Abstract
Systemic endectocidal drugs, used to control nematodes in humans and other vertebrates, can be toxic to Anopheles spp. mosquitoes when they take a blood meal from a host that has recently received one of these drugs. Recent laboratory and field studies have highlighted the potential of ivermectin to control malaria parasite transmission if this drug is distributed strategically and more often. There are important theoretical benefits to this strategy, as well as caveats. A better understanding of drug effects against vectors and malaria ecologies are needed. In the near future, ivermectin and other endectocides could serve as potent and novel malaria transmission control tools that are directly linked to the control of neglected tropical diseases in the same communities.
The birth of endectocides
Endectocides are drugs with endoparasitocidal and ectoparasitocidal activity. Avermectins, the most effective and well-developed class of endectocides, are 16-membered ring macrocyclic lactones produced from the fermentation broth of an actinomycete. Ivermectin (IVM) is a semi-synthetic avermectin derivative that was first licensed in 1981 as a veterinary drug. It has since become one of the most successful drugs discovered owing to its broad spectrum activity against nematodes and ectoparasites, high potency, long pharmacokinetic persistence in blood and lymph, and excellent safety in vertebrates [1]. IVM is one of the primary drugs used to control the filarioid nematodes Onchocerca volvulus and Wuchereria bancrofti through human mass drug administrations in endemic areas. The drug alleviates disease in individuals as well as reduces nematode transmission in the community by killing the transmissible microfilaria stages and impairing the development and fecundity of adult worms in the human [2]. IVM accomplishes this by interfering with the neuromuscular physiology of invertebrates (Box 1).
Box 1. Anthelmintics, insecticides, and ligand-gated ion channels.
Several classes of both anthelmintic drugs and insecticides selectively target invertebrate ligand-gated ion channels [41, 42]. These members of the Cys-loop ligand-gated ion channel (LGIC) superfamily are located at both synaptic and extrasynaptic sites on invertebrate nerve and muscle cells, and open (or gate) in response to ligands to allow ions to flow across the cell membrane. Each channel exists as a pentamer of subunit proteins, which are comprised of an extensive N-terminal extracellular domain that binds the ligand, and four transmembrane domains (TM1–TM4) [43]. The LGIC genes of insects encode channel subunit families defined by their corresponding ligands, including nicotinic acetylcholine receptors (nAChRs), and glutamate, glycine, histamine and γ-amino butyric acid (GABA)-gated channels [44] (Table I). The nAChRs (not listed in Table I) mediate excitatory neurotransmission in insects by allowing cations (primarily Na+ and K+) to pass through their channel, and they have an expanded and distinct gene family [45]; neonicotinoids are important insecticides that agonize these channels. Insect GABA-gated channels are antagonized by established insecticide classes such as the cyclodienes (e.g. dieldrin) and phenylpyrazoles (e.g. fipronil) [42]. These and the other channels in Table I are mostly thought to be permeable to anions (primarily Cl−) [46], and so they mediate inhibitory signals that act by hyperpolarizing the cell membrane potential; however, Gisselman et al. [47] reported that Drosophila GRD and LCCH3 subunits can form excitatory heteromultimeric cation channels.
The avermectins specifically hyperpolarize the membrane potential of invertebrate postsynaptic neurons and muscle fibers through agonization of the inhibitory glutamate-gated chloride channels uniquely used by insects to regulate neuromuscular transmission [36]. This causes flaccid muscle paralysis that can lead to death of the insect [48]. There is also evidence that IVM agonizes pHCls in flies and scabies mites [49, 50] and HisCls in flies [51], and some evidence that GABA and GluCl subunits can associate to form heteromultimeric channels [52]. Whether heteromultimeric channels can form in mosquitoes and how such channels would be regulated between two different ligands is not clear. These issues might affect the susceptibility of mosquitoes to IVM and potential cross-resistance between IVM and other insecticides like dieldrin. Furthermore, GluCl and GABA-receptor subunits have splice isoforms stemming from exon 3, which generates the crucial ligand binding domain in the N terminus [53]. Interestingly, GluCl and pHCl exist as single genes in Dipterans, while the GABA-, glycine- and histidine-gated subunit encoding genes have mostly expanded into 2 or more paralogues within species of Diptera (Table I).
One primary reason for the excellent safety profile of IVM is that GluCls do not exist in mammals [48]. GluCls’ orthologous mammalian channel subunits are the distantly-related glycine-gated cation channel subunits, and while these can be activated by IVM at high concentrations [54], they exist only in the central nervous system and are sequestered behind the blood-brain barrier. The blood-brain barrier is not easily crossed by large macrolide molecules like IVM due to their affinity for P-glycoprotein xenobiotic transporters abundant in the brain’s vascular endothelium [55].
