Abstract
Background
Diabetes mellitus is a major independent risk factor for cardiovascular disease (CVD), but high cardiovascular risk in diabetes mellitus patients is not completely explained by clustering traditional risk factors. Recently, associations between diabetic polyneuropathy (DPN) and macrovasculopathy have been suggested. We aimed to assess associations between DPN and cardiovascular complications in type 2 diabetic patients.
Methods
Microvascular and cardiovascular complications were evaluated in 1,041 type 2 diabetic patients.
Results
In patients with DPN, the age, prevalence of hypertension, diabetes duration, systolic blood pressure, pulse pressure, and hemoglobin glycation (HbA1c) levels were significantly higher, while the high density lipoprotein cholesterol (HDL-C) levels were lower than in those without DPN. The prevalence of CVD was higher in patients with DPN. In multivariate analysis, DPN was independently associated with CVD (odds ratio, 1.801; 95% confidence interval, 1.009 to 3.214).
Conclusion
Our results showed that DPN was associated with a high prevalence of cardiovascular disease in type 2 diabetic patients, but further studies are needed to investigate the causative nature of associations between DPN and CVD.
Keywords: Cardiovascular diseases; Diabetes mellitus, type 2; Diabetic neuropathies
INTRODUCTION
Type 2 diabetes mellitus is the major cause of blindness, end-stage renal disease, and non-traumatic amputation. Diabetes mellitus is also a major independent risk factor for cardiovascular disease such as ischemic heart disease and stroke [1]. This high cardiovascular risk is not completely explained by clustering traditional risk factors. In this regard, additional linkage between diabetes mellitus and increased cardiovascular morbidity and mortality might be related to the development of microvascular complications, such as albuminuria, decreased glomerular filtration rate, retinopathy, and cardiovascular autonomic neuropathy [2-4].
Diabetic polyneuropathy (DPN) is one of the microvascular complications of diabetes that is attributed to chronic hyperglycemia and is a common cause of morbidity in patients with diabetes mellitus [5]. The major morbidity associated with DPN is foot ulceration, predisposition to gangrene, and limb loss [5]. Calcification of the media of arterial walls is common in patients with diabetes mellitus. Recently, some studies have reported that DPN is associated with medial arterial calcification in diabetes mellitus [6,7]. Young et al. [7] reported that medial arterial calcification correlated with vibration perception threshold. Medial arterial calcification is known to be associated with coronary heart disease and all-cause mortality [8,9]. Thus, DPN might be directly related to cardiovascular disease. However, relationships between DPN and cardiovascular disease in type 2 diabetic patients are not fully understood. In the present study, we aimed to assess associations between DPN and cardiovascular complications in type 2 diabetic patients.
METHODS
This study was an observational study designed to investigate the prevalence of chronic complications in type 2 diabetic patients. The data was obtained from September 2008 to August 2009. The study was approved by the Ethics Committee of Chonnam National University Hospital. All participants gave informed consent. A total of 1,041 patients with type 2 diabetes mellitus diagnosed after the age of 35 years who visited our hospital to evaluate chronic diabetic complications were investigated. Patients having any of the following characteristics were excluded from the study: active liver disease, glucocorticoid use, positive GAD autoantibodies, malignancy or primary neurologic disorders (previous spinal injury, a history of lumbar or cervical discopathies, carpal tunnel syndrome, alcoholic neuropathy, inherited neuropathy). A history and physical examination, including measurements of blood pressure, height, and body weight, were performed. Body mass index was calculated as weight (kg) divided by the square of height (m2). Blood pressure was measured twice in a sitting position at a 5-minute interval after 30 minutes of rest, and the mean values were calculated. Pulse pressure was calculated as the difference between systolic and diastolic blood pressures. Hypertension was considered if the patient had a blood pressure greater than 140/90 mm Hg or used antihypertensive drugs. Smoking was defined as never/past or current.
