Abstract
Despite the high prevalence of chronic rhinosinusitis (CRS) worldwide, the exact pathogenesis of the disease remains unknown. Even with therapeutic intervention, treatment response is often only partial and frequently ineffective. The inability to define exact disease phenotypes in relation to specific disease mechanisms has led to a broad based approach with both anti-inflammatory and anti-microbial intervention. The clinical efficacy of such current therapeutic strategies is highlighted and the urgent need for further robust therapeutic intervention studies in CRS is discussed in this article.
Keywords: Rhinosinusitis; anti-inflammatory, antibiotics
INTRODUCTION
Chronic rhinosinusitis (CRS) is a common condition associated with significant morbidity and healthcare costs. In the USA alone, the prevalence is estimated at 14% of the population with significant economic consequences related to 73 million days off work. The annual medical cost is a staggering 2.4 billion US dollars.1 Current therapeutic strategies remain inadequate. Thus there is an urgent unmet clinical need.
Rhinosinusitis is defined as inflammation of the nose and paranasal sinuses characterised by two or more symptoms, one of which should be nasal obstruction (blockage, congestion) or nasal discharge (anterior/posterior) along with either facial pressure/pain or reduction or loss of smell.2 Confirmation of sinus-nasal mucosal inflammation via either endoscopic inspection or imaging is recommended to confirm the clinical diagnosis. Chronicity is arbitrarily defined by the persistence of symptoms beyond 12 weeks.
The evolutionary changes which led man to an upright stance have meant that sinus ventilation and drainage occur against gravity and are dependent upon patency of the slit-like ostiomeatal complex area. Thus anything compromising this can act as a predisposing factor to rhinosinusitis.
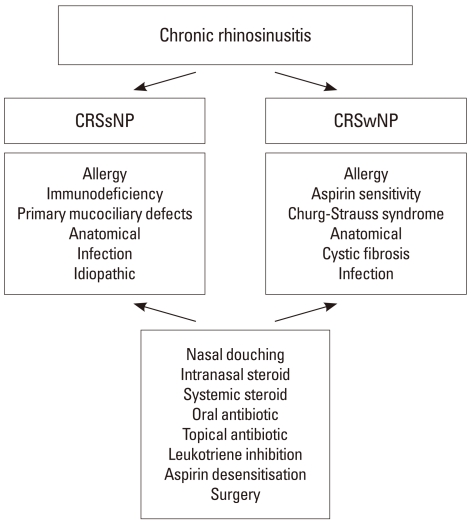
CRS is not a distinct disease entity but an 'umbrella' term that groups together a spectrum of disorders with distinct immuno-pathological mechanisms.3 The exact pathogenesis remains poorly defined and treatments options are still limited and often ineffective. Currently, CRS is subclassified into two distinct UKsubtypes termed CRS without nasal polyps (CRSsNP) and CRS with nasal polyps (CRSwNP) (Figure). We will not discuss fungal rhinosinusitis in this article. Whilst this sub-classification allows clinicians to view two CRS groups with distinct yet related disease mechanisms separately, overall this classification remains too simplistic. A significant proportion of patients still remain symptomatic despite maximal treatment. A therapeutic approach that incorporates defined clinical phenotypes with exact disease pathogenesis, an approach that is now more standard in allergic rhinitis is urgently needed.4
Figure.
Summary of therapeutic approaches to chronic rhinosinusitis. Chronic rhinosinusitis (CRS) can be broadly divided into CRS without nasal polyps (CRSsNP) and CRS with nasal polyps (CRSwNP). Further phenotyping of disease to identify exacerbating co-factors or distinct forms of disease such as immunodeficiency, aspirin sensitivity or vasculitis is needed, in to order to appropriately guide therapy. The approach with nasal douching, steroid therapy and antibiotics can often benefit most forms of CRS, but different subtypes are more responsive than others. Particularly individuals with aspirin sensitivity may benefit from leukotriene receptor inhibition and intranasal aspirin desensitisation. Surgical correction of anatomical abnormalities that may predispose to exacerbation should be addressed.
Immunopathology
Chronic rhinosinusitis without nasal polyps (CRSsNP)
CRSsNP represents up to 60% of CRS cases and incorporates a heterogeneous group of disorders which can be distinct but yet can often sometimes overlap in aetiology and exacerbating factors. These include allergen driven IgE mediated inflammation, defects in both humoral and mucosal host-defence and nasal structural abnormalities. The overall immunopathogenesis is incompletely understood. Th1 dominance with a more significant neutrophilic inflammation is seen, with an associated Th1 cytokine profile of IFN-γ, TNF-α, IL-1, IL-3, IL-6, and IL-8.5,6 Immunoregulatory factors such as TGF-β1 is increased in expression and the key transcription factor implicated in Foxp3+ T regulatory cell populations appear similar to the normal nasal mucosa.7 Why such severe immune dysregulation occurs, and what sustains inflammation is currently unknown, but it is likely that there are additional immunological factors that may promote disease severity will emerge.
Tissue structural change (termed remodelling) is also present in CRSsNP with basement membrane thickening, excess goblet cell numbers, subepithelial glandular hyperplasia and hypertrophy, along with altered and excess extracellular matrix deposition.7 The exact mechanisms that lead to tissue remodelling remain unknown but one can speculate the mucosal injury-repair cycle is poorly regulated and driven to excess as a result of the inflammatory burden. However, intrinsic defects in tissue repair mechanisms may still exist in CRS and contribute to remodelling. Whether such structural change promotes and sustains the chronic inflammation in CRS is not known but very possible. It is also likely that remodelling contributes to disease severity in CRS with excess mucus production, tissue oedema with ostial obstruction and impaired mucociliary function. Extrapolating from asthma studies current anti-inflammatory strategies are often ineffective in relation to neutrophilic inflammation and have no definite effect on airway remodelling.
Chronic rhinosinusitis with nasal polyps (CRSwNP)
CRSwNP is a more distinct immunological disease. Th2 dominance with excess, IL-5, IL-4, and IL-13 expression is observed.6 IL-33 excess is associated with a recalcitrant CRS subtype.8 Mucosal eosinophilia is present in abundance.6 Th17 cells are now also implicated, especially in Chinese polyps, with increased IL-17 expression related to eosinophil count and a more severe sino-nasal CT score.9 FoxP3 expression is minimal suggesting a lack of T regulatory cell induced immunoregulation.6 In addition there is a distinct local immune response to Staphylococcus aureus enterotoxins (SAE) which act as superantigens, leading to polyclonal T cell and B cell activation, with excessive local IgE production to not only SAE but other extrinsic and intrinsic allergens and cytokine secretion. However, CRSwNP from a Chinese cohort demonstrated excessive neutrophilic inflammation with skewing towards Th1/Th17 inflammation.10 Such findings highlight the complexity of unravelling the exact events that lead to disease and the current immaturity we have in our overall understanding of such processes.
