Abstract
Purpose
To determine the survival benefit of postoperative chemoradiation therapy for elderly patients with resected gastric adenocarcimona.
Methods
We identified 1,023 individuals age 65 years and older (median=76) who underwent gastrectomy for non-metastatic stage IB–IV gastric adenocarcioma diagnosed between 2000–2002 in the linked Surveillance, Epidemiology and End Results-Medicare database. We examined factors associated with receiving postoperative chemoradiation and analyzed the survival benefit associated with receiving postoperative chemoradiation.
Results
Thirty percent of patients received adjuvant chemoradiation. On multivariable analysis, younger age (p<0.0001), lymph node involvement (p<0.0001), and more recent diagnosis (p=0.0284) were associated with receiving chemoradiation. There was a trend towards increased use among patients with less comorbidity (p=0.0515). The median follow-up was 25.5 months and 62% died. On multivariable survival analysis, older patients (p< 0.0001), those with lymph node involvement (p<0.0001), T3 or T4 disease (p=0.0472), higher grade disease (p=0.0355), and more comorbidity (p=0.0411) were more likely to die. After adjustment for other factors, receipt of adjuvant chemoradiation therapy did not significantly increase survival (HR 0.90; 95% CI 0.72–1.12; p=0.3453) and did not increase survival in a multivariable analysis that included propensity scores (p=0.2090).
Conclusion
We did not detect a survival benefit, suggesting some elderly patients with resected gastric adenocarcinoma may not gain a survival benefit from the administration of adjuvant chemoradiation. The analysis had limitations and the results are hypothesis generating. Future gastric cancer trials should enroll more elderly patients and stratify patients by age to better understand the impact of treatment regimens on older patients.
Keywords: Gastric cancer, Aged, SEER-Medicare, Chemotherapy, Radiotherapy
Introduction
In a landmark trial, Intergroup (INT) 0116, postoperative chemotherapy and radiation therapy improved survival for patients with resected gastric adenocarcimona.1 However, the effectiveness of postoperative chemoradiation for elderly individuals treated outside of a controlled clinical trial setting is not known. It is important to understand the potential benefit for older patients because gastric cancer mostly affects older individuals; the average age at diagnosis is 71 years and almost two thirds of those diagnosed with gastric cancer are above 65.2 Older patients diagnosed with cancer are less likely to receive standard treatment compared to younger patients, even when such treatments are potentially curative.3–5 Indeed, older patients with gastric cancer are less likely to receive any type of treatment for their cancer compared to younger patients6 and older patients who undergo gastrectomy for gastric adenocarcinoma are less likely to receive postoperative radiation therapy.7
The determination of treatment effectiveness in the elderly may substantially impact postoperative chemoradiation utilization among elderly patients with locally advanced gastric adenocarcinoma. Therefore, this study used the linked Surveillance, Epidemiology and End Results Medicare database (SEER-Medicare) to examine clinical and sociodemographic factors associated with receiving postoperative chemoradiation for resected gastric adenocarcimona among individuals age 65 and older and to evaluate the survival benefit associated with receiving postoperative chemoradiation. We hypothesized that postoperative chemoradiation would confer a survival benefit, but this survival benefit would be less than the benefit demonstrated in the INT-0116 trial.
Methods
Data sources
Patients were identified from the linked SEER-Medicare database. The catchments for the seventeen SEER tumor registries comprise 25% of the US population8 and the registries participating in the SEER program capture approximately 97% of incident cancers.9 The registries collect data on patient demographics, primary tumor site, tumor morphology and stage at diagnosis, first course of treatment, and the date and cause of death. The linked Medicare data include inpatient and outpatient medical claims and physician billings8, and were utilized to determine radiation and chemotherapy treatment, comorbid illnesses, and treatment for metastatic disease. The SEER-Medicare database used for this analysis contained SEER diagnoses through 2002, Medicare claims through 2005, gastric cancer specific mortality through 2003 and vital status follow-up through February 2005.
Cohort selection
The cohort contained individuals age 65 and older diagnosed with non-metastatic invasive American Joint Committee on Cancer (AJCC, 6th edition) Stage IB to IV gastric adenocarcioma in a SEER region between January 1, 2000 and December 31 2002 and who underwent gastrectomy as initial therapy. We selected this date range because the results of the INT-0116 trial were disseminated in 200010 and the results of the next landmark gastric cancer trial, the MAGIC trial, were initially presented in early 2003.11 Individuals within our study cohort met basic criteria for enrollment in the INT-0116 trial (resected tumor (T) 2 to T4 disease or lymph node involvement)1. We excluded subjects who died within one month of gastrectomy, subjects with a prior cancer diagnosis because prior treatment can impact adjuvant therapy recommendations, and subjects who developed metastatic disease within 6 months of gastrectomy. Subjects who did not have continuous Medicare enrollment (both Part A and Part B) and those who were enrolled in a health maintenance organization (HMO) any time from 13 months before diagnosis (for use in comorbidity assessment) through 6 months after gastrectomy were excluded because they did not have complete claims data. In total, 1,023 subjects met our inclusion criteria.
Variables and their measurement
The primary outcomes were receipt of adjuvant chemoradiation within 6 months post-gastrectomy and overall survival. Medicare claims identified adjuvant chemotherapy administration and have previously been shown to correlate well with chemotherapy receipt.12 Medicare claims used to capture gastrectomy, general chemotherapy administration and general radiation therapy administration are detailed in Table 1. Both SEER and Medicare were used to identify radiation therapy receipt within 6 months after the first gastrectomy claim to ensure a comprehensive assessment of radiation treatment.13 In the SEER database, patients are coded as receiving radiation therapy or recommendation for radiation therapy as a component of the first course of treatment.
Table 1.
