Abstract
T-cell activation is subject to tight regulation to avoid inappropriate responses against self-antigens. Here we show that genetic deficiency in an ubiquitin ligase, Peli1, causes hyper activation of T cells and renders T cells refractory to suppression by T regulatory cells and transforming growth factor (TGF)-β. As a result, Peli1 knockout mice spontaneously develop autoimmunity, characterized by multiorgan inflammation and autoantibody production. Peli1 deficiency results in accumulation of nuclear c-Rel, a member of the NF-κB family of transcription factors with pivotal roles in T-cell activation. Peli1 negatively regulates c-Rel by mediating its K48 ubiquitination. These results identify Peli1 as a critical factor in the maintenance of peripheral T-cell tolerance and reveal a novel mechanism of c-Rel regulation.
INTRODUCTION
T cells are primary players in adaptive immune responses against invading pathogens. Upon antigen engagement, the T cell receptor (TCR) signals T cells activation in cooperation with signals delivered by costimulatory molecules, most importantly CD28 1. Activated CD4+ and CD8+ T cells differentiate into various effector T cells that participate in pathogen clearance either directly or indirectly, by providing help for activation of other immune cells. In contrast to their efficient response to foreign antigens, T cells are normally tolerant to antigens of self-tissues, thus preventing the development of autoimmunity 2. T cell tolerance to self-antigens is controlled by different mechanisms. In addition to the developmental deletion or inactivation of autoreactive T cells by mechanisms of central tolerance 2, peripheral T cells are tightly controlled by both extrinsic and intrinsic factors 3. Among the extrinsic factors, T regulatory (Treg) cells suppress naïve T-cell activation through both physical interaction and secretion of immunosuppressive cytokines, such as TGFβ and IL-10 4–7. Intrinsic factors include various molecules that negatively regulate the TCR and CD28 signals 8. Thus, autoimmunity may occur due to either Treg defects or impaired negative regulation of TCR-CD28 signaling.
Several negative regulators of the TCR-proximal signaling have been described 9, although relatively less is known about the regulation of downstream signaling events. A critical downstream signaling event triggered by the TCR-CD28 signals is activation of the NF-κB pathway, a family of transcription factors required for T cell activation and differentiation 10, 11. Mammalian NF-κB is composed of five members, RelA, RelB, c-Rel, p50, and p52, which form various dimeric complexes and transactivate target genes via binding to an enhancer element, κB. In resting T cells, NF-κB proteins are sequestered in the cytoplasm by inhibitory proteins, termed IκBs. Canonical pathway of NF-κB activation involves phoshorylation of IκBα by the IκB kinase (IKK) and subsequent IκBα degradation, which triggers the nuclear translocation of NF-κB dimers. An important NF-κB family member involved in T cell activation is c-Rel, which mediates cytokine production, proliferation, and differentiation of T cells 12–17. Deficiency in c-Rel renders T cells more susceptible to tolerance induction 18. In contrast to the rapid and transient nature of RelA activation, the induction of c-Rel nuclear translocation is delayed and more persistent and critically dependent on CD28 costimulation 19–21. Although RelA is subject to tight control by IκBα-mediated feedback regulation, the negative regulation of c-Rel activation remains unclear.
Ubiquitination has emerged as a critical mechanism that regulates T-cell activation 9, 22. The Cbl family of ubiquitin ligases mediates lysine (K) 48 ubiquitination and degradation of TCR-proximal signaling factors, thereby negatively regulating T-cell activation 23, 24. On the other hand, ubiquitin ligases that catalyze K63-linked ubiquitin chains mediate IKK activation and positively regulate NF-κB signaling 22. More recently, a new family of E3 ligases, termed Peli (or Pellino), has been shown to catalyze formation of both K63 and K48 ubiquitin chains 25–27. Mammalian Peli family is composed of three members, Peli1, Peli2, and Peli3, which share strong sequence homology and structural domains 28, 29. The E3 ubiquitin ligase function of Peli proteins is dependent on their C-terminal RING domain 25–27. In vitro studies suggest that Peli proteins interact with IRAK1 and mediate activation of NF-κB and MAP kinases by Toll-like receptors (TLRs) and interleukin 1 receptor (IL-1R) 28, 29. In addition, Peli1 has an essential role in mediating NF-κB activation by TRIF-dependent TLRs, such as TLR3 and TLR4, although Peli1 is dispensable for NF-κB activation by the MyD88-dependent TLRs and IL-1R 30. Since Peli1 possesses both K63 and K48 E3 ligase activities, it remains an intriguing question whether it mediates distinct biological functions.
In the present study, we describe a new function of Peli1 in the regulation of T-cell activation and homeostasis. We found that Peli1 serves as a critical negative regulator of T cell activation and prevents the development of autoimmunity. This function of Peli1 is mediated through targeting c-Rel for K48 ubiquitination and degradation. Peli1 deficiency does not affect the activation of IKK but causes accumulation of nuclear c-Rel in the activated T cells. Consequently, the Peli1-deficient T cells are hyperresponsive to TCR-CD28 stimulation, and the Peli1 knockout (KO) mice develop autoimmunity. These data establish Peli1 as a critical factor that controls T cell homeostasis and peripheral tolerance and highlight a novel mechanism of c-Rel regulation. Since Peli1 does not effect upstream TCR signaling, it may be exploited as a therapeutic target for manipulating T cell responses.
RESULTS
Peli1 is a negative regulator of T cell activation
As an approach to assess the physiological functions of Peli1, we searched for its expression profile in the BioGps database (http://biogps.gnf.org). Peli1 showed abundant expression in lymphocytes, and neither Peli2 nor Peli3 displayed such an expression pattern. Real-time RT-PCR confirmed that Peli1 was abundantly expressed in lymphocytes, particularly T cells (Supplementary Fig. 1a). Furthermore, the level of Peli1 was induced along with activation of T cells by agonistic anti-CD3 and anti-CD28 antibodies (Supplementary Fig. 1b, c).
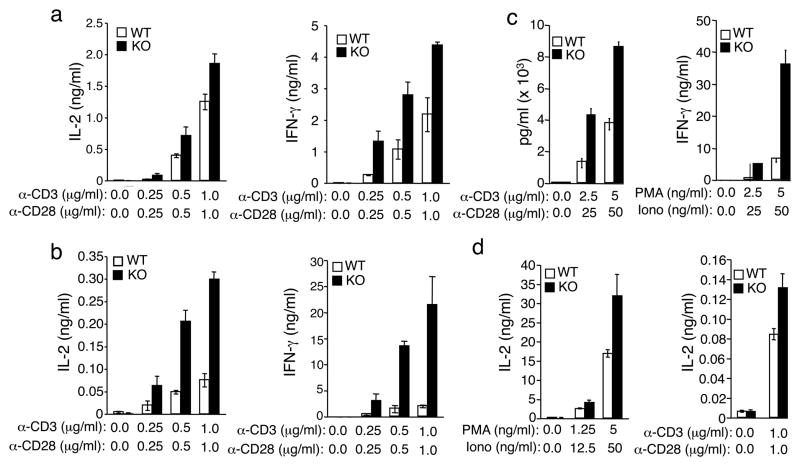
To assess the function of Peli1 in T cell activation, we stimulated the wild-type (Peli1+/+) and Peli1 knockout (Peli1−/−) T cells in vitro using anti-CD3 and anti-CD28. In contrast to its positive role in TRIF-dependent TLR signaling 30, Peli1 displayed a potent negative function in T cell activation. The Peli1−/− CD4+ T cells were hyperresponsive to CD3-CD28 stimulation, as revealed by their heightened production of the major T cell cytokines IL-2 and interferon-γ (IFN-γ) (Fig. 1a). This phenotype was even more striking in Peli1−/− CD8+ T cells (Fig. 1b). In response to both anti-CD3 and anti-CD28 and T cell mitogenes (PMA plus ionomycin) activation, the Peli1−/− CD8+ T cells produced higher amounts of IL-2 and IFN-γ than the wild-type CD8+ T cells (Fig. 1b, c). Moreover, the hyperresponsive phenotype was also readily detected with purified naïve Peli1−/− T cells (Fig. 1d and data not shown).
