Introduction
Fluorescent dyes are a family of optical reporters widely used in studying biomolecules in cells1 and animals2. With an appropriate fluorescent tag, the location and quantity of the target biomolecules or bioprocess can be tracked conveniently. Most dyes fluoresce continuously and show no difference in fluorescence property before and after labels; therefore any leftover unreacted free dye could interfere with the dye attached to the molecules of interest, significantly lowering the signal to background contrast. An alternate way to label target molecules without worrying about the background issue is to use fluorogenic fluorochromes, whose emission wavelength or intensity shift or change after the labeling reaction3. Taking advantage of these unique fluorescent properties could minimize the high background issue. Although the benefit of using the fluorogenic fluorochromes is obvious, the approach has not been applied widely for at least two reasons; limited available fluorogenic fluorochromes, and the non-selective reactivity of these dyes. Without specific chemical reactivity, labeling particular molecule remains challenging.
The ‘click’ reaction between an azide and an alkyne groups has recently been used to label various biomolecules in cells4-7 and even animals8,9. The small size of the azide and alkyne groups enables them to ‘trick’ some nature synthases, and thus modified amino acids10, nucleosides11-13 and monosaccharide14 with an azide or alkyne reactive group have been reported as the tagging residues for proteins15, nucleic acids13 and carbohydrates16. Since azide and alkyne reactive groups are not normally expressed in biological systems, the ‘click’ reaction is specific. Many existing dyes have been engineered with a reactive azide or alkyne functional group for labeling7,17; however this modification only solves half of the problem, i.e. specific reactivity. The background signal remains an issue.
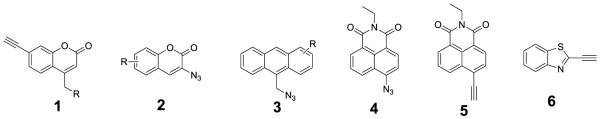
A new kind of ‘click-on’ fluorogenic dyes has lately been reported to overcome the aforementioned problems18. The ‘click-on’ fluorogenic dyes were designed to have low or no baseline fluorescence by masking the core fluorophore with an electron-donating azide group or electron-withdraw alkyne group. After the ‘click’ reaction, the newly forms triazole conjugating structure delocalizes the local electrons and restore fluorescence. To date, only a handful of successful examples, derived from coumarin (1, 2)18-21, anthracene (3)22 and naphthalimide (4, 5)23 have been reported (Figure 1). More ‘click-on’ dyes that fluoresce at different wavelength are needed for various biomolecule labeling and applications.
Figure 1.
Example for existing fluorogenic dyes
Recently, we also reported an alkyne-containing benzothiazole ‘click-on’ dye (6), which has extremely low initial fluorescence, and its ‘click-on’ product shows 158-fold increase in fluorescence24. However, its low excitation and emission maxima which are approximately 290 nm and 360 nm, respectively, have restricted its biological application. In order to improve this shortcoming, we hypothesized that an electron-donating group such as dimethylamino or methoxyl group could enhance the effect of conjugating the parental aromatic benzothiazole ring, shifting the excitation and emission spectra into the visible range.
Result and discussion
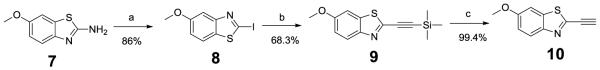
To add a methoxyl group at the 6-position of benzothiazole, 6-methoxybenzothiazole-2-alkyne 10 was synthesized using 7 as starting material (Scheme 1). Following a previous developed protocol24, 7 was initially transformed with 86% yield into iodobenzothiazole 8 via Diazotization-Sandmeyer iodination in the presence of p-toluenesulfonic acid in acetonitrile25. The protected acetylenic benzothiazole 9 was obtained with 68.3% yield after a cross-coupling reaction of iodobenzothiazole 8 and a terminal alkyne with catalytic quantities of Pd(PPh3)2Cl2 under a basic condition26,27. The prepared 9 was deprotected with KF in MeOH at room temperature for 3h, and then purified by a silica gel column to obtain 10 with 99% yield.
