
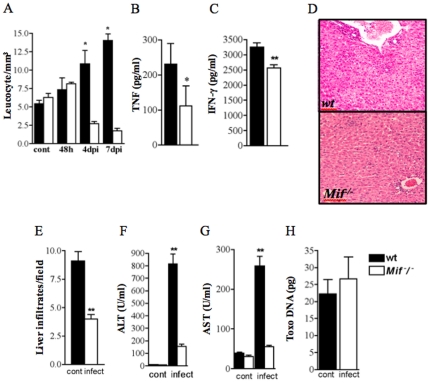
Systemic inflammatory responses are regulated by MIF and enhance T. gondii-induced pathology
The systemic inflammation during T. gondii infection resembles bacterial sepsis. Considering the critical role of MIF in the pathogenesis of experimental sepsis, we investigated several markers of systemic inflammation in T. gondii-infected wt and Mif−/− mice. Leukocyte counts were higher in wt mice at day 4 and 7 post-infection when compared to Mif−/− mice (Fig. 6A). Given that systemic inflammation during per oral infection are induced by pro-inflammatory mediators such as IFN-γ and TNF-α, we investigated the concentrations of these cytokines in the plasma of infected wt and Mif−/− mice. The concentrations of both cytikones were higher in wt compared to Mif−/− mice (Fig. 6B, C). Thrombosis was often associated with vascular inflammatory infiltration in the livers of wt and Mif−/− mice (Fig. 6D). Wt mice had increased numbers of mononuclear cell infiltrates in perivascular areas and scattered at the hepatic parenquima (Fig. 6D, E). In contrast, infected Mif−/− mice had preserved liver parenquima and reduced mononuclear cell infiltration (Fig. 6D, E). Wt mice presented higher alanine aminotransferase and aspartate aminotransferase plasma concentrations compared to Mif−/− mice, an indication of liver damage and dysfunction (Fig. 6F, G). Moreover, T. gondii DNA levels were similar in wt and Mif−/− mice (Fig. 6H). The findings suggest that T.gondii-induced systemic response is in part dependent on MIF, while parasite replication and dissemination is not affected by MIF.
Figure 6. T. gondii-induced sepsis-like response is partially dependent of MIF.
(A) Leukocyte counts were determined in peripheral blood at indicated times. (B) TNF-α and (C) IFN-γ concetrations in the plasma of wt and Mif −/− mice (n = 3–5) 9 dpi were measured by ELISA. (D) Histopathologycal analysis of the liver at 9 dpi from wt and Mif −/− mice. (E) Quantitative analysis of liver sections (3 slides/animal) of infected wt and Mif −/− mice mice was performed. Liver damage was also determined by quantifying serum concentrations of biochemical marker as (F) alanine aminotransferase (ALT), and (G) aspartate aminotransferase (AST). (H) Toxo DNA concentration was determined in the liver of wt and Mif −/− mice. One representative experiment out of six experiments is shown. Data of three to five mice/group are given as as means ± SEM and p values were determined by t-test (* p≤0.05, ** p≤0.01). Cont, control not infected.
Discussion
In the present study we demonstrated that MIF increases the inflammatory response and tissue damage due to T. gondii oral infection in susceptible C57BL/6 mice. Mif−/− mice had reduced intestinal and systemic inflammation surviving more compared to wt mice, despite of an increase in intestinal parasite burden. This reduced inflammatory response in the intestine of Mif−/− mice was associated with decreased expression of inflammatory cytokines and MMP-9 comapared to wt mice. Despite of the lower expression of IL-23 compared to wt mice in ileal explants, IL-22 expression was siginificantly increased in Mif−/− mice. The originally local inflammatory response in the small intestine of T. gondii-infected wt mice had systemic repercussions similar to sepsis, including increased plasma concentrations of inflammatory cytokines, leukocytosis and liver damage, all less pronounced in Mif−/− mice. Together, these results indicate that MIF is involved in the response to T. gondii infection of the small intestine, increasing the ileitis and the systemic inflammatory response.