Figure 1. Peli1-deficient T cells are hyper-responsive to TCR and CD28 signals.
(a– d) Enzyme-linked immunosorbent assay (ELISA) of IL-2 and IFN-γsecretion by CD4+ T cells (a), CD8+ T cells (b, c), or naïve CD4+ T cells (d) purified from splenocytes of age-and sex-matched wild-type (WT) and Peli1−/− (KO) 7 week old mice and stimulated for 48 h with the indicated doses of plate-bound anti-CD3, anti-CD3 plus anti-CD28, or PMA plus ionomycin. Data are representative of 4 independent experiments performed using multiple mice.
We examined T cell proliferation using dilution of the CFSE dye. Both total and naïve Peli1−/− CD8+ T cells displayed markedly enhanced proliferation ability (Supplementary Fig. 2a, b). Notably, the loss of Peli1 rendered T cells capable of responding to TCR stimulation in the absence of CD28 ligation. Whereas wild-type T cells were only weakly stimulated at low doses of anti-CD3 antibody, the Peli1−/− T cells displayed strong proliferation ability when stimulated with even low doses of CD3 antibody. The effect of Peli1 deficiency on CD4+ T cell proliferation was less prominent (data not shown). Taken together, these results demonstrate a critical role for Peli1 in negatively regulating T cell activation.
Peli1 deficiency causes spontaneous activation of T cells in vivo
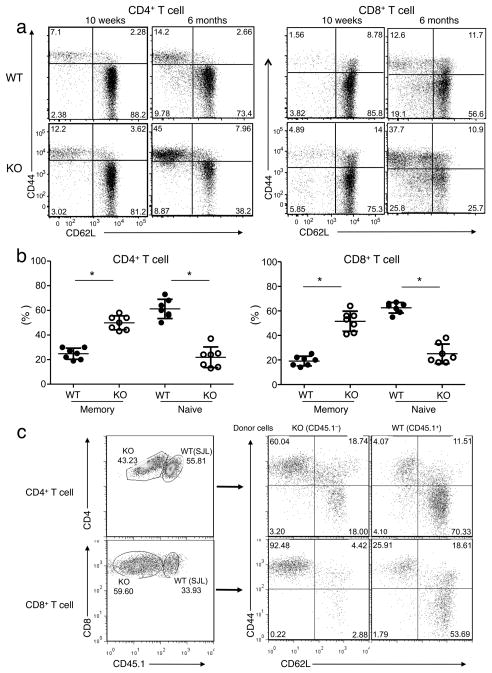
To assess the role for Peli1 in regulating T cell homeostasis in vivo, we analyzed the activation status of CD4+ and CD8+ T cells based on their surface expression of activation markers, CD44 and CD62L. In wild-type mice, the majority of T cells isolated from the spleen were in a naïve state, characterized by a CD44loCD62Lhi phenotype (Fig. 2a)., Peli1−/− mice did not show obvious abnormality in the frequency of naïve and memory T cells at an young age (5–8 weeks; data not shown) and only displayed a moderate increase in memory CD44hiCD62Llo T cells at an older age (10 weeks;Fig. 2a). However, at 6 months of age or older, Peli1−/− mice had a higher frequency of memory T cells and a concomitant decrease in naïve T cells in the spleen (Fig. 2a, b). A similar phenotype was detected in peripheral lymph nodes (Supplementary Fig. 3), although it was moderate in the mesentery lymph nodes (Supplementary Fig. 3). The absolute number of memory T cells was also higher in the spleen and peripheral lymph nodes of the Peli1−/− mice (Supplementary Fig. 4). These results suggest that Peli1 plays a critical role in maintaining the homeostasis of T cells in vivo, in line with the in vitro hyperresponsiveness of the Peli1-deficient T cells.
Figure 2. Peli1 has a cell autologous role in regulating T cell homeostasis in vivo.
(a) Frequency of naïve (CD44loCD62Lhi) and memory (CD44hiCD62Llo) CD4+ and CD8+ T cells in total splenocytes from age- and sex-matched Wild-type (WT) and Peli1−/− (KO) mice (10 week and 6 month old) gated on CD3+CD25 cells. Data are representative of 5 independent experiments with multiple mice. (b) Spleen CD4+ and CD8+ memory and naïve T cells were measured as in a using age-matched wild-type and Peli1−/− mice (6–8 month old). Data are presented as mean value of multiple mice (each circle represents an individual mouse). *P<0.001 (two-tailed unpaired t test). (c) Bone marrow cells (2 × 106) from Peli1−/− (CD45.1−; backcrossed to B6 background) and wildtype B6.SJL (CD45.1+) mice were mixed in 1:1 ratio and adoptively transferred into γ-irradiated Rag1−/− mice. After 10 weeks, recipient mice were sacrificed for flow cytometry analyses using peripheral lymph node cells. The frequency of Peli1−/− and wild-type (SJL) CD4+ and CD8+ T cells were determined based on expression of CD45.1 (left panels). The frequency of memory and naïve T cells within the Peli1−/− and wild-type (SJL) CD4+ and CD8+ T-cell populations were determined based on CD44 and CD62L markers (naïve: CD44loCD62Lhi; memory: CD44hiCD62Llo). Data are representative of four recipients of each group.
Peli1 is a T cell intrinsic regulator of activation
To examine whether the role of Peli1 in T cell regulation is cell intrinsic, we performed mixed bone marrow adoptive transfer experiments. Under such experimental conditions, wild-type and Peli1−/− T cells develop and are homeostatically maintained in the same in vivo environment. Ten weeks following mixed bone marrow adoptive transfer, we analyzed the frequency of memory and naïve T cells derived from the Wild-type and Peli1−/− bone marrow. Compared to wild-type T cells, Peli1−/− T cells had a marked increase in memory T cells (CD44hiCD62Llo) and concomitant decrease in naïve T cells (CD44loCD62Lhi) (Fig. 2c). This phenotype was detected in different peripheral lymphoid organs, including peripheral lymph nodes (Fig. 2c), spleen (Supplementary Fig. 5a), and mesentery lymph nodes (Supplementary Fig. 5b). Therefore, Peli1 has a T cell intrinsic role in the negative regulation of T cell activation.
Peli1−/− T cells are refractory to suppression by Tregs and TGF-β
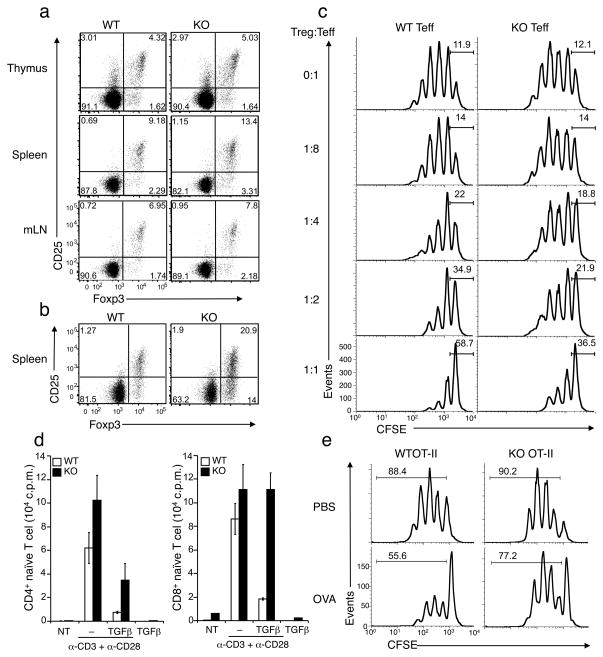
Since Treg cells play a critical role in maintaining peripheral T cell tolerance, we asked whether Peli1 influences Treg development. We analyzed the frequency of Tregs in the thymus and the peripheral lymphoid organs (spleen and lymph nodes) based on their expression of the transcription factor Foxp3 and the surface expression of CD4+ and CD25. Peli1−/− mice did not show a reduction in the frequency of Treg cells (Fig. 3a). We detected a slightly higher frequency of Treg cells in both the thymus and the peripheral lymphoid organs of young Peli1−/− mice (Fig. 3a, b). This phenotype became more prominent in older Peli1−/− mice (6 months; Fig. 3b), probably due to the higher percentage of memory T cells in the mutant mice. Notwithstanding, these results suggest that Peli1 deficiency does not cause a defect in Treg development.
