Abstract
TNFAIP2 is a protein upregulated in response to TNF signaling but its cellular expression and function in normal and neoplastic tissues remains largely unknown. Here we use standard immunohistochemical techniques to demonstrate that TNFAIP2 is normally expressed by follicular dendritic cells, interdigitating dendritic cells, and macrophages but not by lymphoid cells in secondary lymphoid tissues. Consistent with this expression pattern, we found strong TNFAIP2 staining of tumor cells in 4/4 cases (100%) of follicular dendritic cell sarcoma and in 3/3 cases (100%) of histiocytic sarcoma. Although TNFAIP2 is not expressed by the small and intermediate-size neoplastic B-cells comprising follicular lymphoma, small lymphocytic lymphoma, mantle cell lymphoma, or marginal zone lymphoma, we observed strong TNFAIP2 staining of the large, neoplastic cells, in 31/31 cases (100%) of classical Hodgkin lymphoma, 12/12 cases (100%) of nodular lymphocyte predominant Hodgkin lymphoma, and 27/31 cases (87%) of primary mediastinal (thymic) large B cell lymphoma. In contrast, TNFAIP2 was expressed by the malignant cells in only 2/45 cases (4%) of diffuse large B cell lymphoma, not otherwise specified, 2/18 cases (11%) of Burkitt lymphoma, and 1/19 cases (5%) of anaplastic large cell lymphoma. Further analysis indicates that TNFAIP2, as a single diagnostic marker, is more sensitive (sensitivity= 87%) and specific (specificity= 96%) than TRAF1, nuclear cRel, or CD23 for distinguishing the malignant B-cells of primary mediastinal (thymic) large B cell lymphoma from those of its morphologic and immunophenotypic mimic, diffuse large B cell lymphoma, not otherwise specified. Thus, TNFAIP2 may serve as a useful new marker of dendritic and histiocytic sarcomas whose aberrant expression in the malignant cells of classical Hodgkin lymphoma and primary mediastinal (thymic) large B cell lymphoma serves to distinguish these tumors from other large cell lymphomas in routine clinical practice.
Keywords: TNFAIP2, Hodgkin Lymphoma, primary mediastinal (thymic) large B cell lymphoma, immunohistochemistry
INTRODUCTION
Major categories of large cell lymphomas include classical Hodgkin lymphoma (cHL), nodular lymphocyte predominant Hodgkin lymphoma (NLPHL), anaplastic large cell lymphoma (ALCL), primary mediastinal (thymic) large B cell lymphoma (PMBL), and diffuse large B cell lymphoma not otherwise specified (DLBCL).(22). Whereas established diagnostic criteria can be used to reliably distinguish cHL, NLPHL, and ALCL from DLBCL in most, but not all cases, establishing the diagnosis of PMBL is frequently problematic.(13, 22) The malignant B-cells of PMBL and DLBCL show extensive morphologic and phenotypic similarities including a sheet-like growth pattern of large lymphoid cells and the expression pan-B lineage markers such as CD19, CD20, CD79a, and PAX5. Other routine phenotypic markers that are useful for the subclassification of B-cell lymphomas such as Bcl6, IRF4/MUM1, and CD30 can expressed by the malignant B cells of both PMBL and DLBCL. MAL, TRAF1, nuclear cRel, and CD23 are biomarkers that have been reported to facilitate the distinction of PMBL and DLBCL.(2, 3, 13) (17) However, the routine use of each of these markers presents challenges to the practicing pathologist- from the lack of commercially available antibodies (MAL), to the lack of sensitivity (TRAF1, nuclear cRel) or specificity (CD23) for PMBL relative to DLBCL. Thus the identification of novel immunophenotypic markers that reliably distinguish PMBL from DLBCL and which are amenable to routine surgical pathology practice remains an ongoing effort.
Tumor necrosis factor- alpha inducible protein-2 (TNFAIP2) was originally identified as a gene transcript induced in endothelial cells following stimulation with TNFα.(18, 24) Subsequent work has shown that TNFAIP2 is induced in a variety of cell types in response a spectrum of pro-inflammatory stimuli, including LPS and IL-1.(4) In mouse embryos, TNFAIP2 transcripts are abundant in developing myocardium, liver, and the aorta, but in the adult mouse, TNFAIP2 transcripts are restricted to lymphoid organs such as the spleen, tonsil and Peyer’s patches.(25) In humans, TNFAIP2 has been identified as a retinoic acid target gene in acute promyelocytic leukemia.(16) However, the normal tissue and cellular distribution of TNFAIP2 protein in mice and humans remains largely unknown. Moreover, TNFAIP2 shows no significant homology with any proteins of defined biologic function, and a TNFAIP2 deficient mouse has yet to be reported. As a result the biologic roles of TNFAIP2 remain poorly understood.
In this report, we used a specific antibody and standard immunohistochemical staining (IHC) techniques to determine the cellular distribution of TNFAIP2 protein in secondary lymphoid tissues- a tissue type showing high TNFAIP2 transcript levels in mice.(25) We show that TNFAIP2 protein is largely restricted to the cell body, cellular processes and, to a variable extent, in the nucleus of follicular dendritic cells, interdigitating dendritic cells, and macrophages. TNFAIP2 is not expressed by non-neoplastic lymphoid cells in reactive lymphoid tissues. In examining select dendritic cell, histiocytic and lymphoid malignancies, we found that TNFAIP2 is robustly expressed by the tumor cells comprising follicular dendritic cell sarcoma (FDCS) and histiocytic sarcoma (HS) but not expressed by the small to intermediate-sized neoplastic B cells comprising follicular lymphoma (FL), mantle cell lymphoma (MCL), marginal zone lymphoma (MZL) and small lymphocytic lymphoma (SLL). In addition, we found that TNFAIP2 is highly expressed by the malignant Reed-Sternberg (RS) cells and variants of the nodular sclerosis and mixed cellularity subtypes of cHL and by the lymphocyte predominant (LP) cells (also known as lymphocytic and histiocytic (L&H) cells) of NLPHL. We also found that the malignant B-lymphocytes comprising the vast majority of PMBL express TNFAIP2 whereas the malignant cells of DLBCL and ALCL do not. Upon further analysis we find that TNFAIP2 is superior to TRAF1, nuclear cRel, or CD23 as an isolated biomarker for distinguishing the malignant cells of PMBL from DLBCL. Thus TNFAIP2 is a novel marker of normal and neoplastic dendritic cells and histiocytes and we propose that the aberrant expression of TNFAIP2 by the malignant cells of cHL and PMBL can serve as a useful marker for distinguishing these tumor types from their morphologic and immunophenotypic mimics in routine surgical pathology practice.