Blood samples were collected after a 10 to 12 hour overnight fast. The plasma glucose level was measured using the hexokinase method (Hitach 7600-110; Hitachi Co., Tokyo, Japan), and HbA1c was measured using ion exchange liquid chromatography (HLC-723-GHbV; Tosoh, Tokyo, Japan). Levels of total cholesterol (AU 5400; Olympus, Tokyo, Japan), high density lipoprotein cholesterol (HDL-C) (AU5400), low density lipoprotein cholesterol (LDL-C) (AU5400), and triglycerides (AU5400) were measured. High-sensitivity C-reactive protein was measured using the BNTM system (N High Sensitivity CRP; Dade Behring, Marburg, Germany).
Urinary albumin excretion was determined using the urinary albumin: creatinine ratio (UAE) in random urine samples. The urinary albumin concentration was measured using the immunoturbidimetric method with a commercial kit (Randox, Antrim, UK). Nephropathy was defined as UAE ≥300 mg/gCr in two of three urine collections. To evaluate retinopathy, an ophthalmologist performed fundoscopy after pupillary dilation. In addition to a clinical neurological examination, peripheral neuropathy was assessed through the measurement of peripheral nerves with an electroneuromyographic device (Medelec Synergy; Oxford Instruments, Oxford, UK). Motor and sensory amplitudes, conduction velocities, F responses and latencies were recorded. If two or more different nerves showed abnormal findings greater than two standard deviations from the normal range in three or more of four parameters, the patient was determined as having neurophysiologically diagnosed peripheral polyneuropathy [10,11]. Deep breathing (E/I ratio), Valsalva and posture tests were used to assess cardiovascular autonomic function with the Monitor one nDx (QMed Inc., Entontown, NJ, USA). Cardiovascular autonomic neuropathy was diagnosed in patients having at least two abnormal tests: an abnormal E/I ratio based on age-adjusted norms, a Valsalva ratio <1.2 or a 30:15 ratio <1.03.
Coronary heart disease and cerebrovascular disease were defined as cardiovascular disease. A diagnosis of coronary heart disease was based on a history of myocardial infarction, angina pectoris or ischemic heart disease, coronary artery angioplasty, or pathological findings coded using Minnesota codes 1.1 to 3, 4.1 to 4, and 5.1 to 3 in the resting electrocardiogram [12]. Cerebrovascular disease (CVD) was defined according to medical records, if pathological findings such as ischemic stroke on computed tomography or an area of low signal intensity measuring at least 3 mm on T1-weighted images and a hyperintense lesion on T2-weighted images on magnetic resonance imaging of the brain had been documented [13].
Statistical analysis
Data were expressed as the mean±standard deviation, unless otherwise stated. A χ2 test was used to determine significance, if any, in the differences measured between groups for each variable. Continuous variables were analyzed using Mann-Whitney U test or a Student's t-test. Multivariate logistic regression analyses were performed to determine associations between DPN and other diabetic complications. Using the logistic regression model with the forward Wald method, multivariate analyses were performed with identified independent variables and factors previously reported to have independent associations in order to analyze the association of DPN with cardiovascular disease. Statistical analysis was performed using SPSS software version 17.0 (SPSS Inc., Chicago, IL, USA). A P value of less than 0.05 was considered statistically significant.
