Abstract
Introduction
3,4-Methylenedioxymethamphetamine (MDMA) use is increasing, enhancing the need for its detection in clinical, workplace, pain management, and driving under the influence of drugs testing programs. Oral fluid is an important alternative matrix for drug testing, but little is known about MDMA detection windows in oral fluid.
Aims
To characterize MDMA and metabolite disposition in expectorated oral fluid following controlled MDMA administration.
Methods
Placebo, low (1.0 mg/kg), and high (1.6 mg/kg) oral MDMA doses were given double-blind in random order in separate sessions to 29 healthy adults with histories of MDMA use. 1286 expectorated oral fluid specimens collected up to 7 days after dosing were analyzed for MDMA, 3,4-methylenedioxyamphetamine (MDA), 4-hydroxy-3-methoxymethamphetamine (HMMA), and 4-hydroxy-3-methoxyamphetamine (HMA) by gas chromatography mass spectrometry. Limits of quantification were 5 ng/mL for MDMA and MDA and 10 ng/mL for HMA and HMMA.
Results
MDMA was the primary analyte detected, with concentrations up to 12,000 ng/mL in 872 specimens (67.8%). MDA was quantified in 656 specimens (51.0%) at concentrations <403 ng/mL, and was never present without concurrent MDMA. HMA and HMMA were not detected. 59.8, 58.6 and 54.9% of specimens were MDMA-positive at the Talloires (20 ng/mL), Driving under the Influence of Drugs, Alcohol and Medicines (DRUID, 25 ng/mL), and proposed US Substance Abuse and Mental Health Services Administration (50 ng/mL) confirmation cutoffs, respectively. MDMA was first observed in oral fluid 0.25-1.25 h after dosing; MDA was initially detected at 0.5-1.75 h. In general, MDMA and MDA windows of detection were 47 and 29 h, respectively, although a few specimens were positive up to 71 and 47 h.
Conclusion
Oral fluid monitoring efficiently detects single, recreational 70-150 mg MDMA use for 1-2 days. These controlled administration data provide a scientific basis for interpreting MDMA oral fluid test results.
Keywords: Oral Fluid, Saliva, Alternative Matrix, MDMA, DUID
Introduction
3,4-methylenedioxymethamphetamine (MDMA, ecstasy) and 3,4-methylenedioxy-methamphetamine (MDA) are illicit drugs widely abused around the world for their stimulant and euphoric effects.1-3 The 2009 National Survey on Drug Use and Health estimated that 2.8 million Americans age 12 and older tried MDMA at least once in the past year, and 0.3% of the American population used MDMA in the prior month.4 The 2007 National Roadside Survey of Alcohol and Drug Use by Drivers reported that oral fluid samples from 11.1% of daytime and 14.6% of randomly stopped nighttime drivers were drug positive.5 Stimulants such as methamphetamine and/or MDMA and their metabolites accounted for 1.6% and 3.2% of the positive specimens collected, respectively. Thus, there is a strong clinical, public health, and safety need for valid and efficient methods of detecting and monitoring MDMA use.
There is continued interest in oral fluid as an alternate matrix to blood and urine to monitor illicit drug use. Oral fluid requires a less invasive means of specimen collection, and collection under gender-neutral direct observation reduces the possibility of adulteration, substitution, or dilution. 6-8 There also is evidence that oral fluid drug concentrations are reasonably correlated with blood concentrations. 9, 10 The Substance Abuse and Mental Health Administration (SAMHSA) proposed guidelines in 2004 for workplace drug testing of oral fluid in the United States. 11 Similar guidelines were proposed by the European initiative Driving Under the Influence of Drugs, Alcohol and Medicines (DRUID). 12 A September 2006 meeting of international experts in Talloires, France suggested guidelines for future research on drugged driving and proposed oral fluid drug cutoff concentrations. 13
Oral fluid testing is growing in the United States, Australia, and Europe; however, there are few controlled MDMA administration studies with expectorated oral fluid to guide MDMA oral fluid interpretation. Expectorated MDMA oral fluid concentrations collected approximately 1 h post ingestion of authentic MDMA tablets (57 ± 20 mg) varied from 33 – 3533 ng/mL in 19 recreational MDMA users. 14 Samyn et al. administered 75 mg MDMA to 12 healthy volunteers on 3 separate occasions at 2-week intervals. 14 Expectorated oral fluid had MDMA concentrations from 50 – 6982 ng/mL up to 5 h after administration, with maximum concentrations (Cmax) between 2 – 3 h. A similar controlled double-blind administration study collected 108 specimens from volunteers after 75 or 100 mg MDMA. Oral fluid concentrations collected with the OraSure Intercept® device 5.5 h later were less than 3079 ng/mL. Unfortunately, additional specimens were not collected, preventing determination of MDMA oral fluid detection windows. 15
Navarro et al. collected non-stimulated oral fluid specimens 1.5, 4, 6, 10 and 24 h after a single 100 mg MDMA dose to 8 participants. 16 MDMA concentrations were highest at the first collection (1.5 h) and detectable for 10 h; all specimens were negative at 24 h. Mean MDA concentrations were highest between 4 – 6 h, in concentrations less than 50 ng/mL.
