Abstract
Lysosomal acid lipase (LAL) cleaves cholesteryl esters and triglycerides to generate free fatty acids and cholesterol in lysosomes. LAL deficiency causes expansion of CD11b+GR-1+ immature myeloid cells, loss of T cells and impairment of T cell function. To test how myeloid cell LAL controls myelopoiesis and lymphopoiesis, a myeloid-specific doxycycline-inducible transgenic system was used to re-introduce human LAL (hLAL) expression into LAL gene knock-out (lal−/−)mice. Expression of hLAL in myeloid cells of lal−/− mice reversed abnormal myelopoiesis in the bone marrow starting at the granulocyte-macrophage precursors (GMP) stage and reduced systemic expansion of myeloid-derived suppressor cells (MDSCs). Myeloid hLAL expression inhibited reactive oxygen species production and arginase expression in CD11b+GR-1+ cells of lal−/− mice. Structural organization of the thymus and spleen was partially restored in association with reduced infiltration of CD11b+GR-1+ cells in these mice. In the thymus, reconstitution of myeloid cell LAL restored development of thymocytes at the double-negative DN3 stage. Myeloid cell LAL expression improved the proliferation and function of peripheral T cells. In vitro co-culture experiments showed that myeloid hLAL expression in lal−/− mice reversed CD11b+GR-1+ myeloid cell suppression of CD4+ T cell proliferation, T cell signaling activation, and lymphokine secretion. Blocking Stat3 and NFκB p65 signaling by small molecule inhibitors in MDSCs achieved the similar effect. Injection of anti-Gr-1 antibody into lal−/− mice to deplete MDSCs restored T cell proliferation. These studies demonstrate that LAL in myeloid cells plays a critical role in maintaining normal hematopietic cell development and balancing immunosuppression and inflammation.
Keywords: LAL, MDSCs, T cell suppression, myeloid-specific transgenic mice
Introduction
Lysosomal acid lipase (LAL) hydrolyzes cholesteryl esters and triglycerides to generate free fatty acids and cholesterol in lysosomes of cells. Our previous studies demonstrated that LAL plays a critical role in maintaining homeostasis of immune cells, and that disruption of LAL expression leads to pathogenic phenotypes in multiple organs (1–6). In LAL knock-out mice (lal−/− mice), LAL deficiency blocks common lymphoid progenitor (CLP) development in the bone marrow (2) and impairs T cell development in the thymus (1) to affect lymphopoiesis. As a result, peripheral T cell levels are dramatically reduced. Lal−/− T cells display defects after T cell receptor (TCR) stimulation, including a failure to upregulate CD69 expression, severely diminished T cell proliferation, and decreased expression of T cell cytokines in response to anti-CD3 Ab plus anti-CD28 Ab, or phorbol-12-myristate-13-acetate and ionomycin (1).
Conversely, LAL deficiency significantly increases systemic myeloid cell expansion, especially a population of CD11b+/Gr-1+ myeloid-derived suppressive cells (MDSCs) in multiple organs. Aberrant growth and differentiation of myeloid cells in lal−/− mice arise from dysregulated production of progenitor cells in the bone marrow (2). Immature myeloid cells in lal−/− mice infiltrate multiple organs, including the thymus, spleen, lung, liver and small intestine (1, 5, 7). It is conceivable that infiltration of MDSCs in these organs influences the localized tissue microenvironments and host immunity. For example, MDSCs are well known to function as T cell suppression (8–10). Infiltration of MDSCs into the thymus and spleen may affect T cell development and maturation. At least in the in vitro study, MDSCs from lal−/− mice show strong inhibition on proliferation and function of T cells (2). Therefore, abnormal T cell development, homeostasis, and function may be related to MDSCs expansion in lal−/− mice. Importantly, disorganization of the thymus and spleen structures is observed in lal−/− mice with a massive presence of myeloid cells (1). The link between T cell deficiency and MDSCs infiltration and expansion in lal−/− mice has not been studied.
To further determine if the aberrant development and function of LAL deficiency-induced myeloid cells affect T cells, a previously generated triple transgenic mouse model, in which myeloid-specific doxycycline-inducible wild type hLAL is expressed in lal−/− mice under the control of the c-fms promoter, was used (7). Previously, we showed that restricted-expression of hLAL in myeloid cells significantly reversed tissue inflammation, aberrant gene expression and pathogenic phenotypes in the lung, liver and small intestine of lal−/− mice. In this report, we showed that myeloid expression of hLAL in triple mice reversed abnormal myeloid progenitor development and differentiation in the bone marrow, reduced MDSCs expansion and infiltration into the thymus and spleen, corrected abnormal T cell development in the thymus, and restored T cell proliferation and function in the spleen. These new results demonstrate that LAL in myeloid cells is critical for the development, homeostasis and function of myeloid cells and T cells. Disruption of this pathway leads to systemic inflammation and tissue pathogenesis.
Materials and Methods
Animal Care
All scientific protocols involving the use of animals have been approved by the Institutional Animal Care and Use Committee of Indiana University School of Medicine and followed guidelines established by the Panel on Euthanasia of the American Veterinary Medical Association. Protocols involving the use of recombinant DNA or biohazardous materials have been reviewed by the Biosafety Committee of Indiana University School of Medicine and followed guidelines established by the National Institutes of Health. Animals were housed under Institutional Animal Care and Use Committee-approved conditions in a secured animal facility at Indiana University School of Medicine.
Fluorescence activated cell sorting (FACS) analysis
Single cell suspensions from the bone marrow, spleen and blood were prepared as previously described (11). Approximately 1 to 2 × 106 cells from various organs were blocked with FcR blocking antibodies in FACS buffer (BD Pharmingen, San Diego, CA) followed by incubation with isotype control or primary antibodies. For 6-color hematopoietic progenitor cell analysis and sorting (12), a previously described procedure was followed (2). For measurement of intracellular signaling molecules, the assays were performed according to the protocols previously described (11). Anti-phospho-Erk, P38, NFkB, Stat1, Stat3 and Stat6 were purchased from Cell Signaling Technology.
Double immunofluorescence staining
The thymus and spleen were washed with PBS and dehydrated by a series of increasing ethanol concentrations, followed by paraffin embedding. Five μm tissue sections were doubly stained with rabbit anti-Flag antibody (1:100, Sigma, St Louis, MO), rabbit anti-GR-1 antibody and CD11b mouse antibody (eBioscience). A Cy2-conjugated donkey anti-rabbit IgG and a Cy3-conjugated donkey anti-mouse IgG (Jackson ImmunoResearch, West Grove, PA) were used as the secondary antibodies. Primary antibodies or secondary antibodies alone served as control and showed no positive staining.
Real Time PCR
Real-Time PCR analysis was performed as previously described (13) using the Taqman Reverse Transcription Kit and SYBR Green PCR Master Mix (Applied Biosystems, Foster City, CA). GAPDH primers were used as an endogenous control for normalizing all cDNA samples. The reactions were analyzed using the StepOnePlu Real-Time PCR System (Applied Biosystems).
Primers for Real-Time PCR:
mBid
Upstream: 5′-GGCGTCTGCGTGGTGATT-3′
Downstream: 5′-ACAAGCTGTGTAGCTCCAAGCA-3′
mCasp3
Upstream: 5′-TGCTTACTCTACAGCACCTGGTTACT-3′
Downstream: 5′-TGAACCACGACCCGTCCTT-3′
mCasp6
Upstream: 5′-CGGGCAGGTGAAAGTAAAACA-3′
Downstream: 5′-GGATCGAACACTTCCCTACTTTTG-3′
mCasp8
Upstream: 5′-CGCCAAGAGAACAAGACAGTGA-3′
Downstream: 5′-CGAGGTTTGTTCTTCATTTGGTAA-3′
mCasp9
Upstream: 5′-AACGACCTGACTGCCAAGAAA-3′
Downstream: 5′-GGTTCCGGTGTGCCATCTC-3′
mFasL
Upstream: 5′-CGAGGAGTGTGGCCCATT T-3′
Downstream: 5′-TCCAGAGGGATGGACCTTGA-3′
mIL-1β
Upstream: 5′-TTGACGGACCCCAAAAGATG- 3′
Downstream: 5′-CAGGACAGCCCAGGTCAAA-3′
mIL-2
Upstream: 5′-AAACTAAAGGGCTCTGACAACACA-3′
Downstream: 5′-CACCACAGTTGCTGACTCATCA-3′
mIL-4
Upstream: 5′-TTGAACGAGGTCACAGGAGAAG-3′
Downstream: 5′-AGGACGTTTGGCACATCCA-3′
mIL-6
Upstream: 5′-GAGGCTTAATTACACATGTTC-3′
Downstream: 5′-TGCCATTGCACAACTCTTTTCT-3′
mIL-10
Upstream: 5′-GCTGCGGACTGCCTTCA-3′
Downstream: 5′-TCCAGCTGGTCCTTTGTTTGA-3′
mIL-13
Upstream: 5′-AGCAACATCACACAAGACCAGACT-3′
Downstream: 5′-CCAGGTCCACACTCCATACCA-3′
mIL-16
Upstream: 5′-GCTCCCTGCATGGTGACAA-3′
Downstream: 5′-CACCCTGTTCTGTCCCTTTGA-3′
mIL-17
Upstream: 5′-TCTGTGTCTCTGATGCTGTTGCT-3′
Downstream: 5′-CGCTGCTGCCTTCACTGTAG-3′
mIFNγ
Upstream: 5′-CAAGGCGAAAAAGGATGCAT-3′
Downstream: 5′-CTGGACCTGTGGGTTGTTGAC-3′
mTNFα
Upstream: 5′-CCCCAAAGGGATGAGAAGTTC-3′
Downstream: 5′-TGAGGGTCTGGGCCATAGAA-3′
GAPDH
Upstream: 5′-GGCCATCAAGCCAGAGCTT-3′
Downstream: 5′-CCAAACCATCACTGACACTCAGA-3′
Isolation of MDSCs
Spleen single cells were placed in anti-CD11b Ab-coated culture dishes and incubated for 3 hours at 37°C in a humidified 5% CO2 atmosphere. Cells were gently washed with PBS. Adherent cells were incubated with biotin-labeled primary anti-GR-1 antibody for 20 min, then followed by 20 min incubation of anti-biotin secondary antibody beads in PBS. Labeled cells were selected on MS column using the magnetic-activated cell sorting technology (Miltenyi Biotech, Auburn, CA).