Scheme 1.
Reagents and conditions: (a) 1. p-MeC6H4SO3H, MeCN, RT-10°C, 2. NaNO2, H2O, 10min, 10-15°C. 3 KI, RT, overnight. (b) 1.ethynyltrimethylsilane, CuI, PdCl2(PPh3)2, NEt3, DCM, 2. NaHCO3, H2O. (c) KF, MeOH, RT, 3h.
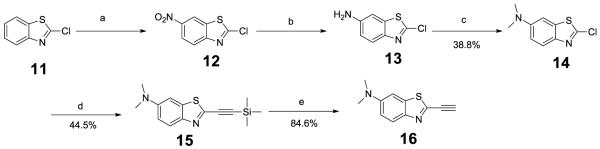
To prepare benzothiazole with dimethylamino at 6-position, 6-dimethylbenzothiazole alkyne 16 was prepared from 11 (Scheme 2). Nitration of 11 was done with a mixture of concentrated sulfuric acid and fuming nitric acid to give 12. The nitro group at the 6-position was then reduced with iron powder in MeOH under catalytic quantities of acetic acid to afford 6-amino-compound 1328. Reductive amination of 13 with formaldehyde and triacetoxyborohydride in DCM formed 6-dimethyl compound 14. Compound 16 was prepared, using a similar procedure for 10, from 14 via cross-coupling and deprotection reaction with 44.5% and 84.6% yield, respectively.
Scheme 2.
Reagents and conditions: (a) HNO3, H2SO4. (b) Fe, EtOH, AcOH, 55°C, 3h. (c) 36% formaldehyde, DCM, NaBHAc3, HOAc, RT, 2h. (d) 1.ethynyltrimethylsilane, CuI, PdCl2(PPh3)2, NEt3, DCM, 2. NaHCO3, H2O. (e) KF, MeOH, RT, 3h.
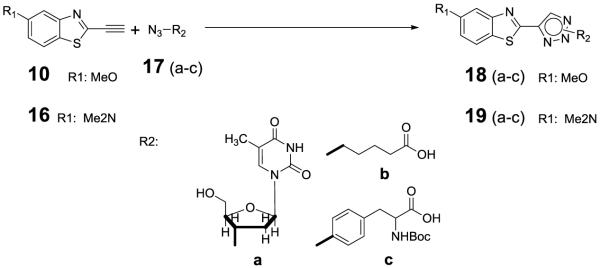
The reactivity and ‘click-on’ fluorogenic properties of the prepared compounds 10 and 16 were tested by performing a [3+2] cycloaddition, also known as ‘click’ reaction, with model analogs 17(a~c), representative analogs of nucleoside, amino acids and linker (Scheme 3). Cu2+/TBTA (Tris[(1 - benzyl - 1H - 1,2,3 - triazol - 4 - yl)methyl] amine) was used as the catalyst in DMF/H2O for 1h at room temperature. Near quantitative conversion was obtained for all reactions (Supporting material).
Scheme 3.
Model ‘Click’ reactions Reagents and conditions: CuSO4, sodium ascorbate, TBTA, DMF/H2O(3/1), RT, 30min
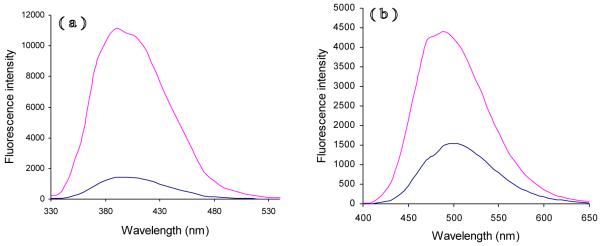
As expected, a red-shift was observed as an electron-donating group was added to the 6-position of benzothiazol. In aqueous buffer, the emission maxima of 6-methoxyl dye 10 and 6-dimethylamino dye 16 was approximately 390 nm and 500 nm, respectively. More red-shift was achieved with dimethylamino dye 16. The emission maximum of the parent benzothiazole 6 is approximately 360 nm. Importantly, both dyes 10 and 16 were ‘clickable’ fluorogenic dyes whose ‘clicked’ products had a significant increase in fluorescence intensity (Figure 2). The quantum yield of 18b was 0.36, but that of its parent compound 10 is only 0.05 in EtOH. The fluorescence intensity of 18b (10μM) in pH=7.0 PBS buffer was 7.9-fold stronger than that of the parent dye 10 under the same condition. The quantum yield of 19b and its parent compound 16 in EtOH were 0.65 and 0.49, respectively. The fluorescence intensity of 19b (10μM) in pH=7.0 BPS buffer was 3.5-fold stronger than that of the parent dye 16. Interestingly, the click reaction had little effect on wavelength.