This study characterized MDMA and metabolite disposition in expectorated oral fluid following controlled oral MDMA administration, with the aim of establishing time course of initial detection, peak concentrations, duration of detection, and excretion rates of MDMA and metabolites in oral fluid. These pharmacokinetic data will improve interpretation of oral fluid drug concentrations in clinical, drug treatment, law enforcement, and workplace drug testing programs.
Materials and Methods
PARTICIPANTS AND STUDY DESIGN
The National Institute on Drug Abuse Institutional Review Board approved the study and all participants provided written informed consent. Prior to admission, participants underwent comprehensive medical and psychological evaluations, including self-reported drug use history. Eligibility criteria included lifetime use of at least five MDMA tablets and at least one in the prior three months. Participants resided on a secure clinical research unit for up to 23 days under 24-h medical surveillance to ensure safety and to prevent additional drug use. Placebo, low (1.0 mg/kg), and high (1.6 mg/kg) oral MDMA doses were given double-blind in counter-balanced order, with a minimum of 7 days between doses. For safety reasons, the maximum MDMA dose was 150 mg. Participants received a single capsule containing a 50:50 racemic mixture of d,l-MDMA HCl; identical placebo capsules contained only lactose.
CHEMICALS AND REAGENTS
MDMA-d0, MDA-d0, MDMA-d5, and MDA-d5, were purchased from Cerilliant Corporation (Round Rock TX, USA). HMMA-d0 and HMA-d0 were obtained from Lipomed Inc. (Cambridge, MA, USA). ACS reagent grade Tris [hydroxymethyl] aminomethane base, Tris [hydroxymethyl] aminomethane hydrochloride, triethylamine (99.5% purity), p-hydroxymethamphetamine (pholedrine), and GC grade n-heptane were acquired from Sigma-Aldrich (St. Louis, MO, USA). ACS reagent grade potassium phosphate monobasic and potassium phosphate dibasic, concentrated hydrochloric acid, acetic acid, ammonium hydroxide, and HPLC grade solvents were from JT Baker (Phillipsburg, NJ, USA). Heptafluorobutyric acid anhydride (HFAA) was from Regis Technologies, Inc. (Morton Grove, IL, USA), filtration columns (RFV02F4P) for preparing oral fluid samples for solid phase extraction were from United Chemical Technologies (Bristol, PA, USA), and SPEC C18AR/MP1, 3mL reservoir/30 mg bed mass, mixed mode cation exchange solid phase extraction columns were obtained from Varian Inc. (Lake Forest, CA, USA).
ORAL FLUID COLLECTION
Oral fluid was collected prior to admission and up to 143 h post-dose, at the following 31 time points: (−0.25, 0.25, 0.5, 0.75, 1.0, 1.25, 1.5, 1.75, 2.0, 2.25, 2.5, 2.75, 3.0, 3.5, 4.0, 4.5, 5.0, 7.0, 9.0, 11, 13, 15, 23, 29, 34, 39, 47, 71, 95, 119, and 143 h). Specimens were collected by non-stimulated expectoration into 50 mL polypropylene tubes, centrifuged at 1800 g, and stored frozen (−20°C) until analysis.
EXTRACTION/QUANTITATIVE ANALYSIS
Quantitative analysis for MDMA, MDA, HMMA, and HMA in expectorated oral fluid was performed with minor modifications according to a previously published method. 17 Gas chromatography mass spectrometry (GCMS) injection volume was reduced to 1 μL to prevent saturation of the detector at elevated concentrations without sacrificing signal response at the limit of quantification (LOQ). In addition, two calibration curves, utilizing a 1/x2 weighted least squares model, were established to encompass the wide range of drug concentrations expected. These modifications permitted analyte quantification in a single analysis across three orders of magnitude. Low calibration curves were 5 – 500 ng/mL for MDMA and MDA; and 10 – 500 ng/mL for HMA and HMMA. High calibration curves were 500 – 4000 ng/mL for all analytes.
Briefly, working internal standard (MDMA-d5, MDA-d5 and pholedrine) and 2 mL 0.1 M potassium phosphate buffer (pH 6.0) were added to 400 μL expectorated oral fluid. After mixing and centrifugation, the filtrate was decanted onto preconditioned solid phase extraction columns. Columns were washed, dried, and analytes eluted with 1.5 mL of ethyl acetate:methanol:ammonium hydroxide (78:20:2, v/v/v). Acidified methanol was added prior to drying to reduce evaporative loss of volatile analytes. The extract was reconstituted in 100 μL 0.1M triethylamine in heptane, 10 μL HFAA and derivatized at 60°C for 20 min. A back extraction was performed by adding 200 μL 0.1M potassium phosphate buffer, pH 7.4. Samples were centrifuged and organic (upper) layers transferred to autosampler vials for analysis by electron impact GCMS in selective ion monitoring mode. Instrument parameters are presented in detail in Scheidweiler and Huestis. 17
Within and between-run imprecision were calculated at three control concentrations across each linear range with coefficients of variation of <8.4%. Total imprecision (%CV) was less than 12.1%. 18 Bias at the same concentrations (15, 60, 400 and 800, 1500, 3000 ng/mL) was less than ±9.9%.
