Abstract
Purpose
Based on the vascular theory of glaucoma pathogenesis, we wanted to evaluate the effect of Ginkgo biloba extract (GBE) on peripapillary blood flow in patients with normal tension glaucoma (NTG).
Methods
Thirty patients with NTG were randomly placed in the GBE-treated or control groups. The GBE-treated group received 80 mg GBE orally, twice a day for four weeks, and the control group received a placebo twice a day for four weeks. Complete ocular examinations including visual field, Heidelberg retina flowmeter, and systemic examinations were performed on the first study day and on the day treatment was completed.
Results
After GBE treatment, the mean blood flow, volume, and velocity increased at almost all points, and there was a statistically significant increase in blood flow at almost all points, in comparison to the placebo. Blood volume significantly increased only in the superior nasal and superior temporal neuroretinal rim areas. GBE also significantly increased blood velocity in areas of the inferior temporal neuroretinal rim and superior temporal peripapillary area.
Conclusions
GBE administration appears to have desirable effect on ocular blood flow in NTG patients.
Keywords: Ginkgo biloba extract, Heidelberg retina flowmeter, Normal tension glaucoma, Ocular blood flow
Although the pathogenesis of glaucoma remains obscure, both a mechanical theory (intraocular pressure [IOP] causes structural damage to the nerve head) and vascular theory (tissue ischemia from impaired blood flow causes glaucomatous damage) have been proposed [1,2]. Recently, many reports support the vascular theory, which suggests that vascular factors may be involved in the pathogenesis of glaucoma. These factors include systemic hypotension, vasospasm (migraine, Raynaud's disease), cardiovascular disease, autoimmune disease, hemorrhagic abnormalities and cerebral microvascular ischemia [3].
Normal tension glaucoma (NTG) is a form of glaucoma in which optic nerve damage and glaucomatous visual field loss occurs without the IOP exceeding the normal range. Treatment of glaucoma has almost always focused on lowering the IOP, but some patients with NTG continue to suffer a visual field defect despite sufficient control of IOP. Therefore, pharmacologic treatment of the vascular mechanism underlying glaucoma should be emphasized in NTG patients.
Ginkgo biloba extract (GBE) consists of flavonoid glycosides, terpene lactone (ginkgolides), and other organic acids [4]. GBE is widely used in treating vascular diseases such as peripheral circulation diseases and cerebral insufficiency [5], because of its favorable effect on blood circulation. Flavonoids can dilate blood vessels by increasing the release of endothelium-derived relaxing factor and prostacyclin (PGI2) from vascular endothelial cells, and decrease blood viscosity by antagonizing platelet activating factor [6]. Moreover, flavonoid glycosides act as antioxidants by scavenging free radicals [7]. These properties support the therapeutic value of GBE in treating NTG.
There have been reports on the effect of GBE on ocular blood flow in healthy individuals; Chung et al. [8] reported that GBE significantly increased end diastolic flow velocity in the ophthalmic artery of healthy volunteers. In Korea, Lee et al. [9] confirmed that GBE increases microcircular blood velocity, flow, and volume in healthy individuals.
However, there has been little investigation into the effect of GBE on ocular blood flow in NTG patients. We aimed to evaluate the short-term effects of GBE on peripapillary retinal blood flow in patients with NTG.
Materials and Methods
Patients
For this trial, we recruited 30 patients who were diagnosed as having NTG at Gangnam Severance Hospital between January 2003 and December 2004. The study followed the tenets of the Declaration of Helsinki and was approved by the local Institutional Review Board. All patients provided informed consent.
The eligibility criteria were as follows: 1) patients between the ages of 18 and 80, 2) patients with unilateral or bilateral NTG with glaucomatous visual field defect and/or abnormal excavation of optic nerve head (ONH) ratio, IOP < 21 mmHg with open angle, 3) patients with no medical history of glaucoma, and in cases of patients taking medications with any effect on blood flow, no change of dose and direction of medication for at least two months before trial, and no cessation of medication more than two weeks before the trial (e.g. calcium channel blocker, ACE inhibitor, angiotensin II receptor blocker, β-blocker), 4) patients with no known diabetes, and 5) patients who had not taken vitamin compounds for more than one month.
