Abstract
Human mesenchymal stem cells (MSCs) from bone marrow are a heterogeneous ensemble of progenitors and lineage-committed cells, with a broad range of regenerative properties. Ex vivo expansion to produce sufficient quantities of MSCs is essential for most therapeutic applications. The present study resolves the relationship between proliferation potential of MSCs and their potency. Clonal analysis generated single-cell derived colonies of MSCs that were classified according to their trilineage potential to exhibit adipo- (A), chondro-(C) and osteogenesis (O) as a measure of potency. Multipotent OAC clones were highly proliferative with colony-forming efficiencies that ranged from 35% to 90%; whereas, O clones formed colonies with an efficiency of 5% or less (P<0.01). Similar trends were evident during ex vivo expansion: for example, the median specific growth rate was 0.85 day−1 (20 h doubling time) for cultures inoculated with OAC clones and was 5-fold less for inocula of O clones (P<0.01). OA and OC clones had similar proliferation potentials. More than 75% of cells in subconfluent cultures inoculated with O clones stained positive for senescence-associated β-galactosidase activity vs. less than 10% for OAC clones (P<0.001). Apoptotic cells were in the minority for all potency groups. Preliminary data generated during clonal analysis suggest that osteogenic potential of MSCs to produce mineralized matrix is a function of potency, as well. These results are discussed in the context of the preparation of efficacious MSC therapies by ex vivo expansion.
Keywords: mesenchymal stem cells, potency, proliferation, osteogenesis, senescence, apoptosis
Introduction
Mesenchymal stem cells (MSCs) are a heterogeneous population of cells containing highly regenerative progenitors (Russell et al., 2010) with application as stem cell therapies and in tissue engineering (Barrilleaux et al., 2006; Salem and Thiemermann, 2010). These adult stem cells differentiate into multiple mesenchymal cell lineages (Prockop, 1997) and secrete trophic factors to regulate a variety of cellular processes, including fibrosis and the immune system (Caplan and Dennis, 2006). With this broad range of regenerative properties, MSCs are capable of repairing damaged tissues of mesenchymal and non-mesenchymal origins alike (Horwitz et al., 1999; Ortiz et al., 2003). Although MSCs have been harvested from diverse tissues (Crisan et al., 2008), the versatility and accessibility of MSCs derived from bone marrow stroma make them the standard for many therapeutic applications (Bianco et al., 2001).
Due to their scarcity in tissues (Veyrat-Masson et al., 2007), MSCs must be expanded ex vivo to obtain sufficient amounts of progenitors for most clinical applications (Ringdén et al., 2006). The rapid expansion of human bone marrow MSCs has been extensively documented (D’Ippolito et al., 2004; Sotiropoulou et al., 2006), but these studies focused on the overall growth characteristics of the cultures. Far less is known about the underlying cell populations that constitute heterogeneous MSC cultures. Populations of MSCs that amplify at different rates have been detected (Colter et al., 2001; Gronthos et al., 2003); however, there are a limited number of conflicting reports about the relationship between the potency of MSC populations to differentiate into specific lineages and their proliferation potential (Karystinou et al., 2009; Mareddy et al., 2007). The content of multipotent progenitors in heterogeneous MSC cultures affects their efficacy to repair damaged tissue: administration of a clonal population of multipotent MSCs to repair infarcted myocardium resulted in greater cardiac function than was achieved with the parent MSC culture from which the clone was derived (Zhang et al., 2006). Given the importance of MSC proliferation and potency to tissue regeneration, the present study resolves discrepancies in the literature about the relationship between these two parameters by analysis of clonal cultures of human bone marrow MSCs of known potency.
There are numerous challenges to isolating MSCs of a specified potency. Recent attempts to identify an immunophenotype that distinguishes MSCs of different potencies have had only limited success (Battula et al., 2009; Hachisuka et al., 2007). Sorting MSCs according to light scattering properties during flow cytometry provides a partial enrichment of multipotent cells (Smith et al., 2004). These obstacles can be overcome by characterizing MSCs by their functional ability to differentiate. The potency of individual MSC clones has been evaluated in this manner (Muraglia et al., 2000); however, a high-capacity format (≥96-well microplates) is required to obtain statistically significant results. Our research group developed a high-capacity assay which is unique in its ability to (1) quantify the potency of MSC clones in terms of their trilineage potential to exhibit adipo-, chondro- and osteogenesis, and (2) cryopreserve a template of clones of known potency for future use (Russell et al., 2010).
The current investigation demonstrates the utility of the high-capacity assay to resolve regenerative properties of MSCs as a function of potency, focusing initially on proliferation potential. Two aspects of proliferation potential are examined in this work: the efficiency of human bone marrow MSCs to form colonies when plated at clonogenic levels and the capacity of these adult stem cells for ex vivo expansion. Our findings are interpreted in terms of the extent of senescence and cell death in distinct clonal populations of MSCs at different stages of lineage commitment. The significance of these results is discussed in the context of developing effective treatment strategies with MSCs to regenerate damaged tissue.
Materials and Methods
MSC Cultivation
Primary MSCs were harvested from 2 ml of iliac crest bone marrow aspirate from a healthy adult volunteer under a protocol approved by the Tulane Institutional Review Board as previously described (Sekiya et al., 2002). Plastic-adherent MSCs prior to expansion are designated as passage P0. All cell culture supplies herein were obtained from Invitrogen (Carlsbad, CA) except where noted, and 100 U/ml penicillin and 100 μg/ml streptomycin were added to all media. For routine cultivation, MSCs were inoculated at 50–100 cells/cm2 in complete culture medium (CCMA) of α-MEM with 2 mM L-glutamine, supplemented with 17% FBS (Hyclone, Logan, UT) and an additional 2 mM L-glutamine (Barrilleaux et al., 2009). Cultures were maintained in a 37 ºC humidified incubator at 5% CO2 with complete medium exchange every 3–4 days and were subcultured at 50% confluence with 0.25% trypsin/1 mM EDTA.
Immunophenotyping
Trypsinized MSCs in CCMA were washed by centrifugation and resuspension in 2X PBS at 1–2 × 106 cells/ml. Aliquots of 100 μl cell suspension were incubated in the dark for 20 min at 25 °C with one of the fluorochrome-conjugated, anti-human monoclonal antibodies listed in Supplemental Table 1 at the manufacture’s recommended concentration. Labeled samples were washed in 3X PBS and analyzed with a FC500 flow cytometer (Beckman Coulter, Fullerton, CA). Isotype controls were run in parallel at the same concentration as each antibody.
High-Capacity Clonal Assay of MSC Potency
A high-capacity assay to quantify the potency of MSC clones (Fig. 1) was developed in our laboratory (Russell et al., 2010) and is summarized below:
Figure 1.
