Abstract
Oral fluid collection is non-invasive and easily observed making it an attractive matrix for objectively determining smoking status. Despite large inter-subject variability, cotinine oral fluid concentrations correlate with cigarettes smoked per day (CPD). Few studies, however, assessed nicotine markers in oral fluid other than cotinine; other markers might improve smoking status assessment and/or time of last cigarette.
Materials and Methods
Smoking histories and oral fluid specimens were collected from non-treatment-seeking light (1–10 CPD) and heavy smokers (>10 CPD), and from environmentally exposed and nonexposed nonsmokers who provided written informed consent for this Institutional Review Board-approved study. Nicotine, cotinine, hydroxycotinine (OH-cotinine) and norcotinine oral fluid concentrations were quantified via liquid chromatography tandem mass spectrometry (LCMSMS).
Results
Comparison of 1, 3 and 10ng/mL oral fluid LCMSMS cutoffs demonstrated that 10ng/mL cutoffs performed optimally for cotinine, OH-cotinine, nicotine and norcotinine identifying 98, 97, 88 and 15% of self-reported smokers; 1% nonsmokers had >10ng/mL cotinine. No self-reported nonsmoker had >10ng/mL OH-cotinine, nicotine or norcotinine. Norcotinine was only identified in smokers’ oral fluid. Oral fluid nicotine, cotinine and nicotine/cotinine ratios were negatively correlated with time of last smoking (r=−0.53, −0.23, and −0.51; p<0.05) and CPD (r=0.35, 0.26 and 0.33; p<0.01), respectively.
Discussion and Conclusion
OH-cotinine performed slightly better than cotinine for distinguishing smokers from nonsmokers and should be considered as an additional oral fluid smoking indicator. Further research is required to determine if oral fluid norcotinine is a marker for distinguishing light and heavy smokers. Moderate correlations suggest nicotine, cotinine and nicotine/cotinine ratios may be useful for determining smoking recency in “spot samples” collected during nicotine cessation treatment.
Introduction
All biological tissues and fluids for monitoring an individual’s drug intake have strengths and limitations. Urine, the traditional matrix for detecting drug exposure, correlates poorly with acute drug effects, and specimens are more easily adulterated. Many drug effects correlate with blood drug concentrations, but collection is invasive requiring trained medical staff. Oral fluid, a mixture of secretions from the major and minor salivary glands and gingival crevices, is advancing as an alternative matrix for monitoring drug use in clinical and treatment settings 1. Oral fluid can be collected with numerous commercially available collection devices or simply via expectoration into a tube 1, 2. Collection is non-invasive, easily observed and has minimal chance of adulteration. The primary mechanism for drug distribution into oral fluid is passive diffusion of non-ionized, unbound drug from blood 1, 2. Limited specimen volume, low drug concentrations and contamination via smoked or oral drug administration are limitations of oral fluid testing 1.
Although smoking is decreasing in the United States, according to the 2009 National Survey on Drug Use and Health, 58.7 million Americans aged 12 years or older smoked cigarettes in the past month 3. Furthermore, the Center for Disease Control and Prevention lists tobacco smoking as the single largest preventable cause of death in the United States 4. Nicotine is extensively metabolized, primarily to cotinine via a two-step process involving cytochrome P450s 2A6 (CYP2A6) and 2B6 and aldehyde oxidase 5, 6. 3′-Hydroxycotinine (OH-cotinine) and norcotinine also are formed by CYP2A6-mediated metabolism 5, 6. Phase II glucuronide conjugation occurs with nicotine, cotinine and OH-cotinine 5, 6. Nicotine’s plasma half-life is relatively short (1.5–3.5 h) 7, while cotinine and OH-cotinine half-lives are 6–22 and 5–8 h, respectively, after intravenous administration of deuterated analogs 8, 9.
Oral fluid cotinine, the most prevalent nicotine marker, was extensively investigated as a smoking indicator in clinical research and smoking treatment studies 10–18. CYP2A6 activity is highly variable 5, 13, resulting in large inter-subject variability in cotinine oral fluid concentrations. However, oral fluid cotinine concentrations were significantly correlated with self-reported number of cigarettes smoked per day, r=0.52, p<0.001 19 and with nicotine tolerance scores assessed by the modified Fagerstrom Tolerance Questionnaire (FTQ), r=0.40, p<0.001 20. Oral fluid cotinine also correlated with all of the items in the Hooked on Nicotine Checklist (r=0.11–0.47, p<0.05), except for “Did you feel more irritable because you couldn’t smoke”, r=0.05, p>0.05 21. Other tobacco markers are less well studied. The OH-cotinine/cotinine oral fluid ratio is helpful to determine nicotine clearance and CYP2A6 activity 13, 22, 23. There may be additional markers to estimate time of last use and assess smoking patterns.
We simultaneously quantified nicotine, cotinine, OH-cotinine and norcotinine by liquid chromatography tandem mass spectrometry (LCMSMS) in oral fluid specimens from 1) nonsmokers with no passive exposure 2) nonsmokers with any passive exposure in the past month 3) smokers reporting 1–10 cigarettes per day (CPD) and 4) smokers reporting greater than 10 CPD. We evaluated multiple cutoffs for the four analytes to best distinguish these 4 groups, and investigated correlations of nicotine and metabolite oral fluid concentrations with times of last smoking and daily cigarette consumption.
Methods
Participants
Non-treatment-seeking smokers and nonsmokers were recruited; heavy smokers were defined as smoking >10 CPD and light smokers as smoking 1–10 CPD. Nonsmokers reporting any passive smoke exposure at home or work during the previous month were defined as environmentally exposed nonsmokers, and nonsmokers reporting no passive smoke exposure in the past month as nonexposed nonsmokers.
Exclusion criteria included chronic pulmonary disease and cannabis smoking greater than 5 times in the past 2 weeks or within the past 24 h. Additional exclusion criteria for smokers included: current interest in decreasing smoking or quitting; treatment for tobacco dependence within the past 3 months; use of nicotine replacement products, bupropion, or varenicline in the past 3 months; and current use of tobacco products other than cigarettes. Additional exclusion criteria for nonsmokers included use of any tobacco or nicotine product in the past 3 months.
This study protocol was approved by the National Institute on Drug Abuse Intramural Research Program Institutional Review Board and participants provided written informed consent.
