Abstract
A reported association between estrogen and selenium status may be important in the regulation of selenium metabolism. In this study the effect of estrogen status on the metabolism of orally administered 75Se-selenite and tissue selenium status was investigated. Female Sprague Dawley rats were bilaterally ovariectomized at 7 weeks of age and implanted with either a placebo pellet (OVX) or pellet containing estradiol (OVX+E2), or were sham operated (Sham). At 12 weeks of age, 60 μCi of 75Se as selenite was orally administered to OVX and OVX+E2 rats. Blood and organs were collected 1, 3, 6, and 24h after dosing. Estrogen status was associated with time dependent differences in distribution of 75Se in plasma, RBC, liver, heart, kidney, spleen, brain, and thymus and incorporation of 75Se into plasma selenoprotein P (Sepp1) and glutathione peroxidase (GPx). Estrogen-treatment also significantly increased selenium concentration and GPx activity in plasma, liver, and brain, selenium concentration in RBC, and hepatic Sepp1 and GPx1 mRNA. These results suggest that estrogen status affects tissue distribution of selenium by modulating Sepp1 as this protein plays a central role in selenium transport.
Keywords: selenium, estrogen, selenoprotein P, glutathione peroxidase, ovariectomized rats
1. INTRODUCTION
A relationship between estrogen status and selenium metabolism has been suggested. Selenium concentrations in blood [1,2], liver [3,4], and brain [5] have been reported to differ in adult male and female mammals and blood glutathione peroxidase (GPx) activity declines during pregnancy in rats [6] and humans [7,8], suggesting that estrogen modulates selenium metabolism. Blood selenium and GPx activity were positively correlated with estrogen concentrations during the rat estrous cycle [9] and the human menstrual cycle [10]. Also, estrogen administration significantly increased erythrocyte GPx activity in both premenopausal [11] and postmenopausal women [12,13]. 17β-estradiol administration increased the amount of GPx4 mRNA, but not GPx1 mRNA or GPx3 mRNA, in the bovine oviduct [14]. GPx mRNA expression in hepatoma H4IIE cells exposed to the dietary phytoestrogen daidzein was also increased by 40% [15], but not by the addition of 17β-estradiol to cultures of human endothelial cells [16].
Twenty-five human selenoproteins have been identified including multiple isoforms of GPx [17]. Selenoprotein P (Sepp1) has attracted much attention in light of its role in the transport of selenium from liver to other tissues [18,19]. Sepp1 synthesis is regulated by dietary selenium intake as inadequate selenium is associated with decreased plasma Sepp1 in rats [20] and humans [21] and significantly lower Sepp1 mRNA levels in rat liver and kidney [22]. The potential influence of estrogen status on Sepp1 expression and activity has not been previously examined.
Sex-based differences in the health effects of selenium have been reported in a number of epidemiologic studies [23]. Results from prospective human studies indicate that anticarcinogenic effects of selenium are generally greater in men than in women [24], suggesting endocrine influences on the health-promoting effects of selenium. Although previous research has focused on the effect of estrogen status or estrogen administration on selenium concentration and GPx activity in plasma and erythrocytes, more specific investigations of the impact of estrogen on distribution and metabolism of selenium in multiple tissues are limited. The purpose of this study was to investigate the effects of estrogen status on the apparent absorption, tissue distribution and incorporation of selenium into selenoproteins. The effect of estrogen status on selenium status also was determined by determining selenium concentrations and GPx activity in different tissues, hepatic levels of Sepp1 mRNA and GPx mRNA, and plasma Sepp1.
2. MATERIALS AND METHODS
2.1. Animals and diets
Female Sprague Dawley rats (Taconic, Germantown, NY) weighing 45–55 g were fed the casein-based AIN-93G diet containing 150 μg total Se/kg diet as sodium selenate (Research Diets, New Brunswick, NJ) beginning at weaning (3 weeks) and throughout the study. At 7 weeks of age, rats were randomly divided into three groups. The first two groups underwent ovariectomy with (OVX+E2, n=22) or without (OVX, n=22) estrogen replacement. The third group was sham operated (Sham, n=24) and designed for 6 rats to be killed on each day of the estrous cycle. Estrous cycles were determined by examining vaginal smears as described previously [9]. Rats in the OVX+E2 group were implanted with a subcutaneous pellet containing 1.5mg 17β-estradiol for release over 60 days (Innovative Research of America, Sarasota, FL). Rats in the OVX and Sham groups were subcutaneously implanted with a placebo pellet. The animals were housed in individual plastic cages in a room with controlled temperature (20–22°) and a 12h light-dark cycle with lights off from 1800 to 0600 h. Animals were fed between 0800 and 0900h. Because OVX+E2 rats had lower food intakes than the other groups, the OVX+E2 group was given free access to diet, and the OVX and Sham groups were pair-fed the mean intake of the OVX+E2 group during the previous day in order to maintain similar caloric and selenium intake among animals. Food intakes were recorded daily and body weights were recorded weekly. Animals were fasted overnight before sacrifice. All procedures involving animals were approved by the Institutional Animal Care and Use Committee of The Ohio State University.
2.2. Experimental design
The effect of estrogen on the metabolism of 75Se-selenite was studied in the OVX+E2 and OVX groups because they were expected to have the higher and lower plasma concentrations of estrogen respectively, than those for the Sham group. At 12 weeks of age, the OVX and OVX+E2 groups were administered 60 μCi of 75Se-sodium selenite with specific activity of 1,400 Ci/g (University of Missouri Research Reactor Facility, Columbia, MO) in 1.0 ml 150 mM NaCl by gavage. Four rats in each group were killed 1, 3, 6, and 24 h after 75Se administration. Rats were anesthetized by brief exposure to carbon dioxide prior to terminal cardiac puncture for collection of blood into syringes containing 10 mg Na2EDTA in 100 μl saline. Liver, kidney, heart, brain, lung, thymus, spleen, and the gastrointestinal tract (GI tract, including esophagus, stomach, duodenum, small intestine, large intestine, rectum, and the anus) and its contents were collected. Another set of rats from the OVX (n=6), OVX+E2 (n=6), and Sham (n=24) groups that did not receive 75Se were also killed at 12 weeks of age to collect blood, liver, kidney, heart, and brain for determination of total selenium content, GPx activity, hepatic levels of GPx and Sepp1 mRNAs, and plasma Sepp1. All samples were stored at −80° before analysis.
2.3. Plasma 17β-estradiol and ceruloplasmin activity
Concentration of plasma 17β-estradiol was determined using a double-antibody estradiol 125I radioimmunoassay kit (Diagnostic Products Corp., Los Angeles, CA). Because the copper containing protein ceruloplasmin has been shown to be upregulated by estrogen [25], ceruloplasmin enzyme activity served as a positive control for estrogen-mediated alteration of trace element metabolism. Ceruloplasmin activity was measured according to Schosinsky et al. [26] with o-dianisidine dihydrochloride as the substrate.