PHARMACOKINETIC AND STATISTICAL ANALYSIS
Visual data inspection and evaluation by Kolmogorov-Smirnov tests indicated non-normal data distribution. Therefore, paired statistical comparisons were conducted via non-parametric Wilcoxon signed-rank tests using IBM PASW version 18.0 for Windows (SPSS Inc., Chicago, IL, USA). Comparisons were considered significant if p<0.05. Area under the curve (AUC0-last) and terminal elimination half-lives were calculated employing a non-compartmental model with WinNonlin version 5.2 (Pharsight Inc., Mountain View, CA, USA). Analysis of covariance regression was performed with Prism 5.02 (Graphpad, Inc., Ja Jolla, CA, USA) to test whether MDA/MDMA trends differed after low and high dose MDMA. The time of first detection (Tfirst) was the time when the first specimen was ≥ LOQ, and the time of last detection (Tlast) was the time when the last specimen after dosing was ≥ LOQ. Only data from the 8 continuous-stay participants were included in the analysis of times of first and last detection, Cmax and time of maximum concentrations (Tmax). Only specimens positive for both analytes were included in MDA/MDMA ratio analysis.
Results
HUMAN PARTICIPANTS
Twenty-nine MDMA users were enrolled (18 male, 11 female; 21 African American, 7 Caucasian, 1 Hispanic). Mean (SD) age was 23.4 (4.4) years (range, 18 - 35); mean weight 72.9 (14.6) kg (43.2 - 100.0). Two participants whose weight exceeded 93.75 kg were administered the maximum high dose (150 mg) for safety reasons.
Eight participants completed the study in a single 23-day continuous stay; dosing sessions for the remaining 21 participants were completed in three shorter stays at least a week apart. Sixteen of these completed fMRI scanning to evaluate brain activity following MDMA dosing. Oral fluid collection could not be done while participants were in the scanner from 1.5 to 4 h after dosing, precluding inclusion of these data in specific pharmacokinetic analyses such as determination of Cmax and Tmax. Separate-stay participants were re-evaluated prior to each session to ensure continued study eligibility and remained on the unit for 1-7 days after each dose. Five participants withdrew from the study prior to receiving all doses.
OVERALL ORAL FLUID RESULTS
1286 oral fluid specimens from 29 subjects were analyzed. MDMA was the primary analyte detected (67.8% of specimens), with concentrations up to 6,507 and 11,986 ng/mL after 1.0 and 1.6 mg/kg, respectively. MDA was quantified in 51.0%, with maximum concentrations (Cmax) of 151 and 403 ng/mL after low and high doses, respectively. There was considerable intra- and inter-subject variability in MDMA and MDA oral fluid concentrations. MDA was never present without concurrent MDMA. HMMA and HMA were not detected in any specimen.
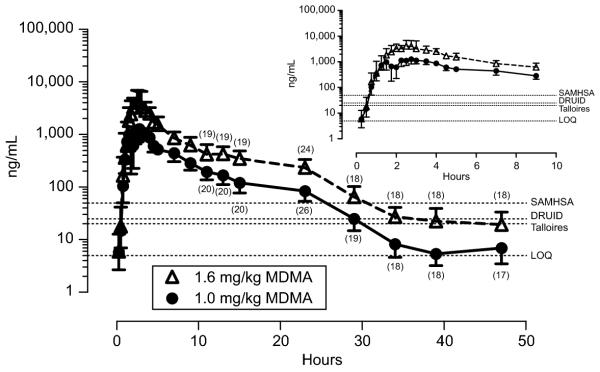
Median MDMA Cmax of 1443 ng/mL (range 0 – 2655) and 4137 ng/mL (range 1486 – 11986) were observed after low (N=11) and high (N=8) doses, respectively (Fig. 1). MDMA concentrations rapidly increased for the first 2.25 – 2.5 hours, then slowly decreased, with all specimens collected 23 and 34 h after low and high doses ≥ LOQ (Figure 3). The majority of specimens collected almost two days (47 h) after a single low or high MDMA dose (53% and 94%, respectively) were quantifiable.
Figure 1.
Mean 3,4-methylenedioxymethamphetmine (MDMA) concentrations in oral fluid collected via expectoration 0.25 - 47h after single oral doses of 1.0 or 1.6mg/kg MDMA to healthy adult MDMA users. N=27 and 25 for 1.0 and 1.6mg/kg doses, respectively for collections 0.25 -1.25 and 4.5 - 9h. N=11 and 8 for low and high doses respectively for specimens collected 1.5 – 4h. Participants qualifying for the fMRI portion of the study were in the scanner from 1.5 - 4h precluding oral fluid collection. Numbers in parentheses indicate observations after 11 - 47h. Error bars are 95% confidence intervals. Dashed lines indicate the limit of quantification (LOQ) (5ng/mL) and 3 recommended cutoff concentrations: International meeting in Talloires, France (Sept., 2006) (20ng/mL); Driving Under the Influence of Drugs, Alcohol and Medicines (DRUID, 25ng/mL); and proposed US Substance Abuse and Mental Health Services Administration (SAMHSA, 50 ng/mL).