METHODS
Case selection
Previously diagnosed cases of FDCS (n=4), HS (n=3), cHL (n=31), NLPHL (n=12), (non-mediastinal) DLBCL (n=45), PMBL (n=31), ALCL (n=19), FL (n=18), SLL (n=8), MCL (n=13), MZL (n=14), Burkitt lymphoma (18), B lymphoblastic leukemia/lymphoma (13), T lymphoblastic leukemia/lymphoma (10), angioimmunoblastic T-cell lymphoma (7), peripheral T-cell lymphoma, NOS (10), extranodal NK/T-cell lymphoma, nasal type (5), and reactive lymphadenopathy due to infectious mononucleosis (4), toxoplasmosis (1) and cat scratch disease (3) were obtained from the files of the Department of Pathology at Brigham and Women’s Hospital, Boston, MA, with institutional internal review board approval. Diagnoses were established according to the criteria of the WHO classification using morphology and a standard set of immunohistochemical studies.(22) Cases of classified as PMBL were confirmed, as part of this study, to satisfy the combination of clinical, radiologic, morphologic and phenotypic criteria for this entity. (20) These included radiologic identification an isolated mediastinal mass in a young or middle aged individual with or without local extension into adjacent tissues and organs, a morphologic pattern showing sheets of large atypical lymphoid cells without or without a dense sclerotic background and scattered thymic remnants, and positive staining of tumor cells for mature B lymphoid markers and an absence of CD15 expression. In situ hybridization for Epstein-Barr encoded small RNAs (EBER) was performed in a subset of cases of cHL by standard methods.(23). The status of ALK in cases of ALCL was established by either immunohistochemical staining for ALK protein (using clone ALK1, DAKO USA, Carpentaria, CA) or by fluorescent in situ-hybridization using standard, commercial reagents (Abbott molecular, Abbott Park, IL).(11)
Immunohistochemistry
IHC was performed using 5 μm thick formalin or B+ fixed, paraffin-embedded (FFPE) tissue sections. Slides were soaked in xylene, passed through graded alcohols and put in distilled water. Slides were then pre-treated with 10-mM citrate, pH 6.0 (Zymed, South San Francisco, CA) in a steam pressure cooker (Decloaking Chamber, BioCare Medical, Walnut Creek, CA) as per manufacturers instructions followed by washing in distilled water. All further steps were performed at room temperature in a hydrated chamber. Slides were pre-treated with Peroxidase Block (DAKO USA, Carpentaria, CA) for 5 minutes to quench endogenous peroxidase activity.
Primary mouse anti-TNFAIP2 antibody (clone F-6, catalogue number sc-28318, 1:200 dilution; Santa Cruz Biotechnology, Santa Cruz, CA) was applied in DAKO diluent (DAKO) for 1 hour at room temperature. Slides were washed in 50-mM Tris-Cl, pH 7.4, and anti-murine horseradish peroxidase-conjugated antibody solution (Envision+ detection kit, DAKO) was applied for 30 minutes. After further washing, immunoperoxidase staining was developed using a diaminobenzidine (DAB) chromogen kit (DAKO) per the manufacturer and counterstained with Harris hematoxylin (Polyscientific, Bay Shore, NY).
Immunostaining for TRAF1 was performed using mouse monoclonal antibody clone H-3 (Catalog # sc-6253, Santa Cruz Biotechnology, Santa Cruz, CA), immunostaining for cRel was performed using rabbit polyclonal antibody (catalog # PC139, Calbiochem/EMD Chemicals, Gibbstown, NJ), and immunostaining for CD23 was performed using mouse monoclonal antibody (clone 1B12, catalog # MHM6, DAKO, 1:25 dilution, heat mediated retrieval) according to a standard IHC procedure that has been validated in our clinical diagnostic laboratory and others. (8) (13, 14).
Reactivity for TNFAIP2 was determined and scored independently by two hematopathologists (SK and SJR). For each stained slide, the percentage of tumor cells showing positive staining for TNFAIP2 was recorded. Intra-tumoral macrophages and dendritic cells served as internal controls for staining. Intensity of tumor cell staining for TNFAIP2 was scored as follows: (−) = no staining detected, (1+) = weak staining, (2+) = moderate staining, (3+) = strong staining. A case was scored as positive if at least 50% of the tumor cells stained positive for TNFAIP2 with an intensity of 1+, 2+, or 3+. Positive staining cells showed reactivity for TNFAIP2 in both the cytoplasm and nucleus.
Reactivity for TRAF1 and nuclear cRel was determined as described.(13) Briefly, positive staining of tumor cells at an intensity scored as 2+ or 3+ in >20% of tumor cells was considered positive staining for TRAF1. Staining that obscured nuclear detail and in excess of cytoplasmic staining in >50% of the tumor cells was considered positive staining for nuclear cRel.