Figure 2.
Emission spectra of benzothiazole derivatives (10μM) in 3ml pH=7 buffer solution containing: (a) Compound 10 (blue) and 18b (red) (λex=316nm); (b) Compound 16 (blue) and 19b (red) (λex=375nm).
Dimethylamino series, compounds 16 and 19, had a long Stoke shift, 140-150 nm, in PBS buffer, while the Stoke shift of methoxyl series, compounds 10 and 18, was approximately 70 nm. Solvent effect was also more significant for the dimethylamino series (Table 1). The Stoke shift for compounds 19 in methanol was shortened to 100 -110 nm. In contrast, the 6-methoxy series only showed approximately 10 nm difference between the two solvents.
Table 1.
UV absorption and emission spectra of compounds 10, 16, 18(a-c) and 19(a-c)
| λ max(abs) (nm) | λ max(em) (nm) | Φ | Stoke shift (nm) |
|
|---|---|---|---|---|
| 10 | 316(MeOH) 316 (H2O) |
379 (MeOH) 393 (H2O) |
0.05* | 63 77 |
| 18a | 316(MeOH) 316 (H2O) |
381 (MeOH) 392 (H2O) |
65 76 |
|
| 18b | 315(MeOH) 315(H2O) |
379 (MeOH) 391 (H2O) |
0.36* | 64 76 |
| 18c | 314(MeOH) 314 (H2O) |
381 (MeOH) 390 (H2O) |
67 76 |
|
| 16 | 364(MeOH) 343(H2O) |
460 (MeOH) 500 (H2O) |
0.49** | 96 157 |
| 19a | 356(MeOH) 350(H2O) |
466 (MeOH) 492 (H2O) |
110 142 |
|
| 19b | 355(MeOH) 335(H2O) |
459 (MeOH) 493 (H2O) |
0.65** | 104 158 |
| 19c | 352(MeOH) 332(H2O) |
459 (MeOH) 491 (H2O) |
0.63** | 107 159 |
Quantum yield condition:
Solvent: EtOH, λex: 330 nm; slit: 4 nm, λem: 345-550 nm; slit: 4 nm, Inc.: 1 nm; reference compound: 9,10- diphenylanthracence, Φ = 0.90
Solvent: EtOH, λex: 360 nm, slit: 2 nm, λem: 375-650 nm; slit: 2 nm, Inc.: 1 nm; reference compound: 7-diethylamino-4-methylcoumarin, Φ = 0.73.
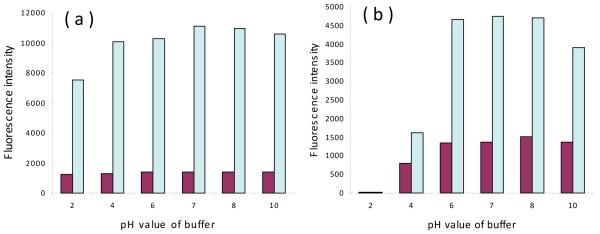
The fluorescent property of compounds 10 and 16, and their corresponding ‘clicked’ dyes 18b and 19b was further investigated in different pH condition. As seen in Figure 3, compounds 10 and 18b were relatively pH stable, while compounds 16 and 19b showed clear pH-dependent. From Figure 3b, the fluorescence intensities of compound 16 and 19b were down to zero at pH lower than 2. The fluorescence intensity of compound 16 and 19b increased with increasing pH, reaching the maxima at pH 6-8. The fluorescence intensity of 19b dropped considerably on pH increase to 10; but the fluorescence intensity of 16 decreased only slightly. This clear pH dependence could be due to protonation of the dimethylamino group. In contrast, the methoxyl group cannot be protonated as easy as the amino group, thus dyes 10 and 18b are more pH stable.
