Abstract
Macrophages play a central role in innate immune responses, in disposal of cholesterol, and in tissue homeostasis and remodeling. To perform these vital functions macrophages display high endosomal/lysosomal activities. Recent studies have highlighted that acid sphingomyelinase (ASMase), which generates ceramide from sphingomyelin, is involved in modulation of membrane structures and signal transduction in addition to its metabolic role in the lysosome. In this review, we bring together studies on ASMase, its different forms and locations that are necessary for the macrophage to accomplish its diverse functions. We also address the importance of ASMase to several disease processes that are mediated by activated macrophages.
Keywords: Sphingomyelin, Acid sphingomyelinase, Macrophage, Ceramide, Lysosome, Niemann–Pick disease
Introduction
The fluid mosaic model of cell membranes, first proposed in 1972 [1], postulated that the plasma membrane could be described as a two-dimensional liquid, in which receptors and other membrane-associated proteins are randomly diffused. However, later studies showed that sphingolipids, an ubiquitous class of structurally diverse bioactive lipids, are involved in membrane structures [2]. Sphingolipids have an asymmetrical distribution in the membrane and a tendency to associate with one another through hydrogen bonds [3, 4], which forced a revision of the fluid mosaic model. Sphingomyelin (SM) is the most abundant sphingolipid found in eukaryotic cell membranes. Acid sphingomyelinase (ASMase, EC 3.1.4.12), primarily found in lysosomes, hydrolyzes SM, resulting in the generation of ceramide. Thus, ASMase plays a central role in transmembrane signaling as well as in lysosomal metabolic functions.
The macrophage is a very heterogeneous cell that differentiates from hematopoietic progenitors [5] which can form dendritic cells, osteoclasts, and monocytes as well as macrophages. Monocytes circulate in the blood, but when activated are present in tissues as macrophages [6]. These cells are active in both innate and adaptive immune responses and in reverse cholesterol transport [7], and are involved in normal tissue homeostasis through the ingestion and clearance of apoptotic bodies [8, 9] and in resorption of bone during normal bone remodeling [10]. A classically activated macrophage requires both interferon-gamma and either the induction of tumor necrosis factor alpha (TNF-α), or the engagement of toll-like receptor (TLR) typically by a microbial product [11]. In response, the macrophage secretes inflammatory cytokines such as TNF-α and interleukin-1β (IL-1β), and chemokines that drive an immune reaction. As a primary phagocytic cell type, macrophages can ingest and process many different types of particles. Hence, this cell type has high endosomal/lysosomal activities. This review focuses on studies addressing the role of ASMase in macrophage biology, with special emphasis on the involvement of this enzyme in disease processes. This review also briefly addresses the role of ASMase in cell types that can influence macrophage functionality, such as endothelial cells.
Sphingomyelinases
The first enzyme characterized to hydrolyse SM to ceramide was isolated from rat livers and had an optimal activity at pH 4.5–5.0 [12]. Later studies showed that this enzyme, ASMase, was also present in most human tissues [13]. Since then, three different SMases have been described and characterized primarily based on their optimal pH: neutral, alkaline and acid SMases.
Neutral sphingomyelinase (nSMase) is encoded by three different genes generating three different forms of the enzyme. The first form, nSMase 1, has been shown to be involved in the induction of T-cell receptor-mediated apoptosis [14], and in heat stress-induced apoptosis of zebrafish embryonic cells [15]. However, its role remains undefined as knockout (KO) mice for nSMase 1 do not exhibit detectable defects in lipid storage nor in sphingolipid metabolism [16]. The enzyme nSMase 2 has been shown to mediate stress-induced apoptosis in daunorubicin-induced cell death of the breast cancer cell line MCF-7 [17], and in cigarette smoke-induced apoptosis of bronchial epithelial cells [18]. It may also function in the cell cycle by causing arrest in the G0/G1 phase [19], and in inflammation [20]. The nSMase 2 enzyme has a function in controlling embryonic and post-natal development, as shown in the KO mouse which displays a dwarf-phenotype and delayed puberty [21]. A role for nSMase 2 in macrophage growth has been implicated by the discovery of nSMase 2 mutations in several myeloid leukemias [22]. The relatively recent description of nSMase 3 has not so far shown significant functional data, but it appears to play a role in the response of MCF-7 cells to TNF-α stimulation [23].
Alkaline SMase (Alk-SMase) is secreted in the intestinal lumen and mucosa and appears to be primarily involved as the first step in the digestion of SM present in food [24, 25]. However, it likely plays other roles as Alk-SMase has anti-proliferative effects on HT-29 colon carcinoma cells [26], and a mutation has been shown to be associated with the development of human colon cancer [27]. The presence of macrophages in inflammatory bowel disease suggests a function for Alk-SMase in regulatory activities [24].
ASMase is the most studied sphingomyelinase although its regulation is still incompletely understood. ASMase has been found in almost all cell types that have been studied, including macrophages, with the endothelium being a particularly rich source [28]. While the optimal activity at acidic pH led researchers to characterize ASMase as a lysosomal protein [29], a secreted form, secretory ASMase (S-ASMase), has also been described [30]. Secretory ASMase is generated through differential processing and trafficking of the same precursor as L-ASMase [31, 32]. A major biochemical difference between the two forms is the requirement for exogenous zinc in S-ASMase activation, whereas L-ASMase, by virtue of its trafficking to and localization in the lysosome, does not require exogenous zinc for its activation [32]. In the next section, differences in the roles of L- and S-ASMase are discussed.
Lysosomal versus secretory ASMase
Lysosomes are heterogeneously sized membrane-bound structures that contain acid hydrolases [33]. These organelles have been described as ‘the stomach of the cell’ as they are able to digest material such as oligosaccharides and lipoproteins into their constituent parts that can be reused by the host cell [34]. Recently published data have shown that lysosomal stability in fibroblasts depends on ASMase activity [35]. ASMase is located in the luminal leaflet of endosomes, lysosomes and phagosomes. It has been shown that maintenance of lysosomal stability requires binding of heat shock protein 70 (HSP70) to an endolysosomal anionic phospholipid bis(monoacylglycero) phosphate, which is necessary for lysosomal SM metabolism [35]. Interestingly, the conversion of SM to ceramide in the lysosome changes its shape and facilitates its fusion to other membrane structures [36–39]. Furthermore, at the immunological synapse where secretory granules (also known as secretory lysosomes) containing perforin and granzymes fuse to the plasma membrane of T cells, L-ASMase is required to reduce the volume of the granules to better expel their contents [40]. L-ASMase activity asymmetrically generates ceramide in the luminal leaflet and leads to increased surface tension and a contraction of the granule [40, 41]. The ability of L-ASMase in changing the shape of the granule can thus reduce its volume and diminish its carrying capacity [40, 41]. Moreover, fusion of lysosomes to the plasma membrane was found to be an important part of wound healing mediated by ASMase [42]. When the plasma membrane is compromised by insults such as bacterial pore-forming proteins, calcium influx causes rapid lysosomal exocytosis at the site of the wound. L-ASMase delivered to the outer leaflet of the plasma membrane converts SM to ceramide, which in turn generates microdomains that bud inwards forming endosomes internalizing the lesion and resulting in a newly intact plasma membrane [42].
The function of S-ASMase is not clearly defined. S-ASMase levels in the serum were found to be upregulated by TNF-α, IL-1β [28], lipopolysaccharide (LPS), and oxidative stress [43]. In patients with chronic heart failure, high levels of S-ASMase correlated with the development of cardiac cachexia, which has a worse prognosis [44, 45]. A correlation between high levels of serum S-ASMase activity and hemophagic lymphohistiocytosis (HLH) has been described [46]. HLH is a disease where macrophages and T-cells become hyperactivated and infiltrate organs where they subsequently secrete very large quantities of cytokines (hypercytokinemia) [47]. Symptoms include fever, splenomegaly and cytopenias, and if left untreated can prove to be fatal. The origin of the HLH-associated S-ASMase is unknown, as are its effects. However, it can be envisaged that, through the conversion of SM to ceramide on the macrophage cell surface, S-ASMase may promote an inflammatory response and perpetuate the secretion of inflammatory cytokines.
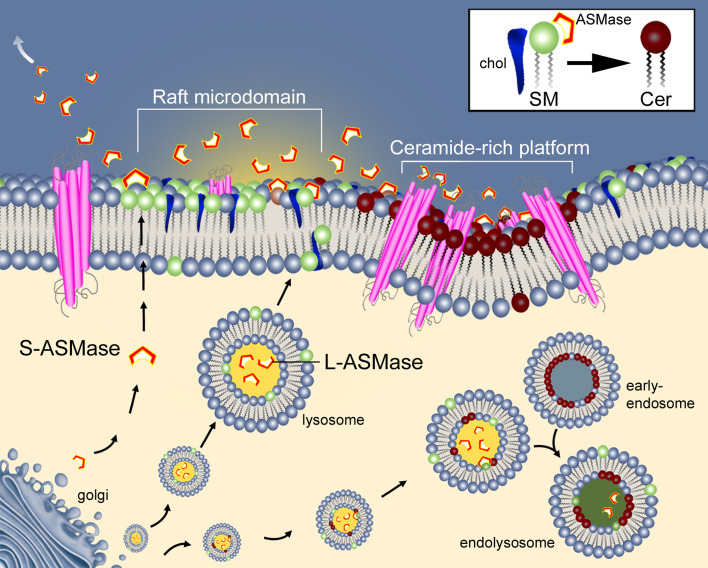
Despite the optimal pH of L-ASMase and S-ASMase being acidic, ASMase can be active at a less than optimal pH [30, 48]. A possible mechanism that may maintain enhanced ASMase activity could be that acidic microdomains on the exterior of the plasma membrane may be formed through lysosomal fusion with the plasma membrane and the release of its acidic contents. Local changes in membrane topology generated by the SM-derived ceramide could stabilize the acidic environment long enough for sustained ASMase activity by providing a membrane-bounded, partially enclosed region (Fig. 1).
Fig. 1.