Procedure
Smoking histories, breath CO, and urine and oral fluid specimens were collected. Time of day was not standardized for specimen collection. Breath CO readings were obtained with a Vitalograph breath CO instrument having ±3ppm accuracy across a 0–199ppm reporting range (Vitalograph, Lenexa, KS). Two oral fluid specimens were collected from each participant. Expectorated oral fluid specimens were analyzed by NicAlert® (Nymox Corp., Hasbrouck Heights, NJ) and oral fluid specimens collected with the Quantisal™ collection device (Immunalysis, Pomona, CA) were analyzed by LCMSMS. NicAlert is an immunochromatographic dipstick test directed against cotinine with 12–40% OH-cotinine cross-reactivity 24. One end of the test strip containing gold particles coated with monoclonal cotinine antibodies is placed in the expectorated oral fluid specimen for 20 seconds. Color change indicates gold particle migration distance on the test strip; migration distance indicates analyte concentration.
LCMSMS Nicotine and Metabolites Oral Fluid Assay
Nicotine, cotinine, OH-cotinine and norcotinine concentrations in oral fluid collected with the Quantisal device were quantified by LCMSMS according to a previously validated method 25. Extraction efficiencies from oral fluid were 56–101%, n=5. Limits of detection (LOD) and quantification (LOQ) were 0.5 and 1.0ng/mL for nicotine, 0.1 and 0.2ng/mL for cotinine, and 0.3 and 0.5ng/mL for OH-cotinine, and 0.7 and 1.0ng/mL for norcotinine, respectively. Upper limits of linearity (ULOL) were 2000ng/mL for all analytes. Calibration curves were constructed with 6–10 calibrators for each analyte in oral fluid. Inter-assay analytical recoveries (n=20; bias) were 97–111% of target concentrations and inter-assay imprecision (n=20), expressed as percent coefficient of variation, were less than 12% for nicotine and metabolites. Matrix effects (ion suppression) were less than 29% for all analytes.
Statistical Analysis
Graphpad Prism 5.02 (Graphpad Software, Inc., La Jolla, CA) was used for all statistical comparisons with p<0.05 significance threshold. Differences between heavy smokers, light smokers, environmentally exposed and nonexposed nonsmokers were examined with one-way analysis of variance with follow-up t-test comparisons (Table 1). Differences in categorical variables (e.g., sex) were examined with Pearson chi-square tests (Table 1). Participant demographic group differences between smokers and nonsmokers were examined with t-tests.
Table 1.
Self-reported demographics, smoking and exposure historiesa.
| Nonexposed Nonsmokers (n=46) | Exposed Nonsmokers (n=36) | Light Smokers (n=44) | Heavy Smokers (n=45) | Group Differences | Smokers vs. Nonsmokers | |
|---|---|---|---|---|---|---|
| Mean age (SD) yearsb | 30.9 (11.5) | 36.4 (12.8) | 39.3 (10.5) | 38.0 (9.6) | p=0.002 | p=0.002 |
| Mean years of education (SD)b | 13.7 (2.0) | 12.8 (2.3) | 12.9 (2.0) | 12.4 (1.7) | p=0.012 | p=0.032 |
| Gender (% female) | 43.5 | 38.9 | 29.5 | 35.6 | ns | ns |
| Race (%) | p=0.03 | ns | ||||
| African American | 71.7 | 91.7 | 68.3 | 55.6 | ||
| Asian | 2.2 | 0 | 4.5 | 0 | ||
| Caucasian | 23.9 | 8.3 | 22.7 | 42.2 | ||
| Other | 2.2 | 0 | 4.5 | 2.2 | ||
| Ethnicity (% Hispanic) | 4.3 | 0 | 6.8 | 2.2 | ns | ns |
|
| ||||||
| Mean time since last cigarette(min, SD) | NA | NA | 142.6 (216.1) | 111.1 (133.6) | ns | NA |
| Mean cigarettes/day (SD)c | NA | NA | 8.7 (1.7) | 18.1 (6.1) | p<0.001 | NA |
| Mean years of smoking (SD) | NA | NA | 20.1 (12.8) | 19.5 (10.0) | ns | NA |
|
| ||||||
| Passive smoke exposure-home (%) | 0 | 91.7 | 43.2 | 53.3 | ||
| Frequency exposed at home (%) | p<0.001 | p=0.004 | ||||
| Daily | 0 | 41.7 | 34.1 | 44.4 | ||
| Weekly, less than daily | 0 | 38.9 | 9.1 | 8.9 | ||
| Monthly, less than weekly | 0 | 11.1 | 0 | 0 | ||
| Never or rarely | 100.0 | 8.3 | 56.8 | 46.7 | ||
| Mean h exposed per day (SD) | NA | 2.2 (3.1) | 4.8 (3.3) | 5.2 (4.1) | p=0.004 | p=0.001 |
| Mean h since last exposure at home (SD) | NA | 35.0 (39.5) | 18.5 (30.1) | 11.1 (13.6) | p=0.015 | p=0.005 |
|
| ||||||
| Passive smoke exposure-work (%) | 0 | 13.9 | 9.1 | 20.0 | ||
| Frequency exposed at work (%) | ns | ns | ||||
| Daily | 0 | 8.3 | 9.1 | 15.6 | ||
| Weekly, less than daily | 0 | 5.6 | 0 | 4.4 | ||
| Monthly, less than weekly | 0 | 0 | 0 | 0 | ||
| Never or rarely | 100.0 | 86.1 | 90.9 | 80.0 | ||
| Mean hours exposed per day (SD) | NA | 1.4 (1.8) | 3.1 (3.5) | 3.4 (3.0) | ns | ns |
| Mean hours since last exposure at work (SD) | NA | 43.4 (46.0) | 24.1 (24.8) | 31.6 (28.2) | ns | ns |
Heavy smokers were defined as smoking >10 cigarettes/day (CPD) and light smokers as smoking 1–10 CPD. Nonsmokers reporting any passive smoke exposure at home or work during the previous month were defined as exposed nonsmokers, and nonsmokers reporting no passive smoke exposure in the past month as nonexposed nonsmokers.
Significant difference between nonexposed and exposed nonsmokers.
Significant difference between light and heavy smokers.