T cell proliferation assay
CD4+ T cells were isolated from the spleen of various mouse groups and purified with anti-CD4 monoclonal antibody (mAb)–coated magnetic beads and MACS-LS columns according to the manufacturer’s instructions. Isolated CD4+ cells were labeled with carboxyfluorescein diacetate succinimidyl diester (CFSE; Molecular Probes). Labeled cells were cultured and stimulated with 1 μg/ml plate-bound anti-CD3 Ab and 1 μg/ml soluble anti-CD28 Ab (BD Pharmingen) for 4 days at 37°C in a humidified 5% CO2 incubator. CFSE profiles of CD4+ T cell proliferation was evaluated by FACS. Activation of cultured CD4+ T cells was assessed by expression of surface activation marker CD69 with anti-CD69 antibody by FACS.
Annexin V Binding
Dual staining with FITC-Annexin V and propidium iodide (PI) was performed to detect cells undergoing apoptosis using an Annexin V-FITC kit (BD Biosciences, Bedford, MA) following a procedure previously described (1). Cultured and stimulated CD4+ T cells were stained with surface markers and washed twice with PBS. After resuspension of labeled cells in Annexin V-binding buffer containing FITC-conjugated Annexin V, PI was added into samples and incubated on ice for 10 minutes. Cells were analyzed on LSRII within 1 hour. Viable cells were defined by FITC negative (FITC−) and PI negative (PI−) population. Early apoptotic cells were defined by FITC positive (FITC+) and PI− population. Nonspecific binding was blocked by pre-incubating cells with rat IgG (10 μg/ml) and anti-FcII/III.
Lin− bone marrow cell purification
A previously described procedure was used (2). Briefly, bone marrow cells were isolated from various mouse groups. Erythrocytes were lysed. Bone marrow cells were labeled with a cocktail of biotin-coupled antibodies raised against lineage-specific antigens: CD11b, GR-1, B220, TER-119, and CD3ε (mouse lineage panel Kit; BD Pharmingen, San Diego, CA) for 20 min at 4 °C. Unlabeled cells were separated on a depletion column using the magnetic-activated cell sorting technology according to the manufacturer’s instruction (Miltenyi Biotech, Auburn, CA). This method resulted in > 95% Lin− cells.
In vitro MDSC suppression assay and lymphokine measurement
CD4+ T cells were isolated from the spleen of wild type (lal+/+) mice with anti-CD4 monoclonal antibody (mAb)–coated magnetic beads and MACS-LS columns according to the manufacturer’s instructions. Isolated wild type CD4+ cells were labeled with CFSE and cultured with 1 μg/ml plate-bound anti-CD3 Ab and 1 μg/ml soluble anti-CD28 Ab for 4 days in the presence or absence of CD11b+/Gr-1+ cells from various mouse groups at 37°C in a humidified 5% CO2 incubator. The ratio between MDSCs:CD4+ T cells was1:5. Proliferation of CD4+ T cells was evaluated as CFSE dilution by FACS. T cell activation was also assessed with anti-CD69 antibody. The lymphokine concentrations in the cultured medium were measured by OptEIA ELISA kits according to the protocols by Pharmingen (San Diego, CA) or (R&D Systems, Minneapolis, MN).
Measurement of pCD3ξ-chain
CD4+ T cells isolated from the wild type mouse spleen were co-cultured (106/ml T cells per well) with CD11b+/Gr-1+ cells from various mouse groups for 4 days in RPMI 1640 medium containing 1 μg/ml plate-bound anti-CD3 Ab, and anti-CD28 Ab at 37°C in a humidified 5% CO2 incubator. Activation of intracellular pCD3ξ-chain in CD4+ T cells was measured by FACS using anti-mouse-phosphoCD3ξ Ab (BD Pharmingen). Rat-IgG2 Ab (BD Pharmingen) was used as isotype control.
Arginase activity
Arginase activity was measured as previously described (14). Briefly, cells were lysed for 30 min at room temperature with 50μl of 0.1% Triton X-100 PBS containing 5μg pepstatin, 5μg aprotinin and 5μg antipain protease inhibitors. Subsequently, 50 μl of 10 mM MnCl2 and 50 μl of 50 mM Tris-HCl (PH7.5) were added, and the enzyme was activated by heating for 10 min at 56°C. Arginine hydrolysis (100 μl) was conducted by incubating the lysate with 100 μl of 0.5 M L-arginine (pH 9.7) at 37°C for 60–120 min. The reaction was stopped with 400 μl of H2SO4 (96%)/H3PO4 (85%)/H2O (1/3/7, v/v/v). The urea concentration was measured at 540 nm after addition of 25 μl of 9% α-isonitrosopropiophenone (dissolved in 100% ethanol), followed by heating at 95°C for 45 min and 10min in dark. One unit of enzyme activity is defined as the amount of enzyme that catalyzes the formation of 1 μmol urea per min.
Reactive oxygen species (ROS) production
MDSCs were isolated from the spleen of lal+/+ mice (WT), lal−/− mice (KO), 3-month doxycycline-treated (Tg/KO ON) or untreated (Tg/KO OFF) Tg/KO triple mice using cell sorting. The purity of cell populations was >95%. Oxidation-sensitive dye DCFDA (Molecular Probes/Invitrogen), was used to measure ROS production. Cells were incubated at 37°C in pre-warmed RPMI 1640 in the presence of 2.5 μM DCFDA for 20 min. Cells were then labeled with APC-conjugated anti-Gr-1 and PE-conjugated anti-CD11b Abs on ice and analyzed by flow cytometry.
Stat3 and NFκB inhibition
Stat3 inhibitor cucurbitacin B (C8499) and NFkB inhibitor ammonium pyrrolidinedithiocarbamate (PDTC, P8765) were purchased from Sigma.
MDSCs depletion study
MDSCs were depleted in vivo by i.p injection of 50μg anti-mouse Gr-1 Ab (RB6-8C5; eBioscience) per mouse once every 3 days. After two weeks, splenocytes were harvested and analyzed by FACS.
Statistical analysis
The results were mean values of at least three independent experiments. A paired Student’s t test or ANOVA was used to evaluate the significance of the differences. Statistical significance was set at P< 0.05.
Results
Myeloid expression of hLAL-Flag fusion protein in Tg/KO triple mice
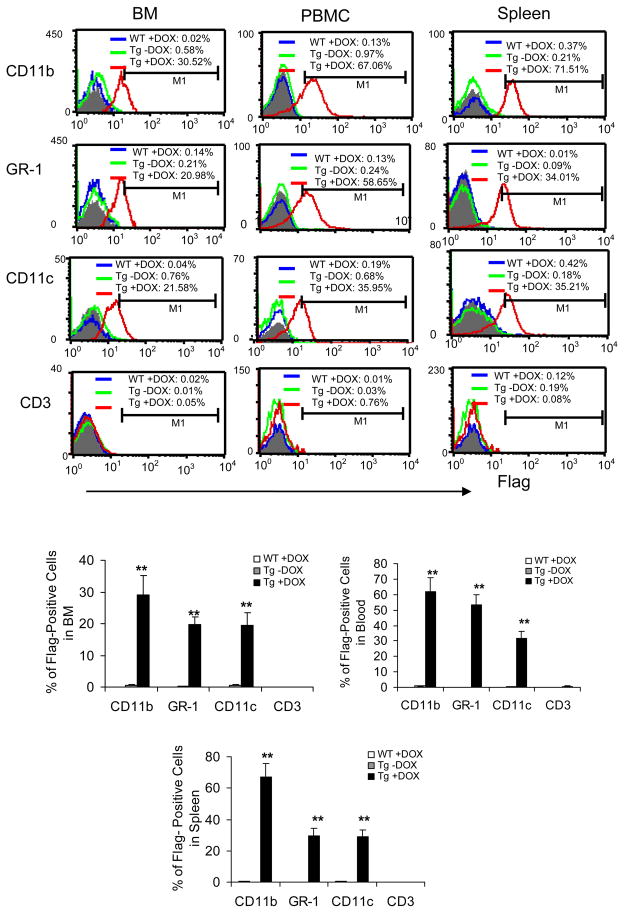
A previously established c-fms-rtTA/(TetO)7-CMV-hLAL; lal−/− (refer as Tg/KO thereafter) triple mouse model (7) was treated with or without doxycycline for 3 months and analyzed by FACS to assess the profile of hLAL-Flag fusion protein expression. Single-cell suspensions from the bone marrow, blood and spleen were doubly stained with fluorochrome-conjugated Flag antibody and antibodies specific for macrophages, dendritic cells (DCs), neutrophils, or T cells. CD11b+ myeloid cells, Gr-1+ neutrophils and CD11c+ DCs showed significant hLAL-Flag expression in all tested organs of doxycycline-treated triple mice compared with organs of untreated triple mice and doxycycline-treated wild type mice (Figure 1). There was no hLAL-Flag protein expression in CD3+ T lymphocytes regardless of doxycycline treatment. This result confirmed that hLAL-Flag fusion protein expression in Tg/KO triple mice was myeloid cell specific. No hLAL-Flag fusion protein was detected in lal+/+ mice regardless of doxycycline treatment, suggesting that induction of hLAL-Flag fusion protein was not caused by doxycycline alone. The morphological shape of CD11b+ myeloid cells in doxycycline-treated lal−/− triple mice was different from those in untreated lal−/− triple mice.