Medicare Billing Codes
| Variable | Billing Codes |
|---|---|
| Gastrectomy | ICD-9-CM procedure 43.5x-43.99 |
| CPT 43620–43622, 43631–43634 | |
| General chemotherapy administration | ICD-9-CM diagnosis V58.1 |
| ICD-9-CM procedure 99.25 | |
| HCPCS C1166, C1167, C1178, C9110, C9205, C9207, C9213–C9216, C9411, C9414–C9419, C942x, C9430–C9438, G0355, G0356, G0359–G0362, J7150, J85xx–J87xx, J8999, J9xxx, Q0083–Q0085, S9325–S9329, S933x–S937x, S9494–S9497 | |
| CPT 9651x–9654x, 964xx | |
| Revenue center 0331, 0332, 0335 | |
| DRG 410 | |
| BETOS O1D | |
| Radiation therapy administration | ICD-9-CM diagnosis V58.0 |
| ICD-9-CM procedure 92.2x | |
| HCPCS S8049 | |
| CPT 77xxx, 79xxx; revenue center 0330, 0333, 0339. | |
| DRG 409 | |
| BETOS P7A | |
| Inpatient (MEDPAR) indicator for receipt of radiology oncology services, and MEDPAR indicator for receipt of radiology therapeutic services. | |
| Diagnosis of metastatic disease | ICD-9-CM diagnosis 196–199 |
BETOS: Berrenson-Eggers Type Of Service
CPT: Current Procedural Terminology
DRG: Diagnosis Related Groups
HCPCS: Health Care Financing Administration Common Procedure Coding System
ICD-9-CM: International Classification of Diseases 9th Revision Clinical Modification
Explanatory variables evaluated for association with receiving adjuvant chemoradiation therapy and with survival included: diagnosis year, tumor characteristics (size, number of lymph nodes involved), clinical characteristics (age at diagnosis, comorbidities), sociodemographic factors (ethnicity, socioeconomic status, region of the country), type of treating institution (academic vs. community hospital), and distance to nearest radiation treatment facility. Diagnosis year was categorized into six month blocks. Tumor stage was categorized by AJCC 6th edition tumor (T) stage, which is based on the depth of penetration (T1, T2, T3 and T4). The number of involved regional lymph nodes were grouped according to AJCC 6th edition regional lymph node (N) staging as none (N0), 1 to 6 (N1), 7 to 15 (N2), and more than 15 nodes (N3). We identified comorbidties by collecting diagnostic billing codes for specific health conditions during the year before diagnosis of gastric cancer using the Deyo implementation of the Charlson score applied to both inpatient and outpatient claims.14–16 Subjects were categorized as receiving their gastrectomy in a teaching hospital if there was a bill for indirect medical education during their stay. Distance to nearest radiation treatment facility was determined by an established algorithm that calculated the distance from the zip code of the patient’s residence to that of the closest radiation therapy facility.17
Statistical Analysis
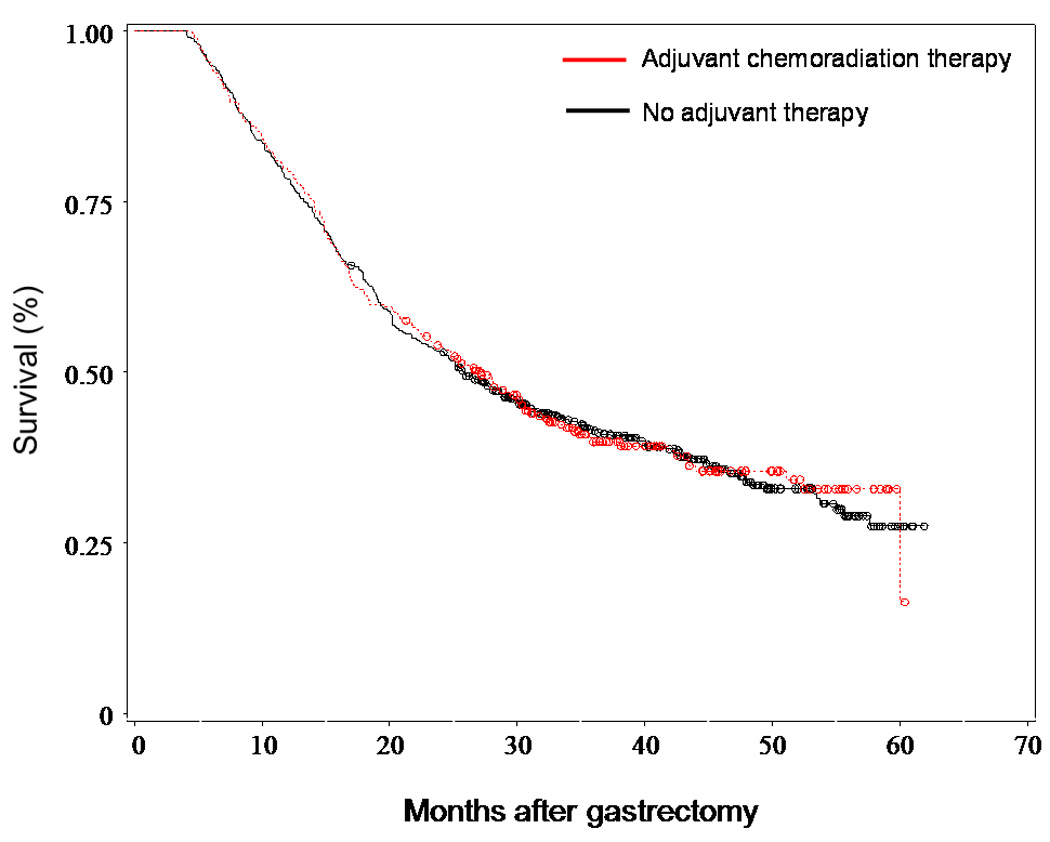
Descriptive statistics were generated for the study cohort. The study subjects were stratified by adjuvant treatment received: a) no adjuvant chemotherapy or radiation therapy; b) either radiation therapy or chemotherapy; and c) both chemotherapy and radiation therapy. Chi-square tests and kruskal-wallis test were used to compare categorical and continuous variables across treatment groups, respectively. The crude association of each potential explanatory variable with the outcome of receiving chemoradiation therapy was examined using univariate logistic regression. The independent association of an explanatory variable was examined using a multivariable logistic regression model constructed using forward and backward elimination. Subjects with missing data for T stage (n=10), N stage (n=74), grade (n=17) or distance from radiation facility (n=1) were excluded from univariate and multivariable models including these variables. The survival of the subjects who received both adjuvant chemotherapy and radiation therapy was compared to the survival of the subjects who received no adjuvant therapy. Individuals who received either only chemotherapy or only radiation were removed from the survival analysis (n=131). Survival, calculated from date of gastrectomy, was examined using multivariable and propensity-based Cox-proportional hazard regression models that included all explanatory variables. Propensity scores were created to