Graphical abstract
Abbreviations: TTX, tetrodotoxin; CTX GVIA, ω-conotoxin GVIA; ICC, interstitial cells of Cajal; FLC, fibroblast-like cells; SMC, smooth muscle cells; TP, thromboxane prostanoid; VIP, vasoactive intestinal polypeptide
Keywords: Nonadrenergic noncholinergic relaxation, Rat gastric fundus, KV7 channels, KCNQ channels, Retigabine, XE-991
Abstract
Voltage-dependent type 7 K+ (KV7) channels play important physiological roles in neurons and muscle cells. The aims of the present study were to investigate the motor effects of KV7 channel modulators in the rat gastric fundus and the expression of KV7 channels in this tissue.
Muscle tone and electrical field stimulation (EFS)-evoked relaxations of precontracted longitudinal muscle strips of the rat gastric fundus were investigated under nonadrenergic noncholinergic conditions by organ bath studies. Gene expression was studied by real-time PCR and tissue localization of channels was investigated by immunohistochemistry.
The KV7 channel blocker XE-991 induced concentration-dependent contractions, with mean pD2 and Emax of 5.4 and 48% of the maximal U46619-induced contraction, respectively. The KV7 channel activators retigabine and flupirtine concentration-dependently relaxed U46619-precontracted strips, with pD2s of 4.7 and 4.4 and Emax of 93% and 91% of the maximal relaxation induced by papaverine, respectively. XE-991 concentration-dependently inhibited retigabine-induced relaxation with a pIC50 of 6.2. XE-991 and DMP-543, another KV7 channel blocker, increased by 13–25% or reduced by 11–21% the relaxations evoked by low- or high-frequency EFS, respectively. XE-991 also reduced the relaxation induced by vasoactive intestinal polypeptide (VIP) by 33% of controls. Transcripts encoded by all KV7 genes were detected in the fundus, with 7.4 and 7.5 showing the highest expression levels. KV7.4 and 7.5 channels were visualized by confocal immunofluorescence in both circular and longitudinal muscle layers.
In conclusion, in the rat proximal stomach, KV7 channels appear to contribute to the resting muscle tone and to VIP- and high-frequency EFS-induced relaxation. KV7 channel activators could be useful relaxant agents of the gastric smooth muscle.
1. Introduction
In mammalian cells, K+ channels are involved in several physiological processes, such as neurotransmitter and hormone release, motor activity of muscle cells, regulation of water-electrolyte balance and epithelial secretion [1]. Opening of K+ channels hyperpolarizes the resting membrane potential and decreases cell excitability. Many K+ channel subtypes in different cell types of the gastrointestinal tract (epithelial, smooth muscle cells [SMC], interstitial cells of Cajal [ICC], fibroblast-like cells [FLC] and neurons) are involved in gut secretory and motor activities. By differentially controlling the membrane resting potential of SMC–ICC–FLC syncytial apparatus in the various gut segments, K+ channels participate to the region-dependent differences in basal muscle tone levels [2]. Opening of K+ channels is among the signal transduction mechanisms activated by the neurotransmitters released from the inhibitory motor neurons [2]. Voltage-dependent K+ (KV) channels are particularly expressed in the GI tract; indeed, KV1.2, 1.5, 1.6, 2.2, 4.1, 4.3, 7, 11.1 and 12.1 transcripts and/or proteins have been shown in the GI tract, with KV1.2 and 2.2 playing a dominant role in regulating SMC contractility [2].
The KV7 channel subfamily includes 5 members (KV7.1–7.5), each with distinct expression pattern and functional roles. KV7.1 channels mediate the slowly activating K+ current (IKs) involved in the late repolarizing phase of the action potential in cardiomyocytes [3]. In neuronal cells, KV7.2, 7.3 and 7.5 represent the molecular basis of the M current (IKM), a slowly activating and deactivating current inhibited by muscarinic receptors stimulation, that modulates cell excitability and firing pattern [4,5]. KV7.4 channels have been first described in the inner ear and auditory neurons [6] and more recently in skeletal muscle cells [7]. KV7 channels have been shown to regulate vascular, gastrointestinal and genitourinary smooth muscle activity [8–11]. In the gastrointestinal tract, acetylcholine has been long known to increase the membrane excitability of toad [12] and guinea-pig [13] gastric SMC through the inhibition of a voltage-dependent K+ current active at resting membrane potential, having properties similar to neuronal IKM. This evidence might suggest that KV7 channels are expressed in the SMC–ICC–FLC syncytial apparatus of the stomach and their inhibition mediate acetylcholine-induced electrophysiological effects. The expression of KV7 channels and the motor effects of KV7 channel modulators have been investigated in the mouse colon [9]. In the same study, all KV7 channel gene transcripts have been shown to be expressed in both gastric fundus and antrum in the mouse, with KV7.4 and 7.5 showing the highest levels [9]. Only the expression of KV7.1–7.3 channel genes has been investigated in the rat stomach [14]. KV7.1 and 7.3 channel mRNAs were detected in the whole stomach and their levels were relatively high in the antrum but very low in the fundus [14].
The proximal stomach plays an important “reservoir” function, i.e. it accommodates high volumes of food bolus with small increases in intraluminal pressure. The accommodative gastric function occurs mostly through the active reflex neural nonadrenergic noncholinergic (NANC) relaxation of the smooth muscle. In vitro preparations of the rat proximal stomach passively and progressively relax under a constant load during the equilibration period and generally stabilize on a very low basal muscle tone. In addition, they show a very low phasic muscle activity. In vivo, gastric tone appears to be maintained by vagally mediated cholinergic input [15]. The most probable neurotransmitters released by the inhibitory motor neurons are nitric oxide (NO) and vasoactive intestinal polypeptide (VIP). NO is mainly responsible for the rapid beginning and the high speed of the initial phase of the relaxation, whereas VIP and its related peptide, peptide histidine isoleucine (PHI), are mainly involved in the long duration of the inhibitory response evoked by high-frequency neuronal activation [16]. At least a third component, probably produced by a non-purinergic neurotransmitter acting via apamin-sensitive mechanisms, seems also to be present [17]. It is well known that NO and VIP mainly act through the activation of soluble guanylate cyclase and VPAC2 receptors followed by stimulation of adenylate cyclase through Gs protein, respectively. However, the final molecular mechanisms linked to the relaxation have not been fully elucidated in the rat gastric fundus. In addition, the ion channels contributing to the membrane resting potential of the SMC–ICC–FLC syncytial apparatus of the rat gastric fundus have not been definitively characterized. In particular, a characterization of the role of KV7 channels in the motor activity of rat proximal stomach has never been performed. In this study, we investigated the effects of KV7 channel modulators on the resting muscle tone and on the NANC relaxation of the rat gastric fundus. The effects of KV7 channel blockade on the relaxations induced by NO and VIP were also evaluated. The results of the present study indicate that KV7 channels play important roles in the maintenance of the low muscle tone in resting conditions and in the VIP-induced relaxation in the rat gastric fundus. Altogether, the results obtained provide the first functional demonstration of a critical control exerted by KV7 channels over rat proximal stomach motor activity, revealing a novel pharmacological target for therapeutic interventions against gastric motor disturbances.
2. Methods
2.1. Policy and ethics
This study was approved by the institutional Ethical Committee for the Animal Experimentation of the Catholic University. The work described in this article was carried out in accordance with the Directive 2010/63/EU of the European Parliament and of the Council on the protection of animals used for scientific purposes. In addition, this paper fulfils the Uniform Requirements for Manuscripts Submitted to Biomedical Journals of ICMJE.
2.2. Motor activity studies
2.2.1. General methods
Wistar rats of either sex, weighing 180–320 g, were fasted overnight with free access to water, afterwards killed by decapitation and exsanguinated. The gastric fundus was removed and two longitudinal muscle strips (3 × 20 mm) were prepared according to the method of Vane [18] in a Krebs solution of the following composition (mM): NaCl 118.5, KCl 4.8, CaCl2 1.9, KH2PO4 1.2, MgSO4 1.2, NaHCO3 25 and glucose 10.1 (pH 7.4). The strips were mounted between parallel platinum electrodes (22 mm long, 4 mm wide and 5.5 mm apart) and suspended in Krebs solution maintained at 37 °C and bubbled with a 95/5 O2/CO2 mixture inside 5-ml organ baths. The strips were connected to isotonic transducers (model 7006; Ugo Basile Biological Research Apparatus, Comerio, Italy) under a 1-g load. Smooth muscle activity was recorded on a computer using the PowerLab data acquisition system (ADInstruments, Castle Hill, Australia). Isolated EFSs, consisting of rectangular and bipolar pulses of constant duration (1 ms) and amplitude (120 mA), were performed via platinum plate electrodes by a stimulator (model 6012; Palmer Bioscience, now Harvard Apparatus Ltd., Edenbridge, UK) linked in series with a 4-channel constant-current unit (model Multiplexing Pulse Booster; Ugo Basile Biological Research Apparatus). Tissues were initially allowed to equilibrate for 40 min in Krebs solution. After this period, in all experimental series, the bath solution also contained atropine (1 μM) and guanethidine (5 μM) (to achieve NANC conditions). Strips were allowed to equilibrate for 20 more min in this bath solution. The incubation medium was always changed every 10 min (during the equilibration period and in between drug administration and/or periods of EFS).
2.2.2. Study of the motor effects induced by U46619
In a first series of experiments, the strips were exposed to consecutive 5-min incubations with increasing concentrations of 9,11-dideoxy-9α,11α-methanoepoxy prostaglandin F2α (U46619, 1 nM to 1 μM), a selective thromboxane receptor (TP) agonist, to investigate the concentration–response relationship for this muscle contracting agent and set the maximal contractile capacity of the strips. Strips were allowed to recover to basal tone prior to the subsequent U46619 concentration. Contractions were expressed as percentages of the effect produced by the maximal concentration used (1 μM).