The exclusion criteria included patients with closed or shallow angle glaucoma, contact lens wearers, history of ocular surgery or Argon laser trabeculoplasty, history of ocular inflammation or infection in the 12 weeks before the trial, hypersensitivity to ginkgo extracts, pregnant or breast-feeding women, alcoholism, or addiction to medications.
Experimental design
This study followed a randomized, double-masked, placebo-controlled clinical trial design. The 30 patients were randomly assigned to one of the two groups (GBE-treated or placebo). Eligible participants were randomly allocated to two groups a using blocked randomization (block size = 10) list produced in advanced and prescriptions were prepared. The prescribed medication was dispensed at the clinic immediately after he or she consented to this trial. Clinicians and patients were not aware of the treatment being administered.
The GBE-treated study group (15 patients) received 80 mg GBE (flavonoid glycosides 19.2 mg) orally, two times a day for four weeks, and the control group (15 patients) received the placebo orally, two times a day for four weeks. Participants were asked to maintain their dosages of any medications that could influence blood flow during the study period.
Complete ocular examinations including visual field, Heidelberg retina flowmeter (HRF; Heidelberg Engineering, Heidelberg, Germany) and systemic examinations, were performed on the first study day and upon completion of the treatment.
Blood flow measurement
Blood flow measurements were made by confocal scanning laser Doppler flowmetry (SLDF) [10] using the HRF. We obtained two-dimensional visualization of the retina and ONH microcirculation with blood flow parameters including flow, volume, and velocity with HRF.
The two-dimensional planes measured by HRF were scanned repeatedly in a linear fashion. Each line had 256 pixels (10 µm) and was scanned 128 times with a line-sampling rate of 4,000 Hz. In total, 64 vertical lines were measured in this process and the total data acquisition time was 2.048 seconds. After completion of retinal perfusion maps, mean flow, volume, and velocity can be calculated in any of the retinal areas. Flow describes the distance gone by all moving cells inside the sample volume per unit time. Volume is proportional to the mean number of photon collisions with moving cells in a sample volume of the tissue, and velocity can be calculated from the equation of 'velocity = flow / volume' [10].
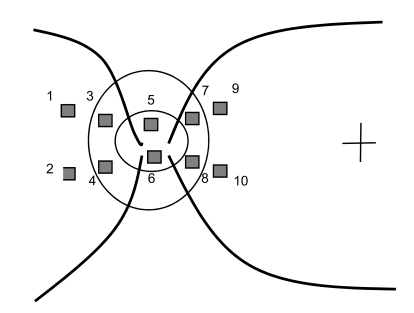
In this study, all measurements were made without pupillary dilation. The areas measured were 10° × 2.5° around the disc. Three separate perfusion images were acquired for each eye by the same experienced optometrist. Perfusion parameters (volume, flow, velocity) were measured at two nasal peripapillary retina areas, two nasal neuroretinal rim areas, two optic cup areas, two temporal neuroretinal rim areas, and two temporal peripapillary retina areas with a size of 10 × 10 pixels (Fig. 1). The averages of the measurements from the three images were used for statistical analysis.
Fig. 1.
Schematic diagram showing locations where perfusion perimeters were measured. 1, Superior nasal peripapillary retina area; 2, inferior nasal peripapillary retina area; 3, superior nasal neuroretinal rim area; 4, inferior nasal neuroretinal rim area; 5, superior optic cup area; 6, inferior optic cup area; 7, superior temporal neuroretinal rim area; 8, inferior temporal neuroretinal rim area; 9, superior temporal peripapillary retina area; 10, inferior temporal peripapillary retina area.
Statistical analysis
Baseline demographic and clinical parameters were compared between treatment groups using Fisher's exact test for categorical variables and Wilcoxon rank sum test for continuous variables. Between-group comparisons before and after medication were performed using Wilcoxon rank sum test for study endpoints (peripapillary blood flow measurements).
In addition, paired-t tests were utilized to compare differences before and after medication with baseline values within treatment groups. A p-value less than 0.05 was accepted as statistically significant.