Figure 3. Peli1 deficiency renders naïve T cells refractory to suppression by Tregs and TGFβ.
(a, b) Flow cytometry to determine the frequency of Tregs (percentage of CD3+CD4+ cells) in the indicated immune organs of 10 weeks (a) or 6 months (b) old mice. Data are representative of three independent experiments. (c) Naïve CD4+ T cells (Teffector) prepared from Wild-type (WT) and Peli1−/− (KO) mice (7 week) were activated in vitro in the presence of the indicated ratios (Treg-to-Teffector) of wild-type Treg cells. Teffector cell proliferation was analyzed based on CFSE dilution, showing the percentage of non-divided cells. Data are representative of two independent experiments. (d) Naïve CD4+ and CD8+ T cells were cultured for 48 h without (NT) or with the indicated inducers and subjected to proliferation assay based on 3H-thymidine incorporation. Data are representative of three independent experiments. (e) Wild-type OT-II and Peli1−/− OT-II mice were given 20 mg of oral OVA or PBS daily for 4 days. CD4+ T cells from spleen were stimulated in vitro with OVA peptide-pulsed APCs and subjected to proliferation assay based on CFSE dilution.
The strength of TCR-CD28 signaling impacts target T cell sensitivity to Treg suppression 5. Because Peli1−/− T cells are hyperresponsive to TCR-CD28 stimulation (Fig. 1), we examined whether the mutant T cells are more resistant to Treg-mediated suppression. Wild-type or Peli1−/− naïve CD4+ T cells were activated in the presence of increasing ratios of Treg cells derived from wild-type mice, and the proliferation of the target T cells was measured based on CFSE dilutions. As expected, the proliferation of wild-type CD4+ T cells was suppressed by the Treg cells in a dose dependent manner (Fig. 3c), while Peli1−/− T cells were less sensitive to Treg-mediated suppression (Fig. 3C). On the other hand, loss of Peli1 did not affect the effector function of Treg cells (Supplementary Fig. 6).
Because Treg cells are known to inhibit target cell activation by secreting TGF-β 31, 32, we examined the effect of TGF-β on CD3 and CD28-mediated T cell activation. TGF-β effectively suppressed the activation of wild-type T cells (Fig. 3d), while under the same conditions Peli1−/− T cells were markedly less susceptible to TGF-β-mediated suppression (Fig. 3d). This phenotype was particularly prominent for CD8+ T cells, although it was also detected for CD4+ T cells (2.8 fold inhibition of Peli1−/− as compared to 7.5 fold inhibition of wild-type CD4+ T cells; Fig. 3d).
Peripheral T-cell tolerance also involves the induction of anergy, as a result of TCR stimulation in the absence of costimulation 41. We examined the role of Peli1 in regulating T cell anergy using a well-characterized in vivo oral tolerance model. We crossed wild-type and Peli1−/− mice to transgenic mice expressing the ovalbumin-specific TCR (OT-II) and induced T cell tolerance by feeding the mice with OVA peptide. As expected, OVA peptide induced T cell anergy in Wild-type OT-II mice, as indicated by a 37.1% inhibition of antigen-stimulated T cell proliferation (Fig. 3e). Peli1-deficiency reduced, although did not completely block (14.4% inhibition), the induction of T cell anergy (Fig. 3e). Collectively, these data suggest that Peli1 deficiency renders CD4+ and CD8+ T cells less susceptible to suppression by Treg cells and TGF-β as well as to anergy induction, a phenotype that has also been observed with T cells lacking some other negative regulators of T cell activation5, 33, 34.
Peli1−/− mice spontaneously develop autoimmunity
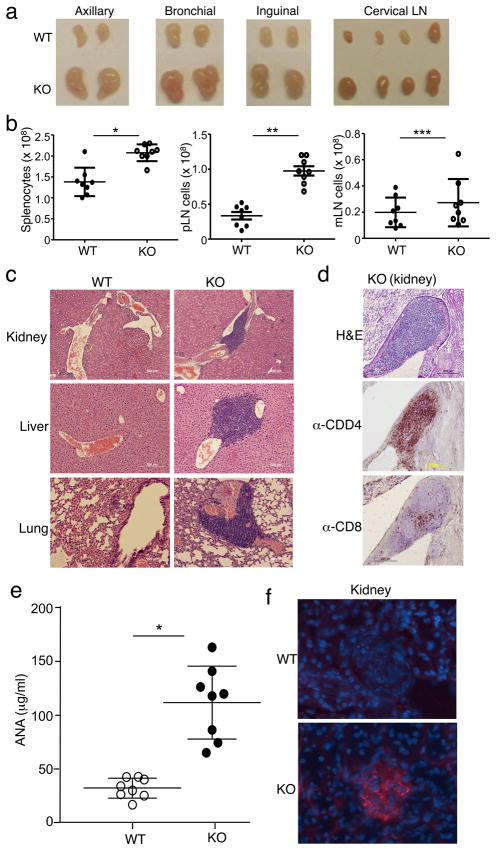
Since T cell abnormal activation is often associated with autoimmunity, we examined potential autoimmune phenotypes of the Peli1−/− mice. The peripheral lymph nodes of the mutant mice were enlarged as early as 10 weeks of age (Fig. 4a). At the age of 6 months, mutant mice also displayed moderate splenomegaly (Supplementary Fig. 7). Moreover, old Peli1−/− mice has increased cellularity in the spleen and peripheral lymph nodes, although this phenotype was moderate in mesentery lymph nodes (Fig. 4b). Histology analyses detected severe immune cell infiltration in multiple organs, including kidney, liver, and lung (Fig. 4c). Both CD4+ and CD8+ T cells were readily detected by immunohistochemistry assays in the inflammatory lesions of the Peli1−/− mice (Fig. 4d), and the lesions also contained B220+ B cells (Supplementary Fig. 8). These results are typical of autoimmune diseases associated with aberrant T cell responses. To further examine the autoimmune status of the Peli1−/− mice, we investigated the presence of serum autoantibodies and immune complex deposition in kidney glomeruli. Peli1−/− mice contained significantly higher amounts of antinuclear antibodies (ANA) in the serum (Fig. 4E) and also displayed severe immune complex deposition in the kidney glomeruli (Fig. 4F).
Figure 4. Peli1−/− mice spontaneously develop autoimmune symptoms.
(a) Lymph nodes isolated from age- and sex-matched Wild-type (WT) and Peli1−/− (KO) mice (10 week old). (b) Total cell numbers were counted in the spleen, peripheral lymph nodes (pLN), and mesentery lymph nodes (mLN) of 6 month old mice. Data are presented as mean value of multiple mice (each circle represents one mouse). *P<0.0002, **P<0.0001, and ***p=0.3457 (two-tailed unpaired t test). (c) Hematoxylin-eosin staining of the indicated tissue sections from 6 month old WILD-TYPE and KO mice, showing immune cell infiltrations. Original magnification, 100x (kidney) and 200x (liver and lung). (d) Kidney tissue sections were subjected to either hematoxylin-eosin staining or immunohistochemistry using anti-CD4 or anti-CD8 antibodies. Original magnification, 20x. Data in c and d are representative of multiple mice. (e) ANA ELISA of sera collected from age-matched wild-type and Peli1−/− mice (8 each; 10 to 24 month old). *P<0.001 (two-tailed unpaired t test). (f) Immunofluorescence staining of kidney tissue sections of wild-type and Peli1−/− mice to detect immune-complex deposits (red). Glomeruli were visualized by DAPI counterstaining (blue). Original magnification, 40x. Data are representative of 4 wild-type and 4 Peli1−/− mice.