RESULTS
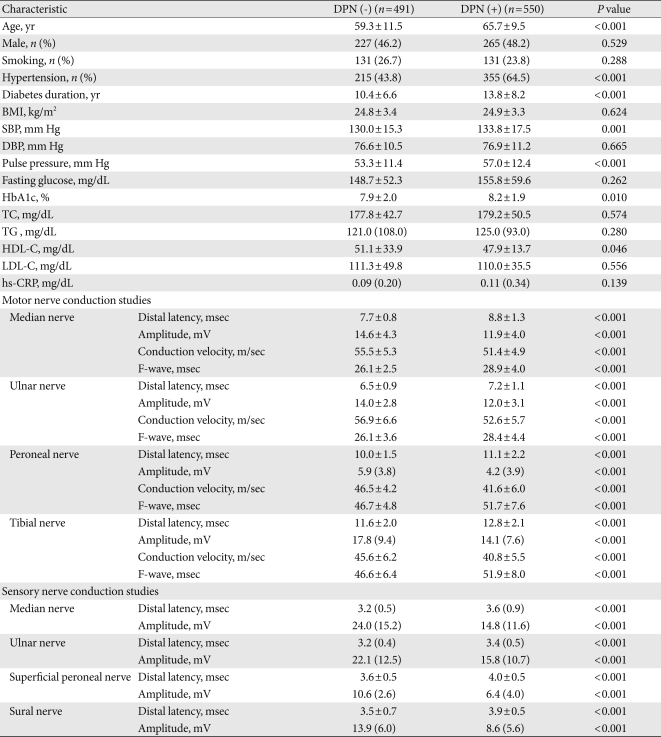
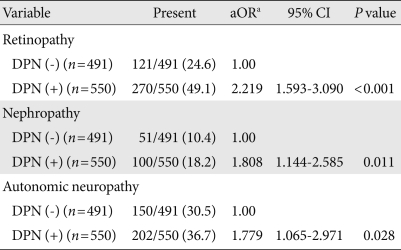
The mean age of the participants was 62.6±11.1 years; diabetes duration and HbA1c level were 12.2±7.7 years and 8.1±2.0%, respectively. The treatment methods for diabetes were: lifestyle modification alone (6.8%), oral hypoglycemic agents alone (61.2%), insulin dosing (11.8%), or a combination of oral hypoglycemic agents and insulin (20.2%). The clinical findings and laboratory data of type 2 diabetic patients, with and without DPN, are shown in Table 1. Of a total of 1,041 type 2 diabetic patients, 550 patients were in the DPN group. In patients with DPN, the age, prevalence of hypertension, diabetes duration, systolic blood pressure, pulse pressure, and HbA1c level were significantly higher, while the HDL-C level was significantly lower compared to those in patients without DPN. After age adjustment, there were also significant differences in systolic blood pressure, diabetes duration, pulse pressure and HbA1c between patients with and without DPN (P<0.05, respectively). In motor and sensory nerve studies, there were significant differences between patients with and without DPN (Table 1). The prevalence of diabetic retinopathy, nephropathy, or autonomic neuropathy was higher in patients with DPN, remaining significant after adjustment for age, diabetes duration, and hypertension (Table 2).
Table 1.
Characteristics of type 2 diabetic patients with or without diabetic polyneuropathy
Data are mean±standard deviation or median (interquartile range). Number in parenthesis is percentage. Analyzed using Mann-Whitney U test, Student's t-test or χ2 test.
DPN, diabetic polyneuropathy; BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; HbA1c, hemoglobin glycation; TC, total cholesterol; TG, triglyceride; HDL-C, high density lipoprotein cholesterol; LDL-C, low density lipoprotein cholesterol; hs-CRP, high-sensitivity C-reactive protein.
Table 2.
Associations of diabetic polyneuropathy (DPN) with other microvascular complications in type 2 diabetic patients
Number in parenthesis is percentage. Analyzed using χ2 test and multivariate logistic regression.
OR, odds ratio; CI, confidence interval.
aAdjusted for age, diabetes duration and hypertension.
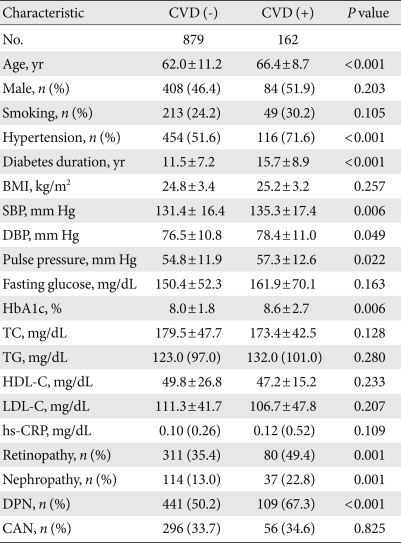
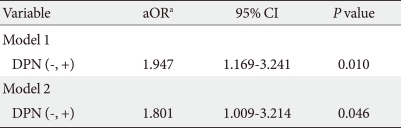
CVD was significantly associated with increased age, greater prevalence of hypertension, longer diabetes duration, a wider range in pulse pressure, and higher HbA1c level (Table 3). The prevalence of diabetic retinopathy, nephropathy or DPN was higher in patients with CVD. To identify the significant independent determinants for CVD in all patients, logistic regression analyses were performed. In univariate analysis, DPN was associated with CVD (odds ratio [OR], 2.043; 95% confidence interval [CI], 1.434 to 2.910). For the multivariate analysis, we included the identified independent variables and factors previously reported to have independent associations with CVD. Here, DPN was independently associated with CVD (OR, 1.947; 95% CI, 1.169 to 3.241) (Table 4). When retinopathy, nephropathy, and autonomic neuropathy were also included in this model (model 2), DPN was also independently associated with CVD (OR, 1.801; 95% CI, 1.009 to 3.214).
