Abstract
Background
Follistatin-like 1 (FSTL1) is an extracellular glycoprotein that is found in human serum. Recent work suggests that FSTL1 is secreted in response to ischemic injuries and that its overexpression is protective in the heart and vasculature.
Methods and Results
Here, we examined serum FSTL1 levels in patients with chronic heart failure with left ventricular (LV) ejection fraction <40% (n=86). The distribution of the sample, from these chronic heart failure patients, was separated into three tertiles of low, medium and high FSTL1 levels. Serum FSTL1 levels were increased 56% above age- and gender-matched, healthy controls. Diabetes mellitus, brain natriuretic peptide level, left atrial size, LV posterior wall thickness, LV end-diastolic diameter and LV mass were significant determinants of FSTL1 serum levels by bivariate analysis. After controlling for significant covariates, FSTL1 levels predicted LV hypertrophy (as measured by LV mass index) by multivariate linear regression analysis (P<0.001). Unadjusted survival analysis demonstrated increased mortality in patients with increasing FSTL1 levels (P=0.09). After adjusting for significant parameters, patients with increased FSTL1 remained at the highest risk of death [hazard ratio (95% confidence limits) 1.028, (0.98 and 1.78)]; (P=0.26). To determine whether elevated FSTL1 may be derived from the myocardium, FSTL1 protein expression was measured in samples from explanted, failing (n=18) and non-failing human hearts (n=7). LV failing hearts showed 2.5-fold higher FSTL1 protein levels than non-failing control hearts (P<0.05).
Conclusions
Elevated serum FSTL1 in human heart failure patients was associated with LV hypertrophy. Further studies on the role of FSTL1 as a biomarker in chronic systolic heart failure are warranted.
Keywords: follistatin-like 1, systolic heart failure, left ventricular hypertrophy
Follistatin-like 1 (FSTL1), also referred to as TSC36 (TGF-β-stimulated clone 36), is an extracellular glycoprotein that has a follistatin-like domain1. Members of the follistatin family function to regulate the TGF-β superfamily proteins through their ability to function as binding partners and antagonize the binding of ligands to the receptors. Recently it was shown that DIP2A (Disco-interacting protein 2 homolog A) functions as a FSTL1 receptor2. Although FSTL1 is reported to suppress cancer growth and invasion in animal models3, and modulate inflammation and allograft survival4, 5; little is known about its function in cardiovascular disease. We previously found that FSTL1 is upregulated and secreted in cardiovascular injury models where it promotes myocyte survival and ischemia-induced revascularization6, 7.
Widera et al. found that FSTL1 levels are increased in acute coronary syndrome (ACS) and associated with all-cause mortality8. In patients with end-stage heart failure (HF) on left ventricular (LV) assist devices (LVAD), cardiac FSTL1 gene expression was increased and subsequently declined when LV function improved9. Collectively, these findings suggest that FSTL1 can serve as a cardiac tissue-derived marker of cardiovascular disease. To explore the role of FSTL1 in the pathogenesis of human systolic HF, we measured serum FSTL1 levels in patients with chronic LV systolic dysfunction to determine its relationship with measures of LV cardiac remodeling. Additionally we measured FSTL1 protein levels from the LV of patients undergoing heart transplantation due to end-stage HF. More precisely, we sought to test the following: (1) Serum FSTL1 levels are elevated in the HF patients compared to controls using dataset 1; (2) FSTL1 protein expression is increased in explanted failing LV human tissue using dataset 2 and (3) in chronic systolic HF patients there is an association between serum FSTL1 levels and clinical markers of cardiac remodeling using dataset 3.
Methods
Subjects
Eighty six patients with chronic HF and LV ejection fraction (LVEF) <40% were recruited, between 2001–2004, from an ambulatory HF clinic at Boston Medical Center. Data was recorded at enrollment and the analysis reflects the measurements of 86 patients. A medical history was obtained to document etiology, symptoms by New York Heart Association (NYHA) functional class and coexisting diseases. Routine laboratory results (e.g., electrolytes and blood count) and concomitant cardiac medications were recorded. Body mass index (BMI) was calculated as the ratio of weight to squared height. Twenty-one healthy volunteers of similar age and gender were used for comparison. The Boston Medical Center Institutional Review Board approved the study. All subjects gave written, informed consent. LV systolic dysfunction etiology was defined as: 1) ischemic: prior history of myocardial infarction (electrocardiogram/positive troponin), results of a positive non-invasive stress test, or cardiac catheterization; 2) hypertensive: documented history of pharmacologically treated hypertension; 3) idiopathic: having no identifiable cause of the cardiomyopathy and 4) other: including valvular, alcohol-induced and familial.
