Abstract
Reperfusion of ischemic organs induces a potent inflammatory response initiated by the generation of reactive oxygen species (ROS) that directly damage tissue and promote leukocyte infiltration and activation that also mediate tissue injury. We recently found that radiation-induced tissue injury, which is caused by radiation induced ROS, is attenuated by administration of CBLB502, a pharmacologically optimized derivative of the Toll-like receptor 5 (TLR5) agonist flagellin. Therefore, we tested the ability of CBLB502 to attenuate injury in a murine model of acute ischemic renal failure. CBLB502 given 30 minutes before imposition of bilateral renal pedicle occlusion provided marked protection against the renal dysfunction and inflammation that follows reperfusion of ischemic kidneys, including marked decreases in leukocyte infiltration, proinflammatory cytokine production, and tubular injury. Importantly, CBLB502 given within 30 minutes after ischemic kidney reperfusion reproduced the protective effects of pretreatment with the TLR5 agonist, indicating a window following reperfusion in which CBLB502 administration abrogates acute renal ischemic failure. Bone marrow reconstituted chimeras were used to show that the protective effects of CBLB502 could be delivered by intact MyD88 signaling on renal parenchymal cells. Consistent with this, antibody staining of kidney sections indicated that cells lining the renal vasculature expressed TLR5. Overall, these results indicate the use of TLR5 agonists as mitigators and protectants of acute renal ischemic failure.
Keywords: TLR 5, inflammation, ischemia-reperfusion, neutrophils
Introduction
Reperfusion of organs and other tissues subjected to long periods of ischemia induces an intense inflammatory response that directs leukocyte infiltration with subsequent tissue injury (1–4). Ischemia-reperfusion injury continues to be a major clinical problem causing significant morbidity and mortality in transplantation as well as in other surgeries (5–9). A critical component initiating ischemic injury is the generation of reactive oxygen species (ROS) by endothelial cells during reperfusion (10, 11). These oxygen radicals directly stimulate the endothelium and other cells to produce the chemoattractants IL-8, CXCL1, CXCL2 and complement cleavage products C5a that direct the infiltration and activation of neutrophils, macrophages and in some cases CD4 T cells into the ischemic tissue (12–14). Furthermore, IL-8 and CXCL1 binding to CXCR1 and CXCR2 on neutrophils stimulate the release of granules containing ROS, proteases, and cytokines that mediate tissue damage (15, 16). Strategies either depleting neutrophils prior to reperfusion or inhibiting their infiltration into ischemic tissues during reperfusion have been extremely effective in attenuating tissue injury of ischemic organs in animal models (13, 14, 17–20).
Recent interest is focused on the role of a group of pattern recognition receptors, Toll-like receptors (TLR), expressed on leukocytes and on tissue parenchymal cells as important sensors of infection and tissue damage (21, 22). In addition to microbial-derived ligands, TLR2 and TLR4 also bind molecules released by mammalian cells in response to stress or injury (23–28). These molecules include HMGB1, heat shock proteins, hyaluronan fragments and heparin sulfate. There is accumulating evidence that these TLR agonists activate leukocytes to express inflammatory functions mediating tissue damage in ischemia-reperfusion injury. Several studies have documented the absence or attenuation of injury when ischemia-reperfusion injury is imposed to a variety of organs in mice with targeted deletions in genes encoding TLR2 or TLR4, (29–34). These results implicate TLR2-and TLR4-mediated inflammation as a critical component of the tissue injury induced by acute ischemic injury. Whether other TLRs play a role in promoting or regulating tissue injury in ischemia-reperfusion injury is poorly understood.
Imposition of a short period of ischemia to heart, liver, lung and other organs provides a window of several hours of protection to the imposition of a longer, more severe period of ischemia (35, 36). This ischemic pretreatment effect is mediated through the activation of NF-κB to induce synthesis of protective molecules including heme oxygenase-1 that neutralize ROS and suggests the tissue protective effects induced by a low-level inflammatory insult. We have recently demonstrated that the TLR5 agonist flagellin is a potent radioprotectant and radiomitigator in mouse and primate models (37). The administration of a pharmacologically optimized flagellin derivative named Protectant CBLB502 activated a protective response that decreased ROS accumulation, reminiscent of the protection induced during ischemic pretreatment. These findings led us to test the effect of CBLB502 in a murine model of acute renal ischemic injury. The results indicate that administration of the TLR5 agonist prior to imposition of ischemia or immediately following reperfusion of the ischemic kidney provides protection from acute renal ischemic injury with marked inhibition of neutrophil infiltration during reperfusion and the absence of increases in serum creatinine that are diagnostic markers of renal dysfunction. The results indicate that provision of TLR5 signaling abrogates the production of key mediators that direct the infiltration and activation of neutrophils and other leukocytes into the ischemic organ.
Materials and Methods
Animals
Wild type C57BL/6, B6.MyD88−/− and MOLF mice were purchased from the Jackson Laboratory (Bar Harbor, ME). Adult males 8–12 weeks old were used in these studies. Mice were maintained under pathogen-free conditions according to NIH guidelines and their use was approved by the Cleveland Clinic Institutional Animal Care and Use Committee.
Reagents
TLR5 agonist Protectant CBLB502 was produced by Cleveland BioLabs, Inc. (Buffalo, NY) and was stored frozen in PBS. The structure and TLR5-binding properties of CBLB502 have been described in detail (37). Briefly, CBLB502 is a recombinant protein of Salmonella enterica serovar Dublin flagellin that retains the N- and C-terminal domains responsible for specific binding and activation of TLR5 and is missing the globular domain encoded by a central part of the gene through substitution with a short artificial linker peptide. The resulting protein is half as long and 50 times less immunogenic than native flagellin, while retaining its TLR5-dependent NF-κB-activating capacity. CBLB502 demonstrated strong radioprotective activity when injected i.v., i.m. or s.c. into mice and Rhesus macaques (0.2 and 0.04 mg/kg, respectively) (37). Trace amounts of lipopolysaccharide (LPS) were removed from the purified protein using detoxigel (Pierce, Rockford, IL) according to the manufacturer’s protocol.
Acute renal ischemic injury
Renal ischemia/reperfusion (I/R) injury was performed in mice as previously detailed (13, 38). Briefly, mice were given 20 U sodium heparin i.p. 20 minutes before surgery. The mice were anesthetized with phenobarbital and kept warm under a 60-Watt light bulb until surgery. Under aseptic conditions, the abdominal cavity was opened with a midline incision and the bilateral renal pedicle was occluded non-traumatically with a microvascular clamp (World Precision Instruments, Sarasota, FL). The wound was temporarily closed with 4-0 silk suture and mice were placed on a heat pad under a 60-Watt light bulb with the sensor tip of the Traceable™ Certificate Memory Monitoring Thermometer (Fisher Scientific, Pittsburgh, PA) placed into the abdominal cavity to ensure temperature maintenance at 32°C during the 45-minute ischemic period. After removal of the clamp, immediate and complete renal reperfusion was visually confirmed and the peritoneal cavity was closed. Sham-operated mice were treated in an identical manner except for bilateral clamping of the renal pedicle. Mice were given various doses of CBLB502 in 200 μl of PBS i.v., or PBS alone as a control, at various times before or following ischemia.