2.4. 75Se in tissues, cytosol, and membrane
75Se was determined in plasma, RBC, and organs with a Cobra II auto-gamma counter (Packard Instrument Company, Meriden, CT). As organs were not perfused during collection, contamination of erythrocytes and plasma was estimated via hemoglobin (Hb) concentrations and subtracted from the totals in organs. For organs in which the 75Se activity differed between the OVX and OVX+E2 groups, the distribution of 75Se in total membrane (organelle) and cytoplasmic compartments was determined. Organs were homogenized in 50 mM phosphate buffer (25% homogenate) containing 10% sucrose, pH 6.3, using a Brinkman Polytron homogenizer (Brinkman Instruments Co., Westbury, NY). An aliquot of the homogenate was centrifuged at 4° for 90 min at 110,000 × g (Ti 50 rotor, Beckman Model L7-65, Palo Alto, CA) and supernatant (cytosol) was removed to measure 75Se activity. Activity was normalized by protein concentration determined by the bicinchoninic acid method (BCA protein assay kit, Pierce, Rockford, IL). 75Se activity in the membrane fraction was estimated by subtracting activity in cytosol from the total activity in homogenate.
2.5. Determination of 75Se in selenoproteins
The incorporation of 75Se into selenoproteins was determined using sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Proteins in plasma and cytosol were separated with 5% stacking and 12% resolving gels. 10 μl samples containing 50–100 μg protein was loaded to each well. Gels were sliced according to the known molecular weights of well characterized selenoproteins and the 75Se activity in gel slices was measured by gamma ray spectrometry (Packard Cobra II Auto Gamma; Packard Instrument Company, Meriden, CT). This procedure provides a convenient method to examine the 75Se distribution among selenoproteins without the use of antibodies, and has been used to identify selenoproteins with molecular weights from 12 kDa to 75 kDa [27]. Total protein 75Se was defined as the sum of 75Se activity of every band with molecular weight between 12 kDa and 75 kDa. Here we investigated the distribution of 75Se into Sepp1 and GPx by using this method.
2.6. Selenium Status
Selenium concentrations in diet, plasma, RBC, liver, kidney, heart, and brain were measured by the gas chromatography technique of McCarthy et al. [28] using an Agilent 6890 Series gas chromatograph with a 225 Durabond Megabor column and an electron capture detector maintained at the following temperatures: oven (column) 190°C, front inlet (injector) 220°C, and front detector 300°C. Nitrogen (~60 psi) and helium (~80 psi) were used as carrier gases. Data were integrated using an Agilent 6890 Series Integrator. GPx activity of plasma, RBC, liver, kidney, brain, and heart was determined by the coupled assay of Paglia and Valenine [29] using a Shimadzu UV-visible recording spectrophotometer (model UV160U). The activity was expressed as units per gram of protein (hemoglobin for RBC). One unit of activity is equivalent to 1 μmol NADPH oxidized per minute at 37°C. The protein concentration in plasma and cytosol was measured by the bicinchoninic acid method (Pierce, Rockford, IL) with the Shimadzu UV-visible recording spectrophotometer (model UV160U). The hemoglobin concentration of RBC was determined with the Shimadzu UV-visible recording spectrophotometer (model UV160U) using Drabkin’s reagent. Plasma Sepp1 concentration was determined by a competitive radioimmunoassay using a modified method of Hill et al. [30].
2.7. Hepatic levels of Sepp1 mRNA and GPx1 mRNA
Total RNA was extracted from the liver of rats in the OVX (n=6) and OVX+E2 (n=6) groups using a RNAqueous-4PCR kit (Ambion, Austin, Texas). First stand cDNA was synthesized from 1 μg RNA samples using the SuperScript First-Strand Synthesis System for RT-PCR (Invitrogen, Carlsbad, CA). To quantify the cDNA of Sepp1 and GPx1 mRNA, quantitative real-time PCR analysis was performed in an Mx3000P QPCR® system (Stratagene, La Jolla, CA) using the published primers for Sepp1 [31], GPx1[32], and GAPDH [33]. Temperature cycling conditions were 95°C for 2 min, 45 cycles of 95°C for 30 s, and 60°C for 1 min. The amounts of GAPDH mRNA were used to normalize expression.
2.8. Statistical methods
Data were reported as means±SE. The Student t-test was used to detect the differences between OVX+E2 and OVX groups. Analysis of Variance (ANOVA) was utilized to determine the differences of the indices at different times after administering 75Se, and to determine the differences of the indices among treatment groups in the non-radioactive study. Differences were considered significant at P<0.05. When significant difference among the means was indicated, Tukey’s post hoc probability test was performed to determine which means differed significantly. All statistical analyses were performed using SPSS V18 (SPSS Inc., Chicago, IL).
3. RESULTS
3.1. Body weights
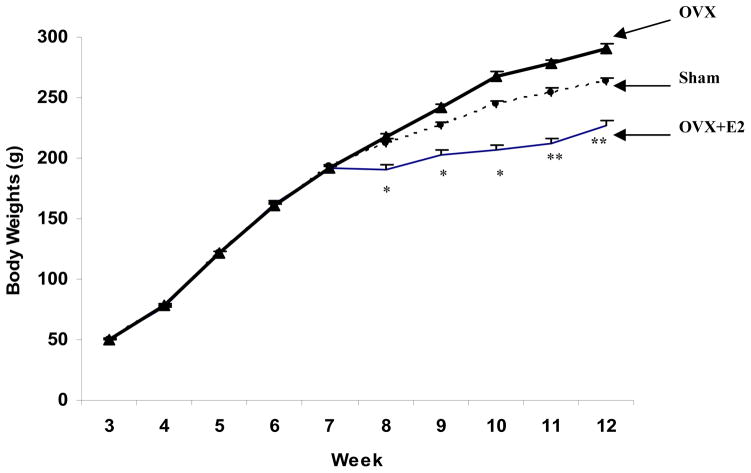
Body weights for the three experimental groups prior to the ovariectomy and sham operations at 7 weeks of age were not significantly different (P > 0.05, Fig. 1). Chronic administration of estrogen to ovariectomized rats (OVX+E2) decreased body weight gain during the first post-operative week. Rats in the OVX group had the most rapid increase in body weight, followed by the Sham group, and then the OVX+E2 group despite using a pair-feeding paradigm. At weeks 8–10, body weights of the OVX and Sham groups were not significantly different, but significantly greater than that of the OVX+E2 group (P < 0.05). At weeks 11 and 12, body weight differed significantly among the three groups with OVX > Sham > OVX+E2 (P < 0.05).
Fig.1.
Body weights of female SD rats pre- and post-surgery. Surgery was performed at 7 weeks of age. Rats were randomized into three treatment groups: OVX (n=22); OVX+E2 (n=22); and Sham (n=24). Data are means ± SEM. * Body weights of OVX+E2 group were significantly less than that of the other two groups (P < 0.05) at weeks 8–10. ** At weeks 11 and 12, body weights of OVX >Sham >OVX+E2 (P < 0.05).