Figure 1. Expression profile of hLAL-Flag fusion protein in Tg/KO triple mice.
A representative and statistical analysis of Flag expression in CD11b+, Gr-1+, CD11c+ and CD3+ cells from the bone marrow (BM), blood (PBMC) and lung of 3-month doxycycline-treated WT mice (WT +DOX; blue line), doxycycline-treated (Tg +DOX; red line) or -untreated (Tg -DOX; green line) Tg/KO triple mice were stained with anti-Flag Ab in combination with the cell surface markers. In gated cells, the numbers of the Flag+ cells were analyzed by FACS and calculated based on M1, a histogram marker excluding isotypic control. Isotype controls were shown as the shaded areas. Results were mean ± SD from 5 mice in each groups (n=5). **P < 0.01.
hLAL expression reversed abnormal increase of GMP and MDSCs in Tg/KO triple mice
Abnormal development of hematopoietic progenitor cells causes significant expansion of CD11b+GR-1+ immature myeloid cells in lal−/− mice (2). To test whether LAL expression in myeloid cells reverses this problem, lal+/+ mice, lal−/− mice, 3-month doxycycline-treated (Tg ON) or -untreated (Tg OFF) c-fms-rtTA/(TetO)7-CMV-hLAL bitransgenic mice and 3-month doxycycline-treated (Tg/KO ON) or -untreated (Tg/KO OFF) Tg/KO triple mice were used. The percentage and total numbers of hematopoietic progenitor LSK (IL7Rα−Lin−Sca-1+c-Kit+), LK (IL7Rα−Lin−c-Kit+Sca-1−), CMP (IL7Rα−Lin−Sca-1+c-Kit+CD34+FcRII/IIIlow), GMP (IL7Rα−Lin−Sca-1+c-Kit+CD34+FcRII/III+) and MEP (IL7Rα−Lin−Sca-1+c-Kit+CD34−FcRII/III−) populations were analyzed with FACS. Similar to that reported before (2), frequencies of LSK, LK and GMP were increased in lal−/− mice compared to lal+/+ wild type mice in the bone marrow. No change was observed in c-fms-rtTA/(tetO)7-CMV-hLAL bitransgenic mice regardless of doxycycline treatment. Tg/KO triple mice without doxycycline treatment showed similar progenitor frequencies compared with lal−/− mice. But, Tg/KO mice with doxycycline treatment reduced GMP progenitor frequencies close to the level of lal+/+ mice (Figure 2A,B). LK and LSK frequencies were slightly affected with no significance after doxycycline treatment in triple mice. Doxycycline treatment also reversed the MEP decrease in triple mice (Figure 2A). CMP showed relatively no change in all groups. These results indicate that myeloid-specific expression of hLAL is able to rescue hematopoietic developmental defects at the GMP developmental stage, but not at the earlier LK, LSK and CMP stages in the bone marrow.
Figure 2. Reverse of abnormal hematopoietic progenitor development and MDSCs expansion by hLAL in Tg/KO triple mice.
A. The percentage and total numbers of LSK, LK, CMP, GMP and MEP populations in the bone marrow of lal+/+ mice, lal−/− mice, 3-month doxycycline-treated (Tg ON) or -untreated (Tg OFF) c-fms-rtTA/(TetO)7-CMV-hLAL bitransgenic mice and 3-month doxycycline-treated (Tg/KO ON) or -untreated (Tg/KO OFF) Tg/KO triple mice were analyzed by FACS. Results were mean ± SD from 4 mice in each groups (n=4). *P < 0.05; **P < 0.01. LK: IL7Rα−Lin−c-Kit+Sca-1− progenitors; LSK: IL7Rα−Lin−/Scal+/c-kit+ progenitors; CMP: Common myeloid progenitors; GMP: Granulocyte-monocyte progenitors; MEP: Megakaryocyte –erythroid progenitor. lal+/+ and lal−/− served as control; B. A representative and statistical analysis of CD11b+/GR-1+ cells in the bone marrow, blood and spleen from lal+/+ mice (WT), lal−/− mice (KO), 3-month doxycycline-treated (Tg ON) or -untreated (Tg OFF) c-fms-rtTA/(TetO)7-CMV-hLAL bitransgenic mice and 3-month doxycycline-treated (Tg/KO ON) or -untreated (Tg/KO OFF) Tg/KO triple mice. Results were mean ± SD from 5 mice in each groups (n=5). *P < 0.05; C. Statistical analysis of Ly6C+ and Ly6G+ cells in gated CD11b+ cells of the bone marrow, blood and spleen from lal+/+ mice (WT), lal−/− mice (KO), and 3-month doxycycline-treated (Tg/KO ON) or -untreated (Tg/KO OFF) Tg/KO triple mice. Results were mean ± SD from 5 mice in each groups (n=5). *P < 0.05.
To see if correction of the GMP abnormality reverses abnormal myelopoiesis in the bone marrow and other organs, the bone marrow, blood (PBMC) and spleen were harvested from lal+/+ mice (WT), lal−/− mice (KO), c-fms-rtTA/(tetO)7-CMV-hLAL bitransgenic mice (Tg), and Tg/KO mice. MDSCs were stained with fluorochrome-conjugated anti-CD11b and anti-Gr-1 antibodies for FACS analysis. In the blood, the percentage numbers of CD11b+Gr-1+ MDSCs were drastically increased in lal−/− mice compared to lal+/+ mice, similar to previous reports (2). The numbers of CD11b+Gr-1+ MDSCs in c-fms-rtTA/(tetO)7-CMV-hLAL bitransgenic mice were similar to those in lal+/+ mice regardless of doxycycline treatment. Tg/KO mice without doxycycline treatment showed modestly decreased MDSCs (19.34%) compared with those in lal−/− mice (31.84%). However, doxycycline treatment for 3 months further decreased MDSCs (7.40%), closer to those in lal+/+ mice (4.97%) (Figure 2B). The same trend was also observed in the bone marrow and spleen (Figure 2B), except that there was no statistical difference in the bone marrow. Since bitransgenic mice with or without doxycycline treatment showed very little differences from those in lal+/+ mice, bitransgenic mice were not further analyzed. Only lal+/+ and lal−/− mice were used as controls in the following studies. MDSCs can be divided into monocytic (CD11b+Ly6C+) and granulocytic MDSC (CD11b+, Ly6G+). Both populations were further analyzed for LAL effect. In gated CD11b+ cells, Gr-1+ cells appeared as Ly6C+Ly6G+ double positive in most organs, except in the lung where a significant portion of Gr-1+ cells only appeared as Ly6G+ positive (Figure 2C).
hLAL expression reversed malformation and malfunction of MDSCs in Tg/KO mice
To test the differentiation ability of bone marrow progenitor cells into MDSCs, Lin− (lineage negative) bone marrow cells were isolated from lal+/+ mice (WT), lal−/− mice (KO) and doxycycline-untreated (Tg/KO OFF) and -treated (Tg/KO ON) Tg/KO mice and cultured in vitro. Five days after GM-CSF and IL-4 stimulation, CD11b+GR-1+ population in cultured cells were increased in lal−/− mice (79.75%) compared with those in lal+/+ mice (38.49%). In Tg/KO triple mice, the CD11b+GR-1+ population from doxycycline-treated mice (60.76%) was partially reduced compared with those from doxycycline-untreated mice (79.00%) (Figure 3A). A similar study was performed in Lin+ and whole bone marrow subpopulations. The same observation was made in whole bone marrow cells, but not in Lin+ bone marrow cells (Figure 3A). Therefore, LAL deficiency in Lin− bone marrow cells was partially responsible for MDSCs homeostasis.