Echocardiography
Two-dimensional and Doppler echocardiography were performed at baseline as previously described10 using the Vingmed Vivid Five System (GE Vingmed, Milwaukee, WI) with a 2.5-Mhz phased-array transducer. Echocardiograms were performed and analyzed in a blinded manner. Measurements of systolic and diastolic chamber dimensions and wall thickness were obtained from 2-D imaging according to the recommendations of the American Society of Echocardiography11. The standard cube formula was utilized in order to calculate LV mass12.
Biomarker Analysis
Blood samples were collected and serum decanted. Samples were stored at −80°C. Brain natriuretic peptide (BNP) levels were measured by the ADVIA Centaur assay (Siemens Healthcare Diagnostics). Routine laboratory analysis was performed at the Boston Medical Center Clinical Laboratory.
Serum FSTL1 measurements
Serum FSTL1 levels were determined by quantitative Western blotting. Serum were added to 10-fold volumes of blue loading buffer (Cell Signaling Technology Inc., USA), and separated on SDS-PAGE (Lonza Group Ltd., Switzerland). Proteins were transferred onto PVDF (GE Healthcare UK Ltd., England) and probed with the anti-human FSTL1 antibody (1/2000; R&D Systems Inc., USA) followed by the incubation with the anti-goat IgG-HRP secondary antibody (1/1000; Santa Cruz Biotechnology Inc., USA). ECL Western blotting detection reagents and analysis system (GE Healthcare UK Ltd., England) were used for the detection of the protein signal. The signal intensities were standardized by recombinant human FSTL1 protein (R&D Systems Inc., USA) and quantified by using Image J software (the National Institutes of Health, USA. At a molecular weight of 50 kDa, the FSTL1 band intensity was measured by densitometry and after adjusting to a standard curve created from three different doses of recombinant human FSTL1 protein, was expressed as arbitrary units (arb. u.).
Human myocardial tissue procurement
Failing LV tissues were obtained from patients with end-stage, nonischemic dilated cardiomyopathy (DCM; n=9) and ischemic cardiomyopathy (ICM; n=9) at the time of cardiac transplantation. For comparison non-failing (NF) human LV tissues were obtained from organ donors (n =7) with no known history of cardiac disease who could not be transplanted for technical reasons. All subjects or organ donor family members gave written consent for tissue donation. The study was reviewed and approved by the Ethical Committee of the University Medical Center Hamburg-Eppendorf (Az. 532/116/9.7.1991).
Tissue western blot analysis
Membranes were blocked with 5% (w/v) dried milk in 100 mmol/l Tris, pH 7.5, 0.1% (v/v) Tween 20 and 150 mmol/l NaCl (TBST) for 1 h prior to overnight incubation at 4 °C with the primary antibodies. Primary antibodies were used against α-actinin (1/1000; clone EA-53, Sigma, Saint Louis, Missouri, USA) and against FSTL1 (1/2000; Abcam, Cambridge, UK). Immunoblots were developed with anti-mouse or anti-goat IgG-horseradish peroxidase, subjected to Enhanced Chemiluminescence detection reagents (Thermo Scientific, Rockford, USA) and exposed to film for appropriate times. Densitometry signals on X-ray films were evaluated with Chemie Genius2 Bio Imaging System with Gene Tools software (Syngene).
Statistical Analysis
Summary statistics are presented as mean±standard deviation for continuous variables and as number (percentage) for categorical variables. To address hypothesis 1, Student’s t test was used to compare serum FSTL1 levels between chronic, systolic HF patients and normal controls. These groups were matched a priori for age and gender. To address hypothesis 2, one-way ANOVA was used to compare FSTL1 protein expression among three explanted human myocardial tissue groups: dilated cardiomyopathy (DCM), ischemic cardiomyopathy (ICM) and non-failing donor hearts (NF). To assess hypothesis 3, multivariable regression models were used to allow us to study the association between FSTL1 and clinical markers while adjusting for confounders. To this end, we first carried out bivariate analyses in which FSTL1 levels were divided into three risk groups based on the tertiles of the observed FSTL1 distribution, where Group 1 included patients with FSTL1<14.3 arbitrary units (arb. u.), Group 2 included patients with FSTL1 between 14.3 arb. u. and 20.5 arb. u. and Group 3 included patients with FSTL1≥20.5 arb. u. Demographics, clinical characteristics and echocardiography parameters were compared among the above groups using ANOVA, or chi-square test, as appropriate. We specified a priori that a factor must show a potential association (i.e., P<0.10) with FSTL1 by bivariate analysis in order to be tested in a multivariable regression model. We then used a backward selection procedure with 0.2 level of alpha to keep variables in the model. In the multivariable regression models FSTL1 was operationalized as a continuous variable. Linear multivariable regression was used for the LV mass index outcomes while Cox multivariable regression was used for the time to death. Patients that survived through the end of the study period were considered censored observations. The assumption of proportional hazards was assessed prior to attempting Cox modeling. A value of P≤0.05 was considered statistically significant. All reported P-values are 2-tailed and all confidence intervals are computed at the 95% level. All analyses were performed using SAS 9.2 (SAS Institute Inc., Cary, NC, USA).