RESULTS
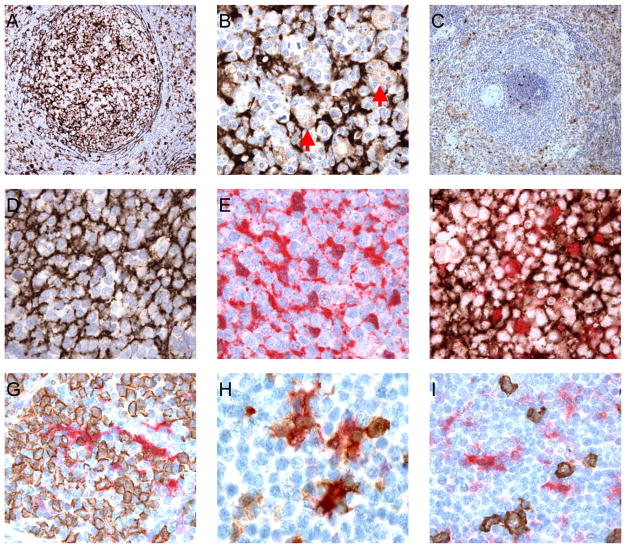
Immunohistochemical analysis for TNFAIP2 revealed moderate to strong staining of a subset of cells within reactive human tonsil, lymph node and spleen (Figure 1) and normal thymus (Figure 1S-A). High power examination of these tissues indicated that the largest collections of positive staining cells were localized to reactive germinal centers of secondary follicles in the typical pattern of follicular dendritic cells (Figure 1B). The positive staining cells were elongated and spindled with small and inconspicuous nuclei. TNFAIP2 expression in these cells localized to the cytoplasm and, to a variable extent, the nuclei. Double labeling reactive tonsil for the follicular dendritic cell marker CD23 and TNFAIP2 revealed colocalization of these two markers (Figure 1D–F). Within germinal centers, macrophages filled with apoptotic debris (tingible bodies) weakly stained for TNFAIP2 (Figure 1B, arrows). Within the inter-follicular regions of the secondary lymphoid tissues, scattered small, spindled cells, consistent with interdigitating dendritic cells as well as scattered larger cells with oval nuclei and abundant cytoplasm consistent with macrophages stained for TNFAIP2 (Figure 1A). Double staining for the interdigitating dendritic cell marker S100 and TNFAIP2 showed colocalization of the proteins within cells (Figure 1H). In contrast to the dendritic cells and macrophages, the lymphoid cells within the germinal centers, colonizing the mantle zones, and in spleen, within the marginal zones, were uniformly negative for TNFAIP2 (Figure 1A–C). Double labeling tonsil with the B cell marker CD20 (Figure 1G, I) or the T cell marker CD3 (not shown) with TNFAIP2 revealed distinct cell populations.
Figure 1.
Reactive tonsil (A, 400×; B, 1000×) and spleen (C, 200×) stained for TNFAIP2 (brown coloration) and showing a distribution of positive staining consistent with dendritic cells and macrophages (B, red arrows= macrophages). Small lymphocytes within germinal centers (A, B), mantle zones (A, C), splenic marginal zones (C), and interfollicular areas (A) are negative for staining. Germinal center from a lymph node (D–F, 1000×) stained for CD23 (D, F; brown coloration) and TNFAIP2 (E, F; red coloration), and showing positive staining of follicular dendritic cells for TNFAIP2. Germinal center from a lymph node (G, 400x) stained for CD20 (brown coloration) and TNFAIP2 (red coloration), and showing no staining of the germinal center B cells for TNFAIP2. The interfollicular region of a lymph node (H, I; 1000×) stained for S100 (H; brown coloration) or CD20 (I; brown coloration) and TNFAIP2 (H, I; red coloration) and showing staining of intedigitating dendritic cells (H) but not the interfollicular B-cells (I) for TNFAIP2.
To confirm that TNFAIP2 expression is absent in the non-neoplastic lymphoid cells comprising reactive tissues, we additionally stained biopsy samples of normal thymus (Figure 1S-A), Kikuchi’s lymphadenitis (1S-B), and lymph nodes with patterns and/or phenotypes consistent with toxoplasmosis (Figure 1S-C), and acute EBV infection (Figure 1S-D). In each case, TNFAIP2 staining highlighted macrophages and follicular and interdigitating dendritic cells but was negative in the reactive lymphoid cells.
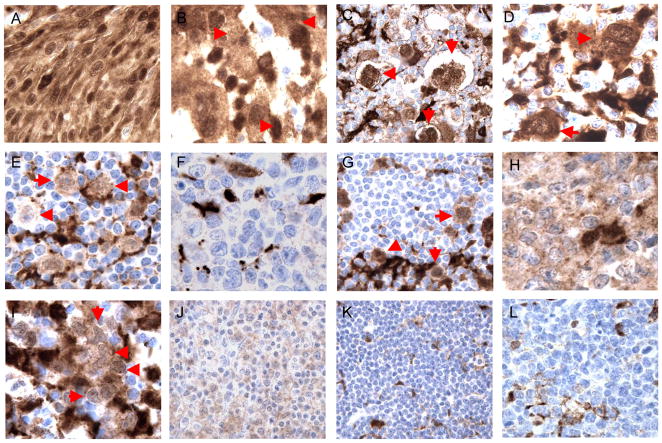
The strong expression of TNFAIP2 by follicular and interdigitating dendritic cells as well as macrophages in normal secondary lymphoid tissues raised the possibility that TNFAIP2 might serve as a useful marker for neoplasms derived from these cell types. Follicular dendritic cell sarcoma (FDCS) and histiocytic sarcoma (HS) are exceedingly rare entities. However, we assembled a group of 7 tumors from our institution and found that the tumor cells in 4 of 4 cases of FDCS and 3 of 3 cases of HS were positive for TNFAIP2 (Figure 2A and B, respectively; Table 1). These results suggest that TNFAIP2 may serve as a useful new marker for identifying these tumor types.