The role of acid sphingomyelinase (ASMase) depends upon its location. Lysosomes containing lysosomal ASMase (L-ASMase) fuse to the plasma membrane at a raft microdomain-containing sphingomyelin (SM) and cholesterol (chol). This exposes the enzyme to SM present on the surface. Hydrolysis of SM to ceramide forms a ceramide-rich platform that facilitates transmembrane signaling, or can initiate endocytosis through the creation of an early endosome. Maturation of the endosome and fusion with lysosomes forms endolysosomes allowing the processing of ingested material. The possible roles of secretory ASMase (S-ASMase) are also shown in its capacity to affect SM hydrolysis away from the macrophage or to initiate the formation of a ceramide rich platform
ASMase biochemistry and cellular function
ASMase plays many different roles depending upon its location in the cell. SM in cellular membranes has a high affinity for cholesterol with which it associates in raft microdomains [49]. This association allows the compartmentalization of cellular processes by concentrating specific proteins (such as Src tyrosine kinase family and caveolins), and may also have a role in sequestering and temporary storage of cholesterol [50, 51]. Ceramide generated by hydrolysis of SM by SMases, however, has a lower affinity for cholesterol and displaces it from SM-rich microdomains [52], and has a higher tendency to aggregate forming ceramide-rich platforms [53, 54]. Ceramide-rich platforms are liquid-ordered domains that spatially reorganize receptors into clusters upon stimulation, and as a consequence there is a high concentration of signaling molecules in the platform that facilitates and enhances the subsequent activation of intracellular messengers [55, 56]. ASMase activation and translocation have been shown to be integral in the induction of receptor-mediated apoptosis. The mechanisms behind how a transmembrane receptor can activate and translocate ASMase are still unclear. However, recent publications have presented biochemical models that help to link receptor stimulation to ASMase activation. It should be noted that, while different models of ASMase activation are described, they are not necessarily mutually exclusive.
ASMase activation and the induction of apoptosis
ASMase activation has been shown to be integral in the induction of apoptosis through the signaling of TNF-α [57], Fas/CD95 [58], and TNF-related apoptosis-inducing ligand (TRAIL) [59]. CD95 is a cell surface molecule that, when stimulated, results in a rapid increase in SMase-propelled ceramide generation leading to apoptosis [60]. During CD95-induced apoptosis, ceramide generated by ASMase is localized to the mitochondrial membrane and the endoplasmic reticulum [61, 62]. However, ASMase-deficient B-cells, normally resistant to CD95-induced apoptosis, can become sensitive to CD95-mediated apoptosis if a significant number of CD95 receptors are cross-linked simultaneously [58]. This suggests that ASMase activation is required to promote enhanced cross-linking of surface receptors to initiate intracellular signaling events. ASMase activation is also implicated in lung development [63], in the signaling of stress responses to bacteria and viruses [40, 64], and in the apoptotic response of endothelial cells to ionizing radiation [65, 66], to UV light [67], and to chemotherapeutic drugs [68].
ASMase activation and translocation by receptor-mediated signaling
Upon activation by transmembrane signaling or through oxidative stress [69], ASMase translocates to the outer leaflet of the plasma membrane where it encounters SM and converts it to ceramide [58, 70]. The pool from which the plasma membrane-associated ASMase originates is still not clear, although it has been assumed by several groups that the source originates from the fusion of vesicles to the plasma membrane [71, 72]. The mechanism behind ASMase translocation in response to receptor cross-linking, also known as receptor-mediated ASMase activation, is also obscure. Fusion of vesicles containing L-ASMase to the plasma membrane can occur within seconds of stimulation [71–73]. This has been assumed to be the source of the ASMase present on the outer leaflet of the plasma membrane, and was supported by data showing that cytoskeletal disruption can inhibit receptor-mediated ASMase translocation but not its activation [74]. More recent data have shown that, upon cross-linking of the CD95 receptor, the translocation and activation of ASMase to the outer leaflet of the plasma membrane occurs via a syntaxin-4-mediated pathway [75]. Moreover, studies examining the expression of both ASMase and syntaxin-4 showed that they are colocalized [75]. Syntaxin-4 is a t-SNARE (target-Soluble NSF Attachment protein REceptor) associated with exocytosis of secretory granules and fusion of membranes, and provides a plausible mechanism for the translocation of ASMase from the lysosomes [76–78].
Another biochemical model of L-ASMase translocation in receptor-mediated activation has been proposed by Zeidan and Hannun [79] who suggest that protein kinase C δ (PKCδ)-phosphorylation of L-ASMase (at S508) induces both its activation and translocation to the outer leaflet of the plasma membrane. A recent report by the same group has shown that a mutation in the PKCδ phosphorylation site (S508A) inhibits ASMase secretion in response to TNF-α and IL-1β stimulation in MCF-7 cells [80]. It was shown that S-ASMase generates long-chain ceramide molecules in response to TNF-α and IL-1β stimulation, and thus proved that, in at least some situations, S-ASMase can also be responsible for the ceramide generation in the plasma membrane [80]. It is unclear what specific role the long chain ceramides play in this signaling event and whether this is a generalized event or cell-type specific. In addition, a role for S-ASMase has been described in the release of the monocyte attractant chemokine (C–C motif) ligand 5 (CCL5, formerly known as RANTES) from IL-1β-treated MCF-7 [81]. Interestingly, the ceramide generated was deacylated by acid ceramidase to form sphingosine, which was required for CCL5 release. The fact that other signaling events, as previously mentioned, can also increase S-ASMase levels in the plasma strongly suggests that the role of S-ASMase is more diverse and generalized and needs to be more thoroughly characterized.
ASMase activation by reactive oxygen species (ROS)
Ceramide-rich platforms concentrate nicotinamide adenine dinucleotide phosphate-oxidase (NADPH oxidase), which is a source of ROS [82]. ASMase can also be activated via ROS, although a study has shown that ROS generation can also be a product of ASMase activity [83–85]. While these results seem contradictory, they may also be describing a feed-forward mechanism where an initially small concentration of ROS can activate ASMase that will in turn generate more ROS. How ROS can activate ASMase is unknown, but one model has suggested that oxidation of the C-terminal region can activate ASMase [86].
Ionizing and some non-ionizing radiation can also activate ASMase via an unknown pathway that may involve ROS. Studies using ultraviolet-C light (also DNA damaging) in U937 cells showed the formation of ceramide-rich platforms and the translocation of ASMase to these regions [87]. The rapid activation of ASMase by ionizing radiation in endothelial cells (within 30 s) suggests that either membrane structural changes and/or free radicals caused by the ionization of water molecules are responsible for ASMase activation [88].
ASMase in disease
ASMase activity is indispensable in tissue homeostasis. However, there are diseases caused when ASMase activity is either absent or appears to have a detrimental effect, possibly due to chronic activity or altered distribution of the enzyme. Understanding these conditions can help to better understand the role of ASMase in the maintenance of human health.
Niemann–Pick disease A and B
The importance of ASMase in tissue homeostasis is underlined when defective expression of the enzyme was discovered as the cause of Niemann–Pick disease A and B (NPD-A and NPD-B) [89]. NPD-A, characterized by neurodegeneration, is the infantile form of the disease and is lethal by the age of 2–3 years. NPD-B is the later-onset form and distinguished by progressive hepatosplenomegaly, pulmonary insufficiency and heart disease [90]. Common between infants with NPD-A and most patients with NPD-B is interstitial lung disease caused by SM storage in pulmonary macrophages [91, 92]. It has been suggested that excess lipids stored in the NPD macrophages transform them into foam cells [93]. These foam cells secrete inflammatory cytokines that drive fibrosis and diminish lung capacity. The different outcomes of NPD-A and NPD-B can be attributed to residual ASMase activity present in NPD-B [94]. The underlying defect in these two diseases is the accumulation of SM in lysosomal compartments, a feature of which is the accumulation of cholesterol and gangliosides [95]. Mouse models for both NPD-A [96] and NPD-B using ASMase KO mice [97] have been developed and have proved invaluable for research on the functionality of ASMase. For example, L-ASMase activity of 1–14% that of wild-type was enough to rescue ASMase KO mice from the neurologic disease they usually develop [97].
Niemann–Pick disease C
The effects of ASMase extend to diseases that are not primarily caused by defective ASMase. Niemann–Pick disease C (NPD-C) is another lipid-storage disease caused by a defective endosomal protein, Niemann–Pick C2, that binds to a cholesterol-binding protein promoting trafficking of cholesterol from the lumen to late endosomes [98, 99]. Maor and colleagues [100] found that the uptake of oxidized low density lipoproteins (oxLDL) in macrophages of NPD-C patients results in the inhibition of L-ASMase, leading to the accumulation of free cholesterol and cholesteryl esters in lysosomes. Recently, it has also been demonstrated that overexpression of ASMase in NPD-C fibroblasts in vitro corrects the defect in lipid and protein trafficking by overcoming the inhibition of ASMase activity [101]. These findings provide hope that other lipid-storage diseases could be treated by using ASMase, even if the primary defect is a different target.
Atherosclerosis
A key role for extracellular ASMase (possibly S-ASMase) in the development of atherosclerotic plaques has been shown through the retention of lipoproteins in the arterial wall [102]. High levels of S-ASMase in the intima of atherosclerotic plaques have been correlated with the development of atherosclerotic plaques [103]. Endothelial cells are a rich source of S-ASMase which have been implicated in converting SM in LDL particles into ceramide [103, 104]. In the blood vessel, the hydrolysis of SM in LDL particles tends to increase the overall size of the particle and also increase the tendency of the particles to clump into yet bigger conglomerations which could lead to enhanced adhesion to the vascular wall [48]. Furthermore, SM inhibits oxidation of the LDL particle into the toxic oxidized form; therefore, enhanced conversion of SM in LDL to ceramide is deemed unfavorable [105]. Hence, exacerbation of atherosclerotic lesions would appear to demonstrate the deleterious consequences of S-ASMase release. Moreover, ASMase activity has been shown to be increased in vascular smooth muscle cells in response to oxLDL leading to apoptotic cell death [106]. Apoptotic cells can also generate oxLDL by exposing and oxidizing membrane lipids which, by activating ASMase-mediated apoptotic pathways, can induce more cell death in the plaque [107].
Atherosclerotic plaque buildup starts with activated macrophages ingesting modified LDL particles, which further increases macrophage activation. Activated macrophages release cytokines, such as TNF-α, that can cause local inflammation and recruit more macrophages thereby deteriorating the condition. Since the action of S-ASMase causes the LDL particles to become bigger and to clump making them easier for the macrophage to ingest [108], experiments with ASMase KO mice showed reduced lipid buildup through reduced lipoprotein retention in the subendothelium [102]. These findings suggest that targeting of ASMase activity and especially S-ASMase may be beneficial in inhibiting the buildup of plaques. However, it should be noted that patients with NPD-A and NPD-B have high ‘bad’ LDL cholesterol and low ‘good’ HDL cholesterol levels, and that the majority of children with NPD-B have coronary artery calcium deposits, a major risk factor for coronary heart disease [109]. Thus, while defects in ASMase may indeed prevent atherosclerotic lesions developing through processing of LDL, defects in SM-hydrolysis cause major damage through the build-up of lipids.
Free cholesterol generated through the hydrolysis of the stored cholesteryl esters can be externalized by efflux on HDL particles to be transported to the liver where it can be excreted (reverse cholesterol transport) [110, 111]. ASMase KO mice have decreased cholesterol efflux but also, perhaps not surprisingly, defective cholesterol trafficking [112]. Combined with the effect of S-ASMase on LDL particles, a potential role for S-ASMase in the development of atherosclerotic plaques becomes more apparent.