Correlations between nicotine, cotinine, OH-cotinine and norcotinine oral fluid concentrations were evaluated with Spearman correlation tests. Spearman correlation tests also evaluated possible correlations between nicotine and metabolite oral fluid concentrations and time of last cigarette, number of cigarettes smoked today and typical CPD. Differences between nonsmokers, light and heavy smokers’ oral fluid nicotine and metabolite concentrations were evaluated via non-parametric one-way analysis of variance (ANOVA) Kruskal-Wallis tests, expressed by the H statistic, using Prism 5.02 (p<0.05). Follow-up comparisons were conducted with Dunn’s multiple comparisons test with Bonferroni-adjusted significance thresholds equivalent to p<0.05. For statistical comparisons, 0.5 times the analyte LOQ were used for specimens containing concentrations less than the LOQ. Metabolite ratios were computed for participants with specimens exceeding assay LOQ for both analytes.
Results
Participants
Table 1 details participant demographics and smoking characteristics. There were 46 heavy smokers (>10 CPD) and 44 light smokers. Monthly passive smoke exposure was reported by 36 nonsmokers, and no passive exposure by 46 nonsmokers. One self-reported heavy smoker smoked within 30 min of oral fluid collection and had highly elevated nicotine and norcotinine LCMSMS concentrations of 12,820 and 534ng/mL, respectively. Nicotine and norcotinine oral fluid concentrations were inconsistent with all other participant data as evaluated by the Grubb’s test, p<0.05. For this reason, this participant’s data were excluded from all analyses.
Nicotine and Metabolites Oral Fluid Cutoff Concentrations for Determining Smoking Status by LCMSMS
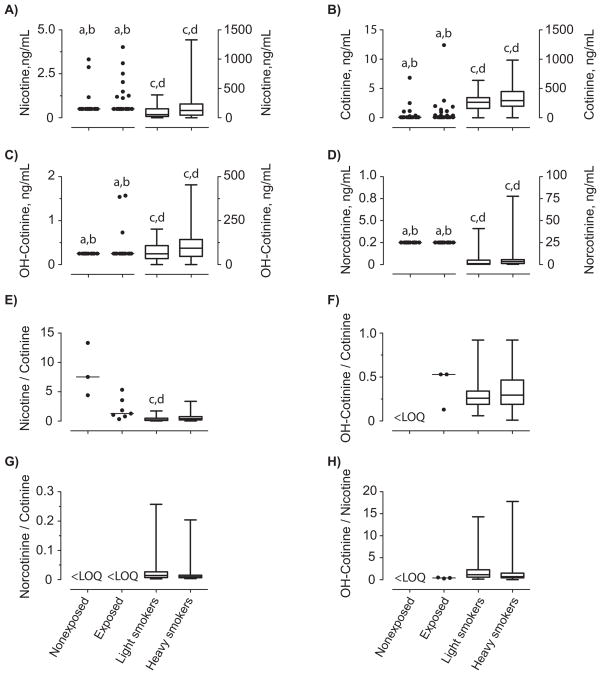
Boxplots showing nicotine, cotinine, OH-cotinine and norcotinine OF concentrations in nonexposed and environmentally exposed nonsmokers, and light and heavy smokers oral fluid specimens are presented in Figure 1. Non-parametric one-way ANOVA Kruskal-Wallis analysis revealed significant group differences for nicotine, cotinine, OH-cotinine, norcotinine, and nicotine/cotinine and OH-cotinine/nicotine ratios (H3=6.6–130.7, p<0.05). Follow-up testing revealed that both nonexposed and exposed nonsmoker nicotine, cotinine, OH-cotinine and norcotinine oral fluid concentrations were significantly lower than those of light and heavy smokers, p<0.05 (Figure 1). The only significant difference observed for nicotine/cotinine ratios was that light smokers ratios were significantly lower than in nonexposed and exposed nonsmokers. There were no significant differences within OH-cotinine/cotinine, norcotinine/cotinine and OH-cotinine/nicotine ratios. There were no significant differences between nonexposed and exposed nonsmokers or light and heavy smokers for nicotine, cotinine, OH-cotinine, norcotinine concentrations or for nicotine/cotinine, OH-cotinine/cotinine, norcotinine/cotinine and OH-cotinine/nicotine ratios (Figure 1).
Figure 1.
Oral fluid concentrations of A) nicotine, B) cotinine, C) hydroxycotinine (OH-cotinine), D) norcotinine, and ratios of oral fluid concentrations E) nicotine/cotinine, F) OH-cotinine/cotinine, G) norcotinine/cotinine and H) OH-cotinine/nicotine in nonexposed and environmentally-exposed nonsmokers, light (≤10 CPD) and heavy smokers (>10 CPD). Ratios were calculated for oral fluid specimens that were positive for both analytes. Oral fluid nicotine and metabolite concentrations were collected with the Quantisal device and quantified by LCMSMS. Nonsmoker data are scatter plots showing all data points with a bar indicating median. For light and heavy smokers, bars show median and range of observations. N=46 for nonexposed nonsmokers, 36 for exposed nonsmokers, 44 for light smokers and 45 for heavy smokers. a different from light smokers, b different from heavy smokers, c different from nonexposed nonsmokers and d different from exposed nonsmokers.
Nicotine, cotinine and OH-cotinine were present in concentrations exceeding 1.0ng/mL in >52% of all oral fluid specimens collected in the study (Table 2). Increasing the cutoff to 10ng/mL for all analytes decreased rates of detection by 11, 5 and 2% for nicotine, cotinine and OH-cotinine, respectively (Table 2). Norcotinine was identified in fewer specimens, exceeding 1.0ng/mL in 33%, and always in concentrations less than 77.6ng/mL (Table 2).
Table 2.
Evaluation of 1, 3 and 10ng/mL nicotine, cotinine, hydroxycotinine (OH-cotinine), and norcotinine liquid chromatography tandem mass spectrometry cutoffson detection rates for oral fluid specimens collected from all study participants a,b.
| A) All study participants (n=171) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Nicotine | Cotinine | OH-cotinine | Norcotinine | Nicotine alone | Cotinine alone | Nicotine & cotinine | Cotinine & OH-cotinine | Nicotine & cotinine & OH-cotinine | |
| ≥1.0ng/mL | 97 | 97 | 89 | 57 | 7 | 6 | 2 | 1 | 88 |
| Positive, % | 57% | 57% | 52% | 33% | 4.1% | 3.5% | 1.2% | 0.6% | 52% |
| Range, ng/mL | 1.2–1330.0 | 1.1–985.0 | 1.5–454.0 | 1.2–77.6 | |||||
| ≥3.0ng/mL | 88 | 89 | 86 | 40 | 2 | 1 | 2 | 2 | 84 |
| Positive, % | 52% | 52% | 50% | 23% | 1.2% | 0.6% | 1.2% | 1.2% | 49% |
| ≥10ng/mL | 78 | 88 | 86 | 13 | 0 | 1 | 1 | 9 | 77 |
| Positive, % | 46% | 52% | 50% | 7.6% | 0 | 0.6% | 0.6% | 5.3% | 45% |
OH-cotinine and norcotinine were never present without co-occurrence of nicotine or cotinine.
