Abstract
Cytotoxic CD4 Th1 cells are emerging as a therapeutically useful T cell lineage that can effectively target tumors, but until now the pathways that govern their differentiation have been poorly understood. We demonstrate that CD134 (OX40) costimulation programs naive self- and virus-reactive CD4 T cells to undergo in vivo differentiation into cytotoxic Th1 effectors. CD137 (4-1BB) costimulation maximized clonal expansion and IL-2 was necessary for cytotoxic Th1 differentiation. Importantly, the T-box transcription factor Eomesodermin (Eomes) was critical for inducing the cytotoxic marker granzyme B. CD134 plus CD137 dual costimulation also imprinted a cytotoxic phenotype on bystanding CD4 T cells. Thus, the present study identifies for the first time a specific costimulatory pathway and an intracellular mechanism relying on Eomes that induces both antigen-specific and bystander cytotoxic CD4 Th1 cells. This mechanism might be therapeutically useful since CD134 plus CD137 dual costimulation induced CD4 T cell-dependent tumoricidal function in a mouse melanoma model.
Introduction
Antigen-primed naïve CD4 T cells can differentiate into numerous helper subsets that secrete a variety of cytokines specialized to coordinate different facets of adaptive immunity (1, 2). CD8 T cells similarly acquire cytokine potential, but inherently develop cytotoxic capacity (1, 3). CD4 T cells can also develop cytotoxic function during in vitro culture (4, 5), and cytotoxic CD4 T cells are induced in vivo in response to certain infections (6, 7). Thus, cytotoxic CD4 T cells might be useful in rejecting MHC class II+ tumors, in particular melanomas that can express MHC class II (8) but have a propensity to down-regulate MHC class I (9). Support for this idea comes from recent data showing that cytotoxic CD4 Th1 cells can eradicate established murine melanoma (10, 11).
Expression of the lytic effector molecules perforin and granzymes in CD8+ CTL depends upon IL-2 signaling (12-14) and the lineage-specific T-box transcription factor Eomesodermin (Eomes) (15, 16). The mechanism that regulates the differentiation of cytotoxic CD4 Th1 cells in vivo has been unknown.
Given the power of TNF family costimulatory molecules to boost both CD4 and CD8 T cell responses (17, 18), we postulated that the costimulators that greatly impact CD8 T cells may also affect CD4 T cells in a similar manner. In particular, combined treatment with agonists to CD134 and CD137 profoundly boosts CD8 T cell responses (19, 20) and elicits tumor immunity in various models (19, 21, 22). Likewise, CD134 costimulation programs CD4 T cells encountering self-antigen to expand and express Th1-associated cytokines (23, 24). Here we show that CD134 costimulation programs CD4 T cells to express lytic molecules and cytotoxic function while CD137 costimulation maximized clonal expansion of the cytotoxic Th1 effector cells. This dual costimulation through CD134 and CD137 enhanced IL-2 signaling in CD4 T cells responding to cognate antigen, and expression of granzyme B was entirely IL-2-dependent.
Notably, in dual costimulated CD4 T cells the Th1 master-regulatory transcription factor T-bet (25) supported two thirds of the potential to synthesize IFN-γ, but none of the granzyme B producing capacity. Rather, Eomes was responsible for programming cytotoxic function because its expression was induced by dual costimulation, and dual costimulation-induced granzyme B expression was impaired in Eomes-/- CD4 T cells. Although these studies identify for the first time specific costimulatory pathways (CD134 plus CD137) that can induce and expand antigen-specific cytotoxic CD4 Th1 cells in vivo, perhaps the most unexpected observation was the “imprinting” of antigen-inexperienced bystanding T cells with similar functional capabilities. Thus, CD134 plus CD137 dual costimulation programs cytotoxic function in both specific and bystanding CD4 T cells through induction of the CD8+ CTL-associated factor Eomes.
Finally, while the potential of CD134 plus CD137 dual costimulation to elicit CD8 T cell-dependent tumor immunity has been established (19, 21, 22), our current study demonstrates the potential of dual costimulation to program CD4 T cells to control a murine melanoma independently of CD8 T cells. CD134 and CD137 costimulatory agonists are currently being developed separately as cancer therapeutics in human clinical trials (26, 27), and our current findings suggest that the application of dual costimulation might be particularly effective in controlling disease progression because it can engage both CD8 and CD4 T cells that directly target tumors.
Materials and Methods
Mice, adoptive transfer and tumor challenge
TCR Tg CD4 T cells specific for an I-Ed-restricted epitope deriving from influenza (PR8 strain) hemagglutinin (HA) (110SFERFEIFPKE120) prepared from CD8-depleted lymph nodes of 6.5 TCR Tg mice (28) on the B10.D2 (H-2d) Thy1.1+ background were adoptively transferred into congenic Thy1.2+ C3-HA Tg mice expressing HA in several parenchymal tissues (self-HA mice, #137 founder line (29)) or Thy1.2+ non-Tg (WT) B10.D2 mice infected with a recombinant vaccinia virus at 106 PFU and treated with or without CD134 and CD137 agonists were recovered from spleens as previously described (24, 30). For experiments using staphylococcal enterotoxin A (SEA), WT C57BL/6 (B6) mice were treated with 1 μg SEA with or without CD134 plus CD137 agonists, and spleens harvested on day 5 to analyze the response of SEA-reactive Vβ3+ and non-reactive Vβ14+ CD4 T cells.
Mice with targeted null mutations in the Il2 (31) and Tbx21 (T-bet) (32) genes on the B6 background were purchased from Jackson Laboratory and backcrossed onto the 6.5 B10.D2 Thy1.1+ background. Thy1.1+ B6 mice were also purchased from Jackson Laboratory. Thy1.2+ B6 conditional Eomes-/- mice (Eomesfloxed/floxed mated to CD4-Cre) (33) and TCR β-/- × δ-/- B6 mice (34) were previously described. The number of Thy1.1+ TCR Tg CD4 T cells adoptively transferred into Thy1.2+ recipient mice was 106 unless otherwise indicated, and their functional responses were analyzed following recovery from recipient spleens at the indicated times as previously described (24, 30, 35). In experiments where WT and gene targeted TCR Tg CD4 T cells were co-transferred, the two populations were distinguished through homozygous vs heterozygous expression of Thy1.1. Agonistic anti-CD137 (25 μg) and/or anti-CD134 (50 μg) mAbs were administered i.p. as previous described (19, 24). As indicated, WT and IL-2-/- TCR Tg CD4 T cells were depleted of CD44high memory cells prior to adoptive transfer using biotinylated anti-CD44 mAb and streptavidin-conjugated magnetic beads (Dynal).
Bone marrow (BM) chimeras were generated as previously described (30) with the following modifications. Lethally-irradiated WT B10.D2 Thy1.2/1.2 mice were rescued with 2 X 105 BM cells from WT B10.D2 Thy1.2/1.2 plus 8 X 105 BM cells from either IL-2+/+ Thy1.1/1.2 or IL-2-/- Thy1.1/1.1 6.5 TCR Tg B10.D2 donors. Irradiated RAG1-/- B6 mice (11) were rescued with 1 X 106 BM cells from WT, T-bet-/- or Eomes-/- B6 donors. All chimeras were reconstituted for at least 6 weeks prior to being used as adoptive transfer donors.
B16-F10 (B16) melanoma cells (1 × 105) were intradermally inoculated into RAG1-/- B6 mice that did or did not receive 1 X 106 polyclonal B6 CD4 T cells (depleted of CD8+, NK1.1+ and B220+ cells via magnetic beads) 5 days earlier and treated with or without anti-CD134 and CD137 as indicated. Tumors were measured 13 days post inoculation using calipers and multiplying perpendicular diameters to calculate surface areas in millimeters squared.
All mouse protocols were approved by The University of Connecticut Health Center's Animal Care and Use Committee.
Flow cytometry
TCR Tg CD4 T cells (CD4+Thy1.1+) were analyzed for expansion and intracellular granzyme B (GzmB) expression directly ex vivo as previously described (24). Where indicated, Gzmb was measured in polyclonal CD4 T cells following 24 h stimulation with plate-bound anti-CD3 mAb. GzmB expression was quantified by mean fluorescence intensity (MFI). Intracellular cytokine expression was analyzed following restimulation with the I-Ed-restricted HA peptide or PMA plus ionomycin in the presence of Brefeldin A for 5 h as previously described (30, 35). Intracellular T-bet staining was performed directly ex vivo (using eBioscience catalog #45-5825) following permeabilization with methanol (36). Eomes staining was also performed directly ex vivo (using eBioscience catalog #12-4875), but following permeabilization with eBioscience Foxp3 staining buffer. CD25 vs pSTAT5 and Foxp3 vs GzmB staining was performed directly ex vivo as previously described (36).