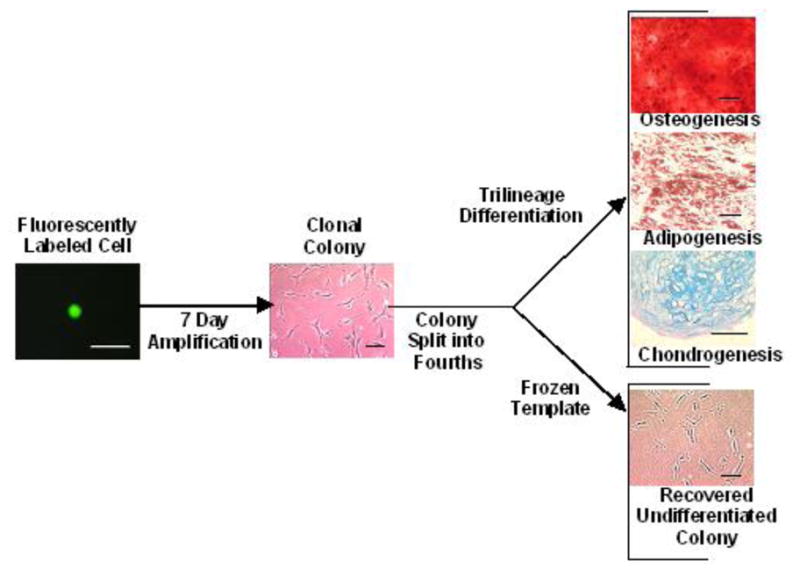
Overview of high-capacity assay to quantify trilineage potential of MSC clonal colonies. A 96-well format was employed to (1) detect fluorescent MSC clones stained with CellTracker Green after limiting dilution, (2) amplify clones for a week and subculture at a 1:4 ratio to generate matched clonal colonies, (3) differentiate three matched colonies per clone to quantify trilineage potential, and (4) cryopreserve in situ the fourth matched colony of each clone for future use. Scale bars: 100 μm.
Cell Cloning
P0 MSCs were labeled for 10–15 min with 5 μM CellTracker Green at 37 ºC in serum-free CCMA, and cloned by limiting dilution into 96-well plates containing 50 μl/well fresh CCMA and 75 μl/well sterile-filtered CCMA conditioned by MSCs. Wells inoculated with a single cell were detected by fluorescent microscopy. Fresh CCMA (50 μl/well) was added 3 days after cloning, and 50 μl/well medium was replaced with CCMA on day 6. P1 clonal colonies (≥300 cells/well) were subcultured at a 1:4 ratio on day 7 to evaluate potency and for cryopreservation.
Evaluation of Potency
The assay quantifies the trilineage potential of MSC clones to exhibit adipo-, chondro- and osteogenesis as a measure of potency. After subculturing, MSC clonal colonies were expanded for 7 days in 96-well microplates containing 150 μl/well CCMA until ∼75% confluent. Osteogenesis was induced by cultivation in low-glucose Dulbecco’s MEM supplemented with 10% FBS, 100 nM dexamethasone (Sigma-Aldrich, St. Louis, MO), 10 mM β-glycerophosphate (Sigma-Aldrich) and 50 μM L-ascorbic acid 2-phosphate (Sigma-Aldrich). After 21 days of differentiation, confluent monolayers were fixed in 4% paraformaldehyde for 20 min and stained with 1% Alizarin Red S (pH 4.2, Sigma-Aldrich) for 20 min to detect mineralized extracellular matrix. Stain was extracted with 100 μl/well of 10% cetylpyridinium chloride in 10 mM sodium phosphate buffer (pH 7.0) for 15 min, and the spectral absorbance was measured at 562 nm. The osteogenic potential of select clones was evaluated with the Alkaline Phosphatase Colorimetric Assay kit (Abcam, Cambridge, MA). One unit of alkaline phosphatase activity is defined as the amount of enzyme required to hydrolyze one micromole of p-nitrophenyl phosphate per minute at pH 9.6 and 25 °C.
To induce adipogenesis, clonal colonies were expanded as described above and cultivated for 21 days in CCMA supplemented with 0.5 μM dexamethasone, 0.5 mM isobutylmethylxanthine (Sigma-Aldrich) and 50 μM indomethacin (Sigma-Aldrich). Lipids were detected by adding 5 μl AdipoRed reagent (Lonza, Walkersville, MD) to the cell monolayer in 200 μl PBS/well. Fluorescence was measured after 10 min with excitation of 485 nm and emission of 535 nm.
For chondrogenesis, clonal colonies were amplified for nearly 2 weeks in 6-well plates containing 2 ml CCMA/well. Pellet cultures were formed by inoculating 2 ± 0.2 × 105 cells/well in 96-well, V-bottom polypropylene microplates (Thermo Fisher Scientific, Waltham, MA) containing 200 μl/well CCMA. The next day, CCMA was replaced with differentiation medium consisting of high-glucose Dulbecco’s MEM supplemented with 100 ng/ml bone morphogenetic protein-2 (R&D Systems, Minneapolis, MN), 10 ng/ml transforming growth factor-β3, 100 nM dexamethasone, 50 μg/ml L-ascorbic acid 2-phosphate, 100 μg/ml pyruvate (Sigma-Aldrich), 40 μg/ml proline (Sigma-Aldrich) and 10 μl/ml ITS+ (BD Biosciences, San Jose, CA). After 21 days of differentiation, the amount of sulfated glycosaminoglycans (GAGs) in digested cell pellets was quantified with 1,9-dimethylmethylene blue (Sigma-Aldrich), using chondroitin sulfate A for calibration (Barbosa et al., 2003). GAG content is reported on a per mass DNA basis by quantitation of DNA in digested pellet samples with Hoechst 33258 (Sigma-Aldrich), employing calf thymus DNA (Sigma-Aldrich) as the calibration standard (Penick et al., 2005).
Cryopreservation of MSC Clones
A master plate of frozen clones was prepared during the initial subculture of P1 clonal colonies by adding 50μl cell suspension to a new 96-well microplate containing 50 μl/well of 2X freezing medium (65% CCMA, 27% FBS and 8% DMSO). Sterile light paraffin oil (100 μl) was added to each well. The plate was frozen in a styrofoam box overnight at −80 ºC, then placed directly in the −80 ºC freezer, and later defrosted in a 37 ºC incubator once potency was evaluated. Select colonies were transferred to 24-well plates containing 1 ml fresh CCMA/well. Subconfluent P2 clonal cultures were frozen in individual cryovials for long-term storage.
Colony-Forming Efficiency and Growth Kinetics
Select frozen MSC clones were thawed and amplified for 3 days (∼2 × 103 cells/clonal colony), and their potency was verified. The efficiency of a P3 amplified clone to form colonies when inoculated at 100 ± 10 cells in a 10-cm tissue-culture dish was evaluated as in Barrilleaux et al. (2009), using crystal violet staining to detect cell colonies. For growth kinetics, P3 MSC clones were inoculated at 100 ± 10 cells/cm2 in 24-well plates containing 0.5 ml CCMA/well, with complete medium exchange every other day. Cell concentration was measured by hemocytometer counting, and specific growth rate was evaluated as in Blanch and Clark (1997).
Cell Senescence and Death
Senescence-associated β-galactosidase activity at pH 6.0 was detected histochemically in subconfluent cultures for growth kinetics, 4 days after inoculation, with the Senescence β-galactosidase Staining kit (Cell Signaling Technology, Danvers, MA). Flow cytometry with the Annexin V-FITC Apoptosis Detection kit (Sigma-Aldrich) was employed to quantify apoptotic cells in pooled MSC clones of a specific potency. Cell samples were prepared by amplifying 7–10 clones of a given potency to 2–3 × 104 cells/clone, verifying the potency of the expanded cultures, and pooling clonal cultures of like potency to form a sample of ∼2 × 105 cells. Loss of membrane integrity in cultures for growth kinetics was detected by the release of glucose-6-phosphate dehydrogenase into conditioned medium between complete medium exchange with the Vybrant Cytotoxicity Assay kit, using lysed MSCs for calibration.