Nicotine and OH-cotinine were never found as the only analytes in a single specimen.
Most specimens (11/19, 9/9 and 2/3) containing 1.0–10ng/mL of nicotine, cotinine and OH-cotinine were from self-reported nonsmokers (Tables 2 and 3). Eleven nonsmokers’ specimens contained greater than 0.5ng/mL cotinine, seven were environmentally exposed and four were not. Norcotinine did not exceed 1.0ng/mL in any self-reported nonsmoker specimen (Table 3). Considering self-reports, the 10ng/mL cutoff produced the fewest false positive specimens, as only one nonsmokers’ oral fluid specimen was positive for any nicotine analyte at this threshold; 12 and 13% of nonsmokers oral fluid specimens exceeded 1ng/mL for cotinine and nicotine, respectively (Table 3).
Table 3.
Evaluation of 1, 3 and 10ng/mL nicotine, cotinine, hydroxycotinine (OH-cotinine), and norcotinine liquid chromatography tandem mass spectrometry cutoffson detection rates for oral fluid specimens collected from participants identified as nonsmokers via A) self-report, B) breath carbon monoxide and C) saliva NicAlert a,b.
| A) Self-reported nonsmokers (n=82) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Nicotine | Cotinine | OH-cotinine | Norcotinine | Nicotine alone | Cotinine alone | Nicotine & cotinine | Cotinine & OH-cotinine | Nicotine & cotinine & OH-cotinine | |
| ≥1.0ng/mL | 11 | 10 | 2 | 0 | 7 | 6 | 2 | 0 | 2 |
| Positive, % | 13% | 12% | 2.4% | 0 | 8.5% | 7.3% | 2.4% | 0 | 2.4% |
| Range, ng/mL | 1.1–4.0 | 1.1–12.4 | 1.5–1.6 | All <1.0 | |||||
| ≥3.0ng/mL | 3 | 2 | 0 | 0 | 2 | 1 | 1 | 0 | 0 |
| Positive, % | 3.7% | 2.4% | 0 | 0 | 2.4% | 1.2% | 1.2% | 0 | 0 |
| ≥10ng/mL | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Positive, % | 0 | 1.2% | 0 | 0 | 0 | 1.2% | 0 | 0 | 0 |
| B) Nonsmokers identified by breath carbon monoxide ≤5ppm (n=85) | |||||||||
| ≥10ng/mL | 1 | 5 | 4 | 0 | 0 | 1 | 0 | 3 | 1 |
| Positive, % | 1.2% | 5.9% | 4.7% | 0 | 0 | 1.2% | 0 | 3.5% | 1.2% |
| C) Nonsmokers identified by saliva NicAlert ≤10ng/mL (n=90) | |||||||||
| ≥10ng/mL | 18 | 20 | 20 | 8 | 0 | 0 | 0 | 2 | 18 |
| Positive, % | 20% | 22% | 22% | 8.9% | 0 | 0 | 0 | 2.2% | 20% |
OH-cotinine and norcotinine were never present without co-occurrence of nicotine or cotinine.
Nicotine and OH-cotinine were never found as the only analytes in a single specimen.
When evaluating detection rates for self-reported smokers at 1.0ng/mL; nicotine, cotinine and OH-cotinine identified similar numbers of smokers (>97%; Table 4). Cotinine and OH-cotinine detection rates were similar with 1, 3 and 10ng/mL cutoffs for self-reported smokers, while detection rates with nicotine decreased 8% when a higher 10ng/mL cutoff was employed (Table 4). Norcotinine was present at lower concentrations than the other analytes, poorly identifying smoking status with 64, 45 and 15% detection rates at 1, 3 and 10ng/mL cutoffs, respectively.
Table 4.
Evaluation of 1, 3 and 10ng/mL nicotine, cotinine, hydroxycotinine (OH-cotinine), and norcotinine liquid chromatography tandem mass spectrometry cutoffson detection rates of oral fluid specimens collected from participants identified as smokers via A) self-report, B) breath carbon monoxide and C) saliva NicAlert a,b.
| A) Self-reported smokers (n=89) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Nicotine | Cotinine | OH-cotinine | Norcotinine | Nicotine alone | Cotinine alone | Nicotine & cotinine | Cotinine & OH-cotinine | Nicotine & cotinine & OH-cotinine | |
| ≥1.0ng/mL | 86 | 87 | 87 | 57 | 0 | 0 | 0 | 1 | 86 |
| Positive, % | 97% | 98% | 98% | 64% | 0 | 0 | 0 | 1.1% | 97% |
| Range, ng/mL | 2.7–1330.0 | 24.7–985.0 | 2.4–454.0 | 1.2–77.6 | |||||
| ≥3.0ng/mL | 85 | 87 | 86 | 40 | 0 | 0 | 1 | 2 | 84 |
| Positive, % | 96% | 98% | 97% | 45% | 0 | 0 | 1.1% | 2.2% | 94% |
| ≥10ng/mL | 78 | 87 | 86 | 13 | 0 | 0 | 1 | 9 | 77 |
| Positive, % | 88% | 98% | 97% | 15% | 0 | 0 | 1.1% | 10% | 86% |
| B) Smokers identified by breath carbon monoxide >5ppm (n=86) | |||||||||
| ≥10ng/mL | 77 | 83 | 82 | 13 | 0 | 0 | 1 | 6 | 76 |
| Positive, % | 90% | 96% | 95% | 15% | 0 | 0 | 1.2% | 7.0% | 88% |
| C) Smokers identified by saliva NicAlert >10ng/mL (n=81) | |||||||||
| ≥10ng/mL | 60 | 68 | 66 | 5 | 0 | 1 | 1 | 7 | 59 |
| Positive, % | 74% | 84% | 82% | 6.2% | 0 | 1.2% | 1.2% | 8.6% | 73% |
OH-cotinine and norcotinine were never present without co-occurrence of nicotine or cotinine.