The peroral infection with T. gondii caused a severe ileitis characterized by intestinal shortening, extensive necrosis, blunting of the villi, loss of Peyer's patches and leukocyte infiltration in the lamina propria [1], [7], [41]. All these pathological parameters were significantly reduced on Mif−/− compared to wt mice. In natural T. gondii infection, gut-associated inflammatory response resembles that observed in Crohn's ileitis and it has been proposed as a model of IBD [1], [7], [41]. An essential role of MIF in experimental colonic inflammation has been demonstrated [20]. It has been also shown that MIF controls the production of inflammatory cytokines including TNF-α and IFN-γ involved in the pathogenesis of intestinal inflammation [42] In the present study, using a model of T. gondii-induced ileal inflammation, we demonstrated a critical role of MIF promoting the intestinal inflammatory response and the expression of TNF-α, IL-12 and IFN-γ.
A recent study showed that during peoral T. gondii infection IL-23 regulates the ileal inflammatory response independent of IL-17 [7]. We also observed that upon T. gondii infection IL-23 expression was upregulated in the intestine while IL-17 was reduced. The inflammatory role of IL-23 was dependent of increased IL-22 production that in turn promoted the ileal inflammation and tissue damage [7]. IL-22, a member of the IL-10 family, is important in epithelial cell homeostasis, in infection and inflammation [43]. In toxoplasmic ileitis, the inflammatory response is a result of IL-22 upregulation induced by IL-23 [7]. In fact, T. gondii-infected IL-22−/− mice have reduced intestinal inflammation and are more resistant than wt. Unexpectaly, we observed a significant increased of IL-22 expression in Mif −/− mice with reduction of IL-23 and TGF-β. Others have previously shown increased IL-22 production by T CD4+ lymphocytes in the absence of IL-23 [44], [45]. Thus our results suggest a previously unrecognized role of MIF inducing IL-23 and inhibiting IL-22 expression. Our results also implies a MIF-dependent pathogenic role of IL-22 in T. gondii-induced ileitis. Interestingly, an anti-inflammatory role of IL-22 in different models of colitis has been previously demonstrated [46]. Future studies will be importat to define the mechanism by which MIF controls IL-23 and IL-22 expression, and how MIF participates in the pathogenic role of IL-22 on T. gondii-induced intestinal inflammation.
We observed that MIF was essential to T. gondii-induced expression of MMP-9, suggesting that MIF could be involved in tissue remodeling/repair at the small intestine during T. gondii peroral infection by over expressing gelatinase B. Several studies have shown that MIF affects tissue remodeling and the expression of different metalloproteinases [40]. Previous studies demonstrated an increased expression and enzymatic activity of metalloproteinases in humans and mice with intestinal inflammation [7], [47]. MMP-2 and MMP-9 are elevated in the intestine of T. gondii-infected animals, however in these model, C57BL/6 mice lacking MMP-2 but not MMP-9 have reduced immunopathology of the small intestine [7]. Thus the exact role of the reduced expression of MMP-9 observed on Mif−/− upon T. gondii infection requires further investigations.
During intestinal parasitic infection, reduced anti-inflammatory responses could decrease parasite burden but intensify tissue damage, causing loss of epithelial barrier integrity and increased bacterial translocation [34]. Our results showed that MIF mediated T. gondii-induced ileitis does not affect IL-4 and increase IL-10 and TGF-β expression. The intestinal inflammatory response induced by T. gondii is complex and intestinal homeostasis requires reciprocal control of both Th1 and TGF-β mediated responses. Consequently, MIF exacerbation of intestinal inflammatory responses might involve not only increased pro-inflammatory responses but also TGF-β-mediated responses. MIF and TGF-β are involved in fibrotic responses in several conditions due to regulation of MMPs [48], [49]. Since both cytokines upregulate MMP-9 expression, it is possible that MIF regulates tissue remodeling in T. gondii-induced ileitis through a TGF-β/MMP-9 pathway.
The reduced inflammatory response and pathological damage in the small intestine of Mif−/− occurred in the context of higher T. gondii burden. It has been recently shown in a model of toxoplasmosis induced by intraperitoneal route that MIF is essentially protective [27]. In this model of systemic infection, Mif−/− had higher numbers of cysts in the brain. The increased susceptibility of Mif−/− mice systemically infected was related with reduced production of inflammatory cytokines, however these animals, in opposition to our findings, developed increased liver damage compared to wt mice [27]. Mif−/− mice of the Balb/c background are also more susceptible to T. gondii when infected by the oral route, presenting increased lethality and parasite burden in the brain and liver [28]. Compared to susceptible hosts such as C57BL/6, the infection of Balb/c mice with T. gondii by the oral route causes a less severe intestinal inflammatory response and a disease that tends to become chronic. These findings suggest that MIF-mediated T. gondii-induced pathological responses might be differently regulated depending on the route and site of infection, and the genetic background of the host.
