Abstract
Background
Childhood trauma has been associated with elevated central corticotropin releasing hormone (CRH) drive in adults meeting general DSM-IV criteria for personality disorder. It is not clear how this may be related to pituitary or adrenal responsiveness in personality disorder. It was hypothesized that high levels of childhood trauma would be associated with blunted cortisol and adrenocorticotropin releasing hormone (ACTH) response to the combined dexamethasone(DEX)/CRH test in adults meeting general DSM-IV criteria for personality disorder.
Method
24 Healthy, medication free adults with personality disorder (N = 16) and a group of healthy controls (N = 8) underwent semi-structured diagnostic interviews and completed the Childhood Trauma Questionnaire (CTQ). Across two separate study sessions separated by at least a week, cerebrospinal fluid (CSF) was sampled by lumbar puncture for measurement of CRH concentration (N = 17), and peripheral blood cortisol and ACTH levels were measured after challenge with DEX/CRH (N = 24).
Results
As hypothesized, high CTQ score was associated with a blunted cortisol and ACTH response to DEX/CRH challenge. Indices of cortisol and ACTH response (peak level and area under the curve (AUC)) to DEX/CRH were in turn significantly negatively correlated with CSF CRH concentration.
Conclusion
Childhood trauma in adults with personality disorder is associated with blunted cortisol and ACTH secretion following DEX/CRH challenge. These effects are independent of depression or posttraumatic stress disorder. Previous work would suggest that blunted pituitary-adrenal response is related to elevated central CRH drive. Corroborating this, CSF CRH levels were significantly and negatively correlated with peak level and AUC of both cortisol and ACTH.
Keywords: Corticotropin releasing hormone/factor, Cortisol, Childhood Trauma, Personality disorder, Posttraumatic stress disorder
1. Introduction
The established association of childhood trauma with the development of personality disorder (Johnson et al., 2006; Johnson et al., 1999) may represent an important clue regarding the biological mechanism of personality disorder. Animal models of the neurobiological effect of disrupted parental care have implicated corticotropin-releasing hormone (CRH) as a mediator of the effect of early life stress on the abnormal development of stress reactivity (Coplan et al., 2001; Plotsky et al., 2005). We have previously shown that in adults meeting DSM-IV general criteria for personality disorder, regardless of specific DSM-IV categorical diagnoses, history of childhood trauma and poor parental bonding predict elevated cerebrospinal fluid (CSF) levels of CRH (Lee et al., 2005; Lee et al., 2006). The relationship between childhood trauma and CRH may be specific to emotional neglect, as measured the Emotional Neglect subscale of the Childhood Trauma Questionnaire (Lee et al., 2005). These results parallel those found in samples that include depressed patients (Carpenter et al., 2004). However, unlike the case of severe depression, the presence of a personality disorder diagnosis itself is not consistently associated with HPA axis abnormalities, even when examining more “affective,” Cluster-B subtypes such as borderline personality disorder (Rinne et al., 2002).
It is not clear if findings of elevated lumbar CSF CRH in adults with histories of childhood maltreatment reflect a chronically elevated “baseline” CRH level, or anticipatory reactivity to lumbar puncture. It is known that pituitary CRH receptors react to chronic CRH stimulation with decreased membrane receptor concentration and uncoupling of the receptor from intracellular adenylate cyclase (Wynn et al., 1988). Such a reaction would be expected to result in decreased sensitivity to exogenous CRH. Confirmation of this prediction was found by Newport and others (2003), who measured ACTH response to CRH infusion and on a separate study session, CSF concentration of CRH sampled by lumbar puncture. In this study, ACTH response to CRH infusion was found to be inversely correlated with CSF concentration of CRH. If CSF CRH were chronically elevated in personality disordered individuals with histories of significant childhood maltreatment, then it would be expected that their pituitary-adrenal response to exogenous CRH would be blunted.
Some of the existing literature would support this prediction. Recent data in psychiatrically normal groups has found that history of childhood trauma is associated in adulthood with decreased pituitary-adrenal response (ACTH and cortisol, respectively) to exogenous CRH (Carpenter et al., 2009; Klassens et al., 2009), as was first noted in adolescent girls with a history of sexual abuse (De Bellis et al., 1994). These results were interpreted to be consistent with peripheral down regulation of CRH receptors in the face of high central CRH “drive”. Similar findings have been reported with PTSD, which may be associated over the long term with blunted pituitary response to exogenous CRH (Ströhle et al., 2008; Smith et al., 1988). On the other hand, childhood trauma in currently depressed patients seems to be associated with enhanced pituitary-adrenal response, possibly due to a depression-related increase in vasopressin drive. This has been found in children with depression (Kaufman et al., 2007) and in adult males and females with depression (Heim et al., 2001; Heim et al., 2007). There may be important, unaccounted for sources of variably however, as not all data fit this pattern (Rasmusson et al., 2001).
In order to more strictly control for the effects of PTSD and MDD, we sought to study the relationship between childhood trauma and pituitary/adrenal response to exogenous CRH in a personality-disordered but non-depressed, non-PTSD sample. We tested the hypothesis that history of exposure to significant childhood trauma would be associated with blunted ACTH and cortisol response to challenge with dexamethasone and CRH (DEX/CRH). Dexamethasone, a synthetic steroid, primarily binds to pituitary glucocorticoid autoreceptors and increases negative feedback on CRH release (Deuschle et al., 1998; Miller et al., 1992; Meijer et al., 1998), diminishing CRH secretion (Galard et al., 2002). This may minimize, to some extent, the possible confounding effects of recent life stress on the state of the HPA axis at the time of CRH challenge, as has been demonstrated in a study of the effects of mild experimental stress on the DEX/CRH (Oshima et al., 2001). Additionally, lumbar puncture was performed on a separate study session in order to obtain cerebrospinal fluid for measurement of central corticotropin-releasing hormone. For the personality disordered group, patients were selected who met DSM-IV general criteria for personality disorder. This study group was chosen over a narrowly defined group in order to maximize the generalizeability of the study while acknowledging that there are no validated biological boundaries between or biomarkers for currently defined personality disorders, Axis II-Axis II comorbidity is common (Lenzenweger et al., 2006), Personality Disorder NOS is in fact one of the most highly prevalent Axis II disorders (Verheul and Widiger, 2004), and considerable heterogeneity is present in even single disorders such as borderline personality disorder (Critchfield et al., 2008).