Nicotine and OH-cotinine were never found as the only analytes in a single specimen.
Most positive specimens contained nicotine, cotinine and OH-cotinine. In 88 cases (51% of total specimens, 91% of cotinine positive specimens); nicotine, cotinine and OH-cotinine exceeded 1.0ng/mL. All six cases with only cotinine, and two cases with only nicotine and cotinine occurred in self-reported nonsmokers.
A 5ppm breath CO cutoff accurately determined smoking status as compared to self-report (Tables 3 and 4). A combination of LCMSMS 10ng/mL oral fluid cutoff and a breath CO 5ppm cutoff yielded more nicotine positive specimens than self-report alone in nonsmokers (Table 3) and fewer nicotine positive specimens than self-report alone in smokers (Table 4). Four of five subjects with a breath CO less than 5ppm and oral fluid specimens containing >10ng/mL cotinine were self-reported smokers. Three participants had a breath CO >5ppm but no nicotine analytes exceeding 10ng/mL, two of whom reported being nonsmokers.
An oral fluid NicAlert cutoff of 10ng/mL performed poorly for differentiating nonsmokers from smokers (Tables 3 and 4). A 10ng/mL NicAlert cutoff yielded 22% false negative and 16% false positive results compared to a 10ng/mL LCMSMS cotinine reference cutoff. Evaluation of additional nicotine analytes by LCMSMS did not improve NicAlert performance (Tables 3 and 4). We also evaluated whether compensating for OH-cotinine cross-reactivity would alter NicAlert performance. LCMSMS cotinine and OH-cotinine concentrations were converted to moles and their specific cross-reactivities added (OH-cotinine cross reactivity 0.4 times that of cotinine); performance was not altered (data not shown).
Applicability of Nicotine and Metabolite Oral Fluid Concentrations as Smoking Markers
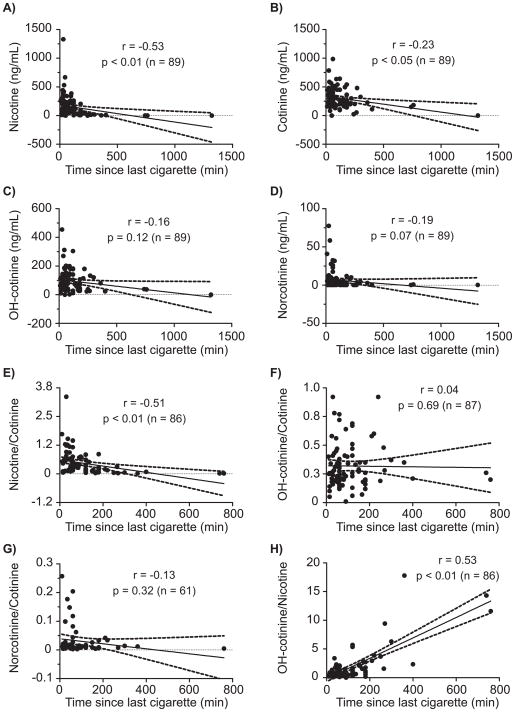
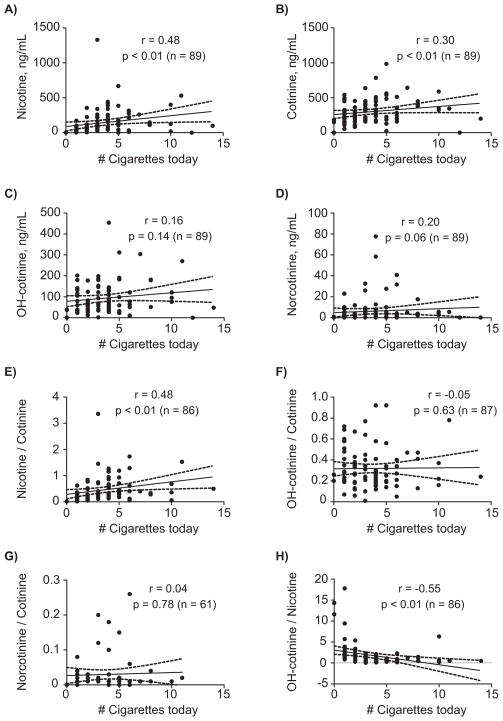
Only nicotine and cotinine oral fluid concentrations and nicotine/cotinine and OH-cotinine/nicotine ratios were significantly correlated with time of last smoking, p<0.05 (Figure 2). Correlation coefficients were −0.53 and −0.23 for nicotine and cotinine, respectively, suggesting that nicotine is a better marker for estimating time of last cigarette. In addition, we observed significant correlations between number of cigarettes smoked today compared to nicotine and cotinine oral fluid concentrations and nicotine/cotinine and OH-cotinine/nicotine ratios, p<0.05 (Figure 3), but not for OH-cotinine and norcotinine concentrations or their ratios (Figure 3). Also, significant correlations between typical CPD and nicotine (r=0.35, p<0.01), cotinine (r=0.26, p<0.01), OH-cotinine (r=0.33, p<0.01) and norcotinine (r=0.26, p<0.01) oral fluid concentrations and nicotine/cotinine ratios (r=0.29, p<0.01) were observed (data not shown). Typical CPD and OH-cotinine/cotinine, norcotinine/cotinine and OH-cotinine/nicotine ratios were not significantly correlated; p>0.05 (data not shown).
Figure 2.
Scatter plots showing correlations between time since last cigarette and A) nicotine, B) cotinine, C) hydroxycotinine (OH-cotinine), D) norcotinine, and oral fluid concentration ratios of E) nicotine/cotinine, F) OH-cotinine/cotinine, G) norcotinine/cotinine and H) OH-cotinine/nicotine in light (≤10 CPD) and heavy smokers (>10 CPD). Ratios were only calculated for oral fluid specimens positive for both analytes. Oral fluid nicotine biomarker concentrations were collected with the Quantisal device and quantified by LCMSMS.
Figure 3.