3.2. Plasma 17β-estradiol concentrations and ceruloplasmin activity
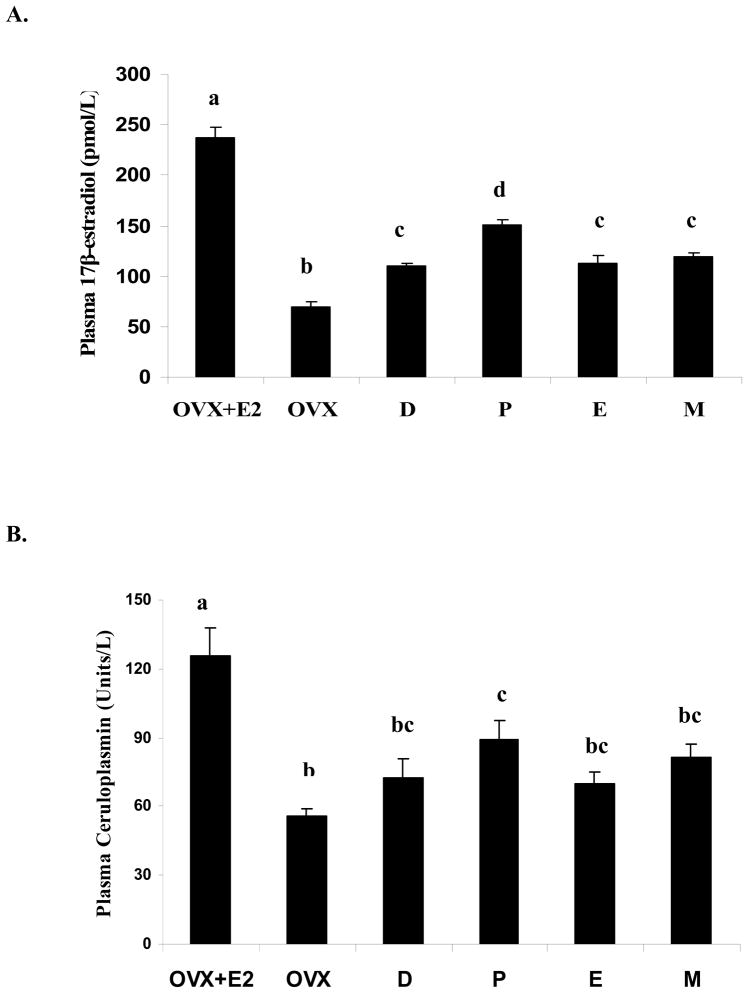
Ovariectomy (OVX) significantly (P < 0.05) reduced plasma 17β-estradiol concentration, whereas estrogen replacement (OVX+E2) significantly (P < 0.05) increased plasma 17β-estradiol concentrations greater than that in sham operated rats (Fig. 2A). Plasma 17β-estradiol levels were significantly greater during proestrus than at other stages of the estrus cycle (P<0.05) in sham-operated rats, which is a typical pattern during the 4-day estrus cycle. Plasma ceruloplasmin activity was positively associated with plasma 17β-estradiol concentration (r=0.78, P<0.01) and significantly (P<0.05) greater in the OVX+E2 group than in the Sham and OVX groups (Fig. 2B). There were no differences in plasma ceruloplasmin activity between the OVX and the Sham groups except during the proestrus stage.
Fig.2.
Plasma concentration of 17β-estradiol (A) and ceruloplasmin activity (B) in treatment groups of female SD rats. The test groups include OVX with estrogen replacement (OVX+E2), OVX with placebo replacement (OVX), and sham-operated with placebo replacement in the diestrus (D), proestrus (P), estrus (E), and metestrus (M) stages. Data are means ± SEM for 6 animals in each group. Bars with different letters differ significantly (P<0.05).
3.3. 75Se activity in tissues
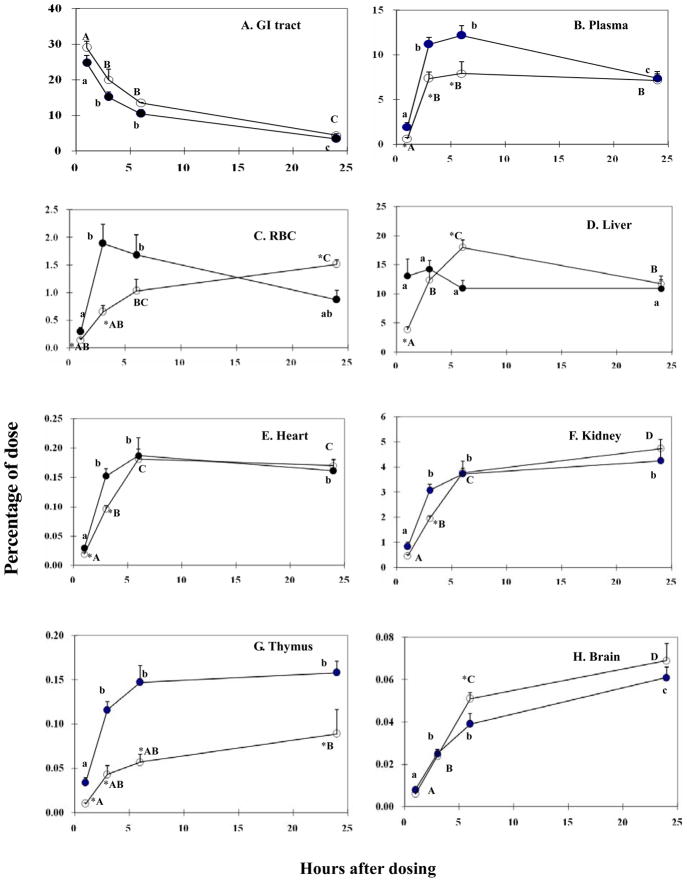
As expected, 75Se activity in the GI tract decreased from 1h to 24h with less than 5% of administered dose remaining in the gut by 24h (Fig. 3A). Because the amount of administered selenium excreted from feces during the first 24h is known to be relatively small [34], our results suggest efficient absorption of 75Se by all treatment groups. There were no significant differences in 75Se activity in the GI tract between the OVX and OVX+E2 groups at any test time. There was a significant increase (P<0.05) of 75Se activity in blood and most organs in both groups from 1h to 3h (Fig. 3). 75Se activity differed (P<0.05) between the OVX+E2 and OVX groups in plasma at 1, 3, 6h (OVX>OVX+E2) (Fig. 3B), RBC at 1, 3 (OVX>OVX+E2), and 24h (OVX+E2>OVX) (Fig. 3C), liver at 1 (OVX>OVX+E2), 6h (OVX+E2>OVX) (Fig. 3D), heart at 1, 3h (OVX>OVX+E2) (Fig. 3E), kidney at 3h (OVX>OVX+E2) (Fig. 3F), spleen at 1h (OVX>OVX+E2, data not shown), thymus at all 4 times (OVX >OVX+E2) (Fig. 3G), and brain at 6h (OVX+E2>OVX) (Fig. 3H).
Fig. 3.
75Se activity in tissues at 1, 3, 6, and 24h after gastric administration of 60 μCi 75SeO32−. (OVX, closed circles; OVX+E2, open circles). Panels: A, GI tract; B, plasma; C, RBC; D, liver; E, heart; F, kidney; G, thymus; H, brain. Data are means ± SEM (n=4). Statistically significant difference (P<0.05) between OVX group and OVX+E2 group are denoted by presence of an asterisk (*). Within the same treatment (OVX or OVX+E2), means that do not share the same letter are significantly different at P<0.05.