Figure 3. Reverse of abnormal MDSCs formation and function by hLAL in Tg/KO triple mice.
A. Myeloid-specific expression of hLAL reduced differentiation of Lin− BM cells in vitro with GM-CSF and IL-4. Whole, Lin−, and Lin+ bone marrow cells were isolated from lal+/+ mice (WT), lal−/− mice (KO), 3-month doxycycline-treated (Tg/KO ON) or -untreated (Tg/KO OFF) Tg/KO triple mice. Cells were cultured with GM-CSF and IL-4 for 5 days. A representative (%) and statistical analysis (total cell numbers) of CD11b+/GR-1+ cells were analyzed by FACS. Results were mean ± SD from 5 mice in each groups (n=5). *P < 0.05; B. CD11b+/GR−1+ cells were isolated from lal+/+ mice (WT), lal−/− mice (KO), 3-month doxycycline-treated (Tg/KO ON) or -untreated (Tg/KO OFF) Tg/KO triple mice. Cells were cultured with GM-CSF and IL-4 for 7 days. CD11c+ and MHCII+ cells were analyzed by FACS. A representative (%) and statistical analysis of CD11c+ and MHCII+ cells was demonstrated. Results were mean ± SD from 5 mice in each groups (n=5). *P < 0.05, **P < 0.01; C. MDSCs were isolated from lal+/+ mice (WT), lal−/− mice (KO), 3-month doxycycline-treated (ON) or -untreated (OFF) Tg/KO triple mice. Cells were cultured with or without 1μg/ml of LPS for 24h. The cultured supernatants were harvested and TGF-β, IL-6, IL-10 and MIP-2 were analyzed by ELISA. Results were mean ± SD from 5 mice in each groups (n=5). *P < 0.05.
To examine whether the expanded GR-1+CD11b+ population from lal−/− mice was an immature proliferating precursor population and sensitive to myeloid growth factors (15), GR-1+CD11b+ cells isolated from the spleen of lal+/+ mice (WT), lal−/− mice (KO) and doxycycline-untreated (Tg/KO OFF) and -treated (Tg/KO ON) Tg/KO mice were cultured in vitro and stimulated with GM-CSF. In the absence of GM-CSF, these cells rapidly died (data not shown). In contrast, 7 days after GM-CSF stimulation, 53.83% of MDSCs differentiated into mature CD11c+MHC class II+ dendritic cells (DCs) in lal−/− mice, and only 6.30% in lal+/+ mice, suggesting that there were more immature GR-1+CD11b+ myeloid cells in the spleen of lal−/− mice than those in lal+/+ mice, and that these precursors possess the ability to differentiate into mature myeloid cells. Most of GR-1+CD11b+ myeloid cells in lal+/+ mice were mature cells (e.g. neutrophils), which lack the ability to convert into DCs. Expression of hLAL in doxycycline-treated Tg/KO mice was able to reduce the immature population. The conversion rate into DCs in doxycycline-treated Tg/KO mice was 15.04% after GM-CSF stimulation compared with 34.99% in untreated mice (Figure 3B). Since DCs are major antigen presenting cells, MHC class II expression was used to confirm the identity of these cells.
Functionally, when in vitro cultured MDSCs from these mice were stimulated with LPS, secretion of immunomodulators TGF-β, IL-6, IL-10 and MIP-2 in the culture medium was significantly increased in lal−/− mice compared with those in lal+/+ mice. Expression of hLAL in doxycycline-treated Tg/KO mice reversed abnormal secretion of these cytokines compared with those in untreated mice (Figure 3C). TGF-β, IL-6 and IL-10 are requisites for T cell suppression and Th2 cell polarization (16).
hLAL expression reversed pathogenic disorganization of the thymus and spleen in Tg/KO mice
LAL deficiency results in disorganization of the thymus and spleen structures in lal−/− mice (1). To determine if this disorganization correlates with infiltration of immature GR-1+CD11b+ cells, the thymus and spleen were harvested from lal+/+ mice (WT), lal−/− mice (KO) and doxycycline-untreated (Tg/KO OFF) and -treated (Tg/KO ON) Tg/KO mice. In lal+/+ mice, the cortical and medullary regions were readily distinguished from one another in the thymus with few GR-1+ and CD11b+ positive cell present. The medullary area was difficult to identify in the thymus of lal−/− mice (KO) (2) and doxycycline-untreated (Tg/KO OFF) Tg/KO mice with massive GR-1+ and CD11b+ positive cell infiltrations. In age-matched doxycycline-treated Tg/KO mice (Tg/KO ON), a readily identifiable medulla area reappeared with significantly reduced numbers of GR-1+CD11b+ double positive cells (Figure 4). In the spleen of lal−/− mice (KO) and doxycycline-untreated Tg/KO mice (Tg/KO OFF), massive accumulation of GR-1+CD11b+ double positive cells were present in the abnormal red pulp area. In age-matched doxycycline-treated Tg/KO mice (Tg/KO ON), the number of GR-1+CD11b+ double positive cells was significantly reduced with restoration of the spleen organization (Figure 4).
Figure 4. Reverse of MDSCs infiltration by hLAL in Tg/KO triple mice.
Double immunofluorescence staining of the thymus and spleen from lal+/+ mice (WT), lal−/− mice (KO), 3-month doxycycline-treated (Tg/KO On) or -untreated (Tg/KO Off) Tg/KO triple mice was performed by Gr-1 (green) and CD11b (red) antibodies. The merged images contain DAPI staining for cell nuclei (blue). Orange color represents double staining of Gr-1 and CD11b. The original magnification was 100 X. C, cortex; M, medullar; RP, red pulp; WP, white pulp. For quantitative characterization, the images (40 x) were transferred by video camera to a computer screen. Six to ten randomly-selected microscopic fields of Gr-1 and CD11b from each mouse were analyzed. Results were mean ± SD from 5 mice in each groups (n=5). *P < 0.05.
hLAL expression reversed abnormal T cell development and peripheral T cell maturation in Tg/KO mice
Our previous studies revealed deficiency in T cell development (in the thymus) and peripheral T cell maturation (mainly in the spleen) in lal−/− mice. In addition to the intrinsic defect in T cells, massive MDSCs infiltration into the thymus and spleen may also contribute to the abnormal T cell development and maturation in lal−/− mice. To test this point, T cell development and maturation in lal+/+ mice (WT), lal−/− mice (KO), doxycycline-untreated (Tg/KO OFF) and -treated (Tg/KO ON) Tg/KO mice were analyzed by FACS.
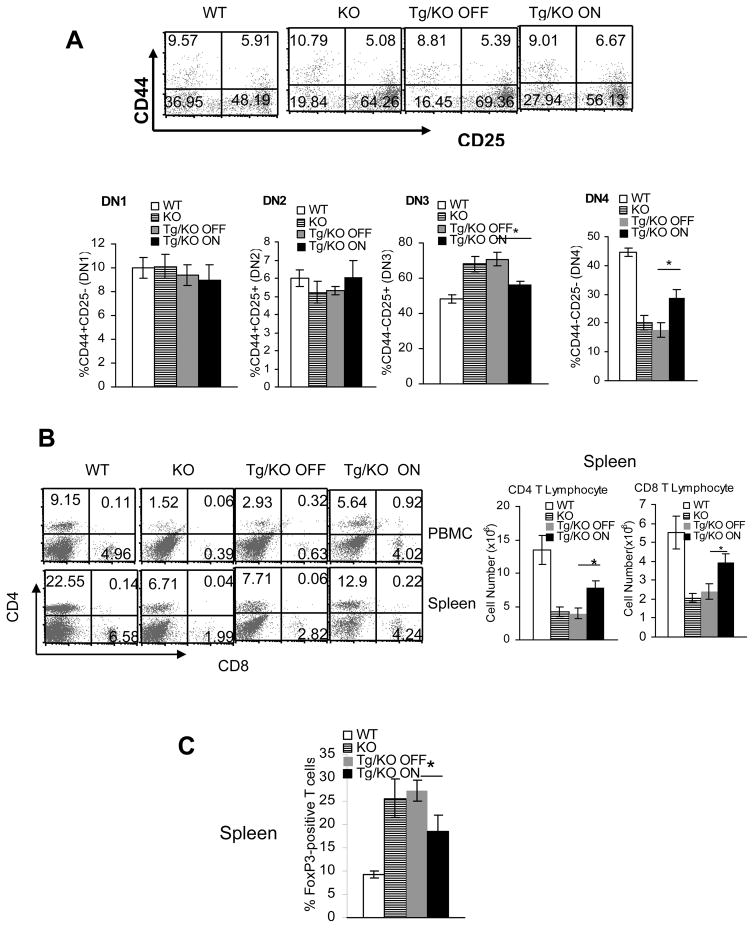
T cell development in the thymus can be divided into CD4−CD8− double-negative (DN), CD4+CD8+ double-positive (DP), and CD4+CD8−/CD4−CD8+ single-positive (SP) stages. Our previous data showed that T cells in almost all these stages were significantly reduced in lal−/− mice compared with those in lal+/+ mice (except that the decrease in CD4+CD8− SP cells was only observed at 9 months of age). We also reported that this blockage in T cell development occurred initially at the DN3 (CD44−CD25+) to DN4 (CD44−CD25−) transition in the thymus (1). When analyzed by CD44 and CD25 markers, myeloid hLAL expression partially reversed this defect. The percentage number of DN3 subset cells was lower (56.13±2.01% vs 69.36±3.91%), whereas the percentage number of DN4 subset cells was higher (27.94±3.06% vs 16.45±2.64%) in the thymus of doxycycline-treated Tg/KO mice compared with those in the thymus of doxycycline-untreated Tg/KO mice (Figure 5A). hLAL expression also partially reversed the deficiency of T cells at other development stages, including DN, DP, and SP stages when analyzed by CD4, CD8 markers (Supplemental Figure 1).