Real-time RT-PCR and chromatin immunoprecipitation (ChIP)
mRNA samples prepared from MACS purified TCR Tg CD4 T cells were analyzed by quantitative SYBR Green-based real-time RT-PCR as previously described (24, 35). Briefly, after reverse transcribing cDNA, T-bet, Eomes, perforin 1 and granzyme B mRNAs were quantified by first normalizing for input sample amounts by calculating the difference in threshold cycle (CT) values relative to HPRT (ΔCT), and then using the ΔΔCT method to calculate the ratio between experimental samples and a representative naive CD4 T cell sample. Primers to T-bet, Eomes and HPRT were previously described (24, 35). Primers to granzyme B were forward, 5′-AATGTGAAGCCAGGAGATGTGTGC and reverse, 5′-CCGAAAGGAAGCACGTTTGGTCTT. Primers to perforin 1 were forward, 5′-GAGCCCCTGCACACATTACTGGAA and reverse, 5′-ACATTCTCAAAGTCCATCT.
Quantitative real-time SYBR Green PCR-based ChIP to measure histone H3 acetylation (AcH3) within the Ifng, Eomes, Prf1 and Gzmb promoters were performed as previously described (24, 37) with the following modifications. DNA immunoprecipitated from fixed and sheered chromatin samples using anti-AcH3 were amplified using primers specific to the various gene promoters. The amount of AcH3 at each promoter was first normalized for input sample amounts by normalizing to the G6PD promoter (ΔCT) and then calculating the fold-difference (ΔΔCT) relative to naive TCR Tg CD4 T cells. Primers corresponding to Ifng (35) and G6PD (24) were previously described. Primers to Eomes were forward, 5′-ACCATGTTCGCAGACTTCAAACCC and reverse, 5′-TATTAATTGGATCGGGTCGCCTGC. Primers to Prf1 were forward, 5′-CAGGGCAGGAAGTAGTAATGATATG and reverse, 5′-CTTCCTCCTCCTTACCTGAAGTC. Primers to Gzmb were forward, 5′-ACTAGATGGTCATGCTTGGTCCTG and reverse, 5′-ATCTTCCCCGGAAGGCCGCCTAGG.
Degranulation assay
Spleen samples were cultured for the indicated times with either 100 μg/ml HA peptide or 5 μg/ml soluble anti-CD3 mAb. FITC-conjugated mAbs to both CD107a and CD107b were present throughout the culture. CD107a/b fluorescence was subsequently analyzed on CD4+Thy1.1+ (specific) and CD4+Thy1.1- (bystander) CD4 T cells.
Lytic assay
Recipient spleen samples containing 5 X 104 TCR Tg CD4 T cells were cultured with 2.5 X 104 A20 cells (38) that had been labeled with 500 nM CFSE and pulsed with cognate HA peptide plus 2.5 X 104 peptide non-pulsed A20 cells labeled with 50 nM CFSE in 96 well plates. Naive WT splenocytes were added so that the total number of cells per well was 106 while maintaining an effector to target (E:T) ratio of 1:1. The proportion of CFSEhigh and CFSElow A20 cells within the live gate (based on forward and side scatter) was measured after 4 h, and the percent specific killing was calculated using the formula: (1 – (% + peptide (CFSEhigh) /% – peptide (CFSElow))) X 100%.
Statistical analysis
All quantitative data are expressed as the mean ± SEM, and p values were calculated using an unpaired two-tailed t test.
Results
CD134 plus CD137 dual costimulation programs cytotoxic CD4 Th1 cell differentiation
We asked whether dual costimulation (DCo) through CD134 and CD137 exerts effects on specific CD4 T cells similar to its known effects on CD8+ CTL. DCo induced HA-specific TCR transgenic (Tg) CD4 T cells transferred into HA-expressing (self-HA) recipients to express high amounts of granzyme B (GzmB) protein (Fig. 1, A and B). Because the serine protease GzmB is a lytic granule component normally expressed in CD8+ CTL and NK cells (39), this result suggested that DCo programs cytotoxic CD4 Th1 cell differentiation. Consistent with this effect being genetically programmed, DCo induced mRNA encoding not only GzmB, but also the pore-forming component of lytic granules perforin 1 (40) (Fig. 1C). Also consistent with the epigenetic profile of CD8+ CTL (41), real-time PCR-based chromatin immunoprecipitation (ChIP) indicated that DCo significantly increased the amount of acetylated histone H3 (AcH3) bound to the Gzmb and Prf1 gene promoters (Fig. 1D). Thus, DCo programmed these genes to undergo epigenetic remodeling towards a transcriptionally permissive configuration. Confirming the fidelity of the ChIP assay, DCo increased AcH3 bound to the Ifng promoter (Fig. 1D), consistent with the enhanced IFN-γ expression (24).
Figure 1.
CD134 plus CD137 DCo programs specific CD4 T cells to express GzmB, perforin and cytotoxic function. HA-specific Thy1.1+ 6.5 TCR Tg CD4 T cells were transferred into Thy1.2+ self-HA recipients treated with anti-CD134 plus CD137 agonistic mAbs (+ DCo) or rat Ig (- DCo), then recovered from spleens on day 4. Representative (A) and total (B, MFI, n=4 per group) intracellular GzmB. C, Real-time RT-PCR analysis of GzmB and perforin 1 mRNAs in MACS-purified specific CD4 T cells, n=4 per group. D, AcH3 bound to the Gzmb, Prf1 and Ifng promoters in purified specific CD4 T cells measured by ChIP, n=5 per group. RT-PCR and ChIP data are expressed as the ratio to naive specific CD4 T cells. E, Degranulation measured via surface CD107a/b expression before and 2 h after restimulation with HA peptide, n=3 per group. F, Specific CD4 T cells treated +/- DCo were cultured 4 h at an E:T of 1:1 with A20 cells pulsed with (CFSEhigh) or without (CFSElow) HA peptide. Representative histograms and bar graph of peptide-specific lysis are based on n=3 per group.
To test whether DCo-programmed specific CD4 T cells are cytotoxic, we measured degranulation of lytic granules via cell surface localization of CD107a (LAMP1) and CD107b (LAMP2) (42). Surface CD107a/b increased 6-fold on DCo-treated specific CD4 T cells following 2 h stimulation with cognate peptide. In contrast, peptide-stimulated DCo-non-treated counterparts expressed substantially less surface CD107a/b (Fig. 1E). Secondly, DCo-treated CD4 T cells induced >50% specific lysis of cognate peptide-pulsed MHC class II+ A20 cells at an effector:target (E:T) ratio of only 1:1 (Fig. 1F). Since DCo increases specific CD4 T cell expansion several-fold (24), this assay was performed in vitro so that lytic activities could be compared at the same E:T ratio. Taken together, our data identify costimulation as a process to induce genetic programming of naïve CD4 T cells into cytotoxic Th1 cells.
We next assessed whether CD134, CD137 or both costimulatory signals are required to program cytotoxic CD4 Th1 cell differentiation. CD137 costimulation alone significantly enhanced expansion (Fig. 2A), expression of IFN-γ, TNF (Fig. 2B) and GzmB (Fig. 2C) in specific CD4 T cells, but CD134 alone induced greater expansion and expression of IFN-γ, TNF and GzmB. DCo did not boost effector molecule expression beyond CD134 alone (Fig. 2, B and C), but it did boost clonal expansion (Fig. 2A). CD134 is thus sufficient to program cytotoxic Th1 differentiation, while addition of CD137 costimulation maximizes clonal expansion of the cytotoxic CD4 Th1 cells.
Figure 2.
CD134 costimulation drives cytotoxic CD4 Th1 differentiation, but CD134 plus CD137 DCo maximizes clonal expansion. TCR Tg CD4 T cells transferred into self-HA mice treated with anti-CD137, -CD134, both or neither agonist (control rat Ig) were recovered from spleens on day 4, n=3 per group. A, Accumulation. B, Representative IFN-γ vs TNF staining after HA peptide restimulation. C, Representative GzmB expression directly ex vivo.