2.2.3. Study of the motor effects induced by KV7 channel blockers
In a second series of experiments, the preparations were first contracted by a submaximal concentration (0.1 μM) of U46619. After 10 min, U46619 was washed out from the incubation medium and the strips were allowed to return to basal tone. Then, the strips were exposed to consecutive 5-min incubations with increasing concentrations of the selective KV7 channel blocker XE-991 (0.5–100 μM) [19]. XE-991 was added cumulatively to the incubation medium. In four strips, the effects of retigabine (1–30 μM), a substance considered to be a selective activator of neuronal KV7 channels [4,20], added cumulatively to the bath medium, were investigated at the top of the concentration-dependent contraction induced by XE-991 (0.5–100 μM). Then, the substance/s was/were washed out from the bath and strip motor activity was recorded for 30–60 min. At this time, U46619 (0.1 μM) was added to the medium for a second time and left in the bath for 10 min.
2.2.4. Study of the motor effects induced by KV7 channel activators
In all following series of experiments, the bath solution also contained U46619 (0.1 μM) (to raise strip tone and so enable recording of relaxant responses). Initially, the strips were stimulated twice with EFS (2 Hz, 30 s) to evaluate the quality and the reproducibility of relaxant responses. A 10-min period elapsed between these two initial EFS.
After 10 more min, the strips were exposed to consecutive incubations with increasing concentrations of retigabine (1–100 μM) or flupirtine (1–144 μM), another substance considered to be a selective activator of neuronal KV7 channels [4]. Since in preliminary experiments the strips fully recovered to basal tone after retigabine washout from the bath, isolated concentration–response curves were performed for this drug. On the contrary, the strips did not fully recover to basal tone after flupirtine washout, so that this second KV7 activator was added cumulatively to the incubation medium. Retigabine and flupirtine were left in the bath until peak relaxations were reached, that required 5 or 10 min, respectively. In some strips, the concentration–response curve for flupirtine was studied in the presence of the voltage-dependent Na+-channel blocker tetrodotoxin (TTX, 1 μM; pre-incubation time: 10 min) or the voltage-dependent N-type Ca2+-channel blocker ω-conotoxin GVIA (CTX GVIA, 30 nM; 10 min). The effects of TTX (1 μM) and CTX GVIA (30 nM) on the relaxation induced by retigabine (100 μM) were also studied. In separate preparations, the effect induced by retigabine (10 or 30 μM) and flupirtine (44 μM) were investigated in the presence of XE-991 (20 μM). Subsequently, to better evaluate the inhibitory effects of XE-991 on retigabine-induced relaxation, the concentration-dependent effects of XE-991 (0.5–20 μM) on the submaximal relaxation induced by retigabine (30 μM) were investigated.
2.2.5. Effects of KV7 channel modulators on the relaxations induced by EFS, NO and VIP
The strips were subjected to two consecutive EFS (pulse train duration: 2 min), at the frequency, respectively, of 2 and 13 Hz. At these parameters, EFS has been shown to induce submaximal responses in the frequency–response curves based on the amplitude or the area under the curve (AUC) of relaxant responses, respectively [21,22]. On the contrary, the amplitude of EFS (13 Hz)-evoked relaxation is a nearly maximal response. Strips were allowed to recover to basal tone prior to the second EFS (usually, 10–15 min elapsed between 2 Hz EFS cessation and 13 Hz EFS beginning). After the induction of these control relaxations and the recovering to basal tone, the strips were exposed to a pharmacological agent and then EFS (2 and 13 Hz) were repeated in its presence. The effects of XE-991 (10–50 μM) and DMP-543 (20 μM), another substance considered to be a selective KV7 channel blocker [4], were evaluated. Since KV7 channel blockers increased strip tone, parallel control strips were studied in which the tone was increased to a similar level by a higher concentration (0.3 μM) of U46619 before the second series of EFS. The effects of XE-991 (20 μM) on the submaximal relaxations produced by 2-min consecutive incubations with NaNO2 (300 μM) in 0.1 N HCl, a tool used to study NO-induced effects, and VIP (10 nM) were also investigated. Pre-incubation time of drugs was 10 min. In some strips, a low concentration (3 μM) of retigabine was added to the bath medium for 2 min just before 2 Hz and 13 Hz EFS and then maintained in the bath during EFS to investigate its effects on EFS-induced relaxations. A group of untreated strips was used at the same time as the strips treated with the different reagents to check for possible effects due to the time elapsed between the two series of EFS or NaNO2 and VIP incubations. Each strip was exposed to a single concentration of a pharmacological agent. In separate preparations, the effect of XE-991 (20 μM) on muscle tone was evaluated with TTX (1 μM) or CTX GVIA (30 nM) in the bath.
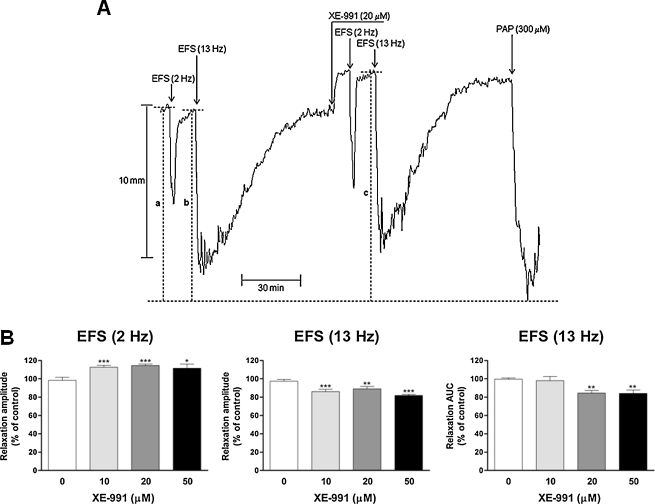
At the end of each experiment, each strip was exposed for 5 min to papaverine (300 μM), which induced maximal relaxation. In the experiments in which the effects of flupirtine were investigated, papaverine was usually added to the medium 10 min after flupirtine washout from the bath. In some experiments, however, 20 or 30 min were waited before strip exposure to papaverine to better evaluate the recovery phase of muscle tone. Relaxant responses induced by EFS (2 Hz), NaNO2 (300 μM) and KV7 channel activators or contractions induced by KV7 channel blockers were calculated as maximal amplitudes. Those induced by EFS (13 Hz) and VIP (10 nM) were calculated as both maximal amplitudes and AUCs, estimated using the software Chart (ADInstruments). For the analysis, amplitudes of relaxations or contractions and AUC of relaxant responses were normalized using some maximal amplitude parameters. The amplitudes of relaxations or contractions produced by KV7 channel activators or blockers, respectively, were expressed as percentages of a maximal amplitude parameter measured from the maximal U46619-induced contraction point (that produced by the first addition of U46619 in the experiments carried out to investigate the concentration-dependent contractile effects induced by XE-991) to the maximal papaverine (300 μM)-induced relaxation point reached at the end of each experiment. As for the relaxant effects induced by EFS or exogenous acidified NaNO2 and VIP, since they can be differentiated as submaximal responses (amplitudes of relaxations induced by 2 Hz EFS and acidified NaNO2 and AUC of relaxant responses induced by 13 Hz EFS and VIP) and nearly maximal responses (amplitudes of relaxant effects induced by 13 Hz EFS and VIP) and the KV7 channel blockers significantly increase the muscle pre-contraction level, we believed that it was not correct to normalize them with respect to a single parameter. Therefore, the submaximal relaxation amplitudes induced by EFS (2 Hz) or acidified NaNO2 were expressed as percentages of a maximal amplitude parameter measured from the maximal U46619-induced contraction point reached before the addition of any test substance to the maximal papaverine (300 μM)-induced relaxation point reached at the end of each experiment. Similarly, the submaximal AUCs (mm min) of the relaxant responses induced by 13 Hz EFS and VIP were divided by the maximal amplitude parameter (mm) used to normalize the submaximal relaxation amplitudes induced by EFS (2 Hz) and acidified NaNO2 and expressed as min [22]. On the contrary, each maximal relaxation amplitude induced by EFS (13 Hz) or VIP was expressed as a percentage of its own maximal amplitude parameter, measured from the U46619-induced contraction point reached at the start of each EFS- or VIP-induced relaxation to the maximal papaverine (300 μM)-induced relaxation point reached at the end of each experiment. An example of the measurement of the different parameters used to normalize the relaxant responses is given in Fig. 4A.
Fig. 4.
Effects of XE-991 on the NANC relaxant responses induced by EFS (2 or 13 Hz, 120 mA, 1 ms, pulse trains of 2 min) of U46619 (0.1 μM)-precontracted longitudinal muscle strips of the rat gastric fundus. (A) Representative tracings showing the effects of XE-991 (20 μM). The submaximal relaxation amplitudes induced by EFS (2 Hz) were expressed as percentages of a maximal amplitude parameter measured from the maximal U46619-induced contraction point reached before the addition of any test substance to the maximal papaverine (300 μM)-induced relaxation point reached at the end of each experiment (parameter indicated with a in the panel). Similarly, the submaximal AUCs (mm min) of the relaxant responses induced by EFS (13 Hz) were divided by this same maximal amplitude parameter (mm) and expressed as min. On the contrary, each maximal relaxation amplitude induced by EFS (13 Hz) was expressed as a percentage of its own maximal amplitude parameter, measured from the U46619-induced contraction point reached at the start of each EFS-induced relaxation to the maximal papaverine (300 μM)-induced relaxation point reached at the end of each experiment (parameters indicated with b and c in the panel, respectively). (B) Mean NANC relaxations evoked by 2 Hz (left graph) or 13 Hz EFS (middle and right graphs) observed in the absence (0 XE-991, time controls) or presence of XE-991 (10–50 μM). Relaxations were measured as peak amplitudes (left and middle graphs) or AUCs (right graph) and are expressed as percentages of control responses. Each point represents the mean ± s.e.m. of responses observed in eight (0, 10 and 20 μM XE-991) or nine (50 μM XE-991) strips. Significant differences between test and control responses: *P < 0.05, **P < 0.01,***P < 0.001.
