Abstract
The overexpression of N-acetylneuraminic acid (Neu5Ac) is closely correlated with malignant transformations. Thus, Neu5Ac is an important target in the design of cancer vaccines. To study the influence of chemical modifications of Neu5Ac on its immunological properties, the α-allyl glycosides of five differently N-acylated neuraminic acid derivatives were prepared. Following selective ozonolysis of their allyl group to form an aldehyde functionality, they were coupled to keyhole limpet hemocyanin (KLH) via reductive amination. Resultant glycoconjugates were studied in C57BL/6 mice. The N-propionyl, N-iso-butanoyl and N-phenylacetyl derivatives of neuraminic acid provoked robust immune responses of various antibody isotypes, including IgM, IgG1, IgG2a and IgG3, whereas N-trifluoropropionylneuraminic acid and natural Neu5Ac were essentially nonimmunogenic. Moreover, the N-phenylacetyl and N-iso-butanoyl derivatives mainly induced IgG responses that are desirable for antitumor applications. These results raise the promise of formulating effective glycoconjugate cancer vaccines via derivatizing sialic acid residues of sialooligosaccharides.
Keywords: carbohydrate, sialic acid, N-acylneuraminic acid, glycoconjugate, immunogenicity, cancer vaccine
Introduction
Sialic acids are a family of neuraminic acid derivatives that are naturally present. The most common sialic acids are N-acetylneuraminic acid (Neu5Ac, 1) and N-glycolylneuraminic acid (Neu5Gc), of which Neu5Gc is expressed in large amounts in all mammals except humans [1].
Sialic acids are unique carbohydrates in that they usually appear at the nonreducing ends of oligosaccharides of natural glycoproteins and glycolipids. Therefore, as the most exposed carbohydrate moieties on cell surfaces, sialic acids play forefront roles in numerous biological and pathological events [2,3]. For example, Siglecs are a class of lectins that bind sialic acids specifically [4,5], and they are critical for cell interactions and signal transductions in the hemopoietic, immune and nerve systems [6]. Siglecs are therefore important templates for new drug design [7]. It has also been established that oncogenic transformations are commonly accompanied by the overexpression of Neu5Ac [8]. Consequently, many tumor antigens are sialooligosaccharides [9], which have become invaluable molecular targets for cancer research, such as in the development of therapeutic cancer vaccines and other immunochemical studies [10-12].
Like all glycans, the biosynthesis of Neu5Ac and sialooligosaccharides is realized by a series of enzymatic reactions. The involved enzymes can tolerate modifications of the substrates, an observation that has been widely used in the enzymatic preparation of carbohydrate analogs and derivatives [13-17]. Chemically modified monosaccharides have also been employed to bioengineer carbohydrate structures on cells [18-24].
Based upon the principles of bioengineering of cell surface Neu5Ac, which was pioneered by Bertozzi [18-22] and Reutter [23,24], we recently proposed a novel strategy to overcome the immunotolerance problem of tumor-associated carbohydrate antigens (TACAs), in the hope of developing new, effective immunotherapies for cancer [25]. The basic strategy is to immunize a cancer patient or animal with a synthetic vaccine that is composed of a TACA analog having artificial sialic acid residues to establish a specific immune response. The patient or animal is then treated with a correspondingly modified precursor of sialic acid to initiate the expression of the artificial TACA in place of the natural ones on tumor cells. The immune response (specific to the artificial TACA) will thereby eliminate bioengineered tumors that are marked by the artificial TACA.
For the new therapy to work, two conditions have to be met. First, biosynthetic machineries of cancer cells must be able to utilize modified precursors to biosynthesize artificial sialo TACA. Second, synthetic vaccines must be able to induce sufficient immune responses. In principle, synthetic carbohydrates, including artificial sialooligosaccharide analogs, are more immunogenic than natural counterparts [26-30]. However, there has been no systematic study of how different functional groups affect the immunological properties of sialic acids. This knowledge will be critical for choosing suitable modification in the design of effective cancer vaccines based upon sialooligosaccharides. In this regard, we prepared a number of artificial sialic acids and their protein conjugates and studied their immunological properties.
Materials and Methods
General methods and materials
NMR spectra were recorded on a Gemini-200 FT NMR or a Gemini-300 FT NMR spectrometer. Proton chemical shifts are reported in ppm (δ) downfield from tetramethylsilane (TMS). Coupling constants (J) are reported in hertz (Hz). Fast atom bombardment mass spectra (FAB-MS) were obtained with a Kratos MS-25RFA spectrometer. Optical rotations were measured on a Perkin-Elmer 241 polarimeter. Thin layer chromatography (TLC) was performed on silica gel 60 GF256 plates detected by charring with phosphomolibdic acid-EtOH or 5% H2SO4-EtOH. Commercial solvents and reagents were directly used without further purification.
Synthesis of 2-O-allyl-5-amino-3,5-dideoxy-D-glycero-α-D-galacto-2-nonulopyranosidonic acid (22)
To a solution of 21 [31] (200 mg, 0.54 mmol) in water (5 mL) was added 5 mL of 20% tetramethylammonium hydroxide in MeOH (20% w/w). After the reaction mixture was refluxed for 6 h, it was neutralized with concentrated HCl and then condensed to dryness under reduced pressure. The residue was dissolved in water (1 mL) and desalted by passing through a Sephadex G10 column (2 cm × 25 cm) with distilled water as eluent. Fractions containing sialic acid, analyzed by the Svennerholm method, were combined and freeze-dried to provide 22 as a white solid (125 mg, 78%), which was used directly in the next step of reaction without further purification. 1H NMR (D2O, 200 MHz): δ 5.93 (1 H, m, CH=CH2), 5.33 (1 H, bd, J 16.8 Hz, CH=CH2), 5.23 (1 H, bd, J 10.2 Hz, =CH2), 4.24 (1 H, dd, J 12.6, 6.2 Hz), 4.05 (1 H, J 10.3, 2.1 Hz), 3.90 (1 H, J 12.0, 2.5 Hz, H-9), 3.77 (1 H, J 11.0, 2.2 Hz, H-7), 3.72 (1 H, dd, J 12.0, 5.7 Hz, H-9′), 3.24 (1 H, J 10.3, 9.7 Hz, H-5), 2.80 (1 H, dd, J 12.5, 3.6 Hz, H-3eq), 1.70 (1 H, t, J 12.6 Hz, H-3ax).
