Abstract
Purpose
Asthma and other allergic disorders have increased over the past decades in nearly all nations. Many studies have suggested the role of vitamin D deficiency in both T-helper1 and T-helper2 diseases; however, the association between vitamin D, allergy, and asthma remains uncertain. In this study, the associations of 25-hydroxy vitamin D3 levels with asthma and with the severity of asthma were evaluated.
Methods
This cross-sectional study was conducted on 50 asthmatic children and 50 healthy controls aged 6-18 years. Serum 25-hydroxy vitamin D3 levels were determined and compared between the two groups. The relationship between serum vitamin D levels and pulmonary function test outcomes and eosinophil counts were examined in asthmatic patients.
Results
Univariate analysis of the relationship between asthma and vitamin D showed that decreased vitamin D levels were associated with significantly increased odds of asthmatic state (P=0.002). In a multivariate analysis after adjustment for age, body mass index, and sex, the relationship between vitamin D and asthma increased. In asthmatic patients, 25-hydroxy vitamin D levels had direct and significant correlations with both predicted FEV1 (R2=0.318; P=0.024) and FEV1/FVC (R2=0.315; P=0.026). There were no associations between vitamin D level and eosinophil counts, duration of disease, and the number of hospitalization or unscheduled visits in the previous year (P>0.05).
Conclusions
These results showed that serum 25-hydroxy vitamin D levels were inversely associated with asthma, and there was a direct and significant relationship between vitamin D levels and pulmonary function test outcomes in asthmatic children. An interventional study in asthmatic patients with low serum vitamin D concentration may establish a causal relationship between asthma and vitamin D.
Keywords: Asthma, vitamin D, allergy
INTRODUCTION
A number of epidemiologic studies have suggested that vitamin D deficiency is associated with an increased incidence of asthma symptoms.1-3 Vitamin D deficiency was thought to be eradicated with the fortification of foods and the apparent disappearance of rickets. However, vitamin D deficiency has reappeared and is associated with many disorders.4 Despite food fortification, multiple studies have shown that vitamin D deficiency is highly prevalent even in sun-replete areas of the world5 and that vitamin D supplementation and fortification of foods in current doses are inadequate to prevent deficiency.6
The existence of associations of vitamin D with asthma and allergy remains uncertain. While some suggest that vitamin D may be protective through prenatal or postnatal exposure, others suggest that vitamin D supplementation may increase the risk of allergy.7 Associations between serum vitamin D levels and asthma severity in children and lung function in adults have been reported.8,9 Higher maternal intake of vitamin D during pregnancy was associated with a decreased risk of recurrence of wheezing in young children.7,10 Ginde et al.11 found an inverse association between serum vitamin D levels and recent upper respiratory tract infections, especially in chronic respiratory diseases such as asthma. On the other hand, a case-control study on adults found that serum vitamin D levels did not differ between asthmatic patients and controls.12
The present study examined the relationship of serum 25 hydroxy vitamin D3 levels with asthmatic state, pulmonary function measures, severity, and control of asthma.
MATERIALS AND METHODS
Study population
This cross-sectional study was carried out on 100 children aged 6 to 18 years who had been referred to the Motahari Clinic of Shiraz University of Medical Sciences in autumn, 2009. The subjects included 50 asthmatic patients (31 males, 19 females; mean age 9.31 years), who were diagnosed based on the following criteria: 1) symptoms of recurrent (i.e., more than two) episodes of wheezing, cough, shortness of breath, or a combination of these; and 2) documented reversibility with bronchodilators.13 Fifty healthy controls (31 males, 19 females; mean age 10.7 years) without a history of any allergic disorders in the child or first-degree relatives were also examined. The two groups were matched for age and sex (31 males, 19 females, aged 6-18 years).
Participants who had a history of consumption of any supplements of vitamin D or drugs that modulate serum vitamin D levels, such as systemic glucocorticoids and anticonvulsants, and those who had chronic diseases were excluded.
A questionnaire completed through an interview included questions regarding age, sex, body mass index, and some outcome measures related to asthma and its severity.
Serum 25(OH) vitamin D levels
Blood samples were collected during the examination and centrifuged, aliquoted, and frozen to -70℃ until required. Serum concentrations of 25(OH) D were assayed using an RIA kit (DRG, Marburg, Germany) after extraction with acetonitrile.