Renal function measurement
Sham operated and mice subjected to bilateral renal I/R injury were anesthetized with isofluorane and bled from the postorbital plexus using a heparin-coated microcapillary tube at 24-hour intervals. The serum was stored at −80°C until measurement. Serum creatinine levels were measured using the Creatinine Kit (Sigma Diagnostics, Inc., St. Louis, MO).
Immunohistochemistry
For immunohistochemistry, retrieved kidneys were halved, embedded in OCT compound (Sakura Finetek U.S.A., Torrence, CA), and immediately frozen in liquid nitrogen. Coronal sections were cut (7 μm), mounted onto slides, dried for 1 hour, and then fixed in acetone for 10 minutes. Slides were immersed in PBS for 10 minutes and in 3% hydrogen peroxide/methanol for 5 minutes at room temperature to eliminate endogenous peroxidase activity. Endogenous biotin activity was blocked with the Biotin Blocking System (DAKO, Carpentaria, CA). After treating with normal rat serum (1:100), anti-mouse Gr-1 mAb (RB6.8C5) diluted 1:100 in PBS with 1% bovine serum albumin (BSA) to detect neutrophils, or 1:50 dilutions of rat anti-mouse CD4 mAb (GK1.5) to detect CD4+ T cells, rat anti-mouse CD8α mAb (53-6.7) to detect CD8+ T cells, or rat anti-mouse macrophage (F4/80) mAb (SEROTEC, Raleigh, NC) was added to the sections. Control slides were incubated with rat IgG. After 1 hour, slides were washed 3X with PBS and incubated for 20 minutes with biotinylated rabbit anti-rat IgG antiserum (Sigma Aldrich) diluted 1:100 in PBS/1% BSA. After 3 washes in PBS, slides were incubated with streptavidin-horseradish peroxidase (DAKO) for 20 min. The DAB (3,3′-diaminobenzidine) substrate-chromagen solution (Vector Laboratories, Inc., Burlingame, CA) was applied to the slides for 0.5–3 minutes. After rinsing in dH2O, slides were counterstained with hematoxylin, washed with dH2O, cover-slipped, and viewed by light microscopy. Images were captured using Image Pro Plus (Media Cybernetics, Silver Spring, MD).
To stain TLR5, 1 μg of anti-TLR5 mAb (ABR-Affinity BioReagents, Inc., Golden, CO) was applied to slides and incubated for 1 hour at room temperature and after washing, biotinylated goat anti-mouse IgG antibody diluted 1:100 was applied to the slides for 30 minutes at room temperature. After applying the DAB, the slides were washed with tap water, dipped for 3 seconds in hematoxylin and then washed. The slides were dehydrated with increasing concentrations of ethanol to 50% and then immersed in citrasolve twice for 10 minutes each. The slides were washed with tap water, cover-slipped, and viewed by light microscopy.
Quantification of leukocyte populations in kidneys
To directly determine the number of neutrophils, macrophages, CD4+ T cells and CD8+ T cells infiltrating ischemic kidneys during reperfusion, one quarter pieces of the retrieved kidney were cut and weighed. Flow cytometry detection of infiltrating cells was performed using a modification of the method of Afanasysev and colleagues (39). Briefly, the weighed kidneys were incubated in 37°C in RPMI 1640 culture medium with 20 mg/ml Type II collagenase (Sigma, St. Louis, MO) for 1 hour and then were pushed through a 70 μm cell strainer using a syringe plunger. The cells were collected and the erythrocytes lysed using ACK Lysing Buffer (GIBCO, Grand island, NY). After 2 washes, viable cells were counted using Trypan blue exclusion. Aliquots of the cells were preincubated with anti-CD16/CD32 Fc receptor antibody (BD Pharmingen, San Diego, CA) for 5 minutes to block nonspecific antibody binding and then samples were incubated with FITC-conjugated anti-CD45 mAb as well as PE-conjugated antibody to detect macrophages (F4/80) or CD8+ T cells (53-6.7) and APC-conjugated antibody to detect neutrophils (RB6.8C5) or CD4+ T cells (GK1.5) (all antibodies from BD Pharmingen) for 30 minutes at 4°C. Cells were analyzed using two-color flow cytometry on a FACSCalibur (BD Biosciences, San Jose, CA). The forward scatter and FL1 (CD45+) channels were used to gate the leukocytes followed by analysis of the specific leukocyte populations. For each sample, 200,000 events were collected. The data were analyzed using CellQuest software (BD Biosciences). Total numbers of each leukocyte population were calculated by: (the total number of viable leukocytes counted) × (% of the leukocyte population counted in the CD45+ cells)/100. The data are reported as number of each leukocyte population/g kidney tissue from sham control and experimental ischemic animals.
RNA extraction and quantitative analysis of chemokines in kidney
One-quarter pieces were cut from harvested kidneys and frozen in liquid nitrogen. Total tissue RNA was extracted using the RNeasy™ Mini Kit (QIAGEN, Valencia, CA) and reverse transcribed using the High-Capacity cDNA Archive Kit (Applied Biosystems, Foster City, CA). Real time PCR was performed on a Prism 7700 Sequence Detection System (Applied Biosystems, Foster City, CA) with test KC/CXCL1, MIP-2/CXCL2 and MCP-1/CCL2 primers and Mrpl32 used as the control (Applied Biosystems, Foster City, CA).
Analysis of tissue chemokine protein levels
Kidneys samples stored in liquid nitrogen were dissolved in 500 μl of PBS with 0.01 M EDTA and a proteinase inhibitor cocktail (10 μg/ml phenylmethyl solfonyl fluoride, 2 μg/ml aprotinin, 2 μg/ml leupeptin, 100 μg/ml Pefabloc SC, and 100 μg/ml chymostatin), and then 1 ml of 1.5% Triton X-100 in PBS was added. After incubation with agitation for 1 hour at 4°C, samples were centrifuged, the supernatant was collected, and the total protein concentration was determined using the BCA™ Protein Assay Kit (Pierce, Rockford, IL). KC/CXCL1, MIP-2/CXCL2 and MCP-1/CCL2 concentrations were measured by sandwich ELISA using Quantikine M Kits (R&D Systems, Minneapolis, MN). To determine the activation of neutrophils during reperfusion of ischemic kidneys, the concentration of myeloperoxidase (MPO) was measured using the Mouse MPO ELISA test kit (Cell Sciences, Canton, MA). Results are reported as concentration of test protein per mg of total tissue protein ±SD.
Generation of reciprocal bone marrow reconstituted chimeras
The tips of femurs and tibias were cut and then flushed with RPMI 1640 and the bone marrow cells collected. Bone marrow recipient mice first received 1100 Rad γ-irradiation and then 3 hours later received 20 × 106 bone marrow cells i.v. Irradiated CD90.1 recipients received bone marrow from congenic CD90.1 donors or vice versa. The reconstituted recipients received antibiotics (0.2 mg/ml sulfamethoxazole and 0.4 mg/ml trimethoprim) in the drinking water from day −1 to 7 for microbial prophylaxis. The recipients were allowed to recover for 8–12 weeks. CD90.1 and CD90.2 expression was measured on peripheral blood leukocytes 8 weeks after bone marrow reconstitution by staining aliquots of the cells with FITC-conjugated 90.2 mAb and PE-conjugated 90.1 mAb to assess chimerism, which was ≥ 85% donor bone marrow derived cells in all reconstituted recipients.