Synthesis of 2-O-allyl-3,5-dideoxy-5-propionylamino-D-glycero-α-D-galacto-2-nonulopyranosidonic acid (3) and 2-O-allyl-3,5-dideoxy-5-iso-butanoylamino-D-glycero-α-D-galacto-2-nonulo-pyranosidonic acid (4)
After 21 (100 mg, 0.33 mmol) was dissolved in a mixture of MeOH (5 mL) and H2O (5 mL) in an ice-water bath, a few drops of 4M aqueous NaOH solution was added to adjust the pH to ca. 10. Then, propionyl or iso-butanoyl anhydride (1.0 mL) was added dropwise within 6 h. Reactions were monitored by TLC. After reactions were complete, concentrated HCl was added to adjust pH to 6. Precipitates were filtrated off and the filtrate was condensed and purified by silica gel column chromatography eluted with CHCl3 and MeOH (4:1) to give 3 and 4 respectively. Compound 3 is a white solid (151 mg, 85%): [α]D −7.0 (c 0.6, H2O). 1H NMR (D2O, 200 MHz): δ 5.90 (1 H, m, CH=CH2), 5.30 (1 H, dd, J 17.3, 1.8 Hz, CH=CH2), 5.20 (1 H, dd, J 10.3, 1.8 Hz, =CH2), 4.20 (1 H, ddt, J 12.1, 6.2, 1.2 Hz), 3.95 (1 H, ddt, J 12.4, 5.8, 1.4 Hz), 3.70 (1 H, dd, J 12.1, 6.0 Hz, H-9), 2.72 (1 H, dd, J 12.5, 4.4 Hz, H-3eq), 2.26 (2 H, q, J 7.6 Hz, CH3CH2), 1.63 (1 H, dd, J 11.5, 12.5 Hz, H-3ax), 1.08 (3 H, t, J 7.6 Hz, CH3CH2). HR-FAB-MS: calc for C15H26NO9 [M + H]+ 364.1608, found 364.1598. Compound 4 is a white solid (103 mg, 83%): [α]D −6.0 (c 0.6, H2O). 1H NMR (D2O, 200 MHz): δ 5.92 (1 H, m, CH=CH2), 5.30 (1 H, dd, J 17.2, 1.6 Hz, =CH2), 5.20 (1 H, dd, J 10.3, 1.6 Hz, =CH2), 4.21 (1 H, ddt, J 12.2, 6.2, 1.2 Hz), 3.97 (1 H, ddt, J 12.1, 5.9, 1.3 Hz), 3.60 (1 H, dd, J 12.4, 6.6 Hz, H-9), 3.50 (1 H, dd, J 8.8, 1.4 Hz, H-7), 2.72 (1 H, dd, J 12.4, 4.2 Hz, H-3eq), 2.50 (1 H, m, J 6.9 Hz, Me2CH-), 1.63 (1 H, dd, J 11.4, 11.4 Hz, H-3ax), 1.08 (3 H, d, J 6.9 Hz, Me2CH-), 1.07 (3 H, d, J 6.9 Hz, Me2CH-). HR-FAB-MS: calc for C16H28NO9 [M + H]+ 378.1764, found 378.1763.
Synthesis of 2-O-allyl-3,5-dideoxy-5-phenylacetamino-D-glycero-α-D-galacto-2-nonulo-pyranosidonic acid (5)
After the mixture of 21 (100 mg, 0.33 mmol) and phenylacetyl chloride (0.5 mL) in MeOH (5 mL) and Et3N (1.0 mL) was stirred at −70 °C for 2 h, solvents were removed in a vacuum. The residue was purified on a silica gel column eluted with CHCl3 and MeOH (4:1) to afford 5 as a white solid (105 mg, 75%). [α]Δ +4.7 (c 0.6, H2O). 1H NMR (D2O, 200 MHz): δ 7.35 (5 H, m, H-Ar), 5.90 (1 H, m, CH=CH2), 5.30 (1 H, dd, J 17.2, 1.7 Hz, =CH2), 5.20 (1 H, dd, J 10.3, 1.8 Hz, =CH2), 4.20 (1 H, ddt, J 12.1, 6.2, 1.3 Hz), 3.96 (1 H, ddt, J 12, 5.8, 1.3 Hz), 3.62 (2 H, s, PhCH2-), 3.50 (1 H, dd, J 12.0, 6.5 Hz, H-9), 2.72 (1 H, dd, J 12.4, 4.3 Hz, H-3eq), 1.63 (1 H, dd, J 11.6, 12.4 Hz, H-3ax). HR-FAB-MS: calc for C20H27NO9 [M + H]+ 426.1764, found 426.1776.
Synthesis of 2-O-allyl-3,5-dideoxy-5-trifluoropropionylamino-D-glycero-α-D-galacto-2-nonulopyranosidonic acid (6)
DCC (210 mg, 1.1 mmol) was added at rt to a solution of trifluropropionic acid (0.091 mL, 1.0 mmol) and HOBt (140 mg, 1.1 mmol) in anhydrous DMF (5 mL). Four hours later, the reaction mixture was filtered off to remove the solid byproduct. Then, 21 (50 mg, 0.16 mmol) in DMF (1 mL) was added to the filtrate, and the reaction was monitored by TLC. Upon disappearance of the starting material 12 h later, solid material was removed by filtration and the filtrate was concentrated to dryness under reduced pressure. The residue was purified on a silica gel column eluted with CHCl3 and MeOH (2:1) to give 6 as a white solid (43 mg, 55%). [α]D −10 (c 0.4, H2O). 1H NMR (D2O, 200 MHz): δ 5.95 (1 H, m, CH=CH2), 5.33 (1 H, dd, J 17.3, 1.8 Hz, =CH2), 5.23 (1 H, dd, J 10.8, 1.2Hz, =CH2), 4.25 (1 H, ddt, J 11, 6.6, 1.2 Hz), 4.02 (1 H, ddt, J 11.0, 6.0, 1.2 Hz), 3.13 (2 H, q, JF-H 11.4 Hz, CF3CH2-), 2.76 (1 H, dd, J 12.6, 4.8 Hz, H-3eq), 1.66 (1 H, dd, J 12.6, 12.0 Hz, H-3ax). HR-FAB-MS: calc for C15H23F3NO9 [M + H]+ 418.1325, found 418.1321.
