Abstract
BACKGROUND
Loss-of-function of Pax2 results in severe defects of the male reproductive system, and Pax2 expression is detected in mouse prostate lobes and human prostatic cancers. However, the role for Pax2 in prostate development remains poorly understood.
METHODS
The expression of Pax2 was examined by in situ hybridization at various developmental stages. Urogenital sinuses (UGS) were dissected out at E18.5 from mouse Pax2 mutants and controls, cultured in vitro or grafted under the renal capsule of CD1 nude mice. The expression of prostate developmental regulatory factors was analyzed by semi-quantitative real-time PCR or immuohistochemistry.
RESULTS
Pax2 is expressed in the epithelial cells of prostate buds. Loss-of-function of Pax2 does not affect the initiation of prostatic buds, but in vitro culture assays show that the prostates of Pax2 mutants are hypomorphic and branching is severely disrupted compared to controls. RT-PCR data from Pax2 mutant prostates demonstrate increased expression levels of dorsolateral prostate (DLP) marker MSMB and ventral prostate (VP) marker SBP and dramatically reduced expression levels of anterior prostate (AP) marker TGM4.
CONCLUSIONS
Pax2 is essential for mouse prostate development and regulates prostatic ductal growth, branching and lobe-specific identity. These findings are important for understanding the molecular regulatory mechanisms in prostate development.
Keywords: Pax2, prostate development, UGS, prostate lobe-specific markers
INTRODUCTION
As a male accessory exocrine secretory sex gland, the prostate originates from the urogenital sinus (UGS), a subdivision of the cloaca. Mouse prostate epithelial buds initiate and grow out from the epithelium of the UGS and protrude into the surrounding mesenchyme of the UGS at approximately embryonic day 16 (E16). After birth, prostatic buds continue to develop and undergo numerous branching events. After branching morphogenesis, the mature prostate is comprised of three lobes, the anterior prostate (AP, also named the coagulating gland), the dorsolateral prostate (DLP) and the ventral prostate (VP) (1). Several markers of prostate lobe-specific epithelial identities have been verified in recent studies, such as Renin1 (Ren1) or Transglutaminase 4 (TGM4) for the AP, Probasin (Pbsn) and Microseminoprotein-β (MSMB) for the DLP and Spermine binding protein (SBP) for the VP (2,3).
Androgens, expressed in the epithelium, activate androgen receptors (AR) in the mesenchyme to induce early prostate morphogenesis. AR expression is detected only in the urogenital sinus mesenchyme (UGM) at early stages of prostate development, and is critical for prostate development (4-6). Estrogens are also involved in prostate growth and differentiation (7,8).
In addition to sex hormones, a variety of other factors are known to regulate aspects of prostate development. The epithelially-expressed transcription factor Nkx3.1 is among the first factors to be transcriptionally activated by androgens. Nkx3.1 is critical for prostatic ductal branching and differentiation and also play important roles in carcinogenesis (9-11). Foxa1 is expressed exclusively in the prostate epithelium and regulates prostate ductal morphogenesis by binding to a highly conserved 37-bp enhancer of the Hoxb13 gene, which is also required for normal prostate differentiation (12-14). In addition, some signaling molecules expressed in prostate epithelial cells, such as Sonic hedgehog (Shh), also impact prostate growth and epithelial differentiation (15,16). During prostate development, epithelial-mesenchymal interactions are important for prostate morphogenesis and differentiation, and mesenchymally-expressed factors, including Fgf10, Bmp4 and Sfrp1, play key roles in prostate growth (17-20).
Pax2 belongs to one of nine members of the paired box (Pax) transcription factor family and is required for kidney development (21-23). Expression of Pax2 during normal prostate development and in human prostatic cancers has been characterized, and its expression can be repressed by androgens (24,25). Urogenital defects in mice mutant for Pax2 have been reported, but defects in prostate can not be easily examined at embryonic stages (26). Here, we reported analyses of the role of Pax2 in prostate morphogenesis. In vitro organ culture assays show ductal branching of Pax2 mutant prostate is perturbed and full development of mature prostatic ducts is inhibited. Markers of prostate lobe-specific epithelial identities are severely disrupted. Expression levels of DLP marker MSMB and VP marker SBP are dramatically increased and expression of AP marker TGM4 is essentially absent in mutant prostatic tissues. Taken together, these results indicate that Pax2 gene plays essential roles in prostate development.
