Abstract
Background
SOX9 is a widely expressed transcription factor playing several relevant functions during development and essential for testes differentiation. It is considered to be the direct target gene of the protein encoded by SRY and its overexpression in an XX murine gonad can lead to male development in the absence of Sry. Recently, a family was reported with a 178 kb duplication in the gene desert region ending about 500 kb upstream of SOX9 in which 46,XY duplicated persons were completely normal and fertile whereas the 46,XX ones were males who came to clinical attention because of infertility.
Methods and results
We report a family with two azoospermic brothers, both 46,XX, SRY negative, having a 96 kb triplication 500 kb upstream of SOX9. Both subjects have been analyzed trough oligonucleotide array-CGH and the triplication was confirmed and characterised through qPCR, defining the minimal region of amplification upstream of SOX9 associated with 46,XX infertile males, SRY negative.
Conclusions
Our results confirm that even in absence of SRY, complete male differentiation may occur, possibly driven by overexpression of SOX9 in the gonadal ridge, as a consequence of the amplification of a gene desert region. We hypothesize that this region contains gonadal specific long-range regulation elements whose alteration may impair the normal sex development. Our data show that normal XX males, with alteration in copy number or, possibly, in the critical sequence upstream to SOX9 are a new category of infertility inherited in a dominant way with expression limited to the XX background.
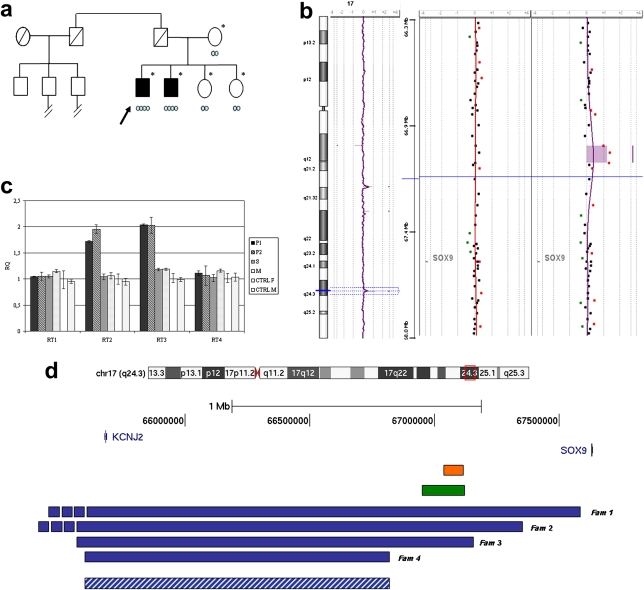
We report on a family with two azoospermic brothers of 47 and 46 years of age, both 46,XX, SRY negative, who have a 96 kb triplication 500 kb upstream of SOX9 (figure 1B,C) that is not present in their two fertile sisters and mother. Interestingly, two of the three paternal cousins of our probands are reported as infertile (figure 1A). The probands' father, who was considered to have been completely normal and the probable carrier of the triplication, died at age of 50 years from a myocardial infarction. The two probands share the same paternal haplotype for the SOX9 region (supplementary table 1), confirming the possibility that the father was indeed the carrier of the same triplication. The two brothers are phenotypically normal males with bilaterally hypotrophic testes, low serum testosterone concentrations (1.0 ng/ml; normal values 1.80–16 ng/ml) and increased follicle stimulating hormone (39.5 mU/ml; normal values 5–25 mU/ml) and luteinising hormone (14.4 mU/ml; normal values 1.5–9.3 mU/ml). A testicular biopsy in one of them showed germinal cell aplasia and mild bilateral gynaecomastia. Both have normal libido and the older brother requested a clinical investigation after 10 years of infertility in spite of continual attempts to have children. A similar family was recently reported by Cox et al.1 They describe three SRY negative azoospermic XX males, two brothers and a paternal uncle, all having a duplication of 178 kb with a distal breakpoint almost coincident to the one we detected in our family. The XY members carrying the duplication were normal fertile males.
Figure 1.
Family tree of the two XX brothers and characterisation of the 96 kb triplication region. (A) Pedigree of the family. Asterisks indicate persons for whom the karyotype was known, the arrow indicates the proband, and light blue circles indicate the copy number of the 96 kb sex reversal critical region. (B) Array comparative genomic hybridisation (CGH) profile (180K, Agilent Technologies, Santa Clara, California, USA) of the whole chromosome 17 of the proband (left), the 17q24.3 region of the proband (right), and one of his healthy sisters (middle). The triplication is indicated by a shadowed area containing three spots with an average log2 ratio of 1.2. (C) Quantitative PCR (qPCR) with specific primer pairs (supplementary table 3). Affected subjects (P1 and P2), one sister (S) and the mother (M), female (CTRL F) and male (CTRL M) controls were analysed. The relative quantitation (RQ) of copy number is indicated on the y axis. RQ of 1 indicates a normal number of copies; RQ of 2 in P1 and P2 shows the presence of a triplication. Altogether array CGH and qPCR analysis defined the proximal breakpoint of the triplication from 67018227 bp (normal) to 67018939 bp (triplicated) and the distal breakpoint from 67114737 bp (triplicated) to 67119234 bp (normal). Genomic positions are referred to the Human Genome March 2006 (NCBI 36, hg18 assembly). (D) SOX9 and its 1.98 Mb upstream region and a schematic illustration of duplications reported by Kurth et al,4 2009 (blue bars), Cox et al,1 2011 (green bar), and the triplication observed in the present family (orange bar). Minimal critical region for brachydactyly–anonychia (Kurth et al,4 2009) is also shown (blue dashed bar).