General procedure for ozonolysis of 2-6
A solution of 2-6 (0.1 mmol each) in MeOH (10 ml) was bubbled with ozone at −78°C for 40 min until a blue color appeared and remained. The solution was kept at −78°C for another 10 min, and then nitrogen was introduced to remove remaining ozone. Me2S (0.5 ml) was added at −78 °C, and the resulting solution was allowed to warm to rt over a period of 1 h and stand for another 1 h before it was condensed in a vacuum. The crude product was purified by passing through a Sephadex G10 column with distilled water as the eluent to give, upon lyophilization, the aldehydes 23-27 as white solids. They were used directly in conjugation reactions without further purification. 2-O-(2-Oxoethyl)-5-acetamino-3,5-dideoxy-D-glycero-α-D-galacto-2-nonulopyranosidonic acid (23, 93%): 1H NMR (D2O, 200 MHz): δ 5.09 (1 H, t, J 4.9 Hz, CHO), 3.40 (1 H, dd, J 10.2, 4.8 Hz, CH2-CHO), 2.70 (1 H, dd, J 12.5, 4.4 Hz, H-3eq), 2.01 (3 H, s, CH3CONH-), 1.67 (1 H, t, J 12.2 Hz, H-3ax). FAB-MS: calc for C13H21NO10 [M+] 351.1, found 351.1. 2-O-(2-Oxoethyl)-5-propionylamino-3,5-dideoxy-D-glycero-α-D-galacto-2-nonulopyranosidonic acid (24, 90%): 1H NMR (D2O, 200 MHz): δ 5.08 (1 H, t, J 4.9 Hz, CHO), 2.70 (1 H, dd, J 12.6, 4.4 Hz, H-3eq), 2.26 (2 H, q, J 7.6, CH3CH2-), 1.70 (1 H, dd, J 12, 12 Hz, H-3ax), 1.08 (3 H, t, J 7.6Hz, CH3CH2-). HR-FAB-MS: calc for C14H23NNaO10 [M + Na]+ 388.1220, found 388.1173; calc for C14H23KNO10 [M + K]+ 404.0959, found 404.0907. 2-O-(2-Oxoethyl)-5-isobutanoylamino-3,5-dideoxy-D-glycero-α-D-galacto-2-nonulopyranosidonic acid (25, 90%): 1H NMR (D2O, 200 MHz): δ 5.13 (1 H, t, J 4.8 Hz, CHO), 2.72 (1 H, dd, J 12.3, 4.4 Hz, H-3eq), 2.50 (1 H, m, J 6.9Hz, Me2CH), 1.71 (1 H, dd, J 12.3, 12.0 Hz, H-3ax), 1.13 (3 H, d, J 6.9 Hz, Me2CH), 1.11 (3 H, d, J 6.8 Hz, Me2CH). HR-FAB-MS: calc for C15H26NO10 [M + H]+ 380.1557, found 380.1551. 2-O-(2-Oxoethyl)-5-phenylacetamino-3,5-dideoxy-D-glycero-α-D-galacto-2-nonulopyranosidonic acid (26, 80%): 1H NMR (D2O, 200 MHz): δ 7.35 (5 H, m, H-Ar), 5.08 (1 H, t, J 4.9 Hz, CHO), 2.72 (1 H, dd, J 12.4, 4.3 Hz, H-3eq), 1.65 (1 H, dd, J 11.6, 12.4 Hz, H-3ax). 2-O-(2-Oxoethyl)-5-trifluoropropionylamino-3,5-dideoxy-D-glycero-α-D-galacto-2-nonulopyranosidonic acid (27, 80%): 1H NMR (D2O, 200 MHz): δ 5.10 (1 H, t, J 4.9 Hz, CHO), 3.20 (2 H, q, JF-H 11.4 Hz, CF3CH2), 2.76 (1 H, dd, J 12.6, 4.8 Hz, H-3eq), 1.66 (1 H, dd, J 12.6, 12.0 Hz, H-3ax). HR-FAB-MS: calc for C14H20F3NNaO10 [M + Na]+ 442.0937, found 442.0884; calc for C14H19NNa2)10 [M − H + 2 Na]+ 464.0757, found 464.0764
General procedure for coupling reactions between 23-27 and KLH or HSA (human serum album)
A solution of 23-27 (6 mg each), KLH or HSA (5 mg) and NaBH3CN (5 mg) in 0.1M NaHCO3 solution (0.4 mL, pH 7.5-8) was allowed to stand at rt in the dark for 4 days with occasional shaking. The reaction mixture was then loaded onto a Bio Gel A 0.5 column (1 cm × 15 cm) and eluted with a 0.1M PBS buffer (I = 0.1, pH = 7.8). Fractions containing the glycoconjugate, characterized by BCA assay for proteins and by the Svennerholm method for sialic acids, were combined, dialyzed against distilled water for 3 days, and lyophilized to give a white powder of the expected glycoconjugates.