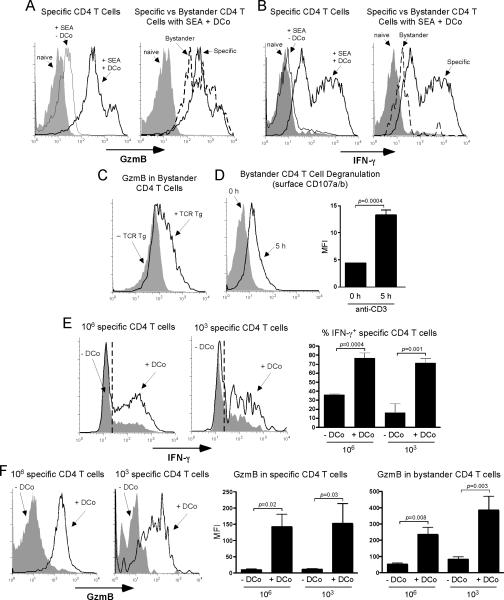
To test if DCo programming of cytotoxic CD4 Th1 cells is a natural phenomenon not limited to artificial TCR Tg adoptive transfer models, we analyzed endogenous CD4 T cell responses to the pathogenic bacterial protein staphylococcal enterotoxin A (SEA). SEA stimulates TCR Vβ3+ but not Vβ14+ CD4 T cells (43). After in vivo stimulation with SEA, DCo programmed specific Vβ3+ CD4 T cells to express high amounts of GzmB (Fig. 3A, left) and IFN-γ (Fig. 3B, left). Thus, DCo programming of cytotoxic CD4 Th1 cells is a general phenomenon. Strikingly, SEA plus DCo induced SEA-non-reactive Vβ14+ “bystander” CD4 T cells to express large amounts of GzmB (Fig. 3A, right), but lower amounts of IFN-γ (Fig. 3B, right).
Figure 3.
DCo induces GzmB in both specific and bystander CD4 T cells. A, B6 mice were treated with 1 μg SEA +/- DCo (“naive” mice received neither SEA nor DCo). On day 5, SEA-specific (Vβ3+) and non-reactive “bystander” (Vβ14+) CD4 T cells in spleens were stained for GzmB directly ex vivo. Histograms are representative of 3-4 replicates per group that were highly comparable. B, IFN-γ staining following in vitro restimulation with PMA + ionomycin in the samples corresponding to panel A. (C and D) Self-HA mice that received Thy1.1+ specific CD4 T cells were treated with DCo (+ specific), while DCo-treated WT mice that were not given specific CD4 T cells served as the control (- specific). Spleens were analyzed on day 4. C, Intracellular GzmB in Thy1.2+ bystander CD4 T cells directly ex vivo, representative of 2-4 replicates per group that were highly comparable. D, Surface CD107a/b on bystander CD4 T cells recovered from self-HA DCo-treated specific CD4 T cell adoptive transfer recipients before (0 h) and 5 h following in vitro restimulation with soluble anti-CD3 mAb. Representative histograms (left) and bar graph (right) are from n=3 per group. (E and F) Either 106 or 103 Thy1.1+ specific CD4 T cells were transferred into WT B10.D2 recipients infected with viral-HA and treated +/- DCo, and recovered from spleens on day 5. E, Intracellular IFN-γ was measured after restimulation with HA peptide. The left and middle panels show representative histograms with a dashed line indicating the cutoff for positive staining determined using an isotype control. The right panel is a graph of the % IFN-γ+ specific CD4 T cells (n=4-5 per group). F, Ex vivo GzmB expression is presented as in panel E, except that the data is expressed as MFI (n=4, 5, 10 and 10 for 106 - DCo, 106 + DCo, 103 - DCo and 103 + DCo, respectively).
In this SEA model the initial frequency of specific Vβ3+ CD4 T cells was ~5% of the overall CD4 T cell repertoire (not shown). To test if GzmB is induced in bystander CD4 T cells when fewer antigen-responsive T cells are initially present, we used the TCR Tg transfer system where the initial frequency is ~0.1% after transfer of 106 cells. Bystander CD4 T cells expressed substantially more GzmB in DCo-treated mice that contained specific CD4 T cells than controls (Fig. 3C). Because bystander CD4 T cells have a diverse TCR repertoire, cytotoxic potential was assessed by degranulation. Importantly, anti-CD3 mAb induced surface CD107a/b expression on these bystander CD4 T cells recovered from DCo-treated recipients (Fig. 3D), albeit at lower magnitude and with slower kinetics compared to specific CD4 T cells (not shown). Thus, although bystander CD4 T cells may be less potent, they do appear to have cytotoxic potential.
HA-specific CD4 T cells transferred into WT mice infected with a recombinant vaccinia virus expressing HA (viral-HA) differentiate into conventional Th1 cells that express IFN-γ (Fig. 3E) but not GzmB (Fig. 3F). The addition of DCo programmed these virally-primed specific CD4 T cells to express GzmB as well as higher amounts of IFN-γ. DCo thus enabled a Th1-inducing virus to induce the cytotoxic Th1 phenotype. Next, the response 106 vs 103 specific CD4 T cells transferred into DCo-treated viral-HA-infected mice was compared to explore whether DCo can program the cytotoxic Th1 profile when the initial specific CD4 T cell frequency is within a physiological range. Adoptive transfer of 103 TCR Tg CD4 T cells seeds recipient secondary lymphoid organs at numbers (~100) approximating individual specificities within the steady state repertoire (44, 45). As expected, 103 specific CD4 T cells transferred into viral-HA-infected DCo-treated recipients accumulated to a lesser degree compared to a 106 transfer group (6.6 X105 ± 2.5 X 105 vs 6.9 X107 ± 8 X 106 at day 5, n=10 and 5 per group, respectively). Perhaps surprisingly, however, in both the 103 and 106 groups DCo significantly boosted expression of IFN-γ (Fig. 3E) and GzmB in specific CD4 T cells (p≤0.03) as well as GzmB in bystander CD4 T cells (p≤0.008) (Fig. 3F). Importantly, GzmB MFI values for specific and bystander CD4 T cells did not differ between the 103 and 106 DCo-treated groups (p≥0.2). Bystander GzmB MFI values also increased in virus-infected DCo-treated mice that did not receive any transferred HA-specific TCR Tg CD4 T cells (not shown), an effect that was presumably mediated by endogenous vaccinia-specific CD4 T cells.
The cellular mechanism mediating cytotoxic CD4 Th1 differentiation
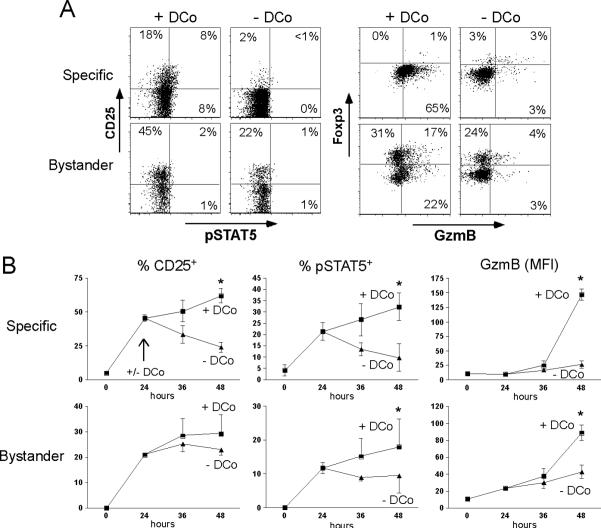
CD134 costimulation enhances IL-2 production by CD4 T cells (46), and IL-2 induces both GzmB (12, 47, 48) and IFN-γ (49, 50). We thus postulated that the CD134 → IL-2 axis might promote cytotoxic CD4 Th1 differentiation. To explore this possibility, expression of CD25 on specific and bystander CD4 T cells treated +/- DCo was examined. Four days following self-HA antigen encounter with DCo CD25 was expressed on 24.6% ± 1.0 (mean ± SEM, n=3) of the specific CD4 T cells, but only 1.7% ± 0.2 were CD25+ without DCo. Even at this later time point some of the DCo-treated CD25+ specific CD4 T cells contained phosphorylated STAT5 (17.5% ± 6.8) (Fig. 4A, 1st and 2nd top panels). CD25 and pSTAT5 expression on specific CD4 T cells peaked 24 h after transfer in the absence of DCo, but expression of both IL-2 signaling molecules increased between 24 and 48 h with DCo. Further, GzmB expression was several-fold higher in DCo-treated compared to non-treated specific CD4 T cells at 48 h (Fig. 4B). DCo thus enhances and prolongs IL-2 signaling, an effect that correlates with the acquisition of GzmB expression. DCo-treated specific CD4 T cells did not express Foxp3 at day 4 (Fig. 4A, 3rd and 4th top panels), consistent with studies where CD134 costimulation blocked iTreg differentiation (51, 52). DCo also increased the proportion of bystander CD4 T cells expressing CD25 and Foxp3 by 1.9- and 1.8-fold, respectively (p≤0.001 in both) (Fig. 4A, bottom panels), and induced GzmB not only in Foxp3- (11.6-fold, p=0.006), but also in Foxp3+ (2.6-fold, p=0.01) bystander CD4 T cells (Fig. 4A, 3rd and 4th bottom panels). Taken together, DCo augments IL-2 signaling followed by induction of GzmB in specific CD4 T cells, and in both Foxp3+ and Foxp3- bystander CD4 T cells.