Figure 3.
Effect of pH on fluorescence intensity ratio of benzothiazole derivatives in different pH PBS buffer. Condition: (a) compound 10 (Brown) and 18b (Cyan), λex: 316nm, 10μM. (b) compound 16 (Brown) and 19b (Cyan), λex: 375nm, 10μM.
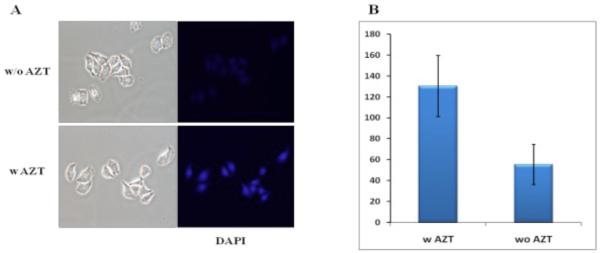
The potential application of the developed ‘click’ fluorogenic dye was then validated in cell culture. For a better fluorescent contrast in biological application, dye 16 which has a long emission maximum and Stoke shift was selected for the study. The azide group was introduced into HeLa cells by incubating with AZT 17a (2μM) for 16 hrs. AZT is a nucleoside analog which is known to be incorporated into DNA. After a thorough washing, cells were incubated with dye 16 and CuSO4 for 3 h, followed by washes, and then reducing agent NaAsc was added to initiate the ‘click’ reaction. Thirty minutes later, the cells were washed and monitored under microscope. After ‘click’ reaction, an approximately 2-fold high fluorescent signal was observed in the AZT treated cells using the common filter set for DAPI (4′, 6-diamidino-2-phenylindole) stain (Figure 4). The fluorescent signal clearly highlight the nucleus, indicating that incorporated AZT reacted with dye 16 and the ‘click’ reaction is able to brighten the fluorescent signal in cells. In a separate experiment, the cells were treated with AZT 17a and dye 16, but CuSO4. The fluorescent signal level of these cells is compatible to it of the cells without AZT 17a.
Figure 4.
‘Click on’ fluorescence images of cells (A), and quantitation of fluorescent signal (B) after reacting with compound 16 (10 μM) in HeLa cells (x20). The fluorescent signal was imaged using the convention DAPI filter set.
Conclusion
We describe the preparation of two 2-ethynyl-benzothiazole derivatized ‘click-on’ fluorogenic dyes. The two derivatives with an electron-donating dimethylamino or methoxyl group at the 6-position showed a 30 to 130 nm red-shift in fluorescence wavelength, compared to the parent benzothiazole fluorogenic dye. The model reactions with functionalized nucleoside and amino acid indicated that the developed ‘click-on’ dye could label various biomolecules, such as nucleic acids, proteins and other molecules.
Supplementary Material
Acknowledgements
We thank the NMR facility at M. D. Anderson Cancer Center for acquiring all NMR data. This research was supported in part by NIH CA135312, and GM094880.