account for unmeasured factors that are associated with receiving chemoradiation that may also influence overall survival. A multivariable logistic regression model with receipt of chemoradiation as the outcome was used to generate the propensity scores. Subjects were stratified into quintiles based on their scores, which were then added as covariates to the multivariable Cox proportional hazards model. In an exploratory analysis, survival outcome among those with stage III or IV disease was examined using a Cox-proportional hazard regression analysis. Interaction terms to test a priori hypotheses regarding the receipt of adjuvant chemoradiation therapy (chemoradiation therapy and age, and chemoradiation therapy and nodal involvement) were studied. For illustrative purposes, unadjusted Kaplan-Meier survival curves were constructed comparing patients who received adjuvant combined chemoradiation therapy to those who received neither adjuvant chemotherapy nor adjuvant radiation therapy. In a sensitivity analysis, gastric-cancer specific survival rather than overall survival was examined using multivariable and propensity-based Cox-proportional hazard regression models.
Statistical analyses were conducted using SAS software, version 9.1.3 for windows (SAS Institute, Cary, NC).
This study was reviewed by the Institutional Review Board at the Dana-Farber Cancer Institute and determined to be exempt.
Results
Descriptive characteristics of the study cohort
Among the 1,023 elderly patients with non-metastatic resected Stage IB to IV gastric adenocarcinoma, 5 % had T1, 65% had T2, and 30% had T3 or T4 disease. Sixty-nine percent of the patients had lymph node involvement (Table 2). The median diagnosis age was 76 years (interquartile range 72 to 81) and the majority of patients were White (72%) or Asian (17%). Adjuvant chemoradiation was administered to 30% (n=309) of subjects during the study period, 57% (n=583) of subjects received no adjuvant therapy and the remainder (n=131) received either adjuvant chemotherapy or adjuvant radiation therapy. Thirty-two percent of subjects diagnosed between July and December 2002 received adjuvant chemoradiation therapy, compared to 21% of subjects diagnosed between January and June 2000.
Table 2.
Characteristics of the 1,023 subjects who underwent gastrectomy for non-metastatic Stage IB–IV gastric adenocarcinoma, stratified by adjuvant treatment.
| Characteristic | All subjects, n=1,023 |
No adjuvant therapy, n=583 |
Either chemotherapy OR radiation therapy, n=131 |
Chemotherapy AND radiation therapy, n=309 |
p-valueb | |
|---|---|---|---|---|---|---|
|
Age at diagnosis (median (IQR)) |
76.3 (71.6–81.0) |
78.8 (73.7–83.6) |
75.1 (69.9–79.0) |
73.0 (70.0–76.7) |
<0.0001 | |
| Gender (n, (%)) | 0.0503 | |||||
| Male | 561 (54.8) | 303 (52.0) | 71 (54.2) | 187 (60.5) | ||
| Female | 462 (45.2) | 280 (48.0) | 60 (45.8) | 122 ( 39.5) | ||
| Race (n, (%)) | 0.0811 | |||||
| White | 737 (72.0) | 419 (71.9) | 96 (73.3) | 222 (71.8) | ||
| Asian | 173 (16.9) | 93 (16.0) | 17 (13.0) | 63 (20.4) | ||
| Other (including black) | 113 (11.1) | 71 (12.2) | 18 (13.7) | 24 (7.8) | ||
| SES Quintile (n, (%)) | 0.0185 | |||||
| 0 | 220 (21.5) | 145 (24.9) | 23 (17.6) | 52 (16.8) | ||
| 1 | 214 (20.9) | 118 (20.2) | 33 (25.2) | 63 (20.4) | ||
| 2 | 197 (19.3) | 119 (20.4) | 17 (13.0) | 61(19.7) | ||
| 3 | 182 (17.8) | 91 (15.6) | 31 (23.7) | 60 (19.4) | ||
| 4 | 210 (20.5) | 110 (18.9) | 27 (20.6) | 73 (23.6) | ||
| Six month diagnosis block (n, (%)) | 0.1393 | |||||
| Early 2000 | 189 (18.5) | 118 (20.2) | 32 (24.4) | 39 (12.6) | ||
| Late 2000 | 160 (15.6) | 87 (14.9) | 19 (14.5) | 54 (17.5) | ||
| Early 2001 | 179 (17.5) | 105 (18.0) | 21 (16.0) | 53 (17.2) | ||
| Late 2001 | 148 (14.5) | 85 (14.6) | 19 (14.5) | 44 (14.3) | ||
| Early 2002 | 176 (17.2) | 95 (16.3) | 17 (13.0) | 64 (20.7) | ||
| Late 2002 | 171 (16.7) | 93 (16.0) | 23 (17.6) | 55 (17.8) | ||
| T categorya (n, (%)) | 0.0002 | |||||
| T1 or T2 | 705 (69.6) | 432 (74.6) | 77 (59.7) | 196 (64.3) | ||
| T3 or T4 | 308 (30.4) | 147 (25.4) | 52 (40.3) | 109 (35.7) | ||
| N categorya (n, (%)) | <0.0001 | |||||
| N0 | 294 (31.0) | 234 (43.5) | 21 (17.7) | 39 (13.4) | ||