Depression
High ASMase activity has been reported in peripheral blood mononuclear cells of patients suffering from clinical depression [113]. Drugs used to successfully treat depression such as desipramine and imipramine also reduce ASMase activity [114] by inhibiting the binding of ASMase to the plasma membrane [115]. The mechanism of ASMase involvement in major depressive disorders is still unknown.
Sepsis
ASMase might also play a role in sepsis-induced apoptosis and organ failure [116]. It has been shown that levels of plasma-associated ASMase were increased in patients that had suffered sepsis, and that mice lacking functioning ASMase had a higher rate of survival in an induced sepsis mouse model [116]. Studies in mice have suggested that the source of S-ASMase was the endothelium [117], but given that sepsis is initiated by infection then a role for macrophages cannot be ruled out.
ASMase and activated macrophages in disease
As has been shown, the upregulation of ASMase activity is required by many different cellular functions. Although understated by the current scientific literature, there are many functions performed by activated macrophages that require ASMase activity. The activity of ASMase can both regulate and provide the mechanisms for macrophages as they respond to disease.
ASMase and differentiation, cytokine secretion
The importance of ASMase to macrophage biology begins early. Monocyte differentiation is dependent on transcription factors sp-1 and ap-2 upregulating ASMase expression [118]. One of the key characteristics of activated macrophages is the secretion of inflammatory cytokines such as TNF-α, a major effector of the innate immune response [119]. TNF-α can be efficiently, although not exclusively, induced through stimulation with a bacterial cell wall component such as LPS [120]. Inhibition of ASMase activity with SMA-7, a synthesized difluoromethlyene analogue of SM suppresses the release of inflammatory cytokines thereby reducing the severity of inflammatory bowel disease in a mouse model of inducible colitis (using dextran sulphate sodium salt) [121]. This suggests a role of ASMase in promoting cytokine secretion; however, this contrasts with the findings of Rozenova et al. [122] who reported that ASMase activity limited the release of TNF-α by LPS-stimulated macrophages by inhibiting the conversion of pro-TNF-α to TNF-α. The seemingly contradictory roles of ASMase in inflammatory cytokine release suggest that the mechanism behind ASMase activation determines whether it will promote or inhibit cytokine, and specifically TNF-α, release. Further studies are needed to elucidate how these differences are enacted.
ASMase and macrophage survival
Macrophages can recognize foreign matter through the use of TLR which forms part of the evolutionarily innate immune response [123]. These receptors recognize patterns that are found in bacteria and fungi, which usually consist of repeating molecular motifs. Binding of LPS to CD14, a component of the innate immune system, can initiate the formation of ceramide-rich platforms through ASMase activation, which then drives the formation of the TLR within the platform [124]. Wang et al. [125] demonstrated that pertussis toxin (PTX), which binds to TLR-4, prolongs macrophage survival by inhibiting ASMase activity. We and others have shown that treatment with LPS, which also binds to TLR-4, activates sphingosine kinase and therefore generates the pro-inflammatory and pro-survival signaling molecule sphingosine 1-phosphate (S1P) [126, 127]. Additionally, Gomez-Muñoz et al. [128] showed that apoptosis induced by the withdrawal of macrophage colony growth factor was inhibited through the addition of S1P in macrophages in whole cells, but not in cell homogenates, possibly via a mechanism involving the protein kinase B (AKT)/phosphoinositol 3-phosphate pathway. The effects of PTX on macrophage survival suggest that ASMase activity can be modulated by bacteria and change both macrophage behavior and the corresponding immune response. The pathways mediating the effects of PTX on macrophage survival appear to be similar to those involved in the inhibition of apoptosis by oxLDL particles, a major factor in the buildup and development of atherosclerosis when ingested by macrophages [129, 130]. Moreover, upregulation of TLR-4 by oxLDL and binding of minimally modified LDL to TLR-4 [131] further demonstrate that shared signaling pathways, some involving ASMase, are important in the development of atherosclerosis. While these results seem at odds with the literature showing cytotoxic effects of oxLDL in macrophages [132–134] and in arterial smooth muscle [135], experiments demonstrating cell survival were performed with low doses of oxLDL, which has been shown to promote cellular growth [129, 134].
ASMase and phagocytosis
Through opsonization, internalization of particles coated with antibodies is facilitated through interactions with the Fc receptors (fragment crystallizable receptors, Fc-Rs), and is perhaps the most characteristic behavior of macrophages. The different Fc-Rs bind to different types of immunoglobulin, so that the Fc-εR binds to IgE, Fc-αR binds to IgA, and Fc-γR binds to IgG [136]. Activated Fc-γRII undergoes ASMase-dependent clustering which then provides enhanced signaling [137]. When U937 human monocytic cells were pre-treated with exogenous bacterial SMase before cross-linking of Fc-γRII, a synergistic increase in ceramide generation was observed [137]. These data imply that secretory bacterial SMases are able to prime a macrophage for enhanced phagocytosis of opsonized bacteria. These findings also suggest that S-ASMases secreted by either endothelial cells or other macrophages may be playing a role in enhancing immune responses.
Once a particle has been phagocytosed, it is entrapped in a phagosome. An interesting consequence of the phagocytosis of small membrane-bound particles formed as a result of apoptosis is that it can also induce ASMase-dependent apoptosis of macrophages and may represent a novel mechanism of immune modulation [138]. However, this mechanism could also represent a means by which a local inflammatory response can be maintained. The phagosome breaks down the contents by sequentially fusing with early and late endosomes, becoming a late phagosome, and then fusing with lysosomes [139, 140]. In studies examining the phagocytosis of Listeria monocytogenes, ASMase was required for proper fusion of lysosomes with the late phagosome [141]. ASMase can also be recruited to the phagolysosome through the action of sortilin [142]. Interestingly, cells defective in ASMase expression are more likely to survive infections by organisms like Neisseria gonorrhoeae and Staphylococcus aureus by virtue of the cells’ inability to ingest the bacteria [143, 144]. Infections by bacteria such as N. gonorrhoeae activate ASMase, and the subsequent production of ceramide is necessary for internalization. The internalization Pseudomonas aeruginosa requires ASMase-generated ceramide rafts [145]. However, patients with cystic fibrosis have defective ASMase activation which can inhibit the internalization of P. aeruginosa, a major cause of morbidity [146]. The pH of some lysosomes from cystic fibrosis patients is more alkaline, which can alter sphingolipid metabolism [147]. The change in pH has been suggested to inhibit ASMase activation in response to P. aeruginosa infection and may be the cause for defective clearance of the bacteria during cystic fibrosis [148].
ASMase and oxidative stress in infection
The generation of ROS is vital to the macrophage response to infection. Early during infection, the macrophage is able to survive the increased ROS concentration due in part to the mitochondrial anti-oxidants, such as manganese superoxide dismutase [149]. During Salmonella enterica infection, an initial drop in L-ASMase activity corresponds to an increase in S-ASMase activity, which correlates with the generation of ROS and cytotoxicity of the invading bacteria [150]. This may be related to the response to P. aeruginosa infection, when macrophages rapidly activate ASMase, which is also associated with the production of ROS and macrophage apoptosis [82]. Thus, the use of ROS by macrophages, controlled in part by ASMase, has a dual role in damaging the infectious organism and in cell activation. In addition, the release of inflammatory cytokines can locally amplify the immune response.
ASMase and dendritic cells
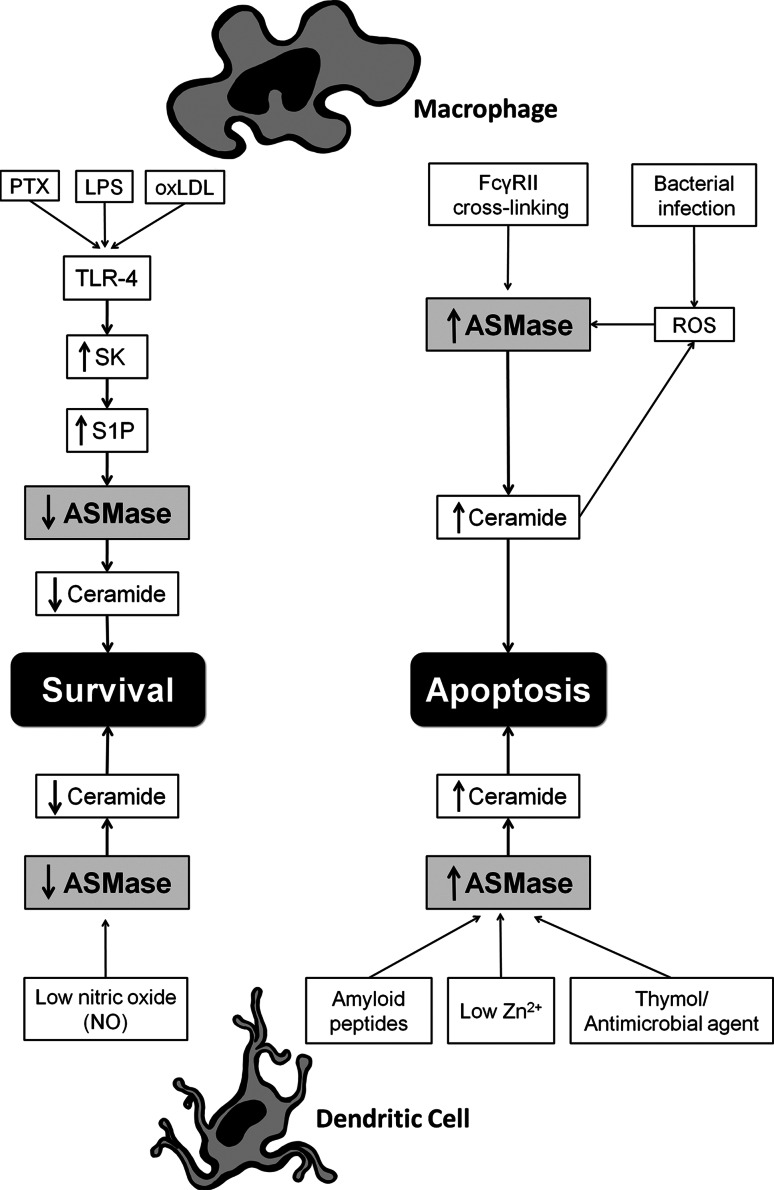
Monocytes can differentiate into dendritic cells (DC) that are able to more efficiently present antigens to T cells, an essential role for the adaptive immune response [151, 152]. Uptake of pathogens by DCs is driven in part by the pattern recognition receptor DC-specific intercellular adhesion molecule-grabbing nonintegrin (DC-SIGN) [153]. It has been shown that DC-SIGN ligation can activate nSMase and L-ASMase and promote the expression of CD150, a receptor required for the uptake of viral particles such as measles virus [154]. Regulation of DC survival could be an effective method for prolonging or shortening a DC-driven immune response. Amyloid peptides were shown to modify DC survival by induction of ASMase-mediated apoptosis [155, 156]. Moreover, a study showed that bacteria are capable of inducing ASMase-dependent apoptosis in immature DCs which correlated with immune suppression during Escherichia coli-induced sepsis [157]. The study also showed that mature DCs express lower levels of ASMase than immature DCs that may protect them from the induction of apoptosis, and perhaps also allow for prolonged antigen presentation at a site of inflammation [157]. Exposure of DCs to nitric oxide (NO), which has many different signaling functions including regulation of immune responses [158], was found to suppress ASMase activity [157]. The mechanism behind NO suppression of ASMase activity was mediated by the activation of protein kinase G via the cyclic guanosine monophosphate pathway [159]. The protective effect of NO only extended to low doses as high concentrations of NO are inherently apoptogenic [160]. Furthermore, thymol, known for its antimicrobial activity, has been shown to influence the generation of ROS [161], which inhibits cell proliferation, and to protect erythrocytes from apoptosis [162]. Stimulation of the DCs with thymol leads to translocation of ASMase from intracellular compartment onto the cell surface, and leads to ceramide formation [163]. Ceramide in turn results in downregulation of Bcl-2 and Bcl-xL (mitochondrial prosurvival signaling molecules), and activation of caspases that initiate apoptosis [163]. A diagram depicting the role of ASMase in activated macrophages and DCs is shown in Fig. 2.