3.4. Distribution of 75Se among subcellular fractions and selenoproteins
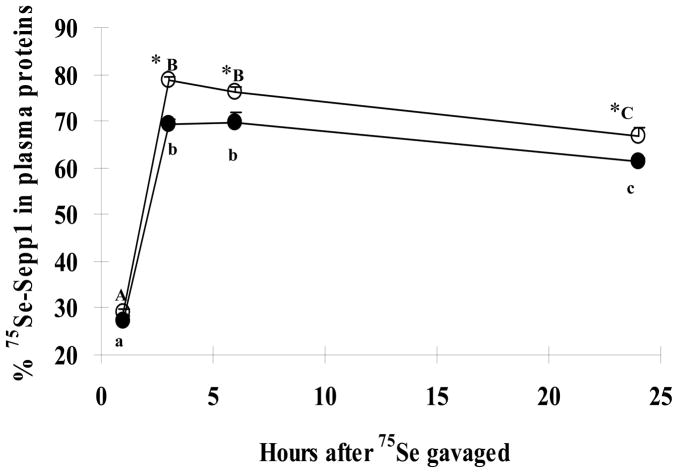
Within organs that differed in total 75Se activity between the OVX and OVX+E2 groups, 75Se activity in both cytosol (Table 1) and membrane fraction (data not shown) of these tissues also differed. The distribution of 75Se between cytosol and the membrane fraction was not significantly affected by chronic estrogen treatment (data not shown). Estrogen treatment also did not affect the fraction of 75Se incorporated into proteins and non-protein bound form in these organs (data not shown). 75Se-GPx activity accounted for 31–38% of the total protein 75Se activity in liver, 28%–31% in heart, and 24%–26% in kidney, spleen, thymus, and brain. In plasma, a greater percentage of 75Se was present in Sepp1 in the OVX+E2 group than in plasma from the OVX group at 3, 6 and 24h (Fig. 4). 75Se was not detected in plasma GPx at 1, 3, and 6h. However, a greater percentage (P < 0.05) of 75Se was associated with plasma GPx at 24h in the OVX+E2 group (8.6%) than for the OVX group (6.5%).
Table 1.
75Se activity in cytosol of organs in female SD rats1
| Organs | Time after dose | OVX | OVX+E2 |
|---|---|---|---|
| Liver | 1h | 22.4 ± 1.8 | 11.9 ± 1.7 * |
| Liver | 6h | 29.9 ± 2.3 | 43.1 ± 3.6 * |
| Heart | 1h | 0.3 ± 0.1 | 0.2 ± 0.1 * |
| Heart | 3h | 3.7 ± 0.7 | 1.8 ± 0.2 * |
| Kidney | 3h | 37.0 ± 4.6 | 28.1 ± 2.4 * |
| Spleen | 1h | 1.8 ± 0.3 | 0.7 ± 0.1 * |
| Thymus | 1h | 0.6 ± 0.2 | 0.4 ± 0.1 * |
| Thymus | 3h | 3.5 ± 0.6 | 2.2 ± 0.5 * |
| Brain | 6h | 0.3 ± 0.1 | 0.7 ± 0.1 * |
Data are means ± SEM (n=4, dpm/μg protein).
Statistical significance between OVX group and OVX+E2 group (P<0.05).
Fig. 4.
75Se activity in plasma Sepp1P at 1, 3, 6, and 24h after administration of 75SeO32− by gavage (OVX, closed circles; OVX+E2, open circles). Data are means ± SEM (n=4). Statistically significance (P<0.05) differences between OVX group and OVX+E2 group at a specific time after dosing are denoted by presence of an asterisk (*). Within the same treatment (OVX or OVX+E2), means that do not share the same letter differ significantly (P<0.05).
3.5. Tissue selenium concentrations and GPx activity
Estrogen status affected selenium concentrations in plasma, RBC, liver, and brain (Table 2). Plasma selenium concentration was significantly (P < 0.05) greater in OVX+E2 than sham-operated rats and plasma selenium was significantly (P <0.05) lower in OVX group than sham-operated rats at all stages of estrus cycle. Plasma selenium concentrations in sham-operated rats were greatest during proestrus. Selenium concentrations in RBC, liver, and brain were significantly higher in OVX+E2 rats than sham-operated rats at all stages of the estrus cycle, except proestrus. OVX rats had significantly lower selenium concentrations in RBC, liver, and brain compared to OVX+E2 and sham-operated rats in the proestrus stage. Selenium concentrations in kidney and heart were independent of plasma estrogen.
Table 2.
| OVX+E2 | OVX | Diestrus | Proestrus | Estrous | Metestrus | |
|---|---|---|---|---|---|---|
| Plasma, μmol/L | 5.61 ± 0.20a | 3.24 ± 0.08d | 4.12 ± 0.23c | 4.84 ± 0.13b | 4.07 ± 0.15c | 4.07 ± 0.18c |
| RBC, μmol/L | 8.13 ± 0.58a | 4.58 ± 0.33 c | 5.21 ± 0.57 bc | 7.03 ± 0.24ab | 4.86 ± 0.39c | 5.09 ± 0.46c |
| Liver, ng/g | 1,760 ± 37a | 1,295 ± 44 c | 1,505 ± 67 bc | 1,692 ± 27ab | 1,482 ± 68bc | 1,370 ± 67c |
| Kidney, ng/g | 1,357 ± 27 | 1,624 ± 47 | 1,563 ± 97 | 1,461 ± 15 | 1,587 ± 87 | 1,494 ± 62 |
| Heart, ng/g | 423 ± 25 | 411 ± 19 | 405 ± 22 | 450 ± 14 | 434 ± 21 | 432 ± 13 |
| Brain, ng/g | 441 ± 9a | 344 ± 9c | 363 ± 21 bc | 421 ± 16ab | 363 ± 21bc | 366 ± 21bc |
Test groups include OVX with estrogen replacement (OVX+E2), OVX with placebo replacement (OVX), and sham-operated with placebo replacement in diestrus (D), proestrus (P), estrus (E), and metestrus (M) stages.
Data are means ± SEM for 6 animals in each group and those not sharing a common superscript within each tissue are significantly different (P<0.05).
Estrogen status affected GPx activity in plasma, liver, and brain (Table 3). GPx activity was significantly (P < 0.05) greater in plasma of OVX+E2 rats compared to plasma of OVX rats and sham-operated rats during all estrous stages except proestrus. There were no significant differences in GPx activity in plasma and liver between OVX rats and sham-operated rats in all estrous stages except proestrus. OVX was associated with decreased GPx activity in brain, with significantly (P < 0.05) lower GPx activity than that of sham-operated rats in proestrus stage and OVX+E2 rats. OVX+E2 rats had greater GPx activity in brain than that of sham-operated rats in all estrous stages except for proestrus.