| N1 | 453 (47.7) | 231 (42.9) | 60 (50.4) | 162 (55.5) | ||
| N2 or N3 | 202 (21.3) | 73 (13.6) | 38 (31.9) | 91 (31.2) | ||
| Gradea (n, (%)) | 0.0046 | |||||
| 1 or 2 | 329 (32.7) | 211 (36.8) | 32 (24.8) | 86 (28.3) | ||
| 3 or 4 | 677 (67.3) | 362 (63.2) | 97 (75.2) | 218 (71.7) | ||
| Charlson (n, (%)) | <0.0001 | |||||
| 0 | 575 (56.2) | 301 (51.6) | 90 (68.7) | 184 (59.6) | ||
| 1 | 273 (26.7) | 154 (26.4) | 23 (17.6) | 96 (31.1) | ||
| 2+ | 175 (17.1) | 128 (22.0) | 18 (13.7) | 29 (9.4) | ||
| Gastrectomy at teaching hospital (n, (%)) | 0.4688 | |||||
| Yes | 552 (54.0) | 306 (52.5) | 76 (58.0) | 170 (55.0) | ||
| No | 471 (46.0) | 277 (47.5) | 55 (42.0) | 139 (45.0) | ||
|
Distance to RT facilitya (miles, median (IQR)) |
5.1 (2.5–12.2) |
5.2 (2.5–12.0) |
5.0 (2.5–13.5) |
5.3 (2.9–13.1) |
0.7486 | |
| SEER Region (n, (%)) | 0.3798 | |||||
| Northeast | 277 (27.1) | 160 (27.4) | 35 (26.7) | 82 (26.5) | ||
| South | 134 (13.1) | 85 (14.6) | 15 (11.5) | 34 (11.0) | ||
| Midwest | 106 (10.4) | 63 (10.8) | 17 (13.0) | 26 (8.4) | ||
| West | 506 (49.5) | 275 (47.2) | 64 (48.9) | 167 (54.1) | ||
Sample size does not add to 1,023 due to missing responses.
Comparison across treatment groups.
Abbreviations: SES=socioeconomic status, T=tumor, N=lymph node, RT=radiation therapy.
Predictors of receiving adjuvant radiation therapy and chemotherapy
On univariate analysis (Table 3), male sex (p=0.0165), younger age (p <0.0001), lymph node involvement (p <0.0001), less comorbidity (p=0.0019), higher socioeconomic status (p=0.0097), and more recent diagnosis (p=0.0161) were associated with increased receipt of adjuvant chemoradiation after gastrectomy (Table 3). Distance to radiation therapy facility was not associated with receiving chemoradiation (p=0.1032).
Table 3.
Univariate predictors of receiving adjuvant combined chemoradiation therapy among the 1,023 subjects who underwent gastrectomy.
| Covariate | Chemoradiation therapy (%) |
Odds Ratio (95% CI) |
p-value | |
|---|---|---|---|---|
| Age at diagnosis (per year increase) | 30.2 | 0.88 (0.85, 0.90) | <0.0001 | |
| 65 to 75 | 45.7 | 1.0 | --- | |
| 75 to 85 | 22.1 | 0.34 (0.25, 0.45) | <0.0001 | |
| 85 and older | 3.3 | 0.04 (0.02, 0.11) | <0.0001 | |
| Gender | ||||
| Male | 33.3 | 1.39 (1.06, 1.83) | 0.0165 | |
| Female | 26.4 | 1.0 | --- | |
| Race | ||||
| White | 30.1 | 1.0 | --- | |
| Asian | 36.4 | 1.33 (0.94, 1.88) | 0.1089 | |
| Other (including black) | 21.2 | 0.63 (0.39, 1.01) | 0.0542 | |
| SES Quintile (per quintile increase) | 30.2 | 1.13 (1.03. 1.24) | 0.0097 | |
| Six month diagnosis block (per 6 month increase) | 1.10 (1.02, 1.19) | 0.0161 | ||
| Early 2000 | 20.6 | 1.0 | --- | |
| Late 2000 | 33.8 | 1.96 (1.21, 3.17) | 0.0061 | |
| Early 2001 | 29.6 | 1.62 (1.01, 2.61) | 0.0478 | |
| Late 2001 | 29.7 | 1.63 (0.99, 2.68) | 0.0555 | |
| Early 2002 | 36.4 | 2.20 (1.38, 3.51) | 0.0010 | |
| Late 2002 | 32.2 | 1.82 (1.13, 2.94) | 0.0135 | |
| T category | ||||
| T1 | 38.0 | 1.0 | --- | |
| T2 | 27.0 | 0.60 (0.33, 1.10) | 0.0977 | |
| T3/T4 | 35.4 | 0.89 (0.48, 1.66) | 0.7209 | |
| N category | ||||
| N0 | 13.3 | 1.0 | --- | |
| N1 | 35.8 | 3.64 (2.47, 5.36) | <0.0001 | |
| N2 | 41.8 | 4.69 (2.96, 7.45) | <0.0001 | |
| N3 | 56.8 | 8.60 (4.34, 17.07) | <0.0001 | |
| Grade | ||||
| 1 | 25.0 | 1.0 | --- | |
| 2 | 26.3 | 1.07 (0.44, 2.61) | 0.8860 | |
| 3/4 | 32.2 | 1.43 (0.60, 3.40) | 0.4254 | |
| Charlson comorbidity score (per score increase) | 30.2 | 0.81 (0.71, 0.93) | 0.0019 | |
| 0 | 32.0 | 1.0 | --- | |
| 1 | 35.2 | 1.15 ( 0.85, 1.56) | 0.3600 | |
| 2+ | 16.6 | 0.42 ( 0.27, 0.65) | 0.0001 | |
| Gastrectomy at teaching hospital | ||||
| Yes | 30.8 | 1.06 (0.81, 1.39) | 0.6554 | |
| No | 29.5 | 1.0 | --- | |
| Distance to RT facility (per additional mile) | 1.00 (1.00, 1.00) | 0.1032 | ||
| Less than 45 | 29.6 | 1.34 (0.85, 2.11) | 0.2118 | |
| 45 or more | 36.0 | 1.0 | --- | |
| SEER Region | ||||
| Northeast | 29.6 | 0.85 (0.62, 1.17) | 0.3287 | |
| South | 25.4 | 0.69 (0.45, 1.06) | 0.0918 | |
| Midwest | 24.5 | 0.66 (0.41, 1.07) | 0.0893 | |
| West | 33.0 | 1.0 | --- | |
Abbreviations: SES=socioeconomic status, T=tumor, N=lymph node, RT=radiation therapy.