Statistical analyses
Significant differences in survival of animals subjected to renal I/R injury were determined by Kaplan-Meier survival analysis. Quantitation of infiltrating cells and levels of cytokine mRNA expression and protein production are reported as mean ±SD for each group of 4–6 animals and the significance of differences in means were analyzed by the Mann-Whitney U test using Prism software (Graph Pad Software, San Diego, CA). A value of P < 0.05 was considered a significant difference in means between groups.
Results
Dose-dependent effect of CBLB502 on acute renal injury
The initial experiments tested the effect of CBLB502 administration given prior to imposition of renal ischemia. Mice were given various doses of CBLB502 i.v. and 30 minutes later were subjected to 45 minutes of bilateral renal pedicle occlusion. In the control group given PBS alone, 80% of the animals expired within 5 days following reperfusion of the ischemic kidneys (Figure 1a). All animals given 5 μg of CBLB502 before imposition of renal ischemia also expired within 5 days following reperfusion. In contrast, all animals given either 1 or 0.5 μg of CBLB502 before renal ischemia survived more than 45 days after which they were sacrificed for histopathological examination. The protective effect of CBLB502 in acute renal ischemia was dose-dependent in that animals given 0.1 or 0.01 μg were not protected against the injury.
FIGURE 1.
CBLB502 given prior to imposition of renal ischemia protects against acute renal ischemic injury. Groups of 5 C57BL/6 mice were subjected to bilateral occlusion of renal pedicles for 45 minutes with one group serving as the sham control. Groups of the mice were given PBS or the indicated dose of CBLB502 i.v. 30 minutes before imposing renal ischemia. (a) The viability of the animals was monitored following reperfusion. (b) The serum creatinine levels at 24 hours post-reperfusion was determined. *P < 0.05
The protective effect was reflected by the low serum creatinine levels 24 hours post-reperfusion in animals given 1.25 or 0.5 μg CBLB502 before renal ischemia (Figure 1b). Animals given the non-protective low doses of CBLB502 (0.1 and 0.01 μg) had serum creatinine levels that were near those observed in the control PBS-treated group.
The use of serum creatinine levels as an indication of renal dysfunction during I/R injury was supported by the histopathology of the ischemic kidneys from animals treated with CBLB502 and surviving control animals 7 days after reperfusion (Figure 2). Surviving control group animals given PBS 30 minutes before imposition of renal ischemia had severe tubular necrosis with caste formation evident and intense leukocytic infiltration. Ischemic kidneys from animals given 0.5 μg CBLB502 30 minutes before ischemia had low levels of infiltrating leukocytes 7 days after reperfusion with relatively normal renal architecture. It was noteworthy that administration of 0.5 μg CBLB502 to sham-operated control animals had no noticeable effect on renal architecture 7 days later.
FIGURE 2.
CBLB502 attenuates leukocytic infiltration and tubular necrosis when given prior to imposition of renal ischemia. Groups of animals were given PBS or 0.5 μg CBLB502 i.v. 30 minutes prior to imposition of bilateral renal pedicle occlusion. A group of animals was also given 0.5 μg CBLB502 and then sham operated. After 45 minutes, the ischemic kidneys were reperfused and were harvested 7 days later. Prepared sections were stained with hematoxylin and representative sections are shown. Magnification, x100.
Neutrophil infiltration and activation reaches peak levels 9–24 hours after reperfusion of ischemic kidneys and is a major mediator of the tissue injury (13). Ischemic kidneys were harvested 9 and 24 hours after reperfusion from animals treated with PBS or 0.5 μg CBLB502 before imposition of ischemia and neutrophil infiltration was assessed by immunohistochemical staining of prepared tissue sections. Marked decreases in neutrophil infiltration into ischemic kidneys were observed both 9 and 24 hours after reperfusion in animals given 0.5 μg CBLB502 (Figure 3).
FIGURE 3.
CBLB502 given prior to imposition of renal ischemia attenuates neutrophil infiltration into ischemic kidneys during reperfusion. Groups of C57BL/6 mice were given PBS or 0.5 μg CBLB502 i.v. 30 minutes prior to imposition of bilateral renal pedicle occlusion. A group of animals was also given PBS and then sham operated. After 45 minutes, the ischemic kidneys were reperfused and were harvested 9 and 24 hours later. Prepared sections were stained with anti-Gr1 mAb to detect neutrophils and representative sections are shown. Magnification, x200.
Direct quantitation of infiltrating leukocyte populations into the ischemic kidneys indicated that 0.5 μg CBLB502 reduced neutrophil infiltration almost to the levels observed in the sham-operated control animals (Figure 4a). The decreased neutrophil infiltration into ischemic kidneys in animals pretreated with 0.5 μg CBLB502 was accompanied by significant decreases in the amount of myeloperoxidase produced 24 hours after reperfusion of the ischemic kidneys (Figure 4b). Decreased CD4 and CD8 T cells and macrophages were observed in ischemic kidneys 24 hours after reperfusion and administration of 0.5 μg of CBLB502 30 minutes before ischemia further decreased the numbers of both CD4 and CD8 T cells (Figure 4a). The effect of renal ischemia/reperfusion on neutrophil numbers in other tissues was also examined. While 10–12 fold increases in neutrophil numbers were observed in the ischemic kidney 24 hours after reperfusion, a 2 fold increase was observed in the spleen and treatment with CBLB502 had a modest effect in decreasing the numbers of neutrophils in the spleen (Table 1). Renal ischemia/reperfusion injury did not provoke an increase in neutrophil numbers in the lung.
Figure 4.
CBLB502 given prior to imposition of renal ischemia attenuates neutrophil but not macrophage infiltration into ischemic kidneys during reperfusion. Groups of 5 C57BL/6 mice were given PBS or 0.5 μg CBLB502 i.v. 30 minutes prior to imposition of bilateral renal pedicle occlusion for 45 minutes. (a) Kidneys were harvested 24 hours after reperfusion from each animal, were digested, and the infiltrating leukocyte populations were quantitated following antibody staining and flow cytometry analyses to detect neutrophils, macrophages, CD4 T cells and CD8 T cells. (b) Kidneys were harvested 9 and 24 hours after reperfusion and prepared tissue homogenates were tested for levels of myeloperoxidase protein. *P < 0.05
Table I.