Figure 3.

Detection rates (% positive) for 3,4-methylenedioxymethamphetamine (MDMA) and 3.4-methylenedioxyamphetamine (MDA) in oral fluid collected via expectoration 0.25 - 71h following single oral doses of 1.0 or 1.6mg/kg MDMA given to healthy adult MDMA users. N=27 and 25 for 1.0 and 1.6mg/kg doses, respectively. Detection rates were calculated with the limit of quantification (5ng/mL), and 3 recommended cutoff concentrations; Talloires: international meeting in Talloires, France (Sept., 2006) (20ng/mL); DRUID: Driving Under the Influence of Drugs, Alcohol and Medicines (25ng/mL); SAMHSA: proposed US Substance Abuse and Mental Health Services Administration (50 ng/mL). Bars are not visible if the detection rate is the same as the higher cutoff concentration.
The overall mean concentration-time profile for MDA was similar to MDMA (Figure 2). Maximum median MDA concentrations (range ng/mL) following low and high doses were 28.2 (12.8 – 58.0) and 80.7 (17.8 – 184.3). Maximum median concentrations occurred 3 h following both doses; 6% and 33% of specimens collected after 47 h were still above 5 ng/mL MDA (Figure 3).
Figure 2.
Mean 3,4-methylenedioxyamphetmine (MDA) concentrations in oral fluid collected via expectoration 0.25 - 47h after single oral doses of 1.0 or 1.6mg/kg MDMA to healthy adult MDMA users. N=27 and 25 for 1.0 and 1.6mg/kg doses, respectively for collections 0.25 -1.25 and 4.5 - 9h. N=11 and 8 for low and high doses respectively for specimens collected 1.5 – 4h. Participants qualifying for the fMRI portion of the study were in the scanner from 1.5 - 4h precluding oral fluid collection. Numbers in parentheses indicate observations after 11 - 47h. Error bars are 95% confidence intervals. Dashed lines indicate the limit of quantification (LOQ) (5ng/mL) and 3 recommended cutoff concentrations: International meeting in Talloires, France (Sept., 2006) (20ng/mL); Driving Under the Influence of Drugs, Alcohol and Medicines (DRUID, 25ng/mL); and proposed US Substance Abuse and Mental Health Services Administration (SAMHSA, 50 ng/mL).
Of 1041 specimens collected after MDMA administration, 698 (67.1%) were positive for MDMA at the proposed SAMHSA 50 ng/mL cutoff. An additional 119 specimens (9.4%) exceeded the SAMHSA criteria (50 ng/mL) for MDA. This did not identify any additional positive specimens because MDA was never present without concurrent MDMA. 94.8% (662) of these MDMA-positive specimens occurred in the first 23 h after dosing; an additional 34 specimens (4.9%) were positive 24 - 47 h after dosing. At the lower 25 ng/mL DRUID cutoff, 683 (92.0%) and 57 (7.7%) of the 742 positive specimens occurred within the first or second day, respectively. A similar pattern was observed with the Talloires cutoff: 99.7% of specimens were above the cutoff within two days of dosing. (Figure 3)
17 specimens (8.1%) collected from two subjects before and after placebo dosing were MDMA positive from previously self-administered drug. Peak MDMA concentrations (259.4 ng/mL) at admission in Subject P decreased rapidly and were negative by 4h. Subject S had MDMA oral fluid concentrations of 6.5 – 28.7 ng/mL for the first 7 h after admission. 13, 10 and 7 of these specimens exceeded the Talloires, DRUID and SAMSHA cutoffs, respectively.
TIMES OF FIRST DETECTION
MDMA was initially detected as early as 0.25 h (the first collection time point) after dosing in the 8 continuous-stay participants, although the initial positive specimen more often occurred at 0.5 or 0.75 h. In comparison, MDA detection was slightly delayed, first appearing from 0.5 – 1.75 h after MDMA administration. There was no significant between dose difference in Tfirst at any cutoff for MDMA or MDA. There were significant differences in MDMA Tfirst at the different cutoffs evaluated (LOQ, Talloires, DRUID and SAMHSA) after the low dose (n=8, p<0.05), whereas all MDMA Tfirst were equivalent after the high dose (Table 1).
Table 1.
