Abstract
LAG-3 (CD223) is a CD4 homolog that is required for maximal regulatory T cell function and for the control of CD4+ and CD8+ T cell homeostasis. Lag3−/− NOD mice developed substantially accelerated diabetes with 100% incidence. Adoptive transfer experiments revealed that LAG-3 was primarily responsible for limiting the pathogenic potential of CD4+ T cells, and to a lesser extent CD8+ T cells. Lag3−/− mice exhibited accelerated, invasive insulitis, corresponding to increased CD4+ and CD8+ T cell islet infiltration and intra-islet proliferation. The frequencies of islet antigen reactive chromogranin A-specific CD4+ T cells and IGRP-specific CD8+ T cells were significantly increased in the islets of Lag3−/− mice, suggesting an early expansion of pathogenic clones which is normally restrained by LAG-3. We conclude that LAG-3 is necessary for regulating CD4+ and CD8+ T cell function during autoimmune diabetes, and thus may contribute to limiting autoimmunity in disease-prone environments.
Keywords: Rodent, T cells, Autoimmunity, Diabetes, LAG-3
INTRODUCTION
Type 1 Diabetes (T1D) is a chronic autoimmune disorder driven primarily by CD4+ T cells in concert with CD8+ T cells, B cells and NK cells (1). Autoimmune diabetes in Non-Obese Diabetic (NOD) mice, a model for TID in humans (2), is initially associated with cellular infiltration of the pancreatic islets (insulitis). Ultimately, the balance between regulatory and pro-inflammatory mediators is lost, leading to the destruction of insulin-secreting β cells and subsequent diabetes (2). The importance of regulatory elements in the early pre-diabetic stages of disease was exposed upon regulatory T cell (Treg) ablation, which results in rapid β cell destruction and diabetes onset (3). However, the regulatory mechanisms responsible for limiting catastrophic disease in autoimmune diabetes-prone mice and patients with T1D remain obscure.
Cell intrinsic negative regulatory molecules, such as CTLA-4, PD-1 and LAG-3 (lymphocyte activation gene-3; CD223), mediate immune regulation with overlapping but distinct characteristics. For instance, whereas PD-1 blockade in NOD mice induces rapid diabetes at any age, disease following CTLA-4 blockade is only induced in neonates (4). LAG-3 is a CD4 homolog that binds to MHC class II molecules with high affinity. It regulates T cell proliferation and homeostasis, CD8+ T cell exhaustion and is required for optimal Treg and NK cell function (5-8). However, Lag3 deletion in C57BL/6 mice resulted in a minor phenotype with no development of spontaneous disease, arguing against a significant role for LAG-3 in controlling autoimmunity (9).
We hypothesized that the function of LAG-3 becomes more critical in autoimmune-prone environments. While recent studies suggested that autoimmune diabetes is accelerated in Lag3−/− NOD, the mechanistic basis for this increased disease is unknown (10). In our study, we determined the effect of LAG-3 loss or blockade on autoimmune diabetes incidence in NOD mice, and asked how this might involve T cells, their functional parameters and/or the frequency of presumptive pathogenic T cell populations.
MATERIALS AND METHODS
Mice and Diabetes Induction
Lag3−/− C57BL/6 mice (9) (obtained from Yueh-Hsiu Chen, Stanford University, Palo Alto, CA, with permission from Christophe Benoist and Diane Mathis, Joslin Diabetes Center, Boston, MA) were bred onto the NOD background for at least 10 generations (100% NOD as determined by SNP microsatellite analysis) or onto the B10.D2 background for at least 10 generations (at Johns Hopkins). NOD.scid and NOD/ShiLtJ mice were obtained from Jackson Laboratories, bred at St. Jude. In some experiments, diabetes was adoptively transferred by injecting NOD.scid female mice i.v. with 107 splenocytes. All animal experiments were in Association for the Accreditation of Laboratory Animal Care-accredited, Helicobacter-free, MNV-free, specific-pathogen-free facilities in the St. Jude Animal Resource Center following national, state and institutional guidelines. Blockade experiments were performed at Biologics Discovery California, Bristol-Myers-Squibb, Milpitas, CA.