Table 3.
Characteristics of type 2 diabetic patients in association with cardiovascular complications
Data are mean±standard deviation or median (interquartile range). Number in parenthesis is percentage. Analyzed using Mann-Whitney U test, Student's t-test or χ2 test.
CVD, cardiovascular disease; BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; HbA1c, hemoglobin glycation; TG, triglyceride; HDL-C, high density lipoprotein cholesterol; LDL-C, low density lipoprotein cholesterol; hs-CRP, high-sensitivity C-reactive protein; DPN, diabetic polyneuropathy; CAN, cardiovascular autonomic neuropathy.
Table 4.
Multivariate logistic regression analysis with CVD as a dependent variable
OR, odds ratio; CI, confidence interval.
aModel 1: adjusted for age, sex, smoking, body mass index, hypertension, HbA1c, diabetes duration, low density lipoprotein cholesterol, high density lipoprotein cholesterol, and pulse pressure; Model 2: model 1 plus retinopathy, nephropathy, and autonomic neuropathy.
DISCUSSION
In this study, our results showed that other diabetic complications, e.g., diabetic retinopathy and nephropathy were more prevalent in type 2 diabetic patients with DPN. Also, we found that there was a strong association between DPN and CVD prevalence in type 2 diabetic patients.
Type 2 diabetic patients with DPN had longer diabetes duration and higher HbA1c levels than those without DPN supporting previous studies [14,15]. Also, DPN was associated with higher prevalence of retinopathy and nephropathy. Several prior studies have suggested that DPN is closely associated with diabetic retinopathy. O'Hare et al. [16] showed that diabetic neuropathy was associated with retinopathy in type 2 diabetes mellitus. Cohen et al. [17] demonstrated that DPN was associated with the high prevalence of both retinopathy and overt proteinuria in type 2 diabetic patients. However, a relationship between DPN and diabetic nephropathy has not been consistently demonstrated. Shaw et al. [18] reported that a significant proportion of type 1 diabetic patients with diabetic nephropathy did not have diabetic peripheral neuropathy. Recently, Karvestedt et al. [19] reported that DPN was more common in type 2 diabetic patients with overt proteinuria than it was in those without overt proteinuria. Results from these prior studies, in conjunction with our findings, suggest a close relationship between neuropathy and nephropathy, as well as between neuropathy and retinopathy.