We compared serum vitamin D levels in the two groups (asthmatic and healthy controls). Additionally, we examined the relationship between vitamin D levels and the following outcomes: eosinophil count, baseline forced expiratory volume in 1 second (FEV1), forced vital capacity (FVC), FEV1/FVC, use of anti-inflammatory drugs in the previous year, any hospitalization or any unscheduled visits for asthma within the past year, and duration and severity of disease.
In a descriptive analysis, we categorized vitamin D levels as deficient (<20 ng/mL), insufficient (≥20 and <30 ng/mL), or sufficient (≥30 ng/mL).4,14
Peripheral blood eosinophil count
A peripheral smear was performed in asthmatic children, and after Wright staining, eosinophils were counted.
Pulmonary-function testing
In asthmatic subjects, spirometry was conducted with a spirometer (Jaeger, flow screen, Würzburg, Germany). The best FEV1, FVC, and FEV1/FVC values were selected for analysis.
Statistical analysis
The data were statistically analyzed using Student's t-test, one way ANOVA, and chi-square (linear by linear correlation) tests, as applicable (with a preset probability of P<0.05). Experimental results are presented as arithmetic mean±SD. Statistical tests were conducted using the SPSS software package, version 16 (SPSS Inc., Chicago, IL, USA) on a personal computer. Additionally, using simple, multiple, and logistic regression analysis, the simultaneous effects of confounding variables such as, age, sex, vitamin D levels, and body mass index (BMI) on the asthmatic state were measured. Normality assumptions of distributions were applicable based on the Kolmogorov-Smirnov test.
RESULTS
In this study, 100 children were examined (50 asthmatic and 50 non asthmatic).
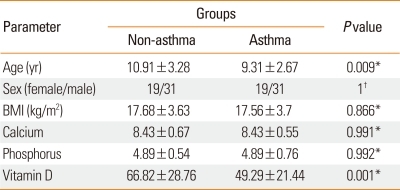
Table 1 shows the characteristics of both non-asthmatic and asthmatic subjects and the differences between the two groups. Mean 25(OH) D levels in the non-asthmatic and asthmatic groups were 66.82 and 49.29, respectively; this difference was statistically significant (Table 1; P=0.001).
Table 1.
Characteristics of patients in asthmatic and non-asthmatic groups
*Two-sample t-test; †Chi-square test.
BMI, body mass index.
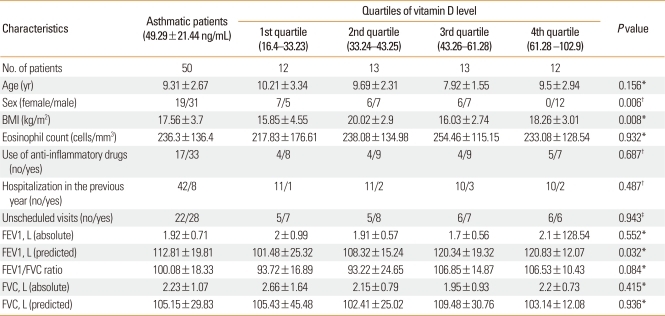
The characteristics of asthmatic participants were stratified according to vitamin D quartiles. As shown in Table 2, in asthmatic subjects, there were statistically significant differences between strata in terms of sex, BMI, and predicted FEV1 (P<0.05). Female sex and lower predicted FEV1 were significant predictors of lower 25(OH) D levels. There were no other statistically significant differences among the quartiles (P>0.05).
Table 2.
Characteristics of asthmatic patients
*One way ANOVA; †linear by linear correlation (chi-square); ‡chi-square test.
BMI, body mass index; FEV1, forced expiratory volume in 1 sec; FVC, forced vital capacity.
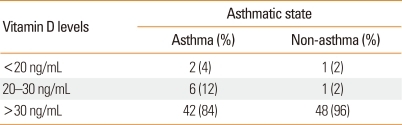
Categorization of vitamin D levels revealed no statistically significant association between levels of vitamin D and asthmatic state (Table 3; linear by linear association=2.82; P=0.094).
Table 3.
Vitamin D levels according to asthmatic state
Chi-square value for linear by linear association=2.82; P=0.094.
Univariate analysis of the relationship between asthmatic state and age, sex, and vitamin D showed that younger age and lower vitamin D levels were associated with significantly increased odds of asthmatic state (P<0.05).