2. METHOD
2.1 Participants
Participants were 24 adults who were either normal controls (n = 8) or personality disordered (PD; n =16). Ten of the PD subjects had Childhood Trauma Questionnaire (CTQ) Total scores ≥ 9 and formed the high trauma subgroup. 6 of the PDs and all of the 8 normal controls had mean CTQ Total scores ≤ 6, and together formed the low trauma subgroup (see Measures below for the rationale regarding CTQ cutoff scores). See Table III for the distribution of specific DSM-IV personality disorder diagnoses across the trauma groups. All procedures were approved by the Institutional Review Board (IRB) and General Clinical Research Center (GCRC) of the University of Chicago. All subjects provided written informed consent, using IRB approved consent forms. Subjects were recruited by advertisements in local newspapers seeking volunteers for biological studies of personality. In order to minimize bias, no mention was made of childhood trauma in the recruiting media. Both personality disordered and normal control subjects were recruited from the same community, using the same recruitment methods. Normal control subjects had no lifetime history of Axis I or Axis II psychpathology, and no history of psychiatric treatment in first degree relatives. Personality disordered subjects met DSM-IV general criteria for personality disorder, but did not meet DSM-IV criteria for PTSD, current MDD, current substance dependence, or lifetime history of psychotic or bipolar mood disorder. Past but not current major depression was allowed. All subjects were free of psychotropic medications for at least 30 days prior to study, had normal thyroid function, urine and blood chemistry, and hematological tests. Additionally all subjects had negative urine drug and alcohol breathalyzer test results at screen and on the days of testing. Due to the important effect that menstrual cycle has on HPA axis function, women of childbearing potential were studied within 10 days of their last menstrual cycle to minimize the chance of studying females in mid-luteal phase. Additional exclusionary criteria were being actively suicidal, current endocrine disorder, being pregnant, and taking oral contraceptives.
2.2 Measures
History of childhood trauma was measured by the Childhood Trauma Questionnaire (CTQ; Bernstein 1988), a 70 item questionnaire assessing the pattern of childhood exposure to childhood abuse and neglect. The CTQ has exhibited good convergent validity with measures of posttraumatic stress disorder, dissociation, alexithymia and depression, and discriminant validity with measures of vocabulary and social desirability (Bernstein et al., 1994). Test-retest reliability over a 2- to 6- month interval of the CTQ is high, with values ranging from .79 to .81 (Bernstein and Fink, 1998, Bernstein et al. 1994). The CTQ Total score reflects contributions from the subscales of Physical and Emotional Abuse, Physical Neglect, Emotional Neglect, and Sexual Abuse. Although in theory, using CTQ Total score to define subgroups could be problematic if individuals varied in the relative distribution of trauma subtypes, in reality the subscale scores are highly correlated with each other. This fact is in accordance with the recognition that subtypes of childhood trauma are associated with common risk factors, such as parental psychopathology. Thus CTQ Total score was selected as the primary independent variable. In order to maximize power, we used an excluded middle design, recruiting subjects only with low and high CTQ scores. Low CTQ score was defined as CTQ Total score ≤ 6; high CTQ was defined as CTQ Total score ≥ 9. As normative data to inform cutoff scores on the 70-item CTQ was not available at the initiation of the study, cutoff scores were chosen to divide a previously studied population of personality disordered adults into thirds based on Total CTQ score (n = 186; Lee et al., 2006, 2007); the lowest third having a mean Total CTQ score comparable to that seen in our population of normal controls and the upper third having a mean Total CTQ score in the same range as that found in clinical populations seeking therapy for childhood abuse issues (Paivio 2001). Using these cutoff scores, subjects with CTQ scores from the upper third have significantly higher cerebrospinal corticotropin releasing hormone (CRH) levels those from the lower third (Lee et al., 2005). This is physiologically significant, in that cerebrospinal fluid CRH levels have previously been found to predict cortisol secretion after CRH challenge (Newport et al., 2003). Using these cutoff scores, our sample included 9 Normal Controls with low CTQ scores, 6 Personality Disorder subjects with low CTQ scores, and 10 Personality Disorder subjects with high CTQ scores (see table III). Of note, none of the normal control subjects had CTQ scores ≥ 9. To preserve statistical power, for exploratory analyses of relationships between CSF CRH and childhood trauma, we limited subtype specific analyses to the Emotional Neglect subscale, which we found previously to be correlated with CSF CRH.
All subjects were evaluated by trained clinical psychologist raters, with Axis I and Axis II diagnoses made according to DSM-IV criteria. Final psychiatric diagnosis was established by team best-estimate consensus, using information from semi-structured interviews by trained clinical raters using the Structured Clinical Interview for Diagnosis of Axis I disorders (SCID) and the Structured Interview for the Diagnosis of DSM Personality Disorder (SIDP; Pfohl et al., 1994).