Endectocide activity against vectors
Initial ectoparasitocidal studies with the avermectins focused on biting flies, bot flies, fleas, lice, mange mites and ticks, and many were found susceptible to the drug. Wilson [3] provided a detailed review of much of this literature 17 years ago, focusing on the prospects of using avermectins in arthropod vector management. His analysis concluded that reducing the ectoparasite abundance on individual animals is a simplistic way to view the broader promise of endectocides for controlling vector-borne diseases. Instead, he argued that the avermectins need to be assessed for their ability to affect all critical variables of vectorial capacity from a vector population, especially reducing the probability of vector lifespan below the extrinsic incubation period (EIP), and reducing the vector feeding frequency through sub-lethal doses (Box 2). Vertebrate blood meals, then, could deliver anti-vector drugs directly into the midguts of vectors to reduce the likelihood of pathogen transmission from the entire vector population. This concept is predicated on high or strategically-targeted coverage of the host population with the drug, and effective drug pharmacokinetics in the host.
Box 2. Effects of mass endectocide administration on mosquito populations.
Ivermectin MDA directly results in the death of a significant proportion of existing biting Anopheles mosquitoes [13, 14], and should increase mortality rates of a proportion of mosquitoes that imbibe a sub-lethal amount of drug [9]. This should have the dual effect of skewing the age structure of the vector population toward the younger age classes that do not participate in transmission and reduce the population density of adult vectors. This was investigated using a previously developed simulation model [14], that incorporated a minor modification of larval density dependence, as described in [56].
| Equation 1 |
p0 = survival of larvae in the absence of density dependent regulation, pd = survival of larvae under density dependent regulation, Lt = number of larvae at time t, α = carrying capacity constant (0.0002) and γ = intraspecific competition constant (1.0) derived from [56].
An ivermectin MDA was simulated to a village in southeastern Senegal covering 80% of the villagers (see text and [14]). The MDA results in the hastened death of a significant proportion of the adult Anopheles gambiae females. Coupled with the emergence of new adult females from the standing larval population, the population age structure shifts toward younger age classes for more than 3 weeks post-MDA (Figure IA). Focusing only on abundance, there is a precipitous but temporary drop in the standing adult female mosquito population size (Figure IB) that is most severe one week post-MDA (26% population reduction). The population remains below pre-MDA levels for up to three weeks post-MDA, but by one month post-MDA the population has rebounded to slightly higher (approximately 4%) than pre-MDA levels (Figure IC). The adult replenishment rate will be influenced by local environmental conditions, including the amount of standing water during the MDA, temperature and weather, so this example is meant to be illustrative rather than predictive. The rebound effect and local environmental conditions are likely to explain the increased bites/person/month observed 28 days after a single MDA [13]. However, given that the majority of the adult female mosquito population is young in the month following MDA, regardless of the population size and local environmental conditions, there will be fewer infectious (sporozoite-transmitting) mosquitoes following treatment [15].
MDAs of ivermectin have been occurring for >15 years in many areas of Africa, and Latin America for onchocerciasis control, and so one may question why those areas have not seen obvious signs of reduced malaria parasite prevalence, incidence or associated disease. These programs only administer MDAs once or twice per year, which will not result in a sustained interruption of the malaria transmission cycle. Furthermore, these MDAs often do not coincide with malaria transmission seasons in the same area. Most importantly, asexual parasitemias in humans can be remarkably stable even in the absence of sporozoite transmission, as is seen in parasitological surveys of humans over transmission seasons [57]. Only sustained reductions in malaria parasite transmission will eventually result in reductions of malaria prevalence or disease.
Mosquito studies
Initial mosquito studies with avermectin/IVM demonstrated that Anopheles species were highly sensitive to these drugs compared to various Aedes and Culex species [4–6]. Studies also demonstrated that sub-lethal concentrations of IVM (for the adult mosquito) could reduce female fecundity, the hatch rate of their eggs, and survival of progeny larvae [5–8]. In laboratory membrane-feeding experiments matching mosquito feeding times to IVM concentrations found in human plasma post-MDA (150 µg/kg), we recorded mortality effects, inhibition of re-blood feeding and compounding mortality effects of sub-lethal doses in colonized Anopheles gambiae s.s. [9]. Direct human blood feeding experiments using colonized mosquitoes and IVM-dosed humans have been conducted with Aedes polynesiensis [10], Anopheles faurauti [11], and recently against An. gambiae s.s. [12]. These studies demonstrated reduced survival of mosquitoes that blood feed on IVM-dosed humans, but reported variable time periods in which mosquitocidal effects could be maintained after drug administration. Bockarie et al. [13] performed the first field demonstration of IVM’s effects on wild mosquitoes, focusing on the transmission of Wuchereria bancrofti. Wild, engorged, indoor-resting Anopheles punctulatus caught from two houses the day after IVM MDA (400 µg/kg) had significantly reduced survivorship compared to those captured from two houses of a control untreated village. In another village, An. punctulatus had significantly reduced survival if caught within three days after residents received an IVM (400 µg/kg) plus diethylcarbamazine (6 mg/kg) MDA compared to those caught pre-MDA, but survivorship was not reduced in mosquitoes collected 28 days post-MDA. The authors also found that the number of bites per person per month significantly increased one month after the MDA (see Box 2 for possible explanation). We recently conducted similar experiments following IVM MDAs for onchocerciasis control (150 µg/kg) in southeastern Senegalese villages [14]. IVM MDA significantly reduced the direct survivorship of An. gambiae s.l. mosquitoes for six days following MDA in villages from three replicate experiments when only ~80% of the villagers were covered in the MDA. The field data also demonstrated that the true IVM MDA effects lasted three times longer than predicted from our laboratory experiments [9]. These data are likely still to be an underestimate because they did not account for IVM sub-lethal effects such as knockdown and inhibited recovery and re-blood feeding that we know occur in the lab. Modeling the field data demonstrated that the basic reproductive rate of malaria (R0) could be significantly reduced if MDA were given at monthly intervals or shorter. Lastly, sporozoite rates were analyzed from these same captured mosquitoes. Mean sporozoite rates in mosquitoes captured from treated villages decreased 79% from before to two weeks after MDA, while sporozoite rates from nearby pair-matched untreated villages increased over the same time periods. Logistic regression analysis of treatment by time confirmed the difference between control and treated villages to be significantly different [15].