We also examined the role of Peli1 in inducible autoimmunity and we used EAE (experimental autoimmune encephalomyelitis) as an experimental model. To avoid a contribution from innate immune cells, which also express Peli1, EAE was induced using a T cell transfer model, in which wild-type and Peli1−/− T cells were transferred into Rag1−/− mice. Consistent with their hyperresponsive phenotype, Peli1−/− T cells induced EAE with much higher disease scores than wild-type T cells (Supplementary Fig. 9a). Consistently, Peli1−/− T cells produced significantly higher frequency of inflammatory TH17 and TH1 cells (Supplementary Fig. 9b). These findings suggest that Peli1 has an important role in preventing T cell mediated autoimmunity.
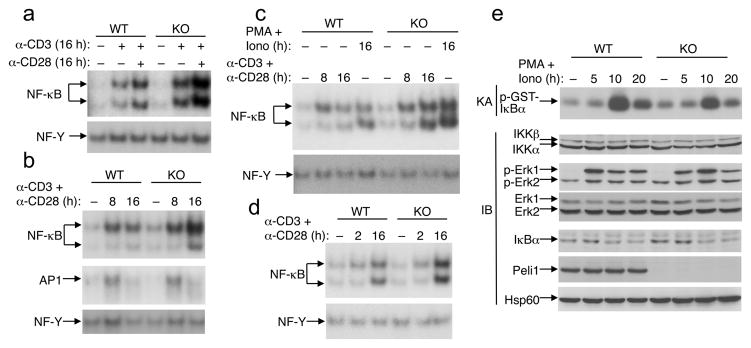
Peli1 deficiency causes hyper-activation of late-phase NF-κB
To obtain molecular insights into Peli1 function, we analyzed the effects Peli1 deficiency on TCR and CD28-stimulated signaling events. We purified T cells from young (5–8 weeks) wild-type and Peli1−/− mice, since at such young ages they did not show differences in the frequency of memory and naïve T cells (data not shown). The activation of NF-κB by anti-CD3 or anti-CD3 plus anti-CD28 was strongly enhanced in Peli1−/− T cells (Fig. 5a). We noticed that the hyper-activation of NF-κB in Peli1−/− T cells was particularly evident for the late time point of cell stimulation, while during the early phase loss of Peli1 had no obvious effect on the activation of NF-κB (Fig. 5b–d). Furthermore, in contrast to the hyper-activation of NF-κB, the activation of another T-cell transcription factor, AP-1, was largely normal (Fig. 5b), thus suggesting a specific role for Peli1 in the control of the NF-κB pathway. Similar results were obtained using purified CD4+ and CD8+ T cells (Fig. 5c and data not shown). Of note, the hyper-activation of NF-κB was also seen in Peli1−/− T cells stimulated with the T cell mitogens PMA and ionomycin (Fig. 5c), which are known to activate the PKC and calcium pathways independently of the TCR-proximal signaling steps, suggesting that Peli1 might not affect TCR proximal signaling. To exclude that the enhanced NF-κB activation in Peli1−/− T cells was not due to the presence of more memory T cells, we repeated the experiment using sorted naïve T cells (Fig. 5d). As in the case of total T cells, Peli1 deficiency promoted the delayed activation of NF-κB without appreciably altering the early phase NF-κB activation.
Figure 5. Peli1 deficiency causes hyper-activation of late-phase NF-κB.
(a) Total T cells isolated from Wild-type (WT) and Peli1−/− mice (7 week old) were either not treated (−) or stimulated (+) with plate-bound anti-CD3 and anti-CD28 antibodies (1 μg/ml). Nuclear extracts were subjected to EMSA using 32P-radiolabeled probes for NF-κB or NF-Y. (b, c) Total T cells (b) or CD8+ T cells (c) were either not treated (NT) or stimulated for the indicated times with anti-CD3 (1 μg/ml) plus anti-CD28 (1 μg/ml) or PMA (5 ng/ml) plus ionomycin (100 ng/ml). Nuclear extracts were subjected to EMSA using probes for the indicated transcription factors. (d) Naïve T cells were stimulated as indicated and subjected to EMSA as in a. (e) Total T cells were stimulated with PMA plus ionomycin, and total cell lysates were subjected to protein kinase assay (KA) or IB to detect IKK activity (panel 1) and ERK phosphorylation (panel 3) or the expression level of the indicated proteins (other panels). Data are representative of 4 independent experiments performed using multiple mice.
To understand how Peli1 negatively regulates NF-κB activation, we examined the effect of Peli1 deficiency on the activation of IKK, the protein kinase mediating activation of NF-κB. Peli1−/− T cells did not display elevated IKK activation in response to T cell mitogens (Fig. 5e). and even more, we detected a moderately reduced level of IKK activation in the Peli1−/− T cells (Fig. 5e). Peli1 deficiency did not promote mitogen-stimulated activation of the MAPK ERK (Fig. 5e). In addition, following TCR and CD28 cross-linking, we did not detect appreciable differences in the activation of Zap-70 or ERK (Supplementary Fig. 10). Taken together with the predominant effect of Peli1 deficiency on late-phase NF-κB activation, these findings suggest that Peli1 may specifically regulate a downstream step in NF-κB activation.
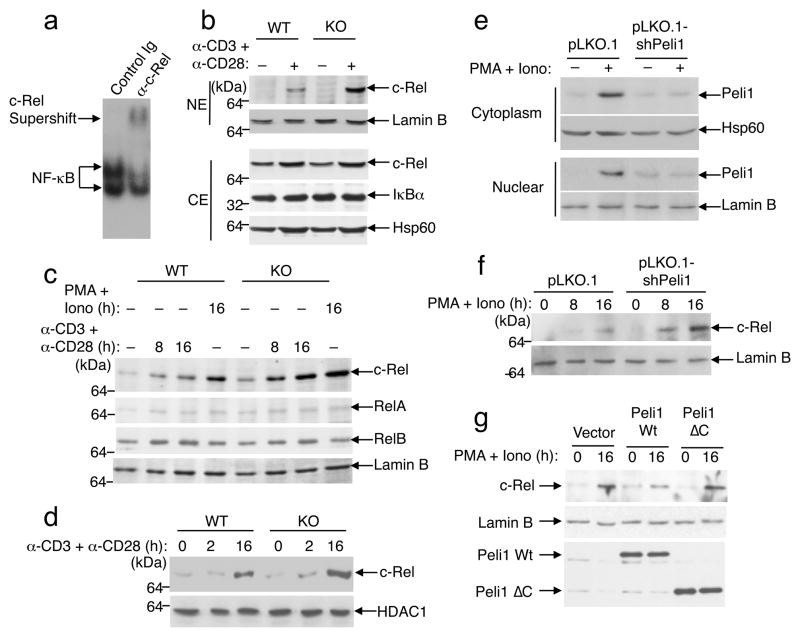
Lost of Peli1 selectively promotes activation of c-Rel
c-Rel is a late-phase NF-κB family protein activated in T cells19 and is crucial for the induction of genes involved in the activation and differentiation of T cells as well as in the prevention of T cell anergy 12, 14, 16, 35. An antibody supershift assay revealed abundant c-Rel in the late-phase NF-κB complex (Fig. 6a). We thus examined the effect of Peli1 deficiency on TCR plus CD28-stimulated nuclear expression of c-Rel and other NF-κB members. Peli1−/− T cells had markedly more nuclear c-Rel than the control wild-type T cells (Fig. 6b). In contrast to c-Rel, the nuclear level of RelA and RelB were comparable between the wild-type and Peli1−/− T cells (Fig. 6c). Hyper-activation of c-Rel was also seen in Peli1−/− T cells stimulated with PMA and ionomycin (Fig. 6c). Moreover, this phenotype was also detected in purified CD4+ and CD8+ T cells (Fig. 6c and data not shown) as well as in naïve T cells (Fig. 6d).
Figure 6. Peli1 negatively regulates c-Rel.