Scatter plots showing correlations between reported number of cigarettes smoked today and A) nicotine, B) cotinine, C) hydroxycotinine (OH-cotinine), D) norcotinine and oral fluid concentration ratios of E) nicotine/cotinine, F) OH-cotinine/cotinine, G) norcotinine/cotinine and H) OH-cotinine/nicotine in light (≤10 CPD) and heavy smokers (>10 CPD). Ratios were only calculated for oral fluid specimens positive for both analytes. Oral fluid nicotine biomarker concentrations were collected with the Quantisal device and quantified by LCMSMS.
Discussion
It should be noted that self-report performed well for identifying smoking status in our cohort. We do not believe that our data suggest that self-report alone is an accurate and reliable indicator of smoking status. There was no pressure or advantage to misreport smoking status; smokers and nonsmokers were recruited for this non-treatment study. This was not a smoking cessation study, and multiple specimens were tested for smoking markers. These factors may have influenced the veracity of self-report.
The high percentage of African Americans in our cohort is a limitation of our study. Ethnic differences in nicotine metabolism occur; higher plasma nicotine concentrations are found in African Americans than Caucasians 6, 26 and nicotine clearance tends to be slower in African Americans than Caucasians 6. The disproportionally high number of African Americans in our cohort may enhance detection rates for differentiating smokers from nonsmokers at higher oral fluid cutoffs than might be observed throughout the general population.
Many cutoffs suggested for nicotine oral fluid testing are extrapolated from plasma results, since research suggested that oral fluid and plasma cotinine concentrations are similar 27. Oral fluid to plasma cotinine concentration ratios were 1.2–1.4 after intravenous cotinine infusion 12 and after one nicotine transdermal administration study 18. However, a recent transdermal nicotine study found oral fluid to plasma cotinine concentration ratios of 2.7, twice as high as those found in the earlier transdermal study 17. The 10ng/mL cotinine cutoff, originally suggested by Benowitz in 1983 for plasma testing 28, appears to perform optimally for distinguishing self-reported smokers from nonsmokers in our cohort, identifying 98% of self-reported smokers and 1% of self-reported nonsmokers as smokers. Passage of smoke-free environmental legislation prompted reducing the proposed cotinine cutoff to 3ng/mL for plasma testing 27. We found that 3ng/mL cotinine oral fluid cutoff performs similar to 10ng/mL for differentiating self-reported smokers from nonsmokers; however, 2% of self-reported nonsmokers (one environmentally-exposed and one nonexposed) were identified as smokers. Decreasing the oral fluid cotinine cutoff concentration from 3 to 1ng/mL classified 8 additional participants as smokers; however all of the additionally identified smokers were self-reported nonsmokers.
With a 3 or 10ng/mL cutoff, OH-cotinine identified the same number of self-reported smokers as cotinine, but while two and one nonsmokers were positive for cotinine, no nonsmokers were positive for OH-cotinine at these cutoffs. These results indicate that OH-cotinine may perform better than cotinine for assessing smoking status with oral fluid, or that both analytes should be monitored. We did not find any advantages for differentiating smokers from nonsmokers by including nicotine at any cutoff concentration. Low norcotinine concentrations in oral fluid were observed; only exceeding 1ng/mL in 33% of specimens. All specimens containing norcotinine were in self-reported smokers. We observed a significant correlation between norcotinine and reported CPD, suggesting that oral fluid norcotinine might be able to assist distinguishing light and heavy smokers. However, smokers’ oral fluid norcotinine concentrations had large inter-subject variability and there was no statistical difference between light and heavy smokers.
Benowitz et al. recently suggested plasma concentrations between 0.05 and 0.49ng/mL identify low-level environmental tobacco smoke, 0.5–2.99ng/mL significant environmental exposure and >3ng/mL active smokers 29. We found 4 and 7 self-reported nonexposed and environmentally exposed nonsmokers had cotinine oral fluid concentrations exceeding 0.5ng/mL, respectively. This could suggest that a 0.5ng/mL cutoff is too high for identifying mild environmentally exposed individuals.
None of the examined biomarkers were effective for distinguishing exposed from nonexposed nonsmokers. It is possible that exposure during the past 30 days as our classification criteria confounds distinction between nonexposed and exposed nonsmokers. However, it should be noted that greater than 80% of our exposed nonsmokers reported weekly or more frequent exposure (Table 1). Therefore, it seems unlikely that the 30 day criterion significantly impacted our ability to distinguish exposed and nonexposed nonsmokers.
We previously reported that sensitivity and specificity were similar for breath CO and self-report for differentiating smokers and nonsmokers in this study 30. Sensitivity and specificity were 98.9 and 97.6%, respectively for self-report combined with 10ng/mL oral fluid cotinine cutoff by LCMSMS as the reference measure for assessing smoking status 30. Sensitivity and specificity were 94.4 and 96.4%, respectively, with a 5ppm breath CO cutoff confirmed with the same cotinine cutoff 30. We tested whether nicotine, OH-cotinine or norcotinine quantified by LCMSMS in oral fluid improved breath CO identification of smokers. We did not observe any increased performance for breath CO verified with multiple nicotine markers than for oral fluid cotinine alone. Breath CO performed well for identifying smokers; 96, 95 and 90% of participants identified as smokers via a 5ppm breath CO cutoff were confirmed as smokers by LCMSMS with a 10ng/mL oral fluid cutoff for cotinine, OH-cotinine and nicotine, respectively. More smokers were misidentified as nonsmokers by breath CO than by self-report; five, four and one participant(s) had breath CO<5ppm and ≥10ng/mL of cotinine, OH-cotinine or nicotine, respectively in oral fluid. No participant who self-reported being a nonsmoker had oral fluid nicotine or OH-cotinine concentrations exceeding 10ng/mL. Norcotinine oral fluid concentrations were low; confirming only 13% of smokers identified by breath CO. Additional oral fluid smoking indicators monitored by LCMSMS failed to improve breath CO performance; lack of pressure to misreport smoking status during this study likely accounts for our observed self-report veracity.
Saliva NicAlert performed poorly for distinguishing smokers from nonsmokers. A previous report documented saliva NicAlert sensitivity of 97% with a 10ng/mL NicAlert cotinine cutoff compared to 10ng/mL oral fluid cutoff via gas chromatography-nitrogen phosphorous detection 31. We observed a sensitivity of 76% for saliva NicAlert with a 10ng/mL cotinine LCMSMS cutoff 30. It is unclear why our results differ. We hypothesized that antibody cross-reactivity with other nicotine analytes could alter NicAlert results as compared to quantitative cotinine concentrations. The NicAlert cotinine specific antibody has 12–40% cross-reactivity with OH-cotinine 24. Inclusion of OH-cotinine with LCMSMS cotinine to compensate for NicAlert antibody cross-reactivity did not improve NicAlert performance.