The promise and pitfalls of using ivermectin for malaria transmission control
Malaria remains a significant threat to human health in the developing world, and the rapid development of new control strategies is essential. Since the failed global malaria eradication campaign of the early 1960s, it has been acknowledged that malaria can only be controlled and potentially eliminated from specific geographical areas with combinations of tools in integrated malaria management programs [16]. The diverse epidemiology of malaria around the world highlights the need for many different integrated management control tools. Those that synergize with existing tools, as well as existing health infrastructures, will make these integrated programs a reality. IVM MDAs may be a powerful and synergistic new tool for integrated malaria and NTD control in defined areas.
The most effective malaria vector control tools are long lasting insecticide treated nets (LLITN) and indoor residual spraying (IRS), both of which are designed to kill endophagic vectors that enter houses or repel them from houses, although there is some evidence that LLITNs have an effect on malaria transmission by exophagic vectors [17]. Targeting endophilic vectors has been the ‘low-hanging fruit’ for malaria vector control because in-home measures focus on a more controllable environment, but it is increasingly recognized that we must control parasite transmission by both exophagic and exophilic vectors if we are ever to have success in the new malaria elimination and eradication campaigns being championed [18]. IVM offers a novel mode of delivery to a mosquito, an oral mosquitocidal drug that will be in a person’s blood, and so it will reach both endophagic and exophagic vectors. Furthermore, adaptive changes (e.g. shifting to crepuscular biting or exophagy) will not protect these mosquitoes from drug exposure, as they have for control via LLITNs or IRS.
A second benefit of IVM is the low potential for mosquito cross-resistance from current malaria control and agricultural operations. Mass DDT and pyrethroid agricultural applications have contributed to the spread of insecticide resistance into mosquito populations, threatening malaria control applications using the same insecticides [19]. Avermectin-family agricultural pesticides, on the other hand, are relatively expensive and rarely used in outdoor applications in the developing world. Furthermore, macrocyclic lactone drugs have a different molecular target and mode of action than insecticides currently used for LLITN and IRS (Box 1). Lastly, there is no evidence to date that pyrethroid resistance mechanisms in vectors cross-targets IVM [20].
Thirdly, IVM MDAs have potential value to inhibit the development of malaria parasite resistance to drugs targeting asexual and sexual stages in the human host. Any escapee parasites harboring drug-resistant alleles must still develop in the mosquito to be spread to new hosts. If IVM MDAs were concomitantly deployed with MDAs of antimalarials, a potentially resistant parasite strain is likely to die in the mosquito host and never be propagated. Importantly, under the new malaria elimination and eradication agendas, development of antimalarials that can be safely used in MDAs has been identified as an enhanced research priority [21].
In our models, IVM MDAs might be able to significantly reduce malaria parasite transmission in a community if given once a month or more frequently [14] (Box 2). Current onchocerciasis control regimens only require MDA once or twice per year to be effective, and community directed treatment programs are now effectively employed to achieve the highest sustained coverage of endemic communities [2]. The threat of drug resistance by nematodes associated with more frequent IVM MDAs deserves consideration (discussed below). Additionally, the logistical demands and costs associated with more frequent IVM MDA must be addressed. The community directed treatment models have proven highly efficacious and cost effective [22, 23]; their enhancement by integration with antimalaria measures (more IVM MDAs, bednet distribution) and control of soil-transmitted helminths via more MDAs (see below) is likely to only enhance their cost: benefit ratio. Merck, through its Mectizan Donation Program, currently covers the majority of the cost of IVM MDAs for onchocerciasis control [23]. It is likely that international health and aid agencies would need to step in to bear the added costs if a massive scale up of IVM MDAs for malaria parasite transmission control were to occur. Most obvious to all of these concerns, IVM MDA may be most manageable in areas with seasonal malaria transmission, such as the Sahel and Sudano-Guinean habits, or during epidemics, but may be less tractable in areas with year-round transmission. However, sustained malaria control or elimination from any continental area is expected to require the application of control tools, expanded health infrastructure, and constant malaria surveillance over decades [16], all of which will be expensive and logistically demanding in their own right. When this fact is taken into consideration, the argument that frequent IVM MDAs might be unsustainable under such needed programs seems weak.