2.3. Gene expression studies
The gastric fundus was removed from male Wistar rats, weighing 280–320 g and fasted overnight with free access to water, and total RNA was isolated by use of the TRI-Reagent (Sigma–Aldrich, Milan, Italy). RNA was treated with DNase-I (1 U μl−1; Sigma–Aldrich) for 15 min at room temperature, followed by spectrophotometric quantification. Final preparation of RNA was considered DNA- and protein-free if the ratio between readings at 260/280 nm was >1.7. Isolated mRNA was reverse-transcribed by use of MuLV high-capacity reverse transcriptase (50 U μl−1; Applied Biosystems, Monza, Italy) in a buffer containing 4 mM dNTP, Random Primers, 1 μl of RNase Inhibitor at 37 °C for 120 min. The cDNA was amplified in reverse transcription-polymerase chain reaction (RT-PCR) using PCR gold buffer, also containing 2 mM MgCl2, 0.8 mM dNTP mix, 0.001 mM forward and reverse primers and 1 U μl−1 Amplitaq Gold (Applied Biosystems). The protocol used for PCR amplification was the following: denaturation at 95 °C for 30 s, annealing at 57 °C for 30 s, and elongation at 72 °C for 30 s (40 cycles). To test the ability of the KV7 primers to specifically recognize the mRNA target for which they were designed, RT-PCR experiments were performed with use of cDNA templates from adult rat brain (KV7.2–7.5) and heart (KV7.1) mRNAs.
Real-time quantitative PCR (qPCR) was carried out by a 7500 fast real-time PCR system (Applied Biosystems) with primers specific for the KV7 channel genes and SYBR Green detection. Samples were amplified simultaneously in a triplicate in one-assay run and the cycle threshold (ct) value for each experimental group was determined. Data normalization was performed by using the ct for the amplification of GAPDH gene, a constitutively expressed gene, as a control. Differences in mRNA content between groups were calculated as normalized values by use of the 2−Δct formula.
2.4. Immunohistochemistry
The rat gastric fundus was fixed in cold paraformaldehyde for 2 h at 4 °C. The preparations were then washed several times in phosphate-buffered saline (PBS) and incubated in sucrose 10%-PBS at 4 °C for cryopreservation. Tissue was subsequently embedded in Tissue-tek OCT compound and frozen at −80 °C. Frozen sections (20 μm) were cut using a cryostat, collected on super-frost glasses and stored at −20 °C for further processing. Slices were washed in PBS and incubated at 4 °C with the following primary antibodies: (i) rabbit anti KV7.1 (1:50, Alomone Labs, Jerusalem, Israel); (ii) rabbit anti KV7.4 (1:100, Abcam, Cambridge, UK); (iii) rabbit anti KV7.5 (1:100, Millipore, Temecula, CA, USA). Sections were washed three times in PBS for 10 min and subsequently incubated for 1 h at RT with an anti-rabbit conjugated to Cy3 (1:100, Jackson Immunoresearch, Suffolk, UK) and chromomycin A3 (1:1000, Sigma, St. Louis, MO, USA), used as a nuclear marker. All antibodies and chromomycin were diluted in PBS containing 10% FBS and 0.1% Triton X-100. Subsequently, sections were washed in PBS, allowed to air dry and then mounted in SlowFade Antifade (Invitrogen – Molecular Probes, Carlsbad, CA, USA) before coverslipping. Coverslips were analyzed using a Zeiss LSM 510 Meta argon/krypton laser scanning confocal microscope. Images were acquired using the multitrack system to avoid crosstalk among channels. The excitation/emission settings were 430/505–550 nm for chromomycin, and 543/560–615 nm for CY3. Images were confocally captured by use of 10X, 20X or 63X-oil immersion objective (PlanApochromat; numerical aperture 1.4), with a maximal confocal zoom factor of 3, fixed box sizes of 512 × 512 pixels, and pinhole below 1 Airy unit. Each image was acquired four times, and the signal was averaged to improve the signal to noise ratio. The colour scheme used was green for chromomycin A3, and red for Cy3-labelled structures.
2.5. Data analysis and statistical procedures
The results were evaluated by means of Student's paired and unpaired t-test (results within and between tissues, respectively). When more than two groups had to be compared, analysis of variance (ANOVA) followed by Bonferroni's test for multiple comparison was performed. All values are presented as means ± SEM. P < 0.05 was considered statistically significant. The GraphPad Prism program (GraphPad Software, San Diego, CA, USA) was used for fitting the concentration–response curves and calculating EC50s and maximal effects.
2.6. Drugs, chemicals reagents and other materials
The following drugs were used: atropine sulphate, ω-conotoxin GVIA, guanethidine sulphate, NaNO2, tetrodotoxin, VIP (Sigma, St. Louis, MO, USA); XE-991 dihydrochloride (Tocris, Bristol, UK; Ascent Scientific, Bristol, UK); flupirtine maleate, DMP-543 (Tocris); papaverine hydrochloride (Merck, Darmstadt, Germany); U46619 (Cayman Chemical, Ann Arbor, MI, USA). Retigabine was kindly provided by ASTA Medica (Radebeul, Germany). Substances were dissolved with bidistilled water, except for flupirtine, retigabine, DMP-543 and U46619, that were dissolved with DMSO at 10 mM, 100 mM and 20 mM, and methanol at 1 mM, respectively. Flupirtine was then diluted with bidistilled water. Retigabine and DMP-543 precipitated if they were then diluted with bidistilled water to 10 mM and 2 mM, respectively. Consequently, retigabine was then diluted at 10 mM and 3 mM with 33% and 25% DMSO, respectively. DMP-543 was then diluted at 2 mM with 50% DMSO. Putative motor effects of DMSO were also investigated.
3. Results
3.1. Motor activity studies
3.1.1. Motor effects of U46619
The selective TP agonist U46619 (1 nM–1 μM) induced concentration-dependent contractions of the gastric fundus strips. The mean pD2 (−log EC50) and maximal peak amplitude (Emax) of U46619-induced concentration–response curve were 7.51 ± 0.1 and 102.9 ± 1.7% of U46619 (1 μM)-induced contraction, respectively (n = 6). U46619 (0.1 μM), the concentration chosen to precontract the strips in the experiments in which the relaxant effects of KV7 modulators were studied, contracted the strips by 75.8 ± 3.2% of the maximum.
3.1.2. Motor effects of KV7 channel blockers
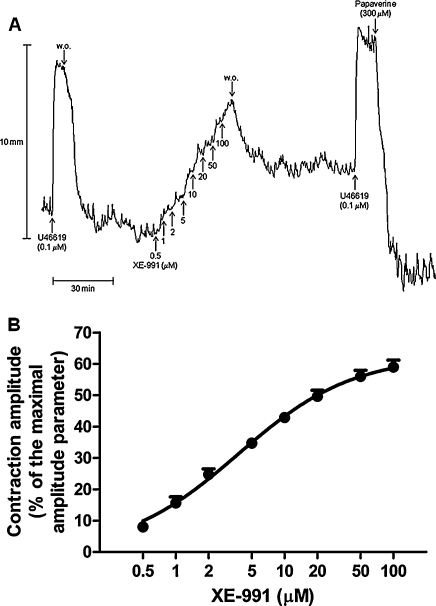
XE-991 (0.5–100 μM) produced concentration-dependent contractions of the gastric fundus strips (Fig. 1A). The mean pD2 and Emax of XE-991-induced concentration-response curve were 5.40 ± 0.06 and 64.0 ± 2.5% of U46619 (0.1 μM)-induced contraction, respectively (n = 13, Fig. 1B). Retigabine, added to the bath medium at the top of XE-991-induced concentration–response curve, did not induce any effect up to 10 μM (n = 4). At the concentration of 30 μM, retigabine reverted XE-991-produced contraction by 13.1 ± 1.9% (n = 4). After XE-991 washout from the bath, the strips recovered to 53.1 ± 4.7% of the maximal contraction induced by XE-991 (Fig. 1A). Including this higher starting tone level remaining after XE-991 washout from the bath medium (i.e. measuring the contraction from the beginning of the concentration–response curve to XE-991), the contraction produced by the second addition of U46619 (0.1 μM) to the medium was 121.0 ± 1.8% of the first one (109.3 ± 3.8% vs. 88.3 ± 3.0%, P < 0.001).
Fig. 1.
Motor effects induced by XE-991 on longitudinal muscle strips of the rat gastric fundus under NANC conditions. (A) Representative tracings showing the concentration-dependent contracting effects of XE-991 (0.5–100 μM). (B) Mean concentration–response curve for contractions induced by XE-991 (0.5–100 μM). Contractions were measured as peak amplitudes. Peak amplitudes are expressed as percentages of a maximal amplitude parameter measured from the maximal U46619-induced contraction point reached before the addition of any test substance to the maximal papaverine (300 μM)-induced relaxation point reached at the end of each experiment. Each point represents the mean ± s.e.m. of responses observed in nine strips.
3.1.3. Motor effects of KV7 channel activators
Since retigabine and flupirtine were dissolved with DMSO, the putative motor effects of this solvent were investigated. DMSO induced reversible relaxations of U46619-precontracted fundus strips. The relaxations produced by DMSO at the concentrations (0.33% and 1.44%) obtained in the bath medium when the effects of retigabine and flupirtine at the maximal concentrations tested (100 and 144 μM, respectively) were investigated were 7.4 ± 1.4% and 22.0 ± 2.7% (n = 4), respectively.