(a) EMSA was performed using nuclear extract from anti-CD3/CD28-stimulated (16 h) Peli1−/− (KO) T cells, in the presence of control Ig or anti-c-Rel antibody. (b) Total T cells from wild-type (WT) and Peli1−/−mice (7 week old) were incubated for 16 h in the absence (−) or presence (+) of plate-bound anti-CD3 (1 μg/ml) and anti-CD28 (1 μg/ml). Nuclear (NE) and cytoplasmic (CE) extracts were subjected to IB to detect the indicated proteins. (c) Purified CD8+ T cells from wild-type and Peli1−/− mice (7 week old) were either not treated (−) or stimulated (+) with anti-CD3 plus anti-CD28 or PMA (5 ng/ml) plus ionomycin (100 ng/ml). Nuclear extracts were subjected to IB. (d) Naïve CD4+ T cells were stimulated as indicated. Nuclear extracts were subjected to IB to detect c-Rel and HDAC1. (e) EL4 T-cells were infected with lentiviral vector pLKO.1 or pLKO.1 encoding Peli1 shRNA. The cells were either not treated (−) or stimulated (+) with PMA (5 ng/ml) plus ionomycin (100 ng/ml) for 8 h, and cytoplasmic and nuclear extracts were subjected to IB. (f) The cells from e were stimulated as indicated and subjected to IB. Data are representative of 4 independent experiments performed using multiple mice. (g) EL4 cells were infected with pRV100G vector or wild-type Peli1 or Peli1ΔC. Following stimulation, nuclear c-Rel and Lamin B and whole-cell Peli1 proteins were detected by IB.
To address whether the detected phenotype was directly caused by the loss of Peli1, we knocked down Peli1 in the EL4 T cell line by shRNA. As seen in primary T cells (Supplementary Fig. 1c), the level of Peli1 was low in untreated EL4 cells but was greatly induced in response to mitogen stimulation (Fig. 6e). Moreover, Peli1 knockdown led to a marked enhancement in c-Rel activation (Fig. 6f), while overexpression of Peli1 in EL4 cells inhibited the activation of c-Rel (Fig. 6g). Of note, modulation of c-Rel activation required the C-terminal RING domain of Peli1, since a mutant lacking this region (Peli1ΔC) did not inhibit c-Rel (Fig. 6g). Collectively, these results demonstrate a role for Peli1 in regulating c-Rel activation by TCR and CD28 signals.
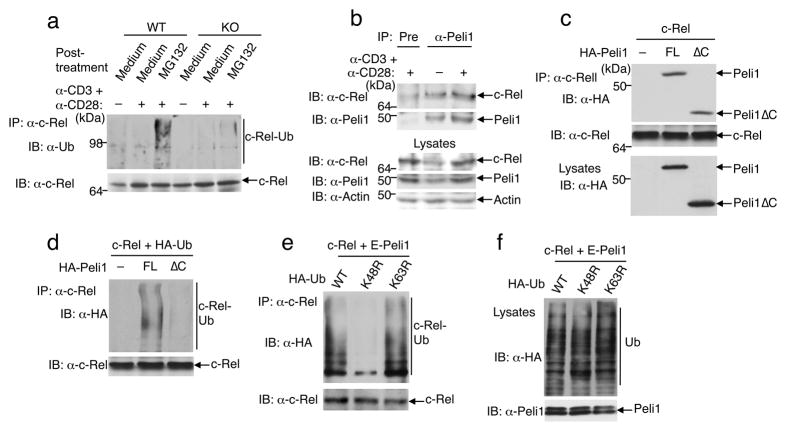
Peli1 mediates K48 ubiquitination of c-Rel
A previous study reported that c-Rel undergoes ubiquitination and degradation in leukemia T cell lines with constitutive NF-κB signaling 36. To examine whether TCR plus CD28 signals induce the ubiquitination of c-Rel and whether this event is regulated by Peli1, we stimulated T cells with anti-CD3 and anti-CD28 antibodies and then incubated them with the proteasome inhibitor MG132 to block degradation of ubiquitinated c-Rel. In wild-type T cells, c-Rel was ubiquitinated in response to TCR plus CD28 stimulation (Fig. 7a). Because the accumulation of ubiquitinated c-Rel was detected only in the presence of the proteasome inhibitor MG132, it is likely that c-Rel undergoes ubiquitin-dependent degradation in activated T cells. However, the induction of c-Rel ubiquitination was largely blocked in the Peli1−/− T cells (Fig. 7a). These results suggest the involvement of ubiquitination in the regulation of c-Rel during normal T-cell activation and establish Peli1 as an essential E3 that mediates c-Rel regulation.
Figure 7. Peli1 induces c-Rel ubiquitination.
(a) Wild-type (WT) and Peli1−/− (KO) T cells were incubated for 16 h in the absence (−) or presence (+) of anti-CD3 plus anti-CD28 and then further incubated for 6 h with medium or MG132. Cell lysates were subjected to c-Rel IP, followed by IB. (b) Wildtype T cells were not treated (−) or stimulated (+) with anti-CD3 plus anti-CD28 for 16 h. Cell lysates were subjected to c-Rel-Peli1ΔcoIP (top two panels) or direct IB (bottom three panels). Data in a and b are representative of 3 independent experiments performed using multiple mice. (c) HEK293 cells were transfected with c-Rel along with full-length (FL) Peli1 or Peli1ΔC. Total cell lysates were subjected to c-Rel-Peli1ΔcoIP (top two panels) or Peli1 IB (bottom panel). (d) 293 cells were transfected as indicated. Immunoprecipitated c-Rel was subjected to IB to detect its conjugation with HA-ubiquitin and its expression level. (e, f) 293 cells were transfected with c-Rel and Etag-Peli1 along with HA-tagged wild-type (WT) or mutant forms of ubiquitin. Cell lysates were subjected to c-Rel ubiquitination (e) or direct IB (f) assays. Data in c-f are representative of 3 independent experiments.
We further examined if a physical interaction occurred between Peli1 and c-Rel. Endogenous Peli1 and c-Rel were co-precipitated by an anti-Peli1 antibody but not by a pre-immune serum (Fig. 7b), suggesting the two proteins interact in T cells. A stronger binding of c-Rel to Peli1 was detected in CD3 plus CD28-stimulated T cells, probably due to the increase in c-Rel and Peli1 protein levels. In HEK293 cells transfected with the two proteins, Peli1 bound to c-Rel and the C-terminal RING domain of Peli1 was dispensable for this association (Fig. 7c and Supplementary Fig. 11). Moreover, Peli1 expression in HEK293 cells led to potent induction of c-Rel ubiquitination (Fig. 7d). Since the ubiquitin ligase function of Peli1 requires its C-terminal RING domain, a Peli1 mutant lacking its C-terminal region (Peli1ΔC) largely lost its ability to induce c-Rel ubiquitination (Fig. 7d), despite its ability to bind c-Rel (Fig. 7c). Similar results were obtained with a T cell line, EL4 (Supplementary Fig. 12). The defective c-Rel ubiquitination by Peli1ΔC was consistent with the inability of Peli1ΔC to inhibit c-Rel activation (Fig. 6g).
Since Peli1 is able to catalyze both K48-and K63-linked ubiquitin chains 26, we examined whether Peli1-induced c-Rel ubiquitination involved K63-or K48-linked ubiquitination chains by employing ubiquitin mutants harboring K to R mutations at K63 (K63R) and K48 (K48R). Whereas the K63R ubiquitin mutant was competent in mediating Peli1-stimulated c-Rel ubiquitination, the K48R ubiquitin mutant was defective in this modification (Fig. 7e). This result was not due to the differential expression of these ubiquitin mutants (Fig. 7f). Thus, Peli1 induces the K48-ubiquitination and degradation of c-Rel in activated T cells.
DISCUSSION
Peli family of proteins are regulators of IL-1R and TLR signaling 28, 29. Peli1 has a critical role in regulating IKK activation by TRIF-dependent TLR signaling, while being largely dispensable for IKK activation by IL-1R and the MyD88-dependent TLRs 30. In the present study, we show that Peli1 is unique in its high levels of expression in lymphocytes and that it plays an important role in the negative regulation of T-cell activation and maintenance of peripheral immune tolerance. In contrast to its role in TLR signaling, Peli1 is largely dispensable for activation of IKK by TCR signals, but mediates the ubiquitination and degradation of c-Rel. Peli1-deficient T cells are hyper-responsive to TCR and CD28 stimulation in vitro and display an activated phenotype in vivo, and Peli1−/− mice develop severe autoimmune symptoms. Although a previous work suggests c-Rel constitutive ubiquitination in transformed T-cell lines 36, whether this event occurs inducibly during normal T-cell activation has remained unknown. Our work not only identified Peli1 as a c-Rel ubiquitin ligase but also demonstrated ubiquitin-dependent degradation of c-Rel as a mechanism to negatively regulate T-cell activation.