Figure 4.
DCo enhances IL-2 signaling. A, Specific CD4 T cells transferred into self-HA mice treated +/- DCo were recovered from spleens on day 4 and directly stained for pSTAT5 vs CD25 and GzmB vs Foxp3. Plots are gated on both Thy1.1+ specific and Thy1.2+ bystander CD4 T cells. The cutoff for positive CD25, GzmB and Foxp3 staining was based on isotype controls, while the cutoff for positive pSTAT5 staining was based on the - DCo groups. B, Time course of CD25, pSTAT5 and GzmB expression on specific and bystander CD4 T cells between 0 and 48 h post-transfer into self-HA mice treated +/- DCo. The arrow in the top left graph indicates that DCo was given at 24 h. N=3 for each 0 and 24 h point and 4 for each 36 and 48 h point. Asterisks indicate p<0.05 for +/- DCo comparisons.
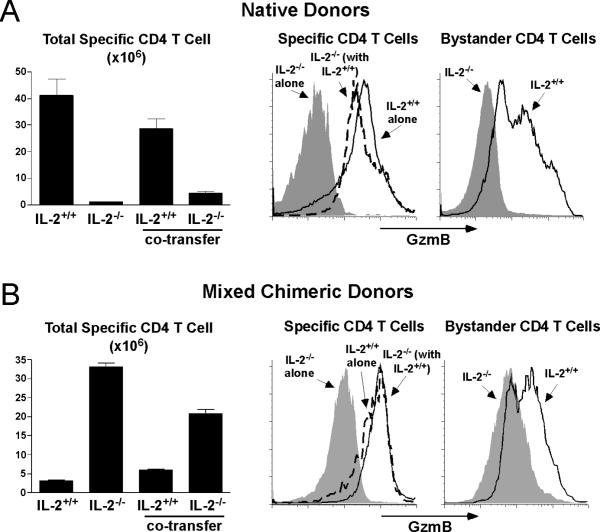
IL-2-/- specific CD4 T cells were used to definitively test if IL-2 facilitates DCo-programmed cytotoxic Th1 differentiation. Due to the absence of Treg cells, IL-2-/- mice contain a disproportionally high frequency of activated/memory T cells (53). Therefore, we used two separate approaches to compare the response of IL-2-/- vs IL-2+/+ specific CD4 T cells. First, CD44high activated/memory cells were removed via magnetic beads from both populations prior to adoptive transfer into DCo-treated self-HA recipients. IL-2-/- specific CD4 T cells expressed substantially less GzmB (Fig. 5A, middle panel) and IFN-γ (not shown) compared to IL-2+/+ counterparts, and they failed to induce GzmB in bystander CD4 T cells (Fig. 5A, right panel). GzmB expression was rescued in the IL-2-/- specific CD4 T cells when they were co-transferred with IL-2+/+ counterparts (Fig. 5A, middle panel). This result was confirmed using a second approach that utilized IL-2-/- and IL-2+/+ specific CD4 T cells that developed in mixed bone marrow (BM) chimeric mice that contained functional Treg cells (Fig. 5B, middle and right panels). Interestingly, IL-2-/- specific CD4 T cells prepared from native donors expanded less than IL-2+/+ counterparts (Fig. 5A, left panel), while the opposite pattern was observed with specific CD4 T cells prepared from mixed BM chimeric donors (Fig. 5B, left panel). Thus, although the expansion of specific CD4 T cells following adoptive transfer was influenced by the environment they developed in, this effect did not impact their ability to undergo cytotoxic Th1 differentiation nor the necessity for IL-2 in this process.
Figure 5.
IL-2 facilitates DCo-induced GzmB expression in both specific and bystander CD4 T cells. A, IL-2-/- Thy1.1/1.2 and IL-2+/+ Thy1.1/1.1 CD44-depleted specific CD4 T cells from native TCR Tg donors were transferred alone or together into DCo-treated self-HA recipients and recovered from spleens on day 4 and directly stained for intracellular GzmB. B, IL-2-/- and IL-2+/+ specific CD4 T cells from mixed BM chimeric donors were transferred and analyzed as in panel A. These mixed BM chimeric donors were generated by rescuing irradiated WT Thy1.2/1.2 hosts with BM from 6.5 TCR Tg donors that were either IL-2-/- Thy1.1/1.1 or IL-2+/+ Thy1.1/1.2 plus WT Thy1.2/1.2 BM that supported normal Treg cell function. In both panels n=3 per group for the bar graphs showing specific CD4 T cell expansion, and GzmB expression plots are highly representative for each group.
Eomesodermin programs cytotoxic CD4 Th1 cell differentiation
To genetically dissect how DCo programs cytotoxic CD4 Th1 differentiation, relevant transcription factors were examined. T-bet drives IFN-γ expression in both CD4 and CD8 T cells, but is not essential in CD8 T cells (32). CD8 T cells express another T-box family member, Eomesodermin (Eomes), that drives IFN-γ, perforin and GzmB expression (15, 16). We show that DCo increased T-bet mRNA (Fig. 6A) and protein (Fig. 6C) in specific CD4 T cells. Importantly, DCo also increased Eomes mRNA (Fig. 6A), AcH3 bound to the Eomes promoter (Fig. 6B) and Eomes protein (Fig. 6C).
Figure 6.
DCo induces T-bet and Eomes in specific CD4 T cells. Specific CD4 T cells transferred into self-HA recipients treated +/- DCo were recovered from spleens on day 4. A, T-bet and Eomes mRNAs were measured by real-time RT-PCR using the samples shown in Fig. 1C. B, AcH3 bound to the Eomes promoter measured by ChIP using the samples shown in Fig. 1D. C, Intracellular T-bet and Eomes histograms, representative of 3 replicates per group that were highly comparable.
To test if T-bet programs cytotoxic CD4 Th1 differentiation the response of Tbx21-/- (T-bet-deficient) (32) specific CD4 T cells to DCo was analyzed. WT and T-bet-/- specific CD4 T cells underwent comparably robust expansion in DCo-treated self-HA recipients (not shown), but T-bet-/- specific CD4 T cells expressed a third the amount of IFN-γ and similar amounts of TNF compared to WT (Fig. 7A). In viral-HA-primed conventional Th1 cells, T-bet-deficiency completely eliminated IFN-γ expression and partially reduced TNF potential (Fig. 7A). GzmB production was not altered in T-bet-/- specific CD4 T cells after DCo (Fig. 7B).
Figure 7.
DCo induces GzmB expression in specific CD4 T cells independently of T-bet. T-bet+/+ (WT) and T-bet-/- specific CD4 T cells were transferred into DCo-treated self-HA or DCo-non-treated viral-HA-infected recipients and recovered from spleens on day 4. IFN-γ vs TNF expression following restimulation with HA peptide (A) and GzmB directly ex vivo (B). Plots are representative of 3-4 replicates per group that were highly comparable.