Neutrophil infiltration into various organs following renal ischemia-reperfusion injury
| # neutrophils/mg tissue | |||||
|---|---|---|---|---|---|
| Group | Pre-treatment | Renal Injury | Kidney | Lung | Spleen |
| 1 | PBS | Sham | 167 ±60 | 71 ±67 | 21518 ±7425 |
| 2 | CBLB502 | Sham | 199 ±59 | 60 ±25 | 18526 ±11784 |
| 3 | PBS | I/R | 2093 ±481 | 79 ±32 | 44452 ±7452 |
| 4 | CBLB502 | I/R | 1158 ±315 | 58 ±41 | 33494 ±7286 |
Groups of 5 C57BL/6 mice were subjected to bilateral occlusion of renal pedicles for 45 minutes with one group serving as the sham control. Groups of the mice were given PBS or 1 μg of CBLB502 i.v. 30 minutes before imposing renal ischemia. Kidneys, lungs and spleens were harvested 24 hours after reperfusion from each animal, were digested, and the infiltrating neutrophils were quantitated following staining with anti-Gr-1 antibody and flow cytometry analyses. Data are indicated as number of neutrophils per mg of target tissue ±standard deviation.
TLR5 agonist decreases pro-inflammatory cytokines during reperfusion of ischemic kidneys
Peak levels of the neutrophil chemoattractants CXCL1 and CXCL2 are induced in ischemic kidneys at 9 hours post-reperfusion and direct neutrophils into the kidneys during acute ischemic injury (13). To begin to investigate mechanisms underlying the CBLB502-mediated decrease in neutrophil infiltration into ischemic kidneys, kidneys were harvested 9 and 24 hours after reperfusion and the mRNA and protein levels of neutrophil and macrophage chemoattractants were determined (Figure 5). Administration of protective doses of CBLB502 (1.25 or 0.5 μg) resulted in significant decreases in mRNA expression and protein levels of CXCL1 and CXCL2 at 9 hours post-reperfusion. Expression of CCL2 mRNA and protein levels were low both 9 and 24 hours after reperfusion and were not further influenced by pretreatment with CBLB502. mRNA levels of the acute phase proteins IL-1β and IL-6 but not TNFα were also significantly decreased in ischemic kidneys at 9 hours post-reperfusion in CBLB502-treated animals (Figure 6).
FIGURE 5.
CBLB502 given prior to imposition of renal ischemia attenuates production of neutrophil chemoattractant chemokines during reperfusion. Groups of 5 C57BL/6 mice were given PBS or 0.5 μg CBLB502 i.v. 30 minutes prior to imposition of bilateral renal pedicle occlusion or sham operation. After 45 minutes, the ischemic kidneys were reperfused and were harvested 9 and 24 hours later and tissue homogenates prepared. In the upper panels, whole cell RNA was isolated and tested by qRT/PCR for expression levels of neutrophil (CXCL1 and CXCL2) and macrophage (CCL2) chemoattractant chemokines. In the lower panels, aliquots of tissue homogenates were tested for levels of chemokine protein by ELISA.
Figure 6.
Effect of CBLB502 given prior to imposition of renal ischemia on expression of acute phase cytokines during reperfusion. Groups of 5 C57BL/6 mice were given PBS or 0.5μg CBLB502 i.v. 30 minutes prior to imposition of bilateral renal pedicle occlusion for 45 minutes. Whole cell RNA was isolated from individual kidneys 1, 9 and 24 hours post-reperfusion and tested by qRT/PCR for expression levels of the acute phase cytokines TNFα, IL-1βand IL-6. *P < 0.05.
Protective effect of TLR5 stimulation during reperfusion of ischemic kidneys
The use of CBLB502 given prior to imposition of ischemia to prevent acute ischemic renal failure in settings of established ischemia is likely to have limited applicability. Therefore, we tested the protective effect of TLR5 stimulation with CBLB502 in acute ischemic injury of kidneys when the agent was given shortly after initiation of reperfusion. Groups of mice were subjected to 45 minutes of bilateral renal pedicle occlusion and were administered 0.5 μg of CBLB502 at various times following declamping. CBLB502 given 30 minutes before bipedicle renal clamping or within 30 minutes after declamping rescued the viability of all mice subjected to the ischemic injury (Figure 7a). CBLB502 given 1 hour or later after initiation of reperfusion failed to rescue any of the mice from the injury. The protective effect of CBLB502 given 30 minutes before declamping or within 30 minutes following declamping was reflected by the low levels of serum creatinine monitored 24 hours after reperfusion of the ischemic kidneys (Figure 7b). The protective effect of CBLB502 given within 30 minutes of reperfusion to mice with renal ischemia was accompanied by significant decreases in inflammatory gene expression in kidneys harvested at 9 hours after reperfusion, including the neutrophil chemoattractants CXCL1 and CXCL2 (Figure 8a) and hemoxygenase-1 and IL-10 (Figure 8b).
FIGURE 7.
CBLB502 given after initiation of reperfusion of ischemic kidneys protects against acute renal ischemic injury. Groups of 5 C57BL/6 mice were subjected to bilateral occlusion of renal pedicles for 45 minutes with one group serving as the sham control. Groups of the mice were given PBS or 0.5 μg of CBLB502 i.v. at the indicated time before or after reperfusion. (a) The viability of the animals was monitored following reperfusion. (b) The serum creatinine levels at 24 hours post-reperfusion was determined.
Figure 8.
CBLB502 given after initiation of reperfusion of ischemic kidneys attenuates the expression of inflammatory components. Groups of 5 C57BL/6 mice were subjected to bilateral occlusion of renal pedicles for 45 minutes with groups given PBS or 0.5 μg of CBLB502 i.v. 30 minutes after reperfusion. (a) Kidneys were harvested 9 and 24 hours after reperfusion and aliquots of tissue homogenates tested for levels of CXCL1 and CXCL2 protein by ELISA. *p < 0.05. (b) The ischemic kidneys from groups of 5 mice given PBS or 0.5 μg CBLB502 30 minutes after reperfusion were harvested 9 hours later and whole cell RNA was isolated and tested by qRT/PCR for expression levels of HO-1 and IL-10. **P < 0.01.
CBLB502 protection requires TLR5 signaling on renal parenchymal cells
It was unclear whether the protection of ischemic kidneys provided by CBLB502 was mediated through effects on infiltrating leukocytes or on parenchymal cells in the kidney. This was addressed by investigating the target source of the protective effect of CBLB502 treatment during reperfusion of ischemic kidneys. Since signaling through TLR5 is dependent upon the function of MyD88, reciprocal radiation-induced/bone marrow reconstituted chimeras were generated between wild-type C57BL/6 mice and B6.MyD88−/−mice, incapable of conducting signals from engaged TLR5 (40).
In preliminary studies, the chimeras were subjected to bilateral renal pedicle occlusion eight weeks after bone marrow reconstitution and given either 0.5 μg CBLB502 or PBS within 30 minutes of reperfusion. Whereas 80% of irradiated MyD88−/− recipients of wild-type bone marrow given CBLB502 during reperfusion expired within 5 days, all irradiated wild-type recipients of MyD88−/− bone marrow given CBLB502 survived more than 15 days (data not shown). In addition, 50% of the animals in each of the groups of chimeras subjected to renal ischemia and then given PBS during reperfusion expired within 3 days.
