Abstract
Alcoholism is a devastating condition that represents a progression from initial alcohol use to dependence. Although most individuals are capable of consuming alcohol in a limited fashion, the development of alcohol dependence in a subset of individuals is often associated with negative emotional states (including anxiety and depression). Since the alleviation of this negative motivational state via excessive alcohol consumption often becomes a central goal of alcoholics, the transition from initial use to dependence is postulated to be associated with a transition from positive to negative reinforcement mechanisms. Vasopressin is a neuropeptide known to potentiate the effects of CRF on the HPA axis, and emerging evidence also suggests a role for centrally located vasopressin acting on V1b receptors in the regulation of stress- and anxiety-like behaviors in rodents. The present study determined state-dependent alterations in vasopressin/V1bR signaling in an animal model of ethanol dependence. The V1bR antagonist SSR149415 dose-dependently reduced excessive levels of ethanol self-administration observed in dependent animals without affecting the limited levels of ethanol drinking in non-dependent animals. Ethanol self-administration reduced V1b receptor levels in the basolateral amygdala of non-dependent animals, a neuroadaptation that could theoretically facilitate the positive reinforcing effects of alcohol. In contrast, V1bR levels were seemingly restored in ethanol-dependent rats, a switch that may in part underlie a transition from positive to negative reinforcement mechanisms with dependence. Together, our data suggest a key role for vasopressin/V1bR signaling in the transition to ethanol dependence.
Keywords: Amygdala, Ethanol Dependence, Vasopressin
Introduction
In the United States, approximately 51% of people over the age of 12 consume alcohol (120 million), and of these current users, 7.7% (18 million) have met the criteria for Substance Abuse or Dependence (Substance Abuse and Mental Health Services Administration, 2008). Thus, while most people consume alcohol recreationally for its positive rewarding effects, some individuals go on to develop problem drinking. The gradual development of excessive alcohol use is typically associated with the recruitment of a negative emotional state, including the emergence of anxiety and depression symptoms (Koob and Le Moal, 1997; Kreek and Koob, 1998; Edwards and Koob, 2010). To study this condition, preclinical models that are able to distinguish and describe neurobiological differences between dependent and non-dependent groups of animals are invaluable in understanding the important transition from initial to habitual drug use (Koob et al., 2004; Kalivas, 2005). In rodents, recreational alcohol drinking is often represented via limited access operant ethanol self-administration, where drinking tends to remain controlled over time, as is seen in non-dependent (recreational) human drinkers. Excessive, compulsive drinking can be produced in this model by the induction of dependence using experimental titration of blood alcohol levels (BALs) following chronic exposure to ethanol vapors. Use of this paradigm has characterized pharmacological agents that are capable of reducing drinking in both non-dependent and dependent groups (e.g., naltrexone; Walker and Koob, 2008), or selectively in dependent animals (e.g., corticotropin-releasing factor (CRF) receptor antagonism; Funk et al., 2007; Richardson et al., 2008). These studies have led to the specific hypothesis that a recruitment and potentiation of brain stress neurotransmission is responsible for driving a negative motivational state supporting ethanol dependence (Gilpin and Koob, 2008).
Several emerging lines of evidence have implicated the neuropeptide vasopressin in the pathophysiology of stress-related behaviors (Surget and Belzung, 2008; Chrousos, 2009). Vasopressin is a nine-amino acid peptide first known to be synthesized in the hypothalamus and transported to the posterior pituitary (Brownstein et al., 1980). Here, it is released into the bloodstream after appropriate stimulation (dehydration) to act on the kidneys for the purposes of water retention, acting on V2 vasopressin receptors (Kaufmann et al., 2000). Vasopressin-synthesizing neurons are also centrally localized (Buijs, 1978; Buijs et al., 1983; De Vries and Buijs, 1983), originating within the paraventricular nucleus (PVN), bed nucleus of the stria terminalis (BNST), medial amygdala (MeA) and suprachiasmatic nucleus (SCN). Vasopressin cell bodies project extensively throughout the limbic system (Veinante and Freund-Mercier, 1997) and play a significant role in regulating various complex behaviors, including aggression, social affiliation, and sexual pair bonding. Its facilitatory role in limbic system plasticity is thought to underlie various aspects of affective processing (Caldwell et al., 2008), and early studies established a specific role for central vasopressin in aversive learning and memory mechanisms (de Wied and Versteeg, 1979; Koob and Bloom, 1982; Le Moal et al., 1984; Engelmann et al., 1996). More recently, the generation of small molecule, receptor subtype-selective antagonists has greatly advanced the characterization of the complex behavior regulated by the two major central vasopressin receptor subtypes: V1a and V1b. While V1a receptors are believed to regulate social behavior and aggression, V1b receptors are thought to play a role in generalized affective-like behaviors, as demonstrated by the anxiolytic- and antidepressant-like effects of the small molecule V1b vasopressin receptor antagonist SSR149415 (nelivaptan, (2S,4R)-1-[(3R)-5-chloro-1-(2,4-dimethoxyphenyl)sulfonyl-3-(2-methoxyphenyl)-2-oxo-indolin-3-yl]-4-hydroxy-N,N-dimethyl-pyrrolidine-2-carboxamide; Griebel et al., 2002). Studies utilizing regional microinjections of SSR149415 have established a role for stress- and/or anxiety-like V1bR signaling in the basolateral/central/medial amygdala (Salome et al., 2006), the lateral septum (Stemmelin et al., 2005), and the dorsal hippocampus (Engin and Treit, 2008).
A role for vasopressin/V1bR signaling in drug dependence was recently hypothesized (Koob, 2008; Zhou et al., 2010). Vasopressin mRNA levels are increased selectively in the amygdala during early spontaneous withdrawal from chronic heroin exposure, and SSR149415 blocks footshock-induced reinstatement of heroin-seeking behavior, suggesting that vasopressin systems in the amygdala may represent a key component of the aversive emotional consequences of opioid withdrawal (Zhou et al., 2008). Given the literature suggesting that V1bR antagonists have anxiolytic-like profiles and the fact that vasopressin and its receptors are highly expressed in the extended amygdala, we hypothesized that vasopressin systems in the extended amygdala or other limbic areas may have a role in the increased alcohol intake associated with dependence. In the present study, we show that a differential regulation of vasopressin/V1b receptor signaling is involved in the transition from limited ethanol use to ethanol dependence, a transformation that is hypothesized to be regulated by activation of vasopressinergic neurons as part of the brain stress system.