Eosinophils can lead to excessive tissue damage, but are now recognised as important in tissue repair but also remodelling.11 The observed denuded epithelium and epithelial shedding seen in CRSwNP is reminiscent of the 'chronic wound scenario' changes seen in asthmatic lower airways, where an activated epithelium can promote inflammation and sustain remodelling by continued signalling to the underlying submucosal mesenchymal cells. The findings of defective epithelial barrier function in asthma have initiated an interest in the role of epithelium in CRS where early reports of deficiency of epithelial proteins implicated in barrier function and antimicrobial defence are emerging.12
Infection
The exact role of infection in the pathogenesis and maintenance of CRS is currently under debate. Infection induced inflammation; bacterial superantigen-immune over-drive and infective osteitis have been considered as contributory in either distinct forms of CRS or as exacerbating factors in CRS with multifactorial aetiologies. In addition, there is recognition of defects in immunity, both innate and humoral. For example CF heterozygotes are over-represented in the CRS population.13 Even when immunoglobulin isotype levels are within the normal range, a high proportion of patients with recalcitrant CRSsNP fail to generate an adequate functional antibody response in response to unconjugated pneumococcal vaccination.14 Low IgG3 levels have been reported in CRS patients compared to population controls.15 Toll-like receptor (TLR)-2 is important for mucosal recognition of gram-positive bacteria and initiation of mucosal defence. TLR-2 expression is lower in CRS populations and low TLR-2 relates to earlier disease relapse post-surgery.16 It is likely that there are other yet unknown defects in humoral immunity in the sinonasal mucosa which may predispose to infection and may even exaggerate mucosal immune leading to disease exacerbation or persistence.17,18 However, the frequent lack of positive bacterial cultures from sinus cavities and variable and non-sustained response to antimicrobial therapy has led to consideration of the role of bacterial biofilms in CRS.
Bacterial biofilms
Biofilm-forming bacteria include Haemophilus, Staphylococcus and Pseudomonas species. A biofilm is considered an organised bacterial community that is characterised by adherence to a mucosal (or foreign body) surface. The bacteria are embedded within an extensive polymeric substance termed a glycocalyx. The latter encases what is often a polymicrobial mixture of bacterial colonies and modulates the bacterial microenvironment, allowing interbacterial signalling (termed quorum sensing) and supports the development of bacterial virulence factors. The glycocalyx affords structural barrier protection and evasion by host-defence systems such as phagocytosis and the complement system. Biofilm formation is confirmed in CRS19 and there is growing evidence that biofilms may contribute to the relapse, persistence and severity of certain CRS subtypes.20,21 Antibiotics can still penetrate biofilms, so the increased resistance to antimicrobial drugs is believed to be related to microbial community sharing of resistance genes and presence of slow bacterial growth conditions (thus slow metabolism).22,23 It is presumed that persistence of infection will allow continued interaction and stimulation of the mucosal immune system, for example such as enterotoxin superantigens from S. aureus leading to polyclonal T cell activation and local hyper-IgE production and activation of innate mucosal 'danger-sensing' signals such the TLR system. The interaction of the biofilm itself in both biophysical and biochemical terms with mucosal tissue can theoretically at least contribute to disease pathogenesis and persistence.
Therapeutic intervention
Douching
A recent Cochrane meta-analysis shows that simply washing the nose with saline solutions is effective in CRS.24 As yet the optimal formulation (normal/hypertonic) has not been determined.
Intranasal steroids
The ability of steroids to attenuate key aspects of the airway inflammatory response whilst supporting induction of important immunoregulatory mechanisms, has established these compounds as first line therapy in treating CRS. Whilst disease in some groups of patients will respond to such therapy, there are significant proportion of patients for whom steroids are ineffective. Thus it has become ever more important to define the exact phenotype of patients who are steroid sensitive, and study in detail the patient groups and the specific immune mechanisms that occur in the steroid unresponsive groups. The failure to define exactly CRS subtypes has led to difficulty in interpreting treatment response to therapy in several studies and as such, systematic review via meta-analysis has been limited. In fact the treatment response for steroid therapy in CRSsNP could not be defined via meta-analysis.
Chronic rhinosinusitis without nasal polyps (CRSsNP)
A systematic review of INS in CRSsNP in 2008 concluded that from published studies that incorporate all the strict criteria needed for analysis were applied, there were no articles that could be used to evaluate evidence for treatment response.25 This is disappointing and demands that well-designed studies are urgently needed. An early study in 1986 looked at the effect of Tixocortol Pivalate (a corticosteroid with topical anti-inflammatory activity equal to that of hydrocortisone) with neomycin or neomycin alone.26 The study population was divided into infective and allergic subtypes. The drug was administered via endosinus irrigation once daily for 11 days. The study confirmed significantly less nasal obstructive symptoms with improved maxillary ostia patency in the group with both the steroid and antibiotic. What this study suggested was that INS effectively treats both types of CRS but concomitant treatment of infection if it is present leads to a significantly better treatment response. The duration of treatment, the lack of a placebo controlled arm to the study and inclusion of heterogeneous CRS patients such as those with nasal polyps weaken the data in terms of any definite conclusion.
As far as we are aware there are only 4 studies since then that have incorporated randomised design (evidence level Ib) and the insights gained are discussed below. A double blind placebo controlled randomised (DBPCR) study in CRS associated with associated chronic mucopurelence evaluated the effects dexamethasone-tramozoline (decongestant) with or without neomycin versus placebo propellant only, over 14 days.27 Both treatment arms were clinically effective, and the presence of an antibiotic made no active difference. This data suggests that sinus drainage as a result of open sinus ostia is a key factor. Improved mucociliary function (measured via the saccharin clearance) improved in the treatment groups. The study is limited again in duration but also in that both atopic and non-atopic individuals were incorporated in the groups which limit numbers per CRS group and makes it difficult to say for which form of CRS such therapy is most effective. The authors state that treatment response was irrespective of IgE status. A DBPCR trial of n=9 (active) and n=13 (placebo) completed therapy over 16 weeks failed to show any effect of the INS fluticasone propionate 400 mcg per day, although two patients per group each had co-existent polyps.28 The study was insufficiently powered to detect significant clinical response.
In case treatment failure in CRS is related to poor delivery of INS into the sinus cavity, direct instillation of budesonide 256 mcg daily into the maxillary antrum over 3 weeks has been undertaken.29 In this DBPCR approach, whilst the patient cohort here is stated as unresponsive to previous medical treatment with conventional regimes, direct sinus installation of drug was associated with prolonged clinical improvement alongside decreased mucosal Th2 inflammation. However all patients here had house-dust mite related perennial rhinitis associated CRS, and thus a distinct immune phenotype that is often steroid responsive. Inhaled budesonide is also effective in CRS30 but again, whilst all patients fulfilled the clinical definition of CRS with persistent symptoms despite antibiotic therapy and nasal polyps were not present, 51% of patients were IgE positive to aeroallergens. Thus, again the exact CRS type that best responds to INS cannot be strictly confirmed from this otherwise well designed study. Fluticasone propionate (FP) is another topically active corticosteroid with established efficacy in seasonal and perennial allergic rhinitis. A recent study evaluated FP 400 mcg BD delivered via a bi-directional flow breath-activated device (Optinose) that can deliver product more effectively to the middle meatus. Whilst only n=10 patients were in the active arm, and again this population of CRSsNP with otherwise difficult to treat was heterogeneous with both atopic and aspirin-sensitive individuals, significant clinical response was seen with treatment.31 Further studies are urgently required, particularly in relation to homogenous CRSsNP populations before the exact therapeutic role of steroid therapy can be fully defined in this group. As far as we are aware there are no studies evaluating the role of systemic steroids in this group.
Chronic rhinosinusitis with nasal polyps (CRSwNP)
Intranasal steroids
The earliest study of intranasal steroids in moderate-severe nasal polyps evaluated beclomethasone dipropionate (BDP) 400 mcg daily in n=35 patients randomised to treatment versus placebo.32 After only 3 weeks there was a statistically significant improvement in nasal symptom scores.
The EPO3S document included 15 studies on the basis of level Ib evidence for the intranasal treatment of CRSwNP, and only 13 were considered of sufficient quality on the basis of strict definition of the patient group with CRSwNP, randomised double-blind placebo controlled design and defined study outcomes.2 The higher number of studies in CRSwNP that fulfil the rigid criteria reflects the better-defined nature of the disease subtype. Within these studies however, there is still a mixed heterogeneity of disease with aspirin-sensitivity, the potential for eosinophil or neutrophil predominance and the role of local infection and superantigen drive with local IgE production to consider.