These studies were extended by generating another set of chimeras and eight weeks after bone marrow reconstitution the chimeras were subjected to bilateral renal pedicle occlusion and given either 0.5 μg CBLB502 or PBS within 30 minutes of reperfusion. The ischemic kidneys were harvested 9 hours after reperfusion to test the induction of CXCL1 and CXCL2 (Figure 9a and b). Consistent with previous results, sham surgery did not induce expression of CXCL1 or CXCL2 in the kidneys of any of the chimeras. In wild-type recipients reconstituted with wild-type bone marrow, administration of 0.5 μg CBLB502 within 30 minutes of reperfusion of ischemic kidneys significantly decreased CXCL1 and CXCL2 mRNA levels, indicating the ability of treatment with the TLR5 agonist to inhibit ischemia/reperfusion induced expression of these chemokines. In irradiated MyD88−/− recipients reconstituted with either MyD88−/− or wild-type bone marrow, low levels of CXCL1 and CXCL2 mRNA was induced during reperfusion of ischemic kidneys indicating the need for MyD88 signaling in kidney parenchymal cells for optima production of these chemokines during reperfusion of the ischemic kidneys. Nevertheless, administration of CBLB502 during reperfusion of these kidneys did not further decrease the mRNA levels of these chemokines. In contrast, ischemic kidneys from wild-type recipients of MyD88−/− bone marrow expressed high levels of CXCL1 and CXCL2 mRNA following reperfusion and these levels were significantly decreased by administration of CBLB502 during reperfusion of the ischemic kidneys. Together these results indicated that the protective effect of CBLB502 in attenuating injury during reperfusion of ischemic kidneys was delivered when TLR5 signaling was intact on wild-type radiation resistant parenchymal cells rather than on bone marrow derived cells.
FIGURE 9.
Reciprocal bone marrow reconstituted chimeras were generated between wild-type C57BL/6 and B6.MyD88−/− mice. Six weeks after the bone marrow transplants, groups of 4 chimeras were subjected to bilateral occlusion of renal pedicles for 45 minutes with two groups serving as sham controls. Groups of the mice were given PBS or 0.5 μg of CBLB502 i.v. 30 minutes after reperfusion of the ischemic kidneys. The ischemic kidneys were harvested 9 hours after reperfusion, whole cell RNA was isolated, and expression levels of CXCL1 and CXCL2 were tested by qRT/PCR.
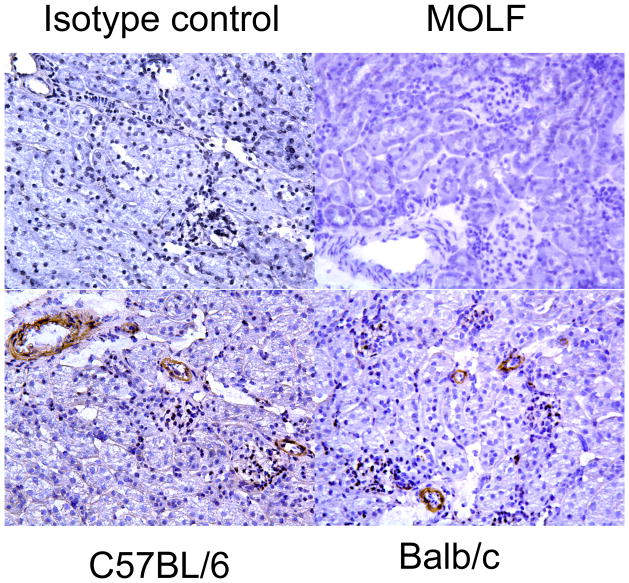
To identify cells that are likely to be targets of CBLB502, we used immunohistochemistry to detect TLR5 expressing cells in normal mouse kidneys. Renal sections from wild type C57BL/6 and BALB/c mice were stained with anti-TLR5 antibody (Figure 10). The cells staining positively were primarily cells in the vasculature and staining was not apparent on renal tubular cells or in glomeruli. As a further control, kidney sections from Moth Eaten mice that have a genetic defect in the expression of TLR 5 (41) did not stain with the anti-TLR5 antibody.
FIGURE 10.
Expression of TLR5 in cells lining the renal vasculature. Kidney sections from C57BL/6, BALB/c and TLR5-deficient MOLF mice were stained to detect TLR5. Representative sections are shown. Magnification, x200.
Discussion
Reperfusion of ischemic organs stimulates the activation of vascular endothelium and other tissue cells to express adhesion molecules and to produce proinflammatory cytokines including TNFα, IL-1, and neutrophil chemoattractant chemokines. Reperfusion of ischemic tissues also induces the rapid production and release of stress proteins and other mediators from injured cells and extracellular matrix components including HMGB1, Hsp 70, hyaluronan fragments, and heparin sulfate (23–28). These molecules are endogenous ligands for TLR2 or TLR4 implicating an important role for the TLR system and MyD88 signaling in mediating inflammation in ischemia-reperfusion injury and other tissue trauma (42, 43). In support of these roles, both TLR2−/− and TLR4−/− mice had attenuated renal ischemic injury including decreased serum creatinine levels, leukocyte infiltration and tubular injury when compared to wild type mice (31, 33, 34).
The current studies indicate that provision of TLR5 signaling can provide protection to acute renal ischemic injury. CBLB502 given 30 minutes before imposition of bilateral renal ischemia resulted in marked decreases in the production of CXCL1 and CXCL2 and in neutrophil infiltration that was accompanied by decreased tubular injury. In addition to the decreased production of the neutrophil chemoattractants, down regulation of HO-1 and IL-10 were also observed during the TLR5 agonist attenuation of reperfusion-induced inflammation. Although HO-1 and IL-10 are considered to be anti-inflammatory mediators, the attenuation of neutrophil infiltration and inflammatory cytokines is likely to have influenced the induction of HO-1 and IL-10 during reperfusion. Importantly, the results also indicate that the protection provided by CBLB502 is as effective when the TLR5 agonist is given before or within 30 minutes after the initiation of reperfusion suggesting a potential clinical use for this strategy in attenuating tissue damage from renal ischemia during trauma, surgery or transplantation. It is important to note that the therapeutic dose window providing this protection to reperfusion of ischemic kidneys is quite narrow. While the results of this study provide proof of principle that a TLR5 agonist can provide protection against ischemic renal failure, further development of the reagent and its administration will be necessary if this approach is to be carried into future clinical use.
Similar to the post-reperfusion use of the TLR5 agonist, pretreatment with the TLR4 agonist LPS or with the TLR9 agonist CpG oligonucleotides can reduce tissue damage following ischemic brain and gut injury and acute cardiac ischemia in mouse models (44–46). The protection provided by LPS pretreatment was not observed in TNFR1−/− mice in the gut model or when TNFα was neutralized with antibody during preconditioning in the brain model. The mechanisms underlying the role of TNFα in the protective ischemic pretreatment with LPS or the TLR9 agonist in these models remain undefined. It is worth noting that CBLB502 treatment did attenuate increases in IL-1 and IL-6 but not TNFα mRNA levels during the ischemic injury suggesting TNFα may also be required for the protective effect of the TLR5 agonist in acute renal ischemic injury. In other models TNFα induced NF-κB activation increases expression of Bcl-2 and MnSOD and such NF-κB induced cell protective mechanisms could conceivably contribute to the decreased tubular injury observed following TLR5 triggering in the current study (51, 52).
