Abstract
Natural killer T (NKT) cells recognize glycolipid antigens presented by CD1d. These cells express an evolutionarily conserved, invariant T cell receptor (TCR), but the forces driving TCR conservation have remained uncertain. Here we show that NKT cells recognize diacylglycerol-containing glycolipids from Streptococcus pneumoniae, the leading cause of community-acquired pneumonia, and group B Streptococcus, which causes neonatal sepsis and meningitis. Furthermore, CD1d-dependent responses by NKT cells are required for activation and host protection. The glycolipid response was dependent on vaccenic acid, which is found at a low level in mammalian cells. Our results show how microbial lipids position the sugar for recognition by the invariant TCR, and most important, they extend the range of microbes recognized by this conserved TCR to several clinically important bacteria.
Natural Killer T (NKT) cells have fascinated immunologists because they have several unique features 1–5. For example, NKT cells are responsive to glycolipids presented by CD1d, a non-polymorphic major histocompatability complex (MHC) class I-like antigen-presenting molecule, as opposed to conventional T cells, which recognize peptide antigens. Furthermore, rather than the diverse antigen receptors expressed by most T cell populations, the majority of NKT cells express an invariant T cell antigen receptor (TCR) α chain formed by a Vα14-Jα18 rearrangement in mice and a Vα24-Jα18 rearrangement in humans. We therefore refer to mouse NKT cells expressing an invariant Vα14 TCR as Vα14i NKT cells, their human counterparts as Vα24i NKT cells, and collectively to this population as iNKT cells. Additionally, rodent and primate iNKT cells recognize the same antigens, and there is interspecies cross reactivity 6. This unusual degree of conservation of antigen recognition suggests that this specificity has a particularly important function.
Many reports have shown that iNKT cells participate in the response to microbial pathogens 1, 2, 5, 7. In some cases, iNKT cells were likely responding not to a microbial glycolipid, but instead they probably were activated by inflammatory cytokines acting alone 8 and/or with self-antigens presented by CD1d 2, 7, 9. By contrast, two types of bacteria were previously shown to have glycolipid antigens for the iNKT cell TCR, Sphingomonas species 10, 11 and Borrelia burgdorferi 12. Helicobacter pylori was also reported to have such antigens 13, although we could not confirm reactivity using synthetic or purified material (J.L.V., A.K., M.K., data not shown). Regardless of this discrepancy, none of these microbes causes lethal diseases, and therefore it is unlikely that they are major drivers for the conservation of the iNKT cell TCR specificity throughout much of mammalian evolution.
Because of this conservation, we reasoned that the iNKT cell TCR would recognize antigens of certain highly pathogenic bacteria. Worldwide, the most lethal bacterial pathogen is Streptococcus pneumoniae (SPN), an agent of pneumonia, bloodstream infections and meningitis in children and the elderly, now estimated to cause 11% of all deaths in children from 1 month – 5 years of age 14. Notably, Vα14i NKT cell deficient Jα18−/− mice challenged with SPN have a dramatic impairment in bacterial clearance from the lung and greatly reduced survival 15. The mechanism is related in part to Interferon-γ (IFN-γ) derived from Vα14i NKT cells, which facilitates bacterial clearance by stimulating Tumor necrosis factor (TNF) and production of the chemokine MIP-2, leading to enhanced neutrophil recruitment to the lung 16.
Here we show CD1d-dependent activation of Vα14i NKT cells in vivo following SPN infection, strongly implicating antigen-dependent activation of these cells. Furthermore, we identified the unique structures of the glycolipids from SPN and another Gram-positive pathogen, group B Streptococcus (GBS), which are recognized by the iNKT cell TCR. Our data demonstrate a requirement in these glycolipid antigens for a fatty acid that is infrequent in mammalian cells. Additionally, we determined the unique binding mode of these antigens to mouse CD1d by solving the crystal structure of the antigen-CD1d complex. We propose that iNKT cell TCR is a particularly useful and conserved specificity in part because it recognizes glycolipids from important pathogens that cause invasive rapid and potentially lethal infections.
Results
Vα14i NKT cells produce IFN-γ after infection
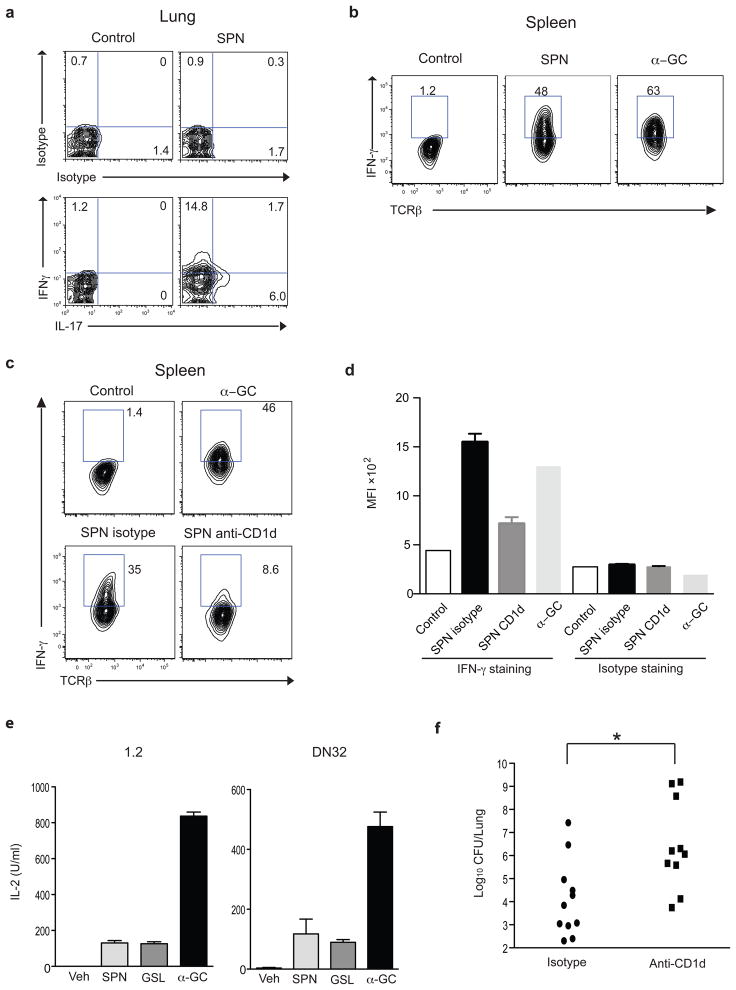
The protective effect of Vα14i NKT cells after SPN infection is dependent upon the ability of liver mononuclear cells (MNC) to produce IFN-γ 16, but several liver cell types can produce IFN-γ, and its production by activated Vα14i NKT cells was not directly demonstrated. We therefore tested if Vα14i NKT cells synthesize cytokines after intratracheal SPN infection. Thirteen hours after SPN infection, lung MNC were stained with α̃GalCer loaded CD1d tetramers, which specifically detect iNKT cells, and the stained cells were analyzed for intracellular cytokines, directly ex vivo, without re-stimulation or brefeldin A treatment. A substantial percentage of Vα14i NKT cells in lung had intracellular IFN-γ or IL-17 at 13h after infection (Fig. 1a and Supplementary Fig. 1a). Intracellular IFN-γ was also observed in Vα14i NKT cells in the spleen at 6h after intravenous (iv) infection with SPN (Fig. 1b and Supplementary Fig. 1b), although under these circumstances intracellular IL-17 was not detectable (data not shown). Similarly, intracellular IFN-γ was also observed for Vα14i NKT cells in the liver after systemic infection (data not shown).
Figure 1. CD1d-dependent cytokine production by Vα14i NKT cells.
(a) Expression of intracellular IFN-γ and IL-17 by α̃GalCer loaded CD1d tetramer+ CD19- lung MNC measured 13h after intratracheal SPN infection. (b) Expression of intracellular IFN-γ by tetramer+ CD19− spleen cells at 6h after i, v, SPN infection. (c, d) Expression of intracellular IFN-γ by tetramer+ CD19− spleen cells at 6h after intravenous SPN infection in mice treated with an anti-CD1d Ab or isotype control. α̃GalCer was administered 1.5 hrs before tissue harvest. Representative data from cells combined from at least five mice (a) or from a control and a α̃GalCer injected mouse and one of three (SPN) mice (b–c) are shown. Similar results were obtained from at least two experiments. (e) Stimulation of Vα14i NKT cell hybridoma clones 1.2 and DN32 with CD11c+ cells from spleen of mice infected 16h earlier. IL-2 was measured by ELISA. Each bar shows mean ± SEM from triplicate wells. Representative data from two independent experiments are shown. (f) Lung CFU of mice treated with either anti-CD1d Ab or isotype control IgG at 3 days after SPN infection. Each symbol represents an individual mouse. Combined data from two independent experiments are shown. *; p<0.05 (Mann-Whitney test).