Recent meta-analysis on the basis of evaluating 6 studies33-38 confirms that INS are effective in CRSwNP.25 Significant improvement in the polyp size score was seen in all six studies. The authors were unable to analyse the overall effect on nasal symptoms due to inconsistency in the methods used to score or record such changes. The authors excluded 7 studies because they could not ascertain the method of statistical analysis fully.39-43 It is therefore useful to comment on individual highlighted studies and identify important areas for further work.
The most recent studies have been in relation to mometasone furoate and confirm that clinical response in CRSwNP is steroid dose related. Two related studies with mometasone furoate at 200 mcg once daily over 16 weeks or once or twice daily over 16 weeks compared to placebo give data on dose response of nasal polyps to MF. Once daily dosing was associated with significant reductions in polyp size along with improved nasal obstruction, rhinorrhoea and sense of smell.37,38 Twice daily dosing when compared to once daily or placebo lead to greater improvement in nasal congestion, loss of smell and nasal discharge (anterior and posterior). Whilst these three studies were well designed and provide useful data, the failure to fully exclude individuals with perennial allergic rhinitis prevents the data relating solely to CRSwNP.
In an early study comparing twice daily FP 200 mcg against BDP 200 mcg BD or placebo n=55 with mild polyps (score<3) over 26 weeks only BDP reached statistical significance in reduction in polyp score and improved symptoms.41 However, both agents were clinically effective. FP group demonstrated an advantage over the BDP and placebo group on having significant greater reduction in the need for rescue medication and less nasal blockage on morning awakening.41 With patients listed for surgical polypectomy and thus a more severe group, FP 200 mcg BD group responded sooner with greater effect compared to the BDP 200 mcg BD assigned patients.44 Only the FP group reached statistical significance for decrease in total polyp score after 12 weeks of treatment. Active treatment here did not prevent the need for surgical intervention. This may suggest that spray forms of INS do not reach the middle meatus region effectively. Spray formulations are deposited mainly into the atrium of the nose. A droplet formulation can spread more extensively throughout the nasal cavity and it has been shown that three drops alone will cover most of the surface area of the nasal passage.45 It has been shown that FP 400 mcg nasules divided between each nostril taken daily42,43 or twice-daily are both clinically effective, although the twice-day regime was seen to be more significantly effective.42 In a second cohort of severe CRSwNP patients, administration of half-nasule contents daily (alternate each nostril daily) over 12 weeks lead to significant decrease in polyp volume and improvement in associated symptoms of congestion, anterior and posterior nasal discharge and smell. Importantly, only 52% of patients proceeded to surgery.46
Both the aqueous and aerosol (powder) formulations of Budesonide (Bud) have been evaluated. Duration of studies has ranged from 4 weeks39 to 8 weeks35,36. In an early DBPCR study of n=19 (small polyps) over 4 months with a total of 400 mcg of Bud as a pump spray in the active arm, changes in polyp size and number along with significantly improved nasal inspiratory flow and symptom scores were confirmed.47 This is a potent steroid and other studies administering in powder form via a turbohaler device (800 mcg or 400 mcg daily, with no significant dose-dependent difference in clinical effect ),33,34 aqueous form 400 mcg daily40 and 800 mcg daily47 are clinically effective.
Systemic steroids
The mechanical obstruction conferred by NPs may potentially prevent the deposition of INS in the nasal mucosa and sinus cavity. In addition, the inflammatory burden maybe so intense in such patients that oral steroid has become first line therapy in such severe individuals. Systematic review48 is again limited to only one study.49 Here, oral prednisolone at 30 mg for 3 days and then 5 mg dose reduction every 2 days over 14 days in a severe CRSwNP group significantly diminished polyp volume with less nasal obstruction and improved olfaction. These improvements were then sustained with intranasal budesonide. In a separate study, high dose prednisolone at 50 mg for 14 days gave marked clinical improvement, highly correlated with the reduction in endoscopy and MRI scores of disease severity. Prednisolone did not confer any significantly greater adverse event compared to a placebo in severe CRSwNP.50
Leukotriene inhibitors
Chronic rhinosinusitis with/without nasal polyps
Open studies suggest that anti-leukotrienes might be of benefit51-53 Anti-leukotriene treatment in 36 patients with CRS with or without NP, added to standard treatment, resulted in statistically significant improvement in scores for headache, facial pain and pressure, ear discomfort, dental pain, purulent nasaldischarge, postnasal drip, nasal congestion and obstruction, olfaction, and fever. Overall improvement was noted by 72% of the patients and side-effects occurred in 11%.54 In 15 aspirin-sensitive asthma triad patients, addition of an anti-leukotriene resulted in 9 with sinusitis experiencing improvement and over-all benefit in 12.55 In 44 patients with nasal polyposis, significant subjective improvement in nasal symptoms occurred in 64% aspirin tolerant and 50% aspirin sensitive patients. Significant improvement in peak flow occurred only in the aspirin tolerant patients, while acoustic rhinometry, nasal inspiratory peak flow and nitric oxide levels were unchanged.51 A prospective double blind comparative study on 40 patients compared the effects of the leukotriene receptor antagonist, monteleukast and beclomethasone nasal spray on the post-operative course of patients with sinonasal polyps. No significant differences were found in the year after surgery.56 It is apparent that certain patients respond well to anti-leukotrienes, whilst others do not. The reasons for this are gradually becoming apparent57 but at present pharmacogenetic testing is not easily available. Thus a simple therapeutic trial for one month with objective and subjective monitoring is suggested, except in those patients where Churg Strauss syndrome is likely.57
Aspirin desentisation
Chronic rhinosinusitis without nasal polyps
There is no evidence.
Chronic rhinosinusitis with nasal polyps
a) Aspirin tolerant
A DBPC trial of topical nasal lysine-aspirin plus FP spray 400 mcg daily conferred no greater benefit than FP alone and reduced quality of life.58 This suggest that the simple anti-inflammatory effect of aspirin is not indicated for all polyps.
b) Aspirin intolerant (aspirin exacerbated respiratory disease)
Systemic aspirin desensitisation or topical lysine-aspirin treatment may be effective.