Image Analysis
Cell images were captured with an Optronics DEI-750 digital camera (Goleta, CA) mounted onto an Olympus IX50 microscope (Center Valley, PA). Projected 2D area of MSCs in culture was traced using a Graphire 4 CTE-640 tablet (Wacom Technology, Vancouver, WA) and analyzed with Image-Pro Plus software (version 6.1, Media Cybernetics, Silver Spring, MD), using the Area and Percent Area options in the Count/Size function to evaluate percent confluence and cell size (Song et al., 2004). To determine percentage of cells positive for β-galactosidase activity, images of stained cells were analyzed with the Background Correction and Optical Density functions. Results are reported as a mean value from 30 randomly selected images per sample.
Statistical Analysis
Differentiation measurements are reported as standardize scores relative to negative control values by centering and scaling each score using the mean and standard deviation for undifferentiated MSCs. To determine appropriate cut-off values for positive differentiation, the R open-source statistical software package (version 2.11, r-project.org) was employed to generate Gaussian kernel density estimates for the distribution of measurements observed for the negative controls. Values corresponding to the 95th percentile from the fitted control densities were selected as the lower limit of detection for differentiation. Differences between two clone groups were evaluated with a Student’s t-test, and among multiple groups with the Kruskal-Wallis nonparametric ANOVA and Dunn’s post test. The threshold of significance was 0.05.
Results
Potency of MSC Clones
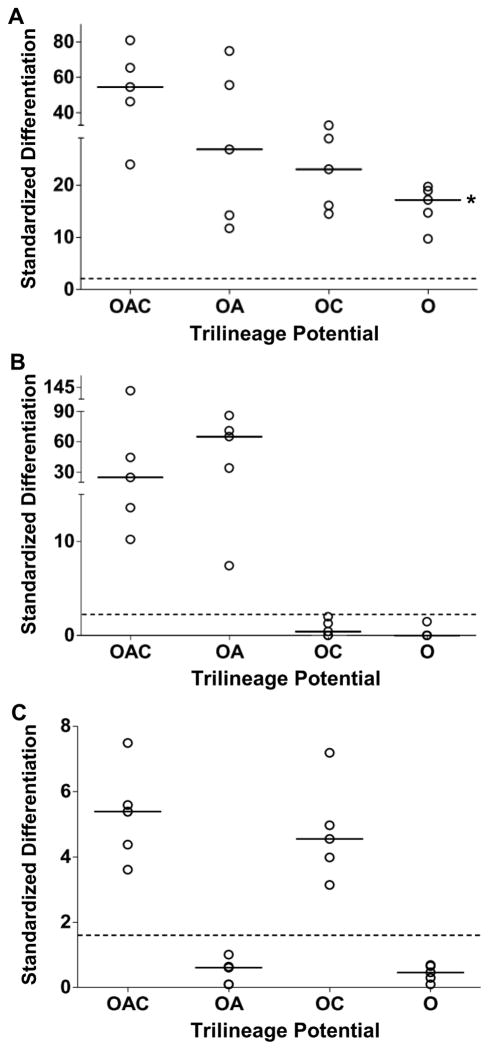
Human marrow-derived MSCs that were cloned in this study satisfied the criteria established by the International Society for Cellular Therapy (Dominici et al., 2006): plastic-adherent MSCs (1) were capable of trilineage differentiation to an adipo- (A), chondro- (C) and osteogenic (O) phenotype, (2) expressed stromal markers CD73, CD90 and CD105, and (3) lacked expression of hematopoietic markers CD11b, CD19, CD34, CD45 and HLA-DR. A comprehensive immunophenotype is presented in Supplemental Table 1. MSC clones of known potency were generated with the high-capacity assay depicted in Figure 1 (Russell et al., 2010). Microplate wells inoculated with a single cell during cloning were detected by labeling MSCs with a fluorescent tracker dye. Single-cell derived colonies were subcultured into four matched clonal cultures: three to evaluate trilineage potential as a measure of potency, and the fourth to cryopreserve undifferentiated clones for future use once potency was known. To evaluate potency, matrix mineralization during osteogenesis, lipid accumulation during adipogenesis and sulfated GAG deposition during chondrogenesis was quantified as a standardized score (Fig. 2). This dimensionless score reports the extent of differentiation as the number of standard deviations from the mean score of the negative control. MSC clones were designated as positive for differentiation when the standardized score exceeded the corresponding value for the 95th percentile of the estimated probability density for the negative control.
Figure 2.
Representative potency of amplified MSC clones. Trilineage potential of clones was verified upon inoculating experimental cultures. Three matched cultures per clone were stained after 21 days in differentiation medium with Alizarin Red S to detect mineralization of extracellular matrix (A); AdipoRed, lipids (B); and 1,9-dimethylmethylene blue, sulfated GAGs (C). Negative control: MSCs in CCMA. Dimensionless differentiation scores were standardized relative to the mean ± standard deviation for the negative controls of 0.19 ± 0.04 absorbance units (A), 2200 ± 400 relative fluorescence units (B), and 0.26 ± 0.07μg GAG/μg DNA (C). Threshold values for positive differentiation (dashed lines) correspond to the 95th percentile of the probability density for the negative controls. Nomenclature: CCMA, cell culture medium with antibiotics; GAG, glycosaminoglycans; OAC, osteo-adipo-chondrogenic; OA, osteo-adipogenic; OC, osteo-chondrogenic; O, osteogenic. Median (__, n=5 clones). *, P < 0.05 vs. OAC clones.
This study examined proliferation potential of clonal MSCs with the following trilineage potentials: osteo-adipo-chondrogenic (OAC), osteo-adipogenic (OA), osteo-chondrogenic (OC) and osteogenic (O). These four categories account for ∼90% of colony-forming MSCs from healthy donors examined to date (Russell et al., 2010). Potency of amplified clones was confirmed upon inoculating experimental cultures (Fig. 2). It is noteworthy that accumulation of mineralized extracellular matrix during osteogenesis is a function of potency (Fig. 2A), with a median differentiation score that was >2-fold higher (n=5) for OAC vs. O clones (P<0.05). The calcification induced in O clones by osteogenic medium (Fig. 2A) does not appear to be dystrophic from cell death (Saldaña et al., 2009) because the mineralization data for these clones correlate well with alkaline phosphatase activity (Supplemental Fig. 1).
Colony Formation and Growth Kinetics
Friedenstein et al. (1974) were the first to describe a fraction of plastic-adherent bone marrow cells, which form isolated colonies from single cells when plated ex vivo at clonogenic levels. To this day, colony-forming efficiency remains a standard measure of the proliferation potential of MSCs. OAC clones were substantially more clonogenic than O clones (Fig. 3). The former formed colonies ex vivo on tissue-culture plastic with an efficiency of 35% to 90% and median value of 65% (n=5). In contrast, the colony-forming efficiency of O clones was ≤5% (P<0.01). OA clones formed colonies with efficiencies that were similar to those for OC clones (median value of 10% to 30%). Colony-forming efficiencies for heterogeneous MSCs from donors typically vary between 20% to 60% (Fischer-Valuck et al., 2010), consistent with the range in Figure 3 for specified potencies.