First detection time for 3,4-methylenedioxymethamphetamine (MDMA) and 3,4-methylenedioxyamphetamine (MDA) in expectorated oral fluid after 1.0 or 1.6 mg/kg oral MDMA administration (n = 8 participants) evaluated with the limit of quantification (LOQ), and cutoff concentrations proposed at the Talloires, France 2006 meeting, the European initiative Driving Under the Influence of Drugs, Alcohol and Medicines (DRUID) and the Substance Abuse and Mental Health Services Administration (SAMHSA).
| First detected (h) a, b, c | ||||||||
|---|---|---|---|---|---|---|---|---|
| MDMA |
MDA |
|||||||
| Cutoff: Concentration: |
LOQ [5 ng/mL] |
Talloires [20 ng/mL] |
DRUID [25 ng/mL] |
SAMHSA [50 ng/mL] |
LOQ [5 ng/mL] |
Talloires [20 ng/mL] |
DRUID [25 ng/mL] |
SAMHSA [50 ng/mL] |
| 1.0 mg/kg | 0.5 2,3,4,† (0.2 - 1.0) |
0.8 1,† (0.5 - 1.2) |
0.8 1,† (0.5 - 1.2) |
0.8 1 (0.5 - 1.5) |
1.1 2,3,† (0.8 – 1.8) |
1.6 1,3,† (1.3 – 3.5) |
2.5 1,2,† (1.5 - 4.0) |
2.8 N/A |
| 1.6 mg/kg | 0.8 † (0.2 - 1.2) |
0.8 † (0.2 - 1.2) |
0.8 † (0.2 - 1.2) |
0.8 † (0.5 - 1.2) |
1.0 2,3,4,† (0.5 - 1.5) |
1.6 1,4,† (0.8 – 2.2) |
1.6 1,4,† (0.8 - 2.2) |
2.2 1,2,3,† (1.5 – 3.0) |
Median (range)
One out of eight participants did not have any specimens exceeding 25 ng/mL of MDA after 1.0 mg/kg MDMA
7 of 8 participants did not have any specimens exceeding 50 ng/mL of MDA after 1.0 mg/kg MDMA
different from LOQ
different from Talloires
different from DRUID
different from SAMHSA
dose-related difference
difference between MDMA and MDA. All statistical comparisons via Wilcoxon signed-rank test, p < 0.05
TIMES OF LAST DETECTION
Tlast at the LOQ for MDMA (low dose: 29-47 h, high dose: 47-71 h) were longer than for MDA (low dose: 23-34 h, high dose: 29-47 h) (Table 2). MDMA Tlast calculated using LOQ, Talloires, DRUID and SAMHSA cutoffs were significantly longer after the high compared with the low dose (All p values <0.05). A similar pattern was observed for MDA. Median MDA Tlast and ranges for the LOQ, Talloires and DRUID cutoffs were significantly longer following the high dose (All p values <0.05). No MDA dose-related difference was observed at the SAMHSA cutoff, as only one out of eight participants had MDA concentrations exceeding the 50 ng/mL cutoff after the low 1.0 mg/kg dose (Table 3). Within-dose comparisons of MDMA and MDA Tlast at Talloires and DRUID cutoffs were equivalent for low and high doses.
Table 2.
Time 3,4-methylenedioxymethamphetamine (MDMA) and 3,4-methylenedioxyamphetamine (MDA) were last detected in expectorated oral fluid after 1.0 or 1.6 mg/kg oral MDMA administration (n = 8 participants) evaluated with the limit of quantification (LOQ), and cutoff concentrations proposed at the 2006 Talloires, France meeting, the European initiative Driving Under the Influence of Drugs, Alcohol and Medicines (DRUID) and the Substance Abuse and Mental Health Services Administration (SAMHSA).
| Last detected (h) a, b, c | ||||||||
|---|---|---|---|---|---|---|---|---|
| MDMA |
MDA |
|||||||
| Cutoff: Concentration: |
LOQ [5 ng/mL] |
Talloires [20 ng/mL] |
DRUID [25 ng/mL] |
SAMHSA [50 ng/mL] |
LOQ [5 ng/mL] |
Talloires [20 ng/mL] |
DRUID [25 ng/mL] |
SAMHSA [50 ng/mL] |
| 1.0 mg/kg | 36.5 2,3,4,*,† (29.0 - 47.0) |
29.0 1,4,*,† (23.0 – 34.0) |
23.0 1,*,† (23.0 - 34.0) |
23.0 1,2,* (13.0 - 29.0) |
23.0 2,3,*,† (23.0 – 34.0) |
12.0 1,*,† (3.0 - 29.0) |
13.0 1,*,† (3.0 - 29.0) |
13.0 N/A |
| 1.6 mg/kg | 47.0 2,3,4,*,† (47.0 – 71.0) |
43.0 1,*,† (29.0 - 47.0) |
43.0 1,*,† (29.0 - 47.0) |
29.0 1,*,† (23.0 - 47.0) |
39.0 2,3,4,*,† (29.0 - 47.0) |
23.0 1,4,*,† (23.0 - 47.0) |
23.0 1,4,*,† (7.0 – 47.0) |
15.0 1,2,3,† (2.75 - 29.0) |
Median (range)
one out of eight participants did not have any specimens exceeding 25 ng/mL of MDA after 1.0 mg / kg MDMA
seven out of eight participants did not have any specimens exceeding 50 ng/mL of MDA after 1.0 mg/kg MDMA
different from LOQ
different from Talloires
different from DRUID
different from SAMHSA
dose-related difference
difference between MDMA and MDA. All statistical comparisons via Wilcoxon signed-rank test, p < 0.05
Table 3.