Sixty-five aspirin-sensitive patients with nasal polyps and asthma underwent oral aspirin desensitization and subsequent daily aspirin treatment over 1 to 6 years (mean 3.1 years). There were significant reductions in sinus infections and an improvement in olfaction. Numbers of sinus and polyp operations and doses of nasal corticosteroids per year were significantly reduced. There were also reductions in hospitalizations for treatment of asthma and use of systemic corticosteroids.59-61
Nucera et al.62 followed three groups of patients with nasal polyposis (half of whom were aspirin sensitive): the first 76 consecutive nasal polypectomy patients given topical nasal lysine aspirin afterwards, the second 49 patients with 40 mg triamcinolone retard ("medical polypectomy") and subsequent nasal lysine aspirin and the third 191 control patients who underwent polypectomy but received no placebo. The group treated with lysine-acetylsalicylate postoperatively had a recurrence rate of 6.9% after l year and 65% after six years post-operatively, while controls experienced recurrence in 51.3% at l year and 93.5% at six years, indicating significant protection against recurrence by lysine-aspirin. Systemic corticosteroid therapy and nasal lysine-aspirin resulted in 33% with unchanged polyp size after three years compared to 15% in the operated-not treated group, but this was not statistically significant.62 A case-controlled trial of treatment with lysine aspirin to one nostril and placebo to the other in 13 patients with bilateral nasal polyposis resulted in delayed polyp recurrence and 8 remained symptom free at 15 months, significantly better than previous treatment with nasal steroid.63
A small DBPCR trial in aspirin sensitive patients did not demonstrate any effect on the nasal airway using 16 mg of intranasal lysine aspirin every 48 hours after 6 months.58 However immunohistochemistry revealed significant decrease of CyslLT 1 receptor in the turbinate mucosa of the patients with active treatment, compared to placebo, so further trials were suggested (REF). Addition of nasal lysine aspirin (60 mg) to the routine regime of 13 previously poorly controlled nasal polyp patients reduced polyp size and did not adversely affect asthma.64 Intranasal lysine acetyl salicylate administration decreases polyp volume in patients with aspirin intolerant asthma.64 An audit of our experience in over 100 patients gradually up-dosed to doses ranging from 60 to 100 mg aspirin reveals benefit in the nasal airway and, after 12 months, in exhaled nitric oxide measures which in turn reflects sustained sinus ventilation, particularly in those with positive skin prick tests. Asthma outcomes were significantly better in 22 patients with aspirin exacerbated respiratory disease who took lysine aspirin over 12 months compared to 20 who were positive on challenge but either refused or rejected therapy because of side effects. These were mostly experienced during up-dosing and included local irritation, increased nasal and sometimes asthma symptoms and occasional gastric irritation (Scadding, manuscript in preparation). The mechanism of desensitisation probably involves reduction of leukotriene receptors and thus attenuation in cellular leukotriene responsiveness.65
Antibiotics
The recognition of CRS as multifactorial in origin and not simply a persistent bacterial infection has lead to a re-evaluation of the role of antibiotics in treatment. It is appreciated that impaired sinus drainage can lead to secondary bacterial infection in all CRS forms, and patients benefit from antibiotic intervention for acute exacerbations of CRS. However, the role of antibiotics beyond this role is currently under debate. The use of antibiotics in CRS can be examined in terms of 'short term' and 'long term' use. However, the evidence base for such use is not clear cut, and has been hampered by clinical studies where the patient groups within CRS are again not clearly defined and often lack radiological confirmation of disease. In addition there are only a limited number of DBPCR trials evaluating such intervention.
Short term treatment
There is no clinical evidence that short term use of antibiotics have any effect in CRS. These findings must interpreted along-side factors such as biofilm formation that may protect bacteria from antibiotic concentrations that lead to bacterial death on the culture plate having no effect in a biofilm niche. Furthermore, only a very limited number of studies exist and the study design is such that only limited interpretation is possible. The earliest study incorporated 15 patients with CRS (with no details of CRS subtype) randomised to either cefaclor or amoxicillin with no placebo arm. After 10 days of treatment no clinical effect was seen in the CRS group.66 There was no statistical evaluation. Legent et al.67 evaluated the effect of ciproxin against amoxicillin/clavulanic acid over 9 days in an undefined heterogeneous population of CRS patients with mucopurulent discharge. The other was an open randomised study with amoxicillin/clavulanic acid against oral cefuroxime over 14 days.68 The lack of placebo arms in these studies diminishes the significance of the clinical response these may have had, and overall were negative studies in that they did not detect statistically significant differences between the treatment arms. The remaining studies are retrospective analysis of patient clinical outcomes in relation to 4 or 6 week antibiotic therapy,69,70 and whilst reporting improvement in clinical parameters and of CT appearance,70 the studies lack methodological robustness to confer any definite conclusion.
Long term treatment
Diffuse panbronchiolitis (DPB) is a disease seen mainly in Japanese populations and is rapidly fatal if untreated. It is characterised by a progressive inflammatory obliteration of the small airways by lymphoid and scar tissue and is frequently associated with Pseudomonas aeruginosa co-infection. It is one of the sino-bronchial syndromes with a high incidence of CRS. The introduction of macrolides for DPB has reduced the mortality of the disease from 90% to around 10%. The remarkable clinical efficacy of erythromycin in DPB, even when infecting microbial strains were antibiotic resistant, immediately suggested that macrolides have additional disease modifying features in the airway. Macrolides are clinically active as a result of their macrocyclic lactone ring. The potent immunosuppressant tacrolimus (FK506) and sirolimus are also macrolide compounds as a result of their macrocyclic lactone structure. Early reports since have suggested that both low dose erythromycin and clarithromycin can benefit primary CRS71 and these reports have been followed up with DBPCR studies evaluating the role of long term macrolides in CRS. The first randomised prospective study evaluated erythromycin over a 3 month period in CRS along with douching and INS, against just surgical intervention followed by INS with nasal douching in patients with persistent CRS.72 Both the CRSsNP and CRSwNP groups demonstrated improvement in the visual analogue score similar to the surgical group at both 6 and 12 months of follow up. Only total nasal volume was greater in the surgery group. This study thus suggests medical treatment for 3 months is indicated in both forms of CRS, and should be considered before surgery. A DBPCR trial with roxithromycin over 3 months versus placebo in a more homogenous population of CRSsNP patients demonstrated significant improvement in olfaction, ciliary function, nasal flow and the SNOT-22 score of treatment.73 Interestingly the group with low serum IgE in particular demonstrated a significant improvement in ciliary function, SNOT-22 along with decreased nasal lavage IL-8 levels. This is interesting as it suggests a particular CRS-sub-phenotype will respond better to long term macrolides therapy and provides a mechanistic insight into immunopathogenesis.
Erythromycin, clarithromycin and roxithromycin are all 14-membered ring compounds and this structure may be relevant as the 16-carbon ring macrolide josamycin has been ineffective in DPB.74 Studies with the 15-membered ring structure compounds such as Azithromycin are awaited. The exact mechanism(s) as to how low-dose long term macrolides may confer treatment efficacy is still under debate. The macrolide mode of action is through the inhibition of bacterial protein biosynthesis. By reversibly binding to the bacterial ribosome 50S subunit, inhibition of peptidyl-tRNA translocation occurs. Macrolides have a propensity to accumulate within leukocytes, and therefore are automatically translocated to sites of infection and inflammation.75 The therapeutic effects of macrolides are not the simple result of the bactericidal effects of 50S ribosomal subunit suppression, as has been shown by the numerous studies in cystic fibrosis.76 Conventionally gram negative bacteria such as pseudomonas are macrolide resistant. Thus effects on both immune modulation and attenuation of bacterial virulence factors are considered important. The immunomodulatory role of macrolides is now evident with in-vivo confirmation on inhibiting neutrophil chemotaxis into tissue in response to LPS.77 and neutrophil activation as evidenced by less free radical production.78 Macrolides (14/15 member ring) directly suppress cytokine transcription via targeting factors NFκ-β and AP-1,79,80 and decrease levels of key neutrophil chemoattractant IL-8, as well as IL-4, IL-5, and GM-CSF.81 LTB4 (neutrophil chemoattractant) production is inhibited by erythromycin at least in DPB.82
Microbial virulence is determined by both extracellular and cell-associated factors. Macrolides can directly inhibit such bacterial survival strategies. Clarithromycin can inhibit biofilm synthesis in P. aeruginosa in a dose-dependent manner again without any bactericidal activity against the bacterium.83 Biofilms facilitate "quorum-sensing", allowing bacteria to effectively coordinate the 'on-off' switch of such virulence genes through the production of auto-inducer molecules. Macrolides act as Pseudomonas quorum-sensing inhibitors.