Measurement of insulitis and diabetes
Insulitis and diabetes was assessed as described previously (11, 12). Briefly, pancreata of NOD mice embedded in paraffin was cut at 4 μm–thick sections at 150-μm step-sections and stained with hematoxylin and eosin at the St. Jude Histology Core Facility. An average of 90–100 islets per mouse were scored in a blinded manner. Two methods of insulitis measurement were used. First, insulitis and peri-insulitis were determined based on the percentage of the islets that possessed leukocyte infiltrate using the following metric: no insulitis (normal islet and no infiltration), peri-insulitis (infiltration on edges of islet or 0–25% of islet infiltrated) or insulitis (infiltration of 25–100% of islet) (11). Second, a defined insulitis score was determined using the method outlined in Current Protocols in Immunology (13). Diabetes incidence was monitored weekly by testing for the presence of glucose in the urine by Clinistix® (Bayer, Elkhart, IN). Mice testing positive by Clinistix® were then tested with a Breeze®2 glucometer (Bayer, Elkhart, IN) for elevated blood glucose levels and were considered diabetic if their blood glucose was >400 mg/dL.
Anti-LAG-3 and anti-PD-1 blockade
Seven week old female NOD mice were treated by i.p. injection with 200μg of chimeric mouse anti-PD-1 (4H2, IgG1), rat anti-mouse LAG-3 (C9B7W, IgG1) or control murine IgG1 (MOPC 21; BioXCell) on days 0, 4 and 7. Mice were tested twice a week for blood glucose levels and were considered diabetic after two consecutive readings above 250 mg/dL.
Islet isolation, flow cytometry and sorting
Islets were isolated as described previously (12). The Chromogranin A (BDC2.5 mimotope) tetramer for CD4+ T cells (AHHPIWARMDA/Ag7) and the IGRP (NRP-V7 mimotope) tetramer for CD8+ T cells (KYNKANVFL/H-2Kd) were obtained from NIH Tetramer Core. Surface antigen and IGRP tetramer staining were performed on ice for 30 minutes. BDC tetramer staining was done for 2 hours at 37°C in cell culture media, in parallel with control CD4 tetramer (LSIALHVGFDH/Ag7). NRP-V7 tetramer enrichment was performed on pooled peripheral lymphoid organs (excluding pancreatic LN) as described previously (14). Briefly, pooled organs were labeled with NRP-V7 tetramer, followed by anti-PE MACS bead (Miltenyi) purification, and the positively selected fraction was counted and analyzed by flow cytometry.
Statistical analysis
Kaplan-Meier test was used to determine significance of diabetes incidence in combination with Log-rank test to determine the P value. The Mann-Whitney non-parametric analysis was used in all other instances to determine significance and the P value.
RESULTS AND DISCUSSION
Accelerated diabetes following loss or blockage of LAG-3
In order to assess the potential role of LAG-3 in autoimmunity, we crossed the Lag3−/− mice onto the autoimmune prone NOD background. Deletion of LAG-3 resulted in rapid diabetes development in both NOD females and males with 100% penentrance at the time when the wild type (WT) female mice exhibited only 15% disease onset; this amounted to 10 and 14 week earlier onset in females and males, respectively (at 50% incidence; Fig. 1A, 1B). Diabetes incidence in heterozygous mice was clearly indicative of haploinsufficiency, suggesting that even a partial reduction in Lag3 gene expression leads to accelerated disease.
Figure 1.
Accelerated diabetes in the absence of LAG-3. Diabetes incidence was monitored in Lag3−/− NOD females (A) and males (B) together with littermate heterozygous Lag3+/– and Lag3+/+(WT) mice (♀ +/+ n=53, +/− n=46, −/− n=29; ♂ +/+ n=40, +/− n=39, −/− n=31). C, NOD WT female mice (7 weeks old) were treated with blocking antibodies to LAG-3, PD-1, control IgG1, or PBS on days 0, 5, and 7 and monitored for diabetes (n=9). D-E, Splenocytes from 5-6 week old mice (D - WT; E - Lag3−/−) were MACS depleted of CD4+ or CD8+ T cells, reconstituted with either CD4+ or CD8+ T cells from age-matched Lag3−/− (D) or WT (E) female mice, and transferred into female NOD.scid recipients (n=11-14).
We further assessed whether the defect observed was due to loss of LAG-3, rather than disruption of an adjacent locus in the targeted mice, by determining the effect of LAG-3 blockade. We also sought to determine whether disruption of LAG-3 function in insulitic, pre-diabetic mice caused accelerated diabetes. Seven week old NOD female mice were given three doses of blocking anti-LAG-3 antibody and monitored for diabetes induction. Anti-PD-1 blockade was included for comparison as such treatment has previously been shown to induce rapid diabetes onset (4). Blocking LAG-3 in WT mice accelerated disease development by ~8 weeks, comparable to the effect seen in the Lag3−/− mice and with a similar incidence to anti-PD-1 treatment, albeit with slightly delayed kinetics (Fig. 1C). Taken together, these data show that LAG-3 genetic deletion or blockade substantially accelerates diabetes incidence and onset, suggesting that LAG-3 limits disease onset and mitigates an ongoing autoimmune response. This contrasts with blockade of CTLA-4 and ICOS that only have a dominant role at earlier stages of insulitis (<4 week old mice) (15).