The safety of frequent IVM administrations would need to be monitored, but reports of frequently-dosed individuals and communities have not been associated with increases in IVM-related toxicity or severe adverse reactions [24, 25]. The primary health concern of widespread IVM MDA in Africa is associated with the presence of high Loa loa microfilaremias in some individuals who may have severe adverse events following drug administration [26]. Current prevention efforts against this rely on a rapid assessment of loasis in endemic areas planned for IVM MDA.
The added benefit of frequent IVM dosing schedules would be the reduction in prevalence and intensities of soil-transmitted helminths (STH) in the same malaria-afflicted communities. IVM is highly efficacious against Strongyloides sterocoralis, variably active against roundworms (Ascaris lumbricoides) and whipworms (Trichuris trichiura), and weakly active against hookworms (Necator americanus and Ancyclostoma duodenale) [27]. The most recent evidence from Nigeria points to significant reduction in the prevalence of roundworms and whipworms among children from villages that have participated in yearly IVM MDA for onchocerciasis control for 13 years [28]. Malaria and STHs are co-endemic across large areas of sub-Saharan Africa [29], and both malaria infections and high STH intensities (particularly hookworms) can result in anemia [30]. Co-infections with malaria and intestinal helminths can increase the likelihood of anemia in children and in pregnant women, and co-infections of women can result in significantly greater risks for adverse birth outcomes [31]. The disease synergies between malaria and STHs, and the possibility of combined control of nematodes and malaria with simple MDA regimens should be a powerful incentive to bundle such regimens into integrated health programs of co-endemic areas.
More frequent endectocide exposure would undoubtedly increase selection on nematode populations to develop drug resistance. There are reports of suboptimal responses of Onchocerca volvulus to IVM, which seem to be associated with genetic selection [32]. Anthelmintic resistance has been a problem in veterinary helminths, where 100% coverage rates and frequent drug administration of sheep flocks and cattle herds can be common. Differential GluCl and P-glycoprotein alleles are associated with IVM resistance in Haemonchus contortus and Cooperia onchophora [33, 34]. The most viable option for limiting the potential for drug resistance under a frequent IVM MDA regimen would likely be combination and/or rotational chemotherapy using other anthelmintic drug classes, such as the benzimidazoles. Similarly, frequent IVM MDAs should not be considered as monotherapy treatment programs that would continue indefinitely, but instead as part of a concerted integrated disease management programs with defined endpoints. Both MDA timing and coverage rates will also influence the selection of resistance alleles in helminths [35]. Human MDAs rarely achieve >80% coverage because of limitations on who can take the drugs, logistics, and non-compliance. This likely creates refugia in untreated people where non-resistant helminths can proliferate, and may out-compete helminths harboring resistance alleles if such alleles confer lower fitness. More frequent IVM MDAs for malaria control might also be limited to the rainy season when mosquitoes are transmitting the most malaria. This season is also when pre-parasitic stages of helminths can best survive outside the host in moist soils, thus better preserving any non-drug selected alleles among the population.
The possibility of IVM resistance developing in mosquitoes is currently theoretical. Drug exposure (both in quantity and in duration) of a mosquito population would be far less compared with helminths that live primarily inside the host, and only female adult mosquitoes would be exposed. Furthermore, the drug exposure of females would be lessened if the malaria vector species had some zoophagic tendencies. A laboratory study of selected IVM resistance in Drosophila was the result of target site mutations, but the flies also had compromised fitness [36]. There are field reports of abamectin resistance in plant pests arising from intensive abamectin spraying operations [37]. Potentially confounding variables are IVM effects on female fecundity, egg hatch rate and larval survival, and the possibility of resistant alleles already being present in current mosquito populations. However, these potential confounders are negatively countered by the variables of incomplete human coverage during MDA, heterogeneity in mosquito blood meal choice, and some mosquito immigration from untreated MDA border areas. On the whole, we think IVM resistance would be less likely to develop in malaria vectors if a frequent but sufficiently-spaced IVM MDA strategy was deployed, and only on a seasonal basis. For example, if IVM MDA were administered every three weeks over the course of a five month malaria transmission season, it would only require six to seven MDAs that could be conducted through community directed treatment programs. Smaller numbers of dry season and early and late wet-season mosquitoes would reproduce without drug pressure, while young, non-infectious adult mosquitoes in the middle of the wet season would still be able to lay eggs over interval periods when drug is not in human blood. This strategy has some of the properties of the ‘evolution-proof’ malaria control strategies proposed by Read and others [38].