Figure 5. Reverse of T cell developmental and maturation defects by hLAL in Tg/KO triple mice.
A. Total cells were isolated from the thymus of lal+/+ mice (WT), lal−/− mice (KO), 3-month doxycycline-treated (Tg/KO On) or -untreated (Tg/KO Off) Tg/KO triple mice. Anti-CD44 and anti-CD25 antibodies were used to define double negative T cell subsets by FACS. A representative and statistical analysis of DN1 (CD44+CD25−), DN2 (CD44+CD25+), DN3 (CD44−CD25+) and DN4 (CD44− CD25−) subsets were demonstrated by FACS. Results were mean ± SD from 4 mice in each groups (n=4). *P < 0.05; B. Peripheral CD4+ and CD8+ profiles in the spleen and blood (PBMC) of lal+/+ mice (WT), lal−/− mice (KO), 3-month doxycycline-treated (Tg/KO On) or -untreated (Tg/KO Off) Tg/KO triple mice were stained with CD4+ and CD8+ antibodies. A representative (%) and statistical analysis (total cell number) of CD4+ and CD8+ populations were performed by FACS. Results were mean ± SD from 6 mice in each groups (n=6). *P < 0.05; C. The percentage numbers of FoxP3+ Treg among total CD4+ T cells from the spleen of lal+/+ mice (WT), lal−/− mice (KO), 3-month doxycycline-treated (Tg/KO On) or -untreated (Tg/KO Off) Tg/KO triple mice were analyzed by FACS. Results were mean ± SD from 6 mice in each groups (n=6). *P < 0.05.
The numbers of T cells localized within other organs were also analyzed using these mouse strains. Compared with 3-month doxycycline-untreated Tg/KO mice, the numbers of CD4+ T cells were increased in the blood (from 2.93% to 5.64%) and the spleen (from 7.71% to 12.90%) of age-matched doxycycline-treated Tg/KO mice. CD8+ T cells were also increased in the blood (from 0.63% to 4.02%) and the spleen (from 2.82% to 4.24%) of doxycycline-treated Tg/KO mice compared with doxycycline-untreated ones (Figure 5B). In contrast, the numbers of CD4+CD25+ T regulatory (Treg) cells were decreased in the spleen of doxycycline-treated Tg/KO mice compared with doxycycline-untreated Tg/KO mice (Figure 5C). Previously, we reported that Treg cells were increased in lal−/− mice (1).
hLAL expression reversed abnormal T cell function in Tg/KO mice
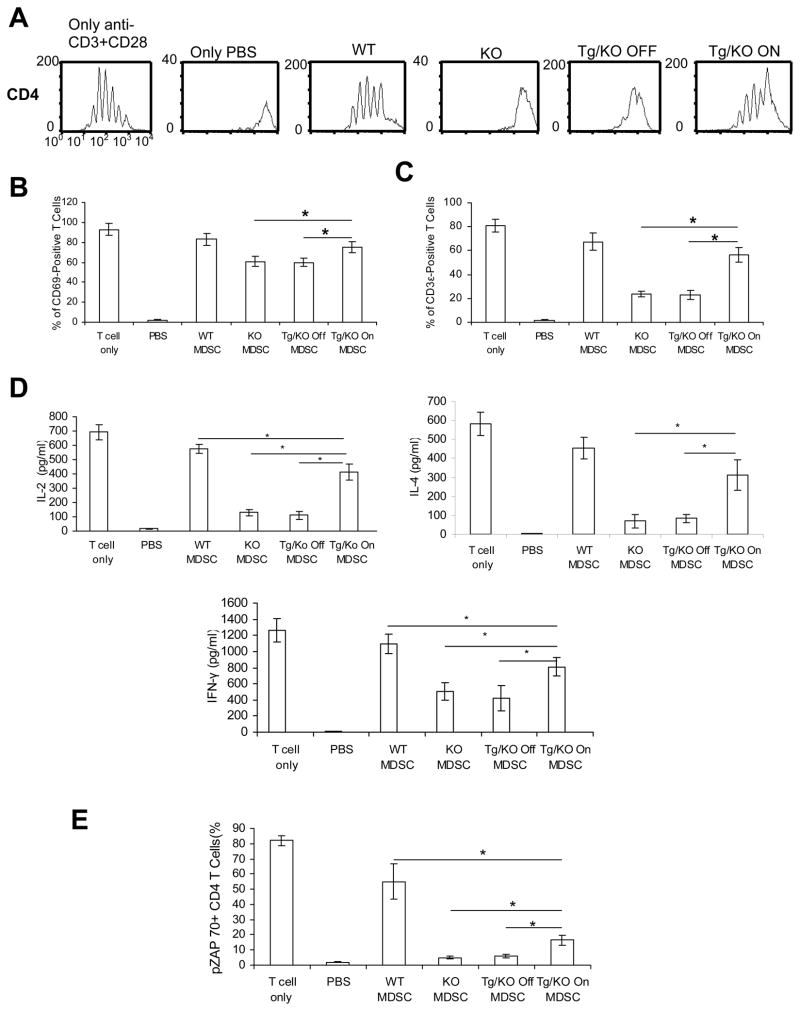
In addition to inhibiting T cell proliferation, LAL deficiency-induced MDSCs expansion may also suppress T cell function, such as response to TCR stimulation. CD4+ splenic T cells from lal+/+ (WT), lal−/− (KO), doxycycline-untreated (Tg/KO OFF) and -treated (Tg/KO ON) Tg/KO mice were cultured in vitro with anti-CD3 Ab plus anti-CD28 Ab. T cell proliferation was measured by CFSE labeling dilution (cell division) and FACS. As demonstrated in Figure 6A, LAL deficiency abolished TCR-stimulated CD4+ T cell proliferation (represented by peaks) in lal−/− mice compared with those in lal+/+ mice. However, myeloid hLAL expression partially restored T cell response to TCR stimulation in doxycycline-treated Tg/KO mice, whereas a minor restoration was observed in doxycycline-untreated ones. Furthermore, myeloid hLAL expression partially restored CD69 expression on T cells from doxycycline-treated (Tg/KO ON) Tg/KO mice compared with those from doxycycline-untreated (Tg/KO OFF) mice (Figure 6B). CD69 is the earliest inducible cell surface glycoprotein during lymphoid activation and a signal-transmitting receptor in lymphocyte proliferation. Myeloid hLAL expression also reduced apoptosis by Annexin V staining, and decreased expression of pro-apoptotic signaling molecules in T cells from doxycycline-treated (Tg/KO ON) Tg/KO mice compared with those from doxycycline-untreated (Tg/KO OFF) mice (Figure 6C). Functionally, expression of multiple CD4+ and CD8+ T cell secreting lymphokines was measured by real-time PCR using sequence-specific oligonucleotide primers after stimulation with anti-CD3 Ab plus anti-CD28 Ab. Myeloid hLAL expression partially restored mRNA levels of Th1 and Th2 lymphokines in doxycycline-treated Tg/KO mice compared with doxycycline-untreated mice. The expression of Th17 lymphokine IL-17 remained relatively unchanged(Figure 6D).
Figure 6. Reverse of T cell proliferation and functional defects by hLAL in Tg/KO triple mice.