Results
FSTL1 and clinical characteristics
Eighty six chronic, systolic HF patients completed the study. Sixty four percent of the patients were black, 60% were males and 80% had hypertension. Mean LVEF was 21±9% and mean LV mass and LV mass index were 284±89 g and 144±39 g/m2, respectively, by echocardiography; thus demonstrating the presence of LV hypertrophy (LVH). Sixty two percent of the controls were black and were age- and gender-matched (58±8 yrs and 57% male, respectively). Controls had no known cardiovascular disease, normal blood pressure and on no cardiovascular medications. Echocardiography demonstrated normal cardiac size and LV function. Mean LVEF was 63±5%, with mean LV mass and LV mass index 169±36 g and 89±18 g/m2, respectively.
Patients were on evidence-based therapy for systolic HF and the mean New York Heart Association (NYHA) was 2.4±0.8 (Table 1). The chronic nature of these HF patients was demonstrated by the duration of HF symptoms at the time of enrollment (49±52 months; range: 1 to 237 months). The majority of patients were overweight/obese with the mean body mass index (BMI) >30 kg/m2. Mean creatinine was 1.6±1.9 mg/dl with a MDRD GFR of 63.9±30 ml/min/1.73m2. Serum FSTL1 levels were assessed by western immunoblots and expressed as arbitrary units (arb. u.). Serum FSTL1 levels were also measured in an age-matched, healthy controls as previously described10. Mean FSTL1 levels were increased in HF patients compared to age and gender-matched controls (19.8±7.2 vs. 12.7±5 arb. u.; P<0.01).
Table 1.
Clinical Characteristics of the Chronic Heart Failure Patients
| Characteristics | N = 86 |
|---|---|
| Age (years) | 60 ± 13 |
| Male | 52 (60 %) |
| Race | |
| Black | 55 (64 %) |
| White | 27 (31 %) |
| Hispanic | 4 (5 %) |
| BMI (kg/m2) | 30.8 ± 6.6 |
| Duration of heart failure (months) at enrollment | 49 ± 52 |
| Systolic blood pressure (mmHg) | 127 ± 22 |
| Diastolic blood pressure (mmHg) | 73 ± 14 |
| Heart rate (beats/minute) | 75 ± 16 |
| Etiology of heart failure | |
| Ischemic | 31 (36 %) |
| Hypertension | 25 (29 %) |
| Idiopathic | 20 (23 %) |
| Other | 10 (12%) |
| Diabetes mellitus | 29 (34 %) |
| Hypertension | 69 (80 %) |
| Chronic Renal Insufficiency (MDRD GFR < 60 mL/min/1.73m2) | 38 (44 %) |
| Active alcohol use | 11 (13 %) |
| Active smoker | 19 (22%) |
| New York Heart Association Functional Class | |
| I | 14 (16 %) |
| II | 33 (38 %) |
| III | 32 (37 %) |
| IV | 7 (8%) |
| Medications | |
| ACE-inhibitor / ARB | 75 (87%) |
| Beta-blocker | 71 (83%) |
| Digoxin | 40 (47%) |
| Spironolactone | 10 (12%) |
| Nitrates | 25 (33%) |
| Hydralazine | 8 (9%) |
| Statin | 42(49%) |
Continuous variables are described by mean ± SD and categorical variables by percentages (in parenthesis). BMI: Body mass index; ACE: Angiotensin converting enzyme; ARB: Angiotensin receptor blocker
FSTL1 and clinical and echocardiography variables
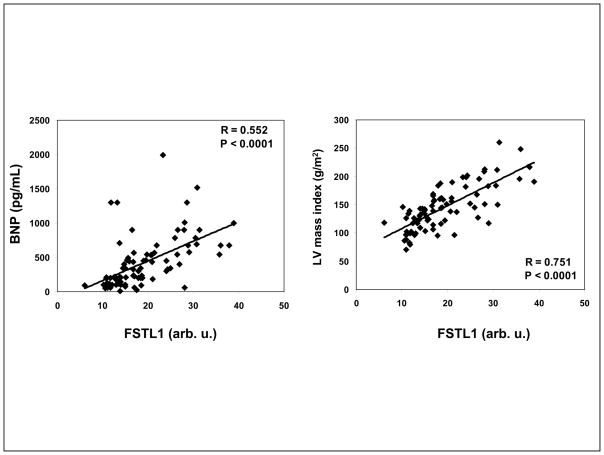
In the chronic, systolic HF patients, FSTL1 and BNP levels were significantly associated by bivariate analysis (P<0.001, Figure 1), with the highest tertile of FSTL1 levels seen with the highest mean BNP levels (Table 2). FSTL1 levels were inversely associated with the presence of diabetes mellitus (BUN; P=0.04) across the tertiles. In addition, FSTL1 was significantly associated with left atrial size (LA), posterior wall thickness, LV end-diastolic diameter (LVEDD), LV end-systolic diameter (LVESD), LV mass and LV mass index (Table 2).