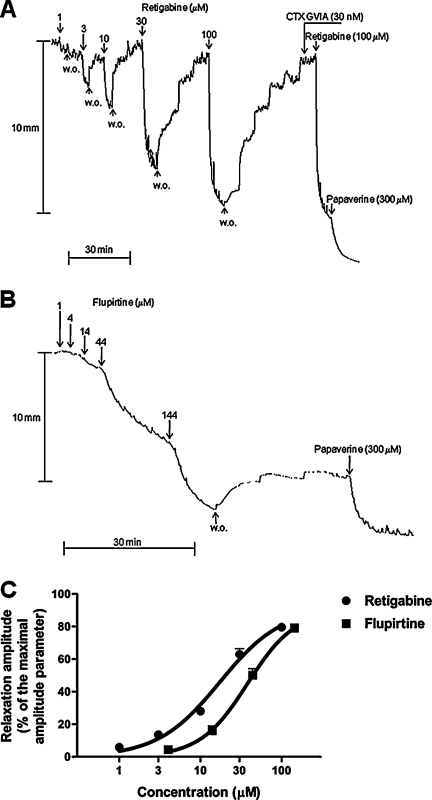
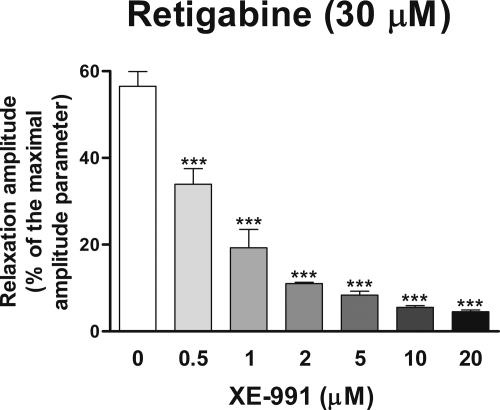
Retigabine (1–100 μM) (Fig. 2A) and flupirtine (4–144 μM) (Fig. 2B) induced concentration-dependent relaxations of U46619-precontracted strips. Flupirtine-induced relaxations displayed a slower progression than those induced by retigabine (Fig. 2A and B). In addition, since in preliminary experiments the strips did not recover to basal tone after flupirtine washout from the bath, the concentration-response relationship for the relaxant effects induced by flupirtine was studied by adding this drug cumulatively to the incubation medium. On the contrary, the strips fully recovered to basal tone after retigabine washout, so that isolated concentration–response curves were performed. The maximal amplitude of the relaxations induced by retigabine and flupirtine, calculated by non linear regression analysis of the concentration–response curves, were 93.6 ± 2.3% (n = 6) and 90.9 ± 2.1% (n = 6) of the maximal amplitude parameter, respectively (Fig. 2C). Thus, the relaxant effects produced by retigabine and flupirtine were much higher than those produced by DMSO. The pD2s (−log EC50) of the concentration-response curves for the relaxations produced by retigabine and flupirtine and were 4.73 ± 0.05 and 4.41 ± 0.06, respectively (Fig. 2C). The strips recovered to 93.1 ± 1.6% (n = 6), 91.1 ± 4.6% (n = 4) and 79.3 ± 0.5% (n = 2) of the maximal relaxation induced by flupirtine 10, 20 and 30 min after flupirtine washout from the bath, respectively (Fig. 2B). XE-991 (20 μM) fully blocked retigabine (10 μM)-induced relaxant effects (n = 4) and almost abolished the relaxations induced by retigabine (30 μM) and flupirtine (44 μM) (7.0 ± 1.3%, n = 4, and 4.1 ± 2.0%, n = 4, respectively). Concentration-response experiments performed to better evaluate the inhibitory effects of XE-991 on retigabine-induced relaxation revealed that XE-991 (0.5–20 μM) concentration-dependently inhibited the relaxant response induced by retigabine (30 μM), with a pIC50 and a maximal inhibitory effect of 6.22 ± 0.05 and 90.1 ± 2.0% of controls (n = 3), respectively (Fig. 3). When re-evaluated 30 min after XE-991 (20 μM) washout from the bath, the relaxation produced by retigabine (30 μM) was 9.4 ± 1.0% of controls. Neither TTX (1 μM) nor CTX GVIA (30 nM) affected the Emax and the pD2 of the flupirtine-induced concentration–response curve (86.3 ± 2.2% and 4.40 ± 0.02, n = 3, with TTX, respectively; 87.3 ± 0.3% and 4.47 ± 0.19, n = 3, with CTX GVIA, respectively). Similarly, the relaxations produced by retigabine (100 μM) were not modified by TTX (1 μM) or CTX GVIA (30 nM, Fig. 2A) (103.6 ± 2.7%, n = 3, and 102.4 ± 3.0%, n = 3, of controls, respectively).
Fig. 2.
Motor effects induced by retigabine and flupirtine on U46619 (0.1 μM)-precontracted longitudinal muscle strips of the rat gastric fundus under NANC conditions. (A) and (B) Representative tracings showing the concentration-dependent relaxant effects of retigabine (1–100 μM) and flupirtine (1–100 μM), respectively. The effect of ω-conotoxin GVIA (30 nM) on retigabine (100 μM)-induced relaxation is also shown. (C) Mean concentration–response curves for relaxations induced by retigabine (1–100 μM) and flupirtine (4–100 μM). Relaxations were measured as peak amplitudes. Peak amplitudes are expressed as percentages of a maximal amplitude parameter measured from the maximal U46619-induced contraction point reached before the addition of any test substance to the maximal papaverine (300 μM)-induced relaxation point reached at the end of each experiment. Each point represents the mean ± s.e.m. of responses observed in six strips.
Fig. 3.
Effects of XE-991 (0.5–20 μM) on retigabine (30 μM)-induced submaximal relaxation of U46619 (0.1 μM)-precontracted longitudinal muscle strips of the rat gastric fundus. Mean retigabine (30 μM)-induced relaxations observed in the absence (0 XE-991, controls) or presence of XE-991 (0.5–20 μM). Relaxations were measured as peak amplitudes. Peak amplitudes are expressed as percentages of a maximal amplitude parameter measured from the maximal U46619-induced contraction point reached before the addition of any test substance to the maximal papaverine (300 μM)-induced relaxation point reached at the end of each experiment. Each point represents the mean ± s.e.m. of responses observed in three strips. Significant differences between test and control responses: ***P < 0.001.
3.1.4. Motor effects of KV7 channel blockers on precontracted strips
In the experiments performed to investigate the effects of K+ channel blockers on the relaxations induced by EFS, XE-991 and DMP-543 further contracted U46619 (0.1 μM)-precontracted strips (Fig. 4A). The contractions produced by XE-991 (10, 20 and 50 μM) and DMP-543 (20 μM) were 14.2 ± 1.7% (n = 8), 24.2 ± 2.3% (n = 8), 23.9 ± 1.9% (n = 9) and 17.3 ± 4.4% (n = 7), respectively. TTX (1 μM) and CTX GVIA (30 nM) did not significantly affect XE-991 (20 μM)-induced contraction of U46619 (0.1 μM)-precontracted strips (27.8 ± 4.1%, n = 6, and 25.3 ± 3.0%, n = 6, in the presence of TTX and CTX GVIA, respectively).
3.1.5. Effects of KV7 channel modulators on EFS-evoked NANC relaxations
The amplitudes of EFS (2 and 13 Hz)-induced relaxations were 61.4 ± 1.4% and 88.9 ± 1.2% of the maximal amplitude parameter, respectively (n = 52). The AUC of EFS (13 Hz)-evoked relaxant response, divided by the maximal amplitude parameter, was 17.1 ± 0.6 min.
In the untreated strips that served for checking possible effects due to the time elapsed between the two series of EFS, the amplitude of the second EFS (2 Hz)-induced relaxation and amplitude and AUC of the second EFS (13 Hz)-evoked relaxant response were not significantly different from those of the first ones (Fig. 4B).
XE-991 (10 μM) significantly increased the amplitude of 2 Hz EFS-induced relaxation (by 12.7 ± 2.1%, n = 8, P < 0.001, of controls; amplitudes before and during strip incubation with XE-991: 56.3 ± 2.8% and 63.4 ± 3.3%, respectively). Higher concentrations (20 and 50 μM) of XE-991 produced similar significant increases (Fig. 4A and B, left graph). XE-991 (10, 20 and 50 μM) significantly inhibited the amplitude of EFS (13 Hz)-evoked relaxation (by 13.9 ± 2.6%, n = 8, P < 0.001, 10.8 ± 2.5%, n = 8, P < 0.01, and 18.2 ± 1.3%, n = 9, P < 0.001, of controls, respectively; P = 0.071, one-way ANOVA, Fig. 4A and B, middle graph). XE-991 (10 μM) did not significantly affect the AUC of 13 Hz EFS-induced relaxation. However, at higher concentrations, XE-991 significantly reduced the AUC of EFS (13 Hz)-evoked relaxant response (by 15.6 ± 2.9%, n = 8, P < 0.01, and 15.8 ± 3.6%, n = 9, P < 0.01, of controls with 20 and 50 μM XE-991, respectively, Fig. 4A and B, right graph). The AUCs of relaxations induced by EFS (13 Hz) in the absence and in the presence of XE-991 (20 μM) were 19.7 ± 1.8 min and 16.4 ± 1.4 min, n = 8, P < 0.01, respectively; those measured before and during incubation with XE (50 μM) were 15.8 ± 0.8 min and 13.3 ± 0.9 min, n = 9, P < 0.01, respectively.
DMP-543 (20 μM) significantly increased the amplitude of EFS (2 Hz)-induced relaxation (by 24.8 ± 3.3%, n = 7, P < 0.001, of controls) and reduced the AUC of EFS (13 Hz)-evoked relaxant effect (by 21.2 ± 4.1%, P < 0.01, of controls). The increase in the amplitude of EFS (2 Hz)-evoked relaxation produced by DMP-543 (20 μM) was significantly greater than that observed with XE-991 (20 μM) (P < 0.05, unpaired t-test). The amplitudes of relaxations evoked by EFS (2 Hz) without or with DMP-543 (20 μM) in the bath were 59.9 ± 2.3% and 74.4 ± 2.2%, n = 7, P < 0.001, respectively. The AUCs of the relaxant responses induced by EFS (13 Hz) in the absence and in the presence of DMP-543 (20 μM) were 16.1 ± 0.8 min and 12.7 ± 0.9 min, P < 0.01, respectively. DMP-543 (20 μM) did not significantly affect the amplitude of EFS (13 Hz)-evoked inhibitory motor response (99.7 ± 1.7% of controls; 86.2 ± 2.6% and 85.7 ± 1.8% before and during incubation with DMP-543, respectively).
Since XE-991 and DMP-543 increased strip tone, control experiments were performed in which some strips were further contracted by a higher concentration (0.3 μM) of U46619 to evaluate whether the higher muscle tone level could affect the relaxant responses evoked by the second series of EFS (2 and 13 Hz). The higher muscle tone produced by U46619 (0.3 μM) (by 23.1 ± 4.6%, n = 6), however, did not significantly affect the relaxant responses induced by EFS (2 and 13 Hz). The responses measured before and after incubation with U46619 (0.3 μM) were 67.1 ± 5.2% and 70.6 ± 6.8%, n = 6, respectively, for the amplitude of EFS (2 Hz)-evoked relaxation, 90.7 ± 4.2% and 87.8 ± 3.5%, respectively, for the amplitude of EFS (13 Hz)-evoked relaxation, and 19.0 ± 1.8 min and 19.1 ± 2.3 min, respectively, for the AUC of EFS (13 Hz)-evoked relaxation.