Fig. 2.
Acid sphingomyelinase plays an important role in the biological functions of macrophages. The stimulation of macrophages with pertussis toxin (PTX) [125], lipopolysaccharide (LPS) [126, 127], and low doses of oxidatively-modified low-density lipoprotein (oxLDL) [131] results in the activation of toll-like receptor-4 (TLR-4), increased sphingosine-kinase (SK) activity, the generation of the pro-inflammatory and pro-survival molecule sphingosine 1-phosphate (S1P), decreased ASMase activity, and prolonged macrophage survival. In contrast, the cross-linking of Fc-γRII leads to an increase in ASMase activity and the generation of ceramide that will lead to apoptosis [124, 137]. Furthermore, bacterial infection increases both ASMase activity and reactive oxygen species (ROS) that lead to apoptosis [150]. The effects of amyloid peptide [156], low Zn2+ [167], and thymol [163] on dendritic cells can induce apoptosis through the increase of ASMase activity and generation of ceramide. However, nitric oxide (NO) suppresses ASMase activity and protects the cells from apoptosis [159]
The depletion or supplementation of Zn2+, the second most prevalent trace element in the body, influences the functions of both innate and adaptive immunity [164, 165]. Shumilina et al. showed that low doses of Zn2+ (up to 100 μM) induce ceramide-mediated apoptosis in ASMase+/+ DCs, but in ASMase−/− DCs higher doses of Zn2+ are required to induce apoptosis. They also showed that the effect of low Zn2+ concentrations on apoptosis critically depends on the presence of functional ASMase [166]. As Zn2+ is also an important ion needed for ASMase activity then this data suggest that it may also be having a direct effect on ASMase.
Conclusions
Macrophages play an important role in maintaining tissue homeostasis and the clearance of infections. Many of the mechanisms necessary for these vital functions appear to be dependent on modulating local membrane structure through the activation of ASMase. Further study and understanding of the role and regulation of ceramide generation in macrophages will be of benefit for a better understanding of macrophage biology and perhaps provide ways to modify macrophage immune responses. Such information could be of use in ensuring the rapid clearance of infectious organisms and also halt or even reverse plaque formation in atherosclerosis.
Acknowledgments
Special thanks to Dr. Y.A. Hannun and his team at MUSC for stimulating discussions and contribution of information to this article. S.M.H. was supported by NIH grant HL079274, NIH (ARRA) grant R01 HL079274 04S1, The Southeastern Clinical and Translational Research Institute (SCTR, formerly GCRC) and the South Carolina COBRE in Lipidomics and Pathobiology (P20 RR17677 from NCRR).
References
- 1.Singer SJ, Nicolson GL. The fluid mosaic model of the structure of cell membranes. Science. 1972;175(23):720–731. doi: 10.1126/science.175.4023.720. [DOI] [PubMed] [Google Scholar]
- 2.Hannun YA, Obeid LM. Principles of bioactive lipid signalling: lessons from sphingolipids. Nat Rev Mol Cell Biol. 2008;9(2):139–150. doi: 10.1038/nrm2329. [DOI] [PubMed] [Google Scholar]
- 3.van Meer G. Lipid traffic in animal cells. Annu Rev Cell Biol. 1989;5:247–275. doi: 10.1146/annurev.cb.05.110189.001335. [DOI] [PubMed] [Google Scholar]
- 4.Simons K, Ikonen E. Functional rafts in cell membranes. Nature. 1997;387(6633):569–572. doi: 10.1038/42408. [DOI] [PubMed] [Google Scholar]
- 5.Geissmann F, Manz MG, Jung S, Sieweke MH, Merad M, Ley K. Development of monocytes, macrophages, and dendritic cells. Science. 2010;327(5966):656–661. doi: 10.1126/science.1178331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van Furth R, Cohn ZA. The origin and kinetics of mononuclear phagocytes. J Exp Med. 1968;128(3):415–435. doi: 10.1084/jem.128.3.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marcel YL, Ouimet M, Wang MD. Regulation of cholesterol efflux from macrophages. Curr Opin Lipidol. 2008;19(5):455–461. doi: 10.1097/MOL.0b013e32830f4a1d. [DOI] [PubMed] [Google Scholar]
- 8.Gordon S. The macrophage: past, present and future. Eur J Immunol. 2007;37(Suppl 1):S9–S17. doi: 10.1002/eji.200737638. [DOI] [PubMed] [Google Scholar]
- 9.Fadeel B, Xue D, Kagan V. Programmed cell clearance: molecular regulation of the elimination of apoptotic cell corpses and its role in the resolution of inflammation. Biochem Biophys Res Commun. 2010;396(1):7–10. doi: 10.1016/j.bbrc.2010.02.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boyce BF, Yao Z, Xing L. Osteoclasts have multiple roles in bone in addition to bone resorption. Crit Rev Eukaryot Gene Expr. 2009;19(3):171–180. doi: 10.1615/critreveukargeneexpr.v19.i3.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mosser DM. The many faces of macrophage activation. J Leukoc Biol. 2003;73(2):209–212. doi: 10.1189/jlb.0602325. [DOI] [PubMed] [Google Scholar]
- 12.Kanfer JN, Young OM, Shapiro D, Brady RO. The metabolism of sphingomyelin. I. Purification and properties of a sphingomyelin-cleaving enzyme from rat liver tissue. J Biol Chem. 1966;241(5):1081–1084. [PubMed] [Google Scholar]
- 13.Schneider PB, Kennedy EP. Sphingomyelinase in normal human spleens and in spleens from subjects with Niemann–Pick disease. J Lipid Res. 1967;8(3):202–209. [PubMed] [Google Scholar]
- 14.Tonnetti L, Veri MC, Bonvini E, D’Adamio L. A role for neutral sphingomyelinase-mediated ceramide production in T cell receptor-induced apoptosis and mitogen-activated protein kinase-mediated signal transduction. J Exp Med. 1999;189(10):1581–1589. doi: 10.1084/jem.189.10.1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yabu T, Imamura S, Yamashita M, Okazaki T. Identification of Mg2+-dependent neutral sphingomyelinase 1 as a mediator of heat stress-induced ceramide generation and apoptosis. J Biol Chem. 2008;283(44):29971–29982. doi: 10.1074/jbc.M805402200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zumbansen M, Stoffel W. Neutral sphingomyelinase 1 deficiency in the mouse causes no lipid storage disease. Mol Cell Biol. 2002;22(11):3633–3638. doi: 10.1128/MCB.22.11.3633-3638.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ito H, Murakami M, Furuhata A, Gao S, Yoshida K, Sobue S, et al. Transcriptional regulation of neutral sphingomyelinase 2 gene expression of a human breast cancer cell line, MCF-7, induced by the anti-cancer drug, daunorubicin. Biochim Biophys Acta. 2009;1789(11–12):681–690. doi: 10.1016/j.bbagrm.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 18.Levy M, Khan E, Careaga M, Goldkorn T. Neutral sphingomyelinase 2 is activated by cigarette smoke to augment ceramide-induced apoptosis in lung cell death. Am J Physiol Lung Cell Mol Physiol. 2009;297(1):L125–L133. doi: 10.1152/ajplung.00031.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marchesini N, Osta W, Bielawski J, Luberto C, Obeid LM, Hannun YA. Role for mammalian neutral sphingomyelinase 2 in confluence-induced growth arrest of MCF7 cells. J Biol Chem. 2004;279(24):25101–25111. doi: 10.1074/jbc.M313662200. [DOI] [PubMed] [Google Scholar]
- 20.Clarke CJ, Snook CF, Tani M, Matmati N, Marchesini N, Hannun YA. The extended family of neutral sphingomyelinases. Biochemistry. 2006;45(38):11247–11256. doi: 10.1021/bi061307z. [DOI] [PubMed] [Google Scholar]
- 21.Stoffel W, Jenke B, Block B, Zumbansen M, Koebke J. Neutral sphingomyelinase 2 (smpd3) in the control of postnatal growth and development. Proc Natl Acad Sci USA. 2005;102(12):4554–4559. doi: 10.1073/pnas.0406380102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim WJ, Okimoto RA, Purton LE, Goodwin M, Haserlat SM, Dayyani F, et al. Mutations in the neutral sphingomyelinase gene SMPD3 implicate the ceramide pathway in human leukemias. Blood. 2008;111(9):4716–4722. doi: 10.1182/blood-2007-10-113068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krut O, Wiegmann K, Kashkar H, Yazdanpanah B, Kronke M. Novel tumor necrosis factor-responsive mammalian neutral sphingomyelinase-3 is a C-tail-anchored protein. J Biol Chem. 2006;281(19):13784–13793. doi: 10.1074/jbc.M511306200. [DOI] [PubMed] [Google Scholar]
- 24.Duan RD, Nilsson A. Metabolism of sphingolipids in the gut and its relation to inflammation and cancer development. Prog Lipid Res. 2009;48(1):62–72. doi: 10.1016/j.plipres.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 25.Zhang Y, Cheng Y, Hansen GH, Niels-Christiansen LL, Koentgen F, Ohlsson L, et al. Crucial role of alkaline sphingomyelinase in sphingomyelin digestion: a study on the enzymeknockout mice. J Lipid Res. 2010;52(4):771–781. doi: 10.1194/jlr.M012880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hertervig E, Nilsson A, Cheng Y, Duan RD. Purified intestinal alkaline sphingomyelinase inhibits proliferation without inducing apoptosis in HT-29 colon carcinoma cells. J Cancer Res Clin Oncol. 2003;129(10):577–582. doi: 10.1007/s00432-003-0466-2. [DOI] [PubMed] [Google Scholar]