Figure 3.
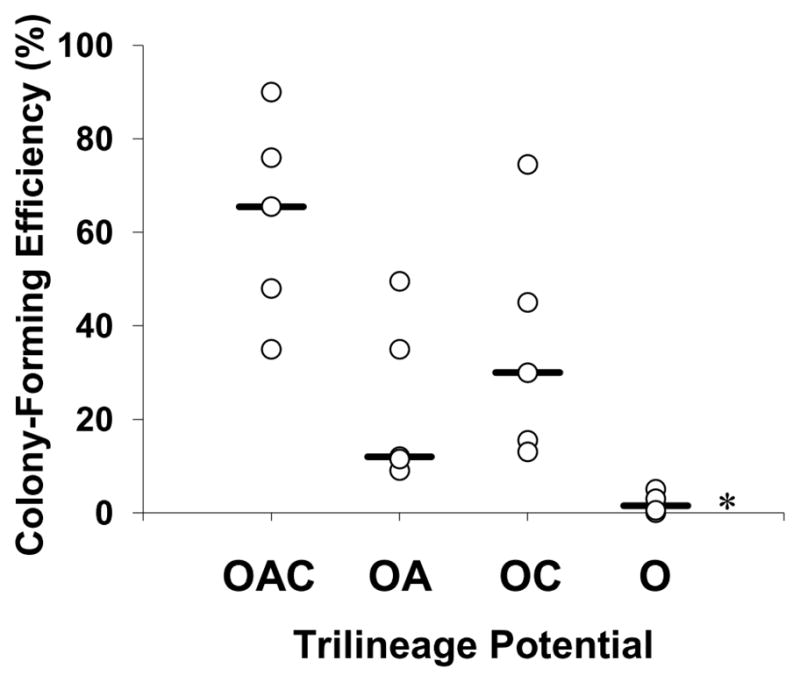
Colony-forming efficiency of MSC clones as a function of trilineage potential. Clones were frozen in situ within 96-well microplates, thawed, and amplified to obtain ∼2 × 103 cells/clone (Passage 3). Potency of the expanded clones was confirmed. Colony-forming efficiency was calculated as the percentage of P3 MSC clones to form colonies when inoculated at 100 ± 10 cells in a 10-cm culture dish containing CCMA. After two weeks of cultivation, colonies were detected by Crystal Violet staining. Nomenclature: See Figure 2 legend. Median (__, n=5 clones). *, P < 0.01 vs. OAC clones.
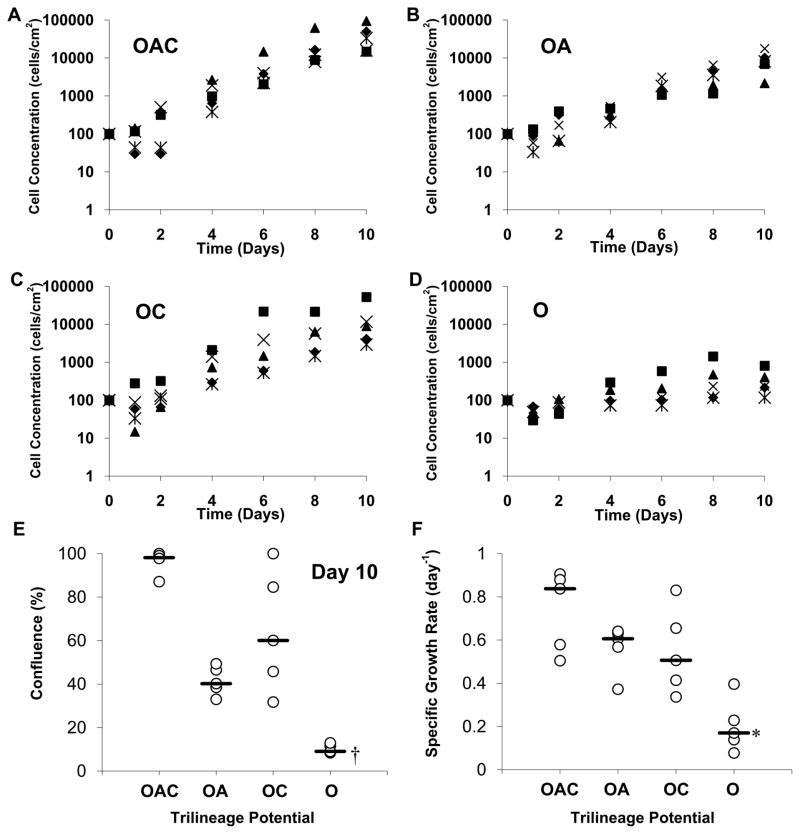
Growth kinetics of MSC clones during ex vivo expansion on tissue-culture plastic is presented in Figure 4. An inoculation density of 100 cells/cm2 was selected because it supports the retention of progenitors in heterogeneous MSCs cultures (Sekiya et al., 2002). There were no significant differences among the four potency groups in the fraction of MSCs that survived in culture 24 h after inoculation and in the duration of the lag phase, with median values of 60% and 1 day (n=20), respectively, for all four groups (Figs. 4A–D). After 10 days of expansion, cultures inoculated with OAC clones expanded ≥ 200-fold (n=5) to 2–10 × 104 cells/cm2 (Fig. 4A) and were 85% to 100% confluent (Fig. 4E). Lineage commitment limited ex vivo expansion of clonal MSCs. When inoculated with O clones, cultures accumulated <103 cells/cm2 (Fig. 4A) and were ≤15% confluent (Fig. 4E) over the same period (P<0.001). Trends in specific growth rates as a function of MSC potency (Fig. 4F) are similar to those for colony-forming efficiency (Fig. 3). Of the four potency groups, inocula of OAC clones exhibited the highest proliferation potential with a median specific growth rate of 0.85 day−1(n=5), equivalent to a 20 h doubling time (Fig. 4F). The median specific growth rate was 5-fold less for cultures inoculated with O clones (P<0.01). For inocula of OA and OC clones, the median specific growth rates were comparable (0.50–0.60 day−1). For heterogeneous MSCs, doubling times ≥30 h are common (Banfi et al., 2000).
Figure 4.
Ex vivo expansion of MSC clones. P3 MSC clones were inoculated at 100 ± 10 cells/cm2 into 24-well plates and cultivated in CCMA for 10 days. Growth profiles of individual MSC cultures inoculated with OAC (A), OA (B), OC (C), and O (D) clones (n=5 clones). Percent confluence (E) on day 10 as determined by image analysis of culture surface (n=30 images/culture). Specific growth rates (F) corresponding to growth profiles in A–D. Nomenclature: See Figure 2 legend. Median (__, n=5 clones). *, P < 0.01 and †, P < 0.001 vs. OAC clones.