Retigabine (3 μM) significantly reduced the amplitude of EFS (2 Hz)-induced relaxation (by 13.6 ± 2.3%, P < 0.001, n = 6, P < 0.01, of controls) and increased the AUC of EFS (13 Hz)-evoked relaxation (by 11.1 ± 2.8%, P < 0.05, of controls). However, when the amplitudes of EFS (2 Hz)-induced relaxations were measured from the point at which retigabine (3 μM) was added to the bath medium, they were not anymore significantly different from control relaxations (105.8 ± 2.9%). Retigabine (3 μM) did not significantly affect the amplitude of the relaxation induced by EFS (13 Hz) (101.6 ± 2.3% of controls). In these experiments, the relaxations produced by the first and the second addition of retigabine (3 μM) to the bath medium were 11.3 ± 1.3% and 12.6 ± 1.5%, respectively.
3.1.6. Effects of KV7 channel blockers on NO- and VIP-induced relaxations
The amplitudes of the relaxant effects produced by acidified NaNO2 (300 μM), a tool used to study NO-induced effects, and VIP (10 nM) were 50.4 ± 2.2% and 89.3 ± 1.9% of the maximal amplitude parameter, respectively (n = 16). The AUC of VIP (10 nM)-evoked relaxant response, divided by the maximal amplitude parameter, was 15.0 ± 0.6 min.
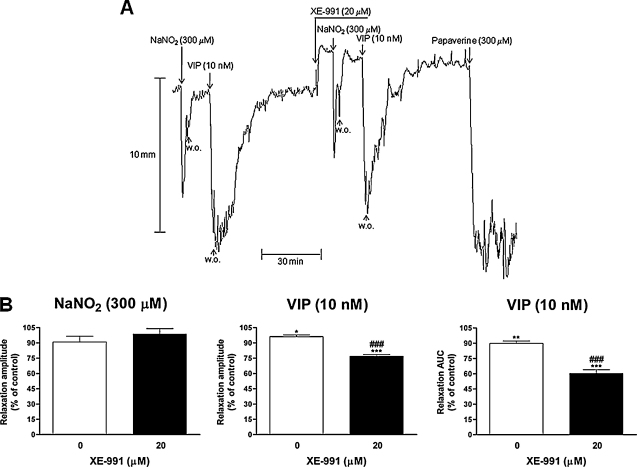
In the untreated strips that served for checking possible effects due to the time elapsed between the two series of consecutive incubations with acidified NaNO2 (300 μM), a tool used to study NO-induced effects, and VIP (10 nM), the amplitude of the second acidified NaNO2 (300 μM)-induced relaxation was not significantly different from that of the first one (90.9 ± 5.7%, n = 9, of controls; 48.3 ± 3.0% and 42.6 ± 1.8%, respectively, Fig. 5B, left graph). On the contrary, the amplitude and the AUC of the relaxant response induced by the second incubation with VIP (10 nM) were significantly different from those of the first one (96.2 ± 1.6%, n = 9, P < 0.05, and 89.8 ± 2.5%, n = 9, P < 0.01, of controls, respectively, Fig. 5B, middle and right graphs). XE-991 (20 μM) did not significantly affect the amplitude of the relaxation induced by acidified NaNO2 (300 μM) (98.7 ± 5.4%, n = 7, of controls; 53.1 ± 3.1% and 52.2 ± 4.0%, respectively, Fig. 5A and B, left graph). However, XE-991 (20 μM) significantly reduced the amplitude and the AUC of the relaxant response produced by VIP (10 nM) (to 76.9 ± 1.7%, n = 7, P < 0.001, and 60.3 ± 3.6%, n = 7, P < 0.001, of controls; both P < 0.001 vs. time controls, unpaired Student's t-test, Fig. 5A and B, middle and right graphs). The amplitudes of relaxations evoked by VIP (10 nM) before and during incubation with XE-991 (20 μM) were 94.4 ± 3.0% and 72.5 ± 2.2%, n = 7, P < 0.001, respectively. The AUCs of the relaxant responses induced by VIP (10 nM) without and with XE-991 (20 μM) in the bath were 14.9 ± 1.0 min and 8.9 ± 0.5 min, P < 0.001, respectively. In this experimental series, the contraction produced by XE-991 (20 μM) was 19.5 ± 1.9%.
Fig. 5.
Effects of XE-991 on the relaxant responses induced by NaNO2 (300 μM) in 0.1 N HCl or VIP (10 nM) of U46619 (0.1 μM)-precontracted longitudinal muscle strips of the rat gastric fundus. (A) Representative tracings showing the effects of XE-991 (20 μM). (B) Mean relaxations evoked by NaNO2 (left graph) or VIP (middle and right graphs) observed in the absence (0 XE-991, time controls) or presence of XE-991 (20 μM). Relaxations were measured as peak amplitudes (left and middle graphs) or AUCs (right graph), normalized for the analysis in a similar way to that described in the legend of Fig. 3 and are expressed as percentages of control responses. Each point represents the mean ± s.e.m. of responses observed in seven (20 μM XE-991) or nine (0 XE-991, time controls) strips. Significant differences between test and control responses: *P < 0.05, **P < 0.01, ***P < 0.001. ###P < 0.001 vs. time controls.
3.2. Gene expression studies
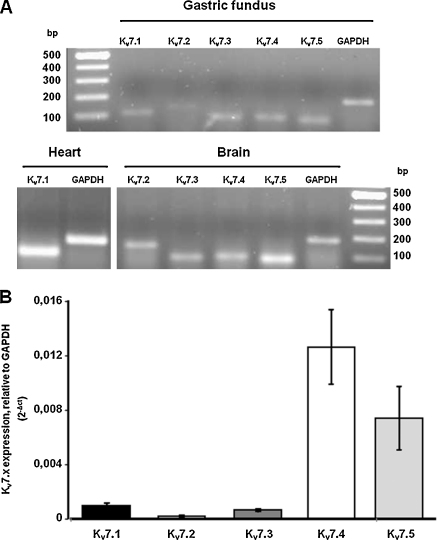
To evaluate the expression of the mRNAs encoding for the various subtypes of KV7 channels and estimate their relative expression levels in the rat gastric fundus, the total RNA isolated from the fundus was amplified in RT-PCR and real-time qPCR experiments using primers specifically designed to recognize all mRNA splicing variants from each KV7 channel gene (Table 1). Transcripts from all known KV7 channel genes were detected in the rat gastric fundus by RT-PCR with 40 cycles (Fig. 6A). However, it is well known that RT-PCR experiments with a high fixed number of cycles can only give qualitative results. Consequently, qPCR experiments were performed to quantify the relative expression levels of KV7 channel genes. KV7.4 and 7.5 channel subtypes showed the highest expression levels (Fig. 6B). The mean cycle thresholds were 27.8, 28.3, 31.5, 32.4, and 34.5 for KV7.4, 7.5, 7.1, 7.3, and 7.2 channel cDNA, respectively. Relative to KV7.4, transcript levels of KV7.1, 7.2, 7.3 and 7.5 channel genes were approximately 8.1%, 1.7%, 5.2% and 58.4%, respectively. In addition to those listed in Table 1, at least one additional set of primer pairs was used to test the expression of each KV7 transcript, with results similar to those shown in Fig. 6A (data not shown).
Table 1.
Sequences of oligonucleotide primers used for PCR.
| Amplified gene | Primer orientation and sequence (5′ → 3′) | Amplicon size (bp) | Reference |
|---|---|---|---|
| KV7.1 | Forward: GGCTCTGGGTTTGCACTG | 106 | Joshi et al. [31] |
| Reverse: CATAGCACCTCCATGCAGTC | |||
| KV7.2 | Forward: GCTTTCCGTATCAAGGGCG | 139 | Lan et al. [54] |
| Reverse: TGCTGACTTTGAGGCCAGG | |||
| KV7.3 | Forward: ACACACCACTGTCCCTCATGTC | 80 | Hadley et al. [55] |
| Reverse: TCTGTCTTGGGAGATGCTGAAG | |||
| KV7.4 | Forward: CCCCGCTGCTCTACTGAG | 86 | Joshi et al. [31] |
| Reverse: ATGACATCATCCACCGTGAG | |||
| KV7.5 | Forward: CGAGACAACGACAGATGACC | 77 | Joshi et al. [31] |
| Reverse: TGGATTCAATGGATTGTACCTG | |||
| GAPDH | Forward: CACCAGCATCACCCCATTT | 157 | Joshi et al. [31] |
| Reverse: CCATCAAGGACCCCTTCATT |
Fig. 6.
Expression of KV7 channel genes in the rat gastric fundus. (A) Agarose gel electrophoresis of RT-PCR products obtained from cDNA amplification of rat gastric fundus mRNA using KV7-selective primers. Amplicon sizes and primer sequences are shown in Table 1. For a comparison, the expression of KV7.1 and KV7.2–7.5 channel genes in rat heart and brain, respectively, was also studied. (B) Quantification of transcripts for KV7 channel subtypes by use of real-time quantitative PCR. Data are expressed as 2−Δct relative to GAPDH gene expression, as described in Section 2. Each bar is the mean ± s.e.m. of four separate determinations.