We observed that Mif−/− mice had reduced signs of systemic inflammation compared to wt mice when infected by the oral route. The reduced systemic inflammation of Mif−/− mice was characterized by lower plasma concentrations of TNF-α and IFN-γ, reduced biochemical and histological parameters of liver damage and lower leukocytosis when compared to wt mice. Considering the pathogenic roles of TNF-α and IFN-γ in T. gondii oral infection, we postulate that enhancement of systemic inflammatory response is mediated by MIF acting in concert with TNF-α and IFN-γ [2], [4], [5]. A deleterious role of MIF was recently observed in a mouse model of lethal dengue virus infection, with reduced systemic inflammatory response in Mif−/− mice compare to wt mice [50]. Although the molecular mechanisms involved in toxoplasmic ileitis are not completely understood, new evidence indicates that co-factors such as Gram-negative intestinal microflora play a role in T. gondii-induced acute ileitis and systemic inflammation through LPS-induced TLR-4 signaling [51]. Since MIF up-regulates the expression of TLR-4 [17], it is possible that the reduced inflammatory response observed in Mif−/− mice upon T. gondii might be a result of reduced TLR-4 activation. Considering that MIF contributes to IBD even in the absence of TLR-4, it is possible that the effect of MIF on T. gondii pathogenesis occurs independently of its effects on TLR-4 expression. Interestingly, MIF also regulates the expression of TLR-11 [28], considered important in the recognition and response to T. gondii [52]. It has been suggest that the reduced expression of TLR-11 in the absence of MIF affects the maturation and activation of dendritic cells consequently hampering a protective immune response to T. gondii [28]. It will be important to characterize the role of TLR-4 and TLR-11 in the MIF-dependent small bowel injury caused by T. gondii in susceptible hosts.
In conclusion, we demonstrated that MIF participates in the pathogenesis of natural T. gondii infection in C57BL/6 mice, promoting an intense ileitis and a robust systemic inflammatory response that resulted in poor outcome during the acute phase. Similar to previous studies, we confirmed a central role of MIF in the control of parasite burden in different models of experimental toxoplasmosis [27], [28]. Although MIF has been regarded as essential in host protection during T. gondii infection, our findings demonstrated a pathogenic role of MIF in natural T. gondii infection in susceptible hosts by exacerbating IL-12, IFN-γ, TNF-α and IL-23 dependent responses. MIF also plays a role in tissue remodeling possibly by regulating TGF-β induced MMP-9. Thus, we propose that MIF actually exerts a bidirectional role in toxoplasmosis. This dichotomy is dependent on the route of infection and the genetic background of the host, and has been also observed with other pro-inflammatory cytokines [2]. In this scenario, MIF seems to compromise host protection by exacerbating intestinal tissue damage and by inducing a sepsis-like response. Previous studies demonstrating a participation of MIF on human T. gondii infection [27], [53], [54] enphasize the importance to understand the diffrential role of MIF on immunity and pathogenesis triggered by T. gondii in distinct tissues.