Figure 1.
In chronic systolic heart failure, serum FSTL1 is significantly and positively associated with BNP (R=0.552; P<0.0001) and LV mass index (R=0.751; P<0.001).
Table 2.
Clinical, Biochemical and Echocardiography Variables across FSTL1 Tertiles (n=86)
| Variable | 1 (FSTL1 < 14.3 arb. u.) | 2 (FSTL1 14.3–20.5 arb. u.) | 3 (FSTL1 >20.5 arb. u.) | P-value |
|---|---|---|---|---|
| Age (years) | 60±12 | 59±15 | 60±14 | 0.96 |
| Men | 17 (61%) | 14 (48%) | 21 (72%) | 0.17 |
| Black | 17 (61%) | 17 (59%) | 21 (72%) | 0.50 |
| BMI (kg/m2) | 28.9±4 | 31.2±6 | 32.2±8.5 | 0.17 |
| NYHA Functional Class | 2.4±1.0 | 2.4±0.7 | 2.4±0.9 | 0.95 |
| Comordid illness | ||||
| Ischemic etiology | 14 (50%) | 9 (31%) | 8 (28%) | 0.47 |
| Diabetes mellitus | 15 (54%) | 8 (28%) | 7 (24%) | 0.04* |
| Hypertension | 23 (82%) | 22 (76%) | 24 (83%) | 0.77 |
| Laboratory Data: | ||||
| Creatinine (mg/dl) | 1.4±0.9 | 1.2±0.5 | 1.7±1.5 | 0.26 |
| MDRD GFR (mL/min/1.73m2) | 67±36 | 65±22 | 61±31 | 0.74 |
| BUN (mg/dl) | 32±22 | 26±14 | 36±28 | 0.22 |
| BNP (pg/ml) | 234±326 | 313±187 | 700±677 | <0.001* |
| Hemoglobin (g/dl) | 12.3±1.5 | 12.9±1.4 | 12.6±1.9 | 0.39 |
| Hemodynamics | ||||
| Systolic blood pressure (mmHg) | 128±21 | 127±20 | 126±26 | 0.954 |
| Diastolic blood pressure (mmHg) | 73±14 | 73±12 | 74±15 | 0.998 |
| Echocardiogram | ||||
| Left atrium (mm) | 4.4±0.7 | 4.7±0.7 | 5.1±0.7 | 0.0014* |
| Interventricular septal thickness (mm) | 0.99±0.2 | 1.02±0.2 | 1.09±0.2 | 0.12 |
| Posterior wall (mm) | 0.93±0.2 | 1.04±0.2 | 1.12±0.2 | 0.0023* |
| Left ventricular end-diastolic diameter (mm) | 5.8±0.9 | 6.2±0.9 | 6.9±1.2 | 0.0002* |
| Left ventricular end-systolic diameter (mm) | 4.6±1.06 | 4.8±1.09 | 5.6±1.38 | 0.011* |
| Left ventricular ejection fraction (%) | 24±9 | 20±8 | 19±9 | 0.09 |
| Left ventricular mass (g) | 217±49 | 276±64 | 358±86 | <0.0001* |
| LVMI (g/m2) | 112±20 | 141±23 | 178±38 | <0.0001* |
Continuous variables are described by mean ± SD and categorical variables by percentages (in parenthesis).
BMI: Body Mass Index; BUN: Blood urea nitrogen; BNP: Brain natriuretic peptide; LVMI: Left ventricular mass index; arb. u.: arbitrary units.