There are many similarities in the molecular and cellular paths leading to tissue failure after ischemia-reperfusion and exposure to toxic doses of ionizing radiation. Both generate accumulation of ROS that serve as primary mediators of acute pathologies. In both situations, the vascular endothelium plays a primary role in the development of tissue damage with involvement as a direct target for injury (e.g., apoptosis) as well as a mediator of the inflammatory response (47, 48). Importantly, stimulation of TLR5 signaling by CBLB502, the derivative of bacterial flagellin, has strong protective effects on radiation-induced injury when applied before or shortly after irradiation (37). It acts by combining at least three protective mechanisms, all mediated by a number of NF-κB-responsive proteins that cause direct suppression of apoptosis (IAPs and Bcl2), scavenge ROS (superoxide dismutase 2 and ferritine) and promote tissue regeneration (cytokines KGF and G-CSF). All of these effects could play tissue protective roles in renal ischemia-reperfusion as well.
Another important property of the TLR5-induced response is the low toxicity of TLR5 agonists at efficacious protective doses. While the effects of agonists of other TLRs are usually seen at doses close to maximal tolerated ones, the efficacious doses of CBLB502 (0.5–1.0 μg) in attenuating renal ischemia-reperfusion injury are less than 0.25% of the maximal tolerated dose, 1.25 μg for a 20 g animal (37). Molecular and cellular mechanisms underlying such significant differences among TLR agonists remain to be determined. The current results are the first to our knowledge to indicate protection from acute renal ischemic failure through the administration of a TLR5 agonist and the first to demonstrate the ability to provide this protection by giving a TLR agonist during reperfusion of the ischemic kidney.
The protection provided by administration of CBLB502 and other TLR agonists in acute renal ischemic injury indicates the presence of these receptors in the kidney and other organs and the targeting of these receptors to obtain this protective effect. Several studies have documented the presence of TLR4 and TLR2 on renal epithelial tubule and mesangial cells and TLR2 on endothelial cells in the renal vasculature (31, 33, 34, 49). Kidney interstitial dendritic cells and glomerular macrophages also express a variety of TLRs including TLR-1, -2, -4, -6, -7, -8, and –9 (50). The current report indicates TLR5 expression on the lining of cells in the renal vasculature but not on other cells in the kidney. This is supported by experiments using irradiation-induced/bone marrow-reconstituted chimeras to show the protective effect of the flagellin requires MyD88 signaling on stromal cells and not bone marrow-derived cells. A similar strategy was used to demonstrate that TLR2 and TLR4 expression on stromal cells in the kidney was required for the injury induced by renal ischemia-reperfusion (31, 34). It is worth noting that both TLR2 and TLR4 as well as IL-1 receptor signaling is functional on renal parenchymal cells in the MyD88−/− bone marrow → irradiated wild-type recipient chimeras and are likely to mediate at least a portion of the inflammation induced by ischemia injury (e.g. the increased CXCL1 and CXCL2) but these inflammatory events are attenuated by TLR5 agonism in these chimeras.
The vascular expression of TLR5 places this receptor at the point at which the inflammation initiated by reperfusion of the ischemic kidney begins. In the radiation protection model the TLR5 agonist rescued the endothelial cells of the small intestine from radiation-induced apoptosis (37), believed to be one of the major sources of intestinal damage by radiation (48). While this mechanism may also play a role in protection from ischemia-reperfusion injury, activation of TLR5 in the renal endothelium also prevents the endothelial cells from triggering an acute inflammatory response. More importantly, expression of TLR5 was not observed on renal tubular epithelial or mesangial cells so that the protective effect mediated through CBLB502 binding to TLR5 on endothelial cells is likely to attenuate the inflammatory effects of ligand induced signaling through TLRs, e.g. TLR2 and TLR4, expressed on these other cells in the kidney.
Overall the results of this study indicate the ability of low doses of the TLR5 agonist CBLB502 to attenuate acute renal ischemic injury and failure. The ability to administer CBLB502 immediately following reperfusion of the ischemic kidney suggests its potential use during reperfusion of transplanted organs as well as to treat other ischemic conditions during surgery.
Acknowledgments
We thank the staff of the Cleveland Clinic Biological Resources Unit for excellent care of the animals used in this study. This work was supported by grants from Cleveland Biolabs, Inc. to R.L.F. and A.V.G., USAMRMC 06137011 from the Department of Defense to R.L.F. and A.V.G. and grant AI080446 from the NIH to A.V.G. and grant AI40459 from the NIH to R.L.F.
Footnotes
This work was supported by grants from Cleveland BioLabs, Inc. to R.L.F. and A.V.G., USAMRMC 06137011 from the Department of Defense to R.L.F. and A.V.G., grant AI080446 from the NIH to A.V.G., and grant AI40459 from the NIH to RLF
Disclosures
AVG and RLF have consulting relationships with Cleveland BioLabs, Inc.
References
- 1.Bonventre JV, Zuk A. Ischemic renal failure: an inflammatory disease? Kidney Int. 2004;66:480–485. doi: 10.1111/j.1523-1755.2004.761_2.x. [DOI] [PubMed] [Google Scholar]
- 2.Carden DL, Granger DN. Pathophysiology of ischaemia-reperfusion injury. J Pathol. 2000;190:255–266. doi: 10.1002/(SICI)1096-9896(200002)190:3<255::AID-PATH526>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 3.de Groot H, Rauen U. Ischemia-reperfusion injury: Processes in pathogenetic networks; A review. Transplant Proc. 2007;39:481–484. doi: 10.1016/j.transproceed.2006.12.012. [DOI] [PubMed] [Google Scholar]
- 4.Rabb H, O’Meara YM, Maderna P, Coleman P, Brady HR. Leukocytes, cell adhesion molecules and ischemic acute renal failure. Kidney Int. 1997;51:1463–1468. doi: 10.1038/ki.1997.200. [DOI] [PubMed] [Google Scholar]