Acknowledgments
We thank Dr. Claudia Paiva (IMPPG, UFRJ, Brazil), Dr. Claudia Benjamin (Department of Pharmacy, UFRJ, Brazil), Steffi CH Hanschke and Dr. João P. Viola (Instituto Nacional do Câncer, RJ, Brazil) for their gifts of reagents and Rosa Arras and Letícia Alves for technical assistance.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was supported by CNPq, FAPERJ and Pronex (Programa de Apoio a Núcleos de Excelência – Pronex/FAPERJ (www.faperj.br/interna.phtml?ctx_cod=1.11)). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Liesenfeld O. Oral infection of C57BL/6 mice with Toxoplasma gondii: a new model of inflammatory bowel disease? J Infect Dis. 2002;185:S96–101. doi: 10.1086/338006. [DOI] [PubMed] [Google Scholar]
- 2.Gazzinelli RT, Wysocka M, Hieny S, Scharton-Kersten T, Cheever A, et al. In the absence of endogenous IL-10, mice acutely infected with Toxoplasma gondii succumb to a lethal immune response dependent on CD4+T cells and accompanied by overproduction of IL-12, IFN-γ, and TNF-α. J Immunol. 1996;157:798–805. [PubMed] [Google Scholar]
- 3.Buzoni-Gatel D, Schulthess J, Menard LC, Kasper LH. Mucosal defenses against orally acquired protozoan parasites, emphasis on Toxoplasma gondii infections. Cell Microbiol. 2006;8:535–544. doi: 10.1111/j.1462-5822.2006.00692.x. [DOI] [PubMed] [Google Scholar]
- 4.Denkers EY, Gazzinelli RT. Regulation and function of T-cell-mediated immunity during Toxoplasma gondii infection. Clin Microbiol Rev. 1998;11:569–588. doi: 10.1128/cmr.11.4.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yap GS, Sher A. Effector cells of both nonhemopoietic and hemopoietic origin are required for interferon (IFN)-gamma and tumor necrosis factor (TNF)-alpha-dependent host resistance to the intracellular pathogen, Toxoplasma gondii. J Exp Med. 1999;189:1083–1092. doi: 10.1084/jem.189.7.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tait ED, Hunter CA. Advances in understanding immunity to Toxoplasma gondii. Mem Inst Oswaldo Cruz. 2009;104:201–210. doi: 10.1590/s0074-02762009000200013. [DOI] [PubMed] [Google Scholar]
- 7.Muñoz M, Heimesaat MM, Danker K, Struck D, Lohmann U, et al. Interleukin (IL)-23 mediates Toxoplasma gondii-induced immunopathology in the gut via matrixmetalloproteinase-2 and IL-22 but independent of IL-17. J Exp Med. 2009;206:3047–3059. doi: 10.1084/jem.20090900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mordue DG, Monroy F, La Regina M, Dinarello CA, Sibley LD. Acute toxoplasmosis leads to lethal overproduction of Th1 cytokines. J Immunol. 2001;167:4574–4584. doi: 10.4049/jimmunol.167.8.4574. [DOI] [PubMed] [Google Scholar]
- 9.Mennechet FJD, Kasper LH, Rachinel N, Li W, Vandewalle A, et al. Lamina propria CD4+T lymphocytes synergize with murine intestinal epithelial cells to enhance proinflammatory responses against intracellular pathogen. J Immunol. 2002;168:2988–2996. doi: 10.4049/jimmunol.168.6.2988. [DOI] [PubMed] [Google Scholar]
- 10.Bernhagen J, Calandra T, Mitchell RA, Martin SB, Tracey KJ, et al. MIF is a pituitary-derived cytokine that potentiates lethal endotoxaemia. Nature. 1993;365:756–759. doi: 10.1038/365756a0. [DOI] [PubMed] [Google Scholar]
- 11.Bucala R. MIF rediscovered cytokine, pituitary hormone, and glucocorticoid-induced regulator of the immune response. FASEB J. 1996;10:1607–1613. doi: 10.1096/fasebj.10.14.9002552. [DOI] [PubMed] [Google Scholar]
- 12.Calandra TJ, Roger T. Macrophage migration inhibitory factor: a regulator of innate immunity. Nature Rev Immunol. 2003;3:791–800. doi: 10.1038/nri1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weber C, Kraemer S, Drechsler M, Lue H, Koenen RR, et al. Structural determinants of MIF functions in CXCR2-mediated inflammatory and atherogenic leukocyte recruitment. Proc Natl Acad Sci U S A. 2008;105:16278–16283. doi: 10.1073/pnas.0804017105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Calandra T, Bernhagen J, Metz CN, Spiegel LA, Bacher M, et al. MIF as a glucocorticoid-induced modulator of cytokine production. Nature. 1995;377:68–71. doi: 10.1038/377068a0. [DOI] [PubMed] [Google Scholar]