On multivariable analysis younger age (p <0.0001), lymph node involvement (p <0.0001), and more recent diagnosis (p=0.0284), were associated with chemoradiation after gastrectomy (Table 4). There was a trend towards the use of chemoradiation among patients with less comorbidity (p=0.0515).
Table 4.
Factors significantly associated with receipt of adjuvant combined chemoradiation therapy on multivariable analysis among the subjects who underwent gastrectomya.
| Covariate | Adjusted Odds Ratio (95% CI) |
p-value | |
|---|---|---|---|
| Age at diagnosis per year increase | 0.87 (0.85, 0.90) | <0.0001 | |
| N category | |||
| N0 | 1.0 | --- | |
| N1 | 3.69 (2.45, 5.56) | <0.0001 | |
| N2 | 4.26 (2.61, 6.96) | <0.0001 | |
| N3 | 7.44 (3.58, 15.47) | <0.0001 | |
| Charlson comorbidity score (per score increase) | 0.87 (0.75, 1.00) | 0.0515 | |
| Six month diagnosis block (per six month block increase) | 1.10 (1.01, 1.21) | 0.0284 | |
Abbreviations: N=lymph node.
Model n=949.
Predictors of survival
The survival of the subjects who received both adjuvant chemotherapy and radiation therapy was compared to the survival of the subjects who received no adjuvant therapy. In total, 62% (554/892) of these subjects died during the follow-up period. The median follow-up was 25.5 months after gastrectomy (range 4 to 62). On Cox proportional hazards multivariable analysis, older patients (p <0.0001) and patients with lymph node involvement (p <0.0001), T3 or T4 disease (p=0.0472), higher grade disease (p=0.0355), and comorbidity (p=0.0411) were more likely to die (Table 5). Additionally, Asian patients were less likely to die than White patients (HR 0.73; 95% CI 0.54–0.97; p=0.0327). After adjustment for other factors, receipt of adjuvant chemoradiation therapy did not significantly increase survival among this elderly population (HR 0.90; 95% CI 0.72–1.12; p=0.3453). The median survival among individuals who received chemoradiation therapy was 25.4 months versus 25.5 months for those who did not. Receipt of adjuvant chemoradiation therapy also did not significantly increase survival in a multivariable analysis that included propensity scores (HR 0.87; 95% CI 0.69–1.09; p=0.2090), or in an analysis limited to patients with stage III or IV disease (HR 0.98; 95% CI 0.70–1.38; p=0.9115). A sensitivity analysis evaluating gastric-cancer survival rather than overall survival determined adjuvant chemoradiation therapy was not associated with improved gastric-cancer survival (p=0.665)).
Table 5.
Multivariable predictors of all cause mortality among the subjects who received either combined chemoradiation therapy or no adjuvant therapya.
| Covariate | Hazard Ratio (95% CI) | p-value | |
|---|---|---|---|
| Treatment | |||
| No adjuvant therapy | 1.0 | --- | |
| Chemotherapy and radiation therapy | 0.90 (0.72, 1.12) | 0.3453 | |
| Age at diagnosis (per year increase) | 1.03 (1.01, 1.04) | 0.0002 | |
| Gender | |||
| Male | 1.02 (0.85, 1.23) | 0.8396 | |
| Female | 1.0 | --- | |
| Race | |||
| White | 1.0 | --- | |
| Asian | 0.73 (0.54, 0.97) | 0.0327 | |
| Other (including black) | 1.13 (0.84, 1.53) | 0.4243 | |
| SES Quintile (per quintile increase) | 1.00 (0.93, 1.06) | 0.9277 | |
| Six month diagnosis block (per block increase) | 0.97 (0.92, 1.03) | 0.3190 | |
| T category | |||
| T1 | 1.0 | --- | |
| T2 | 1.27 (0.81, 1.98) | 0.3023 | |
| T3 orT4 | 1.60 (1.01, 2.55) | 0.0472 | |
| N category | |||
| N0 | 1.0 | --- | |
| N1 | 2.04 (1.62, 2.57) | <0.0001 | |
| N2 | 3.48 (2.62, 4.63) | <0.0001 | |
| N3 | 3.73 (2.38, 5.84) | <0.0001 | |
| Grade | |||
| 1 | 1.0 | --- | |
| 2 | 1.44 (0.75, 2.75) | 0.2720 | |
| 3/4 | 1.98 (1.05, 3.75) | 0.0355 | |
| Charlson comorbidity score (per score increase) | 1.08 (1.00, 1.16) | 0.0411 | |
| Gastrectomy at teaching hospital | |||
| Yes | 0.90 (0.74, 1.08) | 0.2555 | |
| No | 1.0 | --- | |
| Distance to RT facility (per additional mile) | 1.00 (1.00, 1.00) | 0.9661 | |
| SEER Region | |||
| Northeast | 0.96 (0.76, 1.22) | 0.7536 | |
| South | 1.18 (0.89, 1.59) | 0.2548 | |
| Midwest | 1.15 (0.83, 1.58) | 0.3984 | |
| West | 1.0 | --- | |
Model n=809.