Materials and Methods
Animals
Male Wistar rats (n=44, Charles River) initially weighting 250–275g were communally housed (2–3/cage) with food and water available ad libitum. The animals were housed in a temperature-controlled (21.5 C) vivarium and maintained on a 12 h light/dark cycle (lights on at 0800). Animals were handled daily for one week before the onset of operant conditioning. All experiments adhered to the guidelines provided in the NIH Guide for the Care and Use of Laboratory Animals and protocols were reviewed and approved by The Scripps Research Institute’s Institutional Care and Use Committee.
Drug Administration
SSR 149415 was synthesized in the Department of Chemistry at The Scripps Research Institute. It was prepared as a fresh solution each test day in physiological saline (0.9%) containing 5% DMSO (Sigma, St. Louis, MO) and 5% Cremophor EL (Sigma) and injected intraperitonealy thirty minutes before testing.
Operant Chambers
The operant chambers (MedAssociates) used in this study had two retractable levers located 4 cm above a grid floor and 4.5 cm to each side of a two-well acrylic drinking cup that allowed for two solutions to be administered upon pressing of the appropriate lever. Recording of operant responses and subsequent solution delivery were controlled by custom software running on a PC computer. Each lever press resulted in activation of a 15 RPM Razel syringe pump (Sanford, CT) that delivered 0.1 ml of solution to the appropriate well over 0.5 s. Operant chambers were individually housed in ventilated, sound-attenuated cubicles to minimize environmental disturbances.
Acquisition of Operant Self-Administration
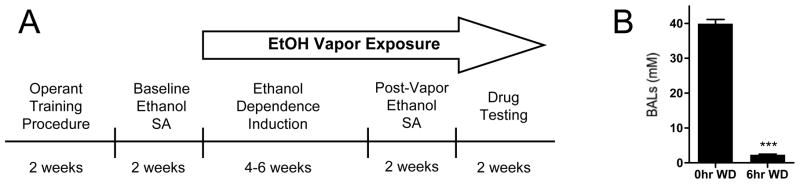
The timeline of experimental procedures is shown in Figure 1. Animals were first trained to self-administer ethanol by a sucrose-fading procedure (Samson, 1986), at the end of which animals were allowed to self-administer 10% (w/v) ethanol and water solutions until stable responding was maintained. Animals were then split into two groups that were matched for ethanol self-administration over their last 5 sessions, with one group designated as “ethanol-dependent” (vapor-exposed) and the other as “non-dependent” (air-exposed).
Figure 1.
Experimental timeline and blood alcohol level cycling. (A) Pharmacological testing and time points for tissue collection for Western analysis occurred after approximately 8–10 weeks of chronic intermittent ethanol vapor exposure. (B) All analyses were conducted when ethanol-dependent animals were in acute (6hr) withdrawal, a time point coinciding with diminished blood alcohol levels (BALs) and increased ethanol self-administration behavior. Asterisks indicate ***p<0.001 significant effect of withdrawal time point.
Ethanol Vapor Chambers
Ethanol vapor exposure has been demonstrated to be a safe, reliable method to titrate blood alcohol levels (BALs) for the induction of ethanol dependence (Gilpin et al., 2008). In this procedure, BALs are adjusted by the experimenter to maintain dependence-inducing BALs without jeopardizing animal health. Rats were made dependent by chronic, intermittent exposure to ethanol vapors (CIEV), and BALs were regulated between 38–49mM. Tail blood samples were taken and analyzed 1–2x week for BAL determination as previously described (Gilpin et al., 2008). Animals underwent cycles of 14 hr on vapor and 10 hr off, with BALs being diminished by 6 hr withdrawal (Figure 1), when behavioral testing and tissue collection occurred. Importantly, at this time point animals exhibit a host of symptoms related to dependence, including both somatic withdrawal signs (Roberts et al., 2000; O’Dell et al., 2004) and negative motivational symptoms (Schulteis et al., 1995), and show excessive alcohol drinking compared to air-exposed animals.
Pharmacological Testing of Ethanol Self-Administration in Dependent and Non-Dependent Animals
Following acquisition of ethanol self-administration and induction of ethanol dependence by four-six weeks of CIEV exposure, dependent and non-dependent (air-exposed) animals were tested until post-vapor intake stabilization was reached (10 sessions). Post-vapor testing (both for behavior and biochemical analysis, see below) was conducted when dependent animals were in acute (6hr) withdrawal. Responses for both ethanol and water (a natural reinforcer) were recorded. After stable responding for ethanol, which included habituation to intraperitoneal injections of vehicle (5% DMSO/5% Cremophor EL in physiological saline), the effects of V1bR antagonism on ethanol self-administration was tested in both ethanol-dependent and non-dependent (air-exposed) rats (n=8/group). Thirty minutes before each test session, animals were injected with the V1bR antagonist SSR149415 (10, 20, 30 mg/kg, IP) or vehicle. This dose range was chosen based on its anxiolytic- and antidepressant-like effects in rodents (Griebel et al. 2002). Each dose (including vehicle alone) was tested in individual sessions conducted twice weekly (on non-cage changing days) according to a Latin square design. Between test sessions, animals were allowed to self-administer ethanol in non-test sessions to reveal any carry-over effects of previous V1b antagonist dosing on subsequent ethanol drinking.