Footnotes
Supporting Information. Details of synthesis, characterization and cell culture validation. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- (1).Hilderbrand SA. Labels and probes for live cell imaging: overview and selection guide. Methods Mol Biol. 2010;591:17–45. doi: 10.1007/978-1-60761-404-3_2. [DOI] [PubMed] [Google Scholar]
- (2).Escobedo JO, Rusin O, Lim S, Strongin RM. NIR dyes for bioimaging applications. Curr Opin Chem Biol. 2010;14:64–70. doi: 10.1016/j.cbpa.2009.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Kucherak OA, Oncul S, Darwich Z, Yushchenko DA, Arntz Y, Didier P, Mely Y, Klymchenko AS. Switchable nile red-based probe for cholesterol and lipid order at the outer leaflet of biomembranes. J Am Chem Soc. 2010;132:4907–4916. doi: 10.1021/ja100351w. [DOI] [PubMed] [Google Scholar]
- (4).Kolb HC, Sharpless KB. The growing impact of click chemistry on drug discovery. Drug Discov. Today. 2003;8:1128–1137. doi: 10.1016/s1359-6446(03)02933-7. [DOI] [PubMed] [Google Scholar]
- (5).Link AJ, Tirrell DA. Cell surface labeling of Escherichia coli via copper(I)-catalyzed [3+2] cycloaddition. J. Am. Chem. Soc. 2003;125:11164–11165. doi: 10.1021/ja036765z. [DOI] [PubMed] [Google Scholar]
- (6).Jao CY, Salic A. Exploring RNA transcription and turnover in vivo by using click chemistry. Proc. Natl. Acad. Sci. U. S. A. 2008;105:15779–15784. doi: 10.1073/pnas.0808480105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Kele P, Li X, Link M, Nagy K, Herner A, Lorincz K, Beni S, Wolfbeis OS. Clickable fluorophores for biological labeling--with or without copper. Org Biomol Chem. 2009;7:3486–3490. doi: 10.1039/b907741c. [DOI] [PubMed] [Google Scholar]
- (8).Prescher JA, Bertozzi CR. Chemistry in living systems. Nat. Chem. Biol. 2005;1:13–21. doi: 10.1038/nchembio0605-13. [DOI] [PubMed] [Google Scholar]
- (9).Chang PV, Prescher JA, Sletten EM, Baskin JM, Miller IA, Agard NJ, Lo A, Bertozzi CR. Copper-free click chemistry in living animals. Proc. Natl. Acad. Sci. U. S. A. 2010;107:1821–1826. doi: 10.1073/pnas.0911116107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Giepmans BN, Adams SR, Ellisman MH, Tsien RY. The fluorescent toolbox for assessing protein location and function. Science. 2006;312:217–224. doi: 10.1126/science.1124618. [DOI] [PubMed] [Google Scholar]
- (11).Diermeier-Daucher S, Clarke ST, Hill D, Vollmann-Zwerenz A, Bradford JA, Brockhoff G. Cell type specific applicability of 5-ethynyl-2′-deoxyuridine (EdU) for dynamic proliferation assessment in flow cytometry. Cytometry A. 2009;75:535–546. doi: 10.1002/cyto.a.20712. [DOI] [PubMed] [Google Scholar]
- (12).Chehrehasa F, Meedeniya AC, Dwyer P, Abrahamsen G, Mackay-Sim A. EdU, a new thymidine analogue for labelling proliferating cells in the nervous system. J Neurosci Methods. 2009;177:122–130. doi: 10.1016/j.jneumeth.2008.10.006. [DOI] [PubMed] [Google Scholar]
- (13).Salic A, Mitchison TJ. A chemical method for fast and sensitive detection of DNA synthesis in vivo. Proc Natl Acad Sci U S A. 2008;105:2415–2420. doi: 10.1073/pnas.0712168105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Baskin JM, Prescher JA, Laughlin ST, Agard NJ, Chang PV, Miller IA, Lo A, Codelli JA, Bertozzi CR. Copper-free click chemistry for dynamic in vivo imaging. Proc Natl Acad Sci U S A. 2007;104:16793–16797. doi: 10.1073/pnas.0707090104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Link AJ, Vink MK, Tirrell DA. Presentation and detection of azide functionality in bacterial cell surface proteins. J Am Chem Soc. 2004;126:10598–10602. doi: 10.1021/ja047629c. [DOI] [PubMed] [Google Scholar]