- 15.Calandra T, Bernhagen J, Mitchell RA, Bucala R. The macrophage is an important and previously unrecognized source of macrophage migration inhibitory factor. J Exp Med. 1994;179:1895–1902. doi: 10.1084/jem.179.6.1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lue H, Kleemann R, Calandra T, Roger T, Bernhagen J. Macrophage migration inhibitory factor (MIF): mechanism of action and role in disease. Microbes Infect. 2002;4:449–460. doi: 10.1016/s1286-4579(02)01560-5. [DOI] [PubMed] [Google Scholar]
- 17.Roger T, David J, Glauser MP, Calandra T. MIF regulates innate immune responses through modulation of Toll-like receptor 4. Nature. 2001;4414:920–924. doi: 10.1038/414920a. [DOI] [PubMed] [Google Scholar]
- 18.Dunne DW, Shaw A, Bockenstedt LK, Allore HG, Chen S. Increased TLR4 expression and downstream cytokine production in immunosuppressed adults compared to non-immunosuppressed adults. PLoS One. 2010;5:e11343. doi: 10.1371/journal.pone.0011343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bozza M, Satoskar AR, Lin G, Lu B, Humbles AA, et al. Target disruption of Migration Inhibitory Factor gene reveals its critical role in sepsis. J Exp Med. 1999;189:341–346. doi: 10.1084/jem.189.2.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.De Jong YP, Abadia-Molina AC, Satoskar AR, Clarke K, Rietdijk ST, et al. Development of chronic colitis is dependent on the cytokine MIF. Nature Immunol. 2001;2:1061–066. doi: 10.1038/ni720. [DOI] [PubMed] [Google Scholar]
- 21.Satoskar AR, Bozza M, Rodriguez-Sosa M, Lin G, David JR. Migration-Inhibitory gene deficient mice are susceptible to cutaneous Leishmania major infection. Infect Immun. 2001;69:1247–1254. doi: 10.1128/IAI.69.2.906-911.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Magalhães ES, Mourao-Sa DS, Vieira-de-Abreu A, Figueiredo RT, Pires AL, et al. Macrophage migration inhibitory factor is essential for allergic asthma but not for Th2 differentiation. Eur J Immunol. 2007;37:1097–1106. doi: 10.1002/eji.200635968. [DOI] [PubMed] [Google Scholar]
- 23.Paiva CN, Arras RH, Magalhães ES, Alves LS, Lessa LP, et al. Migration inhibitory factor (MIF) released by macrophages upon recognition of immune complexes is critical to inflammation in Arthus reaction. J Leukoc Biol. 2009;85:855–861. doi: 10.1189/jlb.0108009. [DOI] [PubMed] [Google Scholar]
- 24.McDevitt MA, Xie J, Shanmugasundaram G, Griffith J, Liu A, et al. Critical role for the host mediator macrophage migration inhibitory factor in the pathogenesis of malarial anemia. J Exp Med. 2006;193:1–12. doi: 10.1084/jem.20052398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Magalhães ES, Paiva CN, Souza HS, Pyrrho AS, Mourão-Sá D, et al. Macrophage migration inhibitory factor is critical to interleukin-5-driven eosinophilopoiesis and tissue eosinophilia triggered by Schistosoma mansoni infection. FASEB J. 2009;23:1262–1271. doi: 10.1096/fj.08-124248. [DOI] [PubMed] [Google Scholar]
- 26.Reyes JL, Terrazas LI, Espinoza B, Cruz-Robles D, Soto V, et al. Macrophage migration inhibitory factor contributes to host defense against acute Trypanosoma cruzi infection. Infect Immun. 2006;72:3571–3576. doi: 10.1128/IAI.01648-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Flores M, Saavedra R, Bautista R, Viedma R, Tenorio EP, et al. Macrophage migration inhibitory factor (MIF) is critical for the host resistance against Toxoplasma gondii. FASEB J. 2008;22:3661–3671. doi: 10.1096/fj.08-111666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Terrazas CA, Juarez I, Terrazas LI, Saavedra R, Calleja EA, et al. Toxoplasma gondii: Impaired maturation and pro-inflammatory response of dendritic cells in MIF deficient mice favors susceptibility to infection. Exp Parasitol. 2010;126:348–358. doi: 10.1016/j.exppara.2010.03.009. [DOI] [PubMed] [Google Scholar]