Estimating time of last smoking may be helpful for verifying self-report during nicotine treatment studies, providing an objective biochemical measure of smoking recency. Thus, we tested whether it might be possible to employ nicotine biomarkers for estimating time of last smoking. We determined for the first time that nicotine (r=−0.53, p<0.01), cotinine (r=−0.23, p<0.05), nicotine/cotinine (r=−0.51, p<0.01) and OH-cotinine/nicotine ratios (r=0.53, p<0.01) were significantly correlated with self-reported time of last smoking. We predicted that nicotine would be the best indicator of recency of smoking given the prolonged clearance of cotinine and OH-cotinine relative to nicotine 5. Furthermore, Lea et al. demonstrated that OH-cotinine/cotinine ratios are stable in active smokers throughout the day 32. Therefore, it is not surprising that nicotine correlated best with time of last smoking. Additional studies are required to develop and evaluate models for estimating time of last smoking by nicotine, cotinine, nicotine/cotinine or OH-cotinine/nicotine oral fluid concentrations.
Oral fluid cotinine predicts CPD 10, 11, 14–16. We tested whether cotinine, nicotine or nicotine metabolites were correlated with CPD. First, we found that nicotine (r=0.48, p<0.01), cotinine (r=0.30, p<0.01), nicotine/cotinine (r=0.48, p<0.01) and OH-cotinine/nicotine ratios (r=−0.55, p<0.01) were significantly correlated, while OH-cotinine, norcotinine, OH-cotinine and norcotinine/cotinine ratios were not significantly correlated with number of cigarettes smoked today. We also found that nicotine, cotinine, OH-cotinine, norcotinine and nicotine/cotinine ratios were significantly correlated with cigarettes smoked in a typical day. This indicates that oral fluid nicotine, cotinine and nicotine/cotinine ratios are objective measures to determine smoking exposure in “spot samples” collected at varying times during smoking cessation studies.
Conclusion
We observed that OH-cotinine performed slightly better than cotinine for distinguishing smokers from nonsmokers. Norcotinine may be useful for distinguishing light from heavy smokers. LCMSMS oral fluid concentrations of nicotine, cotinine, OH-cotinine and norcotinine confirmed similar numbers of smokers determined with 5ppm breath CO cutoff or self-report. Saliva NicAlert poorly identified smokers, and accounting for antibody cross-reactivity did not improve this on-site tests’ performance. Monitoring oral fluid nicotine was better than cotinine for estimating time of last smoking; further studies are necessary to develop and evaluate models for predicting smoking recency. With our non-standardized specimen collection times, we found correlations of oral fluid nicotine, cotinine and nicotine/cotinine ratios with typical CPD and cigarettes smoked today. Thus, oral fluid nicotine, cotinine and nicotine/cotinine ratios are applicable for determining smoking exposure in “spot samples” collected during treatment studies.
Acknowledgments
This research was supported by funds from the National Institutes of Health, Intramural Research Program, National Institute on Drug Abuse.
References
- 1.Bosker WM, Huestis MA. Oral fluid testing for drugs of abuse. Clin Chem. 2009;55:1910–31. doi: 10.1373/clinchem.2008.108670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Crouch DJ. Oral fluid collection: the neglected variable in oral fluid testing. Forensic Sci Int. 2005;150:165–73. doi: 10.1016/j.forsciint.2005.02.028. [DOI] [PubMed] [Google Scholar]
- 3.Substance Abuse and Mental Health Services Administration. NSDUH Series H-38A. DHHS Publication No. SMA 10-4586Findings. Rockville, MD: Department of Health and Human Services (DHHS); 2010. Results from the 2009 National Survey on Drug Use and Health: National Findings Office of Applied Studies. [Google Scholar]
- 4.Centers for Disease Control and Prevention. [Accessed March 28, 2011];Smoking & Tobacco Use [Fast Facts] 2011 March 21; Available at: http://www.cdc.gov/tobacco/data_statistics/fact_sheets/fast_facts/index.htm.
- 5.Hukkanen J, Jacob P, 3rd, Benowitz NL. Metabolism and disposition kinetics of nicotine. Pharmacol Rev. 2005;57:79–115. doi: 10.1124/pr.57.1.3. [DOI] [PubMed] [Google Scholar]
- 6.Benowitz NL, Hukkanen J, Jacob P., 3rd Nicotine chemistry, metabolism, kinetics and biomarkers. Handb Exp Pharmacol. 2009:29–60. doi: 10.1007/978-3-540-69248-5_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Benowitz NL, Jacob P., 3rd Metabolism of nicotine to cotinine studied by a dual stable isotope method. Clin Pharmacol Ther. 1994;56:483–93. doi: 10.1038/clpt.1994.169. [DOI] [PubMed] [Google Scholar]
- 8.Zevin S, Jacob P, 3rd, Benowitz N. Cotinine effects on nicotine metabolism. Clin Pharmacol Ther. 1997;61:649–54. doi: 10.1016/S0009-9236(97)90099-0. [DOI] [PubMed] [Google Scholar]
- 9.Benowitz NL, Jacob P., 3rd Trans-3′-hydroxycotinine: disposition kinetics, effects and plasma levels during cigarette smoking. Br J Clin Pharmacol. 2001;51:53–9. doi: 10.1046/j.1365-2125.2001.01309.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Binnie V, McHugh S, Macpherson L, et al. The validation of self-reported smoking status by analysing cotinine levels in stimulated and unstimulated saliva, serum and urine. Oral Dis. 2004;10:287–93. doi: 10.1111/j.1601-0825.2004.01018.x. [DOI] [PubMed] [Google Scholar]