Western Blot Analysis
In separate groups of drug-naïve animals (n=7–8/group), we measured vasopressin V1b receptor levels in five limbic brain regions previously implicated in the anxiolytic-and antidepressant-like effects of SSR 149415 of ethanol self-administering (dependent and non-dependent) and naïve animals. Naïve control animals were age/batch-matched and housed under identical conditions, were handled twice weekly, but did not undergo any operant training. One day following their last self-administration session (the tenth session post-dependence induction), animals were euthanized under light halothane anesthesia by decapitation. This time point was chosen since it corresponds to the period when animals were normally self-administering ethanol (i.e., six hours post-vapor for ethanol-dependent animals). Rat brains were rapidly dissected and snap frozen in isopentane. Regional tissue samples were obtained with 12–16 gauge punches from chilled coronal brain slices (0.5 mm thick) obtained by the use of a cryostat. Tissue processing for Western blot analysis was conducted as described previously (Edwards et al., 2009). Briefly, tissue samples were homogenized by sonication in lysis buffer (320 mM sucrose, 5 nM HEPES, 50 mM NaF, 1 mM EGTA, 1 mM EDTA, 1% SDS, with Protease Inhibitor Cocktail and Phosphatase Inhibitor Cocktails I and II diluted 1:100; Sigma, St. Louis, MO), boiled for 5 min, and stored at −80°C until determination of protein concentrations by the Lowry method. Samples of 20μg protein were subjected to SDS-polyacrylamide gel electrophoresis on 10% acrylamide gels using a Tris/Glycine/SDS buffer (Bio-Rad, Hercules, CA), followed by electrophoretic transfer to PVDF membranes (polyvinylidene; Amersham, Piscataway, NJ). Membranes were blocked overnight in 5% nonfat milk at 4° C, and incubated in primary antibody for the vasopressin V1b receptor (1:1000; #AVP1B13-S, Alpha Diagnostic International, San Antonio, TX, USA) for 24 hrs at 4° C. This antibody has been extensively characterized in previous reports (Hurbin et al., 2002; Folny et al., 2003; Stemmelin et al., 2005). Membranes were washed and labeled with species-specific peroxidase-conjugated secondary (goat anti-rabbit, 1:10,000; BioRad) for 1 hr at 25° C. Following chemiluminescence detection (ECL plus; Amersham), blots were stripped and reprobed for beta-Tubulin (1:10,000 and 1:5000; Santa Cruz) as internal standards for protein levels. Immunoreactivity was quantified by densitometry (Scion Image) under conditions linear over at least a 3-fold concentration range.
Statistics
Blood alcohol levels between 0hr and 6hr withdrawal from ethanol vapors were analyzed by Student’s t test. The relationship between preferred levels of ethanol self-administration before and after ethanol vapor exposure was determined by Pearson’s linear correlation. The effects of vapor exposure and/or pharmacological challenges on ethanol self-administration were analyzed using a mixed two-way ANOVA with vapor treatment (ethanol or air) as the between-subjects factor and test day or V1bR antagonist dose as the repeated, within-subjects factor. In cases of a significant main effect, post hoc comparisons were performed with Bonferroni’s post hoc tests. Western data were analyzed using a one-way ANOVA, and, in cases of a significant main effect, post hoc comparisons were performed with Newman-Keuls Multiple Comparison tests. All statistical analyses were performed with Prism 4.
Results
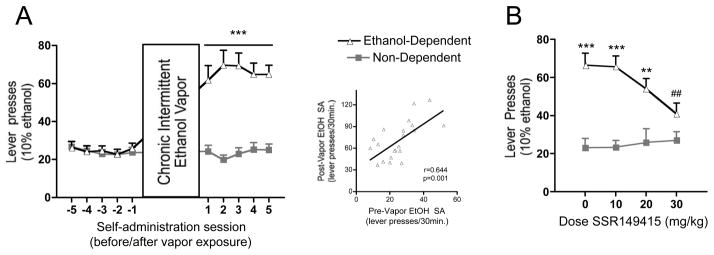
Figure 1A depicts the experimental timeline used in the present study. Animals (n=44) were initially trained to self-administer ethanol via a sucrose-fading procedure. Following stabilized acquisition of ethanol self-administration, half of the rats (ethanol-dependent group, n=22) were made dependent by exposure to intermittent ethanol vapor (14 hr/day for 4–6 weeks) and subsequently tested for ethanol self-administration (10% v/v, FR1, 30 minute sessions) six hours into withdrawal, a time point commensurate with diminished blood alcohol levels (Figure 1B) and heightened anxiety-like behavior (Roberts et al., 2000; O’Dell et al., 2004). The other half of the rats (non-dependent group, n=22) were exposed to air, and tested at the similar time of day as ethanol-dependent animals. All animals were tested on non-cage changing days to facilitate stable ethanol self-administration. Ethanol self-administration behavior five days before and after dependence induction is presented in Figure 2A. Ethanol vapor exposure significantly elevated alcohol drinking in ethanol-dependent animals (group × day interaction, F 9,378 = 25.15). Additionally, there was a positive correlation between individual preferred levels of drinking before and after chronic intermittent vapor exposure (r = 0.644, p =0.001, Figure 2A inset), suggesting a preservation of individual differences in dependent animals following vapor exposure, albeit at magnified levels.
Figure 2.
Ethanol self-administration in ethanol-dependent and non-dependent animals. (A) Induction of ethanol dependence and correlation of limited ethanol self-administration before and excessive drinking after dependence induction following chronic intermittent ethanol vapor exposure. (B) Effects of vasopressin V1bR antagonism on ethanol self-administration, showing a dose-dependent reduction in ethanol drinking in dependent, but not non-dependent, animals. Responses are graphed as mean ± SEM, with correlative data points representing individual averages of the five sessions before vs. five sessions after dependence induction. Asterisks indicate ***p<0.001 significant effect of group × test session (A) or ***p<0.001, **p<0.01 significant effect of group (B). Pound signs indicate ## p<0.01 significant effect of dose in ethanol-dependent group.
After stable responding for ethanol, which included habituation to intraperitoneal injections of vehicle (5% DMSO/5% Cremophor EL in physiological saline), the effects of V1bR antagonism on ethanol self-administration was tested in both ethanol-dependent and non-dependent (air-exposed) rats (n=8/group). Thirty minutes before each test session, animals were injected with the V1bR antagonist SSR149415 (10, 20, 30 mg/kg, IP) or vehicle. This dose range was chosen based on its anxiolytic- and antidepressant-like effects in rodents (Griebel et al. 2002). Each dose (including vehicle alone) was tested in individual sessions conducted twice weekly (on non-cage changing days) according to a Latin square design. Between test sessions, animals were allowed to self-administer ethanol in non-test sessions to reveal any carry-over effects of previous V1bR antagonist dosing on subsequent ethanol drinking, and such effects were not observed. SSR149415 dose-dependently reduced responding for ethanol selectively in dependent rats (Figure 2B, group × dose interaction, F 3,42 = 5.601), reaching significance at the 30 mg/kg dose (q = 6.365, p<0.01 vs. vehicle in ethanol-dependent group, Newman-Keuls Multiple Comparison Test). Although SSR149415 has been demonstrated to exhibit a somewhat limited brain penetrability (Schonberger et al., 2010), antidepressant-like effects of the drug were still observed at 30 mg/kg in hypophysectomized rats (Griebel et al., 2002), indicating a probable action at extrahypothalamic V1bR brain sites at this dose. In contrast, there was no effect of the antagonist on non-dependent animals, suggesting a relative potentiation or sensitization of V1bR signaling in dependent animals. Responses for water were not changed in either group by V1bR antagonism (Table 1). It is worth noting again that the effects of vasopressin (also known as anti-diuretic hormone) on fluid dynamics in the kidney are mediated by vasopressin V2 receptors (Kaufmann et al., 2000).