Figure 2.
Cases of follicular dendritic cell sarcoma (A), histiocytic sarcoma (B), classical Hodgkin lymphoma (C; D), nodular lymphocyte predominant Hodgkin lymphoma (E), diffuse large B cell lymphoma, not otherwise specified (F), T cell/histiocyte rich large B cell lymphoma (G), primary mediastinal (thymic) large B cell lymphoma (H, I), extranodal marginal zone lymphoma (J), small lymphocytic lymphoma (K), and Burkitt lymphoma (L) showing positive (A–E; G–J) and negative (F, K; L) staining for TNFAIP2 in tumor cells (red arrows in B–E, G; I). All images were photographed at 1000×.
Table 1.
TNFAIP2 expression in select lymphomas
| Tumor Type | # Tested | # Positive | % Positive |
|---|---|---|---|
| Follicular dendritic cell sarcoma | 4 | 4 | 100 |
| Histiocytic sarcoma | 3 | 3 | 100 |
| Hodgkin lymphoma | |||
| Classical Hodgkin lymphoma | 31 | 31 | 100 |
| Nodular lymphocyte predominant Hodgkin lymphoma | 12 | 12 | 100 |
| DLBCL | |||
| Not otherwise specified | 45 | 2 | 4 |
| Mediastinal (thymic) large B cell lymphoma | 31 | 27 | 87 |
| T-cell/histiocyte rich large B cell lymphoma | 3 | 3 | 100 |
| Unclassifiable, int. DLBCL and Burkitt lymphoma | 4 | 0 | 0 |
| Burkitt lymphoma | 18 | 2 | 11 |
| Anaplastic large cell lymphoma | 19 | 1 | 5 |
| Additional B-cell neoplasms | |||
| Follicular lymphoma | 18 | 0 | 0 |
| Mantle cell lymphoma | 13 | 1 | 8 |
| Extranodal marginal zone lymphoma | 14 | 1 | 7 |
| Small lymphocytic lymphoma | 8 | 0 | 0 |
| B lymphoblastic leukemia/lymphoma | 13 | 2 | 15 |
| Additional T- and NK-cell neoplasms | |||
| Angioimmunoblastic T-cell lymphoma | 7 | 1 | 14 |
| Peripheral T-cell lymphoma, NOS | 10 | 2 | 20 |
| Extranodal NK/T-cell lymphoma, nasal type | 5 | 1 | 20 |
| T lymphoblastic leukemia/lymphoma | 10 | 3 | 30 |
We had previously observed that select markers of dendritic cells and macrophages, specifically fascin and galectin-1, are also expressed by the malignant RS cells of cHL.(7, 10, 12) To determine whether TNFAIP2 is also expressed by this malignant cell type, we performed IHC on a large cohort of cHL cases. Weak, moderate, or strong staining of, at minimum, 50% of the RS cells of cHL with cytoplasmic and, to some extent, a nuclear pattern was observed in all examined cases (31 of 31 cases; Figure 2C, D; Table 1). These cases included 20 cases of nodular sclerosis Hodgkin lymphoma (NSHL), 5 cases of mixed cellularity Hodgkin lymphoma (MCHL), and 6 cases of cHL not otherwise specified. A subset of the positive staining cases was EBV+ although staining patterns did not correlate with EBV status (data not shown). The non-neoplastic macrophages that are generally part of the inflammatory background of cHL also stained for TNFAIP2 (Figure 2). The non-neoplastic lymphoid cells were negative for antigen expression. We conclude that TNFAIP2 is a robust marker of the RS cells comprising cHL, although care must be taken to distinguish TNFAIP2 expression in RS cells from TNFAIP2 expression in intermixed macrophages.
The neoplastic LP cells (also known as L&H cells) and the non-neoplastic lymphoid infiltrate of NLPHL can resemble the RS cells and reactive background of cHL, respectively(22). To determine whether LP cells express TNFAIP2 we stained a cohort of NLPHL cases and observed positive staining of >50% the neoplastic LP cells in all 12 cases (Figure 2E, Table 1). Non-neoplastic macrophages are less frequent in NLPHL than in cHL and thus positive staining of the LP cells was straightforward to discern. As in cHL the small reactive lymphoid cells surrounding the scattered neoplastic cells were negative for the antigen (Figure 2E). We conclude that the malignant LP cells of NLPHL express TNFAIP2.
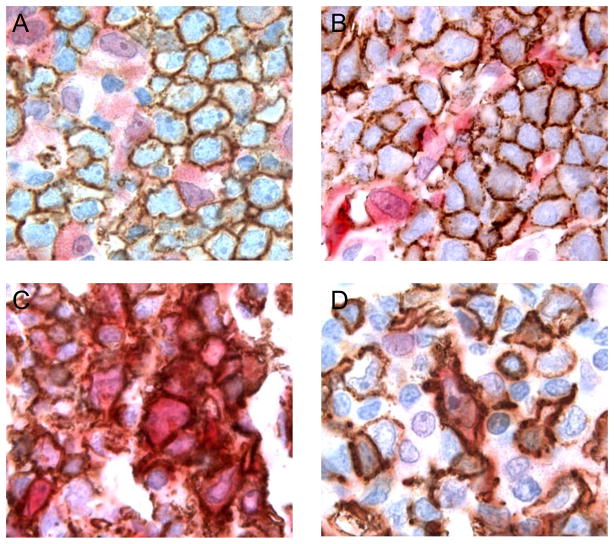
DLBCL is the most common large cell lymphoma and commonly identified by pan-B cell antigen expression by the tumor cells. We stained 45 cases of DLBCL for TNFAIP2 and detected no expression of the antigen in the tumor cells for 43 of 45 cases (Table 1). However the (non-neoplastic) macrophages and dendritic cells interspersed throughout the malignant B cells in most cases of DLBCL were positive for TNFAIP2 and these cells served as an appropriate internal control for staining (Figure 2F). We found no distinguishing clinical or pathologic features among the 2 positive staining cases of DLBCL which would suggest that these 2 cases were mis-classified (data not shown). Double immunohistochemical staining for CD20 and TNFAIP2 in select cases of DLBCL confirmed that TNFAIP2 expression was not localized to the malignant B cells in this tumor type (Figure 3A, B; data not shown). We conclude that TNFAIP2 is very rarely expressed by the malignant B cells of DLBCL.