MATERIALS AND METHODS
In Situ Hybridization
UGS were collected at different stages of gestation, fixed in 4% paraformaldehyde, embedded into Tissue-TEK O.C.T. compound embedding medium and sectioned (12μm). Standard in situ hybridization was performed by using digoxygenin-labeled antisense RNA probes as previously reported (27,28).
Prostate Organ Culture and Renal Capsule Grafting
Pax2 mutant mice used in this study were reported previously (26). In vitro prostate culture was performed as previously described (29). E18.5 UGS from Pax2 mutants and their littermate controls were dissected out and placed on 0.4 μm Millicell filters (Millipore) in 6-well plates with DMEM media containing 2% ITS (Insulin, Transferrin and Selenious Acid, Invitrogen), 25μg/ml gentamycin, 0.25μg/ml amphotericin B and 10-8M 5α-dihydrotestosterone (DHT) for 7 days. Media was replaced every two days. At least five separate samples from Pax2 mutants and controls were analyzed in this study. For in vivo kidney capsule grafts, UGS from Pax2 mutants or their littermate controls were dissected from E18.5 embryos and seminal vesicles were removed. Tissues were then grafted under the renal capsule of at least 8-week-old CD1 male nude mice as described (http://mammary.nih.gov/tools/mousework/Cunha001/index.html). Tissues were collected after one month.
Immunohistochemisty
Tissues were fixed overnight in 4% paraformaldehyde, embedded into Tissue-TEK O.C.T. compound embedding medium, and sectioned (12μm). Tissue sections were incubated overnight at 4°C with goat anti-E-cadherin antibody (1:100, R&D), guinea pig anti-CK8/18 antibody (1:50, American Research Products), and/or Rabbit anti-P63 antibody (1:50, Santa Cruz), rinsed with PBS-Triton (0.1%), and incubated with relevant secondary antibodies for 2-3 hours at room temperature. Images were taken on Olympus BX-51 microscope with an Olympus DP70 Camera.
Real-time PCR
RNA was isolated from pooled E18.5 UGS (n=3) or individual grafted UGS tissue with the Qiagen RNeasy Micro kit (Qiagen). Reverse transcription was performed containing 1μg total RNA, 1mM dNTP mix, 50ng random hexamers, 5mM DTT and 200U Superccript II reverse transcriptase (Invitrogen). Real-time PCR was carried out by using FastStart Universal SYBR Green Master (Roche). Relative expression values were calculated as 2-ΔΔCt and values of controls were normalized to 1. Primers used in our studies are listed in Table 1.
Table I.
Primers used in this research are listed.
| Primer | Forward sequence | Reverse Sequence | Size (bp) |
|---|---|---|---|
| GAPDH | TGTCAGCAATGCATCCTGCA | CCGTTCAGCTCTGGGATGAC | 240 |
| MSMB | TGTCTGATGACTGCATGGATG | GCTCAAAGGCCTAGTAGCGTT | 131 |
| SBP | CAGAGCCCAGAATGTCCTG | AAGCCCTTGATGAACTTGAA | 121 |
| TGM4 | ATCCACGGCCAAGAATCAAGG | CTGCAATGATGAACCCTCCAA | 109 |
| Shh | CAATTACAACCCCGACATCA | GGTCACTCGCAGCTTCACTCC | 142 |
| Bmp4 | GATTGGCTCCCAAGAATCAT | CCTAGCAGGACTTGGCATAA | 114 |
| Fgf10 | CGTCAAAGCCATCAACAGCA | CCTCTATTCTCTCTTTCAGCTTACAGT | 107 |
| Foxa1 | CCATGAACAGCATGACTGCG | TCGTGATGAGCGAGATGTAGGA | 267 |
| Hoxb13 | GATGTGTTGCCAAGGTGAAC | GAGGAGGGTGCTGGACACTGG | 83 |
| Nkx3.1 | CCGAGTCTGATGCACATTTTGAG | CTGTGGCTGCTTGGTGACCTG | 95 |
| Sfrp1 | TCATGCAGTTCTTCGGCTTCTACT | TTGTCGCATGGAGGACACACGGTTGTACC | 145 |
| Sfrp2 | AGGACAACGACCTCTGCATCCC | TTCTTGGTTTTGCAGGCTTCAC | 97 |
Hematoxylin and Eosin Staining
Embryos at E18.5 were fixed overnight at 4°C in 10% formalin solution, dehydrated by graded ethanol and stored in 70% ethanol. Embryos were embedded into paraffin, sectioned at 5 μm and stained with H&E (30).