Table 1.
Water Responses
| Group | SSR 149415 (mg/kg)
|
|||
|---|---|---|---|---|
| Vehicle | 10 | 20 | 30 | |
| Ethanol-Dependent | 13.8 ± 4.4 | 11.3 ± 3.0 | 12.5 ± 4.0 | 13.1 ± 3.7 |
| Non-Dependent | 11.5 ± 5.3 | 13.1 ± 5.1 | 15.5 ± 7.7 | 12.1 ± 6.1 |
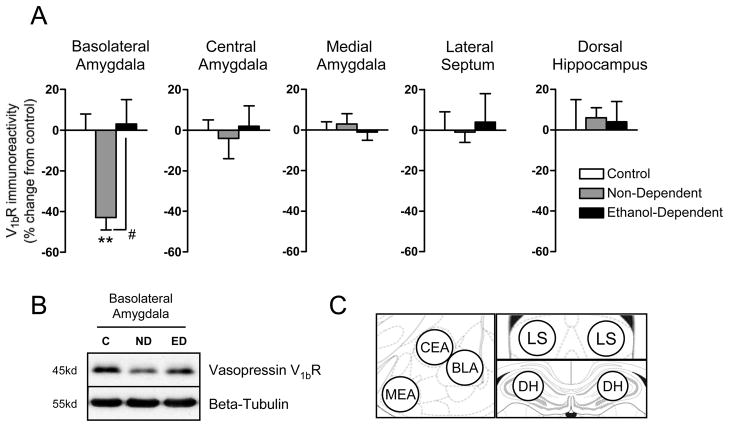
To determine possible neuroadaptations associated with the differential responsiveness to V1bR antagonism between dependent and non-dependent groups, we measured V1bR protein levels in five brain regions previously implicated in SSR149415-mediated reductions in antidepressant-and/or anxiolytic-like behavior in separate groups of ethanol-dependent and non-dependent animals and naïve controls (n=7–8/group). Ethanol self-administration significantly reduced V1bR levels in the basolateral amygdala by 43% without effecting receptor levels in the other four brain regions studied (Figure 3A, F 2, 21 = 6.421; q = 4.201, p < 0.01 non-dependent group vs. naïve controls, Newman-Keuls Multiple Comparison Test). In the ethanol-dependent group, this neuroadaptation was completely reversed, as V1bR levels in dependent animals were up-regulated near levels observed in naïve animals (q = 4.597, p < 0.05 non-dependent vs. dependent group, Newman-Keuls Multiple Comparison Test).
Figure 3.
Regulation of vasopressin V1bR levels by ethanol self-administration. (A) In the basolateral amygdala, V1bR immunoreactivity is significantly reduced in the non-dependent ethanol self-administering group (versus naïve control animals) and seemingly restored in ethanol-dependent animals. (B) Representative immunoblot showing changes in V1bR immunoreactivity with no change in beta-tubulin levels. (C) Diagram of regions collected by the slice/punch technique and analyzed by Western blot. MEA (medial nucleus of the amygdala), CEA (central nucleus of the amygdala), BLA (basolateral nucleus of the amygdala), LS (lateral septum), DH (dorsal hippocampus). Immunoreactivity (% change from naïve control group) is graphed as mean ± SEM. Asterisks indicate **p<0.01 significant decrease in V1bR immunoreactivity in non-dependent group vs. naïve controls. Pound sign indicates p<0.05 significant increase in V1bR immunoreactivity in ethanol-dependent vs. non-dependent group.
Discussion
The transition to ethanol dependence can be thought of as advancement along a timeline, where initial alcohol use is associated with and maintained by positive reinforcement mechanisms. Some individuals go on to escalate their drinking behavior, and at such a point consumption may be driven more so by negative reinforcement, whereby individuals attempt to ameliorate negative withdrawal symptoms via excessive alcohol use (Gilpin et al., 2008). Such a conceptualization posits that distinct stages/levels of drinking may be accompanied by incremental neuroadaptations that underlie these states (Heilig and Koob, 2007; Edwards and Koob, 2010).
In the present study, we used an animal model of ethanol dependence that recapitulates many of the symptoms of alcoholism (Gilpin et al., 2008). In this paradigm, dependent animals display excessive ethanol self-administration at a time point (6–8 hrs WD) commensurate with increased somatic withdrawal signs and anxiety-like behavior (Roberts et al., 2000; O’Dell et al., 2004), decreased brain reward thresholds (Schulteis et al., 1995), and diminished BALs (Figure 1). In comparison, non-dependent animals self-administer limited amounts of alcohol, and model the vast majority of human alcohol consumers. We show here that vasopressin V1bR antagonism reduces ethanol self-administration in dependent animals close to levels observed in non-dependent animals (Figure 2). In comparison, ethanol drinking in non-dependent animals was not altered, suggesting a relative potentiation of vasopressin/V1bR signaling after the induction of ethanol dependence. Supporting this hypothesis, although moderate levels of ethanol self-administration in non-dependent animals were associated with reduced V1bR protein levels in the basolateral amygdala compared to ethanol-naïve animals, V1bR levels were seemingly restored in ethanol-dependent animals (Figure 3). Given the proposed role of V1bR signaling in the BLA in mediating both depression- and anxiety-like behaviors (Salome et al., 2006), a reduction in V1bRs may contribute to the positive rewarding properties (anxiolytic- or antidepressant-like effects) of ethanol self-administration. In ethanol-dependent animals, BLA V1bR levels were up-regulated compared to non-dependent animals, a neuroadaptation that may in fact reflect a switch in motivation for alcohol from positive to negative reinforcement. The precise mechanism for differential V1bR regulation between ethanol-dependent and non-dependent animals is unknown, although there are several candidate possibilities.