Doxycycline is a member of the tetracycline antibiotic group and is commonly used to treat a variety of infections. Doxycycline can also confer immunomodulation at sub-antimicrobial doses and has been used to treat airway diseases such sarcoidosis. Doxycycline is a potent inhibitor of matrix metalloproteases.84 A recent DBPC trial of doxycycline in CRSwNP over 20 days compared to methylprednisolone or placebo85 provides some interesting data. As expected, the inflammatory biomarkers in nasal secretion IL-5 (Th2 cytokine essential for eosinophil recruitment and survival), eosinophil cationic protein (ECP) and IgE were attenuated in the steroid arm, but interestingly myeloperoxidase and MMP-9 along with ECP decreased in the doxycycline arm. Nasal polyp score decreased early on in the steroid group, whilst the moderate but significant decrease in relation to doxycycline was only evident just after 7 days of treatment and sustained up to 12 weeks post-treatment. Only a significant decrease in post-nasal drip was noted with doxycycline, whilst the steroid arm demonstrated significant improvement in congestion, post-nasal drip and olfaction. Further studies are needed.
Topical antibiotics
The findings that serum antibiotic concentration of 1,000 times the minimal inhibitory concentration (MIC) of moxifloxacin that can be achieved using oral therapy was required to inhibit an in-vitro model of S. aureus biofilm growth has led to consideration of topical antibiotic therapy directly to the sinus mucosa.86 In a group of treatment resistant CRS cases with S. aureus culture positive disease, topical irrigation with mupirocin 0.05% improved both symptom scores and endoscopic findings, only after 3 weeks of treatment. Nearly all the participants were culture negative after treatment.87 In recalcitrant CRS this topical approach with mupirocin is undertaken.
CONCLUSION
An understanding of the detailed immune-pathogenesis of the various forms of CRS is needed. This will allow molecular phenotyping into further several distinct mechanistic subtypes that will reveal specific therapeutic approaches. Until then, the current broad-based approach with general anti-inflammatory and anti-microbial strategies will continue. The use of nasal douching appears to be almost uniformly effective and should be the first line therapy.
Footnotes
There are no financial or other issues that might lead to conflict of interest.
References
- 1.Kaliner MA, Osguthorpe JD, Fireman P, Anon J, Georgitis J, Davis ML, Naclerio R, Kennedy D. Sinusitis: bench to bedside. Current findings, future directions. Otolaryngol Head Neck Surg. 1997;116:S1–S20. [PubMed] [Google Scholar]
- 2.Fokkens W, Lund V, Mullol J. European position paper on rhinosinusitis and nasal polyps 2007. Rhinol Suppl. 2007:1–136. [PubMed] [Google Scholar]
- 3.Meltzer EO, Hamilos DL, Hadley JA, Lanza DC, Marple BF, Nicklas RA, Bachert C, Baraniuk J, Baroody FM, Benninger MS, Brook I, Chowdhury BA, Druce HM, Durham S, Ferguson B, Gwaltney JM, Kaliner M, Kennedy DW, Lund V, Naclerio R, Pawankar R, Piccirillo JF, Rohane P, Simon R, Slavin RG, Togias A, Wald ER, Zinreich SJ. Rhinosinusitis: establishing definitions for clinical research and patient care. J Allergy Clin Immunol. 2004;114:155–212. doi: 10.1016/j.jaci.2004.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Min YG. The pathophysiology, diagnosis and treatment of allergic rhinitis. Allergy Asthma Immunol Res. 2010;2:65–76. doi: 10.4168/aair.2010.2.2.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Van Zele T, Claeys S, Gevaert P, Van Maele G, Holtappels G, Van Cauwenberge P, Bachert C. Differentiation of chronic sinus diseases by measurement of inflammatory mediators. Allergy. 2006;61:1280–1289. doi: 10.1111/j.1398-9995.2006.01225.x. [DOI] [PubMed] [Google Scholar]
- 6.Van Bruaene N, Pérez-Novo CA, Basinski TM, Van Zele T, Holtappels G, De Ruyck N, Schmidt-Weber C, Akdis C, Van Cauwenberge P, Bachert C, Gevaert P. T-cell regulation in chronic paranasal sinus disease. J Allergy Clin Immunol. 2008;121:1435–1441. 1441.e1–1441.e3. doi: 10.1016/j.jaci.2008.02.018. [DOI] [PubMed] [Google Scholar]
- 7.Van Bruaene N, Derycke L, Perez-Novo CA, Gevaert P, Holtappels G, De Ruyck N, Cuvelier C, Van Cauwenberge P, Bachert C. TGF-beta signaling and collagen deposition in chronic rhinosinusitis. J Allergy Clin Immunol. 2009;124:253–259. 259.e1–259.e2. doi: 10.1016/j.jaci.2009.04.013. [DOI] [PubMed] [Google Scholar]
- 8.Reh DD, Wang Y, Ramanathan M, Jr, Lane AP. Treatment-recalcitrant chronic rhinosinusitis with polyps is associated with altered epithelial cell expression of interleukin-33. Am J Rhinol Allergy. 2010;24:105–109. doi: 10.2500/ajra.2010.24.3446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Makihara S, Okano M, Fujiwara T, Kariya S, Noda Y, Higaki T, Nishizaki K. Regulation and characterization of IL-17A expression in patients with chronic rhinosinusitis and its relationship with eosinophilic inflammation. J Allergy Clin Immunol. 2010;126:397–400. 400.e1–400.e11. doi: 10.1016/j.jaci.2010.05.014. [DOI] [PubMed] [Google Scholar]
- 10.Zhang N, Van Zele T, Perez-Novo C, Van Bruaene N, Holtappels G, DeRuyck N, Van Cauwenberge P, Bachert C. Different types of T-effector cells orchestrate mucosal inflammation in chronic sinus disease. J Allergy Clin Immunol. 2008;122:961–968. doi: 10.1016/j.jaci.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 11.Kariyawasam HH, Robinson DS. The role of eosinophils in airway tissue remodelling in asthma. Curr Opin Immunol. 2007;19:681–686. doi: 10.1016/j.coi.2007.07.021. [DOI] [PubMed] [Google Scholar]
- 12.Tieu DD, Peters AT, Carter RG, Suh L, Conley DB, Chandra R, Norton J, Grammer LC, Harris KE, Kato A, Kern RC, Schleimer RP. Evidence for diminished levels of epithelial psoriasin and calprotectin in chronic rhinosinusitis. J Allergy Clin Immunol. 2010;125:667–675. doi: 10.1016/j.jaci.2009.11.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang X, Moylan B, Leopold DA, Kim J, Rubenstein RC, Togias A, Proud D, Zeitlin PL, Cutting GR. Mutation in the gene responsible for cystic fibrosis and predisposition to chronic rhinosinusitis in the general population. JAMA. 2000;284:1814–1819. doi: 10.1001/jama.284.14.1814. [DOI] [PubMed] [Google Scholar]