RESULTS
Pax2 expression pattern in UGS
We first examined the spatial and temporal expression profile of Pax2 gene in the UGS. In situ hybridization data showed that Pax2 gene is robustly expressed in epithelial cells of urogenital sinus, seminal vesicles and vas deferens (Fig 1A). Weak Pax2 staining can be observed in epithelial cells of growing prostate buds at E18.5 (Fig. 1A). Expression levels become stronger by P4 in epithelial cells of the prostate and are maintained in the urogenital sinus, seminal vesicles and vas deferens (Fig. 1B). This data is consistent with previous reports (24), and suggests that the Pax2 gene plays pivotal roles in mouse prostate development.
Fig. 1.
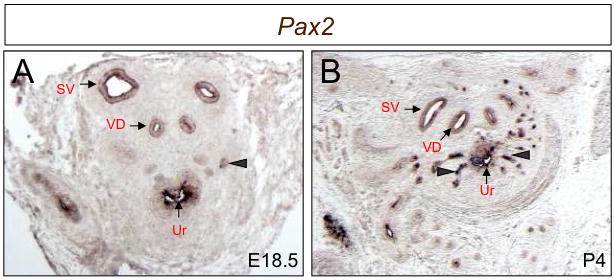
Pax2 is expressed in developing prostate epithelial buds. Pax2 transcripts are visible in epithelial cells of seminal vesicles and vas deferens at E18.5 (A) and P4 (B). Weak staining signals are observed in epithelial cells of E18.5 prostate buds (A, arrowheads) and Pax2 expression in epithelial cells of prostate buds becomes stronger at P4 (B) compared to those at E18.5 (A). SV, seminal vesicles; VD, vas deferens; Ur, urethra.
Pax2 mutant embryos exhibit normal prostatic bud initiation
To begin to evaluate Pax2 function in mouse prostate development, we sectioned through the urogenital systems of Pax2 mutants and littermate controls at E18.5 and analyzed the phenotypes in the urogenital systems by paraffin sectioning and H&E staining. Sections through E18.5 Pax2 male mutants (n=6) show that the vas deferens and seminal vesicles are absent, though the prostate buds initiate normally in mutants compared to controls (n=4) (Fig. 2). These results are consistent with previous reports (26) and show that Pax2 is not involved in the early initiation of prostatic budding, but does not exclude a later role for Pax2 in prostate development.
Fig. 2.
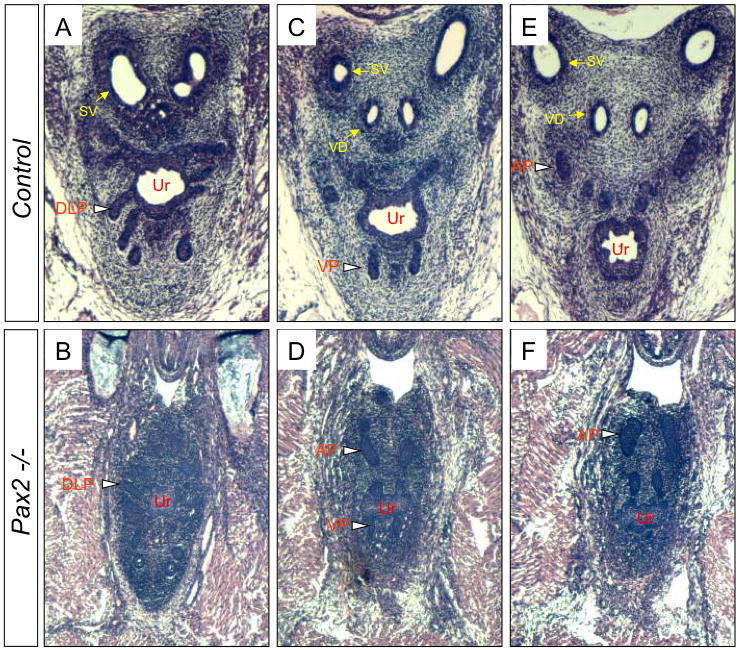
Prostate phenotypes of Pax2 mutants. Serial sections show Pax2 mutants have the normal initiation of prostate buds (blank arrowheads) and lack seminal vesicles and vas deferens (B, D and F) compared to controls (A, C and E). SV, seminal vesicles; VD, vas deferens; Ur, urethra; DLP, dorsolateral prostatic buds; VP, ventral prostatic buds; AP, anterior prostatic buds.