Vα14i NKT cells can be activated by cytokines, particularly IL-12, even in the absence of CD1d antigen presentation and TCR engagement 8, 9, 17. Therefore, we sought evidence that a TCR-dependent response was contributing to the activation of the Vα14i NKT cells after infection. To do this, we injected a blocking anti-CD1d antibody into infected mice. The percentage of IFN-γ positive tetramer+ cells was dramatically decreased by blocking CD1d (Fig. 1c, d and Supplementary Figure 1c), implicating TCR recognition in the activation of the Vα14i NKT cells in vivo in the early phases of SPN infection. To confirm that an antigen that engages the Vα14i NKT cell TCR is formed after infection, we purified CD11c+ cells from spleen of SPN infected mice. These antigen-presenting cells (APCs) were then analyzed for their ability to activate Vα14i NKT cell hybridomas for IL-2 release, a response that is dependent only on TCR engagement. Although Vα14i NKT cell hybridomas are not responsive to IL-12 or LPS (data not shown), we found that APC stimulated Vα14i NKT cell hybridomas IL-2 release, and they were as effective as APC that were pre-loaded with the a synthetic glycosphingolipid antigen from Sphingomonas bacteria (Fig.1e). In order to determine if CD1d-dependent Vα14i NKT cell activation also plays a role in clearance of bacteria, mice that were treated with either anti-CD1d mAb or isotype control were infected intratracheally with SPN, and bacterial numbers in the lungs were determined at 3 days. As shown in Fig. 1f, bacterial colonies were significantly increased by injection of an anti-CD1d mAb. In total, these data suggest that SPN infection of DC in vivo leads to the generation of an antigen that can stimulate the TCR of Vα14i NKT cells and that antigen recognition is important in the clearance of SPN.
Vα14i NKT cell hybridomas respond to bacterial sonicates
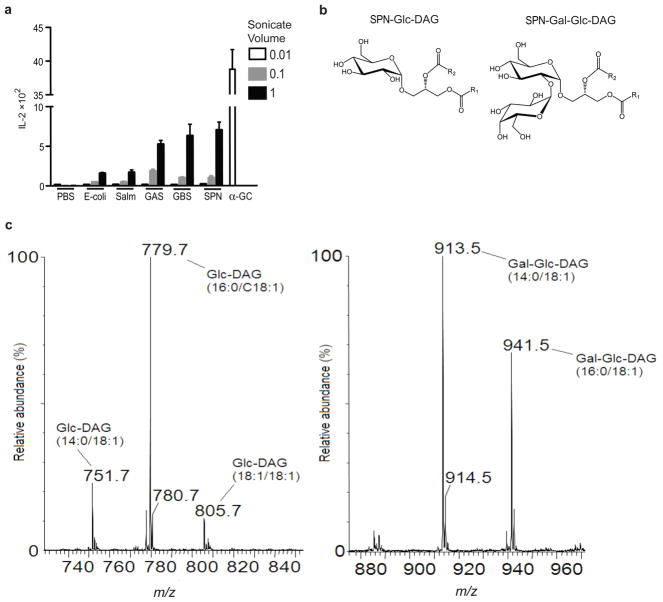
Because iNKT cells can be activated by either self or foreign antigens 2, we determined if SPN contain compounds that can stimulate the iNKT cell TCR. We prepared sonicates from a SPN clinical isolate whose clearance was impaired in Vα14i NKT cell-deficient mice 15. These bacterial sonicates were incubated in microwell plates coated with soluble mouse CD1d molecules. Dose-dependent IL-2 responses to the SPN sonicate were observed using two Vα14i NKT cell hybridomas with different Vβ8.2 rearrangements (Fig. 2a, Supplementary Fig. 2a) and the response was inhibited by an anti-CD1d mAb (Supplementary Fig. 2b). A CD1d reactive hybridoma that does not bear the Vα14i TCR, and which recognizes different glycolipids 18, did not respond to the either bacterial sonicate (Supplementary Fig. 2c).
Figure 2. Structure of SPN glycolipids.
(a) Sonicates of GAS (group A Streptococcus), GBS (group B Streptococcus), SPN (S. pneumoniae) and of two Gram-negative bacteria, E. coli and Salm (Salmonella typhimurium), PBS or α̃GalCer (α̃GC: 5ng/well) were tested in an APC-free assay with Vα14i NKT cell hybridoma 1.2. IL-2 in the culture supernatant was measured by ELISA. The amount of sonicates 0.01, 0.1 and 1 was equivalent to 106, 107, 108 bacteria/well, respectively. Each bar shows mean ± SEM from triplicate wells. (b) Structure of two glycolipids from SPN. One with a single glucose α linked to DAG is SPN glucosyl (Glc) diacylglycerol (DAG) (SPN-Glc-DAG). A second glycolipid contains a disaccharide attached to DAG, with galactose (Gal) α1→2 linked to the glucose sugar (SPN-Gal-Glc-DAG). (c) Electro-spray ionization mass spectrometry analysis of glycolipids with the fatty acid compositions shown in the brackets: SPN-Glc-DAG (left) and SPN-Gal-Glc-DAG (right).
The SPN sonicates were compared to ones prepared from several other bacteria including important Gram-positive pathogens such as group A Streptococcus (GAS), which is estimated to cause over 500 million cases of pharyngitis and 600,000 invasive infections annually worldwide 19, and group B Streptococcus (GBS), the leading cause of life-threatening bacterial infections such as sepsis and meningitis in human newborns 20. When tested in the CD1d coated plate assay, sonicates of GAS and GBS also reproducibly induced IL-2 release from Vα14i NKT cell hybridomas. Escherichia coli and Salmonella typhimurium are widely believed not to have glycolipid antigens for iNKT cells 11, 17, and they gave much weaker responses, although in some assays E. coli sonicates were completely negative (Fig. 2a, and data not shown). Based on these results, we cannot exclude the possibility that there is a weak antigen in S. typhimurium and perhaps in E. coli as well, but clearly these sonicates had a reduced amount of stimulatory activity.
Structure of microbial glycolipids
Crude lipid extracts were prepared from a panel of Gram-positive strains of clinical origin, including SPN strains of serotypes 3, 12 and 17, a GBS serotype IA strain, and a strain of the Gram-positive zoonotic pathogen Streptococcus suis. Lipids from these bacteria were fractioned as described previously 21 and analyzed by electrospray mass spectrometry (ESMS), one- and two-dimensional nuclear magnetic resonance (NMR), and by gas chromatography-mass spectrometry (GCMS). Further details of the analysis of these materials are included as supplementary information (Supplementary Figs. 3–7). Two major fractions of SPN glycolipids were detected, one with a single glucose (Glc) sugar α-linked to diacylglycerol (1,2-di-O-acyl-(α-glucopyranosyl)-(1→3)-glycerol). We abbreviate this as SPN glucosyl (Glc) diacylglycerol (DAG) or SPN-Glc-DAG (Fig. 2b). The second fraction is identical except it contains a disaccharide moiety attached to the DAG, with galactose (Gal) α1→2 linked to the glucose sugar, giving (α-galactopyranosyl)-(1→2)-( α-glucopyranosyl)-(1→3)-glycerol). We abbreviate this as SPN-Gal-Glc-DAG (Fig. 2b). The analysis of SPN-Glc-DAG by ESMS showed two major fatty acids: hexadecanoic (C16) and octadecenoic acid (C18:1) (Fig. 2c). This composition was confirmed by GCMS analysis of fatty acid methyl esters (data not shown). The same fatty acids were found in disaccharide-containing SPN-Gal-Glc-DAG, but this glycolipid also had a significant amount of tetradecanoic acid (C14). Interestingly, the structure of the octadecenoic acid was identified as cis-vaccenic (octadecen-11-oic acid or C18:1(n-7)), which has an unsaturated bond between the 11–12 carbons (Supplementary Fig. 5a). Oleic acid, the more common C18:1 fatty acid in mammalian cells 22, also found in the Borrelia DAG antigen, has a cis unsaturated bond between the 9 and 10 carbons (C18:1 (n-9)). The GBS diacylglycerol glycolipids did not differ from those obtained from SPN, including the presence of vaccenic acid. One of the major purified glycolipids had an α-linked glucose monosaccharide, which we designated as GBS-Glc-DAG, to indicate its strain origin. As in SPN, the other major chemical species has a disaccharide, but with two glucose sugars (Glc α1→2 Glc). This compound, (α-glucopyranosyl)-(1→2)-( α-glucopyranosyl)-(1→3)-glycerol), we abbreviate as GBS-Glc-Glc-DAG. Finally, the DAG glycolipid from S. suis has a monosaccharide with α-linked mannose (Man) (SSu-Man-DAG). It is noteworthy that glucosylated DAG glycolipids are not found only in pathogens. We also analyzed the glycolipid content from a Gram positive commensal organism, Lactobacillus casei (L. casei), which had similar DAG glycolipids with α-linked glucose (data not shown).