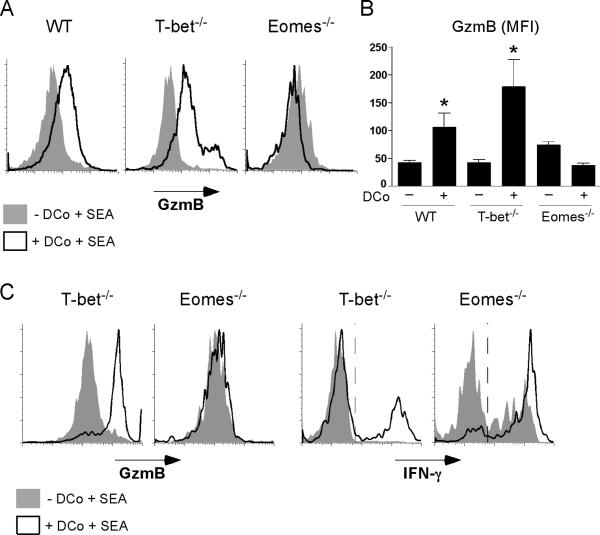
To test if Eomes was responsible for GzmB expression in DCo-treated specific CD4 T cells, WT, T-bet-/- or conditionally Eomes-/- (33) polyclonal T cells were adoptively transferred into TCR γ-/- × γ-/- recipients, and subsequently treated with SEA +/- DCo. As expected, DCo significantly induced GzmB expression in WT and T-bet-/- SEA-specific Vβ3+ CD4 T cells, but importantly DCo failed to induce GzmB in Eomes-/-Vβ3+ CD4 T cells (Fig. 8, A and B). Although T-bet-/- but not Eomes-/- specific CD4 T cells were programmed by DCo to express GzmB, DCo boosted the ability of both T-bet-/- and Eomes-/- specific CD4 T cells to express IFN-γ (Fig. 8C). Taken together, DCo-induced T-bet and Eomes can both contribute to enhancing IFN-γ expression in specific CD4 T cells, but GzmB expression depends on Eomes.
Figure 8.
DCo induces GzmB expression in specific CD4 T cells via Eomes. Lethallyirradiated B6 RAG1-/- mice were reconstituted with B6 BM that was WT, T-bet-/- or conditionally Eomes-/-. Lymphocytes from reconstituted chimeras containing 1 X 106 CD4 T cells were then adoptively transferred into B6 TCR β-/- × δ-/- recipients that were treated the following day with SEA +/- DCo (as in Fig. 3, A and B). Intracellular GzmB was measured directly ex vivo in SEA-specific Vβ3+ CD4 T cells recovered from spleens 5 days following SEA and DCo treatment. A, Representative GzmB histograms. B, Bar graph corresponding to panel A (n=4 per group), * indicates that DCo significantly induced GzmB (p≤0.05) over non-treated. C, DCo-treated and non-treated SEA-specific Vβ3+ T-bet-/- and Eomes-/- CD4 T cells (generated as in panels A and B) were compared for their abilities to express GzmB directly ex vivo and IFN-γ following restimulation with PMA + ionomycin. Plots are representative of 2-3 replicates per group that were highly comparable.
Dual costimulation programs CD4 T cell-dependent tumoricidal activity
DCo can induce CD8 T cell-dependent tumor immunity (19, 21, 22). We currently used the B16-F10 (B16) murine melanoma to test if DCo can program CD4 T cells to mediate tumor immunity. B16 can be induced by IFN-γ to express MHC class II in vivo, thus becoming susceptible to lysis by specific cytotoxic CD4 Th1 cells (10, 11). Non-activated polyclonal CD4 T cells (depleted of CD8+, NK1.1+ and B220+ cells) transferred into RAG-/- recipients were unable to control the growth of B16 melanoma inoculated intradermally 5 days later (Fig. 9, A and B). In RAG-/- recipients given polyclonal CD4 T cells, DCo administered either the day of or 4 days prior to tumor inoculation substantially limited tumor growth (p≤0.006) (Fig. 9, A and B). Importantly, CD4 T cells recovered from DCo-treated mice expressed higher levels of IFN-γ (p=0.01) (Fig. 9C) and GzmB (p=0.009) (Fig. 9D) compared to non-treated counterparts. DCo showed a trend towards limiting tumor growth in RAG-/- mice that did not receive CD4 T cells (Fig. 9B), likely resulting from the activity of NK or other innate immune cells that can respond to CD134 and CD137 costimulation (54, 55). Taken together, DCo can program CD4 T cells to become tumoricidal as part of a multi-pronged anti-tumor immune response.
Figure 9.
DCo programs CD4 T cell-dependent tumoricidal function. RAG1-/- mice were intradermally inoculated with 1 X 105 B16 melanoma cells and tumor size measured 13 days later. As indicated, some mice received 1 X 106 polyclonal CD4 T cells (depleted of CD8+, NK1.1+ and B220+ cells) 5 days prior to tumor inoculation and/or DCo the day of (day 0) or 4 days prior to tumor inoculation (day -4). Asterisks indicate p<0.05 compared to the control group that received neither CD4 T cells nor DCo (open bar). A, N=4 for each group. B, N=8 or 7 for the control and all other groups, respectively. (C and D) CD4 T cells were recovered from the spleens of mice corresponding to panel B on day 14 and intracellular IFN-γ was measured following 5 h in vitro stimulation with PMA plus ionomycin (C) and GzmB was measured following 24 h stimulation with anti-CD3 mAb (D).
Discussion
Cytotoxic CD4 Th1 cells are emerging as a physiologically relevant (6, 7) and therapeutically useful (10, 11) effector T cell lineage. The differentiation process by which these multi-functional effector CD4 T cells develop can be facilitated by IL-2 in vitro (48) and lymphopenia in vivo (10, 11), but the relevant costimulatory pathways and transcriptional networks have until now been poorly understood. Our current study identifies a specific costimulatory pathway that programs cytotoxic CD4 Th1 cells in vivo. CD134 costimulation is sufficient to program the cytotoxic Th1 profile in antigen-primed CD4 T cells, consistent with the ability of CD134 to induce GzmB in CD8 T cells (56). The addition of CD137 (dual) costimulation maximized clonal expansion of these cytotoxic CD4 Th1 cells, suggesting that the combination would be a potent therapeutic regimen for eliciting tumor cytotoxicity.
CD134 plus CD137 dual costimulation increased CD4 T cell expression of the T-box transcription factor T-bet that drives IFN-γ expression in conventional Th1 cells (25), consistent with the increased ability of these CD4 T cells to express IFN-γ. Nevertheless, dual costimulated T-bet-/- specific cytotoxic CD4 Th1 cells exhibited only partially impaired IFN-γ expression. T-bet-/- CD8 T cells can express moderate amounts of IFN-γ because they express the related T-box factor Eomesodermin (Eomes) that also drives granzyme and perforin expression (15, 16). Importantly, dual costimulated specific cytotoxic CD4 Th1 cells expressed Eomes, and Eomes-/- CD4 T cells were unable to be induced by dual costimulation to express granzyme B. Thus, Eomes programs cytotoxic function in dual costimulated CD4 T cells.
In addition to Eomes, IL-2 was also required for cytotoxic Th1 differentiation. Dual costimulation induced specific CD4 T cells to express higher levels of both CD25 and pSTAT5, consistent with IL-2 acting directly on the specific CD4 T cells. That an IL-2-Eomes axis might be critical for cytotoxic CD4 Th1 cell differentiation is consistent with the recent observation that IL-2 induces Eomes in CD8 T cells (14).
Regardless of whether CD134 and/or CD137 costimulation programs cytotoxic CD4 Th1 cell function under physiological conditions such as during viral infection (6, 7), our current finding is clinically relevant. Thus, CD134 and CD137 costimulatory agonists are being developed separately as cancer therapeutics (26, 27), and in the case of MHC class II+ tumors such as B cell lymphomas (57, 58) and melanomas (8) the ability of tumor-reactive CD4 T cells to develop cytotoxic function would allow them to directly kill tumor cells. This would expand their role in tumor immunity beyond their traditionally assumed role as helpers that enhance the function of CD8 T cells and other immune cells (59-61). Indeed, dual costimulation effectively programmed purified polyclonal CD4 T cells to control the growth of B16 melanoma in RAG-/- mice. Consistent with the ability of innate immune cells to respond to CD134 and CD137 costimulation (54, 62-64), dual costimulation elicited moderate control of tumor growth in RAG-/- mice that did not receive T cells (albeit this effect was not statistically significant). Nevertheless, the ability of dual costimulation to engage multiple arms of the immune response (CD8+ CTL, NK cells and cytotoxic CD4 Th1 cells) that can target distinct tumor determinants should be therapeutically beneficial.