- 14.Alqudah M, Graham SM, Ballas ZK. High prevalence of humoral immunodeficiency patients with refractory chronic rhinosinusitis. Am J Rhinol Allergy. 2010;24:409–412. doi: 10.2500/ajra.2010.24.3532. [DOI] [PubMed] [Google Scholar]
- 15.Vanlerberghe L, Joniau S, Jorissen M. The prevalence of humoral immunodeficiency in refractory rhinosinusitis: a retrospective analysis. B-ENT. 2006;2:161–166. [PubMed] [Google Scholar]
- 16.Lane AP, Truong-Tran QA, Schleimer RP. Altered expression of genes associated with innate immunity and inflammation in recalcitrant rhinosinusitis with polyps. Am J Rhinol. 2006;20:138–144. [PMC free article] [PubMed] [Google Scholar]
- 17.Cormier C, Bossé Y, Mfuna L, Hudson TJ, Desrosiers M. Polymorphisms in the tumour necrosis factor alpha-induced protein 3 (TNFAIP3) gene are associated with chronic rhinosinusitis. J Otolaryngol Head Neck Surg. 2009;38:133–141. [PubMed] [Google Scholar]
- 18.Kilty SJ, Bossé Y, Cormier C, Endam LM, Desrosiers MY. Polymorphisms in the SERPINA1 (Alpha-1-Antitrypsin) gene are associated with severe chronic rhinosinusitis unresponsive to medical therapy. Am J Rhinol Allergy. 2010;24:e4–e9. doi: 10.2500/ajra.2010.24.3429. [DOI] [PubMed] [Google Scholar]
- 19.Cryer J, Schipor I, Perloff JR, Palmer JN. Evidence of bacterial biofilms in human chronic sinusitis. ORL J Otorhinolaryngol Relat Spec. 2004;66:155–158. doi: 10.1159/000079994. [DOI] [PubMed] [Google Scholar]
- 20.Bendouah Z, Barbeau J, Hamad WA, Desrosiers M. Biofilm formation by Staphylococcus aureus and Pseudomonas aeruginosa is associated with an unfavorable evolution after surgery for chronic sinusitis and nasal polyposis. Otolaryngol Head Neck Surg. 2006;134:991–996. doi: 10.1016/j.otohns.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 21.Psaltis AJ, Weitzel EK, Ha KR, Wormald PJ. The effect of bacterial biofilms on post-sinus surgical outcomes. Am J Rhinol. 2008;22:1–6. doi: 10.2500/ajr.2008.22.3119. [DOI] [PubMed] [Google Scholar]
- 22.Donlan RM, Costerton JW. Biofilms: survival mechanisms of clinically relevant microorganisms. Clin Microbiol Rev. 2002;15:167–193. doi: 10.1128/CMR.15.2.167-193.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stewart PS, Costerton JW. Antibiotic resistance of bacteria in biofilms. Lancet. 2001;358:135–138. doi: 10.1016/s0140-6736(01)05321-1. [DOI] [PubMed] [Google Scholar]
- 24.Harvey R, Hannan SA, Badia L, Scadding G. Nasal saline irrigations for the symptoms of chronic rhinosinusitis. Cochrane Database Syst Rev. 2007:CD006394. doi: 10.1002/14651858.CD006394.pub2. [DOI] [PubMed] [Google Scholar]
- 25.Joe SA, Thambi R, Huang J. A systematic review of the use of intranasal steroids in the treatment of chronic rhinosinusitis. Otolaryngol Head Neck Surg. 2008;139:340–347. doi: 10.1016/j.otohns.2008.05.628. [DOI] [PubMed] [Google Scholar]
- 26.Cuenant G, Stipon JP, Plante-Longchamp G, Baudoin C, Guerrier Y. Efficacy of endonasal neomycin-tixocortol pivalate irrigation in the treatment of chronic allergic and bacterial sinusitis. ORL J Otorhinolaryngol Relat Spec. 1986;48:226–232. doi: 10.1159/000275873. [DOI] [PubMed] [Google Scholar]
- 27.Sykes DA, Wilson R, Chan KL, Mackay IS, Cole PJ. Relative importance of antibiotic and improved clearance in topical treatment of chronic mucopurulent rhinosinusitis. A controlled study. Lancet. 1986;2:359–360. doi: 10.1016/s0140-6736(86)90051-6. [DOI] [PubMed] [Google Scholar]
- 28.Parikh A, Scadding GK, Darby Y, Baker RC. Topical corticosteroids in chronic rhinosinusitis: a randomized, double-blind, placebo-controlled trial using fluticasone propionate aqueous nasal spray. Rhinology. 2001;39:75–79. [PubMed] [Google Scholar]
- 29.Lavigne F, Cameron L, Renzi PM, Planet JF, Christodoulopoulos P, Lamkioued B, Hamid Q. Intrasinus administration of topical budesonide to allergic patients with chronic rhinosinusitis following surgery. Laryngoscope. 2002;112:858–864. doi: 10.1097/00005537-200205000-00015. [DOI] [PubMed] [Google Scholar]
- 30.Lund VJ, Black JH, Szabó LZ, Schrewelius C, Akerlund A. Efficacy and tolerability of budesonide aqueous nasal spray in chronic rhinosinusitis patients. Rhinology. 2004;42:57–62. [PubMed] [Google Scholar]
- 31.Hansen FS, Djupesland PG, Fokkens WJ. Preliminary efficacy of fluticasone delivered by a novel device in recalcitrant chronic rhinosinusitis. Rhinology. 2010;48:292–299. doi: 10.4193/Rhino09.178. [DOI] [PubMed] [Google Scholar]
- 32.Mygind N, Pedersen CB, Prytz S, Sørensen H. Treatment of nasal polyps with intranasal beclomethasone dipropionate aerosol. Clin Allergy. 1975;5:159–164. doi: 10.1111/j.1365-2222.1975.tb01848.x. [DOI] [PubMed] [Google Scholar]
- 33.Tos M, Svendstrup F, Arndal H, Orntoft S, Jakobsen J, Borum P, Schrewelius C, Larsen PL, Clement F, Barfoed C, Rømeling F, Tvermosegaard T. Efficacy of an aqueous and a powder formulation of nasal budesonide compared in patients with nasal polyps. Am J Rhinol. 1998;12:183–189. doi: 10.2500/105065898781390217. [DOI] [PubMed] [Google Scholar]
- 34.Lildholdt T, Rundcrantz H, Lindqvist N. Efficacy of topical corticosteroid powder for nasal polyps: a double-blind, placebo-controlled study of budesonide. Clin Otolaryngol Allied Sci. 1995;20:26–30. doi: 10.1111/j.1365-2273.1995.tb00007.x. [DOI] [PubMed] [Google Scholar]
- 35.Filiaci F, Passali D, Puxeddu R, Schrewelius C. A randomized controlled trial showing efficacy of once daily intranasal budesonide in nasal polyposis. Rhinology. 2000;38:185–190. [PubMed] [Google Scholar]
- 36.Jankowski R, Schrewelius C, Bonfils P, Saban Y, Gilain L, Prades JM, Strunski V. Efficacy and tolerability of budesonide aqueous nasal spray treatment in patients with nasal polyps. Arch Otolaryngol Head Neck Surg. 2001;127:447–452. doi: 10.1001/archotol.127.4.447. [DOI] [PubMed] [Google Scholar]
- 37.Small CB, Hernandez J, Reyes A, Schenkel E, Damiano A, Stryszak P, Staudinger H, Danzig M. Efficacy and safety of mometasone furoate nasal spray in nasal polyposis. J Allergy Clin Immunol. 2005;116:1275–1281. doi: 10.1016/j.jaci.2005.07.027. [DOI] [PubMed] [Google Scholar]
- 38.Stjärne P, Blomgren K, Cayé-Thomasen P, Salo S, Søderstrøm T. The efficacy and safety of once-daily mometasone furoate nasal spray in nasal polyposis: a randomized, double-blind, placebo-controlled study. Acta Otolaryngol. 2006;126:606–612. doi: 10.1080/00016480500452566. [DOI] [PubMed] [Google Scholar]