Microbial glycolipids stimulate Vα14i NKT cell hybridomas
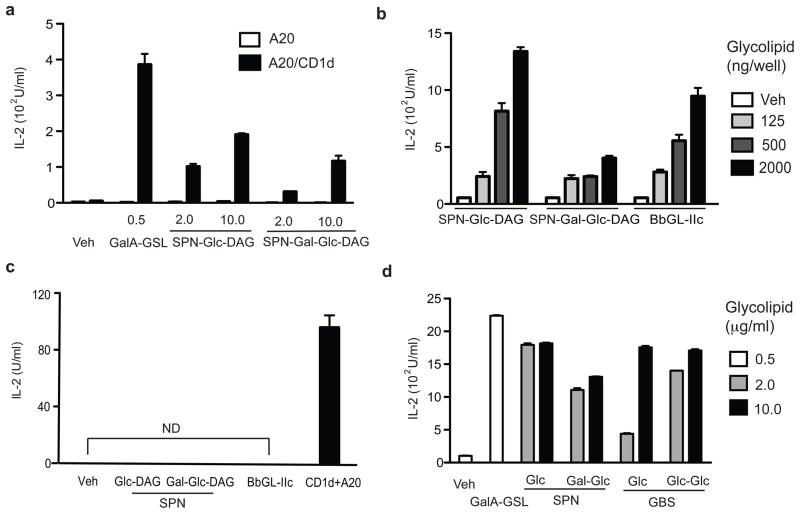
To determine if the purified glycolipids can stimulate iNKT cells, we cultured Vα14i NKT cell hybridomas with CD1d transfectants of A20 B lymphoma cells. CD1d+ cells incubated with SPN-Glc-DAG or SPN-Gal-Glc-DAG induced CD1d-dependent IL-2 release from the hybridomas (Fig. 3a and Supplementary Fig. 8a). To confirm that the purified SPN glycolipids could stimulate the invariant TCR of Vα14i NKT cells, we also tested them in the CD1d coated plate assay. SPN glycolipids stimulated IL-2 release from all four Vα14i NKT cell hybridomas tested when added to CD1d-coated plates (Fig. 3b, Supplementary Fig. 8b, and data not shown). In the coated plate assay, the disaccharide-containing SPN-Gal-Glc-DAG stimulated weaker responses (Fig. 3b). Based on previous work 23, we predicted these compounds might require lysosomal processing to generate a stimulatory monosaccharide antigen. The reduced responses to SPN-Gal-Glc-DAG are consistent with this prediction, and the residual response could be due to contaminating monosaccharide. In agreement with the results from the whole bacterial sonicates, none of these purified glycolipids stimulated two CD1d reactive but non-Vα14i NKT cell hybridomas (Fig. 3c and data not shown), demonstrating specific activation of T cells expressing the invariant TCR. Furthermore, GBS-Glc-DAG, GBS- Glc-Glc-DAG, and the commensal-derived Lactobacillis casei-Glc-DAG also stimulated Vα14i NKT cell hybridomas when cultured with CD1d transfected A20 cells that had been pulsed with these compounds (Fig. 3d, Supplementary Figs. 9a, 9b).
Figure 3. Microbial glycolipids stimulate Vα14i NKT cells in vitro.
(a, b) CD1d-dependent stimulation of Vα14i NKT cell hybridomas by SPN-Glc-DAG and SPN-Gal-Glc-DAG. (a) Cells from Vα14i NKT cell hybridoma 1.2 were cultured with A20 cells (APC) or mouse CD1d transfected A20 cells (A20-CD1d) pulsed with SPN-Glc-DAG (Glc-DAG), SPN-Gal-Glc-DAG (Gal-Glc-DAG) or Sphingomonas GalA-GSL at the indicated concentrations (μg/ml). IL-2 release was measured 20h. (b) The indicated concentrations (ng/well) of SPN-Glc-DAG, SPN-Gal-Glc-DAG or B. burgdorferi glycolipid BbGL-IIc were incubated in wells coated with mouse CD1d and 1.2 hybridoma cells were cultured in the wells for 20h before measuring IL-2 release. (c) Non-Vα14 expressing but CD1d reactive 19 hybridoma cells did not respond to microbial glycolipids (2000 ng/well) in wells coated with CD1d. The 19 hybridoma cells responded to a self-antigen presented by mouse CD1d transfected A20 cells by releasing IL-2. ND; not detected. (d) Two glycolipids from GBS stimulate Vα14i NKT cells. 1.2 hybridoma cells were cultured with A20-CD1d cells that had been pulsed with GBS glycolipids Glc-DAG (Glc) or Glc-Glc-DAG (Glc-Glc) at the indicated concentrations (μg/ml). Each bar shows mean ± SEM from triplicate wells. Representative data from at least two (c) or three (a, b, d) experiments are shown.
Previously it was shown that glycosphingolipid (GSL) antigens containing α-linked glucose and galactose are antigenic, while those containing α-linked mannose were not 24. The DAG lipid from S. suis containing α-linked mannose was not antigenic (Supplementary Fig. 9c). These data on DAG antigens suggest that the recognition of α-linked sugars in the DAG bacterial antigens is similar to the well-characterized recognition of the α-linked carbohydrates in GSLs. Consistent with this, our recent elucidation of the trimolecular structures of the invariant TCR bound to complexes of mouse CD1d with a Sphingomonas GSL and a Borrelia DAG antigen indicate a similar binding mode for the TCR 25.
Glycolipids stimulate Vα14i NKT cells in vivo
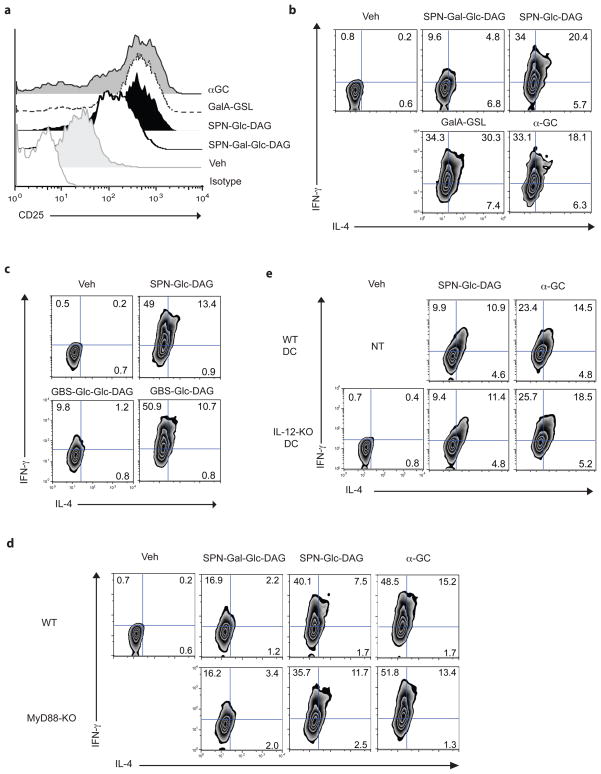
We tested if purified SPN glycolipids could stimulate Vα14i NKT cells in vivo. Bone marrow derived dendritic cells (DCs) that had been pulsed with SPN-Glc-DAG, the disaccharide SPN-Gal-Glc-DAG, or a synthetic version of the natural Sphingomonas galacturonic acid (GalA)-containing GSL 10 were transferred into C57BL/6 mice, and 14h later, activation of Vα14i NKT cells in liver and spleen was analyzed. As a positive control, we transferred DCs pulsed with α-galactosyl ceramide (α̃GalCer), the highly potent synthetic GSL antigen similar to the Sphingomonas antigens 10, 11. Cells staining with α̃GalCer loaded CD1d tetramers were analyzed for expression of activation markers by flow cytometry. The expression of CD25 and CD69 on CD1d tetramer positive cells was increased in mice treated with DCs pulsed with SPN-Glc-DAG compared to mice treated with vehicle pulsed DCs (Fig. 4a and Supplementary Fig. 10a). SPN-Gal-Glc-DAG also could induce increased expression of CD25 and CD69, although to a lesser extent (Fig. 4a and Supplementary Fig. 10a). SPN-Glc-DAG induced intracellular IFN- γ expression by the majority Vα14i NKT cells when they were analyzed directly ex vivo, equivalent to that induced by α̃GalCer (Fig. 4b), indicating that most Vα14i NKT cells respond to this antigen. In order to obtain an optimal response, it should be noted that higher amounts of SPN-Glc-DAG were incubated with the DCs compared to α̃GalCer. Intracellular IL-4 and TNF were also observed in activated liver Vα14i NKT cells, albeit on a smaller percentage of cells, but IL-17 was not detected (Supplementary Fig. 10b, 10c). Decreased cytokine responses were observed with the disaccharide compound (Fig. 4b), in accord with the reduced induction of activation marker expression. Purified glycolipid from GBS induced a similar response in vivo, reflecting its essentially identical structure (Fig. 4c and Supplementary Fig. 11).