Cell Senescence and Death
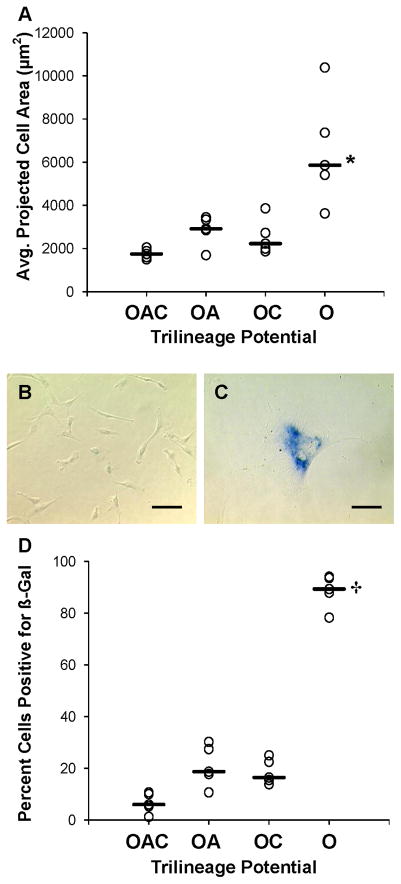
O clones expressed a phenotype indicative of cellular senescence. Cell size was estimated by image analysis of the projected area of MSCs on a subconfluent culture surface (n=30 images/culture). MSC cultures inoculated with O clones contained cells with an average projected area of >3.6 × 103μm2/cell (n=5 cultures) on day 4 (Fig. 5A), equivalent to a cell radius of >34μm (Fig. 5C). In Figure 5D, more than 75% of O clones stained positive for senescence-associated β-galactosidase activity at pH 6.0 (P<0.001). Positive β-galactosidase staining in subconfluent MSCs suggests irreversible growth arrest from senescence rather than a reversible, quiescent state in confluent cells (Severino et al., 2000). Inocula of OAC clones produced cultures with a healthy morphology (Figs. 5A and B) and negligible β-galactosidase staining – less than 10% positive (Fig. 5D). There were no significant differences in Figures 5A and D among OAC, OA and OC clones.
Figure 5.
Phenotyping cellular senescence in MSC clones as a function of trilineage potential. Senescence of MSC cultures depicted in Figure 4 was evaluated on day 4 of cultivation when cells were subconfluent. Cell size (A) was assessed with image analysis as the average projected 2D area of MSCs on the culture surface (n=30 images/culture). Representative phase-contrast images displaying cell size and staining of senescence-associated s-galactosidase (β-Gal) activity at pH 6.0 in MSC cultures inoculated with OAC (B) and O clones (C). Percentage of s-Gal-positive MSCs (D) as determined by image analysis. See Figure 2 legend for additional nomenclature. Median (__, n=5 clones). *, P < 0.01 and †, P < 0.001 vs. OAC clones. Scale bars: 100 μm.
Once MSCs recovered from trypsinization of the inoculum, cell death was negligible in cultures inoculated with OAC, OA and OC clones. Representative data are provided for day 10 of culture (Fig. 6A). The percentage of dead cells in MSC cultures was estimated from the release of glucose-6-phosphate dehydrogenase into conditioned medium from compromised cell membranes. Dead cells represented the minority of total cells present at the end of culture for all four potency groups (Fig. 6A); however, the content of dead cells in culture was statistically higher for inocula of O clones (P<0.01). The percentage of dead cells on day 10 for O clones was between 2% and 40%, with a median value of ∼10% (n=5). These trends were validated for OAC and O clones by flow cytometry (Figs. 6B–D). The majority of cells in both groups were viable: >90% for OAC clones and >70% for O clones (n=7–10 pooled clones/sample) stained negative for annexin V and propidium iodide (Fig. 6D). The primary mode of cell death for O clones was apoptosis: upwards of 25% of the cells were positive for annexin V (n=3 samples).
Figure 6.
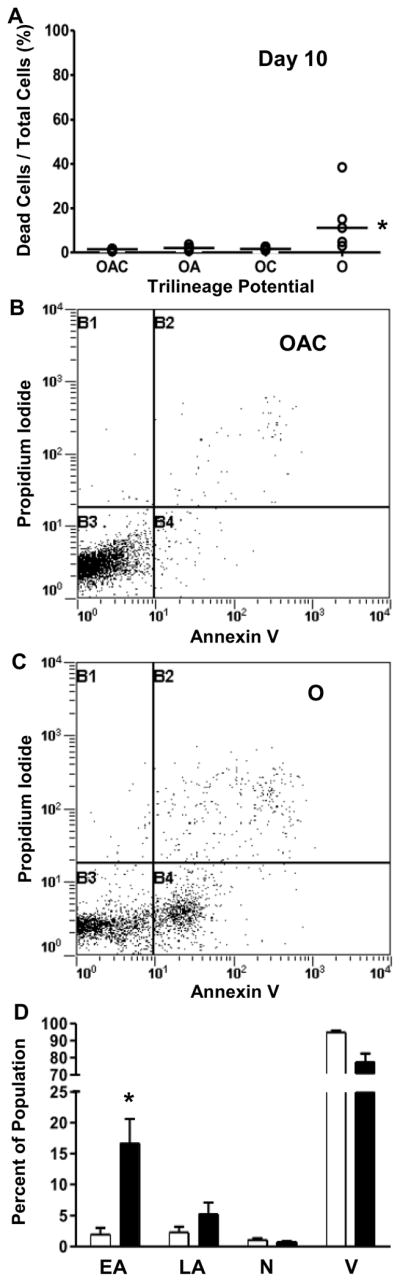
Effect of potency on cell death of MSC clones. Percentage of dead cells (A) in MSC cultures depicted in Figure 4 was estimated on day 10 from release of glucose-6-phosphate dehydrogenase into culture medium from membrane-damaged cells during the preceding two days. Median (__, n=5 clones). For flow cytometric analysis of cell death (B–D), 7–10 clones were amplified to 2-3 × 104 cells/clone, potency of expanded cultures was verified, and cultures of a given potency were pooled to form a sample of ∼2 × 105 cells. Representative bivariate histograms with quadrants delineating Annexin V-positive, propidium iodide-positive, and negative populations of OAC (B) and O (C) clones. Distribution of early apoptotic (EA), late apoptotic (LA), necrotic (N) and viable (V) populations of OAC (outline) and O (solid) clones (D) reported as means ± standard deviations (n=3 samples). See Figure 2 legend for additional nomenclature. *, P < 0.01 vs. OAC clones.
Discussion
Proliferation Potential
Multipotent MSC clones in this study had a significantly higher proliferation potential than their lineage-committed counterparts, as measured by colony-forming efficiency and growth kinetics during ex vivo expansion. For the latter, inocula of OAC clones produced cultures with statistically greater cell accumulation, faster growth rates and less apoptotic cells than inocula of O clones. We did not detect significant differences in proliferation potential between OA and OC clones. These data were obtained with early passage MSCs harvested from a single bone marrow donor. The variance in proliferation among clones of a given potency is consistent with the broad range in the extent of differentiation within a potency group (Fig. 2). The proliferative heterogeneity observed in our clones demonstrates the stochastic variability inherent to the process of cell growth and differentiation, and development of a predictive model of potency on the basis of proliferation parameters would clearly need to account for such variation.