Table 3.
| OVX+E2 | OVX | Diestrus | Proestrus | Estrous | Metestrus | |
|---|---|---|---|---|---|---|
| Plasma | 146 ± 10a | 70 ± 6b | 71 ± 12b | 119 ± 10a | 75 ± 11b | 75 ± 6b |
| RBC | 470 ± 12 | 410 ± 39 | 427 ± 16 | 460 ± 42 | 419 ± 18 | 417 ± 43 |
| Liver | 1,702 ± 60a | 1,202 ± 90b | 1,230 ± 66 b | 1,658 ± 82a | 1,265 ± 96b | 1,290 ± 90b |
| Kidney | 272 ± 22 | 364 ± 48 | 364 ± 43 | 291 ± 18 | 316 ± 26 | 321 ± 41 |
| Heart | 289 ± 31 | 242 ± 27 | 250 ± 21 | 328 ± 26 | 296 ± 24 | 250 ± 34 |
| Brain | 199 ± 9a | 102 ± 7c | 133 ± 13 bc | 169 ± 5ab | 135 ± 14bc | 137 ± 17bc |
Test groups include OVX with estrogen replacement (OVX+E2), OVX with placebo replacement (OVX), sham-operated with placebo replacement in diestrus (D), proestrus (P), estrus (E), and metestrus (M) stages.
Data are means ± SEM (Units/gram protein, Units/gram hemoglobin for RBC) for 6 animals in each group and those not sharing a common superscript within each tissue are significantly different (P<0.05).
3.6. Plasma selenoprotein P concentration
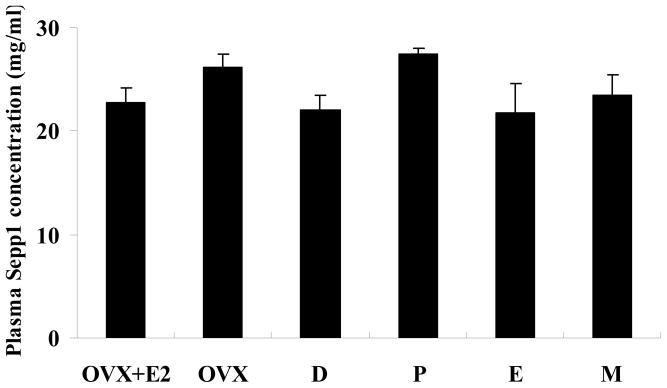
No differences in plasma Sepp1 concentrations were observed among groups. Plasma Sepp1 concentrations (μg/ml, means ± SEM, n=6) were 22.8 ± 1.4, 26.1 ± 1.4, 22.1 ± 1.3, 27.4 ± 0.6, 21.7 ± 2.9, 23.4 ± 2.1 for groups of OVX+E2, OVX, diestrus, proestrus, estrous, and metestrus stages, respectively (Fig. 5).
Fig.5.
Plasma Sepp1 concentration in treatment groups of female SD rats. The test groups include OVX with estrogen replacement (OVX+E2), OVX with placebo replacement (OVX), and sham-operated with placebo replacement in the diestrus (D), proestrus (P), estrus (E), and metestrus (M) stages. Data are means ± SEM for 6 animals in each group. No differences in plasma Sepp1 concentrations were observed among groups (P> 0.05).
3.7. Hepatic levels of Sepp1 and GPx1 mRNA
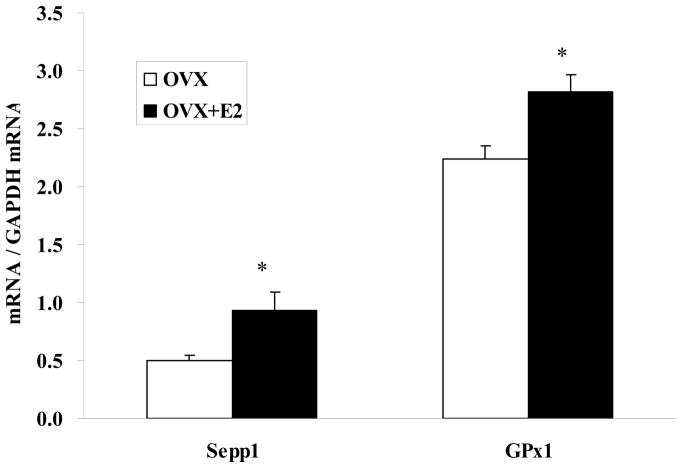
Hepatic Sepp1 mRNA levels were 88% greater in the OVX+E2 group compared to the OVX group (0.94 vs. 0.50, P<0.05) (Fig. 6). Likewise, the amount of GPX1 mRNA in liver of the OVX+E2 group was significantly (P < 0.05) greater than that in liver from the OVX group (2.8 vs. 2.2).
Fig. 6.
Estrogen replacement in OVX rats increases levels of hepatic Sepp1 and GPx1 mRNA. Real-time RT-PCR was carried out with extracted RNA isolated from liver of OVX rats receiving estrogen replacement (OVX+E2) and OVX rats with placebo pellet (OVX). Data are normalized against GAPDH mRNA and presented as means ± SEM for 6 animals in each group. * P<0.05 between OVX and OVX+E2.
4. DISCUSSION
An ovariectomized rat model was used to investigate the effect of estrogen status on selenium metabolism. The results indicate that estrogen affected the tissue distribution and metabolism of an oral dose of 75Se-selenite and increased total Se concentrations and GPx activity in plasma and tissues. Estrogen status for the three groups of rats was verified by measuring 17β-estradiol concentrations in plasma. Ovariectomy significantly decreased 17β-estradiol levels, whereas implantation with estrogen pellets elevated 17β estradiol to a concentration approximately 50% greater than that present during the proestrus phase of the estrus cycle in sham-operated rats (Fig. 2A). The results are also in agreement with previous studies in which estrogen treatment suppressed body weight gain [35,36] and increased plasma ceruloplasmin activity [25] (Fig. 2B). Since rats were pair-fed the same amount of selenium, OVX+E2 rats would have received relatively more selenium per unit body weight, suggesting that estrogen could regulate selenium metabolism via affecting the growth of animals.
The present study is the first to compare the impact of estrogen status on the apparent absorption and distribution of selenium among tissues, subcellular fractions, and selenoproteins. Although estrogen status did not affect selenium apparent absorption, it altered the uptake of 75Se into tissues. A surprisingly quick uptake of 75Se into RBCs (3h) and liver (1h) was observed in OVX rats (Fig. 3). The mechanism for this observation in RBCs and liver needs to be further elucidated. Estrogen also altered the distribution of 75Se in the plasma selenoproteins, Sepp1 and GPx. A greater percentage of 75Se was incorporated into plasma Sepp1 at 3, 6, and 24h in OVX+E2 group than in the OVX group (Fig. 4). Within plasma proteins, 75Se was associated primarily with Sepp1 in plasma. These results agree with previous studies using a similar method in which 68% of 75Se was deposited in Sepp1 when female rats were injected intraperitoneally with 75Se-selenomethione [37]. Previous studies have demonstrated that 75Se in plasma was distributed among Sepp1, GPx, and other un-characterized low molecular weight selenium [38]. In the present study approximately 30% and 70% of plasma 75Se was incorporated into Sepp1 and low molecular weight selenium, respectively, 1 h after gavage. This was followed by a rapid increase in 75Se incorporation into Sepp1 with approximately 75% plasma 75Se present in Sepp1 by 3h. Thereafter, 75Se content in Sepp1 declined suggesting that Sepp1 delivered newly absorbed selenium to other organs, including kidney, the site of plasma GPx synthesis. Though 75Se incorporation into Sepp1 was increased (Fig. 4) by estrogen treatment, plasma total 75Se was decreased (Fig. 3B), suggesting estrogen may affect the partitioning of 75Se among each component of plasma selenium, e.g. some components had increased 75Se incorporation, while others had decreased 75Se incorporation by estrogen treatment. 75Se-GPx was not detected in plasma until 24h. This finding is in agreement with previous studies in which 75Se-GPx was undectable in rat plasma during the first 6h after intravenous injection of 75Se-selenite, whereas 75Se-Sepp1 was detected at 1h [38].