Figure 4. In vivo stimulation of Vα14i NKT cells by purified glycolipids.
(a, b) SPN-Glc-DAG and SPN-Gal-Glc-DAG stimulate expression of CD25 (a) and intracellular cytokines (b) by tetramer+ Vα14i NKT cells. Liver MNC were analyzed 14h after transfer of DCs that had been pulsed with SPN-Gal-Glc-DAG, SPN-Glc-DAG, sphingomonas GalA-GSL or α̃GalCer (20, 20, 10 or 0.1 μg/ml, respectively). (c) Expression of intracellular cytokines by tetramer+ liver MNC at 14h after transfer of DCs that had been pulsed with vehicle (veh), SPN-Glc-DAG, or GBS glycolipids GBS-Glc- DAG or GBS-Glc-Glc-DAG (20 μg/ml). (d) Intracellular IFN-γ and IL-4 expression byα̃GalCer loaded CD1d tetramer positive liver MNC of WT or Myd88−/− mice at 14h after transfer of Myd88−/− -Triflps2/lps2 DCs pulsed with the indicated purified S. pneumoniae antigens (20 μg/ml) or positive control antigen α̃GalCer (α̃GC, 0.1 μg/ml). (e) Expression of intracellular cytokines by tetramer+ liver MNC measured at 4h after transfer into Il12p35−/− mice of Il12p35−/− or WT DCs that had been pulsed with SPN-Glc-DAG, or α̃GalCer (20 or 0.1 μg/ml, respectively). (a–e) Representative data from one of 2 (α̃GalCer in d) or 3 (other panels) mice are shown. Similar results were obtained from at least two independent experiments.
iNKT cells can be activated in the absence of foreign glycolipid antigens by either endogenous antigen(s) presented by CD1d and/or by inflammatory cytokines, such as IL-12, which are produced by APC stimulated with Toll like receptor (TLR) ligands 8, 17. At a relatively early time, 4 h after transfer of Sp-Glc-DAG pulsed DCs into mice, many of the cytokine producing Vα14i NKT cells were double-positive for intracellular IFN-γ andIL-4 (Supplementary Fig. 12a, b) when analyzed immediately ex vivo. This is consistent with TCR-mediated activation of these cells, because IL-4 production has not been observed in Vα14i NKT cells that were only stimulated indirectly by inflammatory cytokines, such as IL-12 8, 17. To confirm that TLR-mediated activation of APC is not required for the activation of Vα14i NKT cells induced by SPN glycolipids, DCs from TLR signaling-defective Myd88−/−-Triflps2/lps2 mice that had been pulsed with SPN-Glc- DAG or SPN-Gal-Glc-DAG were transferred into Myd88−/− mice or wild-type (WT) mice, and the Vα14i NKT cells were stained for intracellular cytokines. DCs from Myd88−/−-Triflps2/lps2 were able to stimulate equal amounts of Vα14i NKT cell production of IFN-γ and IL-4 when responding cells were analyzed from recipient Myd88−/− mice or WT mice (Fig. 4d and Supplementary Fig. 13). Furthermore, the magnitude of the response when the injected DC were deficient for TLR signaling was similar to the response obtained when both donor DCs and recipient mice were wild-type. Consistent with this, IL-12 deficient DCs that had been pulsed with SPN-Glc-DAG also were still capable of inducing cytokines from Vα14i NKT cells when transferred to IL-12 deficient mice (Fig. 4e and Supplementary Fig. 14a, b). Therefore the in vivo activation of Vα14i NKT cells by the purified glycolipids is due to TCR-dependent activation by microbial glycolipid antigens and does not require activation of the innate immune response and IL-12. While activation of Vα14i NKT cells by glycolipid-pulsed APCs does not depend on IL-12, activation following SPN infection does. In agreement with recent work 9, the induction of CD69 expression on Vα14i NKT cells was not different between WT and IL-12 deficient mice after SPN infection, however IFN-γ production by Vα14i NKT cells was significantly lower in Il2p35−/− mice (Supplementary Fig. 15).
Synthetic GlcDAG antigens stimulate iNKT cells
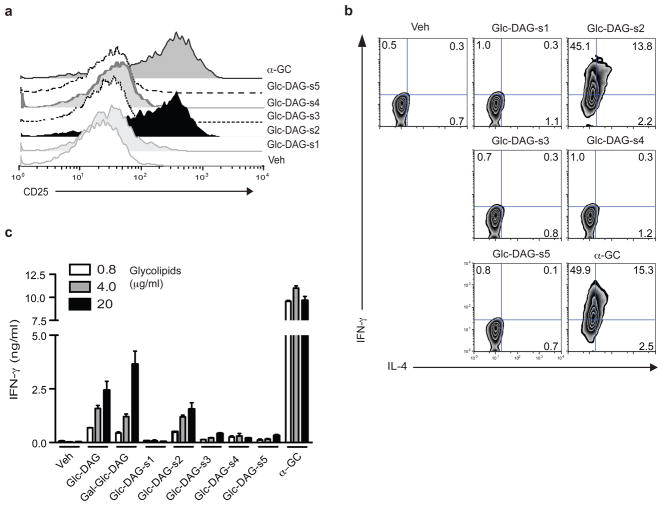
We tested synthetic compounds to verify the identity of the purified material that activated iNKT cells. We synthesized versions of SPN-Glc-DAG containing vaccenic acid in the sn-1 position (Glc-DAG-s1), the sn-2 position (Glc-DAG-s2), which reflects the structure of the natural antigen, or both positions (Glc-DAG-s3), with hexadecanoic acid in the remaining position for Glc-DAG-s1 and – s2 (Table 1).
Table 1.
Fatty acids in synthetic variants of Glc-DAG
| Glycoplipd | R1 | R2 |
|---|---|---|
| Glc-DAG-s1 | C18:1(n-7) | C16:0 |
| Glc-DAG-s2 | C16:0 | C18:1(n-7) |
| Glc-DAG-s3 | C18:1(n-7) | C18:1(n-7) |
| Glc-DAG-s4 | C18:1(n-9) | C16:0 |
| Glc-DAG-s5 | C16:0 | C18:1(n-9) |
To further assess the importance of vaccenic acid, we also tested two compounds with a C18:1 oleic acid. In the hybridoma stimulation assay with CD1d transfected APC, the synthetic version of the naturally occurring antigen, Glc-DAG-s2, was the only one that induced strong IL-2 release (Supplementary Fig. 16a). Compounds with vaccenic acid in the sn-1 position, or linked to both the sn-1 and sn-2 glycerol positions, were ineffective, as were compounds with oleic acid. To test the response of Vα14i NKT cells in vivo, bone marrow DCs were loaded with each of the synthetic compounds, injected into mice, and cells from the recipients were analyzed directly ex vivo, as was done with the purified material. A similar degree of selectivity was observed in this in vivo stimulation assay, as only Glc-DAG-s2 induced surface upregulation of the activation markers CD25 (Fig. 5a) and CD69 (Supplementary Fig. 16b), and intracellular cytokine (Fig. 5b and Supplementary Fig. 16c).
Figure 5. Stringent requirement for vaccenic acid for stimulating iNKT cells.
(a, b) Expression of CD25 (a) and intracellular cytokines (b) by tetramer+ liver MNC measured at 14h after transfer of DCs that had been pulsed with vehicle (veh), synthetic variants of Glc-DAG (20 μg/ml) or α̃GalCer (0.1 μg/ml). Representative data from one of at least three mice are shown. Similar results were obtained from two experiments. (c) Human Vα24i NKT cells recognize purified and synthetic SPN glycolipids. Vα24i NKT cell lines were cultured with human CD1d transfected Hela cells for 24h in the presence of the indicated purified (Glc-DAG and Gal-Glc-DAG) or synthetic glycolipids (μg/ml). Each bar shows mean ± SD from triplicate wells. Representative data from one of five Vα24i NKT cell lines tested are shown.