Figure 3.
Cases of diffuse large B cell lymphoma, not otherwise specified (A, B), and primary mediastinal (thymic) large B cell lymphoma (C, D) stained for CD20 (brown coloration) and TNFAIP2 (red coloration). All images were photographed at 1000×.
A specific subtype of diffuse large B cell lymphoma, termed T-cell/histiocyte rich large B cell lymphoma (T/HRLBCL), shows a mixed inflammatory background reminiscent of cHL. However in contrast to cHL, the malignant cells of T/HRLBCL resemble those of DLBCL, and like DLBCL, express pan-B cell antigens. We examined 3 cases of T/HRLBCL and found the malignant cells of each case to be positive for TNFAIP2 (Figure 2G). All three cases of TCRBCL occurred in patients without a known history of lymphoma, presented as rapidly developing adenopathy, and showed the typical morphologic and immunophenotypic features of the diagnostic entity. Although our finding suggests that this rare type of DLBCL may more closely resemble HL with respect to TNFAIP2 expression, additional, larger cohorts of cases will need to be studied to determine the frequency of TNFAIP2 expression in TCRBCL with more certainty.
The malignant B cells of PMBL morphologically and immunophenotypically resemble DLBCL, however this tumor has clinical, molecular and genetic features that more closely resemble cHL.(13, 15, 20) We tested a large cohort of PMBL cases satisfying the major clinical, radiologic, and pathologic criteria for this entity and readily detected TNFAIP2 expression in the tumor cells in 27/31 cases (87%, Table 1). As for cases of cHL, we found that biopsy tissue stained for TNFAIP2 had to be examined with care to ensure that expression of this antigen was localized to the malignant B-cells in addition the endogenous expression of the protein by the numerous, intermixed macrophages and dendritic cells (Figure 2H, I). Double staining for TNFAIP2 and CD20 in a subset of cases of PMBL confirmed the expression of TNFAIP2 in the tumor cells in this tumor type (Figure 3D, E). We conclude that TNFAIP2 is a robust marker of the malignant cells of PMBL.
Although the tumor cells of Burkitt lymphoma (BL) are generally intermediate in size, occasional cases show large cell size and thus might be considered within the differential diagnosis of a large cell lymphoma. We tested 18 cases of BL and found TNFAIP2 expression in 2 cases (11%). In the remaining cases, there was no staining of the tumor cells, despite positive TNFAIP2 expression by the intermixed tingible body macrophages that are characteristic of this tumor type (Table 1, and Figure 2L). We also tested 4 cases of B-cell lymphoma, unclassifiable, with features intermediated between DLBCL and BL (int. DLBCL/BL). The tumor cells in these cases were uniformly negative for TNFAIP2 expression (Table 1).
The final major category of large cell lymphomas that we examined for TNFAIP2 expression was ALCL. ALCL is a tumor of T-cell origin, and like non-neoplastic T-cells, the malignant cells of ALCL were negative for TNFAIP2 expression in 18 of 19 of cases (Table 1). The ALCLs in our cohort included those with an ALK-rearrangement (10 cases) and without an ALK-rearrangement (9 cases) as determined by IHC or FISH (Table 1, data not shown).
Staining of an additional cohort of low grade B-cell lymphomas, including follicular lymphoma (FL, grades 1 and 2, n=18), mantle cell lymphoma (MCL, n=13), extranodal marginal zone lymphoma (MZL, n=14), small lymphocytic lymphoma (SLL, n=8) revealed expression of TNFAIP2 by the neoplastic cells in only few cases (Table 1; Figure 2J= positive staining MZL, Figure 2K= negative staining SLL). Staining of additional non-Hodgkin lymphomas, including angioimmunoblastic T-cell lymphoma (AIL-T, n=7), peripheral T cell lymphoma not otherwise specified (PTCL, NOS, n=10), extranodal NK/T cell lymphoma, nasal type (NK/T, n=5), and B and T lymphoblastic leukemia/lymphomas (B-LL, n=13; T-LL, n=10 respectively) reveal that TNFAIP2 is rarely, but occasionally expressed in other lymphoid neoplasms (Table 1).
Our data indicated that TNFAIP2 expression is largely restricted to the malignant cells of FDCS, HS, cHL, NLPHL, and PMBL and largely negative in the malignant cells of DLBCL and ALCL. Among these large cell lymphomas, PMBL and DLBCL are often difficult to distinguish by morphologic and immunophenotypic features alone. We therefore examined whether TNFAIP2 could serve as a more reliable diagnostic marker than the expression of TRAF1, nuclear cRel, or CD23 for resolving this differential diagnosis.(13, 17) Antibodies recognizing MAL, another antigen differentially expressed between PMBL and DLBCL, are not commercially available and therefore not tested.(2) We found that the malignant cells in 23/31 cases (74%) of PMBL were positive for TRAF1, 17/30 cases (57%) of PMBL were positive for the expression and nuclear localization of cRel, and 9/28 cases (32%) of PMBL were positive for CD23 (Table 2). The combined expression of TRAF1 and nuclear cRel in the malignant cells was observed in 17/30 cases (57%) of PMBL - a value comparable to that observed in a prior study (13). The overall immunophenotypic profile of the PMBL cases revealed that no marker was entirely sensitive for this tumor type (Table 2). However, among the tested biomarkers, TNFAIP2 proved to be the most sensitive (sensitivity= 87%) and specific (specificity= 96%) marker of tumor cells comprising PMBL relative to (non-mediastinal) DLBCL (Table 3). Among the same sets of cases, we found TNFAIP2 expression to be coordinately expressed with TRAF1 in the vast majority of PMBLs (Table 2). Four cases (13%) of PMBL in our cohort were negative for TNFAIP2, and of these cases, all were positive for TRAF1.