Finally, malaria transmission driven by zoophagic malaria vectors may be particularly amenable to endectocide MDAs. An enhanced zooprophylaxis strategy [39] might be achieved by administration of avermectin endectocides to livestock kept near domiciles. Indeed, Fritz et al. [8] recently demonstrated that IVM administered to cattle significantly affected the survival and fecundity of An. gambiae s.s. blood feeding on these cattle. The pharmacokinetics of IVM in livestock has been optimized by delivery strategies that allow for a longer half life of the drug in animals and sustained release into the blood stream, thus allowing for fewer treatments of the cattle. Both direct injection with non-aqueous preparations and sustained-release implants [40] can achieve these results. Additionally, many other avermectin derivatives have been developed by veterinary pharmaceutical companies for use in livestock and pets (eg. selemectin, doramectin, eprinomectin, moxidectin) which have enhanced pharmacokinetic profiles or reduced toxicity to certain breeds, and which have differential activity against malaria vectors (Ned Walker, unpublished). The added benefit of enhanced zooprophylaxis would be that the owners would have healthier animals by de-worming.
Concluding remarks
We have presented various arguments in favor of developing endectocides, particularly IVM, as novel tools for the control of malaria. These arguments include a novel mode of drug action compared to currently used insecticides, potential to target many vector species regardless of their biting habits, high sensitivity of the vectors to extremely low concentrations of drug, possible reduced potential for developing drug resistance by the mosquito, and cross-activity against intestinal helminths. The arguments against developing these tools include logistical difficulties and higher costs associated with more frequent MDAs, and the potential to foster endectocide resistance in helminth populations. Ultimately, field data must be collected to validate or invalidate the specific arguments, as well as to address unforeseen issues. In our opinion, the promise of endectocides as novel and synergistic malaria control tools is great for many malaria-endemic regions across the globe.
Figure I.
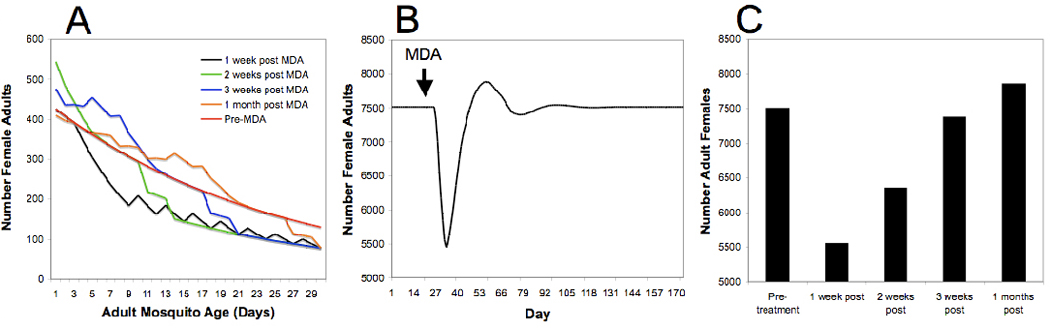
A model of female adult Anopheles gambiae age structure and abundance following an ivermectin mass drug administration. (a) Models of combined age structure and abundance prior to mass drug administration (Pre-MDA, red line), and 1–4 weeks post MDA (black, green, blue and orange lines, respectively). (b) The modeled effect of a single MDA on female adult abundance over time. (c) Discrete number output of the abundance model on a weekly timescale. Derived from [14].
Table I.
The ligand-gated ion channel genes (excluding the nAChR family) of Diptera.
| Ligand | Paralogue name |
Anopheles gambiae |
Aedes aegypti |
Culex quinquefasciatus |
Drosophila melanogaster |
|---|---|---|---|---|---|
| glutamate | GluCl | AGAP001434a | AAEL003003 | CPIJ010616 | FBgn0024963 |
| pH (hydroxyl ions) | pHCl | AGAP005599 | CPIJ002294 | FBgn0036542 | |
| GABA | RDL | AGAP006028 | AAEL008354 | CPIJ008419 | FBgn0004244 |
| LCCH3 | AGAP000038 | AAEL010710 | CPIJ014518 | FBgn0010240 | |
| GRD | AGAP011349 | CPIJ006773 | FBgn0001134 | ||
| FBgn0030707 | |||||
| glycine | AGAP007707 | AAEL001568 | CPIJ017366 | FBgn0029733 | |
| AAEL004513 | CPIJ009348 | FBgn0036727 | |||
| FBgn0039840 | |||||
| histidine | HisCl1 | AGAP012975 | AAEL003028 | CPIJ017412 | FBgn0037950 |
| ort | AGAP001913 | AAEL012248 | CPIJ013943 | FBgn0003011 | |
| CPIJ013955 | |||||
| HisCl2 | AGAP001990 | AAEL006047 | CPIJ006949 | ||
| AAEL015452 | CPIJ002292 | ||||
| AAEL001272 | CPIJ002295 | ||||
| unknown | AGAP010694 | AAEL003958 | CPIJ011023 | ||
| unknown | AGAP000039 | AAEL015091 | FBgn0033558 | ||
Accession numbers for orthologous and paralogous genes were identified in the Vectorbase, Flybase, Ensemble and NCBI databases.