In accord with the results indicating the purified glycolipid did not activate the innate immune system, synthetic Glc-DAG-s2 did not stimulate bone marrow-derived dendritic cells (BMDCs) to increase surface expression of CD1d and costimulatory molecules, including CD40 and CD80, whereas LPS induced the upregulation of these molecules (Supplementary Fig. 17a). Also, APCs pulsed with Glc-DAG-s2 did not increase the autoreactivity of CD1d reactive hybridoma that does not express the invariant TCR (Supplementary Fig. 17b). Furthermore, as for the purified compounds, cytokine release was not dependent on IL-12 secretion, corroborating the requirement for TCR-dependent activation (data not shown). LPS did cause a modest increase in reactivity of the hybridoma lacking the invariant TCR (Supplementary Fig. 17b), although it did not for a Vα14i NKT cell hybridoma. We attribute this increase to augmented CD1d expression, although increased synthesis of the self-antigen for this cell is also possible.
Mouse and human iNKT cells tend to recognize the same glycolipids presented by CD1d 1, 2, 6, 7, although differences in the requirement for particular fatty acids in the B. burgdorferi DAG antigens have been reported 12, 26. We therefore tested several human Vα24i NKT cell lines, which had been expanded in α̃GalCer and IL-2, for reactivity to the purified and synthetic Glc-DAG glycolipids. Vα24i NKT cells secreted IFN-γ when cultured with the purified material, and like mouse Vα14i NKT cells, they responded selectively to Glc-DAG-s2, with vaccenic acid in the sn-2 position (Fig. 5c). Similar results were obtained when four other Vα24i NKT cell lines were tested (data not shown) or when IL-4 release was measured (Supplementary Fig. 18).
The SPN glycolipid has a different CD1d binding mode
Although the α-glucosyl ceramide isomer of α̃GalCer differs from α̃GalCer only by the orientation of the 4′ hydroxyl group of the hexose sugar, it is a weaker antigen 24 with TCR affinity reduced approximately 10-fold 27, 28. Despite this, the glucose-containing antigens, such as Glc-DAG-s2, were approximately as potent as the galactose-containing B. burgdorferi DAG antigen BbGL-IIc, working slightly better on CD1d coated plates (Fig. 3, Supplementary Fig. 8b) but slightly weaker using APCs (Supplementary Figs. 9b and, 16a). A compound containing an α-linked glucose sugar was not antigenic, however, when linked to the same fatty acids as those in the galactose-containing B. burgdorferi antigen, with C18:1 oleic acid in the sn-1 position and C16:0 palmitic acid in sn-2 (Glc-DAG-s4, Fig. 5 and Supplementary Figs. 16, 18). Furthermore, a galactose containing DAG antigens with the same SPN fatty acids were less potent stimulators of iNKT cells (data not shown). Our data therefore indicate that there is an intricate interplay between the lipid and sugar, with stringent requirements for both in determining antigenic potency, at least for the DAG-containing glycolipid antigens.
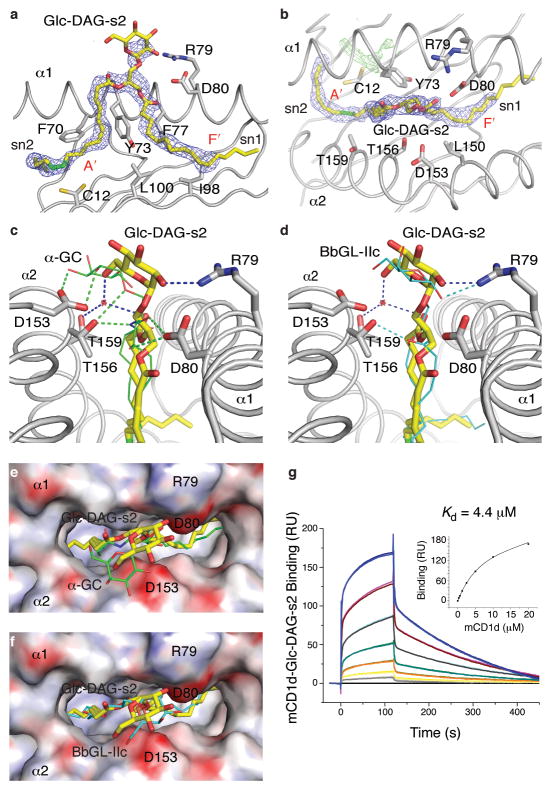
Considering the requirements for a glucose sugar and an unusual fatty acid, in order to understand the basis for the activity of Glc-DAG-s2, we determined the crystal structure of the complex of Glc-DAG-s2 with mouse CD1d at a resolution of 1.7Å (Fig. 6 and Supplementary online material). The data show that the uncommon vaccenic acid at the sn-2 position of Glc-DAG-s2 is bound in the A’ pocket of mouse CD1d, encircling the A’ pole in a clockwise orientation. This is in contrast to most of the other CD1d-glycolipid structures, which have a counterclockwise orientation of a hydrophobic chain in the A’ pocket 29, 30. The sn-1 linked palmitic acid is bound in the F’ pocket while leaving the sn-3 linkedα-anomeric glucose exposed for T cell recognition (Fig. 6a, b). An opposite binding orientation, with the sn1 and sn2 chains in the A’ and F’ pockets respectively, is not supported by the electron density in the region of the glycerol moiety and the polar head-group (Fig. 6a). However, poor electron density is observed at the end of the sn1 chain, most likely a result of the different conformations the acyl chain can adopt in this portion of the F’ pocket, as described for several other mouse CD1d ligands 30. The binding mode of Glc-DAG-s2, which is likely influenced by the position of the cis-unsaturation of the vaccenic acid, differs sharply from the binding of the other known bacterial DAG antigen, B. burgdorferi BbGL-IIc, which has the sn-2 fatty acid in the F’ pocket and the sn-1 C18:1 oleic acid wound in a counter clockwise direction in the A’ pocket 26.
Figure 6. Crystal structure of the mouse CD1d-Glc-DAG-s2 complex.
(a) Conformation of Glc-DAG-s2 in the binding groove. A side view with the α2 helix removed for clarity and the 2Fo-Fc electron density for the ligand (1σ) as a blue mesh. The vaccenic acid unsaturation is in green. (b) Top view of CD1d with the Glc-DAG-s2 ligand in yellow (sugar removed for clarity) and the corresponding 2Fo-Fc electron density in blue, while additional, unmodeled electron density (Fo-Fc map at 3σ in green) is visible at the bottom of the A’ pocket. (c and d) Comparison of the hydrogen-bond network between CD1d and the ligands: (c) Glc-DAG-s2 yellow, α̃GalCer green, PDB code 1Z5L or (d) Glc-DAG-s2 yellow, BbGL-IIc cyan, PDB code 3ILQ. Potential hydrogen bonds shown as dashed lines, blue for Glc-DAG-s2, green for α̃GalCer or cyan for BbGL-IIc. (e and f) Top view onto the molecular surface of the CD1d binding pocket with its electrostatic potentials depicted. The Glc-DAG-s2 ligand is yellow, α̃GalCer in green (e) and BbGL-IIc in cyan (f). (g) Binding response of mouse CD1d loaded with Glc-DAG-s2 to an immobilized Vα14Vβ8.2 TCR as measured by surface plasmon resonance. Binding of increasing concentrations (0.3125-20μM) of the CD1d-DAG antigen complex is shown.
Interestingly, the position of the glucose head group in Glc-DAG-s2 mouse CD1d complexes is considerably different compared to the galactose in the α̃GalCer complexes with mouse CD1d (Fig. 6c), although it does resemble the position of the galactose in the Borrelia antigen BbGL-IIc. Key contacts between Asp153 and the 2′- and 3′-OH groups of the galactose of α̃GalCer are not conserved, but similar to other DAG antigens, instead the sugar of Glc-DAG-s2 sits slightly more upright in the binding groove and farther from Asp153 (Fig. 6c), likely positioned in this way as a result of its different lipid backbone. A 60° counterclockwise rotation of the glucose compared to α̃GalCer, when viewed from on top of the carbohydrate, brings the 2′-OH of the glucose in proximity to Arg79, where it forms the only hydrogen-bond interaction directly with a mouse CD1d amino acid. A further water-mediated hydrogen bond is formed between the 6′ OH of glucose and the backbone oxygen of the sn-2 linked fatty acid with Thr159 of mouse CD1d. Overall, these few interactions between the glucose and mouse CD1d gives rise to only a weak electron density for the glucose head group, suggesting a more flexible and dynamic binding for Glc-DAG-s2. Furthermore, from the perspective of the TCR, the glucose moiety is shifted away from Asp153 and toward the α helix amino acid Arg79, sitting more to the center of the binding groove and similar to BbGL-IIc, in contrast to α̃GalCer binding to mCD1d, where the galactose is in more intimate contact with Asp153 of the α2 helix (Fig. 6c–f). The position of the hexose sugar should be more unfavorable for the TCR in the case of a galactose sugar in α linkage to the same S. pneumoniae DAG lipid, in which case the 4′ OH in the axial position would be tilted even more from the optimal position.