Results
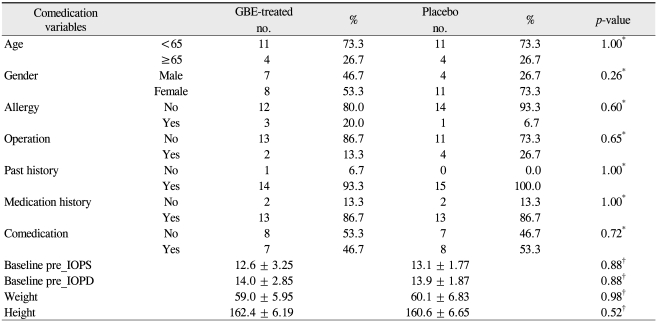
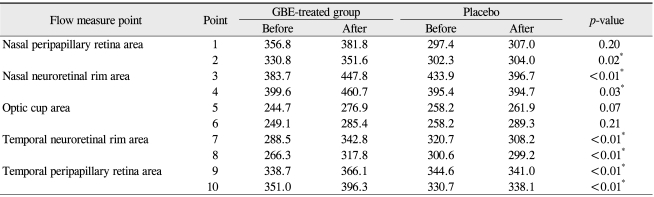
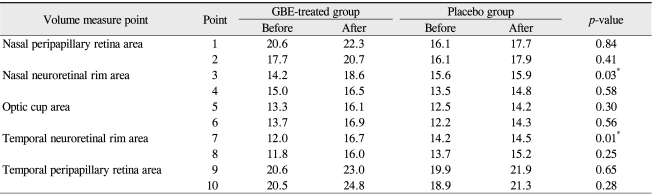
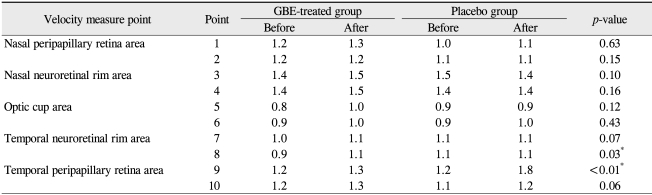
The baseline characteristics are listed in Table 1. No significant differences in demographics were recorded between the two groups. All patients completed the trial and no ocular or systemic side effects were reported in any patients during the trial. No significant modification of IOP or reduction in the mean deviation (MD) and corrected pattern standard deviation (CPSD) values were noted after GBE medication (Table 2). Tables 3, 4, and 5 show the effect of GBE and placebo on ocular blood flow, volume, and velocity, respectively. After GBE treatment, the mean value of blood flow, volume, and velocity increased at almost all points. A statistically significant increase in blood flow in response to GBE compared with placebo was noted at all points, except for the superior nasal peripapillary area (point 1) and optic cup areas (points 5, 6) (Table 3). Blood volume significantly increased after intake of GBE compared to placebo only in the superior nasal and superior temporal neuroretinal rim areas (points 3, 7) (Table 4). GBE also significantly increased blood velocity compared with placebo in the inferior temporal neuroretinal rim (point 8) and superior temporal peripapillary area (point 9) (Table 5).
Table 1.
Characteristics of all patients studied
GBE = Ginkgo biloba extract; IOPS = intraocular pressure of left eye; IOPD = intraocular pressure of right eye.
*Fisher's exact test; †Wilcoxon rank sum test.
Table 2.
Visual field indices and IOP before and after medication
IOP = intraocular pressure; GBE = Ginkgo biloba extract; MD = mean deviation and corrected pattern standard deviation; CPSD = corrected pattern standard deviation.
Table 3.
Blood flow measurements on Heidelberg retina flowmeter before and after medication
GBE = Ginkgo biloba extract.
*Wilcoxon rank sum test.
Table 4.
Blood volume measurements on Heidelberg retina flowmeter before and after medication
GBE = Ginkgo biloba extract.
*Wilcoxon rank sum test.
Table 5.
Blood velocity measurements on Heidelberg retina flowmeter before and after medication
GBE = Ginkgo biloba extract.
*Wilcoxon rank sum test.
Discussion
During the last 150 years, glaucoma has been considered to be a disease resulting from increased IOP, and IOP-lowering therapy is the most common intervention available for treatment of glaucoma. However, with recognition of the fact that marked IOP reduction cannot stop disease progression in all patients, the principles of treating glaucoma are changing. Elevated IOP is not the disease itself, but rather a risk factor for glaucoma [11,12].