CD4+ and CD8+ T cells were isolated from the spleen of lal+/+ mice (WT), lal−/− mice (KO), 3-month doxycycline-treated (Tg/KO On) or -untreated (Tg/KO Off) Tg/KO triple mice. CFSE-labeled CD4+ T cells were cultured in vitro on anti-CD3 plus anti-CD28 antibodies coated plates for 4 days. A. CFSE cell division of CD4+ T cells was measured by FACS; B. CD69 expression on CD4+ T cells was measured by FACS with anti-CD69 and anti-CD4 antibodies. A statistical analysis is presented. Results were mean ± SD from 4 mice in each groups (n=4). *P < 0.05; C. Apoptosis of CD4+ T cells were analyzed by Annexin V staining. mRNA expression levels of pro-apoptotic molecules in CD4+ T cells were measured by Real-Time PCR analysis. Results were mean ± SD from 4 mice in each groups (n=4). *P < 0.05; D. mRNA expression levels of Th1, Th2, and Th17 lymphokines on CD4+ and lymphokines on CD8+ T splenocyte were measured by Real-Time PCR analysis. Results were mean ± SD from 4 mice in each groups (n=4). *P < 0.05.
hLAL expression reversed MDSCs immunosuppressive function in Tg/KO mice
To directly determine if hLAL expression in myeloid cells is responsible for controlling MDSCs immunosuppression of T cell proliferation and function, in vitro MDSCs and T cell co-culturing experiment was performed. CFSE-labeled lal+/+ splenic CD4+ T cells were cultured in vitro and stimulated with anti-CD3 Ab plus anti-CD28 Ab in the presence or absence of CD11b+GR-1+ MDSCs (MDSC: T ratio= 1:5) that were isolated from the spleen of lal+/+ mice (WT), lal−/− mice (KO), or doxcycline-treated (Tg/ON) or -untreated (Tg/OFF) Tg/KO mice. Proliferation of T cells was evaluated as CFSE dilution (cell division) by FACS. MDSCs from lal−/− mice showed strong inhibition on proliferation of lal+/+ T cells that were stimulated with anti-CD3 Ab plus anti-CD28 Ab compared with those from lal+/+ mice. MDSCs from doxcycline-treated Tg/KO triple mice reversed this inhibitory effect, while MDSCs from doxcycline-untreated Tg/KO mice still displayed strong inhibitory activity (Figure 7A). MDSCs from doxycycline-treated (Tg/KO ON) Tg/KO mice also partially restored CD69 expression and pCD3ξ activation (phosphorylation) compared with those from doxycycline-untreated (Tg/KO OFF) mice after TCR stimulation (Figure 7B, C). The TCR-CD3 complex plays a critical role in T cell signaling. Dissociation between TCR and CD3ξ prevents TCR-mediated signaling (17). In addition, expression levels of TCR downstream IL-2, IL-4 and IFN-γ mRNA levels in T cells were partially restored in doxycycline-treated Tg/KO mice compared with those in untreated triple mice after TCR stimulation (Figure 7D), indicating restoration of CD4+ T cell function. When the TCR signaling linked to lymphokine production in CD4+ T cells was investigated, co-culture with MDSCs from lal−/− mice strongly inhibited pZAP-70/Syk activation (phosphorylation) in lal+/+ CD4+ T cells compared with MDSCs from lal+/+ mice. T cell culture with MDSCs from doxcycline-treated Tg/KO mice partially restored phosphorylation of pZAP-70, while MDSCs from doxcycline-untreated mice again inhibited cytokine synthesis following TCR stimulation (Figure 7E). These results suggest that LAL deficiency-induced MDSCs malfunction alters integrity of the TCR complex and its downstream intracellular signaling in CD4+ T cells to down-regulate lymphokine synthesis.
Figure 7. Reverse of the MDSCs immunosuppressive function by hLAL in Tg/KO triple mice.
CD4+ T cells were isolated from the wild type spleen and labeled with CFSE. MDSCs were isolated from the spleen of lal+/+ mice (WT), lal−/− mice (KO), 3-month doxycycline-treated (Tg/KO On) or -untreated (Tg/KO Off) Tg/KO triple mice. CFSE-labeled wild type CD4+ T cells were co-cultured with MDSCs in vitro on anti-CD3 plus anti-CD28 antibodies coated plates for 4 days. The ratio between MDSC:CD4+ T cells was1:5. A. CFSE cell division of CD4+ T cells was measured by FACS; B. CD69 expression on CD4+ T cells was measured by FACS. A statistical analysis is presented. Results were mean ± SD from 3 mice in each groups (n=3). *P < 0.05; C. Phosphorylation of pCD3ξ-chain on CD4+ T cells was measured by FACS. A statistical analysis is presented. Results were mean ± SD from 3 mice in each groups (n=3). *P < 0.05; D. The concentrations of IL-2, IL-4 and IFN-γ in co-cultured medium were measured by ELISA. Results were mean ± SD (n=4). *P < 0.05; E. Phosphorylation of pZAP-70 in CD4+ T cells was measured by FACS with anti-phospho ZAP-70 and anti-CD4 antibodies. Results were mean ± SD (n=4). *P < 0.05.
hLAL expression reversed abnormal ROS production and arginase expression in Tg/KO triple mice
ROS plays a very important role in regulating MDSCs immunosuppressive function on T cells (18). To evaluate ROS production, purified CD11b+GR-1+ cells from the spleen of lal+/+ (WT), lal−/− (KO), doxycycline-untreated (Tg/KO OFF) and -treated (Tg/KO ON) Tg/KO mice were measured for ROS production by DCFDA labeling and FACS. As demonstrated in Figure 8A, LAL deficiency significantly increased ROS production in CD11b+GR-1+ cells of lal−/− mice compared with that in lal+/+ mice (76.94% vs 22.38%). Myeloid hLAL expression partially inhibited ROS production in CD11b+GR-1+ cells of doxycycline-treated Tg/KO mice, compared with that of untreated ones (41.96% vs 72.03%). Increased arginase expression is another important mechanism in regulating MDSCs immunosuppressive function on T cells (19). In the same purified spleen CD11b+GR-1+ cells, LAL deficiency significantly increased arginase expression in lal−/− mice compared with that in lal+/+ mice (Figure 8B). Myeloid hLAL expression partially inhibited arginase expression in CD11b+GR-1+ cells of doxycycline-treated Tg/KO triple mice. Therefore, both ROS production and arginase up-regulation are two critical mechanisms for MDSCs in lal−/− mice.
Figure 8. Reverse of abnormal ROS production and arginase expression by hLAL in Tg/KO triple mice.
A. Myeloid-specific expression of hLAL reduced ROS production in spleen MDSCs. MDSCs were isolated from the spleen of lal+/+ mice (WT), lal−/− mice (KO), 3-month doxycycline-treated (Tg/KO ON) or -untreated (Tg/KO OFF) Tg/KO triple mice, n = 4. Cells were incubated with 2μm DCFDA. A representative of ROS concentration in CD11b+GR-1+ cells was presented by FACS analysis. Results from statistical analysis were mean ± SD from 3 mice in each groups (n=3). *P < 0.05; B. Myeloid-specific expression of hLAL reduced arginase expression in spleen MDSCs. Statistical analysis of arginase activity in CD11b+/GR-1+ cells from the spleen of lal+/+ mice (WT), lal−/− mice (KO), 3-month doxycycline-treated (Tg/KO ON) or -untreated (Tg/KO OFF) Tg/KO triple mice were presented. Results were mean ± SD from 4 mice in each groups (n=5). *P < 0.05.
hLAL expression reversed abnormal activation of Stat3 and NFκB in Tg/KO mice
The functional abnormality of MDSCs in lal−/− mice was also reflected at the activation levels of Stat3 and NFκB p65 signaling molecules as we previously reported (2). Abnormal activation of these molecules serves as hallmarks for MDSCs malformation and malfunction in multiple animal models (2, 11, 20–22). To test if activation of Stat3 and NFκB p65 is required for MDSCs differentiation, inhibitors were used to block these two pathways. First, Lin− bone marrow cells were isolated from lal−/− mice and cultured in vitro. Five days after GM-CSF and IL-4 stimulation in the presence or absence of Stat3 inhibitor cucurbitacin B, or NFkB inhibitor ammonium pyrrolidinedithiocarbamate (PDTC), CD11b+GR-1+ populations were analyzed by FACS. Both cucurbitacin B and PDTC inhibited MDSCs differentiation from Lin− bone marrow cells of lal−/− mice (Figure 9A). Next, in the CFSE labeling study, cucurbitacin B and PDTC reversed suppressive function of spleen MDSCs from lal−/− mice on wild type T cell proliferation (Figure 9B). They also reversed CD69 expression on wild type T cells (Figure 9C). Importantly, expression of hLAL in doxycycline-treated Tg/KO mice significantly reduced activation of Stat3 and NFκB p65 in MDSCs in various compartments compared with those in untreated mice (Figure 9D).
Figure 9. Stat3 and NFκB inhibitors blocked proliferation and function of MDSCs in lal−/− mice.
A. Lin− bone marrow cells were isolated from lal−/− mice. Cells were cultured with GM-CSF and IL-4 for 5 days in the presence of 2μm Cucurbitacin B or 5μm ammonium pyrrolidinedithiocarbamate (PDTC). Statistical analysis of total cell numbers of CD11b+GR-1+ cells were analyzed by FACS. Results were mean ± SD from 6 mice in each groups (n=6), *P < 0.05, **P < 0.01; B. MDSCs were isolated from the spleen of lal−/− mice (KO), and cocultured with 2μm Cucurbitacin B or 5μm PDTC for two days. CFSE-labeled wild type CD4+ T cells were co-cultured with MDSCs in vitro on anti-CD3 plus anti-CD28 antibodies coated plates for 4 days. The ratio between MDSC:CD4+ T cells was1:5. CFSE cell division of CD4+ T cells was measured by FACS. A representative histogram of 5 experiments is presented; C. CD69 expression on CD4+ T cells was measured by FACS in the above study. Results were mean ± SD from 6 mice in each groups, n=6, **P < 0.01; D. Cells from the bone marrow (BM), blood (PBMC) and spleen of lal+/+ mice (WT), lal−/− mice (KO), 3-month doxycycline-treated (Tg/KO ON) or -untreated (Tg/KO OFF) Tg/KO triple mice were stained with CD11b and GR-1 antibodies, followed by the intranuclear staining with pStat3 or pNFκB antibody. Percentages of pStat3 and pNFκB-positive cells from three independent experiments were analyzed by FACS, n = 3, *P< 0.05.