The results of the present study also confirm that measures of selenium status in blood were affected by estrogen. Chronic estrogen treatment significantly (P<0.05) increased plasma GPx activity and RBC content of selenium. These results agree with those of the 75Se tracer study, in which 75Se activity was greater in plasma GPx (8.6% vs. 6.5% of the total protein 75Se activity) and RBC of the OVX+E2 group at 24h (Fig. 3C). As selenium concentrations were measured after 5 weeks of treatment (from 7wk to 12wk), it is not surprising that effects of estrogen treatment on selenium concentrations (Table 2) were opposite that of distribution of ingested 75Se in plasma, RBC, and liver at 1h (Fig 3). At later times, estrogen effects on ingested 75Se distribution were more similar to that of the selenium concentrations. It would be interesting to follow up the 75Se tracer study for a longer time, e.g. up to a few weeks to further determine the patterns of estrogen effects on 75Se distribution. The effect of estrogen on selenium status in human studies has been inconsistent. Whereas some studies have reported that estrogen increased blood selenium and GPx activity in women [10–13], others have not observed an effect of estrogen on selenium status [39–41]. These differences among human studies probably stem from differences in subject age, health status, selenium intake, and lack of control of estrogen status.
Earlier studies reported that hepatic GPx activity is greater in females than in males [1,3,4], suggesting hormonal influences on selenium utilization. In this study both hepatic selenium concentration (Table 2) and GPx activity (Table 3) were greater when estrogen was available in the OVX+E2 group and at the proestrus stage. The results also show that estrogen facilitated post-absorptive 75Se accumulation in liver during the initial 6h and 75Se efflux from liver to other tissues after 6h. Total 75Se in liver was greater in OVX than in OVX+E2 rats at 1h (13.1% vs.3.9% of dose, Fig. 3D), but 75Se activity increased in the OVX+E2 group markedly from 1 to 6h and was greater than in the OVX group (18.0% dose vs. 10.9% dose). Because the liver is the central organ for selenium metabolism, these effects of estrogen on hepatic selenium status have important implications for whole body selenium metabolism. More specifically, real-time RT-PCR showed that estrogen upregulated hepatic mRNA levels of both Sepp1 and GPx1 (Fig. 6). Estrogen-mediated increases in hepatic GPx1 mRNA may increase GPx1 synthesis, and thus greater hepatic GPx activity in OVX+E2 rats. Estrogen treatment had much greater effect on hepatic Sepp1 mRNA levels (50%, Fig. 6) than on plasma 75Se-Sepp1 (10%, Fig. 4) and Sepp1 concentration (no difference, Fig. 5), these differences may due to isoforms of Sepp1 existing in plasma [42], or a posttranscriptional regulation (turnover) of Sepp1. Similarly, estrogen treatment did not have the same extent of effects on hepatic GPx1 mRNA levels (30%, Fig. 6) as those of GPx activity in blood and tissues (Table 3) due to the turnover of GPx. The impact of estrogen on 75Se distribution and hepatic levels of Sepp1 mRNA and GPx mRNA was not investigated in the Sham group, thus limiting our insights into the effects of estrogen fluctuations within a more physiological range. In addition, only two forms of inorganic selenium (sodium selenate and 75Se-sodium selenite) were investigated in this study. Since metabolism of selenium differs greatly by their forms, how estrogen affects metabolism of other forms of selenium, especially those commonly present in foods, needs to be determined in future studies.
Sepp1 synthesis has been shown to be regulated by the selenium supply posttranscriptionally [43] and by cytokines and growth factors transcriptionally [44, 45]. The regulation of Sepp1 synthesis by estrogen has not been reported previously. Estrogen is known to modulate the expression of target genes containing estrogen response elements (ERE) in their promoter region [46]. Although the Sepp1 gene lacks a complete ERE, it contains ERE half-sites (5′-AGGTCA-3′) in the promoter regions. The importance of the estrogen receptor (ER) binding as a monomer to an ERE half-site in some genes remains controversial [47]. The possibility that estrogen regulates expression of Sepp1 by binding of an activated ER to an ERE-half site warrants further investigation. In the absence of an ERE, estrogen may regulate gene expression by a non-classical pathway. Estrogen binding to ER is known to activate transcription factors such as AP1, Sp1 and NF-kB. These proteins bind to cognate recognition sequences in DNA directly and influence the transcription of genes [48, 49]. The Sepp1 promoter contains two binding sites for AP1, and one binding site for Sp1, but no binding site for NF-kB [44,50]. Thus, estrogen may regulate transcription of GPx1 and Sepp1 through binding of AP1 and Sp1 to cognate sites. Additional studies are needed to determine if estrogen may modulate expression of Sepp1 gene expression by direct binding of ER to the ERE-half site or perhaps via activation of transcription factors such as AP1 and Sp1, or by a combination of both pathways.
Footnotes
Presented in part at Experimental Biology 07, April 2007, Washington DC [Zhou, X., Smith, A.M., and Failla, M.L. Estrogen Status Alters Tissue Distribution of Oral Dose of 75Se-Selenite and Liver mRNA Levels of SelP and GPx. FASEB J 2007; 21 (5): 696.4 (Abs).].