Abbreviations: nAChR, nicotinic acetylcholine receptor; GABA, γ-amino-butyric acid.
Acknowledgements
B.D.F., K.C.K., I.M.D.S. and M.S. acknowledge support from NIH grant R21 AI079528, Grand Challenges Explorations grant 51995 from the Bill and Melinda Gates Foundation, and CRC grant 1686174 from Colorado State University.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Campbell WC. Ivermectin and Abamectin. Springer Verlag; 1989. [Google Scholar]
- 2.Cupp EW, et al. Elimination of human onchocerciasis: History of progress and current feasibility using ivermectin (Mectizan((R))) monotherapy. Acta Trop. 2010 doi: 10.1016/j.actatropica.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 3.Wilson ML. Avermectins in arthropod vector management - prospects and pitfalls. Parasitol Today. 1993;9:83–87. doi: 10.1016/0169-4758(93)90210-7. [DOI] [PubMed] [Google Scholar]
- 4.Pampiglioni S, et al. Avermectins, MK-933 and MK-936, for mosquito control. Trans R Soc Trop Med Hyg. 1985;79:797–799. doi: 10.1016/0035-9203(85)90121-x. [DOI] [PubMed] [Google Scholar]
- 5.Gardner K, et al. Effects of ivermectin in canine blood on Anopheles quadrimaculatus, Aedes albopictus and Culex salinarius. J Am Mosq Control Assoc. 1993;9:400–402. [PubMed] [Google Scholar]
- 6.Tesh RB, Guzman H. Mortality and infertility in adult mosquitoes after the ingestion of blood containing ivermectin. Am J Trop Med Hyg. 1990;43:229–233. doi: 10.4269/ajtmh.1990.43.229. [DOI] [PubMed] [Google Scholar]
- 7.Focks DA, et al. Effects of ivermectin (MK-933) on the reproductive rate of Aedes aegypti (Diptera: Culicidae) J Med Entomol. 1991;28:501–505. doi: 10.1093/jmedent/28.4.501. [DOI] [PubMed] [Google Scholar]
- 8.Fritz ML, et al. Toxicity of bloodmeals from ivermectin-treated cattle to Anopheles gambiae s.l. Ann Trop Med Parasitol. 2009;103:539–547. doi: 10.1179/000349809X12459740922138. [DOI] [PubMed] [Google Scholar]
- 9.Kobylinski KC, et al. The effect of oral anthelmintics on the survivorship and re-feeding frequency of anthropophilic mosquito disease vectors. Acta Trop. 2010;116:119–126. doi: 10.1016/j.actatropica.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cartel JL, et al. Cumulative mortality rates in Aedes polynesiensis after feeding on polynesian Wuchereria bancrofti carriers treated with single doses of ivermectin, diethylcarbamazine and placebo. Trop Med Parasitol. 1991;42:343–345. [PubMed] [Google Scholar]
- 11.Foley DH, et al. The potential of ivermectin to control the malaria vector Anopheles farauti. Trans R Soc Trop Med Hyg. 2000;94:625–628. doi: 10.1016/s0035-9203(00)90211-6. [DOI] [PubMed] [Google Scholar]
- 12.Chaccour C, et al. Effect of ivermectin on Anopheles gambiae mosquitoes fed on humans: the potential of oral insecticides in malaria control. J Infect Dis. 2010;202:113–116. doi: 10.1086/653208. [DOI] [PubMed] [Google Scholar]
- 13.Bockarie MJ, et al. Mass treatment with ivermectin for filariasis control in Papua New Guinea: impact on mosquito survival. Med Vet Entomol. 1999;13:120–123. doi: 10.1046/j.1365-2915.1999.00159.x. [DOI] [PubMed] [Google Scholar]
- 14.Sylla M, et al. Mass drug administration of ivermectin in south-eastern Senegal reduces the survivorship of wild-caught, blood fed malaria vectors. Malar J. 2010;9:365. doi: 10.1186/1475-2875-9-365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kobylinski KC, et al. Ivermectin mass drug administration to humans disrupts malaria parasite transmission in Senegalese villages. Am J Trop Med Hyg. doi: 10.4269/ajtmh.2011.11-0160. (In press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Organization WH. Global malaria control and elimination: report of a technical review. World Health Organization; 2008. p. 47. [Google Scholar]
- 17.Charlwood JD, et al. Do bednets reduce malaria transmission by exophagic mosquitoes? Trans R Soc Trop Med Hyg. 2005;99:901–904. doi: 10.1016/j.trstmh.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 18.Ferguson HM, et al. Ecology: a prerequisite for malaria elimination and eradication. PLoS Med. 2010;7:e1000303. doi: 10.1371/journal.pmed.1000303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lines JD. Do agricultural insecticides select for insecticide resistance in mosquitoes? A look at the evidence. Parasitol Today. 1988;4:S17–S20. doi: 10.1016/0169-4758(88)90083-x. [DOI] [PubMed] [Google Scholar]