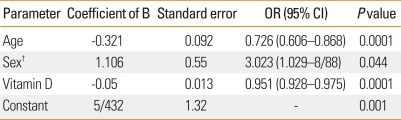
Multivariate analyses of the relationship between asthma and vitamin D levels with adjustment for age and sex were conducted. As shown in Table 4, after controlling for age and sex, a stronger relationship between vitamin D levels and asthmatic state can be seen compared with the univariate analysis. Thus, increased vitamin D levels were associated with a greater decrease in the probability of asthmatic state compared with the univariate analysis (odds ratio [OR]= 0.95 and 0.97).
Table 4.
Association between asthmatic state and age, sex, and Vitamin D levels in multivariate analysis*
*BMI excluded from the model; †male is the reference group.
OR, odds ratio; CI, confidence interval.
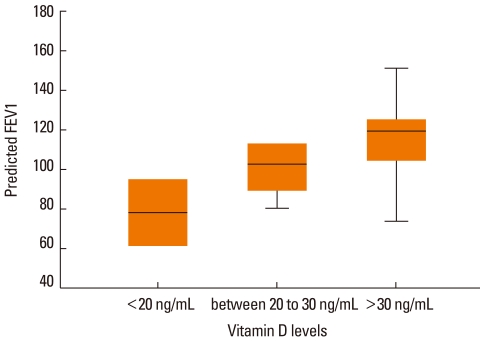
Linear association between vitamin D levels and lung-function parameters revealed that the associations between vitamin D level and predicted FEV1 (R2=0.318; P=0.024) and FEV1/FVC (R2=0.315; P=0.026) were statistically significant and those with baseline FEV1 and baseline and predicted FVC were not (P>0.05). The correlation between vitamin D levels and predicted FEV1 is shown in Figure.
Figure.
Correlation between vitamin D levels and predicted FEV1.
Linear association analysis between vitamin D levels and eosinophil counts and other outcomes (use of anti-inflammatory drugs in the previous year, any hospitalization or any unscheduled visits for asthma within the past year, and duration of disease) revealed no significant association (P>0.05).
DISCUSSION
Vitamin D is involved in the maintenance of immune homeostasis. It has an important role in innate immunity; particularly through the direct induction of antimicrobial peptide (cathelicidin) gene expression.15,16 Vitamin D promotes the induction of T regulatory cells, including the expression of inhibitory cytokines (IL-10 and TGFβ) and control of CD4-positive T lymphocytes (Th1 and Th2).17,18 Vitamin D may reverse resistance to glucocorticoids in steroid resistant asthma19 and potentiate the effect of allergen immunotherapy.20 Observational studies suggest that vitamin D deficiency increases the risk of respiratory infection, which may contribute to the incidence of wheezing illnesses in children and adults and cause asthma exacerbations.21 Polymorphisms in the gene encoding the vitamin D receptor have been associated with asthma phenotypes in two studies.22,23 Additionally, some evidence has suggested the effects of vitamin D on lung growth and development in neonates24 and also on lung function in adults.9 For the reasons noted above, vitamin D may be related to asthma and its severity.
In this study, which was conducted based on the categorization of vitamin D levels, 4% of asthmatic subjects had serum vitamin D levels <20 ng/mL (compared with 2% in the control group), 12% had levels of 20-30 ng/mL (compared with 2% in the control group), and overall, 16% had levels ≤30 ng/mL (compared with 4% in the control group; P=0.094), which was not a statistically significant association. Brehm et al.8 found that 25% of asthmatic patients had serum vitamin D levels <30 ng/mL, and 3.4 % had levels <20 ng/mL. In this study, we compared serum 25(OH) D levels in asthmatic subjects with those in a healthy control group. Although we observed higher prevalence of vitamin D insufficiency and deficiency in asthmatic children, this difference was not statistically significant. However, in the univariate analysis, we found a strong inverse association between serum vitamin D levels and asthmatic state. Also, in the multivariate analysis, after controlling for age and sex, this relationship had increased compared to the univariate analysis (OR=0.95-0.97). Thus, our data confirm the presence of lower vitamin D levels among asthmatic patients.
