Abstract
Acetyl salicylic acid (ASA) is metabolized by UDP-glucuronosyltransferase 1A6 (UGT1A6), cytochrome P4502C9 (CYP2C9), and N-acetyl transferase 2 (NAT2). Variations in the activities of these enzymes may modulate adverse ASA-related symptoms such as urticaria. We examined whether polymorphisms in the UGT1A6, CYP2C9, and NAT2 genes are related to ASA-intolerant urticaria (AIU). The genotypes of 148 subjects with AIU (AIU group) and 260 normal healthy control subjects (NC group) were analyzed with respect to the following single nucleotide polymorphisms: CYP2C9 -1188T>C and CYP2C9*3A1075C; UGT1A6 T181A A>G and UGT1A6 R184S A>C; and NAT2 9796A>T, NAT2 197G>A, NAT2 286G>A, NAT2 9601A>G, and NAT2 9306A>G. There were significant differences in the allele frequencies for the CYP2C9 polymorphisms between the two groups. The frequency of the minor allele CYP2C9 -1188T>C was significantly higher in the AIU group than in the NC group (P=0.005). The frequency of the variant genotype CC was higher in the AIU group compared with the controls in both the co-dominant (P=0.007) and recessive models (P=0.012). The frequency of haplotype 2 [CA] was also significantly higher in the AIU group in both the co-dominant (P=0.006) and dominant models (P=0.012). There was no significant difference in genotype frequencies for any of the UGT1A6 or NAT2 polymorphisms between the two groups. Clinical parameters did not differ according to genotype. These results suggest that the C allele of CYP2C9 -1188T>C may be associated with AIU.
Keywords: Aspirin, Cytochrome P4502C9, Metabolizing enzyme, Urticaria
Aspirin ingestion can induce a wide range of clinically recognized allergic reactions, including acetyl salicylic acid (ASA)-exacerbated respiratory disease (AERD), ASA-intolerant urticaria (AIU), chronic rhinitis, and anaphylaxis. Patients with AIU have high rates of atopy and increased total IgE levels,1 but the pathogenic mechanism of AIU remains unclear. ASA is metabolized by UDP-glucuronosyltransferase 1A6 (UGT1A6), cytochrome P4502C9 (CYP2C9), and N-acetyl transferase 2 (NAT2). Inter-individual differences in the activities of these enzymes may be the underlying cause of adverse ASA-related symptoms such as urticaria.
The two polymorphic enzymes CYP2C9 and UGT1A6 are involved in hydroxylation and glucuronidation of ASA, respectively.2,3 Two known variant alleles of UGT1A6 result in amino acid changes at positions 181 and 184, and a 30-50% reduction in enzyme activity compared with wild type activity.4 Similarly, two variant CYP2C9 alleles, CYP2C9*2 (R144C) and CYP2C9*3 (I359L), produce slow metabolizing enzymes, with 5-30% of wild-type enzyme activity.5 Variations in the enzymes that metabolize ASA may play a role in ASA-related diseases such as urticaria.
Leukotriene overproduction is a possible risk factor for ASA hypersensitivity, including AIU.6 Cysteinyl leukotrienes are inactivated by acetyl coenzyme A-dependent N-acetyltransferase (NAT). Thus, functional alterations in the NAT2 gene may contribute to the risk for ASA-intolerant symptoms such as asthma.7 Genetically-determined rapid and slow acetylators produce variation in the elimination rates of xenobiotics, as well as in the levels of NAT2 in the liver and other tissues. Single nucleotide polymorphisms (SNPs) of the NAT2 gene are markers of atopic asthma, high serum IgE levels, and high blood eosinophil counts.8
Based on the involvement of these enzymes in the metabolism of ASA, we investigated the relationship between polymorphisms in UGT1A6, CYP2C9, and NAT2, and the occurrence of ASA-intolerant urticaria in a Korean population. In total, 148 AIU patients (AIU group) and 260 normal healthy control subjects (NC) were enrolled at the Ajou University Hospital in Suwon, Korea. AIU was defined by a positive result on an ASA oral provocation test, which was performed using 500 mg of ASA (Rhonal; KunWha Pharmaceutical Co., Seoul, Korea).5 A control group