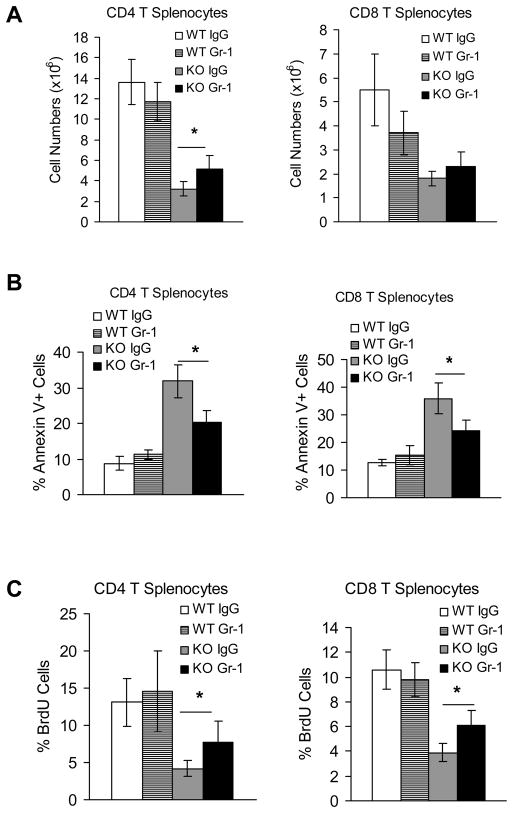
Gr-1 depletion reversed T cell proliferation and decreased T cell apoptosis in lal−/− mice
To address the specific effect of MDSCs on T cell proliferation, anti-Gr-1 antibody was injected into lal−/− mice to deplete MDSCs. After two weeks of injection, CD4+ T cell population was partially recovered (Figure 10A). This observation was associated with decreased apoptosis by Annexin V analysis (Figure 10B), and increased proliferation by BrdU analysis (Figure 10C). Even though there was no significant improvement in CD8+ T cell population, decreased apoptosis and increased proliferation were also observed (Figure 10B,C). This clearly proves that MDSCs is partially responsible of decrease of T cell population in lal−/− mice.
Figure 10. MDSCs depletion partially rescued T cell proliferation in lal−/− mice.
Wild type and lal−/− mice were i.p. injected with anti-mouse Gr-1 every 3 days for two weeks. A. Total CD4+ and CD8+ splenocytes from IgG or Gr-1-injected wild type and lal−/− mice (KO) were stained with CD4+ and CD8+ antibodies. Statistical analysis (total cell number) of CD4+ and CD8+ populations were performed by FACS. Results were mean ± SD from 4–5 mice in each groups (n=4–5). *P < 0.05; B. Apoptosis of CD4+ and CD8+ splenocytes were analyzed by Annexin V staining. Results were mean ± SD from 4–5 mice in each groups (n=4–5). *P < 0.05; C. Proliferation of CD4+ and CD8+ splenocytes were analyzed by BrdU staining. Results were mean ± SD from 4–5 mice in each groups (n=4–5). *P < 0.05.
Discussion
One major manifestation in lal−/− mice is the massive expansion and infiltration of myeloid cells into multiple organs, including the thymus and spleen (2). By contrast, T cell development, proliferation and function are dramatically decreased, leading to impairment of immune surveillance system (1). As a consequence, LAL deficiency results in severe pathogenic phenotypes in multiple organs as we previously reported (1–6). Based on these observations, we hypothesize that LAL in myeloid cells is required for sustaining normal myeloid cell formation and function in multiple steps in multiple compartments. To directly prove that LAL deficiency in myeloid cells is a causative factor for lal−/− pathogenesis, hLAL was specifically introduced into myeloid lineage cells in lal−/− mice by the myeloid-specific c-fms 7.2-kb promoter/intron2 DNA sequence. The rationale for this study is that LAL is a critical enzyme that controls neutral lipid metabolism. Downstream metabolic derivatives of these lipids serve as hormonal ligands for anti-inflammatory nuclear receptors (e.g. peroxisome proliferator-activated receptor-γ, or PPARγ) which belong to a special group of transcription factors. PPARγ is a negative modulator for multiple pro-inflammatory molecules (e.g. IL-6, IL-1β, TNFα, MMP12, Api6 etc) that stimulate MDSCs expansion through intracellular signaling molecule (e.g. Stat3) (21). LAL deficiency leads to insufficient ligands for PPARγ activation and promotes up-regulation of multiple inflammatory molecules. This has been confirmed by the Affymetrix GeneChip microarray study (6). Overexpression of several these PPARγ downstream genes (i.e. MMP12, Api6) causes MDSCs expansion and cancer in vivo (11, 20, 22). By reconstitution of LAL in lal−/− mice, it may restore ligand production that leads to inhibition of pro-inflammatory cytokines and limits MDSCs expansion to prevent T cell apoptosis.
In the bone marrow of lal−/− mice, LAL deficiency caused abnormal development of hematopoietic progenitor cells at multiple stages, including Lin− LSK, LK and GMP populations (Figure 2) (2). Myeloid-specific expression of hLAL corrected the abnormal increase of the GMP population in Tg/KO mice, but not LSK and LK populations (Figure 2). It is more likely that abnormal development of LSK and LK progenitor cells is due to the intrinsic defect. In lal−/− mice, differentiation from Lin− progenitor cells to CD11b+GR-1+ immature cells was abnormally increased. Myeloid hLAL expression successfully reversed this abnormality (Figure 3A). More systematic analysis showed myeloid hLAL expression reversed abnormal expansion of CD11b+GR-1+ MDSCs in the bone marrow, blood and spleen (Figure 2B). MDSCs can be divided into monocytic (CD11b+Ly6C+) and granulocytic MDSC (CD11b+, Ly6G+) (8). Interestingly, most gated CD11b+ cells showed Ly6C+ and Ly6G+ double positive in lal−/− mice (Figure 2C). Taken together, these results clearly support that LAL expression in myeloid lineage cells is critical for normal hematopoiesis and myelopoiesis.
MDSCs are a heterogeneous population of cells consisting of myeloid progenitor cells and immature myeloid cells. In healthy individuals, immature myeloid cells quickly differentiate into mature granulocytes, macrophages or dendritic cells (DCs). In pathological conditions, blockade of the differentiation of immature myeloid cells into mature myeloid cells results in the expansion of this population (8–10). The mechanisms responsible for this disruption are poorly understood. In lal−/− mice, abnormalities in myeloid cells were evident as Stat3 and NFκB intracellular signaling molecules were aberrantly activated (Figure 9D). Inhibition of both molecules by inhibitors resulted in reduced MDSC proliferation and suppressive function (Figure 9A–C). LAL deficiency blocked differentiation of immature myeloid cells to mature DCs (Figure 3C). Myeloid hLAL expression in Tg/KO mice rescued differentiation of immature myeloid cells to mature DCs and reduced abnormal activation of intracellular signaling molecules in MDSCs. Therefore, the LAL pathway in myeloid cells is essential for maintaining differentiation of immature cells to mature cells. Thus, MDSCs expansion in lal−/− mice results from both an abnormal increase in myelopoiesis in the bone marrow and inhibition of differentiation to mature myeloid cells.
Infiltration of MDSCs in the thymus and spleen of lal−/− mice implicates the interrelationship between MDSCs and T cells both in terms of differentiation and function. When MDSCs were depleted by anti-Gr-1 antibody injection, T cell numbers were recovered accordingly (Figure 10). This clearly shows the role of MDSCs in the maturation of T cells in lal−/− mice. LAL inhibits MDSCs suppressive functions to influence T cells at four critical steps. First, LAL expression in monocytic cells blocks MDSCs infiltration into the immune organs such as the thymus and spleen. Loss of the LAL function leads to massive infiltration of MDSCs into these organs, and causing structural disorganization in lal−/− mice (1). Myeloid hLAL expression significantly reduced MDSCs infiltration and partially restored organization of the thymus and spleen (Figure 4). Second, LAL deficiency leads to impaired T cell development in the thymus of lal−/− mice. Myeloid hLAL expression reversed abnormal T cell development in lal−/− thymus (Figure 5 and Supplemental Figure 1). Third, LAL deficiency leads to reduced T cell proliferation in the spleens of lal−/− mice. MDSCs in lal−/− mice generate abnormally high levels of immunomodulators (e.g. cytokines, ROS and arginase etc.) that are involved in suppressing T cell activity (Figure 3C, and Figure 8). Myeloid hLAL expression diminished these immunomodulators and increased T cell maturation and proliferation in lal−/− mice (Figure 6A-C and 7A,B). Fourth, LAL deficiency leads to substantial increase of CD4+FoxP3+ Treg cells in lal−/− mice. It is well known that Treg cells inhibit CD4+ T cell functions such as cytokine production and proliferation (23). Myeloid hLAL expression suppressed Treg cells in lal−/− mice (Figure 5C).