In our study, linear association analysis of serum 25 (OH) D levels and measures of lung function revealed that the direct associations with predicted FEV1 (P=0.024) and also with FEV1/FVC (P=0.026) were statistically significant. These findings suggest the involvement of vitamin D in lung function and the development of airflow limitation. However, the studies by Brehm et al.8 and Litonjua et al.25 reported contradictory data. Vitamin D inhibits the formation of matrix metalloproteinase as well as fibroblast proliferation and influences collagen synthesis; these actions mean that 1,25-dihydroxy vitamin D may influence tissue remodeling and probably lung function.26,27 Black and Scragg9 found that serum 25-OH vitamin D level was positively associated with FEV1 and FVC in the United States' general population.
Brehm et al.8 found an inverse relationship between circulating levels of vitamin D and several markers of allergy and asthma severity such as eosinophil count, IgE levels, asthma exacerbation, airway responsiveness, and skin-test reactivity. Litonjua et al.25 found that children with insufficient levels of 25(OH) D were more likely to have severe exacerbations, but they did not find any association between vitamin D and bronchodilator response or airway hyperresponsiveness. In our study, there were no associations between vitamin D levels and eosinophil counts, course of disease, asthma exacerbation, or anti-inflammatory drug use. This may be due to the smaller number of subjects and unequal sex distribution in both groups.
A number of confounding factors may influence these relationships. One is that the subjects with asthma spend more time indoors, so they may be exposed to less sunlight.2 However, we found a relationship between vitamin D and lung function outcomes that contribute to the severity of asthma, especially FEV1/FVC, which is a marker of airway obstruction. Another factor is the effects of other nutrients, such as vitamin E. Due to the strong evidence linking vitamin D deficiency to Th1 diseases, the role of vitamin E is likely weaker.2
Vitamin D deficiency is highly prevalent even in sun-replete areas of the world.5 Some possible explanations include behavioral factors (e.g. sunscreen use, increased time spent indoors, and clothing coverage) and intrinsic factors such as skin melanin content, ethnicity (dark-skinned person), and decreased cutaneous production of vitamin D3.28
Although there is no consensus regarding optimal 25-hydroxy vitamin D serum levels, based on epidemiologic studies, a desirable level of serum vitamin D, i.e., 25(OH) D, for general health is at least 30 to 40 ng/mL (75 to 100 nmol/L).4,6 It has been suggested that levels higher than 40 ng/mL (100 nmol/L) may be necessary for optimal immune functioning and overall health.29 It seems that current national recommendations of vitamin D (200-600 IU per day, depending on age) are insufficient to bring serum 25(OH)D levels to 75-100 nmol/mL, and, therefore, higher doses up to 700-1,000 IU per day may be necessary.30
In the present study, we found lower serum 25(OH) D levels in asthmatic children compared with controls, and we also found a direct relationship between serum 25(OH) D levels and lung-function measures. An interventional study in asthmatic patients with low serum vitamin D concentrations is now required to establish a causal relationship between asthma and vitamin D level.
Footnotes
There are no financial or other issues that might lead to conflict of interest.
References
- 1.Litonjua AA, Weiss ST. Is vitamin D deficiency to blame for the asthma epidemic? J Allergy Clin Immunol. 2007;120:1031–1035. doi: 10.1016/j.jaci.2007.08.028. [DOI] [PubMed] [Google Scholar]