- 20.Strycharz JP, et al. A new ivermectin formulation topically kills permethrin-resistant human head lice (Anoplura: Pediculidae) J Med Entomol. 2008;45:75–81. doi: 10.1603/0022-2585(2008)45[75:aniftk]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 21.Greenwood BM. Control to elimination: implications for malaria research. Trends Parasitol. 2008;24:449–454. doi: 10.1016/j.pt.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 22.Burnham G, Mebrahtu T. The delivery of ivermectin (Mectizan) Trop Med Int Health. 2004;9:A26–A44. doi: 10.1111/j.1365-3156.2004.01211.x. [DOI] [PubMed] [Google Scholar]
- 23.Goldman AS, et al. National mass drug administration costs for lymphatic filariasis elimination. PLoS Negl Trop Dis. 2007;1:e67. doi: 10.1371/journal.pntd.0000067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Duke BO, et al. Effects of multiple monthly doses of ivermectin on adult Onchocerca volvulus. Am J Trop Med Hyg. 1990;43:657–664. doi: 10.4269/ajtmh.1990.43.657. [DOI] [PubMed] [Google Scholar]
- 25.Guzzo CA, et al. Safety, tolerability, and pharmacokinetics of escalating high doses of ivermectin in healthy adult subjects. J Clin Pharmacol. 2002;42:1122–1133. doi: 10.1177/009127002401382731. [DOI] [PubMed] [Google Scholar]
- 26.Kamgno J, et al. Loa loa microfilarial periodicity in ivermectin-treated patients: comparison between those developing and those free of serious adverse events. Am J Trop Med Hyg. 2009;81:1056–1061. doi: 10.4269/ajtmh.2009.09-0356. [DOI] [PubMed] [Google Scholar]
- 27.Geary TG, et al. Unresolved issues in anthelmintic pharmacology for helminthiases of humans. Int J Parasitol. 2010;40:1–13. doi: 10.1016/j.ijpara.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 28.Gutman J, et al. Effects of annual mass treatment with ivermectin for onchocerciasis on the prevalence of intestinal helminths. Am J Trop Med Hyg. 2010;83:534–541. doi: 10.4269/ajtmh.2010.10-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brooker S, et al. Epidemiology of Plasmodium-helminth co-infection in Africa: populations at risk, potential impact on anemia, and prospects for combining control. Am J Trop Med Hyg. 2007;77:88–98. [PMC free article] [PubMed] [Google Scholar]
- 30.Midzi N, et al. Consequences of polyparasitism on anaemia among primary school children in Zimbabwe. Acta Trop. 2010;115:103–111. doi: 10.1016/j.actatropica.2010.02.010. [DOI] [PubMed] [Google Scholar]
- 31.Yatich NJ, et al. The effect of malaria and intestinal helminth coinfection on birth outcomes in Kumasi, Ghana. Am J Trop Med Hyg. 2010;82:28–34. doi: 10.4269/ajtmh.2010.09-0165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bourguinat C, et al. Genetic selection of low fertile Onchocerca volvulus by ivermectin treatment. PLoS Negl Trop Dis. 2007;1:e72. doi: 10.1371/journal.pntd.0000072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Blackhall WJ, et al. Selection at a P-glycoprotein gene in ivermectin- and moxidectin-selected strains of Haemonchus contortus. Mol Biochem Parasitol. 1998;95:193–201. doi: 10.1016/s0166-6851(98)00087-5. [DOI] [PubMed] [Google Scholar]
- 34.Njue AI, Prichard RK. Genetic variability of glutamate-gated chloride channel genes in ivermectin-susceptible and -resistant strains of Cooperia oncophora. Parasitology. 2004;129:741–751. doi: 10.1017/s0031182004006183. [DOI] [PubMed] [Google Scholar]
- 35.Geerts S, Gryseels B. Anthelmintic resistance in human helminths: a review. Trop Med Int Health. 2001;6:915–921. doi: 10.1046/j.1365-3156.2001.00774.x. [DOI] [PubMed] [Google Scholar]
- 36.Kane NS, et al. Drug-resistant Drosophila indicate glutamate-gated chloride channels are targets for the antiparasitics nodulisporic acid and ivermectin. Proc Natl Acad Sci U S A. 2000;97:13949–13954. doi: 10.1073/pnas.240464697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pu X, et al. Characterisation of abamectin resistance in a field-evolved multiresistant population of Plutella xylostella. Pest Manag Sci. 2009;66:371–378. doi: 10.1002/ps.1885. [DOI] [PubMed] [Google Scholar]