Early developmental regulatory genes are unaltered in Pax2 mutant UGS
Many genes involved in prostatic ductal branching and morphogenesis are well studied (reviewed in (1)). To explore the molecular mechanisms of Pax2 in prostate development and characterize defects in Pax2 mutants at early stages, expression levels of genes functioning in prostate development were examined in E18.5 Pax2 mutant UGS. Real-time PCR data showed expression levels of Shh, Fgf10, Foxa1, Hoxb13, Nkx3.1, Sfrp1 and Sfrp2 are unaltered in E18.5 Pax2 mutant UGS (Fig. 3). Bmp4 expression is reduced in Pax2 mutants at this stage (Fig. 3), indicating possible effects on this pathway.
Fig. 3.
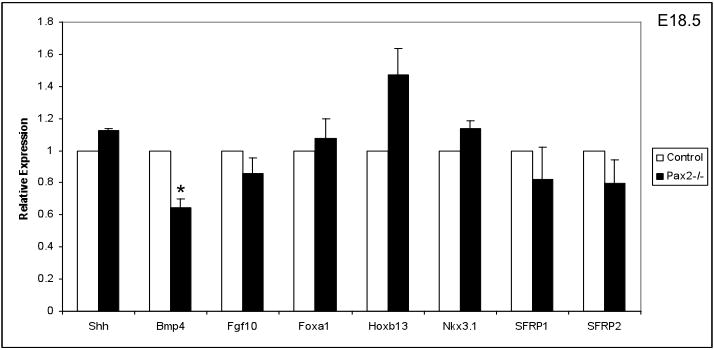
Expression of prostate developmental regulatory genes in Pax2 mutant UGS. Normal expression of Shh, Fgf10, Foxa1, Hoxb13, Nkx3.1, Sfrp1 and Sfrp2 in E18.5 Pax2 mutant UGS were observed. BMP4 expression is slightly reduced in Pax2 mutant UGS at E18.5. Relative expression is shown as mean ± SEM for at least 3 samples. Expression values of controls were normalized to 1. Significant differences between groups were determined by Students’ T test. Asterisk indicates significant differences from controls (P<0.05).
The prostatic ductal branching of Pax2 mutants is disrupted
Since Pax2 mutants do not survive postnatally and the initiation of prostatic buds of Pax2 mutants is normal, in vitro tissue culture was employed to investigate the role of Pax2 in later prostate development. Tissues were collected at E18.5 and cultured for 7 days. Pax2 mutant prostatic tissues were smaller and less developed than controls (Fig. 4A and B). Sections through these tissues demonstrate that prostatic ducts form but ductal branching is significantly decreased in Pax2 mutants (Fig. 4C and D). Ducts in Pax2 mutants are smaller than controls and most of prostate buds are rudimentary (Fig. 4D). Cultured Pax2 heterozygotes do not exhibit any phenotypic abnormalities in these experiments (data not shown). These data indicate that Pax2 is essential for postnatal prostate growth and ductal branching.
Fig. 4.
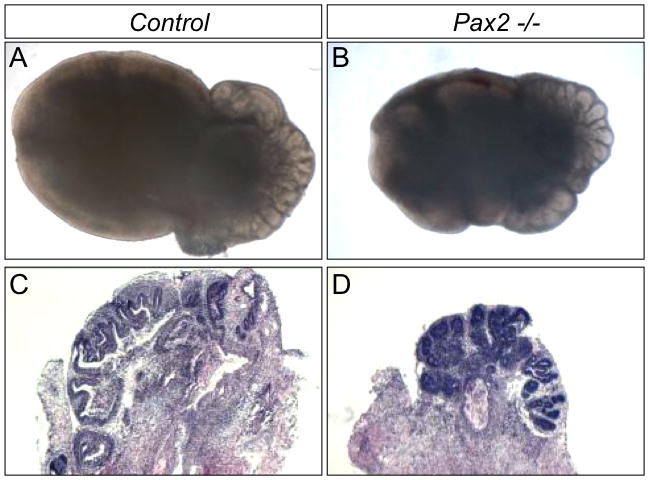
Pax2 mutant UGS collected at E18.5 and cultured for 7 days in vitro. Prostatic tissues in Pax2 mutants are smaller than controls (B and D). H&E staining of sections through cultured prostate tissues show Pax2 mutant prostatic ducts do not fully differentiate, and ductal branching is inhibited (D).