- 27.Wu J, Cheng Y, Nilsson A, Duan RD. Identification of one exon deletion of intestinal alkaline sphingomyelinase in colon cancer HT-29 cells and a differentiation-related expression of the wild-type enzyme in Caco-2 cells. Carcinogenesis. 2004;25(8):1327–1333. doi: 10.1093/carcin/bgh140. [DOI] [PubMed] [Google Scholar]
- 28.Marathe S, Schissel SL, Yellin MJ, Beatini N, Mintzer R, Williams KJ, et al. Human vascular endothelial cells are a rich and regulatable source of secretory sphingomyelinase. Implications for early atherogenesis and ceramide-mediated cell signaling. J Biol Chem. 1998;273(7):4081–4088. doi: 10.1074/jbc.273.7.4081. [DOI] [PubMed] [Google Scholar]
- 29.Fowler S. Lysosomal localization of sphingomyelinase in rat liver. Biochim Biophys Acta. 1969;191(2):481–484. doi: 10.1016/0005-2744(69)90271-x. [DOI] [PubMed] [Google Scholar]
- 30.Schissel SL, Schuchman EH, Williams KJ, Tabas I. Zn2+-stimulated sphingomyelinase is secreted by many cell types and is a product of the acid sphingomyelinase gene. J Biol Chem. 1996;271(31):18431–18436. doi: 10.1074/jbc.271.31.18431. [DOI] [PubMed] [Google Scholar]
- 31.Jenkins RW, Canals D, Hannun YA. Roles and regulation of secretory and lysosomal acid sphingomyelinase. Cell Signal. 2009;21(6):836–846. doi: 10.1016/j.cellsig.2009.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schissel SL, Keesler GA, Schuchman EH, Williams KJ, Tabas I. The cellular trafficking and zinc dependence of secretory and lysosomal sphingomyelinase, two products of the acid sphingomyelinase gene. J Biol Chem. 1998;273(29):18250–18259. doi: 10.1074/jbc.273.29.18250. [DOI] [PubMed] [Google Scholar]
- 33.de Duve C. The lysosome turns fifty. Nat Cell Biol. 2005;7(9):847–849. doi: 10.1038/ncb0905-847. [DOI] [PubMed] [Google Scholar]
- 34.Kolter T, Sandhoff K. Lysosomal degradation of membrane lipids. FEBS Lett. 2010;584(9):1700–1712. doi: 10.1016/j.febslet.2009.10.021. [DOI] [PubMed] [Google Scholar]
- 35.Kirkegaard T, Roth AG, Petersen NH, Mahalka AK, Olsen OD, Moilanen I, et al. Hsp70 stabilizes lysosomes and reverts Niemann–Pick disease-associated lysosomal pathology. Nature. 2010;463(7280):549–553. doi: 10.1038/nature08710. [DOI] [PubMed] [Google Scholar]
- 36.Rutsaert J, Tondeur M, Vamos-Hurwitz E, Dustin P. The cellular lesions of Farber’s disease and their experimental reproduction in tissue culture. Lab Invest. 1977;36(5):474–480. [PubMed] [Google Scholar]
- 37.Utermohlen O, Herz J, Schramm M, Kronke M. Fusogenicity of membranes: the impact of acid sphingomyelinase on innate immune responses. Immunobiology. 2008;213(3–4):307–314. doi: 10.1016/j.imbio.2007.10.016. [DOI] [PubMed] [Google Scholar]
- 38.Goni FM, Alonso A. Sphingomyelinases: enzymology and membrane activity. FEBS Lett. 2002;531(1):38–46. doi: 10.1016/S0014-5793(02)03482-8. [DOI] [PubMed] [Google Scholar]
- 39.Bao JX, Xia M, Poklis JL, Han WQ, Brimson C, Li PL. Triggering role of acid sphingomyelinase in endothelial lysosome-membrane fusion and dysfunction in coronary arteries. Am J Physiol Heart Circ Physiol. 2010;298(3):H992–H1002. doi: 10.1152/ajpheart.00958.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Herz J, Pardo J, Kashkar H, Schramm M, Kuzmenkina E, Bos E, et al. Acid sphingomyelinase is a key regulator of cytotoxic granule secretion by primary T lymphocytes. Nat Immunol. 2009;10(7):761–768. doi: 10.1038/ni.1757. [DOI] [PubMed] [Google Scholar]
- 41.Bogdan C. ASMase: the tailor of cytotoxic T cell granule exocytosis. Nat Immunol. 2009;10(7):683–685. doi: 10.1038/ni0709-683. [DOI] [PubMed] [Google Scholar]
- 42.Tam C, Idone V, Devlin C, Fernandes MC, Flannery A, He X, et al. Exocytosis of acid sphingomyelinase by wounded cells promotes endocytosis and plasma membrane repair. J Cell Biol. 2010;189(6):1027–1038. doi: 10.1083/jcb.201003053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mathias S, Pena LA, Kolesnick RN. Signal transduction of stress via ceramide. Biochem J. 1998;335:465–480. doi: 10.1042/bj3350465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Anker SD, Steinborn W, Strassburg S. Cardiac cachexia. Ann Med. 2004;36(7):518–529. doi: 10.1080/07853890410017467. [DOI] [PubMed] [Google Scholar]
- 45.Doehner W, Bunck AC, Rauchhaus M, von Haehling S, Brunkhorst FM, Cicoira M, et al. Secretory sphingomyelinase is upregulated in chronic heart failure: a second messenger system of immune activation relates to body composition, muscular functional capacity, and peripheral blood flow. Eur Heart J. 2007;28(7):821–828. doi: 10.1093/eurheartj/ehl541. [DOI] [PubMed] [Google Scholar]
- 46.Takahashi T, Abe T, Sato T, Miura K, Takahashi I, Yano M, et al. Elevated sphingomyelinase and hypercytokinemia in hemophagocytic lymphohistiocytosis. J Pediatr Hematol Oncol. 2002;24(5):401–404. doi: 10.1097/00043426-200206000-00016. [DOI] [PubMed] [Google Scholar]
- 47.Gupta S, Weitzman S. Primary and secondary hemophagocytic lymphohistiocytosis: clinical features, pathogenesis and therapy. Expert Rev Clin Immunol. 2010;6(1):137–154. doi: 10.1586/eci.09.58. [DOI] [PubMed] [Google Scholar]
- 48.Schissel SL, Jiang X, Tweedie-Hardman J, Jeong T, Camejo EH, Najib J, et al. Secretory sphingomyelinase, a product of the acid sphingomyelinase gene, can hydrolyze atherogenic lipoproteins at neutral pH. Implications for atherosclerotic lesion development. J Biol Chem. 1998;273(5):2738–2746. doi: 10.1074/jbc.273.5.2738. [DOI] [PubMed] [Google Scholar]
- 49.Brown DA, London E. Structure and function of sphingolipid- and cholesterol-rich membrane rafts. J Biol Chem. 2000;275(23):17221–17224. doi: 10.1074/jbc.R000005200. [DOI] [PubMed] [Google Scholar]
- 50.Rajendran L, Simons K. Lipid rafts and membrane dynamics. J Cell Sci. 2005;118(Pt 6):1099–1102. doi: 10.1242/jcs.01681. [DOI] [PubMed] [Google Scholar]
- 51.Staneva G, Chachaty C, Wolf C, Koumanov K, Quinn PJ. The role of sphingomyelin in regulating phase coexistence in complex lipid model membranes: competition between ceramide and cholesterol. Biochim Biophys Acta. 2008;1778(12):2727–2739. doi: 10.1016/j.bbamem.2008.07.025. [DOI] [PubMed] [Google Scholar]
- 52.Megha, London E. Ceramide selectively displaces cholesterol from ordered lipid domains (rafts): implications for lipid raft structure and function. J Biol Chem. 2004;279(11):9997–10004. doi: 10.1074/jbc.M309992200. [DOI] [PubMed] [Google Scholar]
- 53.Veiga MP, Arrondo JL, Goni FM, Alonso A. Ceramides in phospholipid membranes: effects on bilayer stability and transition to nonlamellar phases. Biophys J. 1999;76(1 Pt 1):342–350. doi: 10.1016/S0006-3495(99)77201-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stancevic B, Kolesnick R. Ceramide-rich platforms in transmembrane signaling. FEBS Lett. 2010;584(9):1728–1740. doi: 10.1016/j.febslet.2010.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Scheel-Toellner D, Wang K, Assi LK, Webb PR, Craddock RM, Salmon M, et al. Clustering of death receptors in lipid rafts initiates neutrophil spontaneous apoptosis. Biochem Soc Trans. 2004;32(Pt 5):679–681. doi: 10.1042/BST0320679. [DOI] [PubMed] [Google Scholar]
- 56.Grassme H, Jendrossek V, Bock J, Riehle A, Gulbins E. Ceramide-rich membrane rafts mediate CD40 clustering. J Immunol. 2002;168(1):298–307. doi: 10.4049/jimmunol.168.1.298. [DOI] [PubMed] [Google Scholar]
- 57.Schutze S, Potthoff K, Machleidt T, Berkovic D, Wiegmann K, Kronke M. TNF activates NF-kappa B by phosphatidylcholine-specific phospholipase C-induced “acidic” sphingomyelin breakdown. Cell. 1992;71(5):765–776. doi: 10.1016/0092-8674(92)90553-O. [DOI] [PubMed] [Google Scholar]
- 58.Grassme H, Jekle A, Riehle A, Schwarz H, Berger J, Sandhoff K, et al. CD95 signaling via ceramide-rich membrane rafts. J Biol Chem. 2001;276(23):20589–20596. doi: 10.1074/jbc.M101207200. [DOI] [PubMed] [Google Scholar]
- 59.Dumitru CA, Gulbins E. TRAIL activates acid sphingomyelinase via a redox mechanism and releases ceramide to trigger apoptosis. Oncogene. 2006;25(41):5612–5625. doi: 10.1038/sj.onc.1209568. [DOI] [PubMed] [Google Scholar]
- 60.Strasser A, Jost PJ, Nagata S. The many roles of FAS receptor signaling in the immune system. Immunity. 2009;30(2):180–192. doi: 10.1016/j.immuni.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ion G, Fajka-Boja R, Kovacs F, Szebeni G, Gombos I, Czibula A, et al. Acid sphingomyelinase mediated release of ceramide is essential to trigger the mitochondrial pathway of apoptosis by galectin-1. Cellular Signal. 2006;18(11):1887–1896. doi: 10.1016/j.cellsig.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 62.Ferrari D, Pinton P, Campanella M, Callegari MG, Pizzirani C, Rimessi A, et al. Functional and structural alterations in the endoplasmic reticulum and mitochondria during apoptosis triggered by C2-ceramide and CD95/APO-1/FAS receptor stimulation. Biochem Biophys Res Commun. 2010;391(1):575–581. doi: 10.1016/j.bbrc.2009.11.101. [DOI] [PubMed] [Google Scholar]
- 63.Longo CA, Tyler D, Mallampalli RK. Sphingomyelin metabolism is developmentally regulated in rat lung. Am J Respir Cell Mol Biol. 1997;16(5):605–612. doi: 10.1165/ajrcmb.16.5.9160843. [DOI] [PubMed] [Google Scholar]