2.3 DEX/CRH Challenge Test
Diagnostic interview and self-report data were collected on the first day of study. Lumbar puncture and DEX/CRH testing occurred on separate study sessions at the General Clinical Research Center (GCRC) of The University of Chicago. The lumbar puncture session always preceded the DEX/CRH session by at least one week. All subjects provided negative urine drug screens, alcohol breathalyzer, and pregnancy tests at the beginning of every study session. Female subjects participated within 10 days of their last menstrual period. The evening before the CRH challenge, subjects were admitted to the GCRC. 16 hours prior to administration of intravenous CRH (11 PM), subjects were administered 1.5 mg of dexamethasone orally by a research nurse. Subjects slept at the GCRC in quiet, non stressful conditions. The next morning, subjects rested and were fed breakfast. At 12 PM, subjects received a heparinized intravenous cannula inserted into a forearm vein of the right arm for repeated blood draws. Blood samples were drawn beginning 60 minutes before CRH infusion, and at 30 minute intervals following CRH infusion. Baseline hormonal measures were sampled at 1:30 p.m. (−150 minutes) and 4:00 p.m. (0 minutes). At 4:00 p.m., 1 mcg/ kg of body weight ovine CRH (oCRH) was administered through the catheter over a 60 second interval. Because peripheral CRH administration stimulates pituitary CRH receptors, the resulting pituitary output of ACTH and adrenal output of cortisol can be simultaneously measured as an index of CRH receptor sensitivity. Blood samples for ACTH and cortisol were drawn at +5, +15, +30, +60, +90, and +120 minutes after oCRH administration. At 6:00 PM, subjects were examined by a physician and discharged from the GCRC. Blood samples for hormone measurements were collected in tubes containing EDTA and centrifuged immediately in a refrigerated centrifuge. Plasma was separated and stored at −70 C until assayed. Samples were assayed for ACTH and Cortisol by the Clinical Research Center (CRC) laboratory (CV% ACTH = 6.7, CV% cortisol = 7.1%). Blood pressure and heart rate were measured hourly beginning from 3:00 PM to 6:00 PM. Subjects additionally completed a visual analogue scale rating of subjective emotional state at −15, +5, +15, +30, +60, +90, and +120.
2.4 Lumbar Puncture
The night before the lumbar sampling, subjects were admitted to the General Clinical Research Center (GCRC). The following morning at 8 am, subjects were placed in lateral decubitus position in their hospital bed. After application of intradermal lidocaine local anesthesia, a 22-guage lumbar puncture needle was inserted through the L3–L4 interspace. Several fractions of CSF totaling 20 cc were obtained and chilled at the bedside. The last 15 cc's were immediately mixed and divided into 15 identical 1 cc aliquots for CSF analysis. The sample were transferred to a −70 C freezer immediately after the procedure until assayed by radio-immunoassay (RIA). After the CSF samples were obtained, the subjects rested supine in bed for 4 hours to minimize risk of post-lumbar puncture headache. CRH was analyzed using 125I-CRH (2000 Ci/mmol/Amersham) and CRH antibody (Linton and Lowry, 1986). The lower detection limit was 3.9 pmol/L and the intra-assay coefficient was 7% (Husum and Mathé, 2002).
2.5 Data Analysis
Statistical analysis of the dependent variables (ACTH, cortisol) was conducted using Repeated Measures ANCOVA, with the between-groups factors of Trauma (High vs. Low), Sex (Male or Female), Personality Disorder (Personality Disorder or Normal Control), the within-groups factor of time, and the covariate of age. Huynh-Feldt significance levels are reported for instances in which assumptions of sphericity are violated. Statistical tests were two-tailed unless indicated otherwise. Exploratory analyses (Repeated Measures ANCOVA) were conducted on physiological measures (blood pressure and heart rate) as well as subjective emotional state as measured by visual analogue scale. In additional exploratory analyses, CSF CRH was correlated with peak ACTH, peak cortisol, and the CTQ subscale Emotional Neglect. A focused analysis of the Emotional Neglect subscale of the CTQ was chosen rather than testing of all subscales to preserve statistical power, given our previous finding of a significant positive correlation between the specific CTQ subscale Emotional Neglect with CSF CRH concentration in adult with personality disorder, but a lack of significant correlation with the other CTQ subscales (Physical and Emotional Abuse, Physical Neglect, and Sexual Abuse; Lee et al., 2005). Given the hypothesized directionality, 1-tailed tests were utilized for this test; all other analyses utilized 2-tailed tests given the lack of strong directional hypotheses.
3. RESULTS
3.1 Adverse Events
There were no serious adverse events. A single subject (High CTQ PD) experienced reactivation of longstanding psoriasis in the form of erythema and itching, beginning immediately after CRH infusion and lasting approximately 40 minutes. This episode was not unexpected given the previous recent history of psoriasis.
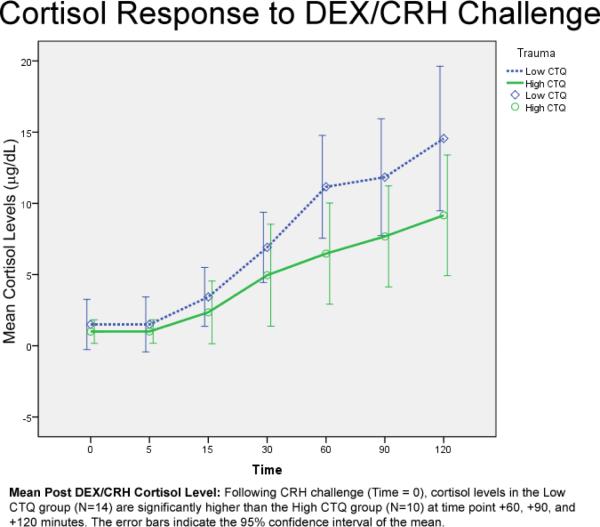
3.2 Cortisol Response to DEX/CRH Challenge
RM-ANCOVA (covarying for age) revealed an overall effect of Trauma (F (1, 20) = 5.896, p = .02), and an interaction for Trauma × Time ((F (2.437, 48.735) = 3.575, p = .03; Huynh-Feldt corrected for nonsphericity; see Figure 1). There were no significant main effects of or interactions with Personality Disorder or Gender. Follow up multivariate analysis of variance probing the interaction between Time and Trauma revealed the High CTQ vs. the Low CTQ group had significantly lower cortisol levels at +60 minutes post CRH infusion (F (1, 24) = 7.260; p = .01), +90 (F (1, 24) = 4.924; p = .038) and +120 minutes (F (1, 24) = 4.460; p = .05), although a trend-difference was apparent as early as +30 minutes (F (1, 24) = 3.902; p = .06). ANCOVA of peak cortisol response, covarying for age, revealed that the high CTQ group was significantly associated with lower peak cortisol post CRH infusion (F (1, 23) = 4.593, p = .04; mean = 9.598 (SD = 5.805) vs. 15.099 (SD = 8.852)). There were no main effects or interactions with Gender or Personality Disorder. ANCOVA of cortisol area under the curve (AUC) post CRH infusion revealed that the high CTQ group was significantly associated with smaller cortisol AUC (F (1, 23) = 5.921, p = .02; mean = 71.292 (SD = 51.212) versus 112.175 (SD = 65.104)). There were no main effects or interactions with Gender or Personality Disorder.