Pharmacokinetic values for 3,4-methylenedioxymethamphetamine (MDMA) and 3,4-methylenedioxyamphetamine (MDA) in expectorated oral fluid following controlled 1.0 and 1.6 mg/kg oral MDMA administration; n = 8 participants, median (range)
| MDMA | ||||
|---|---|---|---|---|
| Dose (mg/kg) |
Cmaxa (ng/mL) |
Tmaxb (h) |
t1/2c (h) |
AUC0 to ∞d (h × ng/mL) |
| 1.0 | 1,643.0 *,# (1,160.0 – 3382.0) |
2.8 (1.3 – 5.0) |
4.6 (3.2 – 11.4) |
8,913.0 *,# (5,861.0 - 27,849.0) |
| 1.6 | 4,760.0 *,# (2,881.0 - 11,985.0) |
2.6 # (1.5 – 4.5) |
7.4 (5.9 -13.4) |
27,957.0 *,#,† (17,221.0 - 78,699.0) |
| MDA | ||||
| 1.0 | 41.0 *,# (23.0 - 151.0) |
4.8 (2.8 – 23.0) |
8.8 (4.6 – 54.0) |
493.0 *,# (310.0 - 1,864.0) |
| 1.6 | 128.0 *,# (50.0 – 403.0) |
4.5 # (2.5 – 15.0) |
8.1 (6.9 – 22.8) |
1,567.0 *,#,† (652.0 - 5,602.0) |
maximum concentration
time maximum concentration occurred
half - life
area under the curve0 to ∞
dose-related difference
significantly greater than predicted by 1.0 mg/kg dose
significant difference between MDMA and MDA. All statistical comparisons via Wilcoxon signed-rank test, p < 0.05
PHARMACOKINETICS OF MDMA AND METABOLITES IN ORAL FLUID
Median MDMA Cmax after low and high doses were 1643 and 4760 ng/mL in the 8 continuous-stay participants, occurring 2.8 and 2.6 h post dose, respectively. There was large inter-subject variability, as shown by the concentration range. Median MDA Cmax were attained 4.8 h (41 ng/mL) and 4.5 h (128 ng/mL) after low and high doses. There were significant dose-related differences for MDMA and MDA maximum concentrations. Median MDMA and MDA Cmax concentrations following the high 1.6 mg/kg dose were approximately 3 times the low 1.0 mg/kg dose, and significantly greater than predicted based on dose alone. Median MDA Cmax were approximately 2.6% median MDMA Cmax. Similarly, high-dose MDMA and MDA AUC 0-last were approximately three times higher than after the low dose, also significantly greater than predicted. Median MDMA half-lives were 4.6 h (range: 3.2 – 11.4) after the low and 7.4 h (range: 5.9 – 13.4) after the high dose. While median MDA half- lives (8.8 and 8.1 h) were longer than MDMA, the differences were not significant, due in part to high inter-subject variability.
METABOLITE TO PARENT DRUG RATIO
Only specimens positive for both analytes, 312 after the low and 288 after the high dose, were included in the MDA/MDMA ratio analysis. Mean percent MDA/MDMA metabolite ratios increased linearly from 0.25 – 29 h, and were significantly higher following the low MDMA dose. Despite the linear relationship, large inter-subject variability was observed following low (6.2 – 22.7%) and high (5.3 – 24.4%) doses. Analysis of covariance regression revealed a statistically significant steeper slope after 1.0 as compared to 1.6 mg/kg MDMA (F1,596 = 22.2, P < 0.0001). Calculated ratios were unable to predict time of last use because the slopes were not equivalent.
DISCUSSION
MDMA and metabolite pharmacokinetics in oral fluid after controlled drug administration and oral fluid testing guidelines proposed by SAMHSA, 11 DRUID, 12 and the Talloires international expert panel were investigated. 13 Our study extended the monitoring period for MDMA and metabolites up to 143 h (from the 24 h in previously published MDMA pharmacokinetic studies), allowing more accurate determination of windows of detection and terminal elimination half-lives.
Collection of expectorated oral fluid samples posed few problems, although some participants experienced “dry-mouth,” commonly associated with MDMA and stimulant use. 6 This did not prevent collection of adequate samples because sample volume requirement was 400 μL. However, dry mouth might limit the usefulness of oral fluid as an assay matrix in situations where larger sample volumes are required for a less sensitive assay.
MDMA was first observed in oral fluid as early as 15 min after each dose and in all participants 1.25 h after dosing. Similar early detection times (first collection) after controlled administration of recreational doses (75-150 mg) were reported in plasma for a few participants, with all specimens MDMA-positive at 0.5 h. 19-21 Buccal contamination from oral drug administration 22 does not appear to have occurred, as the first detection times in oral fluid are delayed in comparison to plasma.
Median MDMA Tmax were approximately 2.8 h after both doses, consistent with previous studies reporting peak oral fluid concentrations approximately 1.5–3 h after oral administration of 1.1–1.4 mg/kg MDMA to healthy volunteers. 14, 16, 23 Peak plasma concentrations also occurred in about 2 h. 16, 19, 24
MDMA half-lives after low and high doses were 3.2–13.4 h, similar to values (5.6 h) reported after a single 100 mg oral dose. 16 MDA half-lives of 4.6 to 54.0 hours are consistent with those reported by Navarro (5.6 – 37.3 h). 16 We observed longer detection windows for MDMA and MDA than previously reported. These data demonstrate the value of longer monitoring periods with restricted drug access to capture the true window of detection of illicit drugs.