DPN is known to predict foot ulceration, lower-extremity amputation, and mortality [20]. The presence of cardiovascular autonomic neuropathy contributes to a higher mortality rate in patients with diabetes mellitus, in part due to asymptomatic (painless) myocardial ischemia and hence delayed diagnosis [2]. Recently, associations between DPN and macrovasculopathy have been suggested [21,22]. Coppini et al. [23] showed that higher vibration perception thresholds were more strongly associated with increased mortality than were other microvascular complications in diabetic patients. Forsblom et al. [24] reported that neurophysiologically diagnosed neuropathy might have a direct role for cardiovascular mortality in type 2 diabetes. In the Detection of Ischemia in Asymptomatic Diabetics (DIAD) study, a significant relation to CVD outcomes such as nonfatal myocardial infarction and cardiac death was found with symptom (numbness) and sign (absent sensation) of peripheral polyneuropathy [25]. The Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial suggested the possible role of cardiac autonomic neuropathy and peripheral polyneuropathy as a strong predictor for CVD mortality [26]. Also, some studies have demonstrated that distal symmetrical neuropathy is associated with medial arterial calcification in diabetes mellitus patients [6,7], which has been reported to be associated with an increase in coronary heart disease and all-cause mortality in type 2 diabetes mellitus patients [8,9]. Yoshida et al. [27] reported that the extent of coronary artery calcification measured by electron beam computed tomography was significantly higher in patients with DPN. In the present study, DPN was associated with a high prevalence of risk factors for macrovascular complications, such as poor metabolic control, dyslipidemia, hypertension, increased pulse pressure, and albuminuria. Also, DPN was associated with a high prevalence of CVD in type 2 diabetes patients. Thus, the results in the present study may support a common pathogenic mechanism as the relationship between neuropathy and macrovascular complications [19,22,28-30], suggesting the roles of vascular and metabolic factors. However, further studies are needed to investigate the mechanism that links DPN with cardiovascular complications.
This study has some limitations. Although nerve electrophysiologic studies are the sensitive, objective and highly reproducible means of investigating diabetic polyneuropathy and have a key role in the elimination of other causes of neuropathy, they have limitations in the assessment of small-fiber dysfunction [10]. Also, because our study was cross-sectional, the causative natures of the associations between DPN and CVD cannot be established. Further investigation is necessary to evaluate whether DPN is associated with incident CVD among type 2 diabetic adults and to determine possible mechanisms between DPN and CVD adverse outcomes.
In conclusion, this study shows that DPN is associated with a high prevalence of cardiovascular disease in type 2 diabetic patients and suggests that type 2 diabetic patients with DPN may warrant a more careful cardiovascular assessment and follow-up.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Grundy SM, Benjamin IJ, Burke GL, Chait A, Eckel RH, Howard BV, Mitch W, Smith SC, Jr, Sowers JR. Diabetes and cardiovascular disease: a statement for healthcare professionals from the American Heart Association. Circulation. 1999;100:1134–1146. doi: 10.1161/01.cir.100.10.1134. [DOI] [PubMed] [Google Scholar]
- 2.Maser RE, Mitchell BD, Vinik AI, Freeman R. The association between cardiovascular autonomic neuropathy and mortality in individuals with diabetes: a meta-analysis. Diabetes Care. 2003;26:1895–1901. doi: 10.2337/diacare.26.6.1895. [DOI] [PubMed] [Google Scholar]