- 64.Utermohlen O, Karow U, Lohler J, Kronke M. Severe impairment in early host defense against Listeria monocytogenes in mice deficient in acid sphingomyelinase. J Immunol. 2003;170(5):2621–2628. doi: 10.4049/jimmunol.170.5.2621. [DOI] [PubMed] [Google Scholar]
- 65.Santana P, Pena LA, Haimovitz-Friedman A, Martin S, Green D, McLoughlin M, et al. Acid sphingomyelinase-deficient human lymphoblasts and mice are defective in radiation-induced apoptosis. Cell. 1996;86(2):189–199. doi: 10.1016/S0092-8674(00)80091-4. [DOI] [PubMed] [Google Scholar]
- 66.Paris F, Fuks Z, Kang A, Capodieci P, Juan G, Ehleiter D, et al. Endothelial apoptosis as the primary lesion initiating intestinal radiation damage in mice. Science. 2001;293(5528):293–297. doi: 10.1126/science.1060191. [DOI] [PubMed] [Google Scholar]
- 67.Zhang Y, Mattjus P, Schmid PC, Dong Z, Zhong S, Ma WY, et al. Involvement of the acid sphingomyelinase pathway in uva-induced apoptosis. J Biol Chem. 2001;276(15):11775–11782. doi: 10.1074/jbc.M006000200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dimanche-Boitrel MT, Meurette O, Rebillard A, Lacour S. Role of early plasma membrane events in chemotherapy-induced cell death. Drug Resist Updat. 2005;8(1–2):5–14. doi: 10.1016/j.drup.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 69.Won JS, Singh I. Sphingolipid signaling and redox regulation. Free Radic Biol Med. 2006;40(11):1875–1888. doi: 10.1016/j.freeradbiomed.2006.01.035. [DOI] [PubMed] [Google Scholar]
- 70.Grassme H, Schwarz H, Gulbins E. Molecular mechanisms of ceramide-mediated CD95 clustering. Biochem Biophys Res Commun. 2001;284(4):1016–1030. doi: 10.1006/bbrc.2001.5045. [DOI] [PubMed] [Google Scholar]
- 71.Jaiswal JK, Andrews NW, Simon SM. Membrane proximal lysosomes are the major vesicles responsible for calcium-dependent exocytosis in nonsecretory cells. J Cell Biol. 2002;159(4):625–635. doi: 10.1083/jcb.200208154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jin S, Yi F, Zhang F, Poklis JL, Li PL. Lysosomal targeting and trafficking of acid sphingomyelinase to lipid raft platforms in coronary endothelial cells. Arterioscler Thromb Vasc Biol. 2008;28(11):2056–2062. doi: 10.1161/ATVBAHA.108.172478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gulbins E, Li PL. Physiological and pathophysiological aspects of ceramide. Am J Physiol Regul Integr Comp Physiol. 2006;290(1):R11–R26. doi: 10.1152/ajpregu.00416.2005. [DOI] [PubMed] [Google Scholar]
- 74.Grassme H, Bock J, Kun J, Gulbins E. Clustering of CD40 ligand is required to form a functional contact with CD40. J Biol Chem. 2002;277(33):30289–30299. doi: 10.1074/jbc.M200494200. [DOI] [PubMed] [Google Scholar]
- 75.Perrotta C, Bizzozero L, Cazzato D, Morlacchi S, Assi E, Simbari F, et al. Syntaxin 4 is required for Acid sphingomyelinase activity and apoptotic function. J Biol Chem. 2010;285(51):40240–40251. doi: 10.1074/jbc.M110.139287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cho WJ, Shin L, Ren G, Jena BP. Structure of membrane-associated neuronal SNARE complex: implication in neurotransmitter release. J Cell Mol Med. 2009;13(10):4161–4165. doi: 10.1111/j.1582-4934.2009.00895.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sudhof TC, Rothman JE. Membrane fusion: grappling with SNARE and SM proteins. Science. 2009;323(5913):474–477. doi: 10.1126/science.1161748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nickel W, Rabouille C. Mechanisms of regulated unconventional protein secretion. Nat Rev Mol Cell Biol. 2009;10(2):148–155. doi: 10.1038/nrm2617. [DOI] [PubMed] [Google Scholar]
- 79.Zeidan YH, Hannun YA. Activation of acid sphingomyelinase by protein kinase Cdelta-mediated phosphorylation. J Biol Chem. 2007;282(15):11549–11561. doi: 10.1074/jbc.M609424200. [DOI] [PubMed] [Google Scholar]
- 80.Jenkins RW, Canals D, Idkowiak-Baldys J, Simbari F, Roddy P, Perry DM, et al. Regulated secretion of acid sphingomyelinase: implications for selectivity of ceramide formation. J Biol Chem. 2010;285(46):35706–35718. doi: 10.1074/jbc.M110.125609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Jenkins RW, Clarke CJ, Canals DN, Snider AJ, Gault CR, Heffernan-Stroud L, et al. Regulation of CC ligand 5/ rantes by acid sphingomyelinase and acid ceramidase. J Biol Chem. 2011;286(15):13292–13303. doi: 10.1074/jbc.M110.163378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhang Y, Li X, Carpinteiro A, Gulbins E. Acid sphingomyelinase amplifies redox signaling in Pseudomonas aeruginosa-induced macrophage apoptosis. J Immunol. 2008;181(6):4247–4254. doi: 10.4049/jimmunol.181.6.4247. [DOI] [PubMed] [Google Scholar]
- 83.Reinehr R, Becker S, Braun J, Eberle A, Grether-Beck S, Haussinger D. Endosomal acidification and activation of NADPH oxidase isoforms are upstream events in hyperosmolarity-induced hepatocyte apoptosis. J Biol Chem. 2006;281(32):23150–23166. doi: 10.1074/jbc.M601451200. [DOI] [PubMed] [Google Scholar]
- 84.Dumitru CA, Zhang Y, Li X, Gulbins E. Ceramide: a novel player in reactive oxygen species-induced signaling? Antioxid Redox Signal. 2007;9(9):1535–1540. doi: 10.1089/ars.2007.1692. [DOI] [PubMed] [Google Scholar]
- 85.Xing YX, Li P, Miao YX, Du W, Wang CB. Involvement of ROS/ASMase/JNK signalling pathway in inhibiting UVA-induced apoptosis of HaCaT cells by polypeptide from Chlamys farreri. Free Radic Res. 2008;42(1):12–19. doi: 10.1080/10715760701762415. [DOI] [PubMed] [Google Scholar]
- 86.Qiu H, Edmunds T, Baker-Malcolm J, Karey KP, Estes S, Schwarz C, et al. Activation of human acid sphingomyelinase through modification or deletion of C-terminal cysteine. J Biol Chem. 2003;278(35):32744–32752. doi: 10.1074/jbc.M303022200. [DOI] [PubMed] [Google Scholar]
- 87.Charruyer A, Grazide S, Bezombes C, Muller S, Laurent G, Jaffrezou JP. UV-C light induces raft-associated acid sphingomyelinase and JNK activation and translocation independently on a nuclear signal. J Biol Chem. 2005;280(19):19196–19204. doi: 10.1074/jbc.M412867200. [DOI] [PubMed] [Google Scholar]
- 88.Truman JP, Garcia-Barros M, Kaag M, Hambardzumyan D, Stancevic B, Chan M, et al. Endothelial membrane remodeling is obligate for anti-angiogenic radiosensitization during tumor radiosurgery. PLoS One. 2010;5(8):e12310. doi: 10.1371/journal.pone.0012310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Brady RO, Kanfer JN, Mock MB, Fredrickson DS. The metabolism of sphingomyelin. II. Evidence of an enzymatic deficiency in Niemann–Pick diseae. Proc Natl Acad Sci USA. 1966;55(2):366–369. doi: 10.1073/pnas.55.2.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Schuchman EH. The pathogenesis and treatment of acid sphingomyelinase-deficient Niemann–Pick disease. Int J Clin Pharmacol Ther. 2009;47(Suppl 1):S48–S57. doi: 10.5414/cpp47048. [DOI] [PubMed] [Google Scholar]
- 91.Minai OA, Sullivan EJ, Stoller JK. Pulmonary involvement in Niemann–Pick disease: case report and literature review. Respir Med. 2000;94(12):1241–1251. doi: 10.1053/rmed.2000.0942. [DOI] [PubMed] [Google Scholar]
- 92.Mendelson DS, Wasserstein MP, Desnick RJ, Glass R, Simpson W, Skloot G, et al. Type B Niemann–Pick disease: findings at chest radiography, thin-section CT, and pulmonary function testing. Radiology. 2006;238(1):339–345. doi: 10.1148/radiol.2381041696. [DOI] [PubMed] [Google Scholar]
- 93.Ferretti GR, Lantuejoul S, Brambilla E, Coulomb M. Case report. Pulmonary involvement in Niemann–Pick disease subtype B: CT findings. J Comput Assist Tomogr. 1996;20(6):990–992. doi: 10.1097/00004728-199611000-00023. [DOI] [PubMed] [Google Scholar]
- 94.Graber D, Salvayre R, Levade T. Accurate differentiation of neuronopathic and nonneuronopathic forms of Niemann–Pick disease by evaluation of the effective residual lysosomal sphingomyelinase activity in intact cells. J Neurochem. 1994;63(3):1060–1068. doi: 10.1046/j.1471-4159.1994.63031060.x. [DOI] [PubMed] [Google Scholar]
- 95.Scandroglio F, Venkata JK, Loberto N, Prioni S, Schuchman EH, Chigorno V, et al. Lipid content of brain, brain membrane lipid domains, and neurons from acid sphingomyelinase deficient mice. J Neurochem. 2008;107(2):329–338. doi: 10.1111/j.1471-4159.2008.05591.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Horinouchi K, Erlich S, Perl DP, Ferlinz K, Bisgaier CL, Sandhoff K, et al. Acid sphingomyelinase deficient mice: a model of types A and B Niemann–Pick disease. Nat Genet. 1995;10(3):288–293. doi: 10.1038/ng0795-288. [DOI] [PubMed] [Google Scholar]
- 97.Marathe S, Miranda SR, Devlin C, Johns A, Kuriakose G, Williams KJ, et al. Creation of a mouse model for non-neurological (type B) Niemann–Pick disease by stable, low level expression of lysosomal sphingomyelinase in the absence of secretory sphingomyelinase: relationship between brain intra-lysosomal enzyme activity and central nervous system function. Hum Mol Genet. 2000;9(13):1967–1976. doi: 10.1093/hmg/9.13.1967. [DOI] [PubMed] [Google Scholar]