Figure 1.
ANCOVA of post-DEX but pre-CRH cortisol levels revealed that covarying for age, Trauma (high vs. low CTQ), but not Personality Disorder, was significantly related to cortisol level (F (1, 23) = 4.542; p = .05), with the high trauma group having lower cortisol levels (mean = .75 (SD = .533) vs. 1.81 (SD = 4.112)). There were no relationships between cortisol peak concentration or AUC with any of the specific personality disorder diagnosis represented in the sample (borderline, narcissistic, obsessive-compulsive, or avoidant).
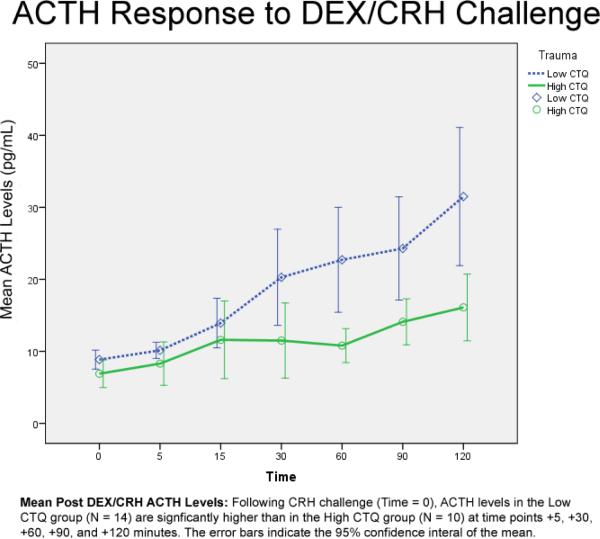
3.3 ACTH Response to DEX/CRH Challenge
RM-ANCOVA (covarying for age) revealed a significant effect of Trauma (F (1, 20) = 8.102; p = .01) and a Trauma × Time interaction (F (3.223, 64.464) = 3.655; p = .02; Huynh-Feldt; see Figure 2). No other main effects (Personality Disorder, Gender) or interactions were significant. Follow up multivariate analysis of variance, covarying for age, revealed the High CTQ vs. the Low CTQ group had significantly blunted ACTH response at +5 (F (1, 23) = 4.41; p = .05), +30 (F (1, 23) = 4.6; p = .04), + 60 (F (1, 23) = 11.12; p = .003), + 90 (F (1, 23) = 6.71; p = .02), and +120 (F (1, 23) = 8.96; p = .007). ANCOVA of peak ACTH response, covarying for age, revealed that the high CTQ group was significantly associated with lower peak ACTH post CRH infusion (F (1, 23) = 8.513, p = .01; mean = 17.9 (SD = 7.549) vs. 33.428 (SD = 16.991)). There were no main effects or interactions with Gender or Personality Disorder. ANCOVA of ACTH area under the curve (AUC) post CRH infusion revealed that the high CTQ group was significantly associated with smaller ACTH AUC (F (1, 23) = 11.456, p < .01; mean = 147.175 (SD = 43.802) versus 261.125 (SD = 110.868)). There were no main effects or interactions with Gender or Personality Disorder.
Figure 2.
ANCOVA of post-DEX but pre-CRH ACTH level, covarying for age, found that neither Personality Disorder nor Trauma significantly predicted ACTH level. There was no association of peak ACTH concentration or AUC with a specific personality disorder diagnosis (borderline, narcissistic, avoidant, or obsessive-compulsive).
3.4 Exploratory Analyses
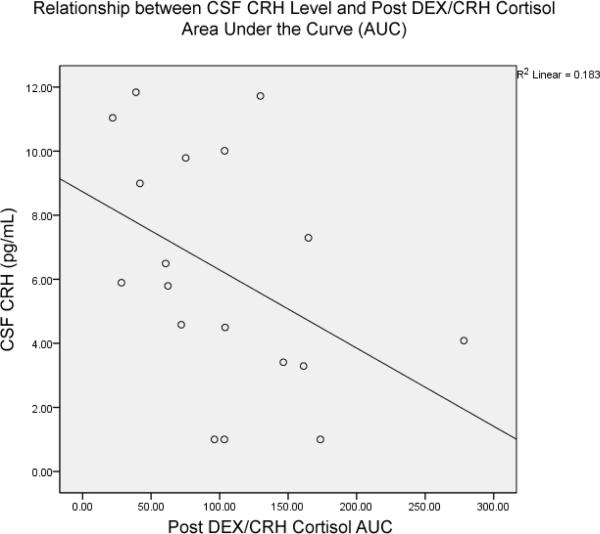
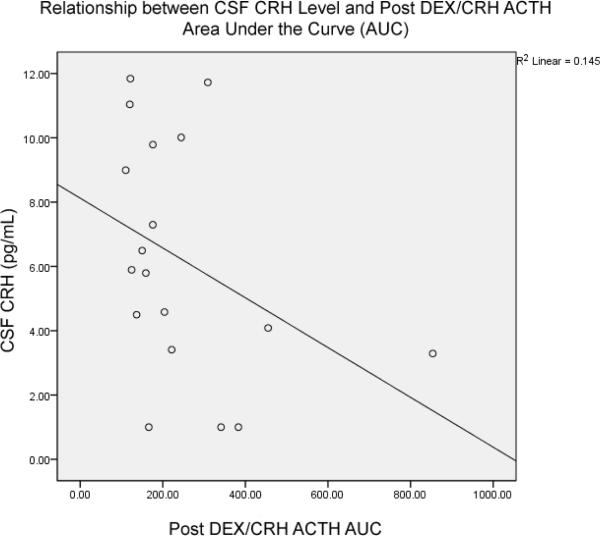
17 out of 24 subjects consented to undergo lumbar puncture. CSF CRH concentration was significantly and inversely correlated with peak ACTH response (r = −.524, p = .02, n = 17; Spearman's 1-tailed) and ACTH AUC (r = −.440, p = .04, n = 17; Spearman's 1-tailed; Figure 3). CSF CRH concentration was significantly negatively correlated with peak cortisol response (r = −.477, p = .036, n = 17; Spearman's 1-tailed) and cortisol AUC (r = −.452, p = .03, n = 17; Spearman's 1-tailed; Figure 4). As hypothesized based on previously reported findings, in this group of subjects CSF CRH concentration was significantly correlated with CTQ Emotional Neglect (r = .434, p = .04, n = 17, Spearman's one-tailed). CRH CRH concentration was not associated with specific diagnosis of borderline, narcissistic, avoidant, or dependent personality disorder.