Analysis of sialic acid loading of glycoconjugates [32]
Accurately weighed samples of glycoconjugates (ca. 0.5 mg each) were dissolved in distilled water (2.0 mL), mixed well with resorcinol reagent (2.0 mL), and heated in boiling water for 30 min. The solutions were then cooled to rt and combined with an extraction solution (1-butanol acetate and 1-butanol, 85:15 v/v, 4.0 mL). The mixture was shaken vigorously and allowed to stand still for ca. 10 min to separate the organic layer from inorganic layer. The upper organic layer was transferred to a 1.0 cm cuvette, and absorbance at 580 nm was determined by an UV/visible spectrometer, using the organic solvents as the blank control. The sialic acid contents of the glycoconjugates were determined with a calibration curve created with standard Neu5Acyl solutions and analyzed under the same conditions. Sialic acid loading of the conjugates was calculated according to the following equation:
Immunization of mice
Six female C57BL/6 mice at the age of eight weeks (Jackson Laboratories, Bar Harbor, ME) were immunized for each N-acylneuraminic acid-KLH glycoconjugate. Immunizations were intraperitoneal with glycoconjugate containing 2 μg of carbohydrate in 200 μl of saline mixed with 200 μl of MPL/TDM Ribi adjuvant (Sigma Chemical, St. Louis, MO) following the manufacturer’s protocol. The mice were boosted with identical immunizations on days 14, 21 and 28 following the initial immunization. The mice were bled by tail vein prior to the initial immunization on day 0 and after immunization on day 27. On day 35, mice were sacrificed and serum was collected by post-ocular orbital bleeding. Bleed was clotted to obtain sera, which were stored at −80 °C.
Protocols for ELISA analysis
ELISA plates were coated with sialic acids conjugated to HSA. These capture reagents allowed detection of antibodies specific for various sialic acid components of the glycoconjugates without detection of antibodies to KLH (the carrier protein used to compose glycoconjugate vaccines). Maxisorp ELISA plates (NuncNalgene, Rochester, N.Y.) were coated overnight at 4 °C with 100 μl of sialic acid-HSA glycoconjugates (1 μg/ml 0.1M bicarbonate buffer), and then washed with PBS. Sera from the six mice per group were pooled, diluted 1:300 to 1:72900 in serial half-log dilutions in PBS with 0.02% azide, and incubated overnight in the coated ELISA plates (100 μl/well). The plates were then washed and incubated with 1:1000 dilution of alkaline phosphatase linked anti-kappa, anti-IgM or anti-IgG2a antibodies, or with 1:2000 dilution of anti-IgG1 or anti-IgG3 antibodies (Southern Biotechnology, Buckingham, AL) for 1 h at rt. Plates were washed and developed with PNPP substrate for colorimetric readout using a BioRad 550 plate reader (BioRad, Hercules, CA) at 405 nM wavelength.
A titer analysis was performed to normalize data and calculate relative immunogenicities of derivatized glycoconjugates. Optical density (OD) values were plotted against dilution values, and a best-fit line was obtained. The equation of this line was used to calculate the dilution value at which an OD of 0.5 was achieved, and antibody titer was calculated as the inverse of this dilution value.
Results and Discussion
Synthesis of sialic acids and their protein conjugates
This research was designed to explore the impact of various chemical modifications of Neu5Ac on its immunogenicity. Thus, N-acyl derivatives (2-6) of neuraminic acid were synthesized and coupled to carrier protein, and their immunological properties were studied.
KLH was employed as the carrier protein to form glycoconjugate immunogens 7-11, since KLH is an effective carrier for experimental cancer vaccines [10-12]. On the other hand, HSA conjugates (12-16) were used as ELISA capture reagents to eliminate detection of antibodies raised by KLH. Regarding the antigens, in addition to natural Neu5Ac that was used essentially as the control, we planned to investigate four artificial N-acyl derivatives of neuraminic acid, namely, N-propionylneuraminic acid (Neu5Pr), N-iso-butanoylneuraminic acid (Neu5iBu), N-phenylacetylneuraminic acid (Neu5PhAc), and N-trifluoropropionylneuraminic acid (Neu5FPr). Although according to the literature Neu5Gc is antigenic [1], because it is a natural sialic acid that is widely spread in mammals other than humans, glycoconjugates of Neu5Gc are of limited interest as cancer vaccines to us.
The designed neuraminic acid derivatives included a range of structural variations relative to natural Neu5Ac. Propionyl group is only one carbon longer than acetyl group, so there is a relatively small structural difference between Neu5Pr and natural Neu5Ac. However, a similar structural change was found in previous studies to substantially improve the immunogenicity of polysialic acid [27,28]. It is thus of interest to us to examine the immunological properties of Neu5Pr. Trifluoropropionyl group is an analog of propionyl group but with its terminal methyl group fully fluorinated. Therefore, a trifluoropropionyl group should be more hydrophobic than a propionyl group, even though they have very similar bulk. We would expect that the higher hydrophobicity of trifluoropropionyl group might improve the immunogenicity of Neu5FPr, because hydrophobic components can often promote the immune response. Phenylacetyl and iso-butanoyl groups are structurally very different from an acetyl group. We would expect that their distinct sizes and hydrophobicity might make them even more immunogenic.
Protein conjugates of various sialic acids were prepared according to the procedure shown in Scheme 1. Our initial attempts to directly hydrolyze the acetyl group of 1 using strong acids and bases failed. The reactions afforded unidentified tars, probably due to the decomposition of 1. The open chain structure of 1 as an α-keto acid is rather sensitive to both acids and bases. We finally conceived a procedure of performing deacetylation with the base-stable glycoside of Neu5Ac and realizing N-modification at a much later stage. In this way, the keto structure could be avoided. We chose to utilize the allyl glycoside because an allyl group can be selectively ozonolyzed to afford an aldehyde functionality, it facilitating later coupling with proteins via reductive amination. Thus, the allyl group also served as a linker.