Table 2.
Phenotype of primary mediastinal large B cell lymphomas tested
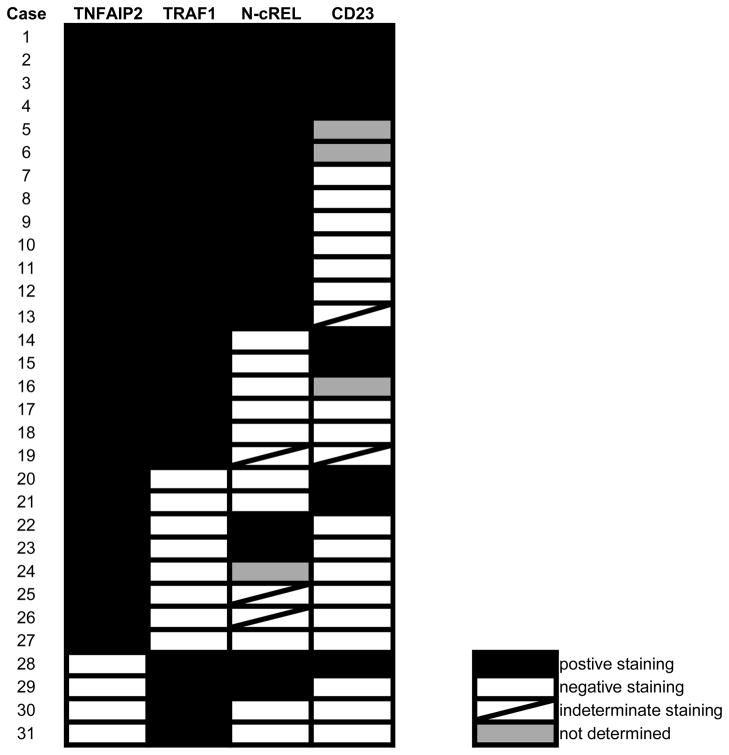
|
Table 3.
Utility of select diagnostic markers for primary mediastinal large B cell lymphoma*
for distinguishing from diffuse large B cell lymphoma, NOS.
Rodig et. al, AJSP, 2007
N-cRel= predominantly nuclear localization of cRel
ND= not determined
DISCUSSION
In this report we demonstrate that TNFAIP2, a protein normally expressed by dendritic cells and macrophages but not by lymphocytes in the secondary lymphoid organs, is strongly expressed by the malignant cells in FDCS, HS, cHL, NLPHL, and PMBL. In contrast, the malignant B cells of DLBCL, with the possible exception of TCRLBCL, are negative for this protein. Similarly the malignant cells of ALCL, a tumor of T cell origin, do not express TNFAIP2, nor do the neoplastic B cells comprising FL, MCL, MZL, and SLL.
The universal expression of TNFAIP2 by the RS cells of cHL and the LP cells of NLPHL reveal TNFAIP2 to be a new diagnostic marker of Hodgkin lymphomas. Although this expression pattern was unexpected, TNFAIP2 is not the first dendritic cell- associated protein found to be upregulated in the malignant cells of Hodgkin lymphoma. Fascin is an intermediate filament protein that is abundant in cells of the macrophage lineage, was discovered 15 years ago to be highly expressed by the RS cells of cHL but not by the neoplastic cells of other large cell lymphomas.(10) At the time, the expression of the fascin in RS cells was considered evidence that this cell type might be derived from dendritic cells- a hypothesis disproven when molecular genetic techniques firmly established a B-cell lineage derivation for these cells. More recently, we reported that galectin-1, an immuno-suppressive lectin normally expressed by macrophages, dendritic cells and endothelial cells, is highly expressed by the RS cells of cHL and by the malignant T cells of ALCL but not by the neoplastic cells of other large cell lymphomas.(7, 12) We demonstrated that galectin-1 expression in RS cells was attributable and dependent upon an AP1 binding site in the galectin promoter and showed that constitutive AP1 signaling is a characteristic of RS cells of cHL and the malignant T cells of ALCL but not the malignant B-cells of PMBL or DLBCL.
Clinical evidence indicates that PMBL is a distinct tumor type from DLBCL.(6, 9) PMBL typically occurs in a younger patient population and with less disseminated disease than DLBCL. In some studies, patients with PMBL demonstrate a more favorable outcome than those with DLBCL.(19) Recently, gene expression profiles of PMBL, cHL and DLBCL confirmed the categorization of PMBL as a distinct tumor type, but unexpectedly, demonstrated that PMBL is more closely related to cHL than DLBCL at the molecular level.(15, 20) For the practicing pathologist, the distinction between PMBL and CHL can be challenging in some cases. Likewise, the distinction between PMBL and DLBCL can be very difficult in the absence of ancillary clinical and radiographic data. Several proteins, detectable by IHC in formalin-fixed paraffin embedded (FFPE) biopsy specimens, have been proposed to distinguish PMBL from DLBCL, including MAL, activated (phosphorylated) STAT6 (p-STAT6), p63, TRAF1, activated (nuclear) cRel and CD23.(2, 5, 13, 17, 26) At this point in time, anti-MAL antibodies are not commercially available and, in our hands, existing antibodies recognizing p-STAT6 are not robust enough for routine evaluation by IHC using FFPE biopsy samples (data not shown). We have also found p63 be a poor discriminator between PMBL and DLBCL (data not shown). In contrast, well validated IHC tests to detect TRAF1, cREL and CD23 are available and we have found them to be useful for establishing the diagnosis of PMBL in routine practice. We found that expression of TNFAIP2, with a sensitivity of 87% and a specificity of 96% for the tumor cells of PMBL, is superior to TRAF1 (sensitivity= 74%, specificity= 88%), nuclear cRel (sensitivity= 57%, specificity= 82%), and CD23 (sensitivity= 32%, specificity= not determined) when the differential diagnosis is between PMBL and DLBCL.