DRUID (25 ng/mL), Talloires (20 ng/mL) and proposed SAMHSA (50 ng/mL) oral fluid guidelines specify confirmation cutoff concentrations for MDMA and MDA. The inclusion of MDA as a target analyte did not identify any additional positive specimens in our study. MDA was never present without concurrent MDMA and metabolite concentrations never exceeded those of parent drug. However, MDA (which can lead to a longer and more intense drug high) was reported in street MDMA samples,25 and MDA can be abused itself. Therefore, we recommend that both analytes be monitored in workplace drug testing, driving under the influence and drug treatment programs. However, it would be unadvisable to require the presence of MDA to confirm MDMA intake, as there would be many false negative tests. Similar detection rates of 72.4, 71.3, and 67.1% were observed at the three proposed cutoffs (Talloires, DRUID, and SAMHSA) after both low and high recreational MDMA doses. Despite the higher SAMHSA cutoff concentration, there appears to be no significant loss in sensitivity.
The metabolite:parent drug ratios observed in our oral fluid study are consistent with MDA:MDMA plasma ratios reported by Kolbrich. 19 Time course plots showed ratios increasing linearly for each dose; although mean MDA/MDMA ratios were higher after the low dose in both matrices. However, considerable intra- and inter-subject variability makes predicting time of last use from these data inappropriate.
These data document non-linear MDMA oral fluid pharmacokinetics. The 1.6-fold difference in MDMA dose was associated with a 3-fold difference in MDMA and MDA AUC0 to ∞. This apparent non-linearity is consistent with observations in plasma from a controlled drug administration study that documented MDMA’s inhibition of its own metabolism. 19 Since MDMA users may consume more than one dose per session, potentially harmful concentrations may accumulate with repeated doses, as bio-availability is much higher and half-lives longer. Farré et al. administered two successive 100 mg doses of MDMA separated by 24 h. Following the second dose, MDMA plasma concentrations were greater than expected by simple accumulation, consistent with metabolic inhibition. 26 In addition, de la Torre observed a 3-fold increase in MDMA dose (50 – 150 mg) was associated with a more than 10-fold change in MDMA AUC0-24. 27
In summary, this controlled MDMA administration study presents pharmacokinetic data on the disposition of MDMA and MDA in expectorated oral fluid, and provides performance characteristics at the DRUID, Talloires and proposed SAMHSA cutoff concentrations, thus providing a scientific basis for policy development for oral fluid testing. These data suggest that oral fluid is a good alternative matrix for monitoring MDMA use, and can efficiently detect a single, recreational (70-150 mg) MDMA dose for one to two days.
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Parrott AC. Human psychopharmacology of Ecstasy (MDMA): a review of 15 years of empirical research. Hum Psychopharmacol. 2001;16:557–577. doi: 10.1002/hup.351. [DOI] [PubMed] [Google Scholar]
- 2.Green AR, Mechan AO, Elliott JM, et al. The pharmacology and clinical pharmacology of 3,4-methylenedioxymethamphetamine (MDMA, “ecstasy”) Pharmacol Rev. 2003;55:463–508. doi: 10.1124/pr.55.3.3. [DOI] [PubMed] [Google Scholar]
- 3.Schifano F. A bitter pill. Overview of ecstasy (MDMA, MDA) related fatalities. Psychopharmacology (Berl) 2004;173:242–8. doi: 10.1007/s00213-003-1730-5. [DOI] [PubMed] [Google Scholar]
- 4.Substance Abuse and Mental Health Services Administration . Results From the 2009 National Survey on Drug Use andHealth: National Findings. Office of Applied Studies; Department of Health and Human Services (DHHS); Rockville, MD: 2010. (NSDUH Series H-38B). DHHS Publication No. SMA 10-4856. [Google Scholar]
- 5.Department of Transportation - National Highway Traffic Safety Administration . 2007 National Roadside Survey of Alcohol and Drug Use by Drivers: Drug Results. Washington, DC: 2008. DOT Publication No. HS 811-249. [Google Scholar]
- 6.Bosker WM, Huestis MA. Oral Fluid Testing for Drugs of Abuse. Clin Chem. 2009;55:1910–1931. doi: 10.1373/clinchem.2008.108670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Drummer OH. Drug testing in oral fluid. Clinical and Biochemical Reviews. 2006;27:147–59. [PMC free article] [PubMed] [Google Scholar]
- 8.Kintz P, Samyn N. Use of alternative specimens: drugs of abuse in saliva and doping agents in hair. Therapeutic Drug Monitoring. 2002;24:239–246. doi: 10.1097/00007691-200204000-00006. [DOI] [PubMed] [Google Scholar]