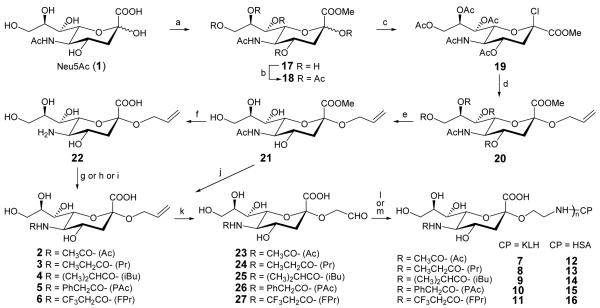
Scheme 1a.
aReagents and conditions: (a) MeOH, H+-resin, >99%; (b) Ac2O, pyridine, DMAP, 95%; (c) HCl, AcOH/Ac2O, >99%; (d) AllOH, silver salicylate, MS (4Å), 93%; (e) 1N NaOMe, MeOH, 93%; (f) 4N Me4NOH, MeOH/H2O, 78%; (g) Pr2O or iBu2O, H2O (pH 10), 83-85%; (h) PhAcCl, MeOH, 75%; (i) CF3CH2COOBt, DMF, 55%; (j) 1.0N NaOH, H2O, rt, 95%; (k) O3, MeOH; then Me2S, 80-90%; (l) NaBH3CN, KLH, buffer (pH 7.5); (m) NaBH3CN, HSA, buffer (pH 7.5).
The synthesis started from commercially available Neu5Ac (Scheme 1). The reactions to prepare 21 were well established [31]. The deacetylation of 21 was achieved by refluxing its aqueous methanol solution in the presence of tetramethylammonium hydroxide. The methyl ester was hydrolyzed concomitantly to give 22 that was purified on a Sephadex G10 column. The NMR spectrum of 22 verified the complete removal of acetyl groups. Upon reaction with propionyl or iso-butanoyl anhydride, 22 was transformed into the allyl glycosides 3 and 4 of Neu5Pr and Neu5iBu, whereas the reaction of 22 with phenylacetyl chloride in methanol gave the allyl glycoside 5 of Neu5PhAc in good yield. The trifluoropropionylation of 22 was carried out by reaction with the N-hydroxybenzotriazole (BtOH) ester of trifluoropropionyl acid. Mild basic treatment of 21 to only remove the methyl group afforded the ally glycoside 2 of Neu5Ac. Thus, the allyl glycosides 2-6 of various sialic acids were obtained from Neu5Ac in 6-7 steps and 35-87% overall yields.
Ozonolysis of 2-6 proceeded smoothly in methanol to give the corresponding aldehydes 23-27. Their 1H NMR spectra clearly showed a proton signal of the hydrated aldehyde at δ 5.10, as well as the disappearance of proton signals of its allyl group at δ 5.90, 5.30 and 5.20. Coupling reactions between 23-27 and KLH or HSA were performed in 0.1N NaHCO3 buffer (pH 7.5-8.0), with 23-27 in excess, according to reported conditions [33]. Conjugation products were easily separated from unreacted carbohydrates by a Bio Gel A 0.5 column. Fractions that were positive on sialic acid analysis (by Svennerholm method) and protein analysis (by BCA method) were combined, dialyzed against distilled water and then lyophilized to afford glycoconjugates 7-16 that were subjected to chemical analysis before their immunological studies.
The sialic acid loading of 7-16 was analyzed by Svennerholm method [32]. So, after standard calibration curves were obtained with synthetic sialic acids, glycoconjugates 7-16 were examined under the same conditions to determine their sialic acid contents, which were then converted to the percentile loadings (see Methods). Table 1 shows the levels of sialic acid loading of all glycoconjugates.
Table 1.
Sialic acid loading of various glycoconjugates
| sample | KLH conjugates |
HSA conjugates |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 7 (Ac) |
8 (Pr) |
9 (iBu) |
10 (PAc) |
11 (FPr) |
12 (Ac) |
13 (Pr) |
14 (iBu) |
15 (PAc) |
16 (FPr) |
|
| Neu5Acyl loading (%) |
15.8 | 13.4 | 10.9 | 12.0 | 9.2 | 23.6 | 23.5 | 17.1 | 15.7 | 11.1 |
Overall, the coupling reactions were very efficient to give glycoconjugates containing 9.2-23.6% of sialic acids, but the loading level of KLH conjugates was generally slightly lower than that of HSA conjugates. In the case of KLH conjugates, a 10% loading signifies that in average about 300 sialic acid residues were attached to each protein molecule. This level of conjugation is usually ideally suitable for vaccine applications.
Immunological studies of KLH conjugates
Our design was to conjugate various sialic acids to KLH to provide T cell “help” for B cell responses [34]. B cells that express membrane-bound antibodies specific for sialic acids should exhibit efficient antibody-enhanced uptake of the glycoconjugates, allowing enhanced B cell presentation of the carrier protein to T cells. This mechanism drives T cell responses that promote the carbohydrate-specific B cells to proliferate more rapidly, undergo isotype class switching and affinity maturation and differentiate into long-lived memory B cells. Thus, conjugation of carbohydrate antigens to carrier protein enhances the magnitude and quality of the antibody response [27].
The immunogenicity of KLH-sialic acid conjugates was assessed in C57BL/6 mice, an immunologically competent inbred strain that has been used in tumor immunology studies [25] and is highly applicable to future studies in this area. The mice received a primary immunization on day 0 and booster immunizations at days 14, 21 and 28 with same antigens. Glycoconjugates were administered in Ribi adjuvant, an oil emulsion system that is approved for use in human trials [35]. The Freund’s adjuvant was avoided because it is not approved for human use.
Following immunization, we examined titers of total antigen-specific serum antibodies and individual titers of antigen-specific IgM, IgG1, IgG2a and IgG3 (Figure 1). These antibody isotypes have different capacities to fix complement and bind Fc receptors. For example, IgG antibodies usually have improved complement fixation and Fc receptor binding ability, leading to antibody dependent cell mediated cytotoxicity [36-39].