Lag3−/− CD4+ T cells alone can recapitulate the Lag3−/− phenotype
Since CD4+ and CD8+ T cell can express LAG-3 and both cell types are instrumental in driving diabetes development, we assessed the relative contribution of each population in the accelerated diabetes observed (1). As the disease environment in NOD mice involves multiple cellular populations, we wanted to assess how the loss of LAG-3 affects CD4+ and CD8+ T cells in the context of WT lymphoid populations. We chose to use splenocytes from young pre-diabetic 5-6 week old mice so that the experiment was not biased by the possibility that a larger number of islet reactive T cells might be present in Lag3−/− NOD mice. Splenocytes from 5-6 week old WT NOD mice were depleted of CD4+ or CD8+ cells and then reconstituted with either Lag3−/− or WT CD4+ or CD8+ cells, respectively, and adoptively transferred into NOD.scid recipients. Reciprocal experiments were also performed where CD4+ or CD8+ T cell-depleted Lag3−/− splenocytes were reconstituted with WT CD4+ or CD8+ T cells, respectively. As a control, depleted Lag3−/− splenocytes were reconstituted with Lag3−/− T cells, and WT splenocytes reconstituted with WT T cells. Importantly, adoptive transfer of splenocytes from Lag3−/− mice into NOD.scid female recipients resulted in accelerated disease compared to WT splenocyte recipients (Fig. 1D).
Interestingly, transfer of Lag3−/− CD4+ T cells in the context of WT splenocytes also resulted in accelerated disease suggesting that loss of LAG-3 expression from CD4+ T cells is sufficient to recapitulate the accelerated diabetes onset and incidence observed in the Lag3−/− splenocyte recipients (Fig. 1D). Likewise, adoptive transfer of WT CD8+ T cells plus CD8-depleted Lag3−/− splenocytes (ie. contained Lag3−/− CD4+ T cells) also resulted in accelerated disease (Fig. 1E). These data suggested that Lag3−/− CD4+ T cells are sufficient to drive the accelerated diabetes onset and incidence observed.
To assess the contribution of Lag3−/− CD8+ T cells, we transferred either Lag3−/− CD8+ T cells plus CD8-depleted WT splenocytes or WT CD4+ T cells plus CD4-depleted Lag3−/− splenocytes (ie. contained Lag3−/− CD8+ T cells). Interestingly, diabetes onset was also accelerated, but in both cases final disease incidence was lower and somewhat comparable to transfer of WT splenocytes (Fig. 1D, E). These data suggest that while loss of LAG-3 on CD8+ T cells can lead to accelerated disease onset this does not translate into increased disease penetrance, inferring that the loss of LAG-3 on CD4+ T cells has a greater impact on disease development.
Accelerated T cell infiltration in the islets of Lag3−/− mice
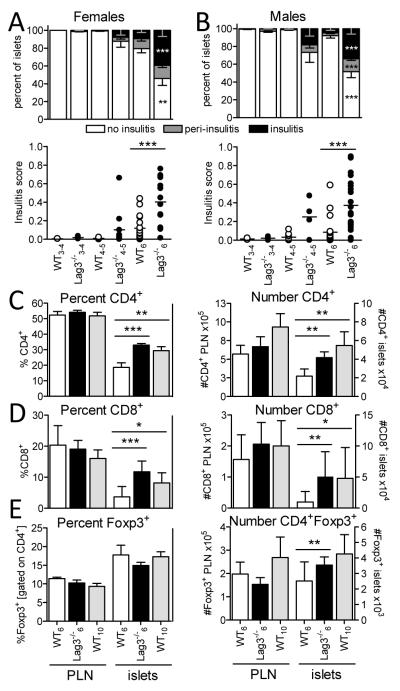
Next we asked if the kinetics of islet infiltration mirrored accelerated diabetes development in Lag3−/− NOD mice. Histological analysis revealed accelerated insulitis in Lag3−/− NOD female and male mice starting as early as 4 weeks of age (Fig. 2A, 2B). Interestingly, insulitis was only accelerated by a few weeks, unlike diabetes which was accelerated by 10-14 weeks, suggesting that once insulitis is initiated the β cell destruction rapidly ensues in the absence of LAG-3.
Figure 2.