Surprisingly, our cohort of cases showed a lower percentage of PMBLs positive for CD23 than previously reported (1, 17) A partial explanation for this difference may be the different choice of antibodies used to detect CD23. Additional studies using defined staining conditions and uniform sets of cases across institutions will be necessary to more rigorously define the origin of these differences. Regardless, our finding that TNFAIP2 is expressed by the tumor cells in 87% PMBLs indicates that this marker exceeds the sensitivity of CD23 even under ideal conditions (70% of cases positive). Furthermore all cases of PMBL tested as part of this study had tumor cells that expressed either TNFAIP2 or TRAF1- a result that suggests that staining for both of these markers is likely to be the most effective means to diagnose PMBL in routine practice.
The basis for TNFAIP2 expression in select lymphomas is unknown. However activation of TNF receptors strongly induces TNFAIP2 expression in several cell types(4, 18, 25), and given the cytokine rich milieu of HL,(21) it is likely that TNFAIP2 expression is related to cytokine-mediated activation of one or more TNF receptor family members expressed at the surface of the malignant cells. Cytokine-mediated upregulation of TNFAIP2 might explain the expression of this protein in cases of T/HRLBCL and PMBL as well. However, macrophages, a major source of TNFα, are commonly found intermixed with the malignant B cells in most DLBCLs. Thus, the abundance of macrophages and dendritic cells in various tumors cannot fully explain the restricted expression pattern of TNFAIP2 to select malignancies. Defining the biologic roles for TNFAIP2 in normal and neoplastic cells is an area of active research, and preliminary work in ovarian cancer suggests that TNFAIP2 may function in signaling pathways that promote intercellular communication (RD and SD, unpublished data). Whether such signaling pathways are active in HL and PMBL is unknown and the subject of future endeavors.
In conclusion, we have shown that TNFα-inducible factor, TNFAIP2, is normally expressed by dendritic cells and macrophages in secondary lymphoid tissues, is expressed by the malignant cells of FDCS and HS, and is aberrantly expressed by the malignant RS cells of cHL, LP cells of NLPHL, and B-cells of PMBL. We anticipate that immunohistochemical staining for TNFAIP2 will serve as a useful ancillary test to establish the diagnosis of PMBL in routine surgical pathology practice in the future.
Supplementary Material
Thymus (A), Kikuchi’s lymphadenitis (B), lymph node with toxoplasmosis (C) and infectious mononucleosis (D) stained for TNFAIP2 (brown coloration) and showing positive staining of macrophages and follicular and interdigitating dendritic cells and no staining of the reactive lymphocytes. Photomicrographs are 1000× (A–C) or 200× (D) original magnification.
Acknowledgments
The authors gratefully acknowledge the technical assistance provided by Donna Skinner, Teri Bowman and Janice Williams. This project was funded, in part, by the Lymphoma Targeting and Testing Center (LTTC) of the Dana-Farber Cancer Institute.
References
- 1.Calaminici M, Piper K, Lee AM, et al. CD23 expression in mediastinal large B-cell lymphomas. Histopathology. 2004;45:619–624. doi: 10.1111/j.1365-2559.2004.01969.x. [DOI] [PubMed] [Google Scholar]
- 2.Copie-Bergman C, Gaulard P, Maouche-Chretien L, et al. The MAL gene is expressed in primary mediastinal large B-cell lymphoma. Blood. 1999;94:3567–3575. [PubMed] [Google Scholar]
- 3.Copie-Bergman C, Plonquet A, Alonso MA, et al. MAL expression in lymphoid cells: further evidence for MAL as a distinct molecular marker of primary mediastinal large B-cell lymphomas. Mod Pathol. 2002;15:1172–1180. doi: 10.1097/01.MP.0000032534.81894.B3. [DOI] [PubMed] [Google Scholar]
- 4.Dixit VM, Green S, Sarma V, et al. Tumor necrosis factor-alpha induction of novel gene products in human endothelial cells including a macrophage-specific chemotaxin. J Biol Chem. 1990;265:2973–2978. [PubMed] [Google Scholar]
- 5.Guiter C, Dusanter-Fourt I, Copie-Bergman C, et al. Constitutive STAT6 activation in primary mediastinal large B-cell lymphoma. Blood. 2004;104:543–549. doi: 10.1182/blood-2003-10-3545. [DOI] [PubMed] [Google Scholar]
- 6.Johnson PW, Davies AJ. Primary mediastinal B-cell lymphoma. Hematology Am Soc Hematol Educ Program. 2008:349–358. doi: 10.1182/asheducation-2008.1.349. [DOI] [PubMed] [Google Scholar]
- 7.Juszczynski P, Ouyang J, Monti S, et al. The AP1-dependent secretion of galectin-1 by Reed Sternberg cells fosters immune privilege in classical Hodgkin lymphoma. Proc Natl Acad Sci U S A. 2007;104:13134–13139. doi: 10.1073/pnas.0706017104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kurtin PJ, Hobday KS, Ziesmer S, et al. Demonstration of distinct antigenic profiles of small B-cell lymphomas by paraffin section immunohistochemistry. Am J Clin Pathol. 1999;112:319–329. doi: 10.1093/ajcp/112.3.319. [DOI] [PubMed] [Google Scholar]