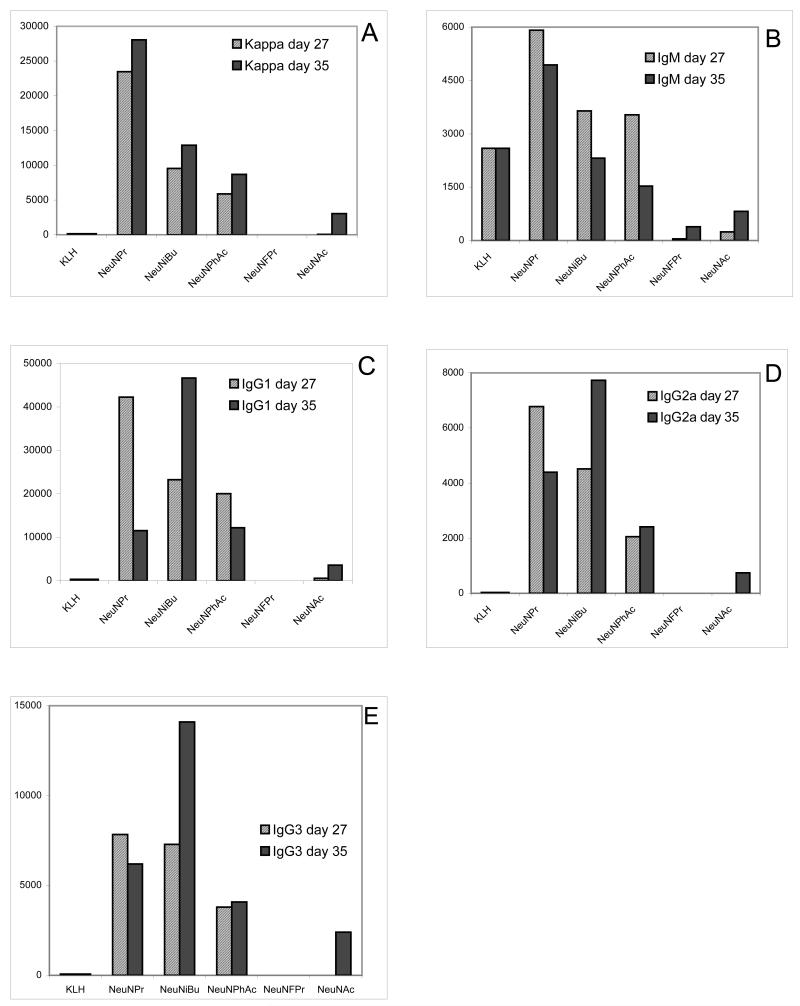
Figure 1.
Titer analysis of antigen-specific antibodies determined by ELISA assay (see Methods). Each represents the titer in pooled serum obtained on indicated day after primary and booster immunizations (see Methods) from six replicate animals.
Panel A of Figure 1 gives total antigen-specific antibody titers assessed by ELISA assays specific for kappa light chain. Kappa light chains are found in association with antibody heavy chains of all isotypes, and as the most common antibody light chain type in mice, they comprise approximately 95% of antibodies [38]. As expected, the artificial sialic acids were much more immunogenic than the natural Neu5Ac. Neu5Pr showed the highest titers, followed by Neu5iBu and Neu5PhAc. To our surprise, however, Neu5FPr produced very low antibody titers.
Panel B of Figure 1 shows antigen-specific IgM titers. IgM has important complement fixing function but is produced by B cells that have not undergone class switching or affinity maturation and have not progressed to a memory phenotype [38]. As seen in the figure, titers of this isotype were generally lower, but the pattern of immunogenicity ranking was similar to that of panel A. Thus, Neu5Pr also gave the highest IgM titers. IgM titers were relatively high for unconjugated KLH, while IgM was the only isotype with a non-zero titer found with Neu5FPr.
Antigen-specific IgG1 titers (panel C) were much higher than IgM. For IgG1 and all other IgG subtypes (panels D and E), Neu5iBu showed the highest titer values followed by Neu5Pr and Neu5PhAc. While these two unnatural sialic acids gave robust serum titers for IgG1, IgG2a and IgG3, Neu5FPr did not give measurable serum titers of these isotypes.
Our results showed that unnatural sialic acids are generally very immunogenic, although Neu5FPr was a poor immunogen and induced only minimal titers of IgM and no detectable IgG response. The reason for the lack of immunogenicity of Neu5FPr is unclear. Neu5Pr, Neu5iBu and Neu5PhAc induced both IgM and IgG responses. Neu5Pr gave the highest titers of total and IgM antibodies, but Neu5iBu and Neu5PhAc produced higher or at least similar levels of IgG antibodies of several subclasses. Since IgG antibodies have more desirable properties (e.g. affinity maturation and memory, which are not found with IgM responses), these results indicate that Neu5iBu and Neu5PhAc are promising candidates for utilization as effective immunogens for therapeutic purposes. We have also noticed that the IgG antibody titers induced by Neu5iBu and Neu5PhAc increased steadily in response to the repeated immunizations, suggesting the potential for prolonged immune responses induced by these antigens. Because IgG antibodies have improved functions, e.g. complement fixation and cell mediated cytotoxicity, they may play a key role in the elimination of tumors in vivo [36,38].
We envision that modifying Neu5Ac in sialo TACAs with iso-butanoyl and phenylacetyl groups may result in promising cancer vaccines, an issue that we are currently pursuing. Our preliminary results [40] have also shown that the phenylacetylated derivative of mannosamine is a very good substrate for Neu5Ac adolase that is involved in the bioengineering of sialic acid and sialoglycoconjugates on cells [41]. These studies indicate great promise for the application of derivatized forms of sialooligosaccharides as immunological targets and guide the choice of derivatizations for further studies of glycoengineering of tumor cells and development of novel and efficient cancer immunotherapies.