3.3. Immunohistochemistry
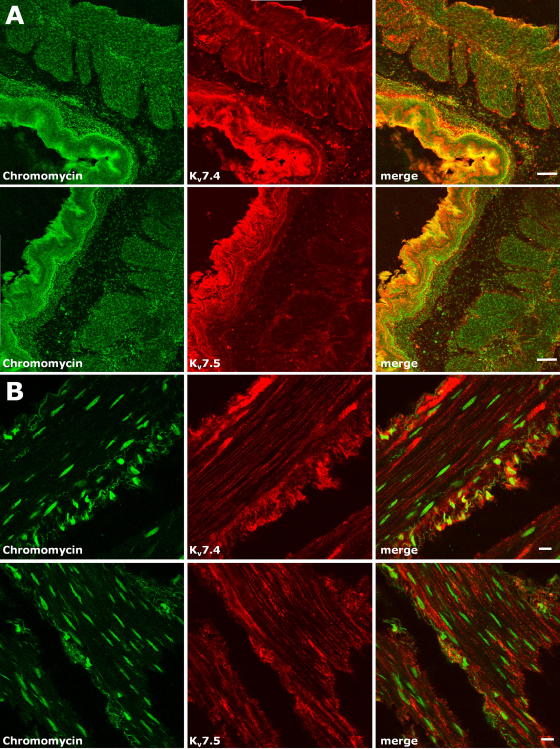
Since gene expression studies revealed that the transcript abundance for KV7.4 and KV7.5 appeared to be the highest among KV7 members, confocal immunofluorescence experiments were carried out using two polyclonal antibodies directed against KV7.4 and KV7.5 to gain insight into the cellular and subcellular localization of KV7.4 and KV7.5 proteins in the rat gastric fundus. The specificity of the antibodies against each target subunit was assessed in immunocytochemical experiments in CHO cells expressing KV7.4 or KV7.5 proteins (data not shown). In rat gastric fundus sections, both KV7.4 and KV7.5 antibodies showed a similar staining pattern, labelling both the epithelial and muscular compartments (Fig. 7A). In the latter, both the circular (internal) and the longitudinal (external) layers appeared to be labelled. KV7.5 staining in the muscular layers appeared fainter than that observed in the epithelial layer. Moreover, in the muscular layers, KV7.4 staining appeared more intense when compared to that for KV7.5. For comparison, staining for KV7.1 was investigated and found to be predominantly, but not exclusively, localized to the epithelial compartment (data not shown). Higher magnification images for both KV7.4 and KV7.5 antibodies revealed that, in the muscular layers, these subunits were mainly localized to the plasma membrane of spindle-shaped smooth muscle cells, although we cannot exclude that part of the immunofluorescence signal also corresponds to neuronal fibres or ICC. No nuclear staining was revealed, as suggested by the lack of co-localization with the DNA-binding marker chromomycin A3 (Fig. 7B). For both KV7.4 and KV7.5 antibodies, membrane staining in smooth muscle cells appeared non-continuous, rather showing puncta of higher expression density, possibly corresponding to regions of subunit clustering.
Fig. 7.
Expression of KV7.4 and KV7.5 channel subunits in the rat gastric fundus. Low (A) and high (B) magnification confocal images of rat gastric fundus cryosections stained for the nuclear marker chromomycin A3 (in green), for KV7.4 or KV7.5 (in red), as indicated; merged images are shown in the rightmost panels. The scale bar is 100 μm in (A), and 10 μm in (B). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of the article.)
4. Discussion
Several K+ channels are expressed in the SMC–ICC–FLC syncytial apparatus of the gastrointestinal tract. Functionally, the most important of them are the delayed rectifier KV1, 2 and 4 channels, large- and small-conductance KCa (BK and SK) channels and ATP-dependent Kir (KATP) channels [2,23]. However, additional types of K+ channels are present in the gastrointestinal SMC–ICC–FLC syncytial apparatus, including the delayed rectifier KV7 (KCNQ) channels [2]. These channels are mostly known for their involvement in the physiology of cardiomyocytes and neurons. The activation of KV7.1 channels produces slowly activating K+ currents (IKs) that are responsible for the late repolarizing phase of the action potential in cardiomyocytes. On the other hand, IKM mediated by KV7.2, 7.3 and 7.5 channels regulates resting membrane potential, firing rate and neurotransmitter release in sympathetic [19], sensory [5] and central [24–26] neurons. Studies on KV7 channels in the smooth muscle were mainly carried out in blood vessels. The first gene expression analysis of vascular KV7 channels was carried out by Ohya et al. [27] in murine portal vein. An extensive profiling of KV7 channel gene expression throughout the murine vasculature followed a few years later [28]. Recently, KV7 channel gene expression has been also shown in human arteries [29]. Many studies evaluated the electrophysiological and functional effects of KV7 channel modulators on blood vessels. Retigabine and flupirtine activate K+ currents in myocytes of murine portal vein [30] and rat pulmonary artery [31] and retigabine-induced effects are prevented by pretreatment with XE991 [30]. Retigabine and flupirtine relax precontracted segments of various mouse blood vessels [28,32] and their effects are blocked by XE-991 [28]. Retigabine and S-1, another KV7 channel activator, relax precontracted human arteries and their effects are reversed by XE-991 [29]. XE-991 and linopirdine, another non-selective KV7 channel blocker, induce significant increases in spontaneous contractile activity in the mouse portal vein [33] and vasoconstriction of different in vitro preparations of rat [31,34–36], mouse [28,34] and human [29] blood vessels. Only a few studies are available on other smooth muscles. KV7 channel activators or blockers reduce or increase the motor activity of rat urinary bladder [11] and mouse and human myometrium, respectively [10,37]. In addition, evidence has been provided that guinea-pig bladder ICC have outward voltage-dependent K+ currents inhibited by XE-991 with a role in the regulation of resting membrane potential and excitability [38]. As for the gastrointestinal smooth muscle, KV7 channel activators or blockers induce inhibitory or excitatory effects on colonic spontaneous motor activity, respectively [9]. In addition, IKs currents have been detected in gastric antrum SMC [14]. In the present study, the effects of KV7 channel modulators on muscle tone and relaxant responses elicited by EFS, NO and VIP were evaluated in the rat gastric fundus. Expression of the transcripts and subunits encoded by the different KV7 genes has been also investigated in this tissue by qPCR and immunohystochemistry, respectively.
At first, the effects of the selective KV7 channel blocker XE-991 [19] on non-precontracted strips of the rat gastric fundus were investigated. XE-991 concentration-dependently contracted the fundus strips with a mean EC50 of approximately 4.6 μM. It has been shown that IC50 values of XE-991 for KV7.1–7.4 and KV7.5 channels are approximately 1–5 μM and 60 μM, respectively, and the actions of this blocker appear to be selective for KV7 channels at these concentrations [8]. Thus, the EC50 of XE-991 in the rat gastric fundus is in the low micromolar range at which this blocker has been shown to inhibit KV7.1–7.4 channels. The mean Emax of XE-991 was 63.2% of the maximal amplitude parameter. Considering that this latter value is similar to that observed with 0.1 μM U46619 (75.8% of the maximal U46619-induced contraction), the maximal effect of XE-991 was approximately 47.9% (63.2% of 75.8%) of the strip maximal contractile potential. XE-991 and DMP-543 also further contracted U46619-precontracted strips by approximately 24% of the maximal amplitude parameter (i.e., approximately 18.2% of the strip maximal contractile potential). Thus, when the muscle is contracted to approximately three quarters of the maximum, XE-991 can still induce a contraction of approximately 38% of that induced when the smooth muscle is relaxed. XE-991-induced contraction was not affected by TTX or CTX GVIA, indicating that it is not produced by neuronal activation or excitatory neurotransmitter release from neuronal terminals. Thus, XE-991 acts very probably at the level of the SMC–ICC–FLC syncytial apparatus. Hypothesizing that XE-991 is acting only on KV7 channels, these findings suggest that, in the SMC–ICC–FLC syncytial apparatus of proximal stomach, a fraction of KV7 channels is in the open state at the membrane resting potential and plays a crucial role in determining the low level of basal muscle tone. This fraction would be higher in strips precontracted to submaximal levels, due to membrane depolarization.
The selective KV7.2–7.5 channel activators retigabine and flupirtine [4,20] produced concentration-dependent relaxations of precontracted fundus strips with EC50 of approximately 19 μM and 40 μM, respectively. Retigabine- and flupirtine-induced relaxations were inhibited in a concentration-dependent manner by XE-991 and were not affected by TTX or CTX GVIA. Thus, they relax the gastric fundus very probably by specifically activating KV7 channels located in the SMC–ICC–FLC syncytial apparatus and not by mechanisms involving non-specific neuronal effects, such as activation of inhibitory motor neurons or neurotransmitter release from their terminals. Retigabine and flupirtine almost completely relaxed the strips, indicating a very significant contribution of KV7 channels in regulating gastric smooth muscle contractility. XE-991-induced contractions and flupirtine-induced relaxations reversed very slowly, leading to a only partial recovery upon their washout from the bath. These results possibly suggest a slow dissociation rate for these drugs from the channels, which would thus remain stabilized in the closed or the open state, respectively, by the two KV7 channel modulators. Retigabine, on the other hand, displayed faster kinetics and its effects were entirely reversed.
The partial reversibility or irreversibility of XE-991-induced effects has been reported in a number of previous studies [33,39]. It is also known that the specific effects of XE-991 on KV7 channels are likely to be maximal at concentrations of 10–20 μM and, when XE-991 concentration is increased above these values, the drug is no longer selective for KV7 channels [8,36,39,40]. Thus, as far as XE-991 is concerned, the following alternative hypothesis may be put forward: on washout of 100 μM XE-991, the recovery to approximately 53% of its maximal contraction may represent reversal of the non-specific effects (and perhaps part of specific effects), with the remaining sustained contraction reflecting its irreversible action on KV7 channels. This hypothesis may be supported by the observation that, 30 min after XE-991 washout from the bath, the relaxant effect of retigabine was still almost fully blocked. In addition, the fact that application of U46619 (0.1 μM) after treatment with XE-991 induced a contraction significantly higher than that produced before XE-991 treatment, is also consistent with this hypothesis. In fact, U46619, when applied for the second time at submaximal concentrations, would be acting on a membrane partially depolarized by the XE-991-produced irreversible KV7 channel blockade. XE-991 was approximately as potent in contracting the rat gastric fundus as in inhibiting the voltage-dependent outward K+ currents in mouse portal vein SMC [33] and enhancing the depolarization-evoked [3H]dopamine release from rat striatal synaptosomes [25]. XE-991 blocks cloned KV7.2, 7.2/7.3, 7.4 and 7.5 channels expressed in Xenopus oocytes, Chinese hamster ovary (CHO) or human embryo kidney (HEK) cells with IC50 of 0.7 μM [19], 0.6 μM [19], 5.5 μM [41] and 65–75 μM [42,43], respectively. Thus, XE-991 EC50 for contracting the rat gastric fundus is very close to its IC50 on cloned KV7.4 channels. Retigabine and flupirtine relax the rat gastric fundus with potencies similar to those found in precontracted rings of rat pulmonary artery (EC50s: 13 μM and 62 μM, respectively) [31]. Retigabine activates cloned KV7.2, 7.3, 7.2/7.3, 7.4, 7.5 and 7.5/7.3 channels expressed in either Xenopus oocytes, CHO or HEK cells with EC50 of 2.5 μM [44], 0.6 μM [44], 1.6 μM [45], 1.4–5.2 μM [44,46], 2–6.4 μM [47] and 1.4 μM [48], respectively. So, retigabine EC50 for relaxing the rat gastric fundus appears closer to its EC50s for activating cloned KV7.4 or 7.5 channels than to those for activating KV7.2 or 7.3 channel subtypes. In our study, gene expression experiments showed that KV7.4 and 7.5 channel genes have the highest expression levels. Compared to the expression level of KV7.4 channel gene, that of KV7.5 channel gene was approximately 60%, whereas those of KV7.1, 7.2 and 7.3 channel genes resulted lower than 10%. A similar scenario has been described in blood vessels [8] and mouse stomach [9]. Very low levels of KV7.1–7.3 channel mRNAs have been detected in the rat gastric fundus also by Ohya et al. [14]. Immunohistochemical studies carried out by confocal immunofluorescence methods showed that KV7.4 and 7.5 channels are localized within the epithelial and muscular layers of the rat proximal stomach. In the muscular layers, both channel subtypes appeared to be mainly localized to the plasma membrane of spindle-shaped smooth muscle cells and the staining for KV7.4 channels appeared more intense when compared to that for KV7.5 channels. Although the present results cannot exclude that part of the immunofluorescence signal within the muscular layers also corresponds to neuronal fibres or ICCs, the fact that TTX or CTX GVIA did not interfere with the effects of XE-991 and retigabine suggests that KV7 channel activators and blockers exert their direct motor effects in the rat gastric fundus by acting preferentially on muscle KV7.4 channels.