Figure 3.
Figure 4.
Analysis of heart rate data from the DEX/CRH challenge session revealed a statistical trend for an effect of Time (F (7, 119) = 2.019, p = .06; heart rate decreased over time), but no effects of PD, Sex, or interactions with each other or with Trauma. A statistical trend was detected for the between-groups factor of Trauma (F (1, 17) = 3.747; p = .07), with the High CTQ group having a lower mean heart rate than the Low CTQ group. Exploratory effects for the effect of CRH on blood pressure revealed no significant effects for systolic blood pressure. For diastolic blood pressure, a statistically significant interaction of time and personality disorder group was detected (F (7, 119) = 2.26; p = .03). Follow up testing revealed that the presence of personality disorder was associated with higher diastolic blood pressure 120 minutes post CRH infusion (F (1, 20) = 4.216; p = .05). Analyses of subjective emotional state, as measured by Visual Analogue Scale, revealed no significant between or within-groups effects.
4. DISCUSSION
This is the first report triangulating the interrelationships between childhood trauma, DEX/CRH response, and CSF CRH levels in personality disorder. Personality disordered patients with histories of significant childhood trauma had blunted cortisol and ACTH response to DEX/CRH challenge. Thus the pattern was not an escape from negative feedback that has been found to characterize some forms of depressive disorder. The findings were not due to concurrent medications, current major depression, PTSD, sex, or age. Nor were the findings due to the presence or absence of personality disorder, as personality disorder itself failed to account for differences in pituitary or adrenal response to DEX/CRH challenge. Blunted cortisol and ACTH response to exogenous CRH is consistent with a theoretical model of pituitary CRH receptor desensitization in the face of chronically increased central CRH drive. Indeed, exploratory analysis from the sub-sample with CSF CRH provided important corroboration, as CSF CRH levels were negatively correlated with measures of pituitary and adrenal responsiveness to DEX/CRH challenge. This would be expected given previous reports of a negative relationship between central CRH level and ACTH following exogenous CRH (Newport et al., 2003; note that in this study dexamethasone was not given prior to CRH challenge). Thus, the results of this study are in line with our previous findings of increased central CRH levels in personality disordered patients who report severe childhood trauma. They are additionally suggestive of tonically increased CRH drive. Indeed, exploratory analysis of the subsample with CSF data provided a replication of our two previous findings of a positive relationship between central CRH concentration and measures of emotional neglect from caregivers in childhood.
4.1 Previous Findings
Our results are comparable with previously reported investigations of the relationship between HPA axis function and childhood trauma (DeBellis et al., 1994; Carpenter and Tyrka 2007; Klaasens et al., 2009). They are most closely comparable Klaassens and others (2009), who also found that childhood trauma was related to blunted cortisol and ACTH response to DEX/CRH challenge in a sample of adults free of current or past Axis I psychiatric disorders. Our results are also generally consistent with findings of blunted ACTH and cortisol response from studies of adults with PTSD (Smith et al., 1989). Major depression may alter the relationship between trauma and pituitary response to exogenous CRH, as has been found in studies that include currently depressed patients (Heim et al.; 2007). A candidate mechanism may be enhanced hypothalamic vasopressin drive in depressives that is able to potentiate the effect of CRH on pituitary receptors (Geokoop et al., 2010). However, definitive proof of this is not yet available and some evidence suggests that in fact PTSD is itself associated with increased vasopressin release (de Kloet et al., 2008). Another possibility is that PTSD and major depressive disorder have divergent effects on negative feedback in the HPA axis, with PTSD associated with increased negative feedback control of the HPA axis (Yehuda et al., 1994; Yehuda et al., 1996). Overall, our findings indicate the childhood trauma in the context of personality disorder has a neuroendocrine profile that resembles that seen in chronic PTSD.
4.2 Limitations
Several important features of the study are worth mentioning. The cross-sectional design precludes causative interpretations, as it not known if history of childhood trauma precedes pituitary CRH receptor sub-sensitivity. This issue could be dealt with using a prospective study design. However, given that personality disorder is not diagnosed in childhood or adolescence, this would require choosing a population with personality disorder-like features, which raises methodological issues that require more attention. Another widely acknowledged limitation of retrospective measures of childhood trauma is the uncertainty regarding the extent to which retrospective accounts of childhood trauma reflect the actual behavior of parents and caregivers versus the subjective experience of them. This is a complex issue, and evidence to date suggest that overly simplistic interpretations of retrospective accounts as either invalid or valid do not reflect the true nature of the childhood environment. The current state of science shows that retrospective accounts are correlated with prospectively collected data (Widom et al., 2005) but are simultaneously affected by genetic factors (Kendler & Baker, 2007). Thus it is possible that some of the relationship between CTQ scores and pituitary CRH receptor sensitivity reflects a common underlying genetic factor, rather than strictly an environmental or developmental effect. This is an important avenue for future exploration. Another limitation of the study is the small size of our sample. If personality disorders are biologically heterogenous, this could increase the likelihood of Type I or Type II error. It must be emphasized that none of the current DSM-IV personality disorders have established biological boundaries, with the possible exception of schizotypal personality disorder. We did not study schizotypal subjects. Thus this remains a theoretical concern, which can only be addressed in the future with either a larger sample size, or with future knowledge of the real biological boundaries of personality disorder. Although a larger sample would be desirable, the study was designed to maximize statistical power while enabling resources to be dedicated to more complete characterization of the sample. This includes in depth assessment of Axis II disorders, use of an excluded middle design, exclusion of subjects on psychotropics or with confounding Axis I conditions, and measurement of central and peripheral indices of HPA axis function. Finally, it is possible that past history of depression may have a scar-like effect on response to DEX/CRH challenge, and represent a confound. Arguing against this is previous research showing that high risk groups have enhanced rather than blunted cortisol and ACTH response to DEX/CRH challenge (Modell et al., 1998). Preliminary analysis in the personality disordered sample revealed that high CTQ score predicted blunted ACTH response even after accounting for past history of depression (results available upon request from the PI). However, in order to validly test the effect of the interaction between past history of depression and trauma in personality disorder on DEX/CRH response, a study design in which high and low trauma groups are counterbalanced with respect to past history of depression would be necessary.