- 2.Weiss ST, Litonjua AA. Maternal diet vs lack of exposure to sunlight as the cause of the epidemic of asthma, allergies and other autoimmune diseases. Thorax. 2007;62:746–748. doi: 10.1136/thx.2007.079707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hyppönen E, Sovio U, Wjst M, Patel S, Pekkanen J, Hartikainen AL, Järvelinb MR. Infant vitamin D supplementation and allergic conditions in adulthood: northern Finland birth cohort 1966. Ann N Y Acad Sci. 2004;1037:84–95. doi: 10.1196/annals.1337.013. [DOI] [PubMed] [Google Scholar]
- 4.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 5.Binkley N, Novotny R, Krueger D, Kawahara T, Daida YG, Lensmeyer G, Hollis BW, Drezner MK. Low vitamin D status despite abundant sun exposure. J Clin Endocrinol Metab. 2007;92:2130–2135. doi: 10.1210/jc.2006-2250. [DOI] [PubMed] [Google Scholar]
- 6.Bischoff-Ferrari HA, Giovannucci E, Willett WC, Dietrich T, Dawson-Hughes B. Estimation of optimal serum concentrations of 25-hydroxyvitamin D for multiple health outcomes. Am J Clin Nutr. 2006;84:18–28. doi: 10.1093/ajcn/84.1.18. [DOI] [PubMed] [Google Scholar]
- 7.Camargo CA, Jr, Rifas-Shiman SL, Litonjua AA, Rich-Edwards JW, Weiss ST, Gold DR, Kleinman K, Gillman MW. Maternal intake of vitamin D during pregnancy and risk of recurrent wheeze in children at 3 y of age. Am J Clin Nutr. 2007;85:788–795. doi: 10.1093/ajcn/85.3.788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brehm JM, Celedón JC, Soto-Quiros ME, Avila L, Hunninghake GM, Forno E, Laskey D, Sylvia JS, Hollis BW, Weiss ST, Litonjua AA. Serum vitamin D levels and markers of severity of childhood asthma in Costa Rica. Am J Respir Crit Care Med. 2009;179:765–771. doi: 10.1164/rccm.200808-1361OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Black PN, Scragg R. Relationship between serum 25-hydroxyvitamin D and pulmonary function in the third national health and nutrition examination survey. Chest. 2005;128:3792–3798. doi: 10.1378/chest.128.6.3792. [DOI] [PubMed] [Google Scholar]
- 10.Devereux G, Litonjua AA, Turner SW, Craig LC, McNeill G, Martindale S, Helms PJ, Seaton A, Weiss ST. Maternal vitamin D intake during pregnancy and early childhood wheezing. Am J Clin Nutr. 2007;85:853–859. doi: 10.1093/ajcn/85.3.853. [DOI] [PubMed] [Google Scholar]
- 11.Ginde AA, Mansbach JM, Camargo CA., Jr Association between serum 25-hydroxyvitamin D level and upper respiratory tract infection in the Third National Health and Nutrition Examination Survey. Arch Intern Med. 2009;169:384–390. doi: 10.1001/archinternmed.2008.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Devereux G, Wilson A, Avenell A, McNeill G, Fraser WD. A case-control study of vitamin D status and asthma in adults. Allergy. 2010;65:666–667. doi: 10.1111/j.1398-9995.2009.02220.x. [DOI] [PubMed] [Google Scholar]
- 13.National Heart, Lung, and Blood Institute, National Asthma Education and Prevention Program. Bethesda: National Heart, Lung, and Blood Institute; 2007. Expert Panel report 3: guidelines for the diagnosis and management of asthma [Internet] Available from: http://www.nhlbi.nih.gov/guidelines/asthma/ [Google Scholar]
- 14.Vieth R, Bischoff-Ferrari H, Boucher BJ, Dawson-Hughes B, Garland CF, Heaney RP, Holick MF, Hollis BW, Lamberg-Allardt C, McGrath JJ, Norman AW, Scragg R, Whiting SJ, Willett WC, Zittermann A. The urgent need to recommend an intake of vitamin D that is effective. Am J Clin Nutr. 2007;85:649–650. doi: 10.1093/ajcn/85.3.649. [DOI] [PubMed] [Google Scholar]
- 15.Wang TT, Nestel FP, Bourdeau V, Nagai Y, Wang Q, Liao J, Tavera-Mendoza L, Lin R, Hanrahan JW, Mader S, White JH. Cutting edge: 1,25-dihydroxyvitamin D3 is a direct inducer of antimicrobial peptide gene expression. J Immunol. 2004;173:2909–2912. doi: 10.4049/jimmunol.173.5.2909. [DOI] [PubMed] [Google Scholar]
- 16.Gombart AF, Borregaard N, Koeffler HP. Human cathelicidin antimicrobial peptide (CAMP) gene is a direct target of the vitamin D receptor and is strongly up-regulated in myeloid cells by 1,25-dihydroxyvitamin D3. FASEB J. 2005;19:1067–1077. doi: 10.1096/fj.04-3284com. [DOI] [PubMed] [Google Scholar]