- 3.Ninomiya T, Perkovic V, de Galan BE, Zoungas S, Pillai A, Jardine M, Patel A, Cass A, Neal B, Poulter N, Mogensen CE, Cooper M, Marre M, Williams B, Hamet P, Mancia G, Woodward M, Macmahon S, Chalmers J ADVANCE Collaborative Group. Albuminuria and kidney function independently predict cardiovascular and renal outcomes in diabetes. J Am Soc Nephrol. 2009;20:1813–1821. doi: 10.1681/ASN.2008121270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ogawa O, Hayashi C, Nakaniwa T, Tanaka Y, Kawamori R. Arterial stiffness is associated with diabetic retinopathy in type 2 diabetes. Diabetes Res Clin Pract. 2005;68:162–166. doi: 10.1016/j.diabres.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 5.Bloomgarden ZT. Diabetic retinopathy and neuropathy. Diabetes Care. 2005;28:963–970. doi: 10.2337/diacare.28.4.963. [DOI] [PubMed] [Google Scholar]
- 6.Jeffcoate WJ, Rasmussen LM, Hofbauer LC, Game FL. Medial arterial calcification in diabetes and its relationship to neuropathy. Diabetologia. 2009;52:2478–2488. doi: 10.1007/s00125-009-1521-6. [DOI] [PubMed] [Google Scholar]
- 7.Young MJ, Adams JE, Anderson GF, Boulton AJ, Cavanagh PR. Medial arterial calcification in the feet of diabetic patients and matched non-diabetic control subjects. Diabetologia. 1993;36:615–621. doi: 10.1007/BF00404070. [DOI] [PubMed] [Google Scholar]
- 8.Everhart JE, Pettitt DJ, Knowler WC, Rose FA, Bennett PH. Medial arterial calcification and its association with mortality and complications of diabetes. Diabetologia. 1988;31:16–23. doi: 10.1007/BF00279127. [DOI] [PubMed] [Google Scholar]
- 9.Lehto S, Niskanen L, Suhonen M, Ronnemaa T, Laakso M. Medial artery calcification. A neglected harbinger of cardiovascular complications in non-insulin-dependent diabetes mellitus. Arterioscler Thromb Vasc Biol. 1996;16:978–983. doi: 10.1161/01.atv.16.8.978. [DOI] [PubMed] [Google Scholar]
- 10.Claus D, Mustafa C, Vogel W, Herz M, Neundorfer B. Assessment of diabetic neuropathy: definition of norm and discrimination of abnormal nerve function. Muscle Nerve. 1993;16:757–768. doi: 10.1002/mus.880160711. [DOI] [PubMed] [Google Scholar]
- 11.Andersen H, Stalberg E, Falck B. F-wave latency, the most sensitive nerve conduction parameter in patients with diabetes mellitus. Muscle Nerve. 1997;20:1296–1302. doi: 10.1002/(sici)1097-4598(199710)20:10<1296::aid-mus12>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 12.Rose GA, Blackburn H. Cardiovascular survey methods. Monogr Ser World Health Organ. 1968;56:1–188. [PubMed] [Google Scholar]
- 13.Saver JL. Proposal for a universal definition of cerebral infarction. Stroke. 2008;39:3110–3115. doi: 10.1161/STROKEAHA.108.518415. [DOI] [PubMed] [Google Scholar]
- 14.Franklin GM, Shetterly SM, Cohen JA, Baxter J, Hamman RF The San Luis Valley Diabetes Study. Risk factors for distal symmetric neuropathy in NIDDM. Diabetes Care. 1994;17:1172–1177. doi: 10.2337/diacare.17.10.1172. [DOI] [PubMed] [Google Scholar]
- 15.Valensi P, Giroux C, Seeboth-Ghalayini B, Attali JR. Diabetic peripheral neuropathy: effects of age, duration of diabetes, glycemic control, and vascular factors. J Diabetes Complications. 1997;11:27–34. doi: 10.1016/s1056-8727(95)00086-0. [DOI] [PubMed] [Google Scholar]
- 16.O'Hare JA, Abuaisha F, Geoghegan M. Prevalence and forms of neuropathic morbidity in 800 diabetics. Ir J Med Sci. 1994;163:132–135. doi: 10.1007/BF02965972. [DOI] [PubMed] [Google Scholar]
- 17.Cohen JA, Jeffers BW, Faldut D, Marcoux M, Schrier RW. Risks for sensorimotor peripheral neuropathy and autonomic neuropathy in non-insulin-dependent diabetes mellitus (NIDDM) Muscle Nerve. 1998;21:72–80. doi: 10.1002/(sici)1097-4598(199801)21:1<72::aid-mus10>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 18.Shaw JE, Gokal R, Hollis S, Boulton AJ. Does peripheral neuropathy invariably accompany nephropathy in type 1 diabetes mellitus? Diabetes Res Clin Pract. 1998;39:55–61. doi: 10.1016/s0168-8227(97)00122-8. [DOI] [PubMed] [Google Scholar]