Non-IOP-dependent risk factors for glaucomatous optic nerve damage have become an area of increasingly active investigation. Specifically, vascular components seem to have an important role in the genesis and progression of NTG. Lee et al. [13] reported that the NTG patients had significantly decreased blood volume, flow, and velocity in the nasal neuroretinal rim and temporal peripapillary retina compared with normal individuals. Moreover, there were statistically significant decreases in peak systolic and diastolic velocities of the ophthalmic artery in normal-tension glaucoma compared to primary open angle glaucoma [14]. These results imply that a local vascular factor might be related to the development of normal-tension glaucoma.
The ONH has a very characteristic blood supply. The superficial layer of the ONH is supplied by small branches of the central retinal artery. The prelaminar region receives its blood supply via branches from recurrent choroid arterioles and the short posterior ciliary arteries. Diffusion from the surrounding choroid is also possible. The circulation to the optic nerve is autoregulated by endothelial, neural, and myogenic mechanisms [15]. Autoregulation refers to the maintenance of constant blood and nutrient supplies by local vascular constriction or dilation of vessels in response to perfusion pressure changes [16]. Dysregulation of ocular vessels can contribute to progression of glaucoma and research has shown that certain systemic medications can be used for regulation of vascular dysfunction in glaucoma patients. Calcium channel blockers can reduce ocular vasospasm in certain glaucoma patients and improve visual field defects by reducing the effect of increased endothelin-1 levels [17,18]. Nitric oxide is another vasoactive molecule that can induce a vasodilatory response. One study demonstrated that a systemic nitroglycerin preparation can induce a protective effect in glaucoma patients who are taking the medication for another reason [19].
GBE also has many properties that should be beneficial in treating non-IOP-dependent risk factors related not only to ocular hemodynamics, but also to neuroprotective action. Neuroprotection refers to the post-injury protection of neurons that are initially undamaged or only marginally damaged by a particular insult [3]. The ultimate object of neuroprotection in glaucoma patients is to prevent retinal ganglion cell death, and this can be achieved by blocking secondary degeneration. Secondary degeneration refers to the spread of degeneration to apparently healthy neurons that are at risk from toxic stimuli (glutamate, calcium, nitric oxide, free radical) released by damaged cells [20-22]. Several animal models have confirmed that the neuroprotective effect of GBE prevents secondary degeneration [23-25].
Many researchers have studied the effects of GBE on the visual field. Quaranta et el. [26] evaluated the effect of GBE on pre-existing visual field damage in patients with NTG, and recorded significant improvement in visual field indices (MD, CPSD) after GBE treatment. Ha and Rho [27] also assessed the effect of oral GBE on the visual field in patients with NTG, and improvement in the visual field through MD, CPSD, and GHT cluster mean threshold was obtained in a NTG patient during at least four years of treatment. In the present study, no significant improvement in MD or CPSD was noted after GBE treatment. However, we employed only one measure of visual field examination before and after medication, and we did not consider the reliability index. Moreover, the follow-up period (four weeks) was too short to influence the visual field. Therefore, it is likely that GBE has beneficial effect on visual field.
Although the mean value of blood flow, volume, and velocity increased at almost all points in this study, no significant increment was noted at the optic cup area. Lamina cribrosa has blood supply from centripetal branches of the short posterior ciliary artery. In addition, blood vessels lie in the fibrous septa and form a dense capillary plexus that makes this part of the ONH a highly vascular structure. The area that has already sufficient blood supply may have minimal effect of GBE. Therefore, we thought that GBE can improve blood circulation more effectively in the area of decreased blood supply.
A limitation of this study is its short-term follow-up period. We cannot comment on the long-term effects of GBE administration, because of the limited duration (one month) of our study. Therefore, further study is required to investigate of the duration of the effect and optimal administration schedule for GBE treatment for NTG patients.
Another limitation of this study is that the reproducibility of HRF measurements in these NTG patients was not obtained. To evaluate reproducibility, the coefficient of variation of the three measurements calculated by SLDF is generally used [28,29]. Although we did not calculate reproducibility, blood flow measurements were obtained several times for each eye at each session by the same experienced investigator to acquire good-quality images. Moreover, the reliability of HRF to measure ocular blood flow has been published, and HRF allows reproducible blood perfusion measurements of retinal and lamina cribrosa areas when a target square of 10 × 10 pixels is used [10].