- 5.Koo DD, Welsh KI, Roake JA, Morris PJ, Fuggle SV. Ischemia/reperfusion injury in human kidney transplantation: an immunohistochemical analysis of changes after reperfusion. Am J Pathol. 1998;153:557–576. doi: 10.1016/S0002-9440(10)65598-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Land W, Messmer K. The impact of ischemia/reperfusion injury on specific and non-specific, early and late chronic events after organ transplantation. Transplant Rev. 1996;10:236–253. [Google Scholar]
- 7.Ojo AO, Wolfe RA, Held PJ, Port FK, Schmouder RL. Delayed graft function: risk factors and implications for renal allograft survival. Transplantation. 1997;63:968–974. doi: 10.1097/00007890-199704150-00011. [DOI] [PubMed] [Google Scholar]
- 8.Terasaki PI, Cecka JM, Gjertson DW, Takemoto S. High survival rates of kidney transplants from spousal and living unrelated donors. N Eng J Med. 1995;333:333–336. doi: 10.1056/NEJM199508103330601. [DOI] [PubMed] [Google Scholar]
- 9.Thadhani R, Pascual M, Bonventre JV. Acute renal failure. N Engl J Med. 1996;334:1448–1460. doi: 10.1056/NEJM199605303342207. [DOI] [PubMed] [Google Scholar]
- 10.Devarajan P. Update on mechanisms of ischemic acute kidney injury. J Am Soc Nephrol. 2006;17:1503–1520. doi: 10.1681/ASN.2006010017. [DOI] [PubMed] [Google Scholar]
- 11.Lefer AM, Tsao PS, Lefer DJ, Ma XL. Role of endothelial dysfunction in the pathogenesis of reperfusion injury after myocardial ischemia. FASEB J. 1991;5:2029–2034. doi: 10.1096/fasebj.5.7.2010056. [DOI] [PubMed] [Google Scholar]
- 12.Linas SL, Whittenburg D, Parsons PE, Repine JE. Ischemia increases neutrophil retention and worsens acute renal failure: role of oxygen metabolites and ICAM-1. Kidney Int. 1995;48:1584–1591. doi: 10.1038/ki.1995.451. [DOI] [PubMed] [Google Scholar]
- 13.Miura M, Fu X, Zhang QW, Remick DG, Fairchild RL. Neutralization of Groα and macrophage inflammatory protein-2 attenuates renal ischemia/reperfusion injury. Am J Pathol. 2001;159:2137–2145. doi: 10.1016/s0002-9440(10)63065-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Takada M, Nadeau KC, Shaw GD, Marquette KA, Tilney NL. The cytokine-adhesion molecule cascade in ischemia/reperfusion injury of the rat kidney. J Clin Invest. 1997;99:2682–2690. doi: 10.1172/JCI119457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baggiolini M, Walz A, Kunkel SL. Neutrophil-activating peptide-1/interleukin-8, a novel cytokine that activates neutrophils. J Clin Invest. 1989;84:1045–1049. doi: 10.1172/JCI114265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jaeschke H, Smith CW. Mechanisms of neutrophil-induced parenchymal cell injury. J Leukoc Biol. 1997;61:647–653. doi: 10.1002/jlb.61.6.647. [DOI] [PubMed] [Google Scholar]
- 17.Colletti LM, Kunkel SL, Walz A, Burdick MD, Kunkel RG, Wilke CA, Strieter RM. Chemokine expression during hepatic ischemia/reperfusion-induced lung injury in the rat. The role of epithelial neutrophil activating protein. J Clin Invest. 1995;95:134–141. doi: 10.1172/JCI117630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cugini D, Azzollini N, Gagliardini E, Cassis P, Bertini R, Colotta F, Norris M, Remuzzi G, Benigni A. Inhibition of the chemokine receptor CXCR2 prevents kidney graft function deterioration due to ischemia/reperfusion injury. Kidney Int. 2005;67:1753–1761. doi: 10.1111/j.1523-1755.2005.00272.x. [DOI] [PubMed] [Google Scholar]
- 19.Klausner JM, I, Paterson S, Goldman G, Kobzik L, Rodzen C, Lawrence R, Valeri CR, Shepro D, Hechtman HB. Postischemic renal injury is mediated by neutrophils and leukotrienes. Am J Physiol. 1989;256:F794–F802. doi: 10.1152/ajprenal.1989.256.5.F794. [DOI] [PubMed] [Google Scholar]
- 20.Seekamp A, Mulligan MS, Till GO, Ward PA. Requirements for neutrophil products and L-arginine in ischemia-reperfusion injury. Am J Pathol. 1993;142:1217–1226. [PMC free article] [PubMed] [Google Scholar]
- 21.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 22.Medzhitov R. Recognition of microorganisms and activation of the immune response. Nature. 2007;449:819–826. doi: 10.1038/nature06246. [DOI] [PubMed] [Google Scholar]
- 23.Johnson GB, Brunn GJ, Kodaira Y, Platt JL. Receptor-mediated monitoring of tissue well-being via detection of soluble heparin sulfate by Toll-like receptor 4. J Immunol. 2002;168:5233–5239. doi: 10.4049/jimmunol.168.10.5233. [DOI] [PubMed] [Google Scholar]
- 24.Park JS, Svetkauskaite D, He Q, Kim JY, Strassheim K, Ishizaka A, Abraham E. Involvement of toll-like receptors 2 and 4 in cellular activation by high mobility group box 1 protein. J Biol Chem. 2004;279:7370–7377. doi: 10.1074/jbc.M306793200. [DOI] [PubMed] [Google Scholar]
- 25.Smiley ST, King JA, Hancock WW. Fibrinogen stimulates macrophage chemokine secretion through Toll-like receptor 4. J Immunol. 2001;167:2887–2894. doi: 10.4049/jimmunol.167.5.2887. [DOI] [PubMed] [Google Scholar]
- 26.Tsung A, Klune JR, Zhang Z, Jeyabalan G, Cao Z, Peng X, Stolz DB, Geller DA, Rosengart MR, Billiar TR. HMGB1 release induced by liver ischemia involves Toll-like receptor 4-dependent reactive oxygen species production and calcium-mediated signaling. J Exp Med. 2007;204:2913–2923. doi: 10.1084/jem.20070247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tsung A, Sahai R, Tanaka H, Nakao A, Fink MP, Lotze MT, Yang H, Li J, Tracey KJ, Geller DA, Billiar TR. The nuclear factor HMGB1 mediates hepatic injury after murine liver ischemia-reperfusion. J Exp Med. 2005;201:1135–1143. doi: 10.1084/jem.20042614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vabulas RM, Ahmad-Nejad P, Ghose S, Kirschning CJ, Issels RD, Wagner H. HSP70 as endogenous stimulus of the Toll/interleukin-1 receptor signal pathway. J Biol Chem. 2002;277:15107–15112. doi: 10.1074/jbc.M111204200. [DOI] [PubMed] [Google Scholar]
- 29.Favre J, Musette P, Douin-Echinard V, Laude K, Henry JP, Arnal JF, Thuillez C, Richard V. Toll-like receptors 2-deficient mice are protected against postischemic coronary endothelial dysfunction. Arterioscler Thromb Vasc Biol. 2007;27:1064–1071. doi: 10.1161/ATVBAHA.107.140723. [DOI] [PubMed] [Google Scholar]
- 30.Kaczorowski DJ, Nakao A, Mollen KP, Vallabhaneni R, Sugimoto R, Kohmoto J, Tobita K, Zuckerbraun BS, McCurry KR, Murase N, Billiar TR. Toll-like receptor 4 mediates the early inflammation response after cold ischemia/reperfusion. Transplantation. 2007;84:1279–1287. doi: 10.1097/01.tp.0000287597.87571.17. [DOI] [PubMed] [Google Scholar]