Supported by OARDC Grant OHO00201 and NIHES02497 (measurement of plasma selenoprotein P concentration by laboratory of RF Burk at Vanderbilt University School of Medicine, Nashville, TN)
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Finley JW, Kincaid RL. Effect of sex and time of sampling on selenium and glutathione peroxidase activity in tissues of mature rats. Bio Trace Elem Res. 1991;3:181–91. doi: 10.1007/BF03032676. [DOI] [PubMed] [Google Scholar]
- 2.Guemouri L, Artur Y, Herbeth B, Jeandel C, Cuny G, Siest G. Biological variability of superoxide dismutase, glutathione peroxidase, and catalase in blood. Clin Chem. 1991;37:1932–37. [PubMed] [Google Scholar]
- 3.Prohaska J, Sunde R. Comparison of liver glutathione peroxidase activity and mRNA in female and male mice and rats. Comp Biochem Physical. 1993;105B:111–16. doi: 10.1016/0305-0491(93)90176-6. [DOI] [PubMed] [Google Scholar]
- 4.Yamamoto T, Ohkuwa T, Itoh H, Sato Y, Naoi M. Effect of gender differences and voluntary exercise on antioxidant capacity in rats. Biochem Biophys Res Commun. 2002;132:437–44. doi: 10.1016/s1532-0456(02)00097-2. [DOI] [PubMed] [Google Scholar]
- 5.Sobocanec S, Balog T, Sverko V, Marotti T. Sex-dependent antioxidant enzyme activities and lipid peroxidation in ageing mouse brain. Free Radic Res. 2003;37:743–48. doi: 10.1080/1071576031000102178. [DOI] [PubMed] [Google Scholar]
- 6.Smith AM, Picciano MF. Evidence for increased selenium requirement for the rat during pregnancy and lactation. J Nutr. l986;ll6:l068–79. doi: 10.1093/jn/116.6.1068. [DOI] [PubMed] [Google Scholar]
- 7.Lopes PA, Santos MC, Vicente L, Rodrigues MO, Pavão ML, Nève J, Viegas-Crespo AM. Trace element status (Se, Cu, Zn) in healthy Portuguese subjects of Lisbon population: A reference study. Biol Trace Elem Res. 2004;101:1–17. doi: 10.1385/BTER:101:1:01. [DOI] [PubMed] [Google Scholar]
- 8.Mistry HD, Wilson V, Ramsay MM, Symonds ME, Broughton Pipkin F. Reduced selenium concentrations and glutathione peroxidase activity in preeclamptic pregnancies. Hypertension. 2008;52:881–888. doi: 10.1161/HYPERTENSIONAHA.108.116103. [DOI] [PubMed] [Google Scholar]
- 9.Smith AM, Cha C, Kimura RE. Plasma selenium and glutathione peroxidase activity fluctuate during the rat estrous cycle. Nutr Res. 1995;15:267–77. [Google Scholar]
- 10.Ha EJ, Smith AM. Plasma selenium and glutathione peroxidase activity increase during the pre-ovulatory phase of the menstrual cycle. J Am Coll Nutr. 2003;22:43–51. doi: 10.1080/07315724.2003.10719274. [DOI] [PubMed] [Google Scholar]
- 11.L’Abbé MR, Collins MW, Trick KD, Laffey PJ. Glutathione peroxidase activity in a healthy Canadian population. Effects of age, smoking and drinking habits, exercise and oral contraceptive use. Trace Elem Med. 1992;9:45–53. [PubMed] [Google Scholar]
- 12.Bednarek-Tupikowska G, Tworowska U, Jedrychowska I, Radomska B, Tupikowski K, Bidzinska-Speichert B, Milewicz A. Effects of oestradiol and oestroprogestin on erythrocyte antioxidative enzyme system activity in postmenopausal women. Clin Endocrinol. 2006;64:463–8. doi: 10.1111/j.1365-2265.2006.02494.x. [DOI] [PubMed] [Google Scholar]
- 13.Massafra C, Gioia D, Fellice C, Musceola M, Longini M, Buonocore G. Gender-related differences in erythrocyte glutathione peroxidase activity in healthy subjects. Clin Endocrinol. 2002;57:663–7. doi: 10.1046/j.1365-2265.2002.01657.x. [DOI] [PubMed] [Google Scholar]
- 14.Lapointe J, Kimmins S, MacLaren LA, Bilodeau JF. Estrogen selectively up-regulates the phospholipids hydroperoxide glutathione peroxidase in the oviducts. Endocrinology. 2005;146:2583–92. doi: 10.1210/en.2004-1373. [DOI] [PubMed] [Google Scholar]
- 15.Rohrdanz E, Ohler S, Tran-Thi QH, Kahl R. The phytoestrogen daidzein affects the antioxidant enzyme system of rat hepatoma H4IIE cells. J Nutr. 2002;132:370–5. doi: 10.1093/jn/132.3.370. [DOI] [PubMed] [Google Scholar]
- 16.Strehlow K, Rotter S, Wassmann S, Adam O, Grohe C, Laufs K, Bohm M, Nickenig G. Modulation of antioxidant enzyme expression and function by estrogen. Circ Res. 2003;93:170–7. doi: 10.1161/01.RES.0000082334.17947.11. [DOI] [PubMed] [Google Scholar]
- 17.Reeves MA, Hoffmann PR. The human selenoproteome: recent insights into functions and regulation. Cell Mol Life Sci. 2009;66:2457–78. doi: 10.1007/s00018-009-0032-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hill KE, Zhou J, McMahan WJ, Motley AK, Atkins JF, Gesteland RF, Burk RF. Deletion of selenoprotein P alters distribution of selenium in the mouse. J Biol Chem. 2003;278:13640–6. doi: 10.1074/jbc.M300755200. [DOI] [PubMed] [Google Scholar]
- 19.Renko K, Werner M, Renner-Müller I, Cooper TG, Yeung CH, Hollenbach B, Scharpf M, Köhrle J, Schomburg L, Schweizer U. Hepatic selenoprotein P (SePP) expression restores selenium transport and prevents infertility and motor-incoordination in Sepp-knockout mice. Biochem J. 2008;409:741–9. doi: 10.1042/BJ20071172. [DOI] [PubMed] [Google Scholar]
- 20.Chittum HS, Hill KE, Carlson BA, Lee BJ, Burk RF. Replenishment of selenium deficient rats with selenium results in redistribution of the selenocysteine tRNA population in a tissue specific manner. Biochim Biophys Acta. 1997;1359:25–34. doi: 10.1016/s0167-4889(97)00092-x. [DOI] [PubMed] [Google Scholar]
- 21.Janghorbani M, Xia Y, Ha P, Whanger PD, Butler JA, Olesik JW, Grunwald E. Effect of dietary selenium restriction on selected parameters of selenium status in men with high life-long intake. J Nutr Biochem. 1999;10:564–72. doi: 10.1016/s0955-2863(99)00039-x. [DOI] [PubMed] [Google Scholar]
- 22.Christensen MJ, Cammack PM, Wray CD. Tissue specificity of selenoprotein gene expression in rats. J Nutr Biochem. 1995;6:367–372. doi: 10.1016/0955-2863(95)80004-v. [DOI] [PubMed] [Google Scholar]
- 23.Schomburg L, Schweizer U. Hierarchical regulation of selenoprotein expression and sex-specific effects on selenium. Biochim Biophys Acta. 2009;1790:1453–62. doi: 10.1016/j.bbagen.2009.03.015. [DOI] [PubMed] [Google Scholar]
- 24.Waters DJ, Chiang EC, Cooley DM, Morris JS. Making sense of sex and supplements: differences in the anticarcinogenic effecs of selenium in men and women. Mutat Res. 2004;551:91–107. doi: 10.1016/j.mrfmmm.2004.02.019. [DOI] [PubMed] [Google Scholar]
- 25.Ganaraja B, Pavithran P, Ghosh S. Effect of estrogen on plasma ceruloplasmin level in rats exposed to acute stress. Indian J Med Sci. 2004;58:150–4. [PubMed] [Google Scholar]