Possible mechanisms of V1bR regulation
Post-transcriptional regulation of V1bR expression represents one mechanism by which receptor levels are regulated, and it is estimated that V1bR content is primarily regulated at the translational level (Volpi et al., 2006). The V1bR gene contains several glucocorticoid response elements, and receptor levels in the pituitary are bi-directionally regulated by circulating glucocorticoids (Aguilera and Rabadan-Diehl, 2000). Restraint stress increases V1bR promoter activation and V1bR expression in the pituitary (Volpi et al., 2002). It is also known that post-transcriptional mechanisms play a major role in regulating V1bR levels, including small upstream open reading frames in V1bR mRNA that code for active peptides capable of inhibiting V1bR translation (Rabadan-Diehl et al., 2007). Overactivation of the extracellular signal-regulated kinase (ERK) cascade also drives V1bR gene transcription (Volpi et al., 2006), and this pathway is activated in the amygdala of ethanol-dependent (but not non-dependent) animals during acute withdrawal (Sanna et al., 2002).
Interactions of Vasopressin Levels and Ethanol
In accordance with the receptor-level changes revealed in the present study, there exists evidence for bidirectional changes in vasopressin peptide levels between initial and chronic ethanol exposure. Although acute ethanol is known to reduce systemic vasopressin release, plasma AVP levels are increased in alcoholics (Beard and Sargent, 1979). Hoffmann et al. (1990) have further speculated that elevated vasopressin levels could result from the stress of ethanol withdrawal, since in one study plasma AVP was increased only in alcoholics showing symptoms of withdrawal (Eisenhofer et al., 1985). Another study revealed that sons of ethanol-dependent fathers are more sensitive to ethanol’s ability to blunt the AVP/ACTH psychosocial stress response, possibly heightening the risk for alcohol use disorders in these individuals (Zimmermann et al., 2004). Treatment of animals with vasopressin both during and after chronic ethanol administration also leads to a long-term maintenance of tolerance to the hypothermic and sedative effects of ethanol (Hoffman et al., 1978; Le et al., 1982; Hoffman et al., 1990), and this effect was linked to a central V1-subtype of vasopressin receptor (Szabo et al., 1988). In contrast, vasopressin-deficient Brattleboro rats fail to develop ethanol tolerance (Pittman et al., 1982). Therefore, it is possible that elevated vasopressin release in dependent individuals during withdrawal could foster a negative emotional state that in turn facilitates an escalation in levels of drinking that lead to alleviation of that state.
Recruitment of amygdala stress-related peptide signaling in ethanol dependence
Our results further demonstrate a role for stress-related peptide signaling in the amygdala in ethanol dependence. The effects of V1bR blockade on excessive ethanol self-administration closely resemble inhibition of the CRF1 receptor system on this behavior (Funk et al., 2007). From a clinical perspective, excessive AVP and CRF signaling through V1b and CRF1 receptors (respectively) may contribute to anxiety and depression, as systemic administration of small-molecule antagonists for these receptors is effective in animal models of these conditions (Hodgson et al., 2007). In addition to CRF, AVP also stimulates the hypothalamic-pituitary-adrenal (HPA) axis by its actions on V1bRs located on anterior pituitary corticotropes (Lolait et al., 2007). While CRF is the predominant HPA axis regulator, AVP synergizes with CRF to release adrenocorticotropin hormone (Antoni, 1993), although whether this synergy exists centrally (i.e., at extrahypothalamic sites) is unknown. Previous studies have demonstrated a recruitment of CRF in the amygdala as a critical element driving the excessive ethanol intake observed during both acute and protracted withdrawal times (Heilig and Koob, 2007). These studies implicated enhanced CRF signaling in discrete amygdala nuclei, including the central (Lack et al., 2005; Funk et al., 2007) and basolateral (Sommer et al., 2008) nuclei as neuroanatomical substrates driving ethanol dependence. The amygdala is regulated by extrinsic (cortical) and intrinsic (BLA) excitatory projections, and it was recently demonstrated that these connections are modulated in opposite manners by oxytocin and vasopressin (Huber et al., 2005), with vasopressin acting to facilitate BLA-CEA communication. Accordingly, either in addition to or in cooperation with CRF, vasopressin activity may represent another mechanism whereby negative reinforcement mechanisms regulate ethanol dependence. Finally, one study found that SSR149415 exhibited significant antagonism at human recombinant oxytocin receptors (OTRs, Griffante et al., 2005). How oxytocin regulates ethanol drinking is unknown, while the effects of this neuropeptide on affective-like behaviors are equivocal. For example, although OTR stimulation inhibits the HPA axis (Neumann et al., 2000), intra-amygdala microinjection of an OTR antagonist produces antidepressant-like effects (Ebner et al., 2005). Thus, future studies to determine the specific roles of these closely related peptides are needed.
Conclusion
In the present study, we demonstrated that limited ethanol self-administration causes a reduction in V1b receptors, a neuroadaptation that was seemingly reversed in ethanol-dependent animals. Further, V1bR antagonism specifically blocked excessive drinking levels in ethanol-dependent animals. Presumably, an ideal therapeutic for alcoholism would not affect either recreational (i.e. non-dependent) drinking or motivation for natural rewards. Given the role of the AVP/V1bR system in anxiety- and stress-related behaviors, reductions in V1b receptors in the basolateral amygdala may be involved in the natural, positive reinforcing effects of alcohol. The restoration of V1b receptor levels in ethanol-dependent animals may in part represent a transitional shift in the motivational state (positive to negative) underlying ethanol self-administration. Importantly, this study extends previous work reporting altered vasopressin signaling in the amygdala of opiate-dependent animals (Zhou et al., 2008), and further supports V1bR antagonism as a valid therapeutic strategy for drug dependence.
Acknowledgments
This work was presented at the 32nd annual meeting of the Research Society on Alcoholism, San Diego, CA, 2009. This research was supported by a grant from the National Institute on Alcohol Abuse and Alcoholism (AA008459 & AA12602, GFK) and a National Research Service Award (AA018250, SE), and by the Pearson Center for Alcoholism and Addiction Research. This is article number 20686 from the Scripps Research Institute. We thank Nick Gilpin, Olivier George, Brendan Walker, Elena Crawford, Yanabel Grant, Molly Brennan, Maury Cole, and Tess Kimber for helpful discussion and technical support pertaining to this study and Mike Arends for editorial assistance.