- 9.Martelli M, Ferreri AJ, Johnson P. Primary mediastinal large B-cell lymphoma. Crit Rev Oncol Hematol. 2008;68:256–263. doi: 10.1016/j.critrevonc.2008.07.020. [DOI] [PubMed] [Google Scholar]
- 10.Pinkus GS, Pinkus JL, Langhoff E, et al. Fascin, a sensitive new marker for Reed-Sternberg cells of hodgkin’s disease. Evidence for a dendritic or B cell derivation? Am J Pathol. 1997;150:543–562. [PMC free article] [PubMed] [Google Scholar]
- 11.Rodig SJ, Mino-Kenudson M, Dacic S, et al. Unique clinicopathologic features characterize ALK-rearranged lung adenocarcinoma in the western population. Clin Cancer Res. 2009;15:5216–5223. doi: 10.1158/1078-0432.CCR-09-0802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rodig SJ, Ouyang J, Juszczynski P, et al. AP1-dependent galectin-1 expression delineates classical hodgkin and anaplastic large cell lymphomas from other lymphoid malignancies with shared molecular features. Clin Cancer Res. 2008;14:3338–3344. doi: 10.1158/1078-0432.CCR-07-4709. [DOI] [PubMed] [Google Scholar]
- 13.Rodig SJ, Savage KJ, LaCasce AS, et al. Expression of TRAF1 and nuclear c-Rel distinguishes primary mediastinal large cell lymphoma from other types of diffuse large B-cell lymphoma. Am J Surg Pathol. 2007;31:106–112. doi: 10.1097/01.pas.0000213334.40358.0e. [DOI] [PubMed] [Google Scholar]
- 14.Rodig SJ, Savage KJ, Nguyen V, et al. TRAF1 expression and c-Rel activation are useful adjuncts in distinguishing classical Hodgkin lymphoma from a subset of morphologically or immunophenotypically similar lymphomas. Am J Surg Pathol. 2005;29:196–203. doi: 10.1097/01.pas.0000149689.75462.ff. [DOI] [PubMed] [Google Scholar]
- 15.Rosenwald A, Wright G, Leroy K, et al. Molecular diagnosis of primary mediastinal B cell lymphoma identifies a clinically favorable subgroup of diffuse large B cell lymphoma related to Hodgkin lymphoma. J Exp Med. 2003;198:851–862. doi: 10.1084/jem.20031074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rusiniak ME, Yu M, Ross DT, et al. Identification of B94 (TNFAIP2) as a potential retinoic acid target gene in acute promyelocytic leukemia. Cancer Res. 2000;60:1824–1829. [PubMed] [Google Scholar]
- 17.Salama ME, Rajan Mariappan M, Inamdar K, et al. The value of CD23 expression as an additional marker in distinguishing mediastinal (thymic) large B-cell lymphoma from Hodgkin lymphoma. Int J Surg Pathol. 18:121–128. doi: 10.1177/1066896909331994. [DOI] [PubMed] [Google Scholar]
- 18.Sarma V, Wolf FW, Marks RM, et al. Cloning of a novel tumor necrosis factor-alpha-inducible primary response gene that is differentially expressed in development and capillary tube-like formation in vitro. J Immunol. 1992;148:3302–3312. [PubMed] [Google Scholar]
- 19.Savage KJ, Al-Rajhi N, Voss N, et al. Favorable outcome of primary mediastinal large B-cell lymphoma in a single institution: the British Columbia experience. Ann Oncol. 2006;17:123–130. doi: 10.1093/annonc/mdj030. [DOI] [PubMed] [Google Scholar]
- 20.Savage KJ, Monti S, Kutok JL, et al. The molecular signature of mediastinal large B-cell lymphoma differs from that of other diffuse large B-cell lymphomas and shares features with classical Hodgkin lymphoma. Blood. 2003;102:3871–3879. doi: 10.1182/blood-2003-06-1841. [DOI] [PubMed] [Google Scholar]
- 21.Skinnider BF, Mak TW. The role of cytokines in classical Hodgkin lymphoma. Blood. 2002;99:4283–4297. doi: 10.1182/blood-2002-01-0099. [DOI] [PubMed] [Google Scholar]
- 22.Swerdlow SH, Campo E, Harris NL, et al. Tumours of Haematopoietic and Lymphoid Tissues. Lyon: IARC; 2008. [Google Scholar]
- 23.Willis SN, Stadelmann C, Rodig SJ, et al. Epstein-Barr virus infection is not a characteristic feature of multiple sclerosis brain. Brain. 2009;132:3318–3328. doi: 10.1093/brain/awp200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wolf FW, Marks RM, Sarma V, et al. Characterization of a novel tumor necrosis factor-alpha-induced endothelial primary response gene. J Biol Chem. 1992;267:1317–1326. [PubMed] [Google Scholar]
- 25.Wolf FW, Sarma V, Seldin M, et al. B94, a primary response gene inducible by tumor necrosis factor-alpha, is expressed in developing hematopoietic tissues and the sperm acrosome. J Biol Chem. 1994;269:3633–3640. [PubMed] [Google Scholar]
- 26.Zamo A, Malpeli G, Scarpa A, et al. Expression of TP73L is a helpful diagnostic marker of primary mediastinal large B-cell lymphomas. Mod Pathol. 2005;18:1448–1453. doi: 10.1038/modpathol.3800440. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Thymus (A), Kikuchi’s lymphadenitis (B), lymph node with toxoplasmosis (C) and infectious mononucleosis (D) stained for TNFAIP2 (brown coloration) and showing positive staining of macrophages and follicular and interdigitating dendritic cells and no staining of the reactive lymphocytes. Photomicrographs are 1000× (A–C) or 200× (D) original magnification.