- 9.Toennes SW, Steinmeyer S, Maurer HJ, et al. Screening for drugs of abuse in oral fluid-correlation of analysis results with serum in forensic cases. Journal of Analytical Toxicology. 2005;29:22–7. doi: 10.1093/jat/29.1.22. [DOI] [PubMed] [Google Scholar]
- 10.Drummer OH. Introduction and review of collection techniques and applications of drug testing of oral fluid. Therapeutic Drug Monitoring. 2008;30:203–206. doi: 10.1097/FTD.0b013e3181679015. [DOI] [PubMed] [Google Scholar]
- 11.Bush DM. The U.S. Mandatory Guidelines for Federal Workplace Drug Testing Programs: current status and future considerations. Forensic Sci Int. 2008;174:111–9. doi: 10.1016/j.forsciint.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 12.Pil K, Raes E, Neste TVd, et al. Toxicological analyses in the DRUID epidemiological studies: analytical methods, target analytes and analytical cut-offs. The European Integrated Project DRUID. 2007 [Google Scholar]
- 13.Walsh JM, Verstraete AG, Huestis MA, et al. Guidelines for research on drugged driving. Addiction. 2008;103:1258–1268. doi: 10.1111/j.1360-0443.2008.02277.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Samyn N, De Boeck G, Wood M, et al. Plasma, oral fluid and sweat wipe ecstasy concentrations in controlled and real life conditions. Forensic Science International. 2002;128:90–97. doi: 10.1016/s0379-0738(02)00157-3. [DOI] [PubMed] [Google Scholar]
- 15.Wood M, Laloup M, Fernandez Mdel M Ramirez, et al. Quantitative analysis of multiple illicit drugs in preserved oral fluid by solid-phase extraction and liquid chromatography-tandem mass spectrometry. Forensic Science International. 2005;150:227–38. doi: 10.1016/j.forsciint.2004.11.027. [DOI] [PubMed] [Google Scholar]
- 16.Navarro M, Pichini S, Farre M, et al. Usefulness of saliva for measurement of 3,4-methylenedioxymethamphetamine and its metabolites: correlation with plasma drug concentrations and effect of salivary pH. Clinical Chemistry. 2001;47:1788–1795. [PubMed] [Google Scholar]
- 17.Scheidweiler KB, Huestis MA. A validated gas chromatographic-electron impact ionization mass spectrometric method for methylenedioxymethamphetamine (MDMA), methamphetamine and metabolites in oral fluid. Journal of Chromatography B. 2006;835:90–99. doi: 10.1016/j.jchromb.2006.03.020. [DOI] [PubMed] [Google Scholar]
- 18.Krouwer JS, Rabinowitz R. How to improve estimates of imprecision. Clinical Chemistry. 1984;30:290–2. [PubMed] [Google Scholar]
- 19.Kolbrich EA, Goodwin RS, Gorelick DA, et al. Plasma pharmacokinetics of 3,4-methylenedioxymethamphetamine after controlled oral administration to young adults. Therapeutic drug monitoring. 2008;30:320–32. doi: 10.1097/FTD.0b013e3181684fa0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de la Torre R, Farre M, Roset PN, et al. Pharmacology of MDMA in humans. Annals of the New York Academy of Sciences. 2000;914:225–237. doi: 10.1111/j.1749-6632.2000.tb05199.x. [DOI] [PubMed] [Google Scholar]
- 21.Mas M, Farre M, de la Torre R, et al. Cardiovascular and neuroendocrine effects and pharmacokinetics of 3,4-methylenedioxymethamphetamine in humans. Journal of Pharmacology and Experimental Therapeutics. 1999;290:136–145. [PubMed] [Google Scholar]
- 22.Cone EJ. Saliva testing for drugs of abuse. Annals of the New York Academy of Sciences. 1993;694:91–127. doi: 10.1111/j.1749-6632.1993.tb18346.x. [DOI] [PubMed] [Google Scholar]
- 23.Pichini S, Navarro M, Farre M, et al. On-site testing of 3,4-methylenedioxymethamphetamine (ecstasy) in saliva with drugwipe and drugread: a controlled study in recreational users. Clinical Chemistry. 2002;48:174–176. [PubMed] [Google Scholar]
- 24.Kraemer T, Maurer HH. Toxicokinetics of amphetamines: metabolism and toxicokinetic data of designer drugs, amphetamine, methamphetamine, and their N-alkyl derivatives. Therapeutic Drug Monitoring. 2002;24:277–289. doi: 10.1097/00007691-200204000-00009. [DOI] [PubMed] [Google Scholar]
- 25.Silcott P, Silcott M. The Book of E : All about Ecstasy. Omnibus Pres; 2000. [Google Scholar]
- 26.Farre M, de la Torre R, Mathuna BO, et al. Repeated doses administration of MDMA in humans: pharmacological effects and pharmacokinetics. Psychopharmacology (Berlin) 2004;173:364–75. doi: 10.1007/s00213-004-1789-7. [DOI] [PubMed] [Google Scholar]
- 27.de la Torre R, Farre M, Ortuno J, et al. Non-linear pharmacokinetics of MDMA (‘ecstasy’) in humans. Journal of Clinical Pharmacology. 2000;49:104–109. doi: 10.1046/j.1365-2125.2000.00121.x. [DOI] [PMC free article] [PubMed] [Google Scholar]