- 17.Gregori S, Giarratana N, Smiroldo S, Uskokovic M, Adorini L. A 1alpha,25-dihydroxyvitamin D(3) analog enhances regulatory T-cells and arrests autoimmune diabetes in NOD mice. Diabetes. 2002;51:1367–1374. doi: 10.2337/diabetes.51.5.1367. [DOI] [PubMed] [Google Scholar]
- 18.Meehan MA, Kerman RH, Lemire JM. 1,25-Dihydroxyvitamin D3 enhances the generation of nonspecific suppressor cells while inhibiting the induction of cytotoxic cells in a human MLR. Cell Immunol. 1992;140:400–409. doi: 10.1016/0008-8749(92)90206-5. [DOI] [PubMed] [Google Scholar]
- 19.Xystrakis E, Kusumakar S, Boswell S, Peek E, Urry Z, Richards DF, Adikibi T, Pridgeon C, Dallman M, Loke TK, Robinson DS, Barrat FJ, O'Garra A, Lavender P, Lee TH, Corrigan C, Hawrylowicz CM. Reversing the defective induction of IL-10-secreting regulatory T cells in glucocorticoid-resistant asthma patients. J Clin Invest. 2006;116:146–155. doi: 10.1172/JCI21759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Taher YA, van Esch BC, Hofman GA, Henricks PA, van Oosterhout AJ. 1alpha,25-dihydroxyvitamin D3 potentiates the beneficial effects of allergen immunotherapy in a mouse model of allergic asthma: role for IL-10 and TGF-beta. J Immunol. 2008;180:5211–5221. doi: 10.4049/jimmunol.180.8.5211. [DOI] [PubMed] [Google Scholar]
- 21.Ginde AA, Mansbach JM, Camargo CA., Jr Vitamin D, respiratory infections, and asthma. Curr Allergy Asthma Rep. 2009;9:81–87. doi: 10.1007/s11882-009-0012-7. [DOI] [PubMed] [Google Scholar]
- 22.Poon AH, Laprise C, Lemire M, Montpetit A, Sinnett D, Schurr E, Hudson TJ. Association of vitamin D receptor genetic variants with susceptibility to asthma and atopy. Am J Respir Crit Care Med. 2004;170:967–973. doi: 10.1164/rccm.200403-412OC. [DOI] [PubMed] [Google Scholar]
- 23.Raby BA, Lazarus R, Silverman EK, Lake S, Lange C, Wjst M, Weiss ST. Association of vitamin D receptor gene polymorphisms with childhood and adult asthma. Am J Respir Crit Care Med. 2004;170:1057–1065. doi: 10.1164/rccm.200404-447OC. [DOI] [PubMed] [Google Scholar]
- 24.Rehan VK, Torday JS, Peleg S, Gennaro L, Vouros P, Padbury J, Rao DS, Reddy GS. 1Alpha,25-dihydroxy-3-epi-vitamin D3, a natural metabolite of 1alpha,25-dihydroxy vitamin D3: production and biological activity studies in pulmonary alveolar type II cells. Mol Genet Metab. 2002;76:46–56. doi: 10.1016/s1096-7192(02)00022-7. [DOI] [PubMed] [Google Scholar]
- 25.Litonjua AA, Hollis BW, Schuemann BK, Celedón JC, Fuhlbrigge AL, Raby BA, Weiss ST. Low serum vitamin D levels are associated with increased asthma exacerbations among children using regular inhaled corticosteroids. J Allergy Clin Immunol. 2008;121:S144. [Google Scholar]
- 26.Koli K, Keski-Oja J. 1alpha,25-dihydroxyvitamin D3 and its analogues down-regulate cell invasion-associated proteases in cultured malignant cells. Cell Growth Differ. 2000;11:221–229. [PubMed] [Google Scholar]
- 27.Dobak J, Grzybowski J, Liu FT, Landon B, Dobke M. 1,25-dihydroxyvitamin D3 increases collagen production in dermal fibroblasts. J Dermatol Sci. 1994;8:18–24. doi: 10.1016/0923-1811(94)90316-6. [DOI] [PubMed] [Google Scholar]
- 28.Litonjua AA. Childhood asthma may be a consequence of vitamin D deficiency. Curr Opin Allergy Clin Immunol. 2009;9:202–207. doi: 10.1097/ACI.0b013e32832b36cd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hollis BW, Wagner CL, Drezner MK, Binkley NC. Circulating vitamin D3 and 25-hydroxyvitamin D in humans: an important tool to define adequate nutritional vitamin D status. J Steroid Biochem Mol Biol. 2007;103:631–634. doi: 10.1016/j.jsbmb.2006.12.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dawson-Hughes B. Impact of vitamin D and calcium on bone and mineral metabolism in older adults. In: Holick MF, editor. Biologic effects of light 2001. Boston: Kluwer Academic Publishers; 2002. pp. 175–183. [Google Scholar]