- (16).Chang PV, Dube DH, Sletten EM, Bertozzi CR. A strategy for the selective imaging of glycans using caged metabolic precursors. J Am Chem Soc. 2010;132:9516–9518. doi: 10.1021/ja101080y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Nagy K, Orban E, Bosze S, Kele P. Clickable long-wave “mega-stokes” fluorophores for orthogonal chemoselective labeling of cells. Chem Asian J. 2010;5:773–777. doi: 10.1002/asia.200900477. [DOI] [PubMed] [Google Scholar]
- (18).Sivakumar K, Xie F, Cash BM, Long S, Barnhill HN, Wang Q. A fluorogenic 1,3-dipolar cycloaddition reaction of 3-azidocoumarins and acetylenes. Org. Lett. 2004;6:4603–4606. doi: 10.1021/ol047955x. [DOI] [PubMed] [Google Scholar]
- (19).Zhou Z, Fahrni CJ. A fluorogenic probe for the copper(I)-catalyzed azide-alkyne ligation reaction: Modulation of the fluorescence emission via 3(n,π*)-1(π, π*) inversion. J. Am. Chem. Soc. 2004;126:8862–8863. doi: 10.1021/ja049684r. [DOI] [PubMed] [Google Scholar]
- (20).Beatty KE, Liu JC, Xie F, Dieterich DC, Schuman EM, Wang Q, Tirrell DA. Fluorescence visualization of newly synthesized proteins in mammalian cells. Angew Chem Int Ed Engl. 2006;45:7364–7367. doi: 10.1002/anie.200602114. [DOI] [PubMed] [Google Scholar]
- (21).Li K, Lee LA, Lu X, Wang Q. Fluorogenic “click” reaction for labeling and detection of DNA in proliferating cells. Biotechniques. 2010;49:525–527. doi: 10.2144/000113463. [DOI] [PubMed] [Google Scholar]
- (22).Xie F, Sivakumar K, Zeng QB, Bruckman MA, Hodges B, Wang Q. A fluorogenic ‘click’ reaction of azidoanthracene derivatives. Tetrahedron. 2008;64:2906–2914. [Google Scholar]
- (23).Sawa M, Hsu TL, Itoh T, Sugiyama M, Hanson SR, Vogt PK, Wong CH. Glycoproteomic probes for fluorescent imaging of fucosylated glycans in vivo. Proc Natl Acad Sci U S A. 2006;103:12371–12376. doi: 10.1073/pnas.0605418103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Qi J, Tung CH. Development of benzothiazole ‘click-on’ fluorogenic dyes. Bioorg Med Chem Lett. 2011;21:320–323. doi: 10.1016/j.bmcl.2010.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Krasnokutskaya EA, Semenischeva NI, Filimonov VD, Knochel P. A new, one-step, effective protocol for the iodination of aromatic and heterocyclic compounds via aprotic diazotization of amines. Synthesis. 2007:81–84. [Google Scholar]
- (26).Van den Hoven BG, Alper H. Remarkable synthesis of 2-(Z)-6-(E)-4H-[1,4]-thiazepin-5-ones by zwitterionic rhodium-catalyzed chemo- and regioselective cyclohydrocarbonylative ring expansion of acetylenic thiazoles. J. Am. Chem. Soc. 2001;123:1017–1022. doi: 10.1021/ja003085c. [DOI] [PubMed] [Google Scholar]
- (27).Schlegel J, Maas G. Propyne iminium salts by N-alkylation of alkynyl imines. Synthesis. 1999:100–106. [Google Scholar]
- (28).Delmas F, Avellaneda A, Di Giorgio C, Robin M, De Clercq E, Timon-David P, Galy JP. Synthesis and antileishmanial activity of (1,3-benzothiazol-2-yl) amino-9-(10H)-acridinone derivatives. Eur J Med Chem. 2004;39:685–690. doi: 10.1016/j.ejmech.2004.04.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.