To further investigate the role of Pax2 in prostate maturation and differentiation at later stages, we employed renal capsule graft assays to allow an examination of continued development. In these experiments, UGS were dissected out at E18.5, grafted under the renal capsule of CD1 nude mice and allowed to develop for one month after grafting. At later stages of prostate maturation, as prostatic ducts begin to canalize, the epithelium is divided into two different types of cells including basal epithelial cells (P63+) and columnar luminal epithelial cells (CK8/18+), and ducts are normally surrounded by multiple cell layers of smooth muscle cells (α actin+, SMA)(reviewed in (1)) (Fig. 5A, C and E). In grafted Pax2 mutant tissues (n=3), normal smooth muscle cells surround prostatic ducts (Fig. 5B), epithelial cells stain positively for E-Cadherin, and CK8/18 and P63 expression is observed (Fig. 5B, D and F), but there is an overabundance of epithelial cells and many of these cells protrude into ductal lumen and appear highly disorganized (Fig. 5B and D). These data show that Pax2 gene plays key roles in prostatic epithelial cell proliferation and organization.
Fig. 5.
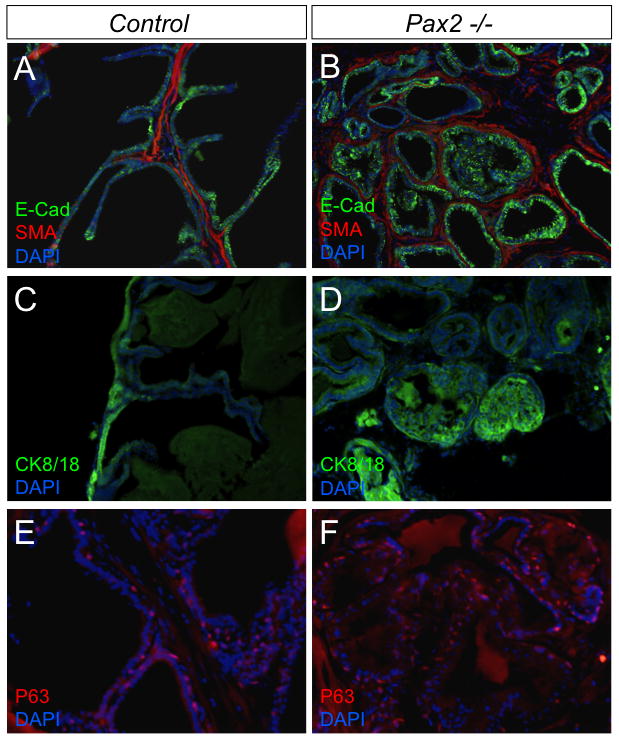
Immunohistochemical staining of grafed Pax2 mutant prostates. The E18.5 UGS were collected and grafted under the renal capsule of CD1 nude mice for 1 month. Grafted tissue sections were immunostained for SMA, E-Cadherin, CK8/18 and P63. Epithelial cells stained positively for E-Cadherin, CK8/18 and P63 could be observed in mutant tissues (B, D and F), but many epithelial cells protrude into some mutant ductal lumens and showed a high level of disorganization (B and D).
To evaluate prostatic epithelial cell differentiation in Pax2 mutants and control tissues, markers of prostate lobe-specific epithelial identities were employed. Previous reports have confirmed Microseminoprotein-β (MSMB) as a marker for the DLP, Spermine binding protein (SBP) as a marker for the VP and Transglutaminase 4 (TGM4) as a marker for the AP (3). The expression of MSMB and SBP displays over 15- and 25-fold increases, respectively, in Pax2 mutants (Fig. 6A and B). However, the expression of AP marker TGM4 is dramatically reduced in Pax2 mutants compared to controls (Fig. 6C). These results demonstrate that Pax2 is required for the normal differentiation of prostatic epithelial cells and suggest Pax2 plays a role in lobe-specific identity.
Fig. 6.
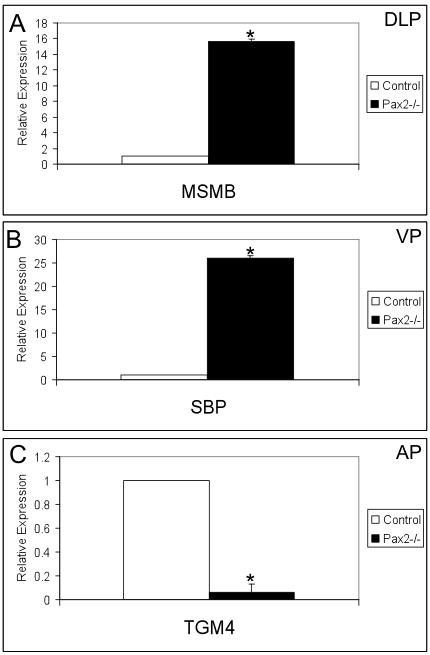
Characterization of grafted Pax2 mutant prostates by using prostate lobe-specific epithelial markers. The expression of MSMB for the DLP and SBP for the VP is increased dramatically (A and B) and TGM4 expression for the AP is dramatically reduced (C) in Pax2 mutant grafts. Three individual samples from Pax2 mutants and controls were analyzed. Asterisk indicates significant differences from controls (P<0.05).