In response to CD134 plus CD137 dual costimulation antigen-specific cytotoxic CD4 Th1 cells “imprint” antigen-inexperienced bystanding CD4 T cells with similar functional capabilities. Antigen-stimulated CD4 T cells can augment proliferation in other antigen-stimulated CD4 T cells (65), but our current result is novel because antigen-stimulated CD4 T cells programmed bystander CD4 T cells to express an effector molecule typically expressed in CD8 T cells and NK cells. The expression of granzyme B in these bystander CD4 T cells is dependent on paracrine IL-2 secreted by antigen-specific CD4 T cells, although other bystander-programming factors may also be involved. An interesting facet of the bystander response is that granzyme B is not only induced in conventional bystander CD4 T cells, but also in Foxp3+ Treg cells that express high affinity IL-2R. Treg cells can exert suppressive function through granzyme B (66, 67), which raises the possibility that one function of the bystander response might be to limit collateral damage inflicted by the robust antigen-specific cytotoxic CD4 Th1 cell response.
The dual costimulation-induced bystander response may have a complex effect during tumor immunotherapy. For instance, polyclonal granzyme-expressing bystander T cells may eliminate residual antigen-loss variant tumor cells that lack expression of vaccine-targeted epitopes (68-72), but their polyclonal nature raises the potential for autoimmune toxicity. In this regard, it was previously noted that careful titration of each costimulatory agonist significantly lowered the effective dose of the other required to elicit optimal specific CD8 T cell responses (20), and thus it might be possible to preserve useful anti-tumor effects while limiting pathogenic side effects by titrating down the dose of each agonist antibody. This strategy would not be possible using single costimulators, and thus represents an in-built advantage of dual costimulation.
Acknowledgments
This work was supported by National Institutes of Health grants RO1 CA109339 (A.J.A. and A.T.V.) and R56 AI057441 (A.J.A.), and H.Z.Q. was supported by T32 AI007080.
Abbreviations used in this article
- AcH3
acetylated histone H3
- BM
bone marrow
- DCo
dual costimulation through CD134 and CD137
- Eomes
Eomesodermin
- GzmB
granzyme B
- MFI
mean fluorescence intensity
- self-HA
transgenic mice expressing hemagglutinin
- Tg
transgenic
- viral-HA
recombinant vaccinia virus expressing hemagglutinin
References
- 1.Murphy KM, Reiner SL. Decision making in the immune system: The lineage decisions of helper T cells. Nat Rev Immunol. 2002;2:933–944. doi: 10.1038/nri954. [DOI] [PubMed] [Google Scholar]
- 2.Zhou L, Chong MM, Littman DR. Plasticity of CD4+ T cell lineage differentiation. Immunity. 2009;30:646–655. doi: 10.1016/j.immuni.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 3.Glimcher LH, Townsend MJ, Sullivan BM, Lord GM. Recent developments in the transcriptional regulation of cytolytic effector cells. Nat Rev Immunol. 2004;4:900–911. doi: 10.1038/nri1490. [DOI] [PubMed] [Google Scholar]
- 4.Misko IS, Pope JH, Hutter R, Soszynski TD, Kane RG. HLA-DR-antigen-associated restriction of EBV-specific cytotoxic T-cell colonies. Int J Cancer. 1984;33:239–243. doi: 10.1002/ijc.2910330212. [DOI] [PubMed] [Google Scholar]
- 5.Williams NS, Engelhard VH. Identification of a population of CD4+ CTL that utilizes a perforin- rather than a Fas ligand-dependent cytotoxic mechanism. J Immunol. 1996;156:153–159. [PubMed] [Google Scholar]
- 6.Appay V, Zaunders JJ, Papagno L, Sutton J, Jaramillo A, Waters A, Easterbrook P, Grey P, Smith D, McMichael AJ, Cooper DA, Rowland-Jones SL, Kelleher AD. Characterization of CD4(+) CTLs ex vivo. J Immunol. 2002;168:5954–5958. doi: 10.4049/jimmunol.168.11.5954. [DOI] [PubMed] [Google Scholar]
- 7.Jellison ER, Kim SK, Welsh RM. Cutting edge: MHC class II-restricted killing in vivo during viral infection. J Immunol. 2005;174:614–618. doi: 10.4049/jimmunol.174.2.614. [DOI] [PubMed] [Google Scholar]
- 8.Topalian SL, Rivoltini L, Mancini M, Markus NR, Robbins PF, Kawakami Y, Rosenberg SA. Human CD4+ T cells specifically recognize a shared melanoma-associated antigen encoded by the tyrosinase gene. Proc Natl Acad Sci U S A. 1994;91:9461–9465. doi: 10.1073/pnas.91.20.9461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferrone S, Marincola FM. Loss of HLA class I antigens by melanoma cells: molecular mechanisms, functional significance and clinical relevance. Immunol Today. 1995;16:487–494. doi: 10.1016/0167-5699(95)80033-6. [DOI] [PubMed] [Google Scholar]
- 10.Xie Y, Akpinarli A, Maris C, Hipkiss EL, Lane M, Kwon EK, Muranski P, Restifo NP, Antony PA. Naive tumor-specific CD4+ T cells differentiated in vivo eradicate established melanoma. J Exp Med. 2010;207:651–667. doi: 10.1084/jem.20091921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Quezada SA, Simpson TR, Peggs KS, Merghoub T, Vider J, Fan X, Blasberg R, Yagita H, Muranski P, Antony PA, Restifo NP, Allison JP. Tumor-reactive CD4+ T cells develop cytotoxic activity and eradicate large established melanoma after transfer into lymphopenic hosts. J Exp Med. 2010;207:637–650. doi: 10.1084/jem.20091918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Verdeil G, Puthier D, Nguyen C, Schmitt-Verhulst AM, Auphan-Anezin N. STAT5-mediated signals sustain a TCR-initiated gene expression program toward differentiation of CD8 T cell effectors. J Immunol. 2006;176:4834–4842. doi: 10.4049/jimmunol.176.8.4834. [DOI] [PubMed] [Google Scholar]
- 13.Redmond WL, Gough MJ, Charbonneau B, Ratliff TL, Weinberg AD. Defects in the acquisition of CD8 T cell effector function after priming with tumor or soluble antigen can be overcome by the addition of an OX40 agonist. J Immunol. 2007;179:7244–7253. doi: 10.4049/jimmunol.179.11.7244. [DOI] [PubMed] [Google Scholar]
- 14.Pipkin ME, Sacks JA, Cruz-Guilloty F, Lichtenheld MG, Bevan MJ, Rao A. Interleukin-2 and inflammation induce distinct transcriptional programs that promote the differentiation of effector cytolytic T cells. Immunity. 2010;32:79–90. doi: 10.1016/j.immuni.2009.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pearce EL, Mullen AC, Martins GA, Krawczyk CM, Hutchins AS, Zediak VP, Banica M, DiCioccio CB, Gross DA, Mao CA, Shen H, Cereb N, Yang SY, Lindsten T, Rossant J, Hunter CA, Reiner SL. Control of effector CD8+ T cell function by the transcription factor Eomesodermin. Science. 2003;302:1041–1043. doi: 10.1126/science.1090148. [DOI] [PubMed] [Google Scholar]
- 16.Intlekofer AM, Takemoto N, Wherry EJ, Longworth SA, Northrup JT, Palanivel VR, Mullen AC, Gasink CR, Kaech SM, Miller JD, Gapin L, Ryan K, Russ AP, Lindsten T, Orange JS, Goldrath AW, Ahmed R, Reiner SL. Effector and memory CD8+ T cell fate coupled by T-bet and eomesodermin. Nat Immunol. 2005;6:1236–1244. doi: 10.1038/ni1268. [DOI] [PubMed] [Google Scholar]
- 17.Croft M. Co-stimulatory members of the TNFR family: keys to effective T-cell immunity? Nat Rev Immunol. 2003;3:609–620. doi: 10.1038/nri1148. [DOI] [PubMed] [Google Scholar]
- 18.Watts TH. TNF/TNFR family members in costimulation of T cell responses. Annu Rev Immunol. 2005;23:23–68. doi: 10.1146/annurev.immunol.23.021704.115839. [DOI] [PubMed] [Google Scholar]