This article is the first prospective study on the effect of GBE on ocular blood flow in NTG. There have not been any previously reported studies on this subject. In this study, GBE administration increased peripapillary blood flow, volume, and velocity, although some of these increases were not statistically significant compared with placebo and the locations differed in terms of the statistically significant increases in blood flow, volume, and velocity.
In conclusion, the results of this study demonstrate that GBE administration can produce desirable effects on peripapillary blood circulation, suggesting that GBE is a beneficial systemic treatment for NTG patients.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Flammer J, Gasser P, Prunte C. The probable involvement of the factors other than intraocular pressure in the pathogenesis of glaucoa. In: Drance SM, Van Buskirk EM, Neufeld AH, editors. Pharmacology of glaucoma. Baltimore: Williams & Wilkins; 1992. pp. 273–283. [Google Scholar]
- 2.Halpern DL, Grosskreutz CL. Glaucomatous optic neuropathy: mechanisms of disease. Ophthalmol Clin North Am. 2002;15:61–68. doi: 10.1016/s0896-1549(01)00012-8. [DOI] [PubMed] [Google Scholar]
- 3.Ritch R. Potential role for Ginkgo biloba extract in the treatment of glaucoma. Med Hypotheses. 2000;54:221–235. doi: 10.1054/mehy.1999.0025. [DOI] [PubMed] [Google Scholar]
- 4.DeFeudis FV. Ginkgo biloba extract (EGb 761): pharmacological activities and clinical applications. Paris: Elsevier; 1991. [Google Scholar]
- 5.Kleijnen J, Knipschild P. Ginkgo biloba for cerebral insufficiency. Br J Clin Pharmacol. 1992;34:352–358. doi: 10.1111/j.1365-2125.1992.tb05642.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koltringer P, Langsteger W, Klima G, et al. Hemorheologic effects of ginkgo biloba extract EGb 761. Dose-dependent effect of EGb 761 on microcirculation and viscoelasticity of blood. Fortschr Med. 1993;111:170–172. [PubMed] [Google Scholar]
- 7.Wimpissinger B, Berisha F, Garhoefer G, et al. Influence of Ginkgo biloba on ocular blood flow. Acta Ophthalmol Scand. 2007;85:445–449. doi: 10.1111/j.1600-0420.2007.00887.x. [DOI] [PubMed] [Google Scholar]
- 8.Chung HS, Harris A, Kristinsson JK, et al. Ginkgo biloba extract increases ocular blood flow velocity. J Ocul Pharmacol Ther. 1999;15:233–240. doi: 10.1089/jop.1999.15.233. [DOI] [PubMed] [Google Scholar]
- 9.Lee DJ, Ahn HB, Rho SH. The effect of Ginkgo biloba to retinal microcirculation. J Korean Ophthalmol Soc. 2002;43:1522–1527. [Google Scholar]
- 10.Michelson G, Schmauss B, Langhans MJ, et al. Principle, validity, and reliability of scanning laser Doppler flowmetry. J Glaucoma. 1996;5:99–105. [PubMed] [Google Scholar]
- 11.Van Buskirk EM, Cioffi GA. Glaucomatous optic neuropathy. Am J Ophthalmol. 1992;113:447–452. doi: 10.1016/s0002-9394(14)76171-9. [DOI] [PubMed] [Google Scholar]
- 12.Levene RZ. Low tension glaucoma: a critical review and new material. Surv Ophthalmol. 1980;24:621–664. doi: 10.1016/0039-6257(80)90123-x. [DOI] [PubMed] [Google Scholar]
- 13.Lee YG, Kim TH, Kim CY, Hong YJ. A comparison of optic nerve head and peripapillary retinal blood flow in normal, primary open angle glaucoma,and normal tension glaucoma. J Korean Ophthalmol Soc. 1999;40:1934–1943. [Google Scholar]