The opposing functions of Peli1 in TLR and TCR signaling pathways are intriguing. However, similar functions have been reported for another E3 ubiquitin ligase, TRAF6. Like Peli1, TRAF6 functions as a negative regulator in T cells, but has a positive role in regulating TLR signaling 34. Precisely how TRAF6 negatively regulates T cell activation is unclear, but TRAF6-deficient T cells have higher Akt activation 34. Our current study revealed that the negative role of Peli1 in T cell activation is not due to IKK hyper-activation but rather involves accumulation of a specific NF-κB protein, c-Rel. Our data suggested that Peli1 mediates K48 ubiquitination of Peli1, in agreement with previous studies showing that Peli1 can catalyze both K48 and K63-linked ubiquitin chains 26. Dual specificity in ubiquitin chain conjugation has been reported for several other E3 ubiquitin ligases, such as the SCFβTrCP, c-IAP1 and c-IAP2, and Parkin 37–40. It remains to be investigated whether Peli1 mediates K48 ubiquitination of additional targets.
T cell activation requires both TCR and CD28 co-stimulatory signals, and stimulation of naïve T cells in the absence of the CD28 signal leads to T cell anergy 41. Accumulating evidence suggests that c-Rel is a critical factor that integrates the TCR and CD28 signals and mediates T cell activation. c-Rel activation is strictly dependent on the CD28 signal 19, 20, and c-Rel is a major transcription factor that binds to the CD28-responsive element in the promoter region of IL-2 and other T-cell cytokine genes 19, 42, 43. Indeed, c-Rel is required for both the initial activation and subsequent differentiation of T cells 12–14, 17, 35. Moreover, c-Rel deficiency renders T cells sensitive to tolerance induction and prevents the development of autoimmune inflammation 14, 18, 35. Consistent with these previous studies, we found that the hyper-activation of c-Rel in Peli1−/− T cells is associated with aberrant T cell activation. In contrast to the requirement for TCR and CD28 co-stimulation for normal T cell activation, Peli1−/− T cells could be activated by the TCR signal in the absence of CD28 co-stimulation. Thus, Peli1, likely through regulating the magnitude of c-Rel activation, controls the threshold of T cell response and maintains the requirement of CD28 co-stimulation, a mechanism that may contribute to prevention of autoimmunity.
We found that Peli1-deficient naïve T cells were less susceptible than control T cells to Treg-mediated suppression. These mutant T cells also displayed a high level of resistance to TGFβ-mediated suppression. This functional phenotype may be due to the hyper-activation of Peli1-deficient T cells, because similar phenotypes have been seen with T cells lacking other negative regulators 33, 34. T cells with hyper-active NF-κB, due to the lack of the IκB-like molecule p105, are also resistant to Treg-mediated suppression 44. It is thus likely that the hyper-activation of c-Rel contributes to the resistance of Peli1 −/−T cells to Treg- and TGFβ-mediated suppression. Of course, our data do not exclude the possible involvement of Peli1 in an additional mechanism of T cell regulation.
In summary, we show that the E3 ubiquitin ligase Peli1 is a novel negative regulator of T cell activation, which contributes to the maintenance of peripheral T cell tolerance. Our data suggest that Peli1 mediates K48 ubiquitination of c-Rel, which appears to prevent aberrant accumulation of this key NF-κB factor during T-cell activation. This is in line with its abundant expression in T cells. Thus, despite their structural homology, the Peli family members possess non-redundant functions, probably due to their differential profile of expression. Our current findings further emphasize the importance of E3 ubiquitin ligases in the regulation of immune tolerance and establish Peli1 as a potential therapeutic target in the treatment of immunological disorders.
METHODS
Mice
Peli1 KO mice (in C57BL/6x129/sv genetic background) were described 30. Where indicated, the mice were backcrossed to C57BL/6 background for 6 generations. For producing experimental animals, Peli1 heterozygous (Peli1+/−) mice were bred to generate age-matched Peli1 homozygous knockout (Peli1−/−) and wild-type mice. B6.SJL, Rag1−/−, Foxp3-GFP, and OTII TCR transgenic mice (in C57BL/6 background) were purchased from The Jackson Laboratory. Mice were maintained in specific pathogen-free facility, and all animal experiments were in accordance with protocols approved by the Institutional Animal Care and Use Committee of the University of Texas MD Anderson Cancer Center.
Plasmids, antibodies and reagents
HA-Peli1 and HA-Peli1ΔC expression vectors (pSG5-KHA2M1 based) were obtained from Dr. Seok Hee Park 45. Peli1ΔC (called Pellino-1 NM in the original paper 45) encodes the N-terminal 280 amino acids and lacks the C-terminal RING domain. Peli1 and Peli1ΔC were subcloned into a modified pRV-GFP retroviral vector (pRV100G). Etag-Peli1 was provided by Dr. Rudi Beyaert, and pcDNA-HA-ubiquitin and GST-IκBα(1–54) were described 30, 46. HA-tagged ubiquitin mutants harboring lysine-to-arginine point mutations (K48R and K63R) were provided by Dr. Zhijian Chen. Lentiviral vector pLKO.1 and pLKO.1-Peli1 shRNA were from Sigma.
Antibodies for p50 (D17), c-Rel (sc-70), c-Rel (sc-71, used for supershift), RelB (N-17), IKKα/β (H-470), IKKγ (FL419), LaminB (C20), HSP60 (H1), ubiquitin (P4D1), Peli1/2 (F-7), Tubulin (TU-02), Zap70 (1E7.2), HDAC1, (ERK (K-23), and phopho-ERK (P-ERK, E-4) were from Santa Cruz. The anti-HA-HRP (3F10) was from Roche, and the anti-p65 (E495) and anti-phospho-Zap70 (Tyr319) were from Cell Signaling. The polyclonal Peli1 antibody used in IP was generated at Cocalico Biologicals (Reamstown, PA) by immunizing rabbits with a recombinant Peli1 protein. Other antibodies were as described 30, 44, 47. The recombinant human TGFβ was purchased from PeproTech. Phorbol 12-myristate 13-acetate (PMA) and ionomycin were from Sigma.
Histology and immunohistochemistry
Organs were processed for hematoxylin-eosin staining and immunohistochemistry as described 44,50. Cryostat sections (6 um) of kidnews were fixed in aceton for 10 min and then stained with Alexa Fluor 594-conjugated goat anti-mouse IgG (invitrogen). The glomeruli were visualized by DAPI counterstaining.
Flow cytometry analyses and cell sorting
Splenic and lymph node cell suspensions were subjected to flow cytometry analyses and cell sorting as previously described 48 using a LSRII flow (BD Bioscience) and FACSAria (BD Bioscience), respectively.
Cell culture, ELISA, and proliferation assays
293T cell transfection and primary T cell isolation from immune organs were as described 30, 44. T cells were stimulated in replicate wells in 96-well plates (1 × 105 cells/well). Cell-culture supernatants were subjected to ELISA (eBioScience), and the cells were labeled for 6 h with 3H-thymidine for proliferation assays. ELISA of serum ANA was performed using a commercial kit (Alpha Diagnostic International). T-cell proliferation assays based on CFSE (carboxyl fluorescent succinimidyl ester) dilution were as described 47.
In vitro Treg assays
Treg cells were isolated from spleen of Foxp3-GFP mice by flow cytometric cell sorting based on cell surface markers (CD4+CD8−CD25+GFP+). The purity is about 97%. For isolating APC, splenocytes of wild-type mice were depleted of CD4+ and CD8+ T cells by MACS sorting. Naïve CD4+ T cells were isolated from spleen of wild-type and Peli1−/− mice by first depleting CD25+ cells and then selecting CD4+ T cells by MACS sorting. The purified naive CD4+ T cells (called Teffector cells) were labeled with 10μM CFSE for 15 min at 37°C, and the CFSE-labeled T cells (5 × 104) were co-cultured with Treg cells (at different Treg-to-Teffector ratios) in the presence of anti-CD3 (1 μg/ml) plus γ-irradiated APCs (5 × 104) in 96-well plates for 4 days. The proliferation of the Teffector cells was analyzed based on CFSE dilution.