Increased insulitis and numbers of infiltrating CD4+ and CD8+ T cells in the islets of Lag3−/− mice. Histological assessment of insulitis performed in female (A) and male (B) Lag3−/− and WT mice at three different ages (3-4 weeks, ♀ n=2-6 ♂ n=2-4; 4-5 weeks, ♀ n=3-11 ♂ n=4-9; and 6 weeks old, ♀ n=16-18 ♂ n=20-22). In the top panel: percent of islets exhibiting no insulitis (white), peri-insulitis (grey) and invasive insulitis (black) (***p≤0.0009, **p=0.0014). Percent and number of CD4+ (C; n=14-23), CD8+ (D; n=5-12), and CD4+Foxp3+ (E; n=9-18) T cells based on flow cytometric analysis of pancreatic LN (PLN) and islets from 6 week old wild type (white), 6 week old Lag3−/− (black) or 10 week old Lag3−/− (grey) female mice (***p≤0.0004, **p≤0.088, *p=0.0399).
LAG-3 is expressed by multiple cell types including CD4+ and CD8+ T cells, NK cells, and pDCs (plasmocytoid dendritic cells) (16). Therefore, we wanted to assess which populations were differentially represented in the pancreatic islets of Lag3−/− NOD mice. We concentrated our analysis on pre-diabetic 6 week old female WT and Lag3−/− mice. However, due to temporal differences in diabetes onset between WT and Lag3−/− mice, in some instances we also included 10 week old WT mice as this is a comparable time point to Lag3−/− mice relative to diabetes onset. The total number of cells infiltrating the islets of 6 week old Lag3−/− mice was increased compared to age matched WT mice but comparable to 10 week old WT controls (Supplemental Fig. 1A). There was a significant increase in the frequency and number of CD4+ and CD8+ T cells in the islets of 6 week old mice, which were comparable to 10 week WT mice (Fig. 2C, D). These differences were limited to the islets as this was not observed in the pancreatic lymph nodes (PLN). While accelerated islet infiltration was observed in Lag3−/− NOD mice, this appeared to plateau as a comparable percentage and number of CD4+ T cells was observed in the islets of 9-10 week old WT and Lag3−/− NOD mice, although the latter were hyperglycemic at this time point (Supplemental Fig. 1B). Taken together, these data suggest that islet infiltration is accelerated in Lag3−/− NOD mice, although the extent and constituency of the islet infiltrate at diabetes onset is similar in WT and Lag3−/− NOD mice. Thus, the accelerated disease onset may be due to early, rapid islet infiltration rather than an increase in the final total T cell numbers.
In contrast to CD4+ and CD8+ T cells, there was no significant difference in the frequency of CD4+Foxp3+ Tregs, although a small increase in the numbers was observed (Fig. 2E). Interestingly, the frequency and number of Tregs were significantly reduced in the islets of diabetic Lag3−/− NOD mice (Supplemental Fig. 1B), consistent with the dramatic Treg reduction seen in late stage disease which is thought to be due to reduced IL-2 at the site of inflammation (17).
We did not observe any significant increase in the frequency or number of NK cells or pDCs in the islets of Lag3−/− NOD mice (data not shown). These data suggest that the absence of LAG-3 results in a selective increase in CD4+ and CD8+ T cells.
Increased T cell proliferation but not effector function in the absence of LAG-3
We hypothesized that enhanced T cell accumulation in the islets of Lag3−/− NOD mice could be due to accelerated trafficking and/or increased proliferation. About 5% of CD4+ or CD8+ T cells in the non-draining LN and PLN expressed Ki67, a marker associated with cell cycle progression (Fig. 3A). The frequency of Ki67+ CD4+ and CD8+ T cells was augmented in the islets of NOD mice, although this did not seem to increase with age. Interestingly, there was a significant increase in the percentage of Ki67+ CD4+ and CD8+ T cells in Lag3−/− NOD mice. A higher percentage of Ki67+ Foxp3+ Tregs, compared with CD4+ and CD8+ T cells, was observed in all organs examined. However, although the frequency of Ki67+ Tregs was substantially higher in the islets, no discernable increase was observed in Lag3−/− mice. These data suggest that increase intra-islet proliferation may contribute to the increased T cell accumulation seen in Lag3−/− NOD mice.
Figure 3.
CD4+ and CD8+ T cells exhibit increased proliferation in the islets of Lag3−/− mice. A, Proliferation was assessed based on intracellular Ki67 staining of CD8+, CD4+Foxp3− and CD4+Foxp3+ T cells in 6 week old Lag3−/− female mice (n=7) and 6 (n=10) or 10 week old (n=5) WT female mice (*p≤0.0229). B, CXCR3 cell surface expression on CD4+ T cells was measured by flow cytometric analysis of 6-7 week old female Lag3−/− mice (n=7; *p=0.0262). C, Intracellular IFNγ expression was assessed in the organs of 6 week old female and 7 week old male Lag3−/− and WT mice after 5 hour stimulation with PMA and ionomycin (n=7-10).