- 19.Lee SJ, Myers L, Muralimohan G, Dai J, Qiao Y, Li Z, Mittler RS, Vella AT. 4-1BB and OX40 dual costimulation synergistically stimulate primary specific CD8 T cells for robust effector function. J Immunol. 2004;173:3002–3012. doi: 10.4049/jimmunol.173.5.3002. [DOI] [PubMed] [Google Scholar]
- 20.Lee SJ, Rossi RJ, Lee SK, Croft M, Kwon BS, Mittler RS, Vella AT. CD134 Costimulation Couples the CD137 Pathway to Induce Production of Supereffector CD8 T Cells That Become IL-7 Dependent. J Immunol. 2007;179:2203–2214. doi: 10.4049/jimmunol.179.4.2203. [DOI] [PubMed] [Google Scholar]
- 21.Cuadros C, Dominguez AL, Lollini PL, Croft M, Mittler RS, Borgstrom P, Lustgarten J. Vaccination with dendritic cells pulsed with apoptotic tumors in combination with anti-OX40 and anti-4-1BB monoclonal antibodies induces T cell-mediated protective immunity in Her-2/neu transgenic mice. Int J Cancer. 2005;116:934–943. doi: 10.1002/ijc.21098. [DOI] [PubMed] [Google Scholar]
- 22.Gray JC, French RR, James S, Al-Shamkhani A, Johnson PW, Glennie MJ. Optimising anti-tumour CD8 T-cell responses using combinations of immunomodulatory antibodies. Eur J Immunol. 2008;38:2499–2511. doi: 10.1002/eji.200838208. [DOI] [PubMed] [Google Scholar]
- 23.Lathrop SK, Huddleston CA, Dullforce PA, Montfort MJ, Weinberg AD, Parker DC. A signal through OX40 (CD134) allows anergic, autoreactive T cells to acquire effector cell functions. J Immunol. 2004;172:6735–6743. doi: 10.4049/jimmunol.172.11.6735. [DOI] [PubMed] [Google Scholar]
- 24.Bandyopadhyay S, Long M, Qui HZ, Hagymasi AT, Slaiby AM, Mihalyo MA, Aguila HL, Mittler RS, Vella AT, Adler AJ. Self-Antigen Prevents CD8 T Cell Effector Differentiation by CD134 and CD137 Dual Costimulation. J Immunol. 2008;181:7728–7737. doi: 10.4049/jimmunol.181.11.7728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Szabo SJ, Kim ST, Costa GL, Zhang X, Fathman CG, Glimcher LH. A novel transcription factor, T-bet, directs Th1 lineage commitment. Cell. 2000;100:655–669. doi: 10.1016/s0092-8674(00)80702-3. [DOI] [PubMed] [Google Scholar]
- 26.Tamada K, Chen L. Renewed interest in cancer immunotherapy with the tumor necrosis factor superfamily molecules. Cancer Immunol Immunother. 2006;55:355–362. doi: 10.1007/s00262-005-0081-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Redmond WL, Weinberg AD. Targeting OX40 and OX40L for the treatment of autoimmunity and cancer. Crit Rev Immunol. 2007;27:415–436. doi: 10.1615/critrevimmunol.v27.i5.20. [DOI] [PubMed] [Google Scholar]
- 28.Kirberg J, Baron A, Jakob S, Rolink A, Karjalainen K, von Boehmer H. Thymic selection of CD8+ single positive cells with a class II major histocompatibility complex-restricted receptor. J Exp Med. 1994;180:25–34. doi: 10.1084/jem.180.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Adler AJ, Huang CT, Yochum GS, Marsh DW, Pardoll DM. In vivo CD4+ T cell tolerance induction versus priming is independent of the rate and number of cell divisions. J Immunol. 2000;164:649–655. doi: 10.4049/jimmunol.164.2.649. [DOI] [PubMed] [Google Scholar]
- 30.Higgins AD, Mihalyo MA, McGary PW, Adler AJ. CD4 cell priming and tolerization are differentially programmed by APCs upon initial engagement. J Immunol. 2002;168:5573–5581. doi: 10.4049/jimmunol.168.11.5573. [DOI] [PubMed] [Google Scholar]
- 31.Schorle H, Holtschke T, Hunig T, Schimpl A, Horak I. Development and function of T cells in mice rendered interleukin-2 deficient by gene targeting. Nature. 1991;352:621–624. doi: 10.1038/352621a0. [DOI] [PubMed] [Google Scholar]
- 32.Szabo SJ, Sullivan BM, Stemmann C, Satoskar AR, Sleckman BP, Glimcher LH. Distinct effects of T-bet in TH1 lineage commitment and IFN-gamma production in CD4 and CD8 T cells. Science. 2002;295:338–342. doi: 10.1126/science.1065543. [DOI] [PubMed] [Google Scholar]
- 33.Intlekofer AM, Banerjee A, Takemoto N, Gordon SM, Dejong CS, Shin H, Hunter CA, Wherry EJ, Lindsten T, Reiner SL. Anomalous type 17 response to viral infection by CD8+ T cells lacking T-bet and eomesodermin. Science. 2008;321:408–411. doi: 10.1126/science.1159806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Babu S, Shultz LD, Klei TR, Rajan TV. Immunity in experimental murine filariasis: roles of T and B cells revisited. Infect Immun. 1999;67:3166–3167. doi: 10.1128/iai.67.6.3166-3167.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Long M, Slaiby AM, Hagymasi AT, Mihalyo MA, Lichtler AC, Reiner SL, Adler AJ. T-bet Down-Modulation in Tolerized Th1 Effector CD4 Cells Confers a TCR-Distal Signaling Defect That Selectively Impairs IFN-gamma Expression. J Immunol. 2006;176:1036–1045. doi: 10.4049/jimmunol.176.2.1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Long M, Adler AJ. Cutting Edge: Paracrine, but Not Autocrine, IL-2 Signaling Is Sustained during Early Antiviral CD4 T Cell Response. J Immunol. 2006;177:4257–4261. doi: 10.4049/jimmunol.177.7.4257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bandyopadhyay S, Qui HZ, Adler AJ. In vitro and in vivo differentiated effector CD8 T cells display divergent histone acetylation patterns within the Ifng locus. Immunol Lett. 2009;122:214–218. doi: 10.1016/j.imlet.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Glimcher LH, Kim KJ, Green I, Paul WE. Ia antigen-bearing B cell tumor lines can present protein antigen and alloantigen in a major histocompatibility complex-restricted fashion to antigen-reactive T cells. J Exp Med. 1982;155:445–459. doi: 10.1084/jem.155.2.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Heusel JW, Wesselschmidt RL, Shresta S, Russell JH, Ley TJ. Cytotoxic lymphocytes require granzyme B for the rapid induction of DNA fragmentation and apoptosis in allogeneic target cells. Cell. 1994;76:977–987. doi: 10.1016/0092-8674(94)90376-x. [DOI] [PubMed] [Google Scholar]
- 40.Pipkin ME, Lieberman J. Delivering the kiss of death: progress on understanding how perforin works. Curr Opin Immunol. 2007;19:301–308. doi: 10.1016/j.coi.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 41.Araki Y, Fann M, Wersto R, Weng NP. Histone acetylation facilitates rapid and robust memory CD8 T cell response through differential expression of effector molecules (eomesodermin and its targets: perforin and granzyme B). J Immunol. 2008;180:8102–8108. doi: 10.4049/jimmunol.180.12.8102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Betts MR, Brenchley JM, Price DA, De Rosa SC, Douek DC, Roederer M, Koup RA. Sensitive and viable identification of antigen-specific CD8+ T cells by a flow cytometric assay for degranulation. J Immunol Methods. 2003;281:65–78. doi: 10.1016/s0022-1759(03)00265-5. [DOI] [PubMed] [Google Scholar]
- 43.Marrack P, Kappler J. The staphylococcal enterotoxins and their relatives. Science. 1990;248:705–711. doi: 10.1126/science.2185544. [DOI] [PubMed] [Google Scholar]
- 44.Moon JJ, Chu HH, Pepper M, McSorley SJ, Jameson SC, Kedl RM, Jenkins MK. Naive CD4(+) T cell frequency varies for different epitopes and predicts repertoire diversity and response magnitude. Immunity. 2007;27:203–213. doi: 10.1016/j.immuni.2007.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Moon JJ, Chu HH, Hataye J, Pagan AJ, Pepper M, McLachlan JB, Zell T, Jenkins MK. Tracking epitope-specific T cells. Nat Protoc. 2009;4:565–581. doi: 10.1038/nprot.2009.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kaleeba JA, Offner H, Vandenbark AA, Lublinski A, Weinberg AD. The OX-40 receptor provides a potent co-stimulatory signal capable of inducing encephalitogenicity in myelin-specific CD4+ T cells. Int Immunol. 1998;10:453–461. doi: 10.1093/intimm/10.4.453. [DOI] [PubMed] [Google Scholar]