DISCUSSION
Our data show that Pax2 plays an important role in prostatic epithelial development and differentiation (24). However, we did not observe defects in initial prostate buds in Pax2 mutants, and early patterning factors expressed in prostate epithelial cells, such as Foxa1, Hoxb13 and Nkx3.1 (9,12,13), exhibit the normal expression in Pax2 mutant UGS, consistent with relatively normal phenotypes of Pax2 mutant prostate buds. Pax2 belongs to the Class III subgroup of Pax family members, consisting of Pax2, Pax5 and Pax8. Pax5 and Pax8 genes are also expressed in E16.5 UGS (24), and it is likely that they function redundantly in early prostate development with Pax2. The generation of compound mutants of Pax2, Pax5 and Pax8 will allow this hypothesis to be tested.
In our present analyses, we found that Pax2 function is important for postnatal-stage prostatic duct growth and branching. Previous studies have shown that other factors are involved in prostatic duct development, such as epithelium-derived Shh and mesenchyme-derived Bmp4, which are required for restricting prostatic ductal growth and morphogenesis (15,16,19), and mesenchyme-derived Fgf10, which is involved in promoting ductal growth (17). Expression of Shh and Fgf10 was unchanged in our Pax2 mutant UGS at E18.5, showing that Pax2 does not appear to function upstream of these signaling pathways to promote prostate growth. A slight decrease of BMP4 expression in Pax2 mutants suggests possible interactions with this pathway.
TGM4, MSMB and SBP encode androgen-dependent prostatic secretory proteins, and are used as markers of prostate lobe-specific epithelial identity (2,3). In our grafted Pax2 mutant prostate tissues, increased expression levels of MSMB and SBP and reduced expression levels of TGM4 indicate a significant defect in establishing prostate lobe identities. Usually, these genes are expressed in completely-developed and quiescent secretory cells. Our data suggests that epithelial cells in the DLP and VP of Pax2 mutants over-proliferate, and epithelial cells in AP of Pax2 mutants do not fully develop or differentiate. Although anterior prostatic buds are normally initiated in Pax2 mutants, later development or differentiation of AP in Pax2 mutants is severely affected. This may be related to previously reported defects in the other anterior accessory gland, seminal vesicles and vas deferens.
The Pax2 phenotype shows partial similarities to Sfrp1 null mutants (18). In Sfrp1 mutants, markers for all these prostate lobe-specific identity are highly up-regulated, suggesting over-proliferation of epithelial cells (18). For Pax2 mutants, the up-regulation is only observed for the DLP and VP. Pax2 mutants exhibit the additional phenotype of an identity defect in the AP. However, Sfrp1 and Sfrp2 expression are not significantly altered in our Pax2 mutants, suggesting different mechanisms for Pax2 in prostate development or these genes are functioning in parallel.
In conclusion, our data shows that Pax2 plays essential roles in normal prostate growth, ductal branching and epithelial cell differentiation. These findings will contribute to understanding molecular regulatory mechanisms in normal prostate patterning and abnormal growth of prostate cancer.
Acknowledgments
We are grateful to Dr. Wade Bushman, Xudong Shi and Min Yu in the University of Wisconsin-Madison for their technical assistance in renal capsule graft and for many useful suggestions on prostate development. This work was supported by NIH DK077045 to D.W.