- 11.Blackford AL, Yang G, Hernandez-Avila M, et al. Cotinine concentration in smokers from different countries: relationship with amount smoked and cigarette type. Cancer Epidemiol Biomarkers Prev. 2006;15:1799–804. doi: 10.1158/1055-9965.EPI-06-0427. [DOI] [PubMed] [Google Scholar]
- 12.Curvall M, Elwin CE, Kazemi-Vala E, et al. The pharmacokinetics of cotinine in plasma and saliva from non-smoking healthy volunteers. Eur J Clin Pharmacol. 1990;38:281–7. doi: 10.1007/BF00315031. [DOI] [PubMed] [Google Scholar]
- 13.Dempsey D, Tutka P, Jacob P, 3rd, et al. Nicotine metabolite ratio as an index of cytochrome P450 2A6 metabolic activity. Clin Pharmacol Ther. 2004;76:64–72. doi: 10.1016/j.clpt.2004.02.011. [DOI] [PubMed] [Google Scholar]
- 14.Etter JF, Vu Duc T, Perneger TV. Saliva cotinine levels in smokers and nonsmokers. Am J Epidemiol. 2000;151:251–8. doi: 10.1093/oxfordjournals.aje.a010200. [DOI] [PubMed] [Google Scholar]
- 15.Figueiredo VC, Szklo M, Szklo AS, et al. Determinants of salivary cotinine level: a population-based study in Brazil. Rev Saude Publica. 2007;41:954–62. doi: 10.1590/s0034-89102006005000048. [DOI] [PubMed] [Google Scholar]
- 16.Jaakkola MS, Ma J, Yang G, et al. Determinants of salivary cotinine concentrations in Chinese male smokers. Prev Med. 2003;36:282–90. doi: 10.1016/s0091-7435(02)00037-3. [DOI] [PubMed] [Google Scholar]
- 17.Miller EI, Norris HR, Rollins DE, et al. Identification and quantification of nicotine biomarkers in human oral fluid from individuals receiving low-dose transdermal nicotine: a preliminary study. J Anal Toxicol. 2010;34:357–66. doi: 10.1093/jat/34.7.357. [DOI] [PubMed] [Google Scholar]
- 18.Norregaard J, Tonnesen P, Simonsen K, et al. Long-term nicotine substitution after application of a 16-hour nicotine patch in smoking cessation. Eur J Clin Pharmacol. 1992;43:57–60. doi: 10.1007/BF02280755. [DOI] [PubMed] [Google Scholar]
- 19.Rubinstein ML, Thompson PJ, Benowitz NL, et al. Cotinine levels in relation to smoking behavior and addiction in young adolescent smokers. Nicotine Tob Res. 2007;9:129–35. doi: 10.1080/14622200601078517. [DOI] [PubMed] [Google Scholar]
- 20.Prokhorov AV, De Moor C, Pallonen UE, et al. Validation of the modified Fagerstrom tolerance questionnaire with salivary cotinine among adolescents. Addict Behav. 2000;25:429–33. doi: 10.1016/s0306-4603(98)00132-4. [DOI] [PubMed] [Google Scholar]
- 21.Huang CL, Cheng CP, Lin HH, et al. Psychometric testing of the Chinese version of the Hooked on Nicotine Checklist in adolescents. J Adolesc Health. 2009;45:281–5. doi: 10.1016/j.jadohealth.2009.02.012. [DOI] [PubMed] [Google Scholar]
- 22.Levi M, Dempsey DA, Benowitz NL, et al. Prediction methods for nicotine clearance using cotinine and 3-hydroxy-cotinine spot saliva samples II. Model application. J Pharmacokinet Pharmacodyn. 2007;34:23–34. doi: 10.1007/s10928-006-9026-0. [DOI] [PubMed] [Google Scholar]
- 23.Levi M, Dempsey DA, Benowitz NL, et al. Population pharmacokinetics of nicotine and its metabolites I. Model development. J Pharmacokinet Pharmacodyn. 2007;34:5–21. doi: 10.1007/s10928-006-9027-z. [DOI] [PubMed] [Google Scholar]
- 24.Nymox Corporation. [Accessed March 28, 2011];NicAlert®: Product Insert. Available at: http://www.nymox.com/default.action?itemid=47.
- 25.Shakleya DM, Huestis MA. Optimization and validation of a liquid chromatography-tandem mass spectrometry method for the simultaneous quantification of nicotine, cotinine, trans-3′-hydroxycotinine and norcotinine in human oral fluid. Anal Bioanal Chem. 2009;395:2349–57. doi: 10.1007/s00216-009-3157-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Caraballo RS, Giovino GA, Pechacek TF, et al. Racial and ethnic differences in serum cotinine levels of cigarette smokers: Third National Health and Nutrition Examination Survey, 1988–1991. JAMA. 1998;280:135–9. doi: 10.1001/jama.280.2.135. [DOI] [PubMed] [Google Scholar]
- 27.Benowitz NL, Bernert JT, Caraballo RS, et al. Optimal serum cotinine levels for distinguishing cigarette smokers and nonsmokers within different racial/ethnic groups in the United States between 1999 and 2004. Am J Epidemiol. 2009;169:236–48. doi: 10.1093/aje/kwn301. [DOI] [PubMed] [Google Scholar]
- 28.Benowitz NL. The use of biologic fluid samples in assessing tobacco smoke consumption. In: Grabowski J, Bell C, editors. Measurement in the Analysis and Treatment of Smoking Behavior. Rockville, MD: National Institute on Drug Abuse; 1983. pp. 6–26. [PubMed] [Google Scholar]
- 29.Benowitz NL, Schultz KE, Haller CA, et al. Prevalence of smoking assessed biochemically in an urban public hospital: a rationale for routine cotinine screening. Am J Epidemiol. 2009;170:885–91. doi: 10.1093/aje/kwp215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marrone GF, Shakleya DM, Scheidweiler KB, et al. Relative performance of common biochemical indicators in detecting cigarette smoking. Addiction. 2011;106:1325–34. doi: 10.1111/j.1360-0443.2011.03441.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cooke F, Bullen C, Whittaker R, et al. Diagnostic accuracy of NicAlert cotinine test strips in saliva for verifying smoking status. Nicotine Tob Res. 2008;10:607–12. doi: 10.1080/14622200801978680. [DOI] [PubMed] [Google Scholar]
- 32.Lea RA, Dickson S, Benowitz NL. Within-subject variation of the salivary 3HC/COT ratio in regular daily smokers: prospects for estimating CYP2A6 enzyme activity in large-scale surveys of nicotine metabolic rate. J Anal Toxicol. 2006;30:386–9. doi: 10.1093/jat/30.6.386. [DOI] [PubMed] [Google Scholar]