- 39.Ruhno J, Andersson B, Denburg J, Anderson M, Hitch D, Lapp P, Vanzieleghem M, Dolovich J. A double-blind comparison of intranasal budesonide with placebo for nasal polyposis. J Allergy Clin Immunol. 1990;86:946–953. doi: 10.1016/s0091-6749(05)80158-7. [DOI] [PubMed] [Google Scholar]
- 40.Vendelo Johansen L, Illum P, Kristensen S, Winther L, Vang Petersen S, Synnerstad B. The effect of budesonide (Rhinocort) in the treatment of small and medium-sized nasal polyps. Clin Otolaryngol Allied Sci. 1993;18:524–527. doi: 10.1111/j.1365-2273.1993.tb00628.x. [DOI] [PubMed] [Google Scholar]
- 41.Holmberg K, Juliusson S, Balder B, Smith DL, Richards DH, Karlsson G. Fluticasone propionate aqueous nasal spray in the treatment of nasal polyposis. Ann Allergy Asthma Immunol. 1997;78:270–276. doi: 10.1016/s1081-1206(10)63180-8. [DOI] [PubMed] [Google Scholar]
- 42.Penttilä M, Poulsen P, Hollingworth K, Holmström M. Dose-related efficacy and tolerability of fluticasone propionate nasal drops 400 microg once daily and twice daily in the treatment of bilateral nasal polyposis: a placebo-controlled randomized study in adult patients. Clin Exp Allergy. 2000;30:94–102. doi: 10.1046/j.1365-2222.2000.00695.x. [DOI] [PubMed] [Google Scholar]
- 43.Keith P, Nieminen J, Hollingworth K, Dolovich J. Efficacy and tolerability of fluticasone propionate nasal drops 400 microgram once daily compared with placebo for the treatment of bilateral polyposis in adults. Clin Exp Allergy. 2000;30:1460–1468. doi: 10.1046/j.1365-2222.2000.00932.x. [DOI] [PubMed] [Google Scholar]
- 44.Lund VJ, Flood J, Sykes AP, Richards DH. Effect of fluticasone in severe polyposis. Arch Otolaryngol Head Neck Surg. 1998;124:513–518. doi: 10.1001/archotol.124.5.513. [DOI] [PubMed] [Google Scholar]
- 45.Hardy JG, Lee SW, Wilson CG. Intranasal drug delivery by spray and drops. J Pharm Pharmacol. 1985;37:294–297. doi: 10.1111/j.2042-7158.1985.tb05069.x. [DOI] [PubMed] [Google Scholar]
- 46.Aukema AA, Mulder PG, Fokkens WJ. Treatment of nasal polyposis and chronic rhinosinusitis with fluticasone propionate nasal drops reduces need for sinus surgery. J Allergy Clin Immunol. 2005;115:1017–1023. doi: 10.1016/j.jaci.2004.12.1144. [DOI] [PubMed] [Google Scholar]
- 47.Holopainen E, Grahne B, Malmberg H, Mäkinien J, Lindqvist N. Budesonide in the treatment of nasal polyposis. Eur J Respir Dis Suppl. 1982;122:221–228. [PubMed] [Google Scholar]
- 48.Patiar S, Reece P. Oral steroids for nasal polyps. Cochrane Database Syst Rev. 2007:CD005232. doi: 10.1002/14651858.CD005232.pub2. [DOI] [PubMed] [Google Scholar]
- 49.Benítez P, Alobid I, de Haro J, Berenguer J, Bernal-Sprekelsen M, Pujols L, Picado C, Mullol J. A short course of oral prednisone followed by intranasal budesonide is an effective treatment of severe nasal polyps. Laryngoscope. 2006;116:770–775. doi: 10.1097/01.mlg.0000205218.37514.0f. [DOI] [PubMed] [Google Scholar]
- 50.Hissaria P, Smith W, Wormald PJ, Taylor J, Vadas M, Gillis D, Kette F. Short course of systemic corticosteroids in sinonasal polyposis: a double-blind, randomized, placebo-controlled trial with evaluation of outcome measures. J Allergy Clin Immunol. 2006;118:128–133. doi: 10.1016/j.jaci.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 51.Ragab S, Parikh A, Darby YC, Scadding GK. An open audit of montelukast, a leukotriene receptor antagonist, in nasal polyposis associated with asthma. Clin Exp Allergy. 2001;31:1385–1391. doi: 10.1046/j.1365-2222.2001.01160.x. [DOI] [PubMed] [Google Scholar]
- 52.Kieff DA, Busaba NY. Efficacy of montelukast in the treatment of nasal polyposis. Ann Otol Rhinol Laryngol. 2005;114:941–945. doi: 10.1177/000348940511401209. [DOI] [PubMed] [Google Scholar]
- 53.Dahlén B, Nizankowska E, Szczeklik A, Zetterström O, Bochenek G, Kumlin M, Mastalerz L, Pinis G, Swanson LJ, Boodhoo TI, Wright S, Dubé LM, Dahlén SE. Benefits from adding the 5-lipoxygenase inhibitor zileuton to conventional therapy in aspirin-intolerant asthmatics. Am J Respir Crit Care Med. 1998;157:1187–1194. doi: 10.1164/ajrccm.157.4.9707089. [DOI] [PubMed] [Google Scholar]
- 54.Parnes SM, Chuma AV. Acute effects of antileukotrienes on sinonasal polyposis and sinusitis. Ear Nose Throat J. 2000;79:18–20. 24–25. [PubMed] [Google Scholar]
- 55.Ulualp SO, Sterman BM, Toohill RJ. Antileukotriene therapy for the relief of sinus symptoms in aspirin triad disease. Ear Nose Throat J. 1999;78:604–606. 608, 613. passim. [PubMed] [Google Scholar]
- 56.Mostafa BE, Abdel Hay H, Mohammed HE, Yamani M. Role of leukotriene inhibitors in the postoperative management of nasal polyps. ORL J Otorhinolaryngol Relat Spec. 2005;67:148–153. doi: 10.1159/000086016. [DOI] [PubMed] [Google Scholar]
- 57.Scadding GW, Scadding GK. Recent advances in antileukotriene therapy. Curr Opin Allergy Clin Immunol. 2010;10:370–376. doi: 10.1097/ACI.0b013e32833bfa20. [DOI] [PubMed] [Google Scholar]
- 58.Parikh AA, Scadding GK. Intranasal lysine-aspirin in aspirin-sensitive nasal polyposis: a controlled trial. Laryngoscope. 2005;115:1385–1390. doi: 10.1097/01.MLG.0000166702.38850.1B. [DOI] [PubMed] [Google Scholar]
- 59.Stevenson DD, Hankammer MA, Mathison DA, Christiansen SC, Simon RA. Aspirin desensitization treatment of aspirin-sensitive patients with rhinosinusitis-asthma: long-term outcomes. J Allergy Clin Immunol. 1996;98:751–758. doi: 10.1016/s0091-6749(96)70123-9. [DOI] [PubMed] [Google Scholar]
- 60.Stevenson DD. Aspirin desensitization in patients with AERD. Clin Rev Allergy Immunol. 2003;24:159–168. doi: 10.1385/CRIAI:24:2:159. [DOI] [PubMed] [Google Scholar]
- 61.Mardiney M, Borish L. Aspirin desensitization for chronic hyperplastic sinusitis, nasal polyposis, and asthma triad. Arch Otolaryngol Head Neck Surg. 2001;127:1287. [PubMed] [Google Scholar]
- 62.Nucera E, Schiavino D, Milani A, Del Ninno M, Misuraca C, Buonomo A, D'Ambrosio C, Paludetti G, Patriarca G. Effects of lysine-acetylsalicylate (LAS) treatment in nasal polyposis: two controlled long term prospective follow up studies. Thorax. 2000;55(Suppl 2):S75–S78. doi: 10.1136/thorax.55.suppl_2.S75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Scadding GK, Hassab M, Darby YC, Lund VJ, Freedman A. Intranasal lysine aspirin in recurrent nasal polyposis. Clin Otolaryngol Allied Sci. 1995;20:561–563. doi: 10.1111/j.1365-2273.1995.tb01603.x. [DOI] [PubMed] [Google Scholar]