FSTL1 and LV mass
Change in LV mass is an indicator of cardiac remodeling. LVH was determined by LV mass indexed to body surface area. In chronic, systolic HF patients, both LV mass and LV mass index were significantly increased across the 3 tertiles of FSTL1 levels with the greatest LV mass seen with the highest tertile of FSTL1 (P<0.001, Figure 1 and Table 2). By multivariate linear regression analysis, FSTL1 level was significantly associated with LVH (LV mass index) (slope =6.7, 95% CI 4.8–8.5) after adjusting for age, diabetes mellitus, BNP, LA and LVEDD (P<0.001). In other words, for each unit increase in FSTL1, our model predicts an increase of 6.7 g/m2 in mean LVMI.
FSTL1 and mortality
Of the 86 chronic, systolic HF patients entered in this study between 2001–2004, 51% had died by 2010. The relationship between FSTL1 levels and survival was therefore investigated. In bivariate analysis we found a marginally significant association between survival and FSTL1 levels (P=0.09). This significance disappeared when adjusting for confounders in multivariable Cox regression (P=0.26). By multivariable Cox regression analysis, after adjusting for, LA and LVEDD, we found that for each unit increase in FSTL1 the hazard of death increased by 2.8%, 95% CI (2.0% – 7.7%).
Measurement of FSTL1 expression from explanted, failing and non-failing (NF) human left ventricle
We measured FSTL1 protein expression in failing and NF heart samples. As shown in Table 3, subjects in the failing group were older than the NF group. There were more females in the NF than in the failing groups. LVEF was <40% in the failing group. In the failing group, patients had DCM or ICM and received standard HF therapy that included diuretics and angiotensin converting enzyme inhibitors or angiotensin receptor blockers. Some were on digoxin, nitrates, dihydropyridine calcium channel blockers and antiarrythmics. None recieved β-blockers. Several patients in both groups received intravenous sympathomimetics such as dopamine or dobutamine and vasopressin.
Table 3.
Clinical Characteristics of Non-failing and Failing Explanted Human Hearts
| Non failing (NF) | DCM | ICM | |
|---|---|---|---|
| N (male/female) | 7 (5/2) | 9 (9/0) | 9 (9/0) |
| Age (yrs) | 38±14 | 47±9 | 58±6 |
| LVEF | normal | ≤ 40% | ≤ 40% |
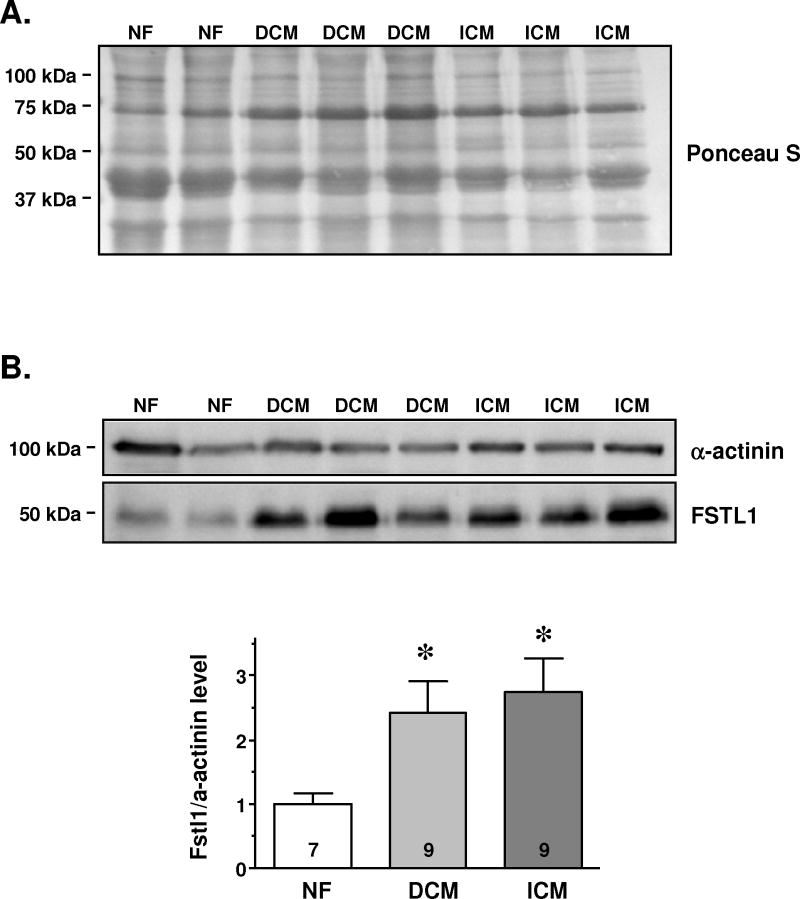
Immunoblots demonstrated significantly increased signal intensity in the failing hearts (~2.5-fold) compared to the NF hearts (NF: 1.0±0.2 vs. DCM: 2.4±0.5 and ICM: 2.7±0.5; P<0.05 vs. NF; Figure 2B lower panel). FSTL1 protein amount was normalized to α-actinin protein expression, which did not differ between groups (Figure 2B upper panel) and Ponceau S staining of blots confirmed equal protein loading (Figure 2A).