- 98.Cheruku SR, Xu Z, Dutia R, Lobel P, Storch J. Mechanism of cholesterol transfer from the Niemann–Pick type C2 protein to model membranes supports a role in lysosomal cholesterol transport. J Biol Chem. 2006;281(42):31594–31604. doi: 10.1074/jbc.M602765200. [DOI] [PubMed] [Google Scholar]
- 99.Infante RE, Wang ML, Radhakrishnan A, Kwon HJ, Brown MS, Goldstein JL. NPC2 facilitates bidirectional transfer of cholesterol between NPC1 and lipid bilayers, a step in cholesterol egress from lysosomes. Proc Natl Acad Sci USA. 2008;105(40):15287–15292. doi: 10.1073/pnas.0807328105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Maor I, Mandel H, Aviram M. Macrophage uptake of oxidized LDL inhibits lysosomal sphingomyelinase, thus causing the accumulation of unesterified cholesterol-sphingomyelin-rich particles in the lysosomes. A possible role for 7-Ketocholesterol. Arterioscler Thromb Vasc Biol. 1995;15(9):1378–1387. doi: 10.1161/01.ATV.15.9.1378. [DOI] [PubMed] [Google Scholar]
- 101.Devlin C, Pipalia NH, Liao X, Schuchman EH, Maxfield FR, Tabas I. Improvement in lipid and protein trafficking in NPC1 cells by correction of a secondary enzyme defect. Traffic. 2010;11(5):601–615. doi: 10.1111/j.1600-0854.2010.01046.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Devlin CM, Leventhal AR, Kuriakose G, Schuchman EH, Williams KJ, Tabas I. Acid sphingomyelinase promotes lipoprotein retention within early atheromata and accelerates lesion progression. Arterioscler Thromb Vasc Biol. 2008;28(10):1723–1730. doi: 10.1161/ATVBAHA.108.173344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Marathe S, Kuriakose G, Williams KJ, Tabas I. Sphingomyelinase, an enzyme implicated in atherogenesis, is present in atherosclerotic lesions and binds to specific components of the subendothelial extracellular matrix. Arterioscler Thromb Vasc Biol. 1999;19(11):2648–2658. doi: 10.1161/01.ATV.19.11.2648. [DOI] [PubMed] [Google Scholar]
- 104.Tabas I. Secretory sphingomyelinase. Chem Phys Lipids. 1999;102(1–2):123–130. doi: 10.1016/S0009-3084(99)00080-8. [DOI] [PubMed] [Google Scholar]
- 105.Subbaiah PV, Subramanian VS, Wang K. Novel physiological function of sphingomyelin in plasma. Inhibition of lipid peroxidation in low density lipoproteins. J Biol Chem. 1999;274(51):36409–36414. doi: 10.1074/jbc.274.51.36409. [DOI] [PubMed] [Google Scholar]
- 106.Deigner HP, Hermetter A. Oxidized phospholipids: emerging lipid mediators in pathophysiology. Curr Opin Lipidol. 2008;19(3):289–294. doi: 10.1097/MOL.0b013e3282fe1d0e. [DOI] [PubMed] [Google Scholar]
- 107.Fruhwirth GO, Hermetter A. Mediation of apoptosis by oxidized phospholipids. Subcell Biochem. 2008;49:351–367. doi: 10.1007/978-1-4020-8831-5_13. [DOI] [PubMed] [Google Scholar]
- 108.Tabas I, Li Y, Brocia RW, Xu SW, Swenson TL, Williams KJ. Lipoprotein lipase and sphingomyelinase synergistically enhance the association of atherogenic lipoproteins with smooth muscle cells and extracellular matrix. A possible mechanism for low density lipoprotein and lipoprotein(a) retention and macrophage foam cell formation. J Biol Chem. 1993;268(27):20419–20432. [PubMed] [Google Scholar]
- 109.McGovern MM, Pohl-Worgall T, Deckelbaum RJ, Simpson W, Mendelson D, Desnick RJ, et al. Lipid abnormalities in children with types A and B Niemann Pick disease. J Pediatr. 2004;145(1):77–81. doi: 10.1016/j.jpeds.2004.02.048. [DOI] [PubMed] [Google Scholar]
- 110.Tall AR. An overview of reverse cholesterol transport. Eur Heart J. 1998;19(Suppl A):A31–A35. [PubMed] [Google Scholar]
- 111.Ohashi R, Mu H, Wang X, Yao Q, Chen C. Reverse cholesterol transport and cholesterol efflux in atherosclerosis. Q J Med. 2005;98(12):845–856. doi: 10.1093/qjmed/hci136. [DOI] [PubMed] [Google Scholar]
- 112.Leventhal AR, Chen W, Tall AR, Tabas I. Acid sphingomyelinase-deficient macrophages have defective cholesterol trafficking and efflux. J Biol Chem. 2001;276(48):44976–44983. doi: 10.1074/jbc.M106455200. [DOI] [PubMed] [Google Scholar]
- 113.Kornhuber J, Medlin A, Bleich S, Jendrossek V, Henkel AW, Wiltfang J, et al. High activity of acid sphingomyelinase in major depression. J Neural Transm. 2005;112(11):1583–1590. doi: 10.1007/s00702-005-0374-5. [DOI] [PubMed] [Google Scholar]
- 114.Albouz S, Le Saux F, Wenger D, Hauw JJ, Baumann N. Modifications of sphingomyelin and phosphatidylcholine metabolism by tricyclic antidepressants and phenothiazines. Life Sci. 1986;38(4):357–363. doi: 10.1016/0024-3205(86)90083-4. [DOI] [PubMed] [Google Scholar]
- 115.Kolzer M, Werth N, Sandhoff K. Interactions of acid sphingomyelinase and lipid bilayers in the presence of the tricyclic antidepressant desipramine. FEBS Lett. 2004;559(1–3):96–98. doi: 10.1016/S0014-5793(04)00033-X. [DOI] [PubMed] [Google Scholar]
- 116.Claus RA, Bunck AC, Bockmeyer CL, Brunkhorst FM, Losche W, Kinscherf R, et al. Role of increased sphingomyelinase activity in apoptosis and organ failure of patients with severe sepsis. FASEB J. 2005;19(12):1719–1721. doi: 10.1096/fj.04-2842fje. [DOI] [PubMed] [Google Scholar]
- 117.Wong ML, Xie B, Beatini N, Phu P, Marathe S, Johns A, et al. Acute systemic inflammation up-regulates secretory sphingomyelinase in vivo: a possible link between inflammatory cytokines and atherogenesis. Proc Natl Acad Sci USA. 2000;97(15):8681–8686. doi: 10.1073/pnas.150098097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Langmann T, Buechler C, Ries S, Schaeffler A, Aslanidis C, Schuierer M, et al. Transcription factors Sp1 and AP-2 mediate induction of acid sphingomyelinase during monocytic differentiation. J Lipid Res. 1999;40(5):870–880. [PubMed] [Google Scholar]
- 119.Calbo E, Garau J. Of mice and men: innate immunity in pneumococcal pneumonia. Int J Antimicrob Agents. 2010;35(2):107–113. doi: 10.1016/j.ijantimicag.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 120.Dentener MA, Bazil V, Von Asmuth EJ, Ceska M, Buurman WA. Involvement of CD14 in lipopolysaccharide-induced tumor necrosis factor-alpha, IL-6 and IL-8 release by human monocytes and alveolar macrophages. J Immunol. 1993;150(7):2885–2891. [PubMed] [Google Scholar]
- 121.Sakata A, Ochiai T, Shimeno H, Hikishima S, Yokomatsu T, Shibuya S, et al. Acid sphingomyelinase inhibition suppresses lipopolysaccharide-mediated release of inflammatory cytokines from macrophages and protects against disease pathology in dextran sulphate sodium-induced colitis in mice. Immunology. 2007;122(1):54–64. doi: 10.1111/j.1365-2567.2007.02612.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Rozenova KA, Deevska GM, Karakashian AA, Nikolova-Karakashian MN. Studies on the role of acid sphingomyelinase and ceramide in the regulation of tumor necrosis factor alpha (TNFalpha)-converting enzyme activity and TNFalpha secretion in macrophages. J Biol Chem. 2010;285(27):21103–21113. doi: 10.1074/jbc.M109.080671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Parker LC, Prince LR, Sabroe I. Translational mini-review series on Toll-like receptors: networks regulated by Toll-like receptors mediate innate and adaptive immunity. Clin Exp Immunol. 2007;147(2):199–207. doi: 10.1111/j.1365-2249.2006.03203.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Cuschieri J, Bulger E, Billgrin J, Garcia I, Maier RV. Acid sphingomyelinase is required for lipid Raft TLR4 complex formation. Surg Infect (Larchmt) 2007;8(1):91–106. doi: 10.1089/sur.2006.050. [DOI] [PubMed] [Google Scholar]
- 125.Wang SW, Parhar K, Chiu KJ, Tran A, Gangoiti P, Kong J, et al. Pertussis toxin promotes macrophage survival through inhibition of acid sphingomyelinase and activation of the phosphoinositide 3-kinase/protein kinase B pathway. Cell Signal. 2007;19(8):1772–1783. doi: 10.1016/j.cellsig.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 126.Wu W, Mosteller RD, Broek D. Sphingosine kinase protects lipopolysaccharide-activated macrophages from apoptosis. Mol Cell Biol. 2004;24(17):7359–7369. doi: 10.1128/MCB.24.17.7359-7369.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Hammad SM, Crellin HG, Wu BX, Melton J, Anelli V, Obeid LM. Dual and distinct roles for sphingosine kinase 1 and sphingosine 1 phosphate in the response to inflammatory stimuli in RAW macrophages. Prostaglandins Other Lipid Mediat. 2008;85(3–4):107–114. doi: 10.1016/j.prostaglandins.2007.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Gomez-Munoz A, Kong J, Salh B, Steinbrecher UP. Sphingosine-1-phosphate inhibits acid sphingomyelinase and blocks apoptosis in macrophages. FEBS Lett. 2003;539(1–3):56–60. doi: 10.1016/S0014-5793(03)00197-2. [DOI] [PubMed] [Google Scholar]
- 129.Hundal RS, Gomez-Munoz A, Kong JY, Salh BS, Marotta A, Duronio V, et al. Oxidized low density lipoprotein inhibits macrophage apoptosis by blocking ceramide generation, thereby maintaining protein kinase B activation and Bcl-XL levels. J Biol Chem. 2003;278(27):24399–24408. doi: 10.1074/jbc.M209179200. [DOI] [PubMed] [Google Scholar]
- 130.Consigny PM. Pathogenesis of atherosclerosis. Am J Roentgenol. 1995;164(3):553–558. doi: 10.2214/ajr.164.3.7863871. [DOI] [PubMed] [Google Scholar]