4.3 Conclusions
The findings from this study corroborate with previous reports on the relationship between childhood trauma and elevated central CRH drive. When combined with data from preclinical models of the effect of early life stress on central CRH function, this work provides evidence for both central and peripheral dysregulation of the stress system in personality disorder with childhood trauma.
Acknowledgements
The procedures were carried out by the research assistants Bennett Barch, and Cara Van Wormer, and the research nurse, Cindy Bogue, R.N.
Role of Funding Source This work was made possible with support an NIMH grant (1RO3MH066888-01: CRF and Childhood Trauma: Behavioral Correlates; PI: Lee), the General Clinical Research Center of The University of Chicago, the Swedish Medical Research Council grant 10414, and the Karolinska Institutet to AAM.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bernstein DP, Fink L. Childhood Trauma Questionnaire. A retrospective self-report: Manual. Psychological Corporation and Harcourt Brace; San Antonio, TX: 1988. [Google Scholar]
- Bernstein DP, Ahluvalia T, Pogge D, Handelsman L. Validity of the childhood trauma questionnaire in an adolescent psychiatric population. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36:340–348. doi: 10.1097/00004583-199703000-00012. [DOI] [PubMed] [Google Scholar]
- Bernstein DP, Fink L, Handelsman L, Foote J, Lovejoy M, Wenzel K, Sapareto E, Ruggiero J. Initial reliability and validity of a new retrospective measure of child aubse and neglect. American Journal of Psychiatry. 1994;151:1132–1136. doi: 10.1176/ajp.151.8.1132. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Licinio J, Darnell A, Krystal JH, Owens MJ, Southwick SM, Nemeroff CB, Charney DS. Elevated CSF corticotropin-releasing factor concentrations in posttraumatic stress disorder. American Journal of Psychiatry. 1997;154:624–629. doi: 10.1176/ajp.154.5.624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter LL, Tyrka AR, Ross NS, Khoury L, Anderson GM, Price LH. Effect of childhood emotional abuse and age on cortisol responsivity in adulthood. Biol Psychiatry. 2009;66:69–75. doi: 10.1016/j.biopsych.2009.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter LL, Tyrka AR, McDougle CJ, Malison RT, Owens MJ, Nemeroff CB, Price LH. Cerebrospinal fluid corticotropin-releasing factor and perceived early-life stress in depressed patients and healthy control subjects. Neuropsychopharmacology. 2004;29:777–784. doi: 10.1038/sj.npp.1300375. [DOI] [PubMed] [Google Scholar]
- Coplan JD, Smith EL, Altemus M, Scharf BA, Owens MJ, Nemeroff CB, Gorman JM, Rosenblum LA. Variable foraging demand rearing: sustained elevations in cisternal cerebrospinal fluid corticotropin-releasing factor concentrations in adult primates. Biological Psychiatry. 2001;50:200–204. doi: 10.1016/s0006-3223(01)01175-1. [DOI] [PubMed] [Google Scholar]
- Critchfield KL, Clarkin JF, Levy KN, Kernberg OF. Organization of co-occuring Axis II features in borderline personality disorder. British Journal of Clinical Psychology. 2008;47:185–200. doi: 10.1348/014466507X240731. [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Chrousos GP, Dorn LD, Helmers K, Kling MA, Trickett PK, Putnam FW. Hypothalamic-pituitary-adrenal axis dysregulation in sexually abused girls. Journal of Endocrinology and Metabolism. 1994;78:249–255. doi: 10.1210/jcem.78.2.8106608. [DOI] [PubMed] [Google Scholar]
- De Koet CS, Vermetten E, Geuze E, Wiegant VM, Westenberg HG. Elevated plasma arginine vasopressin levels in veterans with posttraumatic stress disorder. J Psychiatr Res. 2008;42:192–8. doi: 10.1016/j.jpsychires.2006.11.009. [DOI] [PubMed] [Google Scholar]
- Deuschle M, Schweiger U, Gotthardt U, Weber B, Korner A, Schmider J, Standhardt H, Lammers CH, Krumm B, Heuser I. The combined dexamethasone/corticotropin-releasing hormone stimulation test is more closely associated with features of diurnal activity of the hypothalamo-pituitary-adrenocortical system than the dexamethasone suppression test. Biological Psychiatry. 1998;43:762–766. doi: 10.1016/s0006-3223(97)00276-x. [DOI] [PubMed] [Google Scholar]
- Distel MA, Middeldorp CM, Trull TJ, Derom CA, Willemsen G, Boomsma DI. Life events and borderline personality features: the influence of gene-environment interaction and gene-environment correlation. Psychol Med. 2010:1–12. doi: 10.1017/S0033291710001297. epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Galard R, Catalan R, Castellanos JM, Gallart JM. Plasma corticotropin-releasing factor in depressed patients before and after the dexamethasone suppression test. Biological Psychiatry. 2002;51:463–468. doi: 10.1016/s0006-3223(01)01273-2. [DOI] [PubMed] [Google Scholar]
- Golier JA, Yehuda R, Bierer LM, Mitropoulou V, New AS, Schmeidler J, Silverman JM, Siever LJ. The relationship of borderline personality disorder to posttraumatic stress disorder and traumatic events. American Journal of Psychiatry. 2003;160:2018–2024. doi: 10.1176/appi.ajp.160.11.2018. [DOI] [PubMed] [Google Scholar]