Figure 2.
Determination of FSTL1 protein expression in left ventricular myocardium from explanted hearts, from patients with dilated cardiomyopathy (DCM), ischemic cardiomyopathy (ICM), and non-failing donor hearts (NF). (A.) Ponceau S staining from homogenates of DCM, ICM and NF demonstrate equal protein loading and similar band pattern. (B.) Western blot and statistical analysis of FSTL1 protein expression normalized to α-actinin show higher FSTL1 protein expression in DCM and ICM compared to NF. Samples were run on the same gel. *P<0.05 vs. NF. The numbers in the columns represent the numbers of analyzed hearts.
Discussion
Our study demonstrated the following: 1) serum FSTL1 levels are significantly elevated in patients with chronic LV systolic HF versus age- and gender-matched healthy controls; 2) myocardial FSTL1 protein expression is significantly increased in the LV of explanted failing human hearts; 3) serum FSTL1 levels are predictive of LVH (as measured by LV mass index) in chronic human HF, by multivariate linear regression analysis after adjusting for age, diabetes mellitus, BNP, LA and LVEDD; and 4) chronic systolic HF patients with the highest FSTL1 levels had a higher risk of death. After adjusting for significant covariates, those with the highest serum FSTL1 levels still had a trend for a worse prognosis when compared with patients in the other groups.
In the present study, serum FSTL1 level was strongly associated with LVH, a measure of LV remodeling. Increased LV mass is an independent predictor of incident HF unrelated to prevalent or incident myocardial infarction13. Our cohort consisted of chronic stable HF patients where up to 80% had hypertension as a co-existing illness and 29 % of patients had hypertension as the primary etiology of their cardiomyopathy. Therefore it is not surprising that LV mass was increased. BNP is a measure of LV wall stress14 and in our study, mean BNP levels were markedly elevated in those patients with the highest FSTL1 levels. NT-proBNP levels predict mortality in patients with LVH15. Thus the finding in our study that FSTL1 predicts LVH is intriguing. Consequently what is the significance of the relationship of serum FSTL1 to increased LV mass? LVH (increased LV mass) is a hallmark of hypertensive heart disease and is an important prognostic indicator of adverse cardiovascular outcomes. Additionally it is an important target for monitoring hypertension therapy.
It is unknown whether the serum FSTL1 levels in these chronic systolic HF patients simply reflect the underlying disease process or whether FSTL1 has a maladaptive or compensatory role in modulating the pathogenesis of LV dysfunction. FSTL1 may play a mechanistic role in cardiovascular diseases since we previously demonstrated in experimental studies that it functions to protect against ischemia-reperfusion injury6. We have also shown that FSTL1 is upregulated in ischemic skeletal muscle and that its overexpression promotes revascularization in a murine model of peripheral arterial disease7. FSTL1 also promotes endothelial cell migration and suppresses apoptosis in vitro, indicating that it may function in a paracrine manner to promote endothelial cell function. Consistent with its beneficial effects, it has been reported that FSTL1 mRNA levels were increased in the failing human heart at the time of LVAD implantation and subsequently declined when LV function improved9. Furthermore, elevated FSTL1 mRNA levels correlated with subsequent improved cardiac function after LVAD removal, suggesting an adaptive function of FSTL1.
It is of interest to compare the profile of FSTL1 with that of TNFα, a mediator of inflammation. Elevated serum TNFα level is a harbinger of a poor prognosis in HF16. Myocardial TNFα expression is increased in the failing explanted heart17 and also at the time of LVAD implantation18. However unlike FSTL1, the greatest reductions in myocardial TNFα were seen in patients who were successfully weaned off the LVAD and who did not require cardiac transplantation18. At a mechanistic level, FSTL1 differs from TNFα in that it appears to function as a negative regulator of inflammatory signals in some animal models. The role of FSTL1 as a mediator of inflammation has been suggested by its association with extracellular matrix-related and calcium-binding proteins9. Increased FSTL1 reduces matrix metalloproteases 1 and 3 expression in vitro 19 which may degrade extracellular matrix proteins. Similarly FSTL1 overexpression inhibits pro-inflammatory cytokine expression and improves allograft survival.5 Thus it is conceivable that FSTL1 levels either reflects the inflammatory state of the heart or that it functions as a modulator of myocardial inflammation.