Expression of the homing molecules, CXCR3 chemokine receptor and α4 integrins, have been associated with T cell trafficking into the pancreas (18, 19). A substantially higher percentage of CD4+CD44hi T cells in the PLN expressed CXCR3 compared to other lymphoid organs (Fig. 3B). Interestingly, the frequency of CXCR3+CD4+CD44hi T cells was significantly higher in the PLN of Lag3−/− mice. A high percentage of CD8+CD44hi T cells expressed CXCR3 in all the secondary lymphoid organs tested and this did not differ in the absence of LAG-3 (Supplemental Fig. 1C). Likewise, a high percentage of CD4+ and CD8+ T cells infiltrating the islets expressed the α4 integrin chain, although there was no difference in expression between WT and Lag3−/− mice (Supplemental Fig. 1D). Overall, these data suggest that accelerated islet infiltration in Lag3−/− NOD mice may primarily be due to an increase in CD4+ T cell homing to the pancreas followed by increased proliferation of CD4+ and CD8+ T cells in the islets.
We next assessed whether the effector function of islet-infiltrating T cells in Lag3−/− mice was altered. There was an increased frequency of CD4+IFNγ+ and CD8+IFNγ+ T cells in the islets, compared to secondary lymphoid organs (Fig. 3C). In contrast, minimal differences in the frequency of TNFα+ and IL-2+ T cells were observed (Supplemental Fig. 1E-H, J, K). Also, very low percentages of IL-17+ T cells were present in the islets, questioning the contribution of Th17 cells in autoimmune diabetes (Supplemental Fig. 1I). Interestingly, there was no increase in the frequency of effector cytokine-expressing T cells in the islets of Lag3−/− mice compared with WT mice, suggesting that the accelerated disease observed in Lag3−/− mice is due to increased T cell infiltration/retention/proliferation, and not due to increased T cell effector function.
Increased frequency of islet antigen-specific T cells in Lag3−/− NOD mice
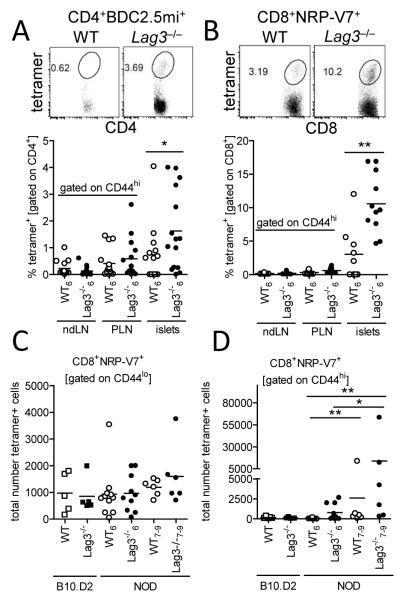
Since Lag3−/− NOD mice exhibited earlier and more extensive T cell infiltration into the islets, we questioned whether the PLN and islets in the Lag3−/− mice had an increased proportion of islet antigen reactive T cells. We utilized MHC:peptide tetramers specific for two major autoantigens, chromogranin A and IGRP, recognized by pathogenic CD4+ and CD8+ T cells, respectively, to determine the frequency of potentially pathogenic clones that may be associated with progression to disease (20, 21). WT and Lag3−/− mice exhibited low frequencies of islet antigen tetramer positive cells within activated CD4+CD44hi and CD8+CD44hi T cell populations in the non-draining and draining LN, but were significantly elevated in the islets, in contrast to non-islet antigen tetramer (Fig. 4A, B, Supplemental Fig. 1L). Importantly, there was a 2-4-fold increase in the frequency of tetramer positive T cells in the islets of Lag3−/− mice compared with WT controls, suggesting that the loss of LAG-3 may have a selective effect on more diabetogenic clones. This increase is relative to the total number of T cells in the islets and thus a preferential expansion of pathogenic clones compared with other infiltrating T cell clones. Thus the actual number of tetramer+ T cells in the islets of Lag3−/− NOD mice is even greater given the increased number of infiltrating T cells observed.
Figure 4.