- Goekoop j.G., de Winter R, Wolterbeek R, Wiegant V. Support for two increased vasopressinergic activities in depression at large and the differential effect of antidepressant treatment. J Psychopharmacol. 2010 doi: 10.1177/0269881110372549. epub ahead. [DOI] [PubMed] [Google Scholar]
- Griep EN, Boersma JW, deKloet ER. Altered reactivity of the hypothalamic-pituitary-adrenal axis in the primary fibromyalgia syndrome. Journal of Rheumatology. 1993;20:469–474. [PubMed] [Google Scholar]
- Heim C, Ehlert U, Hanker JP, Hellhammer DH. Abuse-related posttraumatic stress disorder and alterations of the hypothalamic pituitary-adrenal axis in women with chronic pelvic pain. Psychosomatic Medicine. 1998;60:309–318. doi: 10.1097/00006842-199805000-00017. [DOI] [PubMed] [Google Scholar]
- Heim C, Mletzo T, Purselle D, Musselman DL, Nemeroff CB. The dexamethasone/corticotropin-releasing factor test in men with major depression: role of childhood trauma. Biological Psychiatry. 2008 doi: 10.1016/j.biopsych.2007.07.002. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Heuser I, Yassouridis A, Holsboer F. The combined dexamethasone/CRH test: a refined laboratory test for psychiatric disorders. Journal of Psychiatric Research. 1994;28:341–356. doi: 10.1016/0022-3956(94)90017-5. [DOI] [PubMed] [Google Scholar]
- Hsu DT, Lombardo KA, Herringa RJ, Bakshi VP, Roseboom PH, Kalin NH. Corticotropin-releasing hormone messenger RNA distribution and stress induced activation in the thalamus. Neuroscience. 2001;105:911–921. doi: 10.1016/s0306-4522(01)00239-1. [DOI] [PubMed] [Google Scholar]
- Husum H, Mathe AA. Early life stress changes concentrations of neuropeptide Y and corticotropin-releasing hormone in adult rat brain. Lithium treatment modifies these changes. Neuropsychopharmacology. 2002;27:756–764. doi: 10.1016/S0893-133X(02)00363-9. [DOI] [PubMed] [Google Scholar]
- Johnson JG, Cohen P, Chen H, Kasen S, Brook JS. Parenting behaviors associated with risk for offspring personality disorder during adulthood. Archives of General Psychiatry. 2006;63:579–587. doi: 10.1001/archpsyc.63.5.579. [DOI] [PubMed] [Google Scholar]
- Johnson JG, Cohen P, Kasen S, Ehrensaft MK, Crawford TN. Associations of parental personality disorders and axis I disorders with childrearing behavior. Psychiatry. 2006;69:336–350. doi: 10.1521/psyc.2006.69.4.336. [DOI] [PubMed] [Google Scholar]
- Johnson JG, Cohen P, Brown J, Smails EM, Bernstein DP. Childhood maltreatment increases risk for personality disorders during early adulthood. Arch Gen Psychiatry. 1999;56:607–8. doi: 10.1001/archpsyc.56.7.600. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Perel J, Dahl RE, Moreci P, Nelson B, Wells W, Ryan ND. The corticotropin-releasing hormone challenge in depressed abused, depressed nonabused, and normal control children. Biological Psychiatry. 1997;42:669–679. doi: 10.1016/s0006-3223(96)00470-2. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Baker JH. Genetic influences on measures of the environment: a systematic review. Psychological Medicine. 2007;37:615–626. doi: 10.1017/S0033291706009524. [DOI] [PubMed] [Google Scholar]
- Klaassens ER, van Noorden MS, Giltay EJ, van Pelt J, van Veen T, Zitman FG. Effects of childhood trauma on HPA-axis reactivity in women free of lifetime psychopathology. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2009;33:889–894. doi: 10.1016/j.pnpbp.2009.04.011. [DOI] [PubMed] [Google Scholar]
- Lee R, Geracioti TD, Jr., Kasckow JW, Coccaro EF. Childhood trauma and personality disorder: positive correlation with adult CSF corticotropin-releasing factor concentrations. American Journal of Psychiatry. 2005;162:995–997. doi: 10.1176/appi.ajp.162.5.995. [DOI] [PubMed] [Google Scholar]
- Lee RJ, Gollan J, Kasckow J, Geractioti T, Coccaro EF. CSF corticotropin-releasing factor in personality disorder: relationship with self-reported parental care. Neuropsychopharmacology. 2006;31:2289–2295. doi: 10.1038/sj.npp.1301104. [DOI] [PubMed] [Google Scholar]
- Lenzenweger MF, Lane MC, Loranger AW, Kessler RC. DSM-IV Personality Disorders in the National Comorbidity Survey Replication. Biological Psychiatry. 2007;62:553–564. doi: 10.1016/j.biopsych.2006.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linton EA, Lowry PJ. Comparison of a specific twosite immunoradiometric assay with radioimmunoassay for rat/human CRF-41. Regul Pept. 1986;14:69–84. doi: 10.1016/0167-0115(86)90206-5. [DOI] [PubMed] [Google Scholar]
- Liu J, Yu B, Neugebauer V, Grigoriadis DE, Rivier J, Vale WW, Shinnick-Gallagher P, Gallagher JP. Corticotropin-releasing factor and urocortin I modulat. 2004 doi: 10.1523/JNEUROSCI.5531-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meijer OC, de Lange ECM, Breimer DD, de Boer AG, Workel JO, de Kloet ER. Penetration of dexamethasone into brain glucocorticoid targets is enhanced in mdr1A P-glycoprotein knockout mice. Endocrinology. 1998;139:1789–1793. doi: 10.1210/endo.139.4.5917. [DOI] [PubMed] [Google Scholar]