- 31.Leemans JC, Stokman G, Claessen N, Rouschop KM, Teske GJD, Kirschning CJ, Akira S, van der Poll T, Weening JJ, Florquin S. Renal-associated TLR2 mediates ischemia/reperfusion injury in the kidney. J Clin Invest. 2005;115:2894–2903. doi: 10.1172/JCI22832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shen XD, Ke B, Zhai Y, Gao F, Busuttil RW, Cheng G, Kupiec-Weglinski JW. Toll-like receptor and heme oxygenase-1 signaling in hepatic ischemia/reperfusion injury. Am J Transplant. 2005;5:1793–1800. doi: 10.1111/j.1600-6143.2005.00932.x. [DOI] [PubMed] [Google Scholar]
- 33.Shigeoka AA, Holscher TD, King AJ, Hall FW, Kiosss WB, Tobias PS, Mackman N, McKay DR. TLR2 is constitutively expressed within the kidney and participates in ischemic renal injury through both MyD88-dependent and-independent pathways. J Immunol. 2007;178:6252–6258. doi: 10.4049/jimmunol.178.10.6252. [DOI] [PubMed] [Google Scholar]
- 34.Wu H, Chen G, Wyburn KR, Yin J, Bertolino P, Eris JM, Alexander SI, Sharland AF, Chadban SJ. TLR4 activation mediates kidney ischemia/reperfusion injury. J Clin Invest. 2007;117:2847–2859. doi: 10.1172/JCI31008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bonventre JV. Kidney ischemic preconditioning. Curr Opin Nephrol Hypertension. 2002;11:43–48. doi: 10.1097/00041552-200201000-00007. [DOI] [PubMed] [Google Scholar]
- 36.Reutzel-Selke A, Pratschke J, Martins PN, Denecke C, Jurisch A, Kotsch K, Pascher A, Neuhaus P, Tullius SG. Ischemic preconditioning produces systemic protective and adoptively transferable effects. Kidney Int. 2008;74:622–630. doi: 10.1038/ki.2008.208. [DOI] [PubMed] [Google Scholar]
- 37.Burdelya LG, V, Krivokrysenko I, Tallant TC, Strom E, Gleiberman AS, Gupta D, Kurnasov OV, Fort FL, Osterman AL, DiDinato JA, Feinstein E, Gudkov A. An agonist of Toll-like receptor 5 has radioprotective activity in mouse and primate models. Science. 2008;320:226–230. doi: 10.1126/science.1154986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shimoda N, Fukazawa N, Nonomura K, Fairchild RL. Cathepsin G is required for sustained inflammation and tissue injury after reperfusion of ischemic kidneys. Am J Pathol. 2007;170:930–940. doi: 10.2353/ajpath.2007.060486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Afanasyeva M, Georgakopoulos D, Belardi DF, Ramsundar AC, Barin JG, Kass DA, Rose NR. Quantitative analysis of myocardial inflammation by flow cytometry in murine autoimmune myocarditis: correlation with cardiac function. Am J Pathol. 2004;164:807–815. doi: 10.1016/S0002-9440(10)63169-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hayashi F, Smith KD, Ozinsky A, Hawn TR, Yi EC, Goodlett DR, Eng JK, Akira S, Underhill DM, Aderem A. The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature. 2001;410:1099–1103. doi: 10.1038/35074106. [DOI] [PubMed] [Google Scholar]
- 41.Sebastiani G, Leveque G, Lariviere L, Larousche L, Skamene E, Gros P, Malo D. Cloning and characterization of the murine toll-like receptor 5 (tlr5) gene: sequence and mRNA expression studies in Salmonella-susceptible MOLF/Ei mice. Genomics. 2000;64:230–240. doi: 10.1006/geno.2000.6115. [DOI] [PubMed] [Google Scholar]
- 42.Kanzler H, Barrat FJ, Hessel EM, Coffman RL. Therapeutic targeting of innate immunity with Toll-like receptor agonists and antagonists. Nat Med. 2007;13:552–559. doi: 10.1038/nm1589. [DOI] [PubMed] [Google Scholar]
- 43.Mollen KP, Anand RJ, Tsung A, Prince JM, Levy RM, Billiar TR. Emerging paradigm: Toll-like receptor 4-sentinel for the detection of tissue damage. Shock. 2006;26:430–437. doi: 10.1097/01.shk.0000228797.41044.08. [DOI] [PubMed] [Google Scholar]
- 44.Ha T, Hua F, Liu X, Ma J, McMullen JR, Shiol T, Izumo S, Kelley J, Gao X, Browder W, Williams DL, Kao RL, Li C. Lipopolysaccharide-induced myocardial protection against ischaemia/reperfusion injury is mediated through a PI3K/Akt-dependent mechanism. Cardiovasc Res. 2007;78:546–553. doi: 10.1093/cvr/cvn037. [DOI] [PubMed] [Google Scholar]
- 45.Chen LW, Chang WJ, Chen PH, Liu WC, Hsu CM. TLR ligand decreases mesenteric ischemia and reperfusion injury-induced gut damage through TNF-alpha signaling. Shock. 2008;30:563–570. doi: 10.1097/SHK.0b013e31816a3458. [DOI] [PubMed] [Google Scholar]
- 46.Stevens SL, Ciesielski TM, Marsh BJ, Yang T, Homen DS, Boule JL, Lessov NS, Simon RP, Stenzel-Poore MP. Toll-like receptor 9: a new target of ischemic preconditioning in the brain. J Cereb Blood Flow Metab. 2008;28:1040–1047. doi: 10.1038/sj.jcbfm.9600606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Basile DP. The endothelial cell in ischemic acute kidney injury: implications for acute and chronic function. Kidney Int. 2007;72:151–156. doi: 10.1038/sj.ki.5002312. [DOI] [PubMed] [Google Scholar]
- 48.Paris F, Fuks Z, Kang A, Capodieci P, Juan G, Ehleiter D, Haimovitz-Friedman A, Cordon-Cardo C, Kolesnick R. Endothelial apoptosis as the primary lesion initiating intestinal radiation damage in mice. Science. 2001;293:293–297. doi: 10.1126/science.1060191. [DOI] [PubMed] [Google Scholar]
- 49.Wolfs TG, Buurman WA, van Schadewijk A, de Vries B, Daemens MA, Hiemstra PS, van’t Veer C. In vivo expression of Toll-like receptor 2 and 4 by renal epithelial cells: IFN-gamma and TNF-alpha mediated up-regulation during inflammation. J Immunol. 2002;168:1286–1293. doi: 10.4049/jimmunol.168.3.1286. [DOI] [PubMed] [Google Scholar]
- 50.Anders HJ, Banas B, Schlondorff D. Signaling danger: Toll-like receptors and their potential roles in kidney diseases. J Am Soc Nephrol. 2004;15:854–867. doi: 10.1097/01.asn.0000121781.89599.16. [DOI] [PubMed] [Google Scholar]
- 51.Tamatani M, Che Y, Matsuzaki H, Ogawa S, Okado H, Miyake S, Mizuno T, Tohyama M. Tumor necrosis factor induces Bcl-2 and Bcl-x expression through NFkappaB activation in primary hippocampal neurons. J Biol Chem. 1999;274:8531–8538. doi: 10.1074/jbc.274.13.8531. [DOI] [PubMed] [Google Scholar]
- 52.Wilde GJC, Pringle AK, Sundstrom LE, Mann DA, Lannotti F. Attenuation and augmentation of ischemia-related neuronal death by tumor necrosis factor-alpha in vitro. Eur J Neurosci. 2000;12:3863–3870. doi: 10.1046/j.1460-9568.2000.00273.x. [DOI] [PubMed] [Google Scholar]