Accumulation of islet antigen specific clones in the islets of Lag3−/− NOD mice. Frequency of chromogranin A mimotope (BDC2.5mi) tetramer positive CD4+ (A; n=14-15; *p=0.0495) and IGRP mimotope (NRP-V7) tetramer positive CD8+ (B; n=9-11; **p=0.0024) T cells was assessed in the organs of Lag3−/− mice. Representative islet FACS plots are shown. Numbers of CD44lo precursor (C) and CD44hi activated/expanded (D) IGRP reactive CD8+ T cells were calculated after enrichment for NRP-V7 tetramer+ cells via anti-PE MACS beads and flow cytometric analysis (n=5-9; **p≤0.0022, *p=0.04).
We then posited that the increased frequency of pathogenic clones in the islets of Lag3−/− NOD mice could be due to accelerated or preferential pathogenic clone expansion upon activation in the PLN or islets, or increased precursor frequency of autoreactive clones in Lag3−/− NOD mice. In order to quantify naïve precursors in the periphery of Lag3−/− mice, we employed a previously published method for quantification of small numbers of endogenous antigen-specific T cells (14). We pooled all peripheral lymphoid organs in individual mice excluding draining PLN, and then used anti-PE MACS beads to enrich for IGRP:H-2Kd-PE tetramer+ CD8+ cells. Interestingly, Lag3−/− mice exhibited a similar precursor frequency of naive CD44lo tetramer+ cells in the periphery compared to wild type NOD mice (Fig. 4C, Supplemental Fig. 1M). In order to assess whether there was a strain-specific difference, we also analyzed Lag3−/− and WT B10.D2 mice, an H-2Kd positive, non-autoimmune background. The precursor frequency of CD44lo tetramer+ cells was comparable between Lag3−/− and WT B10.D2 and NOD mice, suggesting that the precursor frequency of potentially pathogenic clones was not increased in the periphery in an autoimmune prone NOD background and/or in the absence of LAG-3. In concordance with these data, there was no difference in the number of IGRP specific thymocytes after tetramer enrichment of single positive CD8+CD3+ thymocytes (data not shown). Unexpectedly, 30% of young (6 week old) Lag3−/− NOD mice had slightly increased numbers of activated CD44hi tetramer+ cells in non-draining peripheral organs, and these cells seemed to accumulate in periphery with age (Fig. 4D, Supplemental Fig. 1M) . Thus, we conclude that LAG-3 primarily functions at a local level where its absence causes increased T cell proliferation following antigen stimulation, which leads to the rapid accumulation of self-reactive T cells and accelerated disease.
Concluding remarks
Our data show that LAG-3 plays a critical role in regulation of autoimmune response in NOD mice by selectively inhibiting antigen-reactive T cell infiltration and expansion in the islets, without affecting the effector phenotype of the cells. In the absence of LAG-3, T cells infiltrate NOD pancreatic islets at a younger age and accumulate faster, processes which are associated with increased CXCR3 expression on CD4+ T cells in the pancreatic LN and increased CD4+ and CD8+ T cell proliferation in the islets of Lag3−/− NOD mice. Given that blockage of LAG-3 after onset of islet infiltration in 7 week old NOD mice results in accelerated diabetes development, our findings suggest that LAG-3 functions throughout disease development. Previous studies have suggested that LAG-3 has a preferential effect on the proliferative capacity of T cells (5, 7, 8), in contrast to PD-1 and CTLA-4 that appear to affect both proliferation and effector function (8, 22). It should be noted that the only known ligand for murine LAG-3 is MHC class II, to which it binds with high affinity (7, 23, 24). Thus, LAG-3 is likely ligated by the macrophages, dendritic cells and B cells that are known to be present in the islet infiltrate and express high levels of MHC class II (1). A particularly interesting finding in our study was the apparent preferential expansion of islet antigen-specific, potentially pathogenic T cell populations. Our previous studies have suggested that only islet antigen-specific T cells can enter the islets (12). Thus, we anticipated that the absence of LAG-3 might affect all infiltrating T cells comparably. However, these data suggest that the absence of LAG-3 may have a greater effect on the more pathogenic T cell clones responsible for initiating and/or driving diabetes onset. Interestingly, we also showed that this preferential expansion of potentially pathogenic clones may be limited to the islets, although these cells can accumulate in the periphery at later stages of disease. Our study suggests that further analysis of the role of LAG-3 in modulating autoimmunity is warranted, particularly given that linkage analysis has suggested that SNPs in the LAG3 locus may confer susceptibility to multiple sclerosis in human patients (25). Furthermore, the therapeutic enhancement of LAG-3 activity, perhaps via agonistic monoclonal antibodies, warrants further investigation as a treatment for T1D.