- 29.Liesenfeld O, Kosek J, Remington JS, Suzuki Y. Association of CD4+ Tcell-dependent interferon γ mediated necrosis of the small intestine with genetic susceptibility of mice to per oral infection with Toxoplasma gondii. J Exp Med. 1996;184:597–607. doi: 10.1084/jem.184.2.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liesenfeld O, Kang H, Park D, Nguyen TA, Parkhe C, et al. TNF-α, nitric oxide and IFN-γ are all critical for development of necrosis in small intestine and early mortality in genetically susceptible mice infected per orally with Toxoplasma gondii. Parasite Immun. 1999;21:365–376. doi: 10.1046/j.1365-3024.1999.00237.x. [DOI] [PubMed] [Google Scholar]
- 31.Suzuki Y, Sher A, Yap G, Park D, Neyer LE, et al. IL-10 is required for prevention of necrosis in the small intestine and mortality in both genetically resistant BALB/c and susceptible C57BL/6 mice following per oral infection with Toxoplasma gondii. J Immunol. 2000;164:5375–5382. doi: 10.4049/jimmunol.164.10.5375. [DOI] [PubMed] [Google Scholar]
- 32.Reischl U, Bretagne S, Kruger D, Ernault P, Costa JM. Comparison of two DNA targets for the diagnosis of Toxoplasmosis by real-time PCR using fluorescence resonance energy transfer hybridization probes. BMC Infect Dis. 2003;3:7. doi: 10.1186/1471-2334-3-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kompalic-Cristo A, Frotta C, Suárez-Mutis M, Fernandes O, Britto C. Evaluation of a real-time PCR assay based on the repetitive B1 gene for the detection of Toxoplasma gondii in human peripheral blood. Parasitol Res. 2007;101:619–625. doi: 10.1007/s00436-007-0524-9. [DOI] [PubMed] [Google Scholar]
- 34.Heimesaat MM, Bereswill S, Fischer A, Niebergall J, Freudenberg M, et al. Gram-negative bacteria aggravate murine small intestinal Th-1 type immunopathology following oral infection with Toxoplasma gondii. J Immunol. 2006;177:8785–8795. doi: 10.4049/jimmunol.177.12.8785. [DOI] [PubMed] [Google Scholar]
- 35.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C (T)). Method Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 36.Roberts CW, Ferguson DJ, Jebbari H, Satoskar A, Bluethmann H, Alexander J. Different roles for interleukin-4 during the course of Toxoplasma gondii infection. Infect Immun. 1996;64:897–904. doi: 10.1128/iai.64.3.897-904.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nickdel MB, Lyons RE, Roberts F, Brombacher F, Hunter CA, et al. Intestinal pathology during acute toxoplasmosis is IL-4 dependent and unrelated to parasite burden. Parasite Immunol. 2004;26:75–82. doi: 10.1111/j.0141-9838.2004.00686.x. [DOI] [PubMed] [Google Scholar]
- 38.Neves ES, Bicudo LN, Curi AL, Carregal E, Bueno WF, et al. Acute acquired toxoplasmosis: clinical-laboratorial aspects and ophthalmologic evaluation in a cohort of immunocompetent patients. Mem Inst Oswaldo Cruz. 2009;104(2):393–6. doi: 10.1590/s0074-02762009000200039. [DOI] [PubMed] [Google Scholar]
- 39.Pakozdi A, Amin MA, Haas CS, Martinez RJ, Haines GK, 3rd, et al. Macrophage migration inhibitory factor: a mediator of matrix metalloproteinase-2 production in rheumatoid arthritis. Arthritis Res Ther. 2006;8:1–14. doi: 10.1186/ar2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Assunção-Miranda I, Bozza MT, Da Poian AT. Pro-inflammatory response resulting from sindbis virus infection of human macrophages: implications for the pathogenesis of viral arthritis. J Med Virol. 2010;82:164–174. doi: 10.1002/jmv.21649. [DOI] [PubMed] [Google Scholar]