Footnotes
The authors declare no conflicts of interest.
Author Contributions
SE conducted all behavioral and biochemical experiments, analyzed the data, and wrote the manuscript. MG and OMG synthesized SSR149415 under the supervision of ER. GFK conceived and designed the study. All authors critically reviewed content and approved the final version for publication.
References
- Aguilera G, Rabadan-Diehl C. Regulation of vasopressin V1b receptors in the anterior pituitary gland of the rat. Experimental Physiology. 2000;85(19S–26S) doi: 10.1111/j.1469-445x.2000.tb00004.x. [DOI] [PubMed] [Google Scholar]
- Antoni FA. Vasopressinergic control of pituitary adrenocorticotropin secretion comes of age. Frontiers in Neuroendocrinology. 1993;14:76–122. doi: 10.1006/frne.1993.1004. [DOI] [PubMed] [Google Scholar]
- Beard JD, Sargent WQ. Water and electrolyte metabolism following ethanol intake and during acute withdrawal from ethanol. In: Majchrowicz E, Noble EP, editors. Biochemistry and Pharmacology of Ethanol. Vol. 2. New York: Plenum Press; 1979. pp. 3–16. [Google Scholar]
- Brownstein MJ, Russell JT, Gainer H. Synthesis, transport, and release of posterior pituitary hormones. Science. 1980;207:373–378. doi: 10.1126/science.6153132. [DOI] [PubMed] [Google Scholar]
- Buijs RM. Intra- and extrahypothalamic vasopressin and oxytocin pathways in the rat. Pathways to the limbic system, medulla oblongata and spinal cord. Cell & Tissue Research. 1978;192:423–435. doi: 10.1007/BF00212323. [DOI] [PubMed] [Google Scholar]
- Buijs RM, De Vries GJ, Van Leeuwen FW, Swaab DF. Vasopressin and oxytocin: distribution and putative functions in the brain. Progress in Brain Research. 1983;60:115–122. doi: 10.1016/S0079-6123(08)64379-4. [DOI] [PubMed] [Google Scholar]
- Caldwell HK, Wersinger SR, Young WS., 3rd The role of the vasopressin 1b receptor in aggression and other social behaviours. Progress in Brain Research. 2008;170:65–72. doi: 10.1016/S0079-6123(08)00406-8. [DOI] [PubMed] [Google Scholar]
- Chrousos GP. Stress and disorders of the stress system. Nature Reviews Endocrinology. 2009;5:374–381. doi: 10.1038/nrendo.2009.106. [DOI] [PubMed] [Google Scholar]
- De Vries GJ, Buijs RM. The origin of the vasopressinergic and oxytocinergic innervation of the rat brain with special reference to the lateral septum. Brain Research. 1983;273:307–317. doi: 10.1016/0006-8993(83)90855-7. [DOI] [PubMed] [Google Scholar]
- de Wied D, Versteeg DH. Neurohypophyseal principles and memory. Fed Proc. 1979;38:2348–2354. [PubMed] [Google Scholar]
- Ebner K, Bosch OJ, Kromer SA, Singewald N, Neumann ID. Release of oxytocin in the rat central amygdala modulates stress-coping behavior and the release of excitatory amino acids. Neuropsychopharmacology. 2005;30:223–230. doi: 10.1038/sj.npp.1300607. [DOI] [PubMed] [Google Scholar]
- Edwards S, Graham DL, Whisler KN, Self DW. Phosphorylation of GluR1, ERK, and CREB during spontaneous withdrawal from chronic heroin self-administration. Synapse. 2009;63:224–235. doi: 10.1002/syn.20601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards S, Koob GF. Neurobiology of dysregulated motivational systems in drug addiction. Future Neurol. 2010;5 (3):393–401. doi: 10.2217/fnl.10.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenhofer G, Lambie DG, Whiteside EA, Johnson RH. Vasopressin concentrations during alcohol withdrawal. British Journal of Addiction. 1985;80:195–199. doi: 10.1111/j.1360-0443.1985.tb03271.x. [DOI] [PubMed] [Google Scholar]
- Engelmann M, Wotjak CT, Neumann I, Ludwig M, Landgraf R. Behavioral consequences of intracerebral vasopressin and oxytocin: focus on learning and memory. Neuroscience & Biobehavioral Reviews. 1996;20:341–358. doi: 10.1016/0149-7634(95)00059-3. [DOI] [PubMed] [Google Scholar]
- Engin E, Treit D. Dissociation of the anxiolytic-like effects of Avpr1a and Avpr1b receptor antagonists in the dorsal and ventral hippocampus. Neuropeptides. 2008;42:411–421. doi: 10.1016/j.npep.2008.04.004. [DOI] [PubMed] [Google Scholar]
- Folny V, Raufaste D, Lukovic L, Pouzet B, Rochard P, Pascal M, Serradeil-Le Gal C. Pancreatic vasopressin V1b receptors: characterization in In-R1-G9 cells and localization in human pancreas. American Journal of Physiology - Endocrinology & Metabolism. 2003;285:E566–576. doi: 10.1152/ajpendo.00148.2003. [DOI] [PubMed] [Google Scholar]
- Funk CK, Zorrilla EP, Lee MJ, Rice KC, Koob GF. Corticotropin-releasing factor 1 antagonists selectively reduce ethanol self-administration in ethanol-dependent rats. Biological Psychiatry. 2007;61:78–86. doi: 10.1016/j.biopsych.2006.03.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilpin NW, Koob GF. Neurobiology of alcohol dependence: focus on motivational mechanisms. Alcohol Research and Health. 2008;31(3):185–195. [PMC free article] [PubMed] [Google Scholar]