- Miller AH, Spencer RL, Pulera M, Kang S, McCewen BS, Stein M. Aderenal steroid receptor activation in rat brain and pituitary following dexamethasone: implications for the dexamethasone suppression test. Biological Psychiatry. 1992;32:850–869. doi: 10.1016/0006-3223(92)90175-y. [DOI] [PubMed] [Google Scholar]
- Modell S, Lauer CJ, Schreiber W, Huber J, Krieg JC, Holsboer F. Hormonal response pattern in the combined DEX-CRH test is stable over time in subjects at high familial risk for affective disorders. Neuropsychopharmacology 1998. 1998;18:253–262. doi: 10.1016/S0893-133X(97)00144-9. [DOI] [PubMed] [Google Scholar]
- Newport DJ, Heim C, Owens MJ, Ritchie JC, Ramsey CH, Bonsall R, Miller AH, Nemeroff CB. Cerebrospinal fluid corticotropin-releasing factor (CRF) and vasopressin concentrations predict pituitary response in the CRF stimulation test: a multiple regression analysis. Neuropsychopharmacology. 2003;28:569–576. doi: 10.1038/sj.npp.1300071. [DOI] [PubMed] [Google Scholar]
- Oshima A, Miyano H, Yamashita S, Owashi T, Suzuki S, Sakano Y, Higuchi T. Psychological, autonomic, and neuroendocrine responses to acute stressors in the combined dexamethasone/CRH test: a study in healthy subjects. Journal of Psychiatric Research. 2001;35:95–104. doi: 10.1016/s0022-3956(01)00010-3. [DOI] [PubMed] [Google Scholar]
- Pavio SC. Stability of retrospective self-reports of child abuse and neglect before and after therapy for child abuse issues. Child Abuse and Neglect. 2001;25:1053–1068. doi: 10.1016/s0145-2134(01)00256-3. [DOI] [PubMed] [Google Scholar]
- Pfohl B, Blum N, Zimmerman M. Structured Interview for the Diagnosis of DSM PDs. U. Iowa. College of Medicine; Iowa City, IA: 1989. [Google Scholar]
- Plotsky PM, Thrivikraman KV, Nemeroff CB, Caldji C, Sharma S, Meaney MJ. Long-term consequences of neonatal rearing on central corticotropin-releasing factor systems in adult male rat offspring. Neuropsychopharmacology. 2005;30:2192–2204. doi: 10.1038/sj.npp.1300769. [DOI] [PubMed] [Google Scholar]
- Rasmusson AM, Lipschitz DS, Wang S, Hu S, Vojvoda D, Bremner JD, Southwick SM, Charney DS. Increased pituitary and adrenal reatvity in premenopausal women with posttraumatic stress disorder. Biological Psychiatry. 2001;15:965–977. doi: 10.1016/s0006-3223(01)01264-1. [DOI] [PubMed] [Google Scholar]
- Rinne T, de Kloet ER, Wouters L, Goekoop JG, DeRijk RH, van den Brink W. Hyperresponsiveness of hypothalamic-pituitary-adrenal axis to combined dexamethasone/corticotropin-releasing hormone challenge in female borderline personality disorder subjects with a history of sustained childhood abuse. Biological Psychiatry. 2002;52:1102–1112. doi: 10.1016/s0006-3223(02)01395-1. [DOI] [PubMed] [Google Scholar]
- Rydmark I, Wahlberg K, Ghatan PH, Modell S, Nygren A, Ingvar M, Asberg M, Heilig M. Neuroendocrine, cognitive, and structural imaging characteristics of women on longterm sickleave with job stress-induced depression. Biological Psychiatry. 2006;60:867–873. doi: 10.1016/j.biopsych.2006.04.029. [DOI] [PubMed] [Google Scholar]
- Smith MA, Davidson J, Ritchie JC, Kudler H, Lipper S, Chappell P, Nemeroff CB. The corticotropin-releasing hormone test in patients with posttraumatic stress disorder. Biological Psychiatry. 1989;26:349–355. doi: 10.1016/0006-3223(89)90050-4. [DOI] [PubMed] [Google Scholar]
- Steele H, Siever L. An attachment perspective on borderline personality disorder: advances in gene-environment considerations. Curr Psychiatry Rep. 2010;12:61–7. doi: 10.1007/s11920-009-0091-0. [DOI] [PubMed] [Google Scholar]
- Ströhle A, Scheel M, Modell S, Holsboer F. Blunted ACTH response to dexamethasone suppression-CRH stimulation in posttraumatic stress disorder. Journal of Psychiatric Research. 2008 doi: 10.1016/j.jpsychires.2008.01.015. epub. ahead of print. [DOI] [PubMed] [Google Scholar]
- Verheul R, Widiger TA. A meta-analysis of the prevalence and usage of the personality disorder not otherwise specified (PDNOS) diagnosis. J Pers Disord. 2004;18:309–19. doi: 10.1521/pedi.18.4.309.40350. [DOI] [PubMed] [Google Scholar]
- Watson S, Gallagher P, Smith MS, Ferrier IN, Young AH. The dex/CRH test- Is it better than the DST? Psychoneuroendocrinology. 2006;31:889–8984. doi: 10.1016/j.psyneuen.2006.03.001. [DOI] [PubMed] [Google Scholar]
- Widom CS, Dutton MA, Czaja SJ, DuMont KA. Development and validation of a new instrument to assess lifetime trauma and victimization history. Journal of Traumatic Stress. 2005;18:519–531. doi: 10.1002/jts.20060. [DOI] [PubMed] [Google Scholar]
- Wynn PC, Harwood JP, Catt KJ, Aguilera G. Corticotropin-releasing factor (CRF) induces desensitization of the rat pituitary CRF receptor-adenylate cyclase complex. Endocrinology. 1988;122:351–358. doi: 10.1210/endo-122-1-351. [DOI] [PubMed] [Google Scholar]