- 41.Munoz M, Liesenfeld O, Heimesaat MM. Immunology of Toxoplasma gondii. Immunol Rev. 2011;240:269–285. doi: 10.1111/j.1600-065X.2010.00992.x. [DOI] [PubMed] [Google Scholar]
- 42.Ohkawara T, Nishihira J, Ishiguro Y. Resistance to experimental colitis depends on cytoprotective heat shock proteins in macrophage migration inhibitory factor null mice. Immunol Lett. 2006;107:148–154. doi: 10.1016/j.imlet.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 43.Aujla SJ, Kolls JK. IL-22: a critical mediator in mucosal host defense. J Mol Med. 2009;87:451–454. doi: 10.1007/s00109-009-0448-1. [DOI] [PubMed] [Google Scholar]
- 44.Ramirez JM, Brembilla NC, Sorg O, Chicheportiche R, Matthes T, et al. Activation of the aryl hydrocarbon receptor reveals distinct requirements for IL-22 and IL-17 production by human T helper cells. Eur J Immunol. 2010;40:2450–2459. doi: 10.1002/eji.201040461. [DOI] [PubMed] [Google Scholar]
- 45.Brembilla NC, Ramirez JM, Chicheportiche R, Sorg O, Saurat JH, et al. In vivo dioxin favors interleukin-22 production by human CD4+ T cells in an aryl hydrocarbon receptor (AhR)-dependent manner. PLoS One. 2011;6:e18741. doi: 10.1371/journal.pone.0018741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Monteleone I, Rizzo A, Sarra M, Sica G, Sileri P, et al. Aryl Hydrocarbon Receptor-Induced Signals Up-regulate IL-22 Production and Inhibit Inflammation in the Gastrointestinal Tract. Gastroenterology. 2011;141:237–248. doi: 10.1053/j.gastro.2011.04.007. [DOI] [PubMed] [Google Scholar]
- 47.Garg P, Vijay-Kumar M, Wang L, Gewirtz AT, Merlin D, et al. Matrix metalloproteinase-9-mediated tissue injury overrides the protective effect of matrix metalloproteinase-2 during colitis. Am J Physiol Gastrointest Liver Physiol. 2009;296:G175–184. doi: 10.1152/ajpgi.90454.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chou YT, Wang H, Chen Y, Danielpour D, Yang YC. Cited2 modulates TGF-beta-mediated upregulation of MMP9. Oncogene. 2006;25:5547–5560. doi: 10.1038/sj.onc.1209552. [DOI] [PubMed] [Google Scholar]
- 49.He XX, Chen K, Yang J, Li XY, Gan HY. Macrophage migration inhibitory factor promotes colorectal cancer. Mol Med. 2009;15:1–10. doi: 10.2119/molmed.2008.00107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Assunção-Miranda I, Amaral FA, Bozza FA, Fagundes CT, Sousa LP, et al. Contribution of macrophage migration inhibitory factor to the pathogenesis of dengue virus infection. FASEB J. 2010;24:218–228. doi: 10.1096/fj.09-139469. [DOI] [PubMed] [Google Scholar]
- 51.Heimesaat MM, Fischer A, Jahn HK, Niebergall J, Freudenberg M, et al. Exacerbation of murine ileitis by Toll-like receptor 4 mediated sensing of lipopolysaccharide from commensal Escherichia coli. Gut. 2007;56:941–948. doi: 10.1136/gut.2006.104497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yarovinsky F, Zhang D, Andersen JF, Bannenberg GL, Serhan CN, et al. TLR-11 activation of dendritic cells by a protozoan profilin-like protein. Science. 2005;308:1626–1629. doi: 10.1126/science.1109893. [DOI] [PubMed] [Google Scholar]
- 53.Ferro EA, Mineo JR, Ietta F, Bechi N, Romagnoli R, et al. Macrophage migration inhibitory factor is up-regulated in human first-trimester placenta stimulated by soluble antigen of Toxoplasma gondii, resulting in increased monocyte adhesion on villous explants. Am J Pathol. 2008;172:50–58. doi: 10.2353/ajpath.2008.070432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.de Oliveira Gomes A, de Oliveira Silva DA, Silva NM, de Freitas Barbosa B, Franco PS, et al. Effect of macrophage migration inhibitory factor (MIF) in human placental explants infected with Toxoplasma gondii depends on gestational age. Am J Pathol. 2011;178:2792–2801. doi: 10.1016/j.ajpath.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]