Abbreviations: SES=socioeconomic status, T=tumor, N=lymph node, RT=radiation therapy.
No significant interaction between age and receipt of adjuvant chemoradiation (p=0.4903) or between nodal involvement and receipt of adjuvant chemoradiation (p=0.2724) was noted on survival analysis.
Discussion
Although a landmark trial demonstrated postoperative chemoradiation improved survival for patients with locally-advanced resected gastric adenocarcimona1, our population-based analysis of 1,023 patients age 65 and older with resected gastric adenocarcinoma found no significant survival benefit from postoperative chemoradiation therapy.
In our study, elderly patients most likely to clinically benefit from adjuvant therapy: those with fewer comorbidities, more advanced disease, and the younger elderly, were indeed more likely to receive adjuvant chemoradiation. Similar treatment patterns have been demonstrated in elderly patients undergoing cancer treatment for prostate, breast, colon, and ovarian cancer.18–21 Patients diagnosed with gastric adenocarcinoma during the later months of the study period were more likely to receive adjuvant chemoradiation therapy, reflecting the dissemination of trial results and adoption of adjuvant chemoradiation into clinical practice. An initial report of the INT-0116 trial was presented in May 200010 and the findings were published in September 2001.1 Prior studies, using receipt of radiation therapy as a proxy for receipt of both radiation therapy and chemotherapy, demonstrated an increase in adjuvant radiation therapy administration among patients of all ages after the May 2000 presentation of study results.7,22 We postulated that patients undergoing gastrectomy at teaching institutions would be more likely to receive adjuvant therapy, potentially reflecting early adoption of the study results at teaching institutions, and that patients living closer to radiation therapy facilities would be more likely to receive adjuvant therapy, since daily travel for radiation treatments would be easier for them. However, neither surgery at a teaching institution nor living closer to a radiation therapy facility were associated with receiving adjuvant therapy.
Studies of patients diagnosed with other types of cancer have demonstrated that older patients are less likely to receive standard cancer treatment than younger patients, even when such treatments are potentially curative.3–5 During our study period, adjuvant chemoradiation was administered to less than one third of the patients. Cancer stage at diagnosis and the perception of elderly as frail are potential explanations for why such a small proportion of the patients in our study received adjuvant chemoradiation. More than half of the patients in our study had a Charlson comorbidity score of zero since patients must be healthy to tolerate a gastrectomy. However, if these elderly patients had a decrease in functional status due to surgery they may no longer be good candidates for adjuvant therapy. Patients may not have received adjuvant therapy because of physician or patient concern about possible treatment toxicity in this elderly population as the acute toxicity of adjuvant chemoradiation reported in the INT-0116 trial was considerable: fifty-four percent of patients experienced grade three or worse National Cancer Institute-Common Toxicity Criteria (NCI-CTC) hematological toxicity; one-third of patients experienced grade 3 or worse NCI-CTC gastrointestinal toxicity.1 Early cancer stage at diagnosis is another potential explanation for why such a small proportion of the elderly patients in our study received adjuvant chemoradiation. Patients in our study had earlier stage disease than the patients in INT-0116, 71% had T1 or T2 and 32% had N0 compared to only 32% with T1 or T2 and 15% with N0 disease in INT-0116. The applicability of the INT-0116 results to patients with early stage disease has been questioned because of the small number of study patients with early stage disease and because of the relatively good prognosis among those with early disease.6,23 The smaller percentage of patients with early-stage disease (T1, T2 or N0) enrolled in the INT-0116 trial compared to our population-based cohort suggests that only a small proportion of such patients were considered for enrollment in INT 0116. This enrollment bias may reflect a preconceived belief that adjuvant therapy is not necessary for early stage patients. Alternatively, the stage distribution in our study may reflect a tendency to forgo surgery in older patients with locally advanced disease.
In our population-based multivariable analysis that adjusted for clinical and demographic differences between treatment groups, the addition of postoperative chemoradiation did not improve survival for elderly patients with resected gastric adenocarcinoma. The median survival was 25.4 months among those who received adjuvant chemoradiation and 25.5 months among those who did not receive combined adjuvant chemoradiation. This is in stark contrast to the nine-month survival benefit, from 26 to 35 months, seen with the addition of chemoradiotherapy in the INT-0116 trial.1,24 The median follow-up in our study was not as long as the follow-up in INT-0116, but it is well beyond the time when the survival curves in INT-0116 diverged. Prior institutional series and population-based studies that suggested improved survival with the addition of adjuvant chemoradiation studied younger patients25,26, did not have information on medical comorbidities which can influence the likelihood of receiving adjuvant chemoradiaiton and truncate survival,25–27 and/or did not have information on chemotherapy administration.27
There are several potential explanations for why our study did not find a survival benefit. First, inadequate radiation therapy may have been administered to patients in our population-based cohort. The radiation field design and treatment planning for gastric cancer is technically challenging. Upon central review of treatment plans in the INT-0116 study, 34% of the radiation treatment plans required a change prior to radiation administration. If these plans were not changed, two-thirds of the deviations would have resulted in undertreatment of patients while one-third had the potential for delivering extremely toxic radiation.28 If radiation treatment caused serious toxicity that aborted treatment before completion or if the radiation treatment target was missed, the patients would not benefit from radiation therapy. We did not have information regarding radiation treatment plans, radiation treatment dose, or whether chemotherapy and radiation therapy courses were completed after initiation of therapy. A consensus statement on appropriate radiation treatment fields was released in 2002, but was not available during the first half of our study period.29 Second, as mentioned previously, the patients in our study had earlier stage disease than INT-0116 patients and may not have benefited from chemoradiation because of the relatively good prognosis among those with early disease. Third, margin status may have impacted survival outcomes. Enrollment in INT-0116 required complete resection with negative margins but margin status is not available in SEER-Medicare. However data suggests that the impact of surgical margin involvement on survival among patients with resected gastric adenocarcinoma who receive adjuvant chemoradiation therapy is minimal.25,30 In our study, the median survival of patients who received no adjuvant treatment in our study was similar to the median survival of patients in the observation arm of INT-0116. Moreover, although margin status can not be examined in our dataset, our results reflect treatment patterns in the U.S. Finally, the demographics of our population-based cohort were different from the INT-0116 trial (median age 60 years); our cohort was older (median age 76) and included a higher proportion of individuals who were Asian. We may not have found a survival benefit in our study because of competing causes of mortality in these elderly patients.