Limitations
Explanted myocardial tissue samples were not matched by age and gender because low numbers, are in general, inherent to explanted human heart tissue studies. Failing, human heart tissues are usually obtained from an older, diseased population. Conversely NF controls are younger (who have died usually from trauma/ motor vehicle accidents) and are healthy enough to be considered as a donor. Similarly samples could not be matched by gender; however, when the 2 female NF controls were removed from the analysis, FSTL1 protein expression still remained significantly greater than NF donor hearts (P<0.05). Despite these limitations, similarly to others9, our data showed that FSTL1 protein expression is increased in failing human myocardium.
Thus taken together, chronic systolic HF is associated with elevated circulating FSTL1 levels. FSTL1 is significantly associated with LVH and its myocardial protein abundance is increased in the failing LV. Future studies are needed to determine its utility as a biomarker in chronic systolic HF and LV remodeling. In patients with ACS, elevated FSTL1 level was associated with poor outcome8. The risk of death was higher in ACS patients presenting with FSTL1 levels above the median than in patients with FSTL1 levels below the median8. Similarly our data suggested that those subjects with the highest FSTL1 levels had decreased survival. The present study is limited by the sample size which is relatively small, and larger prospective studies will be required to better define the role of serum FSTL1 as a prognostic biomarker for HF.
Acknowledgments
Sources of Funding
This work was supported by National Heart Lung and Blood Institute (NHLBI) grants, Bethesda, Maryland, USA, HL102631 (F.Sam) and HL079099 (F.Sam).
Footnotes
Disclosures
None
References
- 1.Shibanuma M, Mashimo J, Mita A, Kuroki T, Nose K. Cloning from a mouse osteoblastic cell line of a set of transforming-growth-factor-beta 1-regulated genes, one of which seems to encode a follistatin-related polypeptide. Eur J Biochem. 1993;217:13–9. doi: 10.1111/j.1432-1033.1993.tb18212.x. [DOI] [PubMed] [Google Scholar]
- 2.Ouchi N, Asaumi Y, Ohashi K, Higuchi A, Sono-Romanelli S, Oshima Y, Walsh K. DIP2A functions as a FSTL1 receptor. J Biol Chem. 2010;285:7127–34. doi: 10.1074/jbc.M109.069468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sumitomo K, Kurisaki A, Yamakawa N, Tsuchida K, Shimizu E, Sone S, Sugino H. Expression of a TGF-beta1 inducible gene, TSC-36, causes growth inhibition in human lung cancer cell lines. Cancer Lett. 2000;155:37–46. doi: 10.1016/s0304-3835(00)00407-9. [DOI] [PubMed] [Google Scholar]
- 4.Kawabata D, Tanaka M, Fujii T, Umehara H, Fujita Y, Yoshifuji H, Mimori T, Ozaki S. Ameliorative effects of follistatin-related protein/TSC-36/FSTL1 on joint inflammation in a mouse model of arthritis. Arthritis Rheum. 2004;50:660–8. doi: 10.1002/art.20023. [DOI] [PubMed] [Google Scholar]
- 5.Le Luduec JB, Condamine T, Louvet C, Thebault P, Heslan JM, Heslan M, Chiffoleau E, Cuturi MC. An immunomodulatory role for follistatin-like 1 in heart allograft transplantation. Am J Transplant. 2008;8:2297–306. doi: 10.1111/j.1600-6143.2008.02398.x. [DOI] [PubMed] [Google Scholar]
- 6.Oshima Y, Ouchi N, Sato K, Izumiya Y, Pimentel DR, Walsh K. Follistatin-like 1 is an Akt-regulated cardioprotective factor that is secreted by the heart. Circulation. 2008;117:3099–108. doi: 10.1161/CIRCULATIONAHA.108.767673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ouchi N, Oshima Y, Ohashi K, Higuchi A, Ikegami C, Izumiya Y, Walsh K. Follistatin-like 1, a secreted muscle protein, promotes endothelial cell function and revascularization in ischemic tissue through a nitric-oxide synthase-dependent mechanism. J Biol Chem. 2008;283:32802–11. doi: 10.1074/jbc.M803440200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Widera C, Horn-Wichmann R, Kempf T, Bethmann K, Fiedler B, Sharma S, Lichtinghagen R, Leitolf H, Ivandic B, Katus HA, Giannitsis E, Wollert KC. Circulating Concentrations of Follistatin-Like 1 in Healthy Individuals and Patients with Acute Coronary Syndrome as Assessed by an Immunoluminometric Sandwich Assay. Clin Chem. 2009;55:1794–800. doi: 10.1373/clinchem.2009.129411. [DOI] [PubMed] [Google Scholar]