Supplementary Material
ACKNOWLEDGEMENTS
We would like to thank John Altman and Richard Willis at the NIH Tetramer Core Facility for providing MHC class I and class II tetramers; Yueh-Hsiu Chen, Christophe Benoist and Diane Mathis for the Lag3−/− mice; Richard Cross, Greig Lennon and Stephanie Morgan for FACS; and Amy McKenna and the staff of the St. Jude Animal Resource Center for maintenance of mouse colonies. At Bristol-Myers-Squibb, the authors wish to thank Mary Rainey and the staff of the Milpitas animal facility for performing the NOD-antibody study and Rangan Vanganipuram, Brian Lee and Shilpa Mankikar for provision of antibodies.
This work was supported by the National Institutes of Health (R01 AI39480, D.A.A.V.), a JDRF Postdoctoral Fellowship (3-2009-594, M.B.), NCI Comprehensive Cancer Center Support CORE grant (CA21765, D.A.A.V.), and the American Lebanese Syrian Associated Charities (ALSAC, D.A.A.V.).
Abbreviations used in the paper
- T1D
Type 1 Diabetes
- LAG-3
lymphocyte activation gene-3
- WT
wild type
- PLN
pancreatic lymph nodes
- Tregs
regulatory T cells
- pDC
plasmocytoid dendritic cell
Footnotes
DISCLOSURES The authors declare competing financial interests.
REFERENCES
- 1.Lehuen A, Diana J, Zaccone P, Cooke A. Immune cell crosstalk in type 1 diabetes. Nat Rev Immunol. 10:501–513. doi: 10.1038/nri2787. [DOI] [PubMed] [Google Scholar]
- 2.Bettini M, Vignali DA. Regulatory T cells and inhibitory cytokines in autoimmunity. Curr Opin Immunol. 2009;21:612–618. doi: 10.1016/j.coi.2009.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Feuerer M, Shen Y, Littman DR, Benoist C, Mathis D. How punctual ablation of regulatory T cells unleashes an autoimmune lesion within the pancreatic islets. Immunity. 2009;31:654–664. doi: 10.1016/j.immuni.2009.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ansari MJ, Salama AD, Chitnis T, Smith RN, Yagita H, Akiba H, Yamazaki T, Azuma M, Iwai H, Khoury SJ, Auchincloss H, Jr., Sayegh MH. The programmed death-1 (PD-1) pathway regulates autoimmune diabetes in nonobese diabetic (NOD) mice. J Exp Med. 2003;198:63–69. doi: 10.1084/jem.20022125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Workman CJ, Cauley LS, Kim IJ, Blackman MA, Woodland DL, Vignali DA. Lymphocyte activation gene-3 (CD223) regulates the size of the expanding T cell population following antigen activation in vivo. J Immunol. 2004;172:5450–5455. doi: 10.4049/jimmunol.172.9.5450. [DOI] [PubMed] [Google Scholar]
- 6.Huang CT, Workman CJ, Flies D, Pan X, Marson AL, Zhou G, Hipkiss EL, Ravi S, Kowalski J, Levitsky HI, Powell JD, Pardoll DM, Drake CG, Vignali DA. Role of LAG-3 in regulatory T cells. Immunity. 2004;21:503–513. doi: 10.1016/j.immuni.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 7.Workman CJ, Vignali DA. Negative regulation of T cell homeostasis by lymphocyte activation gene-3 (CD223) J Immunol. 2005;174:688–695. doi: 10.4049/jimmunol.174.2.688. [DOI] [PubMed] [Google Scholar]
- 8.Blackburn SD, Shin H, Haining WN, Zou T, Workman CJ, Polley A, Betts MR, Freeman GJ, Vignali DA, Wherry EJ. Coregulation of CD8+ T cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nat Immunol. 2009;10:29–37. doi: 10.1038/ni.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miyazaki T, Dierich A, Benoist C, Mathis D. Independent modes of natural killing distinguished in mice lacking Lag3. Science. 1996;272:405–408. doi: 10.1126/science.272.5260.405. [DOI] [PubMed] [Google Scholar]
- 10.Okazaki T, Okazaki IM, Wang J, Sugiura D, Nakaki F, Yoshida T, Kato Y, Fagarasan S, Muramatsu M, Eto T, Hioki K, Honjo T. PD-1 and LAG-3 inhibitory co-receptors act synergistically to prevent autoimmunity in mice. J Exp Med. 208:395–407. doi: 10.1084/jem.20100466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burton AR, Vincent E, Arnold PY, Lennon GP, Smeltzer M, Li CS, Haskins K, Hutton J, Tisch RM, Sercarz EE, Santamaria P, Workman CJ, Vignali DA. On the pathogenicity of autoantigen-specific T-cell receptors. Diabetes. 2008;57:1321–1330. doi: 10.2337/db07-1129. [DOI] [PubMed] [Google Scholar]