Results from this study agree with preliminary data from our laboratory on the proliferation potential of MSCs from a different donor. The median MSC concentration in clonal cultures after six days of ex vivo expansion decreased significantly with lineage commitment (Russell et al., 2010). Similar data were obtained before and after freezing the clones. Combined results from our two studies suggest that the observed loss of proliferation potential with lineage commitment was not donor specific, nor an artifact of cryopreservation.
The findings presented here on colony-forming efficiency aid in interpreting data from our high-capacity assay on MSC heterogeneity. The assay quantifies the percentage of colony-forming cells of a given potency in heterogeneous MSC cultures. OAC clones account for ∼50% of colony-forming MSCs from healthy donors examined to date, as compared with 10% to 20% for O clones (Russell et al., 2010). The prevalence of multipotent MSCs may be due, in part, to their greater colony-forming efficiency relative to more lineage-committed clones.
The correlation between MSC proliferation and potency detected here with clonal analysis is consistent with diminished growth and differentiation of heterogeneous MSC cultures with serial passage. After passage 4–6, the colony-forming efficiency of MSCs and their accumulation during ex vivo expansion is significantly lower than at early passage (Bruder et al., 1997; DiGirolamo et al., 1999). This decrease in proliferation in heterogeneous MSC cultures is accompanied by a substantial reduction, if not complete loss, in adipogenic and chondrogenic potential (DiGirolamo et al., 1999; Kretlow et al., 2008).
Our research provides insight into conflicting reports on the relationship between MSC proliferation and potency obtained by clonal analysis. Consistent with our findings on MSCs from healthy donors, Mareddy et al. (2007) observed that most of their fast-growing MSC clones from bone marrow of osteoarthritic patients exhibited an OAC phenotype; whereas, the majority of the slow-growing clones were more lineage committed. In contrast, a similar investigation was unable to detect a dependence of proliferation on MSC potency (Karystinou et al., 2009). In this case, analysis was confined to OAC and OC clones of MSCs from osteoarthritic and healthy donors. Our results reconcile these discrepancies: we observed significant differences in proliferation potential between OAC and O clones, but not among OAC, OA and OC clones. Lee et al. (2010) reported that highly proliferative MSC clones were multipotent, as in our research; however, they had insufficient quantities of slow-growing clones for a detailed analysis of potency. Our research emphasizes the importance of including lineage-committed clones in the analysis of MSC properties as a function of potency.
The difference in proliferation potential between OAC and O clones is relevant to the cultivation of MSCs and prediction of their therapeutic efficacy. We detected faster growth rates for OAC clones, suggesting that low inoculation densities may enrich the content of this cell population in heterogeneous MSC cultures during ex vivo expansion. This agrees with the inverse correlation of the yield of multipotent MSCs to the inoculation density previously observed (Sekiya et al., 2002; Sotiropoulou et al., 2006). Also, our research demonstrated that OAC clones are statistically more clonogenic than O clones. This supports the use of colony-forming efficiency as a simple measurement to monitor the content of multipotent cells in MSC preparations from different donors (DiGirolamo et al., 1999). The content of fast-growing, multipotent cells profoundly affects the therapeutic efficacy of MSCs. For example, a population of rapidly proliferating MSCs exhibited preferential tissue engraftment relative to more slowly proliferating MSCs (Lee et al., 2006). As such, colony-forming efficiency could be a predictive measure of the efficacy of MSC therapies.
Senescence
O clones expressed a characteristic phenotype of senescence: slow growth rate, large cell size and elevatedβ-galactosidase activity at pH 6.0 in subconfluent cultures. In agreement with our data, there are reports of fast-growing MSCs with a small, spindle-shaped morphology and slow-growing MSCs that were large, flat and cuboid (Colter et al., 2001; Tormin et al., 2009). The yield of small MSCs (classified as recycling stem (RS) cells and type I cells) in heterogeneous cultures is inversely related to the incubation time of each passage (Sekiya et al., 2002). The fraction of large, flat MSCs (also known as type II and mature cells) in culture increases upon serial passage, particularly at high plating densities (Mets and Verdonk, 1981; Neuhuber et al., 2008). Cultures enriched for small MSCs are multipotent; whereas, larger MSCs have diminished differentiation potential (Neuhuber et al., 2008; Sekiya et al., 2002). Furthermore, Mareddy et al. (2007) detected senescence-associated β-galactosidase activity in slow-growing MSCs with limited potency and negligible activity in fast-growing, multipotent MSCs.
These findings have ramifications for the preparation of MSC therapies for donors with appreciable concentrations of senescent cells, particularly for patients with non-union bone fractures and the elderly. MSCs harvested from non-union bone fractures have a greater content of senescent cells and limited osteogenic potential relative to marrow-derived MSCs (Bajada et al., 2009). Similarly, our senescent O clones exhibit limited mineralization during osteogenesis. Recent data suggest that senescent MSCs accumulate in the marrow of the elderly (Wagner et al., 2009; Zhou et al., 2008). For these patients, the loss of proliferation and osteogenic potential in senescent MSCs may compromise the efficacy of autologous stem cell therapies.
Enrichment of robust multipotent cells from heterogeneous MSC cultures containing senescent cells may improve the treatment outcome of autologous MSC therapies by increasing both cell yield after ex vivo expansion and enhancing the integrity of regenerated tissue. As mentioned in the Introduction, an immunophenotype of multipotent MSCs remains poorly defined. High-throughput clonal analysis can be helpful in identifying molecular markers that can distinguish MSCs with different differentiation potential. To this end, we have identified CD146 as a possible biomarker of MSC potency with our high-capacity assay: this cell adhesion molecule is more abundant on the surface of OAC clones than on the parent MSCs from which the clones were derived (Russell et al., 2010). Identification of potency markers may enable consistent and rapid production of efficacious MSC therapies from numerous donors.
Conclusions
Heterogeneous MSC cultures are an ensemble of multipotent and more lineage-committed cells. Clonal analysis of these distinct cell populations resolved the relationship between proliferation potential of MSCs and their potency. OAC clones are highly proliferative in terms of their colony-forming efficiency and capacity for ex vivo expansion, OA and OC clones have similar proliferation potentials, and O clones are slow-growing, senescent and, to a lesser extent, apoptotic. Preliminary data generated during clonal analysis suggest that osteogenic potential is a function of MSC potency, as well. Our results are germane to the ex vivo expansion of MSCs for clinical applications; use of colony-forming efficiency to monitor the content of multipotent cells in MSC therapies and predict their efficacy; enrichment of multipotent MSCs; and preparation of MSC therapies to treat the elderly and non-union bone fractures, when accumulation of senescent MSCs in bone is likely.
Supplementary Material
Acknowledgments
Contract grant sponsor: National Institutes of Health
Contract grant number: R03EB007281
This research was supported with grants from the National Institutes of Health (R03EB007281) and Tulane University.