- 47.Janas ML, Groves P, Kienzle N, Kelso A. IL-2 regulates perforin and granzyme gene expression in CD8+ T cells independently of its effects on survival and proliferation. J Immunol. 2005;175:8003–8010. doi: 10.4049/jimmunol.175.12.8003. [DOI] [PubMed] [Google Scholar]
- 48.Brown DM, Kamperschroer C, Dilzer AM, Roberts DM, Swain SL. IL-2 and antigen dose differentially regulate perforin- and FasL-mediated cytolytic activity in antigen specific CD4+ T cells. Cell Immunol. 2009;257:69–79. doi: 10.1016/j.cellimm.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bream JH, Hodge DL, Gonsky R, Spolski R, Leonard WJ, Krebs S, Targan S, Morinobu A, O'Shea JJ, Young HA. A distal region in the interferon-gamma gene is a site of epigenetic remodeling and transcriptional regulation by interleukin-2. J Biol Chem. 2004;279:41249–41257. doi: 10.1074/jbc.M401168200. [DOI] [PubMed] [Google Scholar]
- 50.Williams CA, Murray SE, Weinberg AD, Parker DC. OX40-mediated differentiation to effector function requires IL-2 receptor signaling but not CD28, CD40, IL-12Rbeta2, or T-bet. J Immunol. 2007;178:7694–7702. doi: 10.4049/jimmunol.178.12.7694. [DOI] [PubMed] [Google Scholar]
- 51.Vu MD, Xiao X, Gao W, Degauque N, Chen M, Kroemer A, Killeen N, Ishii N, Chang Li X. OX40 costimulation turns off Foxp3+ Tregs. Blood. 2007;110:2501–2510. doi: 10.1182/blood-2007-01-070748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.So T, Croft M. Cutting edge: OX40 inhibits TGF-beta- and antigen-driven conversion of naive CD4 T cells into CD25+Foxp3+ T cells. J Immunol. 2007;179:1427–1430. doi: 10.4049/jimmunol.179.3.1427. [DOI] [PubMed] [Google Scholar]
- 53.Malek TR, Yu A, Vincek V, Scibelli P, Kong L. CD4 regulatory T cells prevent lethal autoimmunity in IL-2Rbeta-deficient mice. Implications for the nonredundant function of IL-2. Immunity. 2002;17:167–178. doi: 10.1016/s1074-7613(02)00367-9. [DOI] [PubMed] [Google Scholar]
- 54.Melero I, Johnston JV, Shufford WW, Mittler RS, Chen L. NK1.1 cells express 4-1BB (CDw137) costimulatory molecule and are required for tumor immunity elicited by anti-4-1BB monoclonal antibodies. Cell Immunol. 1998;190:167–172. doi: 10.1006/cimm.1998.1396. [DOI] [PubMed] [Google Scholar]
- 55.Karulf M, Kelly A, Weinberg AD, Gold JA. OX40 ligand regulates inflammation and mortality in the innate immune response to sepsis. J Immunol. 2010;185:4856–4862. doi: 10.4049/jimmunol.1000404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lee SW, Park Y, Song A, Cheroutre H, Kwon BS, Croft M. Functional dichotomy between OX40 and 4-1BB in modulating effector CD8 T cell responses. J Immunol. 2006;177:4464–4472. doi: 10.4049/jimmunol.177.7.4464. [DOI] [PubMed] [Google Scholar]
- 57.Stavely-O'Carroll K, Sotomayor E, Montgomery J, Borrello I, Hwang L, Fein S, Pardoll D, Levitsky H. Induction of antigen-specific T cell anergy: an early event in the course of tumor progression. Proc Natl Acad Sci USA. 1998;95:1178–1183. doi: 10.1073/pnas.95.3.1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Robertson KA, Usherwood EJ, Nash AA. Regression of a murine gammaherpesvirus 68-positive b-cell lymphoma mediated by CD4 T lymphocytes. J Virol. 2001;75:3480–3482. doi: 10.1128/JVI.75.7.3480-3482.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Romerdahl CA, Kripke ML. Role of helper T-lymphocytes in rejection of UV-induced murine skin cancers. Cancer Res. 1988;48:2325–2328. [PubMed] [Google Scholar]
- 60.Hung K, Hayashi R, Lafond-Walker A, Lowenstein C, Pardoll D, Levitsky H. The central role of CD4(+) T cells in the antitumor immune response. J Exp Med. 1998;188:2357–2368. doi: 10.1084/jem.188.12.2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Surman DR, Dudley ME, Overwijk WW, Restifo NP. Cutting edge: CD4+ T cell control of CD8+ T cell reactivity to a model tumor antigen. J Immunol. 2000;164:562–565. doi: 10.4049/jimmunol.164.2.562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Croft M. Control of immunity by the TNFR-related molecule OX40 (CD134). Annu Rev Immunol. 2010;28:57–78. doi: 10.1146/annurev-immunol-030409-101243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Futagawa T, Akiba H, Kodama T, Takeda K, Hosoda Y, Yagita H, Okumura K. Expression and function of 4-1BB and 4-1BB ligand on murine dendritic cells. Int Immunol. 2002;14:275–286. doi: 10.1093/intimm/14.3.275. [DOI] [PubMed] [Google Scholar]
- 64.Pauly S, Broll K, Wittmann M, Giegerich G, Schwarz H. CD137 is expressed by follicular dendritic cells and costimulates B lymphocyte activation in germinal centers. J Leukoc Biol. 2002;72:35–42. [PubMed] [Google Scholar]
- 65.Sporri R, Reis e Sousa C. Newly activated T cells promote maturation of bystander dendritic cells but not IL-12 production. J Immunol. 2003;171:6406–6413. doi: 10.4049/jimmunol.171.12.6406. [DOI] [PubMed] [Google Scholar]
- 66.Gondek DC, Lu LF, Quezada SA, Sakaguchi S, Noelle RJ. Cutting edge: contact-mediated suppression by CD4+CD25+ regulatory cells involves a granzyme B-dependent, perforin-independent mechanism. J Immunol. 2005;174:1783–1786. doi: 10.4049/jimmunol.174.4.1783. [DOI] [PubMed] [Google Scholar]
- 67.Cao X, Cai SF, Fehniger TA, Song J, Collins LI, Piwnica-Worms DR, Ley TJ. Granzyme B and perforin are important for regulatory T cell-mediated suppression of tumor clearance. Immunity. 2007;27:635–646. doi: 10.1016/j.immuni.2007.08.014. [DOI] [PubMed] [Google Scholar]
- 68.Maeurer MJ, Gollin SM, Martin D, Swaney W, Bryant J, Castelli C, Robbins P, Parmiani G, Storkus WJ, Lotze MT. Tumor escape from immune recognition: lethal recurrent melanoma in a patient associated with downregulation of the peptide transporter protein TAP-1 and loss of expression of the immunodominant MART-1/Melan-A antigen. J Clin Invest. 1996;98:1633–1641. doi: 10.1172/JCI118958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jager E, Ringhoffer M, Altmannsberger M, Arand M, Karbach J, Jager D, Oesch F, Knuth A. Immunoselection in vivo: independent loss of MHC class I and melanocyte differentiation antigen expression in metastatic melanoma. Int J Cancer. 1997;71:142–147. doi: 10.1002/(sici)1097-0215(19970410)71:2<142::aid-ijc3>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 70.Yee C, Thompson JA, Byrd D, Riddell SR, Roche P, Celis E, Greenberg PD. Adoptive T cell therapy using antigen-specific CD8+ T cell clones for the treatment of patients with metastatic melanoma: in vivo persistence, migration, and antitumor effect of transferred T cells. Proc Natl Acad Sci U S A. 2002;99:16168–16173. doi: 10.1073/pnas.242600099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhou G, Lu Z, McCadden JD, Levitsky HI, Marson AL. Reciprocal Changes in Tumor Antigenicity and Antigen-specific T Cell Function during Tumor Progression. J Exp Med. 2004;200:1581–1592. doi: 10.1084/jem.20041240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Spiotto MT, Rowley DA, Schreiber H. Bystander elimination of antigen loss variants in established tumors. Nat Med. 2004;10:294–298. doi: 10.1038/nm999. [DOI] [PubMed] [Google Scholar]