- 131.Miller YI, Chang MK, Binder CJ, Shaw PX, Witztum JL. Oxidized low density lipoprotein and innate immune receptors. Curr Opin Lipidol. 2003;14(5):437–445. doi: 10.1097/00041433-200310000-00004. [DOI] [PubMed] [Google Scholar]
- 132.Yuan XM, Li W, Brunk UT, Dalen H, Chang YH, Sevanian A. Lysosomal destabilization during macrophage damage induced by cholesterol oxidation products. Free Radic Biol Med. 2000;28(2):208–218. doi: 10.1016/S0891-5849(99)00220-8. [DOI] [PubMed] [Google Scholar]
- 133.Deigner HP, Claus R, Bonaterra GA, Gehrke C, Bibak N, Blaess M, et al. Ceramide induces aSMase expression: implications for oxLDL-induced apoptosis. FASEB J. 2001;15(3):807–814. doi: 10.1096/fj.15.3.807. [DOI] [PubMed] [Google Scholar]
- 134.Hammad SM, Taha TA, Nareika A, Johnson KR, Lopes-Virella MF, Obeid LM. Oxidized LDL immune complexes induce release of sphingosine kinase in human U937 monocytic cells. Prostaglandins Other Lipid Mediat. 2006;79(1–2):126–140. doi: 10.1016/j.prostaglandins.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 135.Auge N, Maupas-Schwalm F, Elbaz M, Thiers JC, Waysbort A, Itohara S, et al. Role for matrix metalloproteinase-2 in oxidized low-density lipoprotein-induced activation of the sphingomyelin/ceramide pathway and smooth muscle cell proliferation. Circulation. 2004;110(5):571–578. doi: 10.1161/01.CIR.0000136995.83451.1D. [DOI] [PubMed] [Google Scholar]
- 136.Fridman WH. Fc receptors and immunoglobulin binding factors. FASEB J. 1991;5(12):2684–2690. doi: 10.1096/fasebj.5.12.1916092. [DOI] [PubMed] [Google Scholar]
- 137.Abdel Shakor AB, Kwiatkowska K, Sobota A. Cell surface ceramide generation precedes and controls FcgammaRII clustering and phosphorylation in rafts. J Biol Chem. 2004;279(35):36778–36787. doi: 10.1074/jbc.M402170200. [DOI] [PubMed] [Google Scholar]
- 138.Huber LC, Jungel A, Distler JH, Moritz F, Gay RE, Michel BA, et al. The role of membrane lipids in the induction of macrophage apoptosis by microparticles. Apoptosis. 2007;12(2):363–374. doi: 10.1007/s10495-006-0622-7. [DOI] [PubMed] [Google Scholar]
- 139.Haas A. The phagosome: compartment with a license to kill. Traffic. 2007;8(4):311–330. doi: 10.1111/j.1600-0854.2006.00531.x. [DOI] [PubMed] [Google Scholar]
- 140.Rogers LD, Foster LJ. The dynamic phagosomal proteome and the contribution of the endoplasmic reticulum. Proc Natl Acad Sci USA. 2007;104(47):18520–18525. doi: 10.1073/pnas.0705801104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Schramm M, Herz J, Haas A, Kronke M, Utermohlen O. Acid sphingomyelinase is required for efficient phago-lysosomal fusion. Cell Microbiol. 2008;10(9):1839–1853. doi: 10.1111/j.1462-5822.2008.01169.x. [DOI] [PubMed] [Google Scholar]
- 142.Wahe A, Kasmapour B, Schmaderer C, Liebl D, Sandhoff K, Nykjaer A, et al. Golgi-to-phagosome transport of acid sphingomyelinase and prosaposin is mediated by sortilin. J Cell Sci. 2010;123(Pt 14):2502–2511. doi: 10.1242/jcs.067686. [DOI] [PubMed] [Google Scholar]
- 143.Hauck CR, Grassme H, Bock J, Jendrossek V, Ferlinz K, Meyer TF, et al. Acid sphingomyelinase is involved in CEACAM receptor-mediated phagocytosis of Neisseria gonorrhoeae. FEBS Lett. 2000;478(3):260–266. doi: 10.1016/S0014-5793(00)01851-2. [DOI] [PubMed] [Google Scholar]
- 144.Esen M, Schreiner B, Jendrossek V, Lang F, Fassbender K, Grassme H, et al. Mechanisms of Staphylococcus aureus induced apoptosis of human endothelial cells. Apoptosis. 2001;6(6):431–439. doi: 10.1023/A:1012445925628. [DOI] [PubMed] [Google Scholar]
- 145.Grassme H, Cremesti A, Kolesnick R, Gulbins E. Ceramide-mediated clustering is required for CD95-DISC formation. Oncogene. 2003;22(35):5457–5470. doi: 10.1038/sj.onc.1206540. [DOI] [PubMed] [Google Scholar]
- 146.Yu H, Zeidan YH, Wu BX, Jenkins RW, Flotte TR, Hannun YA, et al. Defective acid sphingomyelinase pathway with Pseudomonas aeruginosa infection in cystic fibrosis. Am J Respir Cell Mol Biol. 2009;41(3):367–375. doi: 10.1165/rcmb.2008-0295OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Teichgraber V, Ulrich M, Endlich N, Riethmuller J, Wilker B, De Oliveira-Munding CC, et al. Ceramide accumulation mediates inflammation, cell death and infection susceptibility in cystic fibrosis. Nat Med. 2008;14(4):382–391. doi: 10.1038/nm1748. [DOI] [PubMed] [Google Scholar]
- 148.Zhang Y, Li X, Grassme H, Doring G, Gulbins E. Alterations in ceramide concentration and pH determine the release of reactive oxygen species by Cftr-deficient macrophages on infection. J Immunol. 2010;184(9):5104–5111. doi: 10.4049/jimmunol.0902851. [DOI] [PubMed] [Google Scholar]
- 149.Kamata H, Honda S, Maeda S, Chang L, Hirata H, Karin M. Reactive oxygen species promote TNFalpha-induced death and sustained JNK activation by inhibiting MAP kinase phosphatases. Cell. 2005;120(5):649–661. doi: 10.1016/j.cell.2004.12.041. [DOI] [PubMed] [Google Scholar]
- 150.McCollister BD, Myers JT, Jones-Carson J, Voelker DR, Vazquez-Torres A. Constitutive acid sphingomyelinase enhances early and late macrophage killing of Salmonella enterica serovar Typhimurium . Infect Immun. 2007;75(11):5346–5352. doi: 10.1128/IAI.00689-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392(6673):245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 152.Leon B, Ardavin C. Monocyte-derived dendritic cells in innate and adaptive immunity. Immunol Cell Biol. 2008;86(4):320–324. doi: 10.1038/icb.2008.14. [DOI] [PubMed] [Google Scholar]
- 153.van Kooyk Y, Geijtenbeek TB. DC-SIGN: escape mechanism for pathogens. Nat Rev Immunol. 2003;3(9):697–709. doi: 10.1038/nri1182. [DOI] [PubMed] [Google Scholar]
- 154.Avota E, Gulbins E, Schneider-Schaulies S. DC-SIGN mediated sphingomyelinase-activation and ceramide generation is essential for enhancement of viral uptake in dendritic cells. PLoS Pathog. 2011;7(2):e1001290. doi: 10.1371/journal.ppat.1001290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Eikelenboom P, Veerhuis R, Familian A, Hoozemans JJ, van Gool WA, Rozemuller AJ. Neuroinflammation in plaque and vascular beta-amyloid disorders: clinical and therapeutic implications. Neurodegener Dis. 2008;5(3–4):190–193. doi: 10.1159/000113699. [DOI] [PubMed] [Google Scholar]
- 156.Xuan NT, Shumilina E, Kempe DS, Gulbins E, Lang F. Sphingomyelinase dependent apoptosis of dendritic cells following treatment with amyloid peptides. J Neuroimmunol. 2010;219(1–2):81–89. doi: 10.1016/j.jneuroim.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 157.Falcone S, Perrotta C, De Palma C, Pisconti A, Sciorati C, Capobianco A, et al. Activation of acid sphingomyelinase and its inhibition by the nitric oxide/cyclic guanosine 3′,5′-monophosphate pathway: key events in Escherichia coli-elicited apoptosis of dendritic cells. J Immunol. 2004;173(7):4452–4463. doi: 10.4049/jimmunol.173.7.4452. [DOI] [PubMed] [Google Scholar]
- 158.Ignarro LJ. Nitric oxide. A novel signal transduction mechanism for transcellular communication. Hypertension. 1990;16(5):477–483. doi: 10.1161/01.hyp.16.5.477. [DOI] [PubMed] [Google Scholar]
- 159.Barsacchi R, Perrotta C, Sestili P, Cantoni O, Moncada S, Clementi E. Cyclic GMP-dependent inhibition of acid sphingomyelinase by nitric oxide: an early step in protection against apoptosis. Cell Death Differ. 2002;9(11):1248–1255. doi: 10.1038/sj.cdd.4401095. [DOI] [PubMed] [Google Scholar]
- 160.Perrotta C, De Palma C, Clementi E. Nitric oxide and sphingolipids: mechanisms of interaction and role in cellular pathophysiology. Biol Chem. 2008;389(11):1391–1397. doi: 10.1515/BC.2008.155. [DOI] [PubMed] [Google Scholar]
- 161.Kim DO, Lee CY. Comprehensive study on vitamin C equivalent antioxidant capacity (VCEAC) of various polyphenolics in scavenging a free radical and its structural relationship. Crit Rev Food Sci Nutr. 2004;44(4):253–273. doi: 10.1080/10408690490464960. [DOI] [PubMed] [Google Scholar]
- 162.Mahmud H, Mauro D, Foller M, Lang F. Inhibitory effect of thymol on suicidal erythrocyte death. Cell Physiol Biochem. 2009;24(5–6):407–414. doi: 10.1159/000257433. [DOI] [PubMed] [Google Scholar]
- 163.Xuan NT, Shumilina E, Schmid E, Bhavsar SK, Rexhepaj R, Gotz F, et al. Role of acidic sphingomyelinase in thymol-mediated dendritic cell death. Mol Nutr Food Res. 2010;54(12):1833–1841. doi: 10.1002/mnfr.200900577. [DOI] [PubMed] [Google Scholar]
- 164.Hosea HJ, Rector ES, Taylor CG. Zinc-deficient rats have fewer recent thymic emigrant (CD90 +) T lymphocytes in spleen and blood. J Nutr. 2003;133(12):4239–4242. doi: 10.1093/jn/133.12.4239. [DOI] [PubMed] [Google Scholar]
- 165.Prasad AS. Effects of zinc deficiency on Th1 and Th2 cytokine shifts. J Infect Dis. 2000;182(Suppl 1):S62–S68. doi: 10.1086/315916. [DOI] [PubMed] [Google Scholar]
- 166.Shumilina E, Xuan NT, Schmid E, Bhavsar SK, Szteyn K, Gu S, et al. Zinc induced apoptotic death of mouse dendritic cells. Apoptosis. 2010;15(10):1177–1186. doi: 10.1007/s10495-010-0520-x. [DOI] [PubMed] [Google Scholar]