In summary, an effective method was established to prepare various N-acyl derivatives of neuraminic acid and their protein conjugates, and the immunological properties of these glycoconjugates were studied in detail. It was proved that artificial sialic acids were much more immunogenic than natural Neu5Ac. Even though Neu5Pr, Neu5iBu and Neu5PhAc all generated robust immune responses, Neu5iBu and Neu5PhAc were better immunogens, as they included stronger IgG responses. Therefore, cancer vaccines made of oligosaccharides with iso-butanoyl and phenylacetyl-modified neuraminic acids are excellent targets for further investigations.
Footnotes
This work was supported by a research grant from the NIH/NCI (1R01 CA95142) and a seed grant from the Ohio Cancer Research Associates.
References
- [1].Brinkman-Van der Linden ECM, Sjoberg ER, Juneja LR, Crocker PR, Varki N, Varki A. Loss of N-glycolylneuraminic acid in human evolution. J. Biol. Chem. 2000;275:8633–8640. doi: 10.1074/jbc.275.12.8633. [DOI] [PubMed] [Google Scholar]
- [2].Schauer R. Sialic acids as antigenic determinants of complex carbohydrates. Adv. Exp. Med. Biol. 1988;228:47–72. doi: 10.1007/978-1-4613-1663-3_2. [DOI] [PubMed] [Google Scholar]
- [3].Reutter W, Stasche R, Stehling P, Baum O. The biology of sialic acids: insights into their structure, metabolism and function in particular during viral infection. In: Gabius H-J, Gabius S, editors. Glycosciences. Chapman & Hall; Weinheim: 1997. pp. 245–259. [Google Scholar]
- [4].Wagner M. Sialic acid-specific lectins. Adv. Lectin Res. 1990;3:36–82. [Google Scholar]
- [5].Varki A. Sialic acids as ligands in recognition phenomena. FASEB J. 1997;11:248–255. doi: 10.1096/fasebj.11.4.9068613. [DOI] [PubMed] [Google Scholar]
- [6].Crocker PR. Siglecs: sialic-acid-binding immunoglobulin-like lectins in cell-cell interactions and signalling. Curr. Opin. .Struct. Biol. 2002;12:609–615. doi: 10.1016/s0959-440x(02)00375-5. [DOI] [PubMed] [Google Scholar]
- [7].Von Itzstein M, Thomson RJ. Sialic acids and sialic acid-recognizing proteins: drug discovery targets and potential glycopharmaceuticals. Curr. Med. Chem. 1997;4:185–210. [Google Scholar]
- [8].Takano R, Muchmore E, Dennis JW. Sialylation and malignant potential in tumor cell glycosylation mutants. Glycobiology. 1994;4:665–674. doi: 10.1093/glycob/4.5.665. [DOI] [PubMed] [Google Scholar]
- [9].Hakomori S. Tumor-associated carbohydrate antigens and modified blood group antigens. In: Montreuil J, Vliegenthart JFG, Schachter H, editors. Glycoproteins and Disease. Elsevier; Amsterdam: 1996. pp. 243–276. [Google Scholar]
- [10].Ragupathi G. Carbohydrate antigens as targets for active specific immunotherapy. Cancer Immunol. Immunother. 1998;46:82–87. doi: 10.1007/s002620050316. [DOI] [PubMed] [Google Scholar]
- [11].Hakomori S, Zhang Y. Glycosphingolipid antigens and cancer therapy. Chem. Biol. 1997;3:97–104. doi: 10.1016/s1074-5521(97)90253-2. [DOI] [PubMed] [Google Scholar]
- [12].Helling F, Shang A, Calves M, Zhang S, Ren S, Yu RK, Oettgen HF, Linvingston PO. GD3 vaccines for melanoma: superior immunogenicity of keyhole limpet hemocyanin conjugate vaccines. Cancer Res. 1994;54:197–205. [PubMed] [Google Scholar]
- [13].Unverzagt C. Chemoenzymatic synthesis of a sialylated undecasaccharide-asparagine conjugate. Angew. Chem., Intl. Ed. Eng. 1996;35:2350–2353. [Google Scholar]
- [14].Blixt O, Norberg T. Solid-phase enzymatic synthesis of a sialyl Lewis X tetrasaccharide on a sepharose matrix. J. Org. Chem. 1998;63:2705–2710. doi: 10.1021/jo980074h. [DOI] [PubMed] [Google Scholar]
- [15].Chappell MD, Halcomb RL. Enzyme-catalyzed synthesis of oligosaccharides that contains functionalized sialic acids. J. Am. Chem. Soc. 1997;119:3393–3394. [Google Scholar]
- [16].Ichikawa Y, Shen GJ, Wong CH. Enzyme-catalyzed synthesis of sialyl oligosaccharide with in situ regeneration of CMP-sialic acid. J. Am. Chem. Soc. 1991;113:4698–4700. [Google Scholar]
- [17].Ichikawa Y, Liu JLC, Shen GJ, Wong C-H. A highly efficient multienzyme system for the one-step synthesis of a sialyl trisaccharide: in situ generation of sialic acid and N-acetyllactosamine coupled with regeneration of UDP-glucose, UDP-galactose and CMP-sialic acid. J. Am. Chem. Soc. 1991;113:6300–6302. [Google Scholar]
- [18].Bertozzi CR, Kiessling LL. Chemical glycobiology. Science. 2001;291:2357–2364. doi: 10.1126/science.1059820. [DOI] [PubMed] [Google Scholar]
- [19].Saxon E, Bertozzi CR. Cell surface engineering by a modified Staudinger reaction. Science. 2000;287:2007–2010. doi: 10.1126/science.287.5460.2007. [DOI] [PubMed] [Google Scholar]
- [20].Mahal LK, Bertozzi CR. Engineering cell surfaces: fertile ground for molecular landscaping. Chem. Biol. 1997;4:415–422. doi: 10.1016/s1074-5521(97)90193-9. [DOI] [PubMed] [Google Scholar]