- Gilpin NW, Richardson HN, Cole M, Koob GF. Vapor inhalation of alcohol in rats. Current Protocols in Neuroscience. 2008;9(Unit 9):29. doi: 10.1002/0471142301.ns0929s44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griebel G, Simiand J, Serradeil-Le Gal C, Wagnon J, Pascal M, Scatton B, Maffrand JP, Soubrie P. Anxiolytic- and antidepressant-like effects of the non-peptide vasopressin V1b receptor antagonist, SSR149415, suggest an innovative approach for the treatment of stress-related disorders. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:6370–6375. doi: 10.1073/pnas.092012099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffante C, Green A, Curcuruto O, Haslam CP, Dickinson BA, Arban R. Selectivity of d[Cha4]AVP and SSR149415 at human vasopressin and oxytocin receptors: evidence that SSR149415 is a mixed vasopressin V1b/oxytocin receptor antagonist. British Journal of Pharmacology. 2005;146:744–751. doi: 10.1038/sj.bjp.0706383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilig M, Koob GF. A key role for corticotropin-releasing factor in alcohol dependence. Trends in Neurosciences. 2007;30:399–406. doi: 10.1016/j.tins.2007.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgson RA, Higgins GA, Guthrie DH, Lu SX, Pond AJ, Mullins DE, Guzzi MF, Parker EM, Varty GB. Comparison of the V1b antagonist, SSR149415, and the CRF1 antagonist, CP-154,526, in rodent models of anxiety and depression. Pharmacology, Biochemistry & Behavior. 2007;86:431–440. doi: 10.1016/j.pbb.2006.12.021. [DOI] [PubMed] [Google Scholar]
- Hoffman PL, Ishizawa H, Giri PR, Dave JR, Grant KA, Liu LI, Gulya K, Tabakoff B. The role of arginine vasopressin in alcohol tolerance. Annals of Medicine. 1990;22:269–274. doi: 10.3109/07853899009148939. [DOI] [PubMed] [Google Scholar]
- Hoffman PL, Ritzmann RF, Walter R, Tabakoff B. Arginine vasopressin maintains ethanol tolerance. Nature. 1978;276:614–616. doi: 10.1038/276614a0. [DOI] [PubMed] [Google Scholar]
- Huber D, Veinante P, Stoop R. Vasopressin and oxytocin excite distinct neuronal populations in the central amygdala. Science. 2005;308:245–248. doi: 10.1126/science.1105636. [DOI] [PubMed] [Google Scholar]
- Hurbin A, Orcel H, Alonso G, Moos F, Rabie A. The vasopressin receptors colocalize with vasopressin in the magnocellular neurons of the rat supraoptic nucleus and are modulated by water balance. Endocrinology. 2002;143:456–466. doi: 10.1210/endo.143.2.8643. [DOI] [PubMed] [Google Scholar]
- Kalivas PW. How do we determine which drug-induced neuroplastic changes are important? Nature Neuroscience. 2005;8:1440–1441. doi: 10.1038/nn1105-1440. [DOI] [PubMed] [Google Scholar]
- Kaufmann JE, Oksche A, Wollheim CB, Gunther G, Rosenthal W, Vischer UM. Vasopressin-induced von Willebrand factor secretion from endothelial cells involves V2 receptors and cAMP. Journal of Clinical Investigation. 2000;106:107–116. doi: 10.1172/JCI9516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF. A role for brain stress systems in addiction. Neuron. 2008;59:11–34. doi: 10.1016/j.neuron.2008.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Ahmed SH, Boutrel B, Chen SA, Kenny PJ, Markou A, O’Dell LE, Parsons LH, Sanna PP. Neurobiological mechanisms in the transition from drug use to drug dependence. Neuroscience & Biobehavioral Reviews. 2004;27:739–749. doi: 10.1016/j.neubiorev.2003.11.007. [DOI] [PubMed] [Google Scholar]
- Koob GF, Bloom FE. Behavioral effects of neuropeptides: endorphins and vasopressin. Annual Review of Physiology. 1982;44:571–582. doi: 10.1146/annurev.ph.44.030182.003035. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Drug abuse: hedonic homeostatic dysregulation. Science. 1997;278:52–58. doi: 10.1126/science.278.5335.52. [DOI] [PubMed] [Google Scholar]
- Kreek MJ, Koob GF. Drug dependence: stress and dysregulation of brain reward pathways. Drug & Alcohol Dependence. 1998;51:23–47. doi: 10.1016/s0376-8716(98)00064-7. [DOI] [PubMed] [Google Scholar]
- Lack AK, Floyd DW, McCool BA. Chronic ethanol ingestion modulates proanxiety factors expressed in rat central amygdala. Alcohol. 2005;36:83–90. doi: 10.1016/j.alcohol.2005.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le AD, Kalant H, Khanna JM. Interaction between des-glycinamide9-[Arg8]vasopressin and serotonin on ethanol tolerance. European Journal of Pharmacology. 1982;80:337–345. doi: 10.1016/0014-2999(82)90079-6. [DOI] [PubMed] [Google Scholar]
- Le Moal M, Dantzer R, Mormede P, Baduel A, Lebrun C, Ettenberg A, van der Kooy D, Wenger J, Deyo S, Koob GF. Behavioral effects of peripheral administration of arginine vasopressin: a review of our search for a mode of action and a hypothesis. Psychoneuroendocrinology. 1984;9:319–341. doi: 10.1016/0306-4530(84)90042-8. [DOI] [PubMed] [Google Scholar]
- Lolait SJ, Stewart LQ, Jessop DS, Young WS, 3rd, O’Carroll AM. The hypothalamic-pituitary-adrenal axis response to stress in mice lacking functional vasopressin V1b receptors. Endocrinology. 2007;148:849–856. doi: 10.1210/en.2006-1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann ID, Wigger A, Torner L, Holsboer F, Landgraf R. Brain oxytocin inhibits basal and stress-induced activity of the hypothalamo-pituitary-adrenal axis in male and female rats: partial action within the paraventricular nucleus. Journal of Neuroendocrinology. 2000;12:235–243. doi: 10.1046/j.1365-2826.2000.00442.x. [DOI] [PubMed] [Google Scholar]
- O’Dell LE, Roberts AJ, Smith RT, Koob GF. Enhanced alcohol self-administration after intermittent versus continuous alcohol vapor exposure. Alcoholism: Clinical & Experimental Research. 2004;28:1676–1682. doi: 10.1097/01.alc.0000145781.11923.4e. [DOI] [PubMed] [Google Scholar]
- Pittman QJ, Rogers J, Bloom FE. Arginine vasopressin deficient Brattleboro rats fail to develop tolerance to the hypothermic effects of ethanol. Regulatory Peptides. 1982;4:33–41. doi: 10.1016/0167-0115(82)90106-9. [DOI] [PubMed] [Google Scholar]