References
- 1.Marker PC, Donjacour AA, Dahiya R, Cunha GR. Hormonal, cellular, and molecular control of prostatic development. Dev Biol. 2003;253(2):165–174. doi: 10.1016/s0012-1606(02)00031-3. [DOI] [PubMed] [Google Scholar]
- 2.Cook C, Vezina CM, Allgeier SH, Shaw A, Yu M, Peterson RE, Bushman W. Noggin is required for normal lobe patterning and ductal budding in the mouse prostate. Dev Biol. 2007;312(1):217–230. doi: 10.1016/j.ydbio.2007.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thielen JL, Volzing KG, Collier LS, Green LE, Largaespada DA, Marker PC. Markers of prostate region-specific epithelial identity define anatomical locations in the mouse prostate that are molecularly similar to human prostate cancers. Differentiation. 2007;75(1):49–61. doi: 10.1111/j.1432-0436.2006.00115.x. [DOI] [PubMed] [Google Scholar]
- 4.Charest NJ, Zhou ZX, Lubahn DB, Olsen KL, Wilson EM, French FS. A frameshift mutation destabilizes androgen receptor messenger RNA in the Tfm mouse. Mol Endocrinol. 1991;5(4):573–581. doi: 10.1210/mend-5-4-573. [DOI] [PubMed] [Google Scholar]
- 5.Gaspar ML, Meo T, Bourgarel P, Guenet JL, Tosi M. A single base deletion in the Tfm androgen receptor gene creates a short-lived messenger RNA that directs internal translation initiation. Proc Natl Acad Sci U S A. 1991;88(19):8606–8610. doi: 10.1073/pnas.88.19.8606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.He WW, Kumar MV, Tindall DJ. A frame-shift mutation in the androgen receptor gene causes complete androgen insensitivity in the testicular-feminized mouse. Nucleic Acids Res. 1991;19(9):2373–2378. doi: 10.1093/nar/19.9.2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jarred RA, McPherson SJ, Bianco JJ, Couse JF, Korach KS, Risbridger GP. Prostate phenotypes in estrogen-modulated transgenic mice. Trends Endocrinol Metab. 2002;13(4):163–168. doi: 10.1016/s1043-2760(02)00575-1. [DOI] [PubMed] [Google Scholar]
- 8.Prins GS. Neonatal estrogen exposure induces lobe-specific alterations in adult rat prostate androgen receptor expression. Endocrinology. 1992;130(6):3703–3714. doi: 10.1210/endo.130.6.1597166. [DOI] [PubMed] [Google Scholar]
- 9.Bhatia-Gaur R, Donjacour AA, Sciavolino PJ, Kim M, Desai N, Young P, Norton CR, Gridley T, Cardiff RD, Cunha GR, Abate-Shen C, Shen MM. Roles for Nkx3.1 in prostate development and cancer. Genes Dev. 1999;13(8):966–977. doi: 10.1101/gad.13.8.966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sciavolino PJ, Abrams EW, Yang L, Austenberg LP, Shen MM, Abate-Shen C. Tissue-specific expression of murine Nkx3.1 in the male urogenital system. Dev Dyn. 1997;209(1):127–138. doi: 10.1002/(SICI)1097-0177(199705)209:1<127::AID-AJA12>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 11.Tanaka M, Komuro I, Inagaki H, Jenkins NA, Copeland NG, Izumo S. Nkx3.1, a murine homolog of Ddrosophila bagpipe, regulates epithelial ductal branching and proliferation of the prostate and palatine glands. Dev Dyn. 2000;219(2):248–260. doi: 10.1002/1097-0177(2000)9999:9999<::aid-dvdy1054>3.3.co;2-5. [DOI] [PubMed] [Google Scholar]
- 12.Economides KD, Capecchi MR. Hoxb13 is required for normal differentiation and secretory function of the ventral prostate. Development. 2003;130(10):2061–2069. doi: 10.1242/dev.00432. [DOI] [PubMed] [Google Scholar]
- 13.Gao N, Ishii K, Mirosevich J, Kuwajima S, Oppenheimer SR, Roberts RL, Jiang M, Yu X, Shappell SB, Caprioli RM, Stoffel M, Hayward SW, Matusik RJ. Forkhead box A1 regulates prostate ductal morphogenesis and promotes epithelial cell maturation. Development. 2005;132(15):3431–3443. doi: 10.1242/dev.01917. [DOI] [PubMed] [Google Scholar]
- 14.McMullin RP, Dobi A, Mutton LN, Orosz A, Maheshwari S, Shashikant CS, Bieberich CJ. A FOXA1-binding enhancer regulates Hoxb13 expression in the prostate gland. Proc Natl Acad Sci U S A. 2010;107(1):98–103. doi: 10.1073/pnas.0902001107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Freestone SH, Marker P, Grace OC, Tomlinson DC, Cunha GR, Harnden P, Thomson AA. Sonic hedgehog regulates prostatic growth and epithelial differentiation. Dev Biol. 2003;264(2):352–362. doi: 10.1016/j.ydbio.2003.08.018. [DOI] [PubMed] [Google Scholar]