Mixed bone marrow transfer
Rag1−/− mice were exposed to 900 cGy total body irradiation and, 6 h later, were intravenously transferred with 2×106 bone marrow cells mixed from Peli1−/− (CD45.1−; backcrossed to B6 background for 6 generations) and Wild-type B6.SJL (CD45.1+) mice in 1:1 ratio. After 10 weeks, recipient mice were sacrificed for flow cytometry analysis of Peli1−/− and Wild-type T cells by gating on CD45.1− and CD45.1+ cells.
Oral tolerance induction
Wild-type and Peli1−/− mice were crossed with OTII TCR transgenic mice and intragastrically injected with 20 mg of ovalbumin (Grade V; Sigma, St. Louis, MO) or PBS daily for 4 days. 6 days after the last injection, spleen CD4+ T cells were stimulated in vitro in the presence of irradiated APCs pulsed with OVA323-339 peptide. After 72 h, cell proliferation was measured by flow cytometry based on CFSE dilution.
IB, in vitro kinase assay, and ubiquitination assay
IB and kinase assays were as described 49. For ubiquitination assays, T cells or transfected HEK293 cells were lysed in RIPA buffer supplemented with 4 mM N-ethylmaleimide (NEM). The cell lysates were immediately boiled for 5 min in the presence of 1% SDS and then diluted 10 times with RIPA buffer. Ubiquitinated c-Rel was isolated by IP and detected by IB.
Analysis of TCR-proximal signaling
Spleen T cells were resuspended in RPMI media supplemented with 10% FCS and incubated on ice for 15 min with anti-CD3 (1 μg/ml) plus anti-CD28 (1 μg/ml). The cells were washed once with 500 μl of cold RIPA media and stimulated by crosslinking at 37°C with goat anti-hamster Ig (45 μg/ml) 48.
Statistical analysis
Two-tailed unpaired t test statistical analysis was performed using the Prism software. P value <0.05 and <0.01 means significance and very significance, respectively.
Supplementary Material
Acknowledgments
We thank TIGM for providing the Peli1−/− mice, S. H. Park, R. Beyaert, X. Qin, and Z. Chen for reagents. We also thank the personnel from the flow cytometry core facility (K. Dwyer, K. Ramirez and K. Ackin) and the histology core facility (S. Mudd) of MD Anderson Cancer Center for technical assistance. This study was supported by grants from the US National Institutes of Health (AI057555, AI064639, GM84459, and GM84459-S1).
Footnotes
AUTHOR CONTRIBUTIONS
M.C. and W.J. designed and performed the research and prepared the figures; J.H.C performed the in vitro Treg assays and OVA tolerance assays; Y.X., J.Y., and X.Z. performed the EAE experiment; G.B. performed the EL4 cell Peli1 knockdown and overexpression experiments; Y.H.W. performed the histology and immunohistochemistry; X.C. constructed Peli1 expression vectors; P.L., B.A.R., and P.H. contributed reagents; and S.C.S. designed the research and wrote the manuscript.
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interests.
References
- 1.Smith-Garvin JE, Koretzky GA, Jordan MS. T cell activation. Annu Rev Immunol. 2009;27:591–619. doi: 10.1146/annurev.immunol.021908.132706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goodnow CC, Sprent J, Fazekas de St Groth B, Vinuesa CG. Cellular and genetic mechanisms of self tolerance and autoimmunity. Nature. 2005;435:590–597. doi: 10.1038/nature03724. [DOI] [PubMed] [Google Scholar]
- 3.Tzachanis D, Lafuente EM, Li L, Boussiotis VA. Intrinsic and extrinsic regulation of T lymphocyte quiescence. Leuk Lymphoma. 2004;45:1959–1967. doi: 10.1080/1042819042000219494. [DOI] [PubMed] [Google Scholar]
- 4.von Boehmer H. Mechanisms of suppression by suppressor T cells. Nat Immunol. 2005;6:338–344. doi: 10.1038/ni1180. [DOI] [PubMed] [Google Scholar]
- 5.Sojka DK, Huang YH, Fowell DJ. Mechanisms of regulatory T-cell suppression-a diverse arsenal for a moving target. Immunology. 2008;124:13–22. doi: 10.1111/j.1365-2567.2008.02813.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lu LF, Rudensky A. Molecular orchestration of differentiation and function of regulatory T cells. Genes Dev. 2009;23:1270–1282. doi: 10.1101/gad.1791009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wan YY. Regulatory T cells: immune suppression and beyond. Cell Mol Immunol. 2010;7:204–210. doi: 10.1038/cmi.2010.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wells AD. New insights into the molecular basis of T cell anergy: anergy factors, avoidance sensors, and epigenetic imprinting. J Immunol. 2009;182:7331–7341. doi: 10.4049/jimmunol.0803917. [DOI] [PubMed] [Google Scholar]
- 9.Liu YC. Ubiquitin ligases and the immune response. Annu Rev Immunol. 2004;22:81–127. doi: 10.1146/annurev.immunol.22.012703.104813. [DOI] [PubMed] [Google Scholar]
- 10.Hayden MS, Ghosh S. Shared principles in NF-kappaB signaling. Cell. 2008;132:344–362. doi: 10.1016/j.cell.2008.01.020. [DOI] [PubMed] [Google Scholar]
- 11.Vallabhapurapu S, Karin M. Regulation and function of NF-kappaB transcription factors in the immune system. Annu Rev Immunol. 2009;27:693–733. doi: 10.1146/annurev.immunol.021908.132641. [DOI] [PubMed] [Google Scholar]
- 12.Köntgen F, et al. Mice lacking the c-rel proto-oncogene exhibit defects in lymphocyte proliferation, humoural immunity, and interleukin-2 expression. Genes & Dev. 1995;9:1965–1977. doi: 10.1101/gad.9.16.1965. [DOI] [PubMed] [Google Scholar]
- 13.Liou HC, et al. c-Rel is crucial for lymphocyte proliferation but dispensable for T cell effector function. Int Immunol. 1999;11:361–371. doi: 10.1093/intimm/11.3.361. [DOI] [PubMed] [Google Scholar]
- 14.Hilliard BA, et al. Critical roles of c-Rel in autoimmune inflammation and helper T cell differentiation. J Clinic Invest. 2002;110:843–850. doi: 10.1172/JCI15254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mason NJ, Liou HC, Hunter CA. T cell-intrinsic expression of c-Rel regulates Th1 cell responses essential for resistance to Toxoplasma gondii. J Immunol. 2004;172:3704–3711. doi: 10.4049/jimmunol.172.6.3704. [DOI] [PubMed] [Google Scholar]
- 16.Banerjee D, Liou HC, Sen R. c-Rel-dependent priming of naive T cells by inflammatory cytokines. Immunity. 2005;23:445–458. doi: 10.1016/j.immuni.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 17.Chen G, et al. Regulation of the IL-21 gene by the NF-kappaB transcription factor c-Rel. J Immunol. 2010;185:2350–2359. doi: 10.4049/jimmunol.1000317. [DOI] [PubMed] [Google Scholar]
- 18.Deenick EK, et al. c-Rel phenocopies PKCtheta but not Bcl-10 in regulating CD8+ T-cell activation versus tolerance. Eur J Immunol. 2010;40:867–877. doi: 10.1002/eji.200939445. [DOI] [PubMed] [Google Scholar]
- 19.Maggirwar SB, Harhaj EW, Sun SC. Regulation of the interleukin-2 CD28 responsive element by NF-ATp and various NF-κB/Rel transcription factors. Mol Cell Biol. 1997;17:2605–2614. doi: 10.1128/mcb.17.5.2605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou XY, et al. Molecular mechanisms underlying differential contribution of CD28 versus non-CD28 costimulatory molecules to IL-2 promoter activation. J Immunol. 2002;168:3847–3854. doi: 10.4049/jimmunol.168.8.3847. [DOI] [PubMed] [Google Scholar]