- 26.Schosinsky KH, Lehmann HP, Beeler MF. Measurement of ceruloplasmin from its oxidase activity in serum by use of o-dianisidine dihydrochloride. Clin Chem. 1974;20:1556–63. [PubMed] [Google Scholar]
- 27.Behne D, Scheid S, Kyriakopoulos A, Hilmert H. Subcellular distribution of selenoproteins in the liver of the rat. Biochim Biophys Acta. 1990;1033:219–25. doi: 10.1016/0304-4165(90)90124-f. [DOI] [PubMed] [Google Scholar]
- 28.McCarthy TP, Brodie B, Milner JA, Bevill RF. Improved method for selenium determination in biological sample by gas chromatography. J Chromat. 1981;225:9–16. doi: 10.1016/s0378-4347(00)80238-8. [DOI] [PubMed] [Google Scholar]
- 29.Paglia DE, Valentine WN. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med. 1967;70:158–69. [PubMed] [Google Scholar]
- 30.Hill KE, Xia Y, Akesson B, Boeglin ME, Burk RF. Selenoprotein P concentration in plasma is an index of selenium status in selenium-deficient and selenium-supplemented Chinese subjects. J Nutr. 1996;126:138–45. doi: 10.1093/jn/126.1.138. [DOI] [PubMed] [Google Scholar]
- 31.Tabuchi Y, Kondo T, Suzuki Y, Obinata M. Genes involved in nonpermissive temperature-induced cell differentiation in Sertoli TTE3 cells bearing temperature-sensitive simian virus 40 large T-antigen. Biochem Biophys Res Commun. 2005;329:947–56. doi: 10.1016/j.bbrc.2005.02.065. [DOI] [PubMed] [Google Scholar]
- 32.Knoll KE, Pietrusz JL, Liang M. Tissue specific transcriptome responses in rats with early streptozotocin-induced diabetes. Physiol Genomics. 2005;21:222–9. doi: 10.1152/physiolgenomics.00231.2004. [DOI] [PubMed] [Google Scholar]
- 33.Mikula M, Dzwonek A, Jagusztyn-Krynicka K, Ostrowski J. Quantitative detection for low levels of Helicobacter pylori infection in experimentally infected mice by real-time PCR. J Microbiol Methods. 2003;55:351–9. doi: 10.1016/s0167-7012(03)00166-0. [DOI] [PubMed] [Google Scholar]
- 34.Thomson CD, Robinson MF. Urinary and fecal excretions and absorption of a large supplement of selenium: superiority of selenate over selenite. Am J Clin Nutr. 1986;44:659–63. doi: 10.1093/ajcn/44.5.659. [DOI] [PubMed] [Google Scholar]
- 35.Roesch DM. Effects of selective estrogen receptor agonists on food intake and body weight gain in rats. Physiol Behav. 2006;87:39–44. doi: 10.1016/j.physbeh.2005.08.035. [DOI] [PubMed] [Google Scholar]
- 36.Pantaleão TU, Mousovich F, Rosenthal D, Padrón AS, Carvalho DP, Costa VM. Effect of serum estradiol and leptin levels on thyroid function, food intake and body weight gain in female Wistar rats. Steroids. 2010;75:638–42. doi: 10.1016/j.steroids.2010.03.009. [DOI] [PubMed] [Google Scholar]
- 37.Deagen JT, Bulter JA, Zachara BA, Whanger PD. Determination of the distribution of selenium between glutathione peroxidase, selenoprotein P, and albumin in plasma. Anal Biochem. 1993;208:176–81. doi: 10.1006/abio.1993.1025. [DOI] [PubMed] [Google Scholar]
- 38.Burk RF, Hill KE, Motley AK. Selenoprotein metabolism and function: evidence for more than one function for selenoprotein P. J Nutr. 2003;133:1517S–20S. doi: 10.1093/jn/133.5.1517S. [DOI] [PubMed] [Google Scholar]
- 39.Bureau I, Anderson RA, Arnaud J, Raysiguier Y, Favier AE, Roussel AM. Trace mineral status in post menopausal women: impact of hormonal replacement therapy. J Trace Elem Med Biol. 2002;16:9–13. doi: 10.1016/S0946-672X(02)80003-7. [DOI] [PubMed] [Google Scholar]
- 40.Arnaud J, Arnault N, Roussel AM, Bertrais S, Ruffieux D, Galan P, Favier A, Hercberg S. Relationships between selenium, lipids, iron status and hormonal therapy in women of the SU.VI.M. AX cohort. J Trace Elem Med Biol. 2007;21 (Suppl 1):66–9. doi: 10.1016/j.jtemb.2007.09.025. [DOI] [PubMed] [Google Scholar]
- 41.Breedlove HA, Smith AM, Burk RF, Hill KE, Shapiro CL. Serum selenium measurements in women with early-stage breast cancer with and without chemotherapy-induced ovarian failure. Breast Cancer Res Treat. 2006;97:225–30. doi: 10.1007/s10549-005-9012-z. [DOI] [PubMed] [Google Scholar]
- 42.Ma S, Hill KE, Caprioli RM, Burk RF. Mass spectrometric characterization of full-length rat selenoprotein P and three isoforms shortened at the C terminus. Evidence that three UGA codons in the mRNA open reading frame have alternative functions of specifying selenocysteine insertion or translation termination. J Biol Chem. 2002;277:12749–54. doi: 10.1074/jbc.M111462200. [DOI] [PubMed] [Google Scholar]
- 43.Burk RF, Hill KE, Selenoprotein P. A selenium-rich extracellular glycoprotein. J Nutr. 1994;124:1891–7. doi: 10.1093/jn/124.10.1891. [DOI] [PubMed] [Google Scholar]
- 44.Dreher I, Jakobs TC, Kohrle J. Cloning and characterization of the human selenoprotein P promoter. J Biol Chem. 1997;272:29364–71. doi: 10.1074/jbc.272.46.29364. [DOI] [PubMed] [Google Scholar]
- 45.Mostert V, Wolff S, Dreher I, Köhrle J, Abel J. Identification of an element within the promoter of human selenoprotein P responsive to transforming growth factor-β. Eur J Biochem. 2001;268:6176–81. doi: 10.1046/j.0014-2956.2001.02565.x. [DOI] [PubMed] [Google Scholar]
- 46.McKenna N, Lanz R, O’Malley B. Nuclear receptor coregulators: cellular and molecular biology. Endocr Rev. 1999;20:321–44. doi: 10.1210/edrv.20.3.0366. [DOI] [PubMed] [Google Scholar]
- 47.Das D, Peterson RC, Scovell WM. High mobility group B proteins facilitate strong estrogen receptor binding to classical and half-site estrogen response elements and relax binding selectivity. Mol Endocrinol. 2004;18:2616–32. doi: 10.1210/me.2004-0125. [DOI] [PubMed] [Google Scholar]
- 48.Cerillo G, Rees A, Manchanda N, Reilly C, Brogan I, White A, Needham M. The oestrogen receptor regulates NFkappaB and AP-1 activity in a cell-specific manner. J Steroid Biochem Mol Biol. 1998;67:79–88. doi: 10.1016/s0960-0760(98)00078-8. [DOI] [PubMed] [Google Scholar]
- 49.Porter W, Saville B, Hoivik D, Safe S. Functional synergy between the transcription factor Sp1 and the estrogen receptor. Mol Endocrinol. 1997;11:1569–80. doi: 10.1210/mend.11.11.9916. [DOI] [PubMed] [Google Scholar]
- 50.Al-Taie OH, Seufert J, Mörk H, Treis H, Mentrup B, Thalheimer A, Starostik P, Abel J, Scheurlen M, Köhrle J, Jakob F. A complex DNA-repeat structure within the Selenoprotein P promoter contains a functionally relevant polymorphism and is genetically unstable under conditions of mismatch repair deficiency. Eur J Hum Genet. 2002;10:499–504. doi: 10.1038/sj.ejhg.5200811. [DOI] [PubMed] [Google Scholar]