- 38.Read AF, et al. How to make evolution-proof insecticides for malaria control. PLoS Biol. 2009;7:e1000058. doi: 10.1371/journal.pbio.1000058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bogh C, et al. Zooprophylaxis, artefact or reality? A paired-cohort study of the effect of passive zooprophylaxis on malaria in The Gambia. Trans R Soc Trop Med Hyg. 2002;96:593–596. doi: 10.1016/s0035-9203(02)90320-2. [DOI] [PubMed] [Google Scholar]
- 40.Boyce WM, et al. Use of ivermectin implants for the treatment of psoroptic scabies in free-ranging bighorn sheep. Journal of Zoo and Wildlife Medicine. 1992;23:211–213. [Google Scholar]
- 41.Harder A. Chemotherapeutic approaches to nematodes: current knowledge and outlook. Parasitol Res. 2002;88:272–277. doi: 10.1007/s00436-001-0535-x. [DOI] [PubMed] [Google Scholar]
- 42.Raymond-Delpech V, et al. Ion channels: molecular targets of neuroactive insecticides. Invert Neurosci. 2005;5:119–133. doi: 10.1007/s10158-005-0004-9. [DOI] [PubMed] [Google Scholar]
- 43.Buckingham SD, et al. Insect GABA receptors: splicing, editing, and targeting by antiparasitics and insecticides. Mol Pharmacol. 2005;68:942–951. doi: 10.1124/mol.105.015313. [DOI] [PubMed] [Google Scholar]
- 44.Jones AK, Sattelle DB. The cys-loop ligand-gated ion channel superfamily of the honeybee. Apis mellifera. Invert Neurosci. 2006;6:123–132. doi: 10.1007/s10158-006-0026-y. [DOI] [PubMed] [Google Scholar]
- 45.Jones AK, et al. The nicotinic acetylcholine receptor gene family of the malaria mosquito Anopheles gambiae. Genomics. 2005;85:176–187. doi: 10.1016/j.ygeno.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 46.Lee D, et al. GABA receptors containing Rdl subunits mediate fast inhibitory synaptic transmission in Drosophila neurons. J Neurosci. 2003;23:4625–4634. doi: 10.1523/JNEUROSCI.23-11-04625.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gisselmann G, et al. Drosophila melanogaster GRD and LCCH3 subunits form heteromultimeric GABA-gated cation channels. Br J Pharmacol. 2004;142:409–413. doi: 10.1038/sj.bjp.0705818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bloomquist JR. Chloride channels as tools for developing selective insecticides. Arch Insect Biochem Physiol. 2003;54:145–156. doi: 10.1002/arch.10112. [DOI] [PubMed] [Google Scholar]
- 49.Schnizler K, et al. A novel chloride channel in Drosophila melanogaster is inhibited by protons. J Biol Chem. 2005;280:16254–16262. doi: 10.1074/jbc.M411759200. [DOI] [PubMed] [Google Scholar]
- 50.Mounsey K, et al. Molecular characterisation of a pH-gated chloride channel from Sarcoptes scabei. Invertebrate Neuroscience. 2007;7:149–156. doi: 10.1007/s10158-007-0050-6. [DOI] [PubMed] [Google Scholar]
- 51.Zheng Y, et al. Identification of two novel Drosophila melanogaster histamine-gated chloride channel subunits expressed in the eye. J Biol Chem. 2002;277:2000–2005. doi: 10.1074/jbc.M107635200. [DOI] [PubMed] [Google Scholar]
- 52.Ludmerer SW, et al. Ivermectin and nodulisporic acid receptors in Drosophila melanogaster contain both gamma-aminobutyric acid-gated Rdl and glutamate-gated GluCl alpha chloride channel subunits. Biochemistry. 2002;41:6548–6560. doi: 10.1021/bi015920o. [DOI] [PubMed] [Google Scholar]
- 53.Jones AK, et al. Splice-variant- and stage-specific RNA editing of the Drosophila GABA receptor modulates agonist potency. J Neurosci. 2009;29:4287–4292. doi: 10.1523/JNEUROSCI.5251-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shan Q, et al. Ivermectin, an unconventional agonist of the glycine receptor chloride channel. J Biol Chem. 2001;276:12556–12564. doi: 10.1074/jbc.M011264200. [DOI] [PubMed] [Google Scholar]
- 55.Lespine A, et al. Interaction of macrocyclic lactones with the multidrug transporters: the bases of the pharmacokinetics of lipid-like drugs. Curr Drug Metab. 2009;10:272–288. doi: 10.2174/138920009787846297. [DOI] [PubMed] [Google Scholar]
- 56.Dobson SL, et al. The effect of Wolbachia-induced cytoplasmic incompatibility on host population size in natural and manipulated systems. Proc Biol Sci. 2002;269:437–445. doi: 10.1098/rspb.2001.1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Smith T, et al. Absence of seasonal variation in malaria parasitaemia in an area of intense seasonal transmission. Acta Trop. 1993;54:55–72. doi: 10.1016/0001-706x(93)90068-m. [DOI] [PubMed] [Google Scholar]