This study has additional limitations common to observational studies using administrative data. The data source only captures Medicare patients and has incomplete data on the roughly 15% of patients in managed care. Previous studies suggest that HMO patients tend to have fewer comorbidites than patients in the general Medicare population31 and that practice patterns in HMOs can differ significantly from those in a fee-for service setting32. However, other studies found few significant differences in cancer diagnosis and treatment between managed care and fee for service patients.33 Methods for comorbidity adjustment are still undergoing development and revision.16 Although the SEER-Medicare database is large, gastric cancer is a relatively rare cancer and only 30% of patients received adjuvant chemoradiation therapy; thus our ability to detect significant associations was limited by the size of our study cohort.
We did not detect a survival benefit from the administration of adjuvant chemoradiation therapy in our population-based study. These results suggest that some elderly patients with resected gastric adenocarcinoma may not gain a survival benefit from adjuvant chemoradiation. These findings should be considered hypothesis generating and further investigation is necessary. Randomized trials should enroll more elderly patients with gastric cancer and should stratify patients by age to permit subgroup analysis of the elderly to better understand the impact of treatment regimens on older patients.
Figure 1.
Acknowledgements
The authors acknowledge the efforts of the Applied Research Program, NCI; the Office of Research, Development and Information, CMS; Information Management Services, Inc.; and the Surveillance, Epidemiology, and End Results (SEER) Program tumor registries in the creation of the SEER-Medicare database. The interpretation and reporting of these data are the sole responsibility of the authors.
This work was supported by a National Institutes of Health grant (1K07CA118629, RSP) and a JCRT Foundation Grant (KEH).
References
- 1.Macdonald JS, Smalley SR, Benedetti J, et al. Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N Engl J Med. 2001;345:725–730. doi: 10.1056/NEJMoa010187. [DOI] [PubMed] [Google Scholar]
- 2.Ries LAG, Melbert D, Krapcho M, Stinchcomb DG, Howlader N, Horner MJ, Mariotto A, Miller BA, Feuer EJ, Altekruse SF, Lewis DR, Clegg L, Eisner MP, Reichman M, Edwards BK, editors. Bethesda, MD: National Cancer Institute; SEER Cancer Statistics Review, 1975–2005. http://seer.cancer.gov/csr/1975_2005/, based on November 2007 SEER data submission, posted to the SEER web site, 2008. [Google Scholar]
- 3.Bennett CL, Greenfield S, Aronow H, et al. Patterns of care related to age of men with prostate cancer. Cancer. 1991;67:2633–2641. doi: 10.1002/1097-0142(19910515)67:10<2633::aid-cncr2820671039>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 4.Greenfield S, Blanco DM, Elashoff RM, et al. Patterns of care related to age of breast cancer patients. Jama. 1987;257:2766–2770. [PubMed] [Google Scholar]
- 5.Samet J, Hunt WC, Key C, et al. Choice of cancer therapy varies with age of patient. Jama. 1986;255:3385–3390. [PubMed] [Google Scholar]
- 6.Hundahl SA, Menck HR, Mansour EG, et al. The National Cancer Data Base report on gastric carcinoma. Cancer. 1997;80:2333–2341. doi: 10.1002/(sici)1097-0142(19971215)80:12<2333::aid-cncr15>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 7.Coburn NG, Guller U, Baxter NN, et al. Adjuvant therapy for resected gastric cancer--rapid, yet incomplete adoption following results of intergroup 0116 trial. Int J Radiat Oncol Biol Phys. 2008;70:1073–1080. doi: 10.1016/j.ijrobp.2007.07.2378. [DOI] [PubMed] [Google Scholar]
- 8.Warren JL, Klabunde CN, Schrag D, et al. Overview of the SEER-Medicare data: content, research applications, and generalizability to the United States elderly population. Med Care. 2002;40:IV-3–IV-18. doi: 10.1097/01.MLR.0000020942.47004.03. [DOI] [PubMed] [Google Scholar]
- 9.Zippin C, Lum D, Hankey BF. Completeness of hospital cancer case reporting from the SEER Program of the National Cancer Institute. Cancer. 1995;76:2343–2350. doi: 10.1002/1097-0142(19951201)76:11<2343::aid-cncr2820761124>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 10.Macdonald J, Smalley S, Benedetti J, et al. Postoperative combined radiation and chemotherapy improveds dease-free survival (DEFS) and overall survival (OS) in resected adenocarcinoma of the stomach and GE junction. Results of intergroup study INT-0116 (SWOG 9008) Proc Am Soc Clin Oncol. 2000;19 abstr 1. [Google Scholar]
- 11.Allum W, Cunningham D S. Weeden for the UK NCRI Upper GI Clinical Studies Group. Proc Am Soc Clin Oncol. Vol. 22. 2003. Epsom General Hospital S, UK; Royal Marsden Hospital, Sutton, UK; MRC Clinical Trials Unit, London, UK: Perioperative chemotherapy in operable gastric and lower oesophageal cancer: A randomised, controlled trial (the MAGIC trial, ISRCTN 93793971) abstr 998. [Google Scholar]