References
- Bajada S, Marshall MJ, Wright KT, Richardson JB, Johnson WE. Decreased osteogenesis, increased cell senescence and elevated Dickkopf-1 secretion in human fracture non union stromal cells. Bone. 2009;45:726–735. doi: 10.1016/j.bone.2009.06.015. [DOI] [PubMed] [Google Scholar]
- Banfi A, Muraglia A, Dozin B, Mastrogiacomo M, Cancedda R, Quarto R. Proliferation kinetics and differentiation potential of ex vivo expanded human bone marrow stromal cells: implications for their use in cell therapy. Exp Hematol. 2000;28:707–715. doi: 10.1016/s0301-472x(00)00160-0. [DOI] [PubMed] [Google Scholar]
- Barbosa I, Garcia S, Barbier-Chassefière V, Caruelle J, Martelly I, Papy-García D. Improved and simple micro assay for sulfated glycosaminoglycans quantification in biological extracts and its use in skin and muscle tissue studies. Glycobiology. 2003;13:647–653. doi: 10.1093/glycob/cwg082. [DOI] [PubMed] [Google Scholar]
- Barrilleaux BL, Phinney DG, Prockop DJ, O’Connor KC. Review: Ex Vivo Engineering of Living Tissues with Adult Stem Cells. Tissue Eng. 2006;12:3007–3019. doi: 10.1089/ten.2006.12.3007. [DOI] [PubMed] [Google Scholar]
- Barrilleaux BL, Phinney DG, Fischer-Valuck BW, Russell KC, Wang G, Prockop DJ, O’Connor KC. Small-molecule antagonist of macrophage migration inhibitory factor enhances migratory response of mesenchymal stem cells to bronchial epithelial cells. Tissue Eng Part A. 2009;15:2335–2346. doi: 10.1089/ten.tea.2008.0434. [DOI] [PubMed] [Google Scholar]
- Battula VL, Treml S, Bareiss PM, Gieseke F, Roelofs H, de Zwart P, Müller I, Schewe B, Skutella T, Fibbe WE, Kanz L, Bühring HJ. Isolation of functionally distinct mesenchymal stem cell subsets using antibodies against CD56, CD271, and mesenchymal stem cell antigen-1. Haematologica. 2009;94:173–184. doi: 10.3324/haematol.13740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianco P, Riminucci M, Gronthos S, Robey PG. Bone marrow stromal stem cells: nature, biology, and potential applications. Stem Cells. 2001;19:180–192. doi: 10.1634/stemcells.19-3-180. [DOI] [PubMed] [Google Scholar]
- Blanch HW, Clark DS. Biochemical Engineering. New York: Marcel Dekker; 1997. p. 702. [Google Scholar]
- Bruder SP, Jaiswal N, Haynesworth SE. Growth kinetics, self-renewal, and the osteogenic potential of purified human mesenchymal stem cells during extensive subcultivation and following cryopreservation. J Cell Biochem. 1997;64:278–294. doi: 10.1002/(sici)1097-4644(199702)64:2<278::aid-jcb11>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Caplan AI, Dennis JE. Mesenchymal stem cells as trophic mediators. J Cell Biochem. 2006;98:1076–1084. doi: 10.1002/jcb.20886. [DOI] [PubMed] [Google Scholar]
- Colter DC, Sekiya I, Prockop DJ. Identification of a subpopulation of rapidly self-renewing and multipotential adult stem cells in colonies of human marrow stromal cells. Proc Natl Acad Sci U S A. 2001;98:7841–7845. doi: 10.1073/pnas.141221698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crisan M, Yap S, Casteilla L, Chen CW, Corselli M, Park TS, Andriolo G, Sun B, Zheng B, Zhang L, Norotte C, Teng PN, Traas J, Schugar R, Deasy BM, Badylak S, Buhring HJ, Giacobino JP, Lazzari L, Huard J, Péault B. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3:301–313. doi: 10.1016/j.stem.2008.07.003. [DOI] [PubMed] [Google Scholar]
- DiGirolamo CM, Stokes D, Colter D, Phinney DG, Class R, Prockop DJ. Propagation and senescence of human marrow stromal cells in culture: a simple colony-forming assay identifies samples with the greatest potential to propagate and differentiate. Br J Haematol. 1999;107:275–281. doi: 10.1046/j.1365-2141.1999.01715.x. [DOI] [PubMed] [Google Scholar]
- D’Ippolito G, Diabira S, Howard GA, Menei P, Roos BA, Schiller PC. Marrow-isolated adult multilineage inducible (MIAMI) cells, a unique population of postnatal young and old human cells with extensive expansion and differentiation potential. J Cell Sci. 2004;117:2971–2981. doi: 10.1242/jcs.01103. [DOI] [PubMed] [Google Scholar]
- Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini FC, Krause DS, Deans RJ, Keating A, Prockop DJ, Horwitz EM. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- Fischer-Valuck BW, Barrilleaux BL, Phinney DG, Russell KC, Prockop DJ, O’Connor KC. Migratory response of mesenchymal stem cells to macrophage migration inhibitory factor and its antagonist as a function of colony-forming efficiency. Biotechnol Lett. 2010;32:19–27. doi: 10.1007/s10529-009-0110-6. [DOI] [PubMed] [Google Scholar]
- Friedenstein AJ, Deriglasova UF, Kulagina NN, Panasuk AF, Rudakowa SF, Luria EA, Ruadkow IA. Precursors for fibroblasts in different populations of hematopoietic cells as detected by the in vitro colony assay method. Exp Hematol. 1974;2:83–92. [PubMed] [Google Scholar]
- Gronthos S, Zannettino AC, Hay SJ, Shi S, Graves SE, Kortesidis A, Simmons PJ. Molecular and cellular characterisation of highly purified stromal stem cells derived from human bone marrow. J Cell Sci. 2003;116:1827–1835. doi: 10.1242/jcs.00369. [DOI] [PubMed] [Google Scholar]
- Hachisuka H, Mochizuki Y, Yasunaga Y, Natsu K, Sharman P, Shinomiya R, Ochi M. Flow cytometric discrimination of mesenchymal progenitor cells from bone marrow-adherent cell populations using CD34/44/45(−) and Sca-1(+) markers. J Orthop Sci. 2007;12:161–169. doi: 10.1007/s00776-006-1098-6. [DOI] [PubMed] [Google Scholar]
- Horwitz EM, Prockop DJ, Fitzpatrick LA, Koo WW, Gordon PL, Neel M, Sussman M, Orchard P, Marx JC, Pyeritz RE, Brenner MK. Transplantability and therapeutic effects of bone marrow-derived mesenchymal cells in children with osteogenesis imperfecta. Nat Med. 1999;5:309–313. doi: 10.1038/6529. [DOI] [PubMed] [Google Scholar]
- Karystinou A, Dell’Accio F, Kurth TB, Wackerhage H, Khan IM, Archer CW, Jones EA, Mitsiadis TA, De Bari C. Distinct mesenchymal progenitor cell subsets in the adult human synovium. Rheumatology. 2009;48:1057–1064. doi: 10.1093/rheumatology/kep192. [DOI] [PubMed] [Google Scholar]