- 21.Sen R, Smale ST. Selectivity of the NF-{kappa}B response. Cold Spring Harb Perspect Biol. 2009;2:a000257. doi: 10.1101/cshperspect.a000257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bhoj VG, Chen ZJ. Ubiquitylation in innate and adaptive immunity. Nature. 2009;458:430–437. doi: 10.1038/nature07959. [DOI] [PubMed] [Google Scholar]
- 23.Loeser S, Penninger JM. Regulation of peripheral T cell tolerance by the E3 ubiquitin ligase Cbl-b. Semin Immunol. 2007;19:206–214. doi: 10.1016/j.smim.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 24.Huang F, Gu H. Negative regulation of lymphocyte development and function by the Cbl family of proteins. Immunol Rev. 2008;224:229–238. doi: 10.1111/j.1600-065X.2008.00655.x. [DOI] [PubMed] [Google Scholar]
- 25.Butler MP, Hanly JA, Moynagh PN. Kinase-active interleukin-1 receptor-associated kinases promote polyubiquitination and degradation of the Pellino family: direct evidence for PELLINO proteins being ubiquitin-protein isopeptide ligases. J Biol Chem. 2007;282:29729–29737. doi: 10.1074/jbc.M704558200. [DOI] [PubMed] [Google Scholar]
- 26.Ordureau A, et al. The IRAK-catalysed activation of the E3 ligase function of Pellino isoforms induces the Lys63-linked polyubiquitination of IRAK1. Biochem J. 2008;409:43–52. doi: 10.1042/BJ20071365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schauvliege R, Janssens S, Beyaert R. Pellino proteins are more than scaffold proteins in TLR/IL-1R signalling: a role as novel RING E3-ubiquitin-ligases. FEBS Lett. 2006;580:4697–4702. doi: 10.1016/j.febslet.2006.07.046. [DOI] [PubMed] [Google Scholar]
- 28.Schauvliege R, Janssens S, Beyaert R. Pellino proteins: novel players in TLR and IL-1R signalling. J Cell Mol Med. 2007;11:453–461. doi: 10.1111/j.1582-4934.2007.00040.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moynagh PN. The Pellino family: IRAK E3 ligases with emerging roles in innate immune signalling. Trends Immunol. 2009;30:33–42. doi: 10.1016/j.it.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 30.Chang M, Jin W, Sun SC. Peli1 facilitates TRIF-dependent Toll-like receptor signaling and proinflammatory cytokine production. Nat Immunol. 2009;10:1089–1095. doi: 10.1038/ni.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li MO, Wan YY, Sanjabi S, Robertson AK, Flavell RA. Transforming growth factor-beta regulation of immune responses. Annu Rev Immunol. 2006;24:99–146. doi: 10.1146/annurev.immunol.24.021605.090737. [DOI] [PubMed] [Google Scholar]
- 32.Rubtsov YP, Rudensky AY. TGFbeta signalling in control of T-cell-mediated self-reactivity. Nat Rev Immunol. 2007;7:443–453. doi: 10.1038/nri2095. [DOI] [PubMed] [Google Scholar]
- 33.Wohlfert EA, Callahan MK, Clark RB. Resistance to CD4+CD25+ regulatory T cells and TGF-beta in Cbl-b−/− mice. J Immunol. 2004;173:1059–1065. doi: 10.4049/jimmunol.173.2.1059. [DOI] [PubMed] [Google Scholar]
- 34.King CG, et al. TRAF6 is a T cell-intrinsic negative regulator required for the maintenance of immune homeostasis. Nat Immunol. 2006;12:1088–1092. doi: 10.1038/nm1449. [DOI] [PubMed] [Google Scholar]
- 35.Liou HC, Smith KA. The roles of c-rel and interleukin-2 in tolerance: a molecular explanation of self-nonself discrimination. Immunol Cell Biol. 2011;89:27–32. doi: 10.1038/icb.2010.120. [DOI] [PubMed] [Google Scholar]
- 36.Chen E, et al. Degradation of proto-oncoprotein c-Rel by the ubiquitin-proteasome pathway. J Biol Chem. 1998;273:35201–35207. doi: 10.1074/jbc.273.52.35201. [DOI] [PubMed] [Google Scholar]
- 37.Lim KL, et al. Parkin mediates nonclassical, proteasomal-independent ubiquitination of synphilin-1: implications for Lewy body formation. J Neurosci. 2005;25:2002–2009. doi: 10.1523/JNEUROSCI.4474-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kumar KG, et al. Site-specific ubiquitination exposes a linear motif to promote interferon-alpha receptor endocytosis. J Cell BIol. 2007;179:935–950. doi: 10.1083/jcb.200706034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Varfolomeev E, et al. IAP antagonists induce autoubiquitination of c-IAPs, NF-kappaB activation, and TNFalpha-dependent apoptosis. Cell. 2007;131:669–681. doi: 10.1016/j.cell.2007.10.030. [DOI] [PubMed] [Google Scholar]
- 40.Bertrand MJ, et al. cIAP1 and cIAP2 facilitate cancer cell survival by functioning as E3 ligases that promote RIP1 ubiquitination. Mol Cell. 2008;30:689–700. doi: 10.1016/j.molcel.2008.05.014. [DOI] [PubMed] [Google Scholar]
- 41.Appleman LJ, Boussiotis VA. T cell anergy and costimulation. Immunol Rev. 2003;192:161–180. doi: 10.1034/j.1600-065x.2003.00009.x. [DOI] [PubMed] [Google Scholar]
- 42.Bryan RG, et al. Effect of CD28 signal transduction on c-Rel in human peripheral blood T cells. Mol Cell Biol. 1994;14:7933–7942. doi: 10.1128/mcb.14.12.7933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shapiro VS, Truitt KE, Imboden JB, Weiss A. CD28 mediates transcriptional upregulation of the interleukin-2 (IL-2) promoter through a composite element containing the CD28RE and NF-IL-2B AP-1 sites. Mol Cell Biol. 1997;17:4051–4058. doi: 10.1128/mcb.17.7.4051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chang M, Lee AJ, Fitzpatrick L, Zhang M, Sun SC. NF-kappa B1 p105 regulates T cell homeostasis and prevents chronic inflammation. J Immunol. 2009;182:3131–3138. doi: 10.4049/jimmunol.0803637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee YS, et al. Smad7 and Smad6 bind to discrete regions of Pellino-1 via their MH2 domains to mediate TGF-beta1-induced negative regulation of IL-1R/TLR signaling. Biochem Biophys Res Commun. 2010;393:836–843. doi: 10.1016/j.bbrc.2010.02.094. [DOI] [PubMed] [Google Scholar]
- 46.Xiao G, Harhaj EW, Sun SC. NF-kappaB-inducing kinase regulates the processing of NF-kappaB2 p100. Mol Cell. 2001;7:401–409. doi: 10.1016/s1097-2765(01)00187-3. [DOI] [PubMed] [Google Scholar]
- 47.Jin W, Zhou XF, Yu J, Cheng X, Sun SC. Regulation of Th17 cell differentiation and EAE induction by the MAP3K NIK. Blood. 2009;113:6603–6610. doi: 10.1182/blood-2008-12-192914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Reiley WW, et al. Regulation of T cell development by the deubiquitinating enzyme CYLD. Nat Immunol. 2006;7:411–417. doi: 10.1038/ni1315. [DOI] [PubMed] [Google Scholar]
- 49.Uhlik M, et al. NF-kappaB-inducing kinase and IkappaB kinase participate in human T-cell leukemia virus I Tax-mediated NF-kappaB activation. J Biol Chem. 1998;273:21132–21136. doi: 10.1074/jbc.273.33.21132. [DOI] [PubMed] [Google Scholar]
- 50.Reiley WW, et al. Deubiquitinating enzyme CYLD negatively regualtes the ubiquitin-dependent kinase Tak1 and prevents abnormal T cell responses. J Exp Med. 2007;204:1475–1485. doi: 10.1084/jem.20062694. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.