Avid TCR binding to SPN complexes with mouse CD1d
Surface plasmon resonance binding studies, using a refolded Vα14i NKT cell TCR, support the notion that the glucose sugar for this class of antigens gives rise to a relatively strong antigenic response comparable to the Borrelia antigens with galactose sugars. The equilibrium binding constant (Kd) for Glc-DAG-s2 is 4.4±0.4μM, slightly better than that of the galactose containing BbGL-IIc, which is 6.2μM 26. Compared to Borrelia BbGL-IIc, the TCR binding is slower (ka=1.38±0.06×103M−1s−1), while the dissociation is also much slower (kd=6.05±0.3×10−3s−1) (Figure 6g). We conclude that the unique hydrophobic chains of the SPN antigens contribute to the TCR epitope, despite being buried in the CD1d groove, because the orientation of lipid binding to the two pockets of mouse CD1d defines the position of the exposed sugar. Additionally, the changed orientation of the sugar likely permits glucose-containing antigens to be preferred over galactose, unlike in either GSL antigens with ceramide lipids, or in the B. burgdorferi DAG antigens with C18:1 oleic acids.
Discussion
Here we show that the TCR expressed by mouse and human iNKT cells recognizes unique glycolipid antigens from SPN and GBS, which are among the most serious and widespread bacterial pathogens. Importantly, the iNKT cell response to these glycolipids is conserved in humans. Previous work demonstrated that Vα14i NKT cells are important for host protection 15, and we now demonstrate that Vα14i NKT cells produced IFN-γ and IL-17 rapidly in vivo in the lung after SPN infection. This in vivo cytokine synthesis was greatly reduced by treatment with an anti-CD1d mAb, and APC from infected mice could activate Vα14i NKT cell hybridomas to produce IL-2, a response that requires TCR engagement. Together, these results strongly suggest that iNKT cells produce cytokines in vivo after SPN infection due to recognition of an antigen(s) presented by CD1d. Additionally, in agreement with the increased susceptibility of Jα18−/− 15 and Cd1d−/− 9 mice to SPN infection, treatment with an anti-CD1d mAb increased the SPN colony count in the lung. Therefore, these data suggest that not only is Vα14i NKT cell cytokine production in vivo dependent on TCR engagement, but also, so are the host protective effects of Vα14i NKT cell activation.
We cannot exclude the possibility, however, that some of the CD1d-dependent in vivo activation of iNKT cells is due to self-antigens presented by CD1d. A recent paper provided evidence suggesting that the predominant response of Vα14i NKT cells to bacterial infections, including SPN, is stimulated by IL-12 from activated APC, leading to the secretion of IFN-γ, but not IL-4, by the Vα14i NKT cells 9. Microbes known to have antigens and those probably lacking one, gave similar responses. This led to the suggestion that antigen-independent or self-antigen dependent responses are likely to be dominant over foreign antigen responses. IL-12 also can synergize with responses to relatively weak foreign antigens, and DAG antigens are more than two orders of magnitude weaker than α̃GalCer 26. We also found that the in vivo response to SPN was dominated by IFN-γ, although we detected IL-17 synthesis by lung Vα14i NKT cells, likely reflecting the increased presence of Vα14i NKT cells committed to IL-17 production there 31. This is potentially important, because of the reported role for IL-17 in the host response to SPN 32.
It should be possible to distinguish the relative contributions of self- and foreign antigens to Vα14i NKT cell activation by removing the expression of either one. However, the structure of the predominant self-antigens remains controversial, and some recent results suggest that they may be diverse 33. On the microbial side, results from targeted mutagenesis in SPN of the gene for the enzyme that links the glucose sugar to DAG suggest that inactivation of this gene is a lethal mutation. Similarly, GBS strains that are mutant for genes involved in unsaturated fatty acid synthesis required supplementation with more than one type of fatty acid for their growth, which also apparently allow the formation of antigens (J.L.V., S.D., S.U., V.N., unpublished data). Therefore although we cannot unambiguously distinguish the contributions of self and microbial antigens to CD1d-mediated Vα14i iNKT cell activation in vivo, it is highly likely that the antigens defined here, which have micromolar affinity for the invariant TCR once bound to CD1d, do make a contribution to Vα14i NKT cell cytokine production.
Previous studies have identified glycosylated diacyl glycerol antigens in S. pneumoniae 9, 21 although the complete structures, and their antigenic activity, were not tested 9. The bacterial DAG lipid antigens we have defined have an unusual sn-2 linked fatty acid. Because the aliphatic hydrocarbon chains are buried in the groove, one might suppose their structure is largely irrelevant to determining antigenic potency. Our data demonstrate, however, that not only does the exposed sugar contribute to activation, but also that the microbial lipid also makes a critical contribution. We found that the position of a single unsaturated bond in vaccenic acid linked to the sn-2 position of the glycerol is an important feature for defining the potency of these glucose-containing antigens when compared to several closely related synthetic variants. Interestingly, vaccenic acid is uncommon in mammalian cells 22. The carbohydrate portion, linked to the sn-3 position of DAG, is also of interest. It is either a glucose monosaccharide, or a disaccharide with glucose linked to the DAG moiety. It was surprising that glucose is preferred as the sugar linked to DAG over galactose, because this was not true in the context of other glycolipid antigens. We propose that because of the presence of vaccenic acid positioning the DAG antigen, SPN Glc-DAG-s2 is presented by mouse CD1d in a fashion more tilted up away from CD1d and toward the TCR, so that the initial TCR interaction with a galactose-containing antigen, which contains an axial 4”-OH, may be disfavored. This is consistent with the slower TCR association we found for binding to Glc-DAG-s2 complexes with CD1d. It is highly likely, however, that on binding the antigen-CD1d complex, the TCR will flatten the orientation of the Glc-DAG-s2 sugar in order to maintain the conserved binding mode found for other antigens25,28,33. Regardless of the mechanism of antigen recognition, our work establishes that there is interplay between the lipid, sugar and CD1d in forming an epitope, with stringent requirements for both the lipid and sugar structures. These stringent requirements suggest that a microbe could avoid iNKT cell activation through subtle changes in biosynthesis of either glycolipid component.
The ability of the iNKT cell TCR to recognize diverse antigens in a conserved manner has been referred to as pattern recognition 34. The original concept of pattern recognition referred to microbial associated molecular pattern (MAMP), i.e. a structural feature of fundamental importance to microbes not found in the responding mammalian host 35. We propose that the invariant TCR expressed by iNKT cells recognizes a new type of MAMP, defined by a hexose sugar α-linked to a lipid, usually one with two hydrophobic tails, such as in ceramide or DAG. Furthermore, the abundance of these antigens, and the likely requirement for microbial viability, demonstrate their fundamental importance, similar to other MAMPs.
In summary, we report for the first time that the invariant TCR expressed by iNKT cells can recognize glycolipids from clinically important pathogens with worldwide distributions that cause invasive diseases with high lethality in the absence of antibiotic therapy. The specificity of these responses is conserved between mice and humans, and in mice the CD1d-presented antigens are required for Vα14i NKT cell activation and host protection. Therefore, we propose that the invariant Vα TCR is a particularly useful one, and evolutionarily conserved, in part because of its capability to recognize a set of widely distributed glycolipids that are an essential part of a number of microbes, including pathogens.
Protein Data Bank accession number
The mCD1d-Glc-DAG-s2 structure; 3T1F
Methods
Reagents
α̃GalCer and GM-CSF were kindly provided by Kyowa Hakko Kirin Co., Ltd. (Tokyo, Japan). The Sphingomonas galacturonic acid (GalA) containing glycosphingolipid (GalA-GSL) and B. burgdorferi glycolipid BbGL-IIc were synthesized as described previously 12, 36. Antibodies for staining included CD19 (1D3), CD25 (3C7), CD44 (IM7), CD69 (H1.2F3), IL-4 (BVD4-1D11), TCRβ (H57-597), IFN-γ (XMG1.2), TNF-α (MP6-XT22) and IL-17a (TX11–18H10)
Mice
C57BL/6 mice and IL-12p35−/− mice on the C57BL/6 background were from the Jackson Laboratory. MyD88−/− mice 37 and MyD88−/−-TrifLps2/Lps2 mice 38 on the C57BL/6 background were gifts of Drs. Shizuo Akira (Osaka University) and Bruce Beutler (Scripps Research Institute), respectively. All mice were housed under specific pathogen free conditions and the experiments were approved by the Institutional Animal Care and Use Committees of the La Jolla Institute of Allergy & Immunology and the National Institute of Infectious Diseases, Japan.