- [21].Yarema KJ, Mahal LK, Bruehl RE, Rodriguez EC, Bertozzi CR. Metabolic delivery of ketone groups to sialic acid residues. Application to cell surface glycoform engineering. J. Biol. Chem. 1998;273:31168–31179. doi: 10.1074/jbc.273.47.31168. [DOI] [PubMed] [Google Scholar]
- [22].Mahal KL, Yarema KJ, Bertozzi CR. Engineering chemical reactivity on cell surfaces through oligosaccharide biosynthesis. Science. 1997;276:1125–1128. doi: 10.1126/science.276.5315.1125. [DOI] [PubMed] [Google Scholar]
- [23].Kayser H, Geile CC, Paul C, Zeitler R, Reutter W. Incorporation of N-acyl-2-amino-2-deoxy-hexoses into glycosphingolipids of pheochromocytoma cell line PC12. FEBS. 1992;301:137–140. doi: 10.1016/0014-5793(92)81233-c. [DOI] [PubMed] [Google Scholar]
- [24].Schmidt C, Stehling P, Schnitzer J, Reutter W, Horskorte R. Biological engineering of neural cell surfaces by the synthetic N-propanoyl-substituted neuraminic acid precursor. J. Biol. Chem. 1998;273:19146–19152. doi: 10.1074/jbc.273.30.19146. [DOI] [PubMed] [Google Scholar]
- [25].Liu T, Guo Z, Yang Q, Sad S, Jennings HJ. Biochemical engineering of surface α(2-8)polysialic acid for immunotargeting cancer cells. J. Biol. Chem. 2000;275:32832–32836. doi: 10.1074/jbc.C000573200. [DOI] [PubMed] [Google Scholar]
- [26].Lemieux GA, Bertozzi CR. Modulating cell surface immunoreactivity by metabolic induction of unnatural carbohydrate antigens. Chem. Biol. 2001;8:265–275. doi: 10.1016/s1074-5521(01)00008-4. [DOI] [PubMed] [Google Scholar]
- [27].Jennings H. N-propionylated group B meningococcal polysaccharide glycoconjugate vaccine against group B meningococcal meningitis. Int. J. Infect. Dis. 1997;1:158–164. [Google Scholar]
- [28].Jennings HJ, Sood RK. Synthetic glycoconjugates as human vaccines. In: Lee YC, Lee RT, editors. Neoglycoconjugates: Preparation and Applications. Academic Press; San Diego: 1994. pp. 325–371. [Google Scholar]
- [29].Ritter G, Boosfeld E, Calves MJ, Oettgen HF, Old LJ, Livingston PO. Antibody response after immunization with ganglioside GD3, GD3 lactones, GD3 amide and GD3 gangliosidol in the mouse. GD3 lactone I induces antibodies reactive with human melanoma. Immunobiology. 1990;182:32. doi: 10.1016/S0171-2985(11)80581-4. [DOI] [PubMed] [Google Scholar]
- [30].Ritter G, Boosfeld E, Asluri S, Calves MJ, Oettgen HF, Old LJ, Livingston PO. Antibody response after immunization with ganglioside GD3, GD3 congeners (lactones, amide and gangliosidol) in patients with malignant melanoma. Int. J. Cancer. 1991;48:379–385. doi: 10.1002/ijc.2910480312. [DOI] [PubMed] [Google Scholar]
- [31].Roy R, Laferriere C. Synthesis of protein conjugates and analogs of n-acetylneuraminic acid. Can. J. Chem. 1990;68:2045–2054. [Google Scholar]
- [32].Svennerholm L. Estimation of sialic acids. II. Colorimetric resorcinol-hydrochloric acid method. Biochim. Biophys. Acta. 1957;24:604–611. doi: 10.1016/0006-3002(57)90254-8. [DOI] [PubMed] [Google Scholar]
- [33].Guo Z, Jennings HJ. Protein-polysaccharide conjugation. Methods in Molecular Medicine. 2001;66:49–54. doi: 10.1385/1-59259-148-5:49. Meningococcal Vaccines. [DOI] [PubMed] [Google Scholar]
- [34].Lanzavecchia A. Antigen-specific interaction between T and B cells. Nature. 1985;314:537–539. doi: 10.1038/314537a0. [DOI] [PubMed] [Google Scholar]
- [35].Evans JT, Cluff CW, Johnson DA, Lacy MJ, Persing DH, Baldridge JR. Enhancement of antigen-specific immunity via the TLR4 ligands MPL adjuvant and Ribi.529. Expert Rev. Vaccines. 2003;2:219–229. doi: 10.1586/14760584.2.2.219. [DOI] [PubMed] [Google Scholar]
- [36].Casadevall A, Pirofski LA. Antibody-mediated regulation of cellular immunity and the inflammatory response. Trends Immunol. 2003;24:474–478. doi: 10.1016/s1471-4906(03)00228-x. [DOI] [PubMed] [Google Scholar]
- [37].Danishefsky SJ, Allen JR. From the laboratory to the clinic: A retrospective on fully synthetic carbohydrate-based anticancer vaccines. Angew. Chem. Int. Ed. 2000;39:837–863. doi: 10.1002/(sici)1521-3773(20000303)39:5<836::aid-anie836>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- [38].Goldsby RA, Kindt TJ, Osborne BA, Kuby J. Antibodies: Structure and function. In: Freeman WH, editor. Fundamental Immunology. Company; New York, N.Y.: 2003. pp. 76–105. [Google Scholar]
- [39].Ravetch JV, Kinet JP. Fc eeceptors. Annu. Rev. Immunol. 1991;9:457–492. doi: 10.1146/annurev.iy.09.040191.002325. [DOI] [PubMed] [Google Scholar]
- [40].Pan Y, Ayani T, Nadas J, Guo Z. Accessibility of N-acyl derivatives of D-mannosamine to N-acetylneuraminic acid aldolase. 2004. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Jacobs CL, Goon S, Yarema KJ, Hinderlich S, Hang HC, Chai DH, Bertozzi CR. Substrate specificity of the sialic acid biosynthetic pathway. Biochemistry. 2001;40:12864–12874. doi: 10.1021/bi010862s. [DOI] [PubMed] [Google Scholar]