LAL deficiency-induced MDSCs expansion not only suppresses T cell proliferation, but also inhibits T cell cytokine production. T cell dysfunction in lal−/− mice is characterized by a failure to respond to TCR stimulation, loss of expression of TCR ξ chain and CD69, inactivation of the pZAP-70/Syk intracellular signaling, and lymphokine production. Myeloid hLAL expression enhanced T cell responses to TCR stimulation by restoring expression of TCR ξ chain and CD69, activation of T cell signaling, and lymphokine production (Figure 6, 7). Although MDSCs play an important role in T cell proliferation and function, intrinsic defect due to LAL deficiency in T cells may also contribute to T cell malformation and malfunction. This may explain why myeloid hLAL expression only partially corrects T cell pathogenic phenotypes.
MDSCs were originally identified due to their role in suppressing immunity to tumors. Studies in lal−/− mice suggest that MDSCs also contribute to other diseases in multiple organs. Myeloid hLAL expression significantly ameliorated pathogenic phenotypes in the liver, small intestine and lung of lal−/− organs as reported previously (7, 21). Yet, in general, LAL serves an anti-inflammatory function by negatively regulating pro-inflammatory cytokines/chemokines, matrix metalloproteinases (MMPs), apoptosis inhibitors and oncogenes through the anti-inflammatory PPARγ pathway (5, 6). When several of these genes (e.g. Api6 and MMP12) were over-expressed in myeloid cells or epithelial cells, they triggered regional or systemic MDSCs expansion, T cell suppression and cancer formation in transgenic mice (11, 20, 22). In summary, neutral lipid metabolism by LAL and it’s downstream pathways in myeloid cells are critical in controlling hematopoiesis, myelopoiesis, and lymphopoiesis.
Supplementary Material
Acknowledgments
We thank Lingyan Wu and Beilin Li for assisting animal work.
This study was supported by National Institutes of Health Grants HL087001 (H. Du), CA138759 (C. Yan), HL061803 and HL067862 (C. Yan and H. Du).
Abbreviation
- Ab
antibody
- CFSE
carboxyfluorescein diacetate succinimidyl diester
- CLP
common lymphoid progenitor
- FACS
Fluorescence activated cell sorting
- GMP
granulocyte-macrophage precursors
- KO
knock-out
- LAL
Lysosomal acid lipase
- MDSCs
myeloid-derived suppressor cells
- PDTC
ammonium pyrrolidinedithiocarbamate
- ROS
Reactive oxygen species
- TCR
T cell receptor
- Tg
transgenic
References
- 1.Qu P, Du H, Wilkes DS, Yan C. Critical roles of lysosomal acid lipase in T cell development and function. Am J Pathol. 2009;174:944–956. doi: 10.2353/ajpath.2009.080562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Qu P, Shelley WC, Yoder MC, Wu L, Du H, Yan C. Critical roles of lysosomal acid lipase in myelopoiesis. Am J Pathol. 2010;176:2394–2404. doi: 10.2353/ajpath.2010.091063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Du H, Duanmu M, Witte D, Grabowski GA. Targeted disruption of the mouse lysosomal acid lipase gene: long-term survival with massive cholesteryl ester and triglyceride storage. Hum Mol Genet. 1998;7:1347–1354. doi: 10.1093/hmg/7.9.1347. [DOI] [PubMed] [Google Scholar]
- 4.Du H, Heur M, Duanmu M, Grabowski GA, Hui DY, Witte DP, Mishra J. Lysosomal acid lipase-deficient mice: depletion of white and brown fat, severe hepatosplenomegaly, and shortened life span. J Lipid Res. 2001;42:489–500. [PubMed] [Google Scholar]
- 5.Lian X, Yan C, Yang L, Xu Y, Du H. Lysosomal acid lipase deficiency causes respiratory inflammation and destruction in the lung. Am J Physiol Lung Cell Mol Physiol. 2004;286:L801–807. doi: 10.1152/ajplung.00335.2003. [DOI] [PubMed] [Google Scholar]
- 6.Lian X, Yan C, Qin Y, Knox L, Li T, Du H. Neutral lipids and peroxisome proliferator-activated receptor-{gamma} control pulmonary gene expression and inflammation-triggered pathogenesis in lysosomal acid lipase knockout mice. Am J Pathol. 2005;167:813–821. doi: 10.1016/s0002-9440(10)62053-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yan C, Lian X, Li Y, Dai Y, White A, Qin Y, Li H, Hume DA, Du H. Macrophage-Specific Expression of Human Lysosomal Acid Lipase Corrects Inflammation and Pathogenic Phenotypes in lal−/− Mice. Am J Pathol. 2006;169:916–926. doi: 10.2353/ajpath.2006.051327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ostrand-Rosenberg S, Sinha P. Myeloid-derived suppressor cells: linking inflammation and cancer. J Immunol. 2009;182:4499–4506. doi: 10.4049/jimmunol.0802740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sica A, Bronte V. Altered macrophage differentiation and immune dysfunction in tumor development. J Clin Invest. 2007;117:1155–1166. doi: 10.1172/JCI31422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Qu P, Du H, Li Y, Yan C. Myeloid-specific expression of Api6/AIM/Sp alpha induces systemic inflammation and adenocarcinoma in the lung. J Immunol. 2009;182:1648–1659. doi: 10.4049/jimmunol.182.3.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weissman IL, Shizuru JA. The origins of the identification and isolation of hematopoietic stem cells, and their capability to induce donor-specific transplantation tolerance and treat autoimmune diseases. Blood. 2008;112:3543–3553. doi: 10.1182/blood-2008-08-078220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li Y, Du H, Qin Y, Roberts J, Cummings OW, Yan C. Activation of the signal transducers and activators of the transcription 3 pathway in alveolar epithelial cells induces inflammation and adenocarcinomas in mouse lung. Cancer Res. 2007;67:8494–8503. doi: 10.1158/0008-5472.CAN-07-0647. [DOI] [PubMed] [Google Scholar]
- 14.Corraliza IM, Campo ML, Soler G, Modolell M. Determination of arginase activity in macrophages: a micromethod. J Immunol Methods. 1994;174:231–235. doi: 10.1016/0022-1759(94)90027-2. [DOI] [PubMed] [Google Scholar]
- 15.Delano MJ, Scumpia PO, Weinstein JS, Coco D, Nagaraj S, Kelly-Scumpia KM, O’Malley KA, Wynn JL, Antonenko S, Al-Quran SZ, Swan R, Chung CS, Atkinson MA, Ramphal R, Gabrilovich DI, Reeves WH, Ayala A, Phillips J, Laface D, Heyworth PG, Clare-Salzler M, Moldawer LL. MyD88-dependent expansion of an immature GR-1(+)CD11b(+) population induces T cell suppression and Th2 polarization in sepsis. J Exp Med. 2007;204:1463–1474. doi: 10.1084/jem.20062602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang B, Pan PY, Li Q, Sato AI, Levy DE, Bromberg J, Divino CM, Chen SH. Gr-1+CD115+ immature myeloid suppressor cells mediate the development of tumor-induced T regulatory cells and T-cell anergy in tumor-bearing host. Cancer Res. 2006;66:1123–1131. doi: 10.1158/0008-5472.CAN-05-1299. [DOI] [PubMed] [Google Scholar]
- 17.Minguet S, Swamy M, Alarcon B, Luescher IF, Schamel WW. Full activation of the T cell receptor requires both clustering and conformational changes at CD3. Immunity. 2007;26:43–54. doi: 10.1016/j.immuni.2006.10.019. [DOI] [PubMed] [Google Scholar]
- 18.Condamine T, Gabrilovich DI. Molecular mechanisms regulating myeloid-derived suppressor cell differentiation and function. Trends Immunol. 32:19–25. doi: 10.1016/j.it.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rodriguez PC, Ochoa AC. Arginine regulation by myeloid derived suppressor cells and tolerance in cancer: mechanisms and therapeutic perspectives. Immunol Rev. 2008;222:180–191. doi: 10.1111/j.1600-065X.2008.00608.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Qu P, Yan C, Du H. Matrix metalloproteinase 12 overexpression in myeloid lineage cells plays a key role in modulating myelopoiesis, immune suppression, and lung tumorigenesis. Blood. 117:4476–4489. doi: 10.1182/blood-2010-07-298380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu L, Wang G, Qu P, Yan C, Du H. Overexpression of dominant negative peroxisome proliferator-activated receptor-gamma (PPARgamma) in alveolar type II epithelial cells causes inflammation and T-cell suppression in the lung. Am J Pathol. 178:2191–2204. doi: 10.1016/j.ajpath.2011.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Qu P, Du H, Wang X, Yan C. Matrix metalloproteinase 12 overexpression in lung epithelial cells plays a key role in emphysema to lung bronchioalveolar adenocarcinoma transition. Cancer Res. 2009;69:7252–7261. doi: 10.1158/0008-5472.CAN-09-0577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang HY, Wang RF. Regulatory T cells and cancer. Curr Opin Immunol. 2007;19:217–223. doi: 10.1016/j.coi.2007.02.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.