- 19.Karvestedt L, Martensson E, Grill V, Elofsson S, von Wendt G, Hamsten A, Brismar K. Peripheral sensory neuropathy associates with micro- or macroangiopathy: results from a population-based study of type 2 diabetic patients in Sweden. Diabetes Care. 2009;32:317–322. doi: 10.2337/dc08-1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carrington AL, Shaw JE, Van Schie CH, Abbott CA, Vileikyte L, Boulton AJ. Can motor nerve conduction velocity predict foot problems in diabetic subjects over a 6-year outcome period? Diabetes Care. 2002;25:2010–2015. doi: 10.2337/diacare.25.11.2010. [DOI] [PubMed] [Google Scholar]
- 21.Elliott J, Tesfaye S, Chaturvedi N, Gandhi RA, Stevens LK, Emery C, Fuller JH EURODIAB Prospective Complications Study Group. Large-fiber dysfunction in diabetic peripheral neuropathy is predicted by cardiovascular risk factors. Diabetes Care. 2009;32:1896–1900. doi: 10.2337/dc09-0554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yokoyama H, Yokota Y, Tada J, Kanno S. Diabetic neuropathy is closely associated with arterial stiffening and thickness in type 2 diabetes. Diabet Med. 2007;24:1329–1335. doi: 10.1111/j.1464-5491.2007.02278.x. [DOI] [PubMed] [Google Scholar]
- 23.Coppini DV, Bowtell PA, Weng C, Young PJ, Sonksen PH. Showing neuropathy is related to increased mortality in diabetic patients: a survival analysis using an accelerated failure time model. J Clin Epidemiol. 2000;53:519–523. doi: 10.1016/s0895-4356(99)00170-5. [DOI] [PubMed] [Google Scholar]
- 24.Forsblom CM, Sane T, Groop PH, Totterman KJ, Kallio M, Saloranta C, Laasonen L, Summanen P, Lepantalo M, Laatikainen L, Matikainen E, Teppo AM, Koskimies S, Groop L. Risk factors for mortality in type II (non-insulin-dependent) diabetes: evidence of a role for neuropathy and a protective effect of HLA-DR4. Diabetologia. 1998;41:1253–1262. doi: 10.1007/s001250051062. [DOI] [PubMed] [Google Scholar]
- 25.Young LH, Wackers FJ, Chyun DA, Davey JA, Barrett EJ, Taillefer R, Heller GV, Iskandrian AE, Wittlin SD, Filipchuk N, Ratner RE, Inzucchi SE DIAD Investigators. Cardiac outcomes after screening for asymptomatic coronary artery disease in patients with type 2 diabetes: the DIAD study--a randomized controlled trial. JAMA. 2009;301:1547–1555. doi: 10.1001/jama.2009.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pop-Busui R, Evans GW, Gerstein HC, Fonseca V, Fleg JL, Hoogwerf BJ, Genuth S, Grimm RH, Corson MA, Prineas R Action to Control Cardiovascular Risk in Diabetes Study Group. Effects of cardiac autonomic dysfunction on mortality risk in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial. Diabetes Care. 2010;33:1578–1584. doi: 10.2337/dc10-0125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yoshida M, Takamatsu J, Yoshida S, Tanaka K, Takeda K, Higashi H, Kitaoka H, Ohsawa N. Scores of coronary calcification determined by electron beam computed tomography are closely related to the extent of diabetes-specific complications. Horm Metab Res. 1999;31:558–563. doi: 10.1055/s-2007-978795. [DOI] [PubMed] [Google Scholar]
- 28.Eaton SE, Harris ND, Ibrahim S, Patel KA, Selmi F, Radatz M, Ward JD, Tesfaye S. Increased sural nerve epineurial blood flow in human subjects with painful diabetic neuropathy. Diabetologia. 2003;46:934–939. doi: 10.1007/s00125-003-1127-3. [DOI] [PubMed] [Google Scholar]
- 29.Zenere BM, Arcaro G, Saggiani F, Rossi L, Muggeo M, Lechi A. Noninvasive detection of functional alterations of the arterial wall in IDDM patients with and without microalbuminuria. Diabetes Care. 1995;18:975–982. doi: 10.2337/diacare.18.7.975. [DOI] [PubMed] [Google Scholar]
- 30.Cameron NE, Eaton SE, Cotter MA, Tesfaye S. Vascular factors and metabolic interactions in the pathogenesis of diabetic neuropathy. Diabetologia. 2001;44:1973–1988. doi: 10.1007/s001250100001. [DOI] [PubMed] [Google Scholar]