XE-991 and DMP-543 produced small but significant increases in the relaxation induced by low-frequency neuronal activation. On the contrary, retigabine, at a concentration (3 μM) that induced a very small relaxation (approximately 11–13% of the maximal parameter), significantly reduced low-frequency EFS-evoked relaxation. These findings seem to suggest that KV7 channels, in addition to their effects on SMC contractility, may also be involved in the repolarization mechanisms of the inhibitory motor neurons responsible for this relaxation, that are mainly nitrergic neurons. We could hypothesize that KV7 channel blockade would delay membrane repolarization, thus increasing the duration of the action potential and consequently neurotransmitter release. However, in the absence of any direct measurement of neuronal action potential, we must acknowledge the speculative nature of the proposed mechanism. On the contrary, XE-991 and DMP-543 significantly reduced the relaxation induced by high-frequency EFS. Such an effect could be generated by pre- or post-junctional actions. To clarify that, the effects of XE-991 were also evaluated on the relaxation induced by the main neurotransmitter responsible for the long duration of high-frequency EFS-induced proximal stomach relaxation, i.e. VIP. XE-991 also reduced VIP-induced relaxation, but did not affect the relaxation induced by NO. These results seem to suggest that KV7 channel activation is a signal transduction mechanism of VIP in the SMC of the proximal stomach and the inhibitory effect of XE-991 on the relaxation evoked by high-frequency EFS can be attributed to the blockade of KV7 channel activation by VIP released from the inhibitory motor neurons. VIP relaxes the rat gastric fundus by activating VPAC2 receptors [49]. It is well known that the main signal transduction mechanism of VPAC2 receptors is the activation of adenylate cyclase through Gs proteins [50]. The increase in intracellular cAMP levels and the consequent activation of protein kinase A (PKA), in turn, might activate KV7 channels. This hypothesis is supported by the fact that IKs or IKM activation by β-adrenergic receptor agonists, cAMP analogs or PKA has been shown in cardiomyocytes and SMC [51–53].
5. Conclusions
Our results seem to indicate that the pharmacological modulation of KV7 channels affects the motility of the rat gastric fundus, with KV7 channels activators evoking significant relaxation, and KV7 channel inhibitors increasing gastric tone in resting conditions. Moreover, KV7 channels also appear to play important roles as mediators of VIP-induced relaxation of the rat gastric fundus, and to participate to the repolarization of the inhibitory motor neurons responsible for the gastric relaxation evoked by low-frequency neuronal activation. Altogether, the results obtained provide the first functional demonstration of a critical control exerted by KV7 channels over rat proximal stomach motor activity, thus revealing a novel pharmacological target for therapeutic interventions against gastric motor disturbances.
Acknowledgements
Supported by Fondi Ateneo of the Catholic University of the Sacred Heart, Rome, and the Fondazione Telethon Italy (GGP07125), the Fondazione San Paolo, Program in Neuroscience 2008, and Regione Molise (Convenzione AIFA/Regione Molise) to MT. The technical help of Dr. Davide Viggiano (Dept. of Health Science, University of Molise, Campobasso, Italy) with the immunohistochemistry experiments is deeply acknowledged.
References
- 1.Gutman G.A., Chandy K.G., Grissmer S., Lazdunsky M., McKinnon D., Pardo L.A. International Union of Pharmacology. LIII. Nomenclature and molecular relationships of voltage-gated potassium channels. Pharmacol Rev. 2005;57:473–508. doi: 10.1124/pr.57.4.10. [DOI] [PubMed] [Google Scholar]
- 2.Sanders K.M. Regulation of smooth muscle excitation and contraction. Neurogastroenterol Motil. 2008;20(Suppl 1):39–53. doi: 10.1111/j.1365-2982.2008.01108.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peroz D., Rodriguez N., Choveau F., Baró I., Mérot J., Loussouarn G. KV7.1 (KCNQ1) properties and channelopathies. J Physiol. 2008;586.7:1785–1789. doi: 10.1113/jphysiol.2007.148254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miceli F.M., Soldovieri M.V., Martire M., Tagliatatela M. Molecular pharmacology and therapeutic potential of neuronal KV7-modulating drugs. Curr Opin Pharmacol. 2008;8:65–74. doi: 10.1016/j.coph.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 5.Brown D.A., Passmore G.M. Neural KCNQ (KV7) channels. Br J Pharmacol. 2009;156:1185–1195. doi: 10.1111/j.1476-5381.2009.00111.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kharkovets T., Hardelin J.-P., Safieddine S., Schweizer M., El-Amraoui A., Petit C. KCNQ4, a K+ channel mutated in a form of dominant deafness, is expressed in the inner ear and the central auditory pathway. Proc Natl Acad Sci USA. 2000;97:4333–4338. doi: 10.1073/pnas.97.8.4333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iannotti F.A., Panza E., Barrese V., Viggiano D., Soldovieri M.V., Taglialatela M. Expression, localization, and pharmacological role of KV7 potassium channels in skeletal muscle proliferation, differentiation, and survival after myotoxic insults. J Pharmacol Exp Ther. 2010;332:811–820. doi: 10.1124/jpet.109.162800. [DOI] [PubMed] [Google Scholar]
- 8.Greenwood I.A., Ohya S. New tricks for old dogs: KCNQ expression and role in smooth muscle. Br J Pharmacol. 2009;156:1196–1203. doi: 10.1111/j.1476-5381.2009.00131.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jepps T.A., Greenwood I.A., Moffatt J.D., Sanders K.M., Ohya S. Molecular and functional characterization of KV7 K+ channel in murine gastrointestinal smooth muscles. Am J Physiol Gastrointest Liver Physiol. 2009;297:G107–G115. doi: 10.1152/ajpgi.00057.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McCallum L.A., Greenwood I.A., Tribe R.M. Expression and function of KV7 channels in murine myometrium throughout oestrous cycle. Pflugers Arch Eur J Physiol. 2009;457:1111–1120. doi: 10.1007/s00424-008-0567-5. [DOI] [PubMed] [Google Scholar]
- 11.Rode F., Svalø J., Sheykhzade M., Rønn L.C.B. Functional effects of the KCNQ modulators retigabine and XE991 in the rat urinary bladder. Eur J Pharmacol. 2010;638:121–127. doi: 10.1016/j.ejphar.2010.03.050. [DOI] [PubMed] [Google Scholar]
- 12.Sims S.M., Singer J.J., Walsh J.V., Jr Cholinergic agonists suppress a potassium current in freshly dissociated smooth muscle cells of the toad. J Physiol. 1985;367:503–529. doi: 10.1113/jphysiol.1985.sp015837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lammel E., Deitmer P., Noack T. Suppression of steady membrane currents by acetylcholine in single smooth muscle cells of the guinea-pig gastric fundus. J Physiol. 1991;432:259–282. doi: 10.1113/jphysiol.1991.sp018384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ohya S., Asakura K., Muraki K., Watanabe M., Imaizumi Y. Molecular and functional characterization of ERG, KCNQ, and KCNE subtypes in rat stomach smooth muscle. Am J Physiol Gastrointest Liver Physiol. 2002;282:G277–G287. doi: 10.1152/ajpgi.00200.2001. [DOI] [PubMed] [Google Scholar]
- 15.Azpiroz F., Malagelada J.R. Importance of vagal input in maintaining gastric tone in the dog. J Physiol. 1987;384:511–524. doi: 10.1113/jphysiol.1987.sp016467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Currò D., Ipavec V., Preziosi P. Neurotransmitters of the non-adrenergic non-cholinergic relaxation of proximal stomach. Eur Rev Med Pharmacol Sci. 2008;12(Suppl 1):53–62. [PubMed] [Google Scholar]
- 17.Currò D., De Marco T., Preziosi P. Evidence for an apamin-sensitive, but not purinergic, component in the nonadrenergic noncholinergic relaxation of the rat gastric fundus. Br J Pharmacol. 2004;143:785–793. doi: 10.1038/sj.bjp.0705993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vane J.R. A sensitive method for the assay of 5-hydroxytryptamine. Br J Pharmacol Chemother. 1957;12:344–349. doi: 10.1111/j.1476-5381.1957.tb00146.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang H.-S., Pan Z., Shi W., Brown B.S., Wymore R.S., Cohen I.S. KCNQ2 and KCNQ3 potassium channel subunits: molecular correlates of the M-channel. Science. 1998;282:1890–1893. doi: 10.1126/science.282.5395.1890. [DOI] [PubMed] [Google Scholar]
- 20.Rundfeldt C., Netzer R. Investigations into the mechanism of action of the new anticonvulsant retigabine. Interaction with GABAergic and glutamatergic neurotransmission and with voltage gated ion channels. Arzneimittelforschung. 2000;50:1063–1070. doi: 10.1055/s-0031-1300346. [DOI] [PubMed] [Google Scholar]
- 21.Currò D., Volpe A.R., Preziosi P. Nitric oxide synthase activity and non-adrenergic non-cholinergic relaxation in the rat gastric fundus. Br J Pharmacol. 1996;117:717–723. doi: 10.1111/j.1476-5381.1996.tb15249.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Currò D., De Marco T., Preziosi P. Involvement of peptide histidine isoleucine in non-adrenergic non-cholinergic relaxation of the rat gastric fundus induced by high-frequency neuronal firing. Naunyn Schmiedebergs Arch Pharmacol. 2002;366:578–586. doi: 10.1007/s00210-002-0633-z. [DOI] [PubMed] [Google Scholar]