- 9.Lara-Pezzi E, Felkin LE, Birks EJ, Sarathchandra P, Panse KD, George R, Hall JL, Yacoub MH, Rosenthal N, Barton PJ. Expression of follistatin-related genes is altered in heart failure. Endocrinology. 2008;149:5822–7. doi: 10.1210/en.2008-0151. [DOI] [PubMed] [Google Scholar]
- 10.Kotlyar E, Vita JA, Winter MR, Awtry EH, Siwik DA, Keaney JF, Jr, Sawyer DB, Cupples LA, Colucci WS, Sam F. The relationship between aldosterone, oxidative stress, and inflammation in chronic, stable human heart failure. J Card Fail. 2006;12:122–7. doi: 10.1016/j.cardfail.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 11.Schiller NB, Shah PM, Crawford M, DeMaria A, Devereux R, Feigenbaum H, Gutgesell H, Reichek N, Sahn D, Schnittger I. Recommendations for quantitation of the left ventricle by two-dimensional echocardiography. American Society of Echocardiography Committee on Standards, Subcommittee on Quantitation of Two-Dimensional Echocardiograms. J Am Soc Echocardiogr. 1989;2:358–67. doi: 10.1016/s0894-7317(89)80014-8. [DOI] [PubMed] [Google Scholar]
- 12.Devereux RB, Alonso DR, Lutas EM, Gottlieb GJ, Campo E, Sachs I, Reichek N. Echocardiographic assessment of left ventricular hypertrophy: comparison to necropsy findings. Am J Cardiol. 1986;57:450–8. doi: 10.1016/0002-9149(86)90771-x. [DOI] [PubMed] [Google Scholar]
- 13.De Simone G, Gottdiener JS, Chinali M, Maurer MS. Left ventricular mass predicts heart failure not related to previous myocardial infarction: the Cardiovascular Health Study. Eur Heart J. 2008;29:741–7. doi: 10.1093/eurheartj/ehm605. [DOI] [PubMed] [Google Scholar]
- 14.Luchner A, Stevens TL, Borgeson DD, Redfield M, Wei CM, Porter JG, Burnett JC., Jr Differential atrial and ventricular expression of myocardial BNP during evolution of heart failure. Am J Physiol. 1998;274:H1684–H1689. doi: 10.1152/ajpheart.1998.274.5.H1684. [DOI] [PubMed] [Google Scholar]
- 15.Garcia S, Akbar MS, Ali SS, Kamdar F, Tsai MY, Duprez DA. N-terminal pro B-type natriuretic peptide predicts mortality in patients with left ventricular hypertrophy. International Journal of Cardiology. 2010;143:349–52. doi: 10.1016/j.ijcard.2009.03.070. [DOI] [PubMed] [Google Scholar]
- 16.Levine B, Kalman J, Mayer L, Fillit HM, Packer M. Elevated circulating levels of tumor necrosis factor in severe chronic heart failure. N Engl J Med. 1990;323:236–41. doi: 10.1056/NEJM199007263230405. [DOI] [PubMed] [Google Scholar]
- 17.Torre-Amione G, Kapadia S, Lee J, Durand JB, Bies RD, Young JB, Mann DL. Tumor Necrosis Factor-alpha and Tumor Necrosis Factor Receptors in the Failing Human Heart. Circulation. 1996;93:704–11. doi: 10.1161/01.cir.93.4.704. [DOI] [PubMed] [Google Scholar]
- 18.Torre-Amione G, Stetson SJ, Youker KA, Durand JB, Radovancevic B, Delgado RM, Frazier OH, Entman ML, Noon GP. Decreased Expression of Tumor Necrosis Factor-alpha in Failing Human Myocardium After Mechanical Circulatory Support : A Potential Mechanism for Cardiac Recovery. Circulation. 1999;100:1189–93. doi: 10.1161/01.cir.100.11.1189. [DOI] [PubMed] [Google Scholar]
- 19.Tanaka M, Ozaki S, Kawabata D, Kishimura M, Osakada F, Okubo M, Murakami M, Nakao K, Mimori T. Potential preventive effects of follistatin-related protein/TSC-36 on joint destruction and antagonistic modulation of its autoantibodies in rheumatoid arthritis. Int Immunol. 2003;15:71–7. doi: 10.1093/intimm/dxg005. [DOI] [PubMed] [Google Scholar]