- 16.Podlasek CA, Barnett DH, Clemens JQ, Bak PM, Bushman W. Prostate development requires Sonic hedgehog expressed by the urogenital sinus epithelium. Dev Biol. 1999;209(1):28–39. doi: 10.1006/dbio.1999.9229. [DOI] [PubMed] [Google Scholar]
- 17.Donjacour AA, Thomson AA, Cunha GR. FGF-10 plays an essential role in the growth of the fetal prostate. Dev Biol. 2003;261(1):39–54. doi: 10.1016/s0012-1606(03)00250-1. [DOI] [PubMed] [Google Scholar]
- 18.Joesting MS, Cheever TR, Volzing KG, Yamaguchi TP, Wolf V, Naf D, Rubin JS, Marker PC. Secreted frizzled related protein 1 is a paracrine modulator of epithelial branching morphogenesis, proliferation, and secretory gene expression in the prostate. Dev Biol. 2008;317(1):161–173. doi: 10.1016/j.ydbio.2008.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lamm ML, Podlasek CA, Barnett DH, Lee J, Clemens JQ, Hebner CM, Bushman W. Mesenchymal factor bone morphogenetic protein 4 restricts ductal budding and branching morphogenesis in the developing prostate. Dev Biol. 2001;232(2):301–314. doi: 10.1006/dbio.2001.0187. [DOI] [PubMed] [Google Scholar]
- 20.Thomson AA, Cunha GR. Prostatic growth and development are regulated by FGF10. Development. 1999;126(16):3693–3701. doi: 10.1242/dev.126.16.3693. [DOI] [PubMed] [Google Scholar]
- 21.Brophy PD, Ostrom L, Lang KM, Dressler GR. Regulation of ureteric bud outgrowth by Pax2-dependent activation of the glial derived neurotrophic factor gene. Development. 2001;128(23):4747–4756. doi: 10.1242/dev.128.23.4747. [DOI] [PubMed] [Google Scholar]
- 22.Dressler GR, Wilkinson JE, Rothenpieler UW, Patterson LT, Williams-Simons L, Westphal H. Deregulation of Pax-2 expression in transgenic mice generates severe kidney abnormalities. Nature. 1993;362(6415):65–67. doi: 10.1038/362065a0. [DOI] [PubMed] [Google Scholar]
- 23.Rothenpieler UW, Dressler GR. Pax-2 is required for mesenchyme-to-epithelium conversion during kidney development. Development. 1993;119(3):711–720. doi: 10.1242/dev.119.3.711. [DOI] [PubMed] [Google Scholar]
- 24.Chen Q, DeGraff DJ, Sikes RA. The developmental expression profile of PAX2 in the murine prostate. Prostate. 2010;70(6):654–665. doi: 10.1002/pros.21099. [DOI] [PubMed] [Google Scholar]
- 25.Khoubehi B, Kessling AM, Adshead JM, Smith GL, Smith RD, Ogden CW. Expression of the developmental and oncogenic PAX2 gene in human prostate cancer. J Urol. 2001;165(6 Pt 1):2115–2120. doi: 10.1097/00005392-200106000-00080. [DOI] [PubMed] [Google Scholar]
- 26.Torres M, Gomez-Pardo E, Dressler GR, Gruss P. Pax-2 controls multiple steps of urogenital development. Development. 1995;121(12):4057–4065. doi: 10.1242/dev.121.12.4057. [DOI] [PubMed] [Google Scholar]
- 27.Capellini TD, Zewdu R, Di Giacomo G, Asciutti S, Kugler JE, Di Gregorio A, Selleri L. Pbx1/Pbx2 govern axial skeletal development by controlling Polycomb and Hox in mesoderm and Pax1/Pax9 in sclerotome. Dev Biol. 2008;321(2):500–514. doi: 10.1016/j.ydbio.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dressler GR, Deutsch U, Chowdhury K, Nornes HO, Gruss P. Pax2, a new murine paired-box-containing gene and its expression in the developing excretory system. Development. 1990;109(4):787–795. doi: 10.1242/dev.109.4.787. [DOI] [PubMed] [Google Scholar]
- 29.Doles JD, Vezina CM, Lipinski RJ, Peterson RE, Bushman W. Growth, morphogenesis, and differentiation during mouse prostate development in situ, in renal grafts, and in vitro. Prostate. 2005;65(4):390–399. doi: 10.1002/pros.20321. [DOI] [PubMed] [Google Scholar]
- 30.Wellik DM, Hawkes PJ, Capecchi MR. Hox11 paralogous genes are essential for metanephric kidney induction. Genes Dev. 2002;16(11):1423–1432. doi: 10.1101/gad.993302. [DOI] [PMC free article] [PubMed] [Google Scholar]