- 23.Vogalis F. Potassium channels in gastrointestinal smooth muscle. J Auton Pharmacol. 2000;20:207–219. doi: 10.1046/j.1365-2680.2000.00183.x. [DOI] [PubMed] [Google Scholar]
- 24.Martire M., Castaldo P., D’Amico M., Preziosi P., Annunziato L., Taglialatela M. M channels containing KCNQ2 subunits modulate norepinephrine, aspartate, and GABA release from hippocampal nerve terminals. J Neurosci. 2004;21:592–597. doi: 10.1523/JNEUROSCI.3143-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martire M., D’Amico M., Panza E., Miceli M., Viggiano D., Lavergata F. Involvement of KCNQ2 subunits in [3H]dopamine release triggered by depolarization and pre-synaptic muscarinic receptor activation from rat striatal synaptosomes. J Neurochem. 2007;102:179–193. doi: 10.1111/j.1471-4159.2007.04562.x. [DOI] [PubMed] [Google Scholar]
- 26.Hansen H.H., Waroux O., SeutinV, JentschTJ, Aznar S., Mikkelsen J.D. KV7 channels: interaction with dopaminergic and serotonergic neurotransmission in the CNS. J Physiol. 2008;586(7):1823–1832. doi: 10.1113/jphysiol.2007.149450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ohya S., Sergeant G.P., Greenwood I.A., Horowitz B. Molecular variants of KCNQ channels expressed in murine portal vein myocytes: a role in delayed rectifier current. Circ Res. 2003;92:1016–1023. doi: 10.1161/01.RES.0000070880.20955.F4. [DOI] [PubMed] [Google Scholar]
- 28.Yeung S.Y.M., Pucovský V., Moffatt J.D., Saldanha L., Schwake M., Ohya S. Molecular expression and pharmacological identification of a role for KV7 channels in murine vascular reactivity. Br J Pharmacol. 2007;151:758–770. doi: 10.1038/sj.bjp.0707284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ng F.L., Davis A.J., Jepps T.A., Harhun M.I., Yeung S.Y., Wan A. Expression and function of the K+ channel KCNQ genes in human arteries. Br J Pharmacol. 2011;162:42–53. doi: 10.1111/j.1476-5381.2010.01027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yeung S.Y.M., Schwake M., Pucovský V., Greenwood I.A. Bimodal effects of the KV7 channel activator retigabine on vascular K+ currents. Br J Pharmacol. 2008;155:62–72. doi: 10.1038/bjp.2008.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Joshi S., Sedivy V., Hodyc D., Herget J., Gurney A.M. KCNQ modulators reveal a key role for KCNQ potassium channels in regulating the tone of rat pulmonary artery smooth muscle. J Pharmacol Exp Ther. 2009;329:368–376. doi: 10.1124/jpet.108.147785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Morecroft I., Murray A., Nilsen M., Gurney A.M., MacLean M.R. Treatment with the KV7 potassium channel activator flupirtine is beneficial in two independent mouse models of pulmonary hypertension. Br J Pharmacol. 2009;157:1241–1249. doi: 10.1111/j.1476-5381.2009.00283.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yeung S.Y.M., Greenwood I.A. Electrophysiological and functional effects of the KCNQ channel blocker XE991 on murine portal vein SMC. Br J Pharmacol. 2005;146:585–595. doi: 10.1038/sj.bjp.0706342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Joshi S., Balan P., Gurney A.M. Pulmonary vasoconstrictor action of KCNQ potassium channel blockers. Respir Res. 2006;7:31–40. doi: 10.1186/1465-9921-7-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mackie A.R., Brueggemann L.I., Henderson K.K., Shiels A.J., Cribbs L.L., Scrogin K.E. Vascular KCNQ potassium channels as novel targets for the control of mesenteric artery constriction by vasopressin, based on studies in single cells, pressurized arteries, and in vivo measurements of mesenteric vascular resistance. J Pharmacol Exp Ther. 2008;325:475–483. doi: 10.1124/jpet.107.135764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhong X.Z., Harhun M.I., Olesen S.P., Ohya S., Moffatt J.D., Cole W.C. Participation of KCNQ (Kv7) potassium channels in myogenic control of cerebral arterial diameter. J Physiol. 2010;588:3277–3293. doi: 10.1113/jphysiol.2010.192823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McCallum L.A., Pierce S.L., England S.K., Greenwood I.A., Tribe R.M. The contribution of KV7 channels to pregnant mouse and human myometrial contractility. J Cell Mol Med. 2011;15:577–586. doi: 10.1111/j.1582-4934.2010.01021.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Anderson U.A., Carson C., McCloskey K.D. KCNQ currents and their contribution to resting membrane potential and the excitability of interstitial cells of Cajal from the guinea pig bladder. J Urol. 2009;182:330–336. doi: 10.1016/j.juro.2009.02.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wladyka C.L., Kunze D.L. KCNQ/M-currents contribute to the resting membrane potential in rat visceral sensory neurons. J Physiol. 2006;575:175–189. doi: 10.1113/jphysiol.2006.113308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Elmedyb P., Calloe K., Schmitt N., Schultz Hansen R., Grunnet M., Olesen S.-P. Modulation of ERG Channels by XE991. Basic Clin Pharmacol Toxicol. 2007;100:316–322. doi: 10.1111/j.1742-7843.2007.00048.x. [DOI] [PubMed] [Google Scholar]
- 41.Søgaard R., Ljungstrøm T., Pedersen K.A., Olesen S.-P., Jensen B.S. KCNQ4 channels expressed in mammalian cells: functional characteristics and pharmacology. Am J Physiol Cell Physiol. 2001;280:859–866. doi: 10.1152/ajpcell.2001.280.4.C859. [DOI] [PubMed] [Google Scholar]
- 42.Schroeder B.C., Hechenberger M., Weinreich F., Kubisch C., Jentsch T.J. KCNQ5, a novel potassium channel broadly expressed in brain, mediates M-type currents. J Biol Chem. 2000;275:24089–24095. doi: 10.1074/jbc.M003245200. [DOI] [PubMed] [Google Scholar]
- 43.Yeung S.Y.M., Lange W., Schwake1 M., Greenwood I.A. Expression profile and characterisation of a truncated KCNQ5 splice variant. Biochem Biophys Res Commun. 2008;371:741–747. doi: 10.1016/j.bbrc.2008.04.129. [DOI] [PubMed] [Google Scholar]
- 44.Tatulian L., Delmas P., Abogadie F.C., Brown D.A. Activation of expressed KCNQ potassium currents and native neuronal M-type potassium currents by the anti-convulsant drug retigabine. J Neurosci. 2001;21:5535–5545. doi: 10.1523/JNEUROSCI.21-15-05535.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wickenden A.D., Yu W., Zou A., Jegla T., Wagoner P.K. Retigabine, a novel anti-convulsant, enhances activation of KCNQ2/Q3 potassium channels. Mol Pharmacol. 2000;58:591–600. doi: 10.1124/mol.58.3.591. [DOI] [PubMed] [Google Scholar]
- 46.Schrøder R.L., Jespersen T., Christophersen P., Strøbæk D., Jensen B.S., Olesen S.-P. KCNQ4 channel activation by BMS-204352 and retigabine. Neuropharmacology. 2001;40:888–898. doi: 10.1016/s0028-3908(01)00029-6. [DOI] [PubMed] [Google Scholar]
- 47.Jensen H.S., Callø K., Jespersen T., Jensen B.S., Olesen S.-P. The KCNQ5 potassium channel from mouse: a broadly expressed M-current like potassium channel modulated by zinc, pH, and volume changes. Mol Brain Res. 2005;139:52–62. doi: 10.1016/j.molbrainres.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 48.Wickenden A.D., Zou A., Wagoner P.K., Jegla T. Characterization of KCNQ5/Q3 potassium channels expressed in mammalian cells. Br J Pharmacol. 2001;132:381–384. doi: 10.1038/sj.bjp.0703861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Robberecht P., De Neef P., Lefebvre R.A. Influence of selective VIP receptor agonists in the rat gastric fundus. Eur J Pharmacol. 1998;359:77–80. doi: 10.1016/s0014-2999(98)00662-1. [DOI] [PubMed] [Google Scholar]
- 50.Alexander S.P.H., Mathie A., Peters J.A. Guide to receptors and channels (GRAC), 4th ed. Br J Pharmacol. 2009;158(Suppl 1):S1–S254. doi: 10.1111/j.1476-5381.2009.00499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sims S.M., Singer J.J., Walsh J.V., Jr Antagonistic adrenergic–muscarinic regulation of M current in smooth muscle cells. Science. 1988;239:190–193. doi: 10.1126/science.2827305. [DOI] [PubMed] [Google Scholar]
- 52.Delmas P., Brown D.A. Pathways modulating neural KCNQ/M (KV7) potassium channels. Nat Rev Neurosci. 2005;6:850–862. doi: 10.1038/nrn1785. [DOI] [PubMed] [Google Scholar]
- 53.Dai S., Hall D.D., Hell J.W. Supramolecular assemblies and localized regulation of voltage-gated ion channels. Physiol Rev. 2009;89:411–452. doi: 10.1152/physrev.00029.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lan W.-Z., Abbas H., Lemay A.-M., Briggs M.M., Hill C.E. Electrophysiological and molecular identification of hepatocellular volume-activated K+ channels. Biochim Biophys Acta. 2005;1668:223–233. doi: 10.1016/j.bbamem.2004.12.010. [DOI] [PubMed] [Google Scholar]
- 55.Hadley J.K., Passmore G.M., Tatulian L., Al-Qatari M., Ye F., Wickenden A.D. Stoichiometry of expressed KCNQ2/KCNQ3 potassium channels and subunit composition of native ganglionic M channels deduced from block by tetraethylammonium. J Neurosci. 2003;23:5012–5019. doi: 10.1523/JNEUROSCI.23-12-05012.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]