- 64.Ogata N, Darby Y, Scadding G. Intranasal lysine-aspirin administration decreases polyp volume in patients with aspirin-intolerant asthma. J Laryngol Otol. 2007;121:1156–1160. doi: 10.1017/S0022215107000515. [DOI] [PubMed] [Google Scholar]
- 65.Sousa AR, Parikh A, Scadding G, Corrigan CJ, Lee TH. Leukotriene-receptor expression on nasal mucosal inflammatory cells in aspirin-sensitive rhinosinusitis. N Engl J Med. 2002;347:1493–1499. doi: 10.1056/NEJMoa013508. [DOI] [PubMed] [Google Scholar]
- 66.Sydnor TA, Jr, Scheld WM, Gwaltney J, Jr, Nielsen RW, Huck W, Therasse DG. Loracarbef (LY 163892) vs amoxicillin/clavulanate in bacterial maxillary sinusitis. Ear Nose Throat J. 1992;71:225–232. [PubMed] [Google Scholar]
- 67.Legent F, Bordure P, Beauvillain C, Berche P. A double-blind comparison of ciprofloxacin and amoxycillin/clavulanic acid in the treatment of chronic sinusitis. Chemotherapy. 1994;40(Suppl 1):8–15. doi: 10.1159/000239310. [DOI] [PubMed] [Google Scholar]
- 68.Namyslowski G, Misiolek M, Czecior E, Malafiej E, Orecka B, Namyslowski P, Misiolek H. Comparison of the efficacy and tolerability of amoxycillin/clavulanic acid 875 mg b.i.d. with cefuroxime 500 mg b.i.d. in the treatment of chronic and acute exacerbation of chronic sinusitis in adults. J Chemother. 2002;14:508–517. doi: 10.1179/joc.2002.14.5.508. [DOI] [PubMed] [Google Scholar]
- 69.McNally PA, White MV, Kaliner MA. Sinusitis in an allergist's office: analysis of 200 consecutive cases. Allergy Asthma Proc. 1997;18:169–175. doi: 10.2500/108854197778984374. [DOI] [PubMed] [Google Scholar]
- 70.Subramanian HN, Schechtman KB, Hamilos DL. A retrospective analysis of treatment outcomes and time to relapse after intensive medical treatment for chronic sinusitis. Am J Rhinol. 2002;16:303–312. [PubMed] [Google Scholar]
- 71.Hashiba M, Baba S. Efficacy of long-term administration of clarithromycin in the treatment of intractable chronic sinusitis. Acta Otolaryngol Suppl. 1996;525:73–78. [PubMed] [Google Scholar]
- 72.Ragab SM, Lund VJ, Scadding G. Evaluation of the medical and surgical treatment of chronic rhinosinusitis: a prospective, randomised, controlled trial. Laryngoscope. 2004;114:923–930. doi: 10.1097/00005537-200405000-00027. [DOI] [PubMed] [Google Scholar]
- 73.Wallwork B, Coman W, Mackay-Sim A, Greiff L, Cervin A. A double-blind, randomized, placebo-controlled trial of macrolide in the treatment of chronic rhinosinusitis. Laryngoscope. 2006;116:189–193. doi: 10.1097/01.mlg.0000191560.53555.08. [DOI] [PubMed] [Google Scholar]
- 74.Wales D, Woodhead M. The anti-inflammatory effects of macrolides. Thorax. 1999;54(Suppl 2):S58–S62. doi: 10.1136/thx.54.2008.s58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tamaoki J. The effects of macrolides on inflammatory cells. Chest. 2004;125:41S–50S. doi: 10.1378/chest.125.2_suppl.41s. [DOI] [PubMed] [Google Scholar]
- 76.Cai Y, Chai D, Wang R, Bai N, Liang BB, Liu Y. Effectiveness and safety of macrolides in cystic fibrosis patients: a meta-analysis and systematic review. J Antimicrob Chemother. 2011;66:968–978. doi: 10.1093/jac/dkr040. [DOI] [PubMed] [Google Scholar]
- 77.Tamaoki J, Takeyama K, Yamawaki I, Kondo M, Konno K. Lipopolysaccharide-induced goblet cell hypersecretion in the guinea pig trachea: inhibition by macrolides. Am J Physiol. 1997;272:L15–L19. doi: 10.1152/ajplung.1997.272.1.L15. [DOI] [PubMed] [Google Scholar]
- 78.Abdelghaffar H, Vazifeh D, Labro MT. Erythromycin A-derived macrolides modify the functional activities of human neutrophils by altering the phospholipase D-phosphatidate phosphohydrolase transduction pathway: L-cladinose is involved both in alterations of neutrophil functions and modulation of this transductional pathway. J Immunol. 1997;159:3995–4005. [PubMed] [Google Scholar]
- 79.Kikuchi T, Hagiwara K, Honda Y, Gomi K, Kobayashi T, Takahashi H, Tokue Y, Watanabe A, Nukiwa T. Clarithromycin suppresses lipopolysaccharide-induced interleukin-8 production by human monocytes through AP-1 and NF-kappa B transcription factors. J Antimicrob Chemother. 2002;49:745–755. doi: 10.1093/jac/dkf008. [DOI] [PubMed] [Google Scholar]
- 80.Desaki M, Takizawa H, Ohtoshi T, Kasama T, Kobayashi K, Sunazuka T, Omura S, Yamamoto K, Ito K. Erythromycin suppresses nuclear factor-kappaB and activator protein-1 activation in human bronchial epithelial cells. Biochem Biophys Res Commun. 2000;267:124–128. doi: 10.1006/bbrc.1999.1917. [DOI] [PubMed] [Google Scholar]
- 81.Cervin A, Wallwork B, Mackay-Sim A, Coman WB, Greiff L. Effects of long-term clarithromycin treatment on lavage-fluid markers of inflammation in chronic rhinosinusitis. Clin Physiol Funct Imaging. 2009;29:136–142. doi: 10.1111/j.1475-097X.2008.00848.x. [DOI] [PubMed] [Google Scholar]
- 82.Oda H, Kadota J, Kohno S, Hara K. Leukotriene B4 in bronchoalveolar lavage fluid of patients with diffuse panbronchiolitis. Chest. 1995;108:116–122. doi: 10.1378/chest.108.1.116. [DOI] [PubMed] [Google Scholar]
- 83.Kondoh K, Hashiba M, Baba S. Inhibitory activity of clarithromycin on biofilm synthesis with Pseudomonas aeruginosa. Acta Otolaryngol Suppl. 1996;525:56–60. [PubMed] [Google Scholar]
- 84.Chung AW, Yang HH, Radomski MW, van Breemen C. Long-term doxycycline is more effective than atenolol to prevent thoracic aortic aneurysm in marfan syndrome through the inhibition of matrix metalloproteinase-2 and -9. Circ Res. 2008;102:e73–e85. doi: 10.1161/CIRCRESAHA.108.174367. [DOI] [PubMed] [Google Scholar]
- 85.Van Zele T, Gevaert P, Holtappels G, Beule A, Wormald PJ, Mayr S, Hens G, Hellings P, Ebbens FA, Fokkens W, Van Cauwenberge P, Bachert C. Oral steroids and doxycycline: two different approaches to treat nasal polyps. J Allergy Clin Immunol. 2010;125:1069–1076.e4. doi: 10.1016/j.jaci.2010.02.020. [DOI] [PubMed] [Google Scholar]
- 86.Desrosiers M, Bendouah Z, Barbeau J. Effectiveness of topical antibiotics on Staphylococcus aureus biofilm in vitro. Am J Rhinol. 2007;21:149–153. doi: 10.2500/ajr.2007.21.3007. [DOI] [PubMed] [Google Scholar]
- 87.Ha KR, Psaltis AJ, Butcher AR, Wormald PJ, Tan LW. In vitro activity of mupirocin on clinical isolates of Staphylococcus aureus and its potential implications in chronic rhinosinusitis. Laryngoscope. 2008;118:535–540. doi: 10.1097/MLG.0b013e31815bf2e3. [DOI] [PubMed] [Google Scholar]