- 12.Warren JL, Harlan LC, Fahey A, et al. Utility of the SEER-Medicare data to identify chemotherapy use. Med Care. 2002;40:IV-55–IV-61. doi: 10.1097/01.MLR.0000020944.17670.D7. [DOI] [PubMed] [Google Scholar]
- 13.Virnig BA, Warren JL, Cooper GS, et al. Studying radiation therapy using SEERMedicare-linked data. Med Care. 2002;40:IV-49–IV-54. doi: 10.1097/00005650-200208001-00007. [DOI] [PubMed] [Google Scholar]
- 14.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 15.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45:613–619. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 16.Klabunde CN, Potosky AL, Legler JM, et al. Development of a comorbidity index using physician claims data. J Clin Epidemiol. 2000;53:1258–1267. doi: 10.1016/s0895-4356(00)00256-0. [DOI] [PubMed] [Google Scholar]
- 17.Punglia RS, Weeks JC, Neville BA, et al. Effect of distance to radiation treatment facility on use of radiation therapy after mastectomy in elderly women. Int J Radiat Oncol Biol Phys. 2006;66:56–63. doi: 10.1016/j.ijrobp.2006.03.059. [DOI] [PubMed] [Google Scholar]
- 18.Hershman D, Jacobson JS, McBride R, et al. Effectiveness of platinum-based chemotherapy among elderly patients with advanced ovarian cancer. Gynecol Oncol. 2004;94:540–549. doi: 10.1016/j.ygyno.2004.04.022. [DOI] [PubMed] [Google Scholar]
- 19.Punglia RS, Weeks JC, Neville BA, et al. Radiation therapy after mastectomy between 1991 and 1999 in elderly women: response to clinical trial information. J Clin Oncol. 2006;24:3474–3482. doi: 10.1200/JCO.2006.05.7844. [DOI] [PubMed] [Google Scholar]
- 20.Schrag D, Rifas-Shiman S, Saltz L, et al. Adjuvant chemotherapy use for Medicare beneficiaries with stage II colon cancer. J Clin Oncol. 2002;20:3999–4005. doi: 10.1200/JCO.2002.11.084. [DOI] [PubMed] [Google Scholar]
- 21.Wong YN, Mitra N, Hudes G, et al. Survival associated with treatment vs observation of localized prostate cancer in elderly men. Jama. 2006;296:2683–2693. doi: 10.1001/jama.296.22.2683. [DOI] [PubMed] [Google Scholar]
- 22.Kozak KR, Moody JS. The survival impact of the intergroup 0116 trial on patients with gastric cancer. Int J Radiat Oncol Biol Phys. 2008;72:517–521. doi: 10.1016/j.ijrobp.2007.12.029. [DOI] [PubMed] [Google Scholar]
- 23.Kelsen DP. Postoperative adjuvant chemoradiation therapy for patients with resected gastric cancer: intergroup 116. J Clin Oncol. 2000;18:32S–34S. [PubMed] [Google Scholar]
- 24.Macdonald JS, Smalley S, Benedetti J, et al. Postoperative combined radiation and chemotherapy improves disease-free survival (DFS) and overall survival (OS) in resected adenocarcinoma of the stomach and gastroesophageal junction: Update of the results of Intergroup Study INT-0116 (SWOG 9008) Gastrointestinal Cancers Symposium. 2004 (abstr 6) [Google Scholar]
- 25.Kassam Z, Lockwood G, O'Brien C, et al. Conformal radiotherapy in the adjuvant treatment of gastric cancer: Review of 82 cases. Int J Radiat Oncol Biol Phys. 2006;65:713–719. doi: 10.1016/j.ijrobp.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 26.Kim S, Lim DH, Lee J, et al. An observational study suggesting clinical benefit for adjuvant postoperative chemoradiation in a population of over 500 cases after gastric resection with D2 nodal dissection for adenocarcinoma of the stomach. Int J Radiat Oncol Biol Phys. 2005;63:1279–1285. doi: 10.1016/j.ijrobp.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 27.Coburn NG, Govindarajan A, Law CH, et al. Stage-specific effect of adjuvant therapy following gastric cancer resection: a population-based analysis of 4,041 patients. Ann Surg Oncol. 2008;15:500–507. doi: 10.1245/s10434-007-9640-0. [DOI] [PubMed] [Google Scholar]
- 28.Macdonald JS. Role of post-operative chemoradiation in resected gastric cancer. J Surg Oncol. 2005;90:166–170. doi: 10.1002/jso.20223. [DOI] [PubMed] [Google Scholar]
- 29.Smalley SR, Gunderson L, Tepper J, et al. Gastric surgical adjuvant radiotherapy consensus report: rationale and treatment implementation. Int J Radiat Oncol Biol Phys. 2002;52:283–293. doi: 10.1016/s0360-3016(01)02646-3. [DOI] [PubMed] [Google Scholar]
- 30.Leong CN, Chung HT, Lee KM, et al. Outcomes of adjuvant chemoradiotherapy after a radical gastrectomy and a D2 node dissection for gastric adenocarcinoma. Cancer J. 2008;14:269–275. doi: 10.1097/PPO.0b013e318178d23a. [DOI] [PubMed] [Google Scholar]
- 31.Riley G, Tudor C, Chiang YP, et al. Health status of Medicare enrollees in HMOs and fee-for-service in 1994. Health Care Financ Rev. 1996;17:65–76. [PMC free article] [PubMed] [Google Scholar]
- 32.Riley GF, Potosky AL, Klabunde CN, et al. Stage at diagnosis and treatment patterns among older women with breast cancer: an HMO and fee-for-service comparison. Jama. 1999;281:720–726. doi: 10.1001/jama.281.8.720. [DOI] [PubMed] [Google Scholar]
- 33.Riley GF, Warren JL, Potosky AL, et al. Comparison of cancer diagnosis and treatment in Medicare fee-for-service and managed care plans. Med Care. 2008;46:1108–1115. doi: 10.1097/MLR.0b013e3181862565. [DOI] [PubMed] [Google Scholar]