- 14.Kim JJ, Youn JW, Lee HB. The measurement of ocular blood flow velocity using doppler ultrasound in normal tension glaucoma patients. J Korean Ophthalmol Soc. 1996;37:993–998. [Google Scholar]
- 15.Flammer J, Orgul S, Costa VP, et al. The impact of ocular blood flow in glaucoma. Prog Retin Eye Res. 2002;21:359–393. doi: 10.1016/s1350-9462(02)00008-3. [DOI] [PubMed] [Google Scholar]
- 16.Harris A, Ciulla TA, Chung HS, Martin B. Regulation of retinal and optic nerve blood flow. Arch Ophthalmol. 1998;116:1491–1495. doi: 10.1001/archopht.116.11.1491. [DOI] [PubMed] [Google Scholar]
- 17.Flammer J, Haefliger IO, Orgul S, Resink T. Vascular dysregulation: a principal risk factor for glaucomatous damage? J Glaucoma. 1999;8:212–219. [PubMed] [Google Scholar]
- 18.Flammer J, Pache M, Resink T. Vasospasm, its role in the pathogenesis of diseases with particular reference to the eye. Prog Retin Eye Res. 2001;20:319–349. doi: 10.1016/s1350-9462(00)00028-8. [DOI] [PubMed] [Google Scholar]
- 19.Zurakowski D, Vorwerk CK, Gorla M, et al. Nitrate therapy may retard glaucomatous optic neuropathy, perhaps through modulation of glutamate receptors. Vision Res. 1998;38:1489–1494. doi: 10.1016/s0042-6989(98)00003-0. [DOI] [PubMed] [Google Scholar]
- 20.Pellegrini-Giampietro DE, Cherici G, Alesiani M, et al. Excitatory amino acid release and free radical formation may cooperate in the genesis of ischemia-induced neuronal damage. J Neurosci. 1990;10:1035–1041. doi: 10.1523/JNEUROSCI.10-03-01035.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dugan LL, Choi DW. Excitotoxicity, free radicals, and cell membrane changes. Ann Neurol. 1994;35(Suppl):S17–S21. doi: 10.1002/ana.410350707. [DOI] [PubMed] [Google Scholar]
- 22.Yoles E, Schwartz M. Potential neuroprotective therapy for glaucomatous optic neuropathy. Surv Ophthalmol. 1998;42:367–372. doi: 10.1016/s0039-6257(97)00123-9. [DOI] [PubMed] [Google Scholar]
- 23.Kobayashi MS, Han D, Packer L. Antioxidants and herbal extracts protect HT-4 neuronal cells against glutamate-induced cytotoxicity. Free Radic Res. 2000;32:115–124. doi: 10.1080/10715760000300121. [DOI] [PubMed] [Google Scholar]
- 24.Marcocci L, Maguire JJ, Droy-Lefaix MT, Packer L. The nitric oxide-scavenging properties of Ginkgo biloba extract EGb 761. Biochem Biophys Res Commun. 1994;201:748–755. doi: 10.1006/bbrc.1994.1764. [DOI] [PubMed] [Google Scholar]
- 25.Schindowski K, Leutner S, Kressmann S, et al. Age-related increase of oxidative stress-induced apoptosis in mice prevention by Ginkgo biloba extract (EGb761) J Neural Transm. 2001;108:969–978. doi: 10.1007/s007020170016. [DOI] [PubMed] [Google Scholar]
- 26.Quaranta L, Bettelli S, Uva MG, et al. Effect of Ginkgo biloba extract on preexisting visual field damage in normal tension glaucoma. Ophthalmology. 2003;110:359–362. doi: 10.1016/S0161-6420(02)01745-1. [DOI] [PubMed] [Google Scholar]
- 27.Ha SJ, Rho SH. The effects of oral Ginkgo biloba extract on visual field change in patients with NTG. J Korean Ophthalmol Soc. 2003;44:2047–2057. [Google Scholar]
- 28.Park KH. Comparison of optic nerve head blood flow in normal-tension glaucoma with asymmetric visual field loss. J Korean Ophthalmol Soc. 1999;40:1318–1323. [Google Scholar]
- 29.Kim SW, Kim CY, Seong GJ. The short term effects of bimatoprost on optic nerve head and peripapillary retinal blood flow. J Korean Ophthalmol Soc. 2004;45:1322–1329. [Google Scholar]