- Rabadan-Diehl C, Martinez A, Volpi S, Subburaju S, Aguilera G. Inhibition of vasopressin V1b receptor translation by upstream open reading frames in the 5′-untranslated region. Journal of Neuroendocrinology. 2007;19:309–319. doi: 10.1111/j.1365-2826.2007.01533.x. [DOI] [PubMed] [Google Scholar]
- Richardson HN, Zhao Y, Fekete EM, Funk CK, Wirsching P, Janda KD, Zorrilla EP, Koob GF. MPZP: a novel small molecule corticotropin-releasing factor type 1 receptor (CRF1) antagonist. Pharmacology, Biochemistry & Behavior. 2008;88:497–510. doi: 10.1016/j.pbb.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts AJ, Heyser CJ, Cole M, Griffin P, Koob GF. Excessive ethanol drinking following a history of dependence: animal model of allostasis. Neuropsychopharmacology. 2000;22:581–594. doi: 10.1016/S0893-133X(99)00167-0. [DOI] [PubMed] [Google Scholar]
- Salome N, Stemmelin J, Cohen C, Griebel G. Differential roles of amygdaloid nuclei in the anxiolytic- and antidepressant-like effects of the V1b receptor antagonist, SSR149415, in rats. Psychopharmacology. 2006;187:237–244. doi: 10.1007/s00213-006-0424-1. [DOI] [PubMed] [Google Scholar]
- Samson HH. Initiation of ethanol reinforcement using a sucrose-substitution procedure in food- and water-sated rats. Alcoholism: Clinical & Experimental Research. 1986;10:436–442. doi: 10.1111/j.1530-0277.1986.tb05120.x. [DOI] [PubMed] [Google Scholar]
- Sanna PP, Simpson C, Lutjens R, Koob G. ERK regulation in chronic ethanol exposure and withdrawal. Brain Research. 2002;948:186–191. doi: 10.1016/s0006-8993(02)03191-8. [DOI] [PubMed] [Google Scholar]
- Schonberger M, Leggett C, Kim SW, Hooker JM. Synthesis of [11C]SSR149415 and preliminary imaging studies using positron emission tomography. Bioorganic & Medicinal Chemistry Letters. 2010;20:3103–3106. doi: 10.1016/j.bmcl.2010.03.108. [DOI] [PubMed] [Google Scholar]
- Schulteis G, Markou A, Cole M, Koob GF. Decreased brain reward produced by ethanol withdrawal. Proceedings of the National Academy of Sciences of the United States of America. 1995;92:5880–5884. doi: 10.1073/pnas.92.13.5880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer WH, Rimondini R, Hansson AC, Hipskind PA, Gehlert DR, Barr CS, Heilig MA. Upregulation of voluntary alcohol intake, behavioral sensitivity to stress, and amygdala crhr1 expression following a history of dependence. Biological Psychiatry. 2008;63:139–145. doi: 10.1016/j.biopsych.2007.01.010. [DOI] [PubMed] [Google Scholar]
- Stemmelin J, Lukovic L, Salome N, Griebel G. Evidence that the lateral septum is involved in the antidepressant-like effects of the vasopressin V1b receptor antagonist, SSR149415. Neuropsychopharmacology. 2005;30:35–42. doi: 10.1038/sj.npp.1300562. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration. Results from the 2007 National Survey on Drug Use and Health: National Findings (Office of Applied Statistics, NSDUH Series H-34, DHHS Publication No. SMA 08-4343) Rockville MD: 2008. [Google Scholar]
- Surget A, Belzung C. Involvement of vasopressin in affective disorders. European Journal of Pharmacology. 2008;583:340–349. doi: 10.1016/j.ejphar.2007.11.065. [DOI] [PubMed] [Google Scholar]
- Szabo G, Tabakoff B, Hoffman PL. Receptors with V1 characteristics mediate the maintenance of ethanol tolerance by vasopressin. Journal of Pharmacology & Experimental Therapeutics. 1988;247:536–541. [PubMed] [Google Scholar]
- Veinante P, Freund-Mercier MJ. Distribution of oxytocin- and vasopressin-binding sites in the rat extended amygdala: a histoautoradiographic study. Journal of Comparative Neurology. 1997;383:305–325. [PubMed] [Google Scholar]
- Volpi S, Liu Y, Aguilera G. Vasopressin increases GAGA binding activity to the V1b receptor promoter through transactivation of the MAP kinase pathway. Journal of Molecular Endocrinology. 2006;36:581–590. doi: 10.1677/jme.1.01995. [DOI] [PubMed] [Google Scholar]
- Volpi S, Rabadan-Diehl C, Cawley N, Aguilera G. Transcriptional regulation of the pituitary vasopressin V1b receptor involves a GAGA-binding protein. Journal of Biological Chemistry. 2002;277:27829–27838. doi: 10.1074/jbc.M201508200. [DOI] [PubMed] [Google Scholar]
- Walker BM, Koob GF. Pharmacological evidence for a motivational role of kappa-opioid systems in ethanol dependence. Neuropsychopharmacology. 2008;33:643–652. doi: 10.1038/sj.npp.1301438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Leri F, Cummins E, Hoeschele M, Kreek MJ. Involvement of arginine vasopressin and V1b receptor in heroin withdrawal and heroin seeking precipitated by stress and by heroin. Neuropsychopharmacology. 2008;33:226–236. doi: 10.1038/sj.npp.1301419. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Proudnikov D, Vuferov V, Kreek MJ. Drug-induced and genetic alterations in stress-responsive systems: Implications for specific addictive diseases. Brain Res. 2010;1314:235–252. doi: 10.1016/j.brainres.2009.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann U, Spring K, Wittchen HU, Himmerich H, Landgraf R, Uhr M, Holsboer F. Arginine vasopressin and adrenocorticotropin secretion in response to psychosocial stress is attenuated by ethanol in sons of alcohol-dependent fathers. Journal of Psychiatric Research. 2004;38:385–393. doi: 10.1016/j.jpsychires.2003.11.009. [DOI] [PubMed] [Google Scholar]