Bacterial strains
To prepare sonicates for immune assays, bacterial strains Streptococcus pneumoniae URF918 (clinical isolate, serotype 3) 15, D39, Group B Streptococcus COH, Group A Streptococcus M1, Salmonella typhimurium, and E. coli MC-1061 were used. Bacterial sonicates were generated by addition of 70% ethanol then washing the bacteria three times with phosphate buffered saline (PBS), followed by re-suspension in PBS at a concentration equivalent to 109 CFU/ml before use. For preparation and analysis of glycolipids, bacterial strains Streptococcus pneumoniae R6 39, Streptococcus agalactiae A909 (courtesy of Dr. M. Antony, University of Birmingham, UK), Streptococcus agalactiae (NCIMB LTD, 701346), Streptococcus suis (NCIMB LTD, 702644), two Group B streptococcal clinical strains belonging to signature types 12 and 17, and Lactobacillus casei (ATCC 393) were used. For glycolipid analysis, bacteria were grown in brain heart infusion broth (Oxoid) or on agar (Oxoid) supplemented with 5% (v/v) defibrinated horse blood at 37°C. Lactobacillus were grown for 16h at 37°C in a MRS broth.
SPN infection
S. pneumoniae URF918 were cultured in Todd-Hewitt broth (BD) at 37°C in a 5%CO2 incubator, harvested at a mid-log phase and then washed twice in PBS. To induce pulmonary infection, mice were anesthetized with isoflurane and restrained on a small board. S. pneumoniae (1 × 106-1 × 107 CFU) were inoculated at 50 μl per mouse by insertion of a 24-gauge catheter into the trachea. To induce systemic infection, S. pneumoniae (1 × 107 CFU) were intravenously injected into mice. For CD1d blocking, mice were treated (i.p.) with 200 μg of anti-CD1d Ab (1B1) or rat IgG2b isotype control 6–24h before and just before infection. At 6h (spleen) or 13 h (lung), lung MNC or spleen cells were collected as previously reported 15, 40, and α̃GalCer loaded CD1d tetramer+ CD19− cells were analyzed directly ex vivo for intracellular cytokines without restimulation or Brefeldin pre-treatment. For measurement of lung CFU, tissues were collected at day 3 after infection, and homogenized in PBS by teasing with a stainless steel mesh. The homogenates were inoculated at 100 μl on 5% sheep blood Mueller-Hinton agar plates and cultured for18 h, followed by colony counting. For isolation of CD11c+ cells, spleens were collected at 16–18h after S. pneumoniae infection or injection of GalA–GSL (20 μg) or α̃GalCer (1μg), and CD11+ cells were purified from spleen cells with CD11c positive selection kit (Stemcell technologies) according to the company’s instruction. The CD11c+ cells (1×105) were cultured with CD1d reactive hybridomas (5×104) for 16–18h, and the supernatants were measured for IL-2 by ELISA (BD Bioscience)
Lipid extraction and purification from bacteria
For lipid purification, bacteria were grown to late exponential phase, and harvested by centrifugation at 1,800 g for 15 min. Lipids were extracted from washed cells and purified as described 41. The lipid extract was examined by TLC on aluminum-backed plates of silica gel 60 F254 (Merck 5554), using CHCl3, CH3OH, H2O (65:25:4, v/v/v). Glycolipids were visualized by spraying the plates with α-naphthol/sulfuric acid followed by gentle charring of plates. Other types of lipids were visualized by spraying with 5% ethanolic molybdophosphoric acid and charring, or by using a Dittmer and Lester reagent that is specific for phospholipids. Lactobacillus lipids were fractionated on a column of DEAE-cellulose. The chloroform-methanol (2;1, v/v) fraction was collected and concentrated.
Cell-free antigen presentation assay
CD1d-reactive hybridomas have been described previously 10, 12. Stimulation of T cell hybridomas on CD1d coated plates was carried out according to published protocols 10, 12, 40. Briefly, the indicated amount of bacterial sonicates or compounds were incubated for 24 h in microwells coated with 1.0 μg of mouse CD1d. After washing, between 5×104 and 1×105 Vα14i NKT cell hybridomas or controls were cultured in the plates for 16–20 h, and IL-2 in the supernatant was measured by ELISA (BD PharMingen).
In vivo responses to microbial glycolipids and flow cytometry
Analyses of activation marker expression and intracellular cytokine production by α̃GalCer-CD1d tetramer+ cells were carried out according to published protocols 10. Mouse DCs were prepared by culturing bone marrow progenitor cells with mouse GM-CSF for 7 days. Mouse DCs were incubated with SPN glycolipids, GBS glycolipids, GalA-GSL or α̃GalCer (20, 20, 10 or 0.1 μg/ml, respectively) for 24 h. After washing with PBS, glycolipid-pulsed DCs (5×105) were injected i.v. into mice. α̃GalCer-CD1d tetramer + liver mononuclear cells were analyzed directly ex vivo 4h or 14 h later for activation markers and for intracellular cytokines. Cells were analyzed by using a FACSCalibur or LSRII (BD Bioscience) instrument with FlowJo software.
Vα24i NKT cell response
Human Vα24i NKT cell lines were generated with modifications to a previous protocol 10. Vα24i+ T cells were isolated from leukopaks using magnetic beads (Miltenyi Biotec) coupled to an anti-Vα24 mAb, and were cultured with irradiated (3,000 Rads) autologous immature DCs in the presence of 100 ng/ml α̃GalCer and 10 IU/ml human recombinant IL-2 (R&D Systems) for 10 days. After a second stimulation with α̃GalCer-pulsed irradiated autologous immature DCs, cell lines were 95% Vα24+. Thirty thousand Vα24i NKT cells were cultured with 3×104 irradiated (10,000 Rads) HeLa cells transfected with human CD1d in the presence of glycolipids. The concentrations of IFN-γ or IL-4 in the supernatants were determined by ELISA (eBioscience) after 24 h.
Supplementary Material
Acknowledgments
This work was supported by NIH grants AI45053, AI71922 (MK), AI074952 (DMZ), AI070258 (MT), F32AI083029 (JLV), grants from the Japan Society for the Promotion of Science and MEXT (22689031), from the Ministry of Health, Labor and Welfare of Japan (H22seisakusouyakuippan012), and the Uehara Memorial Foundation (YK), grants from the Wellcome Trust, the Royal Society-Wolfson Research Merit Award, and from Mr. James Bardrick (GSB), and an investigator award from the Cancer Research Institute (DMZ). We thank Drs. Natsuo Yamamoto, Takeshi Kinjo, Gary D Ainge, Darren Gibson, and Gavin Painter for helpful suggestions, Dr. Noriko Sato for help with glycolipid analysis and Chris Lena for technical assistance.
Footnotes
The authors declare no competing financial interests.
Author contributions
Y.K. and M.K. designed the study, except D.M.Z. designed the crystal structure study and the Biacore assay. Y.K., P.I., J.L.V., E.G., V.N., D.M.Z., and M.K. prepared the manuscript. Y.K., J.L.V. and B.P. performed most of the immunology experiments. P.I., K.K. and A. G.-V., performed the analysis of bacterial glycolipids. P.I., M.I. and C-H.W. synthesized glycolipids. G.B.S. provided informational support. E.G., Y.L. and D.M.Z. determined the crystal structure of the CD1d-Glc-DAG-s2 complex and performed the Biacore assay. X.L. P.R. and M.T. performed the human NKT cell experiments. Y.K., J.L.V., Y.K., A.O., Y.M. and K.K. performed SPN infection experiments. S.D., S.U. and V.N. prepared bacterial sonicates and provided advice on bacterial culture and infection. A.K. made the mouse CD1d protein. H.Y. and P.W.A. prepared bacteria for glycolipid analysis. M.K. provided overall supervision.