SOX9 is a widely expressed transcription factor that has several relevant functions during development and is essential for testes differentiation; it is considered to be the direct target gene of the protein encoded by SRY and its overexpression in an XX murine gonad can lead to male development in the absence of Sry.2 Copy number alterations and translocations within the 1.9 Mb gene desert region upstream of SOX9 are responsible for a number of developmental disorders affecting the skeleton and the genitalia.3 4 No pathogenic variants were detected by sequencing both SOX9 and SOX3 genes in our family. Sox3 was recently shown to upregulate expression of Sox9 via a similar mechanism to Sry and to be responsible for XX male sex reversal in humans through gain-of-function mutations mediated by genomic rearrangements around SOX3, possibly leading to its altered regulation.5 Our identified triplicated genomic region is about half the size of the duplication reported by Cox et al.1 We hypothesise that cis-acting regulatory elements are located within the smaller XX-sex reversal critical region we defined, whose duplication increases SOX9 expression driving testicular differentiation in the absence of SRY.2 We did not detect any significant difference in SOX9 expression in lymphoblasts from our family, suggesting that more appropriate primary cells, which were not possible to obtain from our patients, are needed for such expression studies (supplementary table 2).
The main conundrum are the three families reported by Kurth et al4 in which larger duplications, including the sex reversal critical region, are associated with brachydactyly–anonychia and not with XX sex reversal (families 1, 2 and 3 in figure 1D); probably the answer will lie in the careful identification of enhancer/silencing elements.
Our findings define the shortest region of amplification upstream of SOX9 associated with infertile males having an 46,XX karyotype and being SRY negative. Here again, similar to what detected by Cox et al,1 the triplication does not seem to have any effect on the XY background. Since in most infertile XX males the presence of SRY is not routinely investigated, the situation we describe might be more frequent than expected. However, considering that mutations in SOX9 and related transcription factors (ie, NR5A1) were reported to be responsible for a large spectrum of disorders of sexual development, we cannot exclude the possibility that alterations of the region we narrowed down might result in XX individuals carrying abnormalities or ambiguity of the external genitalia as well. Finally, our findings demonstrate once more that gene desert regions are the treasure we have to discover to solve several unexplained pathogenic conditions; concerning developmental sex abnormalities, a similar situation was reported by Smyk et al in an XY female with a 250 kb deletion upstream of NR0B1 having the same phenotypic effect of NR0B1 duplication.6
Footnotes
Funding: The work was supported by the MIUR 2008XA48SC and Cariplo 2007 to AF and Progetto Regione Lombardia (code SAL/45) to AF and OZ.
Competing interests: None to declare.
Patient consent: Obtained.
Ethics approval: Ethics approval was provided by University of Pavia Ethical Committee, Pavia, Italy.
Contributors: OZ designed the study, and supervised the experiments, AV, RC, RG, EDM, and AF performed the experiments, MGP cared for the patients. All authors wrote the report.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Cox JJ, Willatt L, Homfray T, Woods CG. A SOX9 duplication and familial 46, XX developmental testicular disorder. N Engl J Med 2011;364:91–3 [DOI] [PubMed] [Google Scholar]
- 2.Sekido R, Lovell-Badge R. Sex determination involves synergistic action of SRY and SF1 on a specific Sox9 enhancer. Nature 2008;453:930–4 Erratum in: Nature 2008;456: 824 [DOI] [PubMed] [Google Scholar]
- 3.Benko S, Fantes JA, Amiel J, Kleinjan DJ, Thomas S, Ramsay J, Jamshidi N, Essafi A, Heaney S, Gordon CT, McBride D, Golzio C, Fisher M, Perry P, Abadie V, Ayuso C, Holder-Espinasse M, Kilpatrick N, Lees MM, Picard A, Temple IK, Thomas P, Vazquez MP, Vekemans M, Roest Crollius H, Hastie ND, Munnich A, Etchevers HC, Pelet A, Farlie PG, Fitzpatrick DR, Lyonnet S. Highly conserved non-coding elements on either side of SOX9 associated with Pierre Robin sequence. Nat Genet 2009;41:359–64 [DOI] [PubMed] [Google Scholar]
- 4.Kurth I, Klopocki E, Stricker S, van Oosterwijk J, Vanek S, Altmann J, Santos HG, van Harssel JJ, de Ravel T, Wilkie AO, Gal A, Mundlos S. Duplications of noncoding elements 5' of SOX9 are associated with brachydactyly-anonychia. Nat Genet 2009;41:862–3 [DOI] [PubMed] [Google Scholar]
- 5.Sutton E, Hughes J, White S, Sekido R, Tan J, Arboleda V, Rogers N, Knower K, Rowley L, Eyre H, Rizzoti K, McAninch D, Goncalves J, Slee J, Turbitt E, Bruno D, Bengtsson H, Harley V, Vilain E, Sinclair A, Lovell-Badge R, Thomas P. Identification of SOX3 as an XX male sex reversal gene in mice and humans. J Clin Invest 2011;121:328–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smyk M, Berg JS, Pursley A, Curtis FK, Fernandez BA, Bien-Willner GA, Lupski JR, Cheung SW, Stankiewicz P. Male-to-female sex reversal associated with an approximately 250 kb deletion upstream of NR0B1 (DAX1). Hum Genet 2007;122:63–70 [DOI] [PubMed] [Google Scholar]