- 12.Lennon GP, Bettini M, Burton AR, Vincent E, Arnold PY, Santamaria P, Vignali DA. T cell islet accumulation in type 1 diabetes is a tightly regulated, cell-autonomous event. Immunity. 2009;31:643–653. doi: 10.1016/j.immuni.2009.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leiter EH. The NOD mouse: a model for insulin-dependent diabetes mellitus. Curr Protoc Immunol. 2001 doi: 10.1002/0471142735.im1509s24. Chapter 15:Unit 15 19. [DOI] [PubMed] [Google Scholar]
- 14.Moon JJ, Chu HH, Hataye J, Pagan AJ, Pepper M, McLachlan JB, Zell T, Jenkins MK. Tracking epitope-specific T cells. Nat Protoc. 2009;4:565–581. doi: 10.1038/nprot.2009.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Herman AE, Freeman GJ, Mathis D, Benoist C. CD4+CD25+ T regulatory cells dependent on ICOS promote regulation of effector cells in the prediabetic lesion. J Exp Med. 2004;199:1479–1489. doi: 10.1084/jem.20040179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Workman CJ, Wang Y, El Kasmi KC, Pardoll DM, Murray PJ, Drake CG, Vignali DA. LAG-3 regulates plasmacytoid dendritic cell homeostasis. J Immunol. 2009;182:1885–1891. doi: 10.4049/jimmunol.0800185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tang Q, Adams JY, Penaranda C, Melli K, Piaggio E, Sgouroudis E, Piccirillo CA, Salomon BL, Bluestone JA. Central role of defective interleukin-2 production in the triggering of islet autoimmune destruction. Immunity. 2008;28:687–697. doi: 10.1016/j.immuni.2008.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frigerio S, Junt T, Lu B, Gerard C, Zumsteg U, Hollander GA, Piali L. Beta cells are responsible for CXCR3-mediated T-cell infiltration in insulitis. Nat Med. 2002;8:1414–1420. doi: 10.1038/nm1202-792. [DOI] [PubMed] [Google Scholar]
- 19.Baron JL, Reich EP, Visintin I, Janeway CA., Jr. The pathogenesis of adoptive murine autoimmune diabetes requires an interaction between alpha 4-integrins and vascular cell adhesion molecule-1. J Clin Invest. 1994;93:1700–1708. doi: 10.1172/JCI117153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stratmann T, Martin-Orozco N, Mallet-Designe V, Poirot L, McGavern D, Losyev G, Dobbs CM, Oldstone MB, Yoshida K, Kikutani H, Mathis D, Benoist C, Haskins K, Teyton L. Susceptible MHC alleles, not background genes, select an autoimmune T cell reactivity. J Clin Invest. 2003;112:902–914. doi: 10.1172/JCI18337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lieberman SM, Takaki T, Han B, Santamaria P, Serreze DV, DiLorenzo TP. Individual nonobese diabetic mice exhibit unique patterns of CD8+ T cell reactivity to three islet antigens, including the newly identified widely expressed dystrophia myotonica kinase. J Immunol. 2004;173:6727–6734. doi: 10.4049/jimmunol.173.11.6727. [DOI] [PubMed] [Google Scholar]
- 22.Alegre ML, Shiels H, Thompson CB, Gajewski TF. Expression and function of CTLA-4 in Th1 and Th2 cells. J Immunol. 1998;161:3347–3356. [PubMed] [Google Scholar]
- 23.Workman CJ, Rice DS, Dugger KJ, Kurschner C, Vignali DA. Phenotypic analysis of the murine CD4-related glycoprotein, CD223 (LAG-3) Eur J Immunol. 2002;32:2255–2263. doi: 10.1002/1521-4141(200208)32:8<2255::AID-IMMU2255>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 24.Workman CJ, Dugger KJ, Vignali DA. Cutting edge: molecular analysis of the negative regulatory function of lymphocyte activation gene-3. J Immunol. 2002;169:5392–5395. doi: 10.4049/jimmunol.169.10.5392. [DOI] [PubMed] [Google Scholar]
- 25.Zhang Z, Duvefelt K, Svensson F, Masterman T, Jonasdottir G, Salter H, Emahazion T, Hellgren D, Falk G, Olsson T, Hillert J, Anvret M. Two genes encoding immune-regulatory molecules (LAG3 and IL7R) confer susceptibility to multiple sclerosis. Genes Immun. 2005;6:145–152. doi: 10.1038/sj.gene.6364171. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.