- Kretlow JD, Jin YQ, Liu W, Zhang WJ, Hong TH, Zhou G, Baggett LS, Mikos AG, Cao Y. Donor age and cell passage affects differentiation potential of murine bone marrow-derived stem cells. BMC Cell Biol. 2008;9:60. doi: 10.1186/1471-2121-9-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CC, Christensen JE, Yoder MC, Tarantal AF. Clonal analysis and hierarchy of human bone marrow mesenchymal stem and progenitor cells. Exp Hematol. 2010;38:46–54. doi: 10.1016/j.exphem.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee RH, Hsu SC, Munoz J, Jung JS, Lee NR, Pochampally R, Prockop DJ. A subset of human rapidly self-renewing marrow stromal cells preferentially engraft in mice. Blood. 2006;107:2153–2161. doi: 10.1182/blood-2005-07-2701. [DOI] [PubMed] [Google Scholar]
- Mareddy S, Crawford R, Brooke G, Xiao Y. Clonal isolation and characterization of bone marrow stromal cells from patients with osteoarthritis. Tissue Eng. 2007;13:819–829. doi: 10.1089/ten.2006.0180. [DOI] [PubMed] [Google Scholar]
- Mets T, Verdonk G. In vitro aging of human bone marrow derived stromal cells. Mech Ageing Dev. 1981;16:81–89. doi: 10.1016/0047-6374(81)90035-x. [DOI] [PubMed] [Google Scholar]
- Muraglia A, Cancedda R, Quarto R. Clonal mesenchymal progenitors from human bone marrow differentiate in vitro according to a hierarchical model. J Cell Sci. 2000;113:1161–1166. doi: 10.1242/jcs.113.7.1161. [DOI] [PubMed] [Google Scholar]
- Neuhuber B, Swanger SA, Howard L, Mackay A, Fischer I. Effects of plating density and culture time on bone marrow stromal cell characteristics. Exp Hematol. 2008;36:1176–1185. doi: 10.1016/j.exphem.2008.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz LA, Gambelli F, McBride C, Gaupp D, Baddoo M, Kaminski N, Phinney DG. Mesenchymal stem cell engraftment in lung is enhanced in response to bleomycin exposure and ameliorates its fibrotic effects. Proc Natl Acad Sci U S A. 2003;100:8407–8411. doi: 10.1073/pnas.1432929100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penick KJ, Solchaga LA, Welter JF. High-throughput aggregate culture system to assess the chondrogenic potential of mesenchymal stem cells. Biotechniques. 2005;39:687–691. doi: 10.2144/000112009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prockop DJ. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science. 1997;276:71–74. doi: 10.1126/science.276.5309.71. [DOI] [PubMed] [Google Scholar]
- Ringdén O, Uzunel M, Rasmusson I, Remberger M, Sundberg B, Lönnies H, Marschall HU, Dlugosz A, Szakos A, Hassan Z, Omazic B, Aschan J, Barkholt L, Le Blanc K. Mesenchymal stem cells for treatment of therapy-resistant graft-versus-host disease. Transplantation. 2006;81:1390–1397. doi: 10.1097/01.tp.0000214462.63943.14. [DOI] [PubMed] [Google Scholar]
- Russell KC, Phinney DG, Lacey MR, Barrilleaux BL, Meyertholen KE, O’Connor KC. In vitro high-capacity assay to quantify the clonal heterogeneity in trilineage potential of mesenchymal stem cells reveals a complex hierarchy of lineage commitment. Stem Cells. 2010;28:788–798. doi: 10.1002/stem.312. [DOI] [PubMed] [Google Scholar]
- Saldaña L, Sánchez-Salcedo S, Izquierdo-Barba I, Bensiamar F, Munuera L, Vallet-Regí M, Vilaboa N. Calcium phosphate-based particles influence osteogenic maturation of human mesenchymal stem cells. Acta Biomater. 2009;5:1294–1305. doi: 10.1016/j.actbio.2008.11.022. [DOI] [PubMed] [Google Scholar]
- Salem HK, Thiemermann C. Mesenchymal stromal cells: current understanding and clinical status. Stem Cells. 2010;28:585–596. doi: 10.1002/stem.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekiya I, Larson BL, Smith JR, Pochampally R, Cui J, Prockop DJ. Expansion of human adult stem cells from bone marrow stroma: conditions that maximize the yields of early progenitors and evaluate their quality. Stem Cells. 2002;20:530–541. doi: 10.1634/stemcells.20-6-530. [DOI] [PubMed] [Google Scholar]
- Severino J, Allen RG, Balin S, Balin A, Cristofalo VJ. Is beta-galactosidase staining a marker of senescence in vitro and in vivo? Exp Cell Res. 2000;257:162–171. doi: 10.1006/excr.2000.4875. [DOI] [PubMed] [Google Scholar]
- Smith JR, Pochampally R, Perry A, Hsu SC, Prockop DJ. Isolation of a highly clonogenic and multipotential subfraction of adult stem cells from bone marrow stroma. Stem Cells. 2004;22:823–831. doi: 10.1634/stemcells.22-5-823. [DOI] [PubMed] [Google Scholar]
- Song H, David O, Clejan S, Giordano CL, Pappas-LeBeau H, Xu L, O’Connor KC. Spatial composition of prostate cancer spheroids in mixed and static cultures. Tissue Eng. 2004;10:1266–1276. doi: 10.1089/ten.2004.10.1266. [DOI] [PubMed] [Google Scholar]
- Sotiropoulou PA, Perez SA, Salagianni M, Baxevanis CN, Papamichail M. Characterization of the optimal culture conditions for clinical scale production of human mesenchymal stem cells. Stem Cells. 2006;24:462–471. doi: 10.1634/stemcells.2004-0331. [DOI] [PubMed] [Google Scholar]
- Tormin A, Brune JC, Olsson E, Valcich J, Neuman U, Olofsson T, Jacobsen SE, Scheding S. Characterization of bone marrow-derived mesenchymal stromal cells (MSC) based on gene expression profiling of functionally defined MSC subsets. Cytotherapy. 2009;11:114–128. doi: 10.1080/14653240802716590. [DOI] [PubMed] [Google Scholar]
- Veyrat-Masson R, Boiret-Dupré N, Rapatel C, Descamps S, Guillouard L, Guérin JJ, Pigeon P, Boisgard S, Chassagne J, Berger MG. Mesenchymal content of fresh bone marrow: a proposed quality control method for cell therapy. Br J Haematol. 2007;139:312–320. doi: 10.1111/j.1365-2141.2007.06786.x. [DOI] [PubMed] [Google Scholar]
- Wagner W, Bork S, Horn P, Krunic D, Walenda T, Diehlmann A, Benes V, Blake J, Huber FX, Eckstein V, Boukamp P, Ho AD. Aging and replicative senescence have related effects on human stem and progenitor cells. PLoS One. 2009;4:e5846. doi: 10.1371/journal.pone.0005846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Ge J, Sun A, Xu D, Qian J, Lin J, Zhao Y, Hu H, Li Y, Wang K, Zou Y. Comparison of various kinds of bone marrow stem cells for the repair of infarcted myocardium: single clonally purified non-hematopoietic mesenchymal stem cells serve as a superior source. J Cell Biochem. 2006;99:1132–1147. doi: 10.1002/jcb.20949. [DOI] [PubMed] [Google Scholar]
- Zhou S, Greenberger JS, Epperly MW, Goff JP, Adler C, Leboff MS, Glowacki J. Age-related intrinsic changes in human bone-marrow-derived mesenchymal stem cells and their differentiation to osteoblasts. Aging Cell. 2008;7:335–343. doi: 10.1111/j.1474-9726.2008.00377.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.