References
- 1.Taniguchi M, Harada M, Kojo S, Nakayama T, Wakao H. The regulatory role of Valpha14 NKT cells in innate and acquired immune response. Annu Rev Immunol. 2003;21:483–513. doi: 10.1146/annurev.immunol.21.120601.141057. [DOI] [PubMed] [Google Scholar]
- 2.Brigl M, Brenner MB. CD1: Antigen Presentation and T Cell Function. Annu Rev Immunol. 2004;22:817–890. doi: 10.1146/annurev.immunol.22.012703.104608. [DOI] [PubMed] [Google Scholar]
- 3.Godfrey DI, Berzins SP. Control points in NKT-cell development. Nat Rev Immunol. 2007;7:505–518. doi: 10.1038/nri2116. [DOI] [PubMed] [Google Scholar]
- 4.Bendelac A, Savage PB, Teyton L. The biology of NKT cells. Annu Rev Immunol. 2007;25:297–336. doi: 10.1146/annurev.immunol.25.022106.141711. [DOI] [PubMed] [Google Scholar]
- 5.Cerundolo V, Silk JD, Masri SH, Salio M. Harnessing invariant NKT cells in vaccination strategies. Nat Rev Immunol. 2009;9:28–38. doi: 10.1038/nri2451. [DOI] [PubMed] [Google Scholar]
- 6.Brossay L, et al. CD1d-mediated recognition of an alpha-galactosylceramide by natural killer T cells is highly conserved through mammalian evolution. J Exp Med. 1998;188:1521–1528. doi: 10.1084/jem.188.8.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kronenberg M, Kinjo Y. Innate-like recognition of microbes by invariant natural killer T cells. Curr Opin Immunol. 2009;21:391–396. doi: 10.1016/j.coi.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nagarajan NA, Kronenberg M. Invariant NKT cells amplify the innate immune response to lipopolysaccharide. J Immunol. 2007;178:2706–2713. doi: 10.4049/jimmunol.178.5.2706. [DOI] [PubMed] [Google Scholar]
- 9.Brigl M, et al. Innate and cytokine-driven signals, rather than microbial antigens, dominate in natural killer T cell activation during microbial infection. J Exp Med. 2011;208:1163–1177. doi: 10.1084/jem.20102555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kinjo Y, et al. Recognition of bacterial glycosphingolipids by natural killer T cells. Nature. 2005;434:520–525. doi: 10.1038/nature03407. [DOI] [PubMed] [Google Scholar]
- 11.Mattner J, et al. Exogenous and endogenous glycolipid antigens activate NKT cells during microbial infections. Nature. 2005;434:525–529. doi: 10.1038/nature03408. [DOI] [PubMed] [Google Scholar]
- 12.Kinjo Y, et al. Natural killer T cells recognize diacylglycerol antigens from pathogenic bacteria. Nat Immunol. 2006;7:978–986. doi: 10.1038/ni1380. [DOI] [PubMed] [Google Scholar]
- 13.Chang YJ, et al. Influenza infection in suckling mice expands an NKT cell subset that protects against airway hyperreactivity. J Clin Invest. 2011;121:57–69. doi: 10.1172/JCI44845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O’Brien KL, et al. Burden of disease caused by Streptococcus pneumoniae in children younger than 5 years: global estimates. Lancet. 2009;374:893–902. doi: 10.1016/S0140-6736(09)61204-6. [DOI] [PubMed] [Google Scholar]
- 15.Kawakami K, et al. Critical role of Valpha14+ natural killer T cells in the innate phase of host protection against Streptococcus pneumoniae infection. Eur J Immunol. 2003;33:3322–3330. doi: 10.1002/eji.200324254. [DOI] [PubMed] [Google Scholar]
- 16.Nakamatsu M, et al. Role of interferon-gamma in Valpha14+ natural killer T cell-mediated host defense against Streptococcus pneumoniae infection in murine lungs. Microbes Infect. 2007;9:364–374. doi: 10.1016/j.micinf.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 17.Brigl M, Bry L, Kent SC, Gumperz JE, Brenner MB. Mechanism of CD1d-restricted natural killer T cell activation during microbial infection. Nat Immunol. 2003;4:1230–1237. doi: 10.1038/ni1002. [DOI] [PubMed] [Google Scholar]
- 18.Cardell S, et al. CD1-restricted CD4+ T cells in major histocompatibility complex class II-deficient mice. J Exp Med. 1995;182:993–1004. doi: 10.1084/jem.182.4.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carapetis JR, Steer AC, Mulholland EK, Weber M. The global burden of group A streptococcal diseases. Lancet Infect Dis. 2005;5:685–694. doi: 10.1016/S1473-3099(05)70267-X. [DOI] [PubMed] [Google Scholar]
- 20.Schrag S, Gorwitz R, Fultz-Butts K, Schuchat A. Prevention of perinatal group B streptococcal disease. Revised guidelines from CDC. MMWR Recomm Rep. 2002;51:1–22. [PubMed] [Google Scholar]
- 21.Brundish DE, Shaw N, Baddiley J. The glycolipids from the non-capsulated strain of Pneumococcus I-192R, A.T.C.C. 12213. Biochem J. 1965;97:158–165. doi: 10.1042/bj0970158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nakamura T, et al. Serum fatty acid levels, dietary style and coronary heart disease in three neighbouring areas in Japan: the Kumihama study. Br J Nutr. 2003;89:267–272. doi: 10.1079/BJN2002747. [DOI] [PubMed] [Google Scholar]
- 23.Prigozy TI, et al. Glycolipid antigen processing for presentation by CD1d molecules. Science. 2001;291:664–667. doi: 10.1126/science.291.5504.664. [DOI] [PubMed] [Google Scholar]
- 24.Kawano T, et al. CD1d-restricted and TCR-mediated activation of valpha14 NKT cells by glycosylceramides. Science. 1997;278:1626–1629. doi: 10.1126/science.278.5343.1626. [DOI] [PubMed] [Google Scholar]
- 25.Li Y, et al. The Valpha14 invariant natural killer T cell TCR forces microbial glycolipids and CD1d into a conserved binding mode. J Exp Med. 2010;207:2383–2393. doi: 10.1084/jem.20101335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang J, et al. Lipid binding orientation within CD1d affects recognition of Borrelia burgorferi antigens by NKT cells. Proc Natl Acad Sci U S A. 2010;107:1535–1540. doi: 10.1073/pnas.0909479107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sidobre S, et al. The T cell antigen receptor expressed by Valpha14i NKT cells has a unique mode of glycosphingolipid antigen recognition. Proc Natl Acad Sci U S A. 2004;101:12254–12259. doi: 10.1073/pnas.0404632101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wun KS, et al. A molecular basis for the exquisite CD1d-restricted antigen specificity and functional responses of natural killer T cells. Immunity. 2011;34:327–339. doi: 10.1016/j.immuni.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zajonc DM, Kronenberg M. CD1 mediated T cell recognition of glycolipids. Curr Opin Struct Biol. 2007;17:521–529. doi: 10.1016/j.sbi.2007.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zajonc DM, Wilson IA. Architecture of CD1 proteins. Curr Top Microbiol Immunol. 2007;314:27–50. doi: 10.1007/978-3-540-69511-0_2. [DOI] [PubMed] [Google Scholar]
- 31.Michel ML, et al. Identification of an IL-17-producing NK1.1(neg) iNKT cell population involved in airway neutrophilia. J Exp Med. 2007;204:995–1001. doi: 10.1084/jem.20061551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lu YJ, et al. Interleukin-17A mediates acquired immunity to pneumococcal colonization. PLoS Pathog. 2008;4:e1000159. doi: 10.1371/journal.ppat.1000159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mallevaey T, et al. A molecular basis for NKT cell recognition of CD1d-self-antigen. Immunity. 2011;34:315–326. doi: 10.1016/j.immuni.2011.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Scott-Browne JP, et al. Germline-encoded recognition of diverse glycolipids by natural killer T cells. Nat Immunol. 2007;8:1105–1113. doi: 10.1038/ni1510. [DOI] [PubMed] [Google Scholar]
- 35.Medzhitov R, Janeway CA., Jr Innate immunity: the virtues of a nonclonal system of recognition. Cell. 1997;91:295–298. doi: 10.1016/s0092-8674(00)80412-2. [DOI] [PubMed] [Google Scholar]
- 36.Wu D, et al. Bacterial glycolipids and analogs as antigens for CD1d-restricted NKT cells. Proc Natl Acad Sci U S A. 2005;102:1351–1356. doi: 10.1073/pnas.0408696102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Adachi O, et al. Targeted disruption of the MyD88 gene results in loss of IL-1-and IL-18-mediated function. Immunity. 1998;9:143–150. doi: 10.1016/s1074-7613(00)80596-8. [DOI] [PubMed] [Google Scholar]
- 38.Hoebe K, et al. Identification of Lps2 as a key transducer of MyD88-independent TIR signalling. Nature. 2003;424:743–748. doi: 10.1038/nature01889. [DOI] [PubMed] [Google Scholar]
- 39.Pearce BJ, Iannelli F, Pozzi G. Construction of new unencapsulated (rough) strains of Streptococcus pneumoniae. Res Microbiol. 2002;153:243–247. doi: 10.1016/s0923-2508(02)01312-8. [DOI] [PubMed] [Google Scholar]
- 40.Tupin E, Kronenberg M. Activation of natural killer T cells by glycolipids. Methods Enzymol. 2006;417:185–201. doi: 10.1016/S0076-6879(06)17014-7. [DOI] [PubMed] [Google Scholar]
- 41.Fischer W. The polar lipids of group B Streptococci. I. Glucosylated diphosphatidylglycerol, a novel glycopholipid. Biochim Biophys Acta. 1977;487:74–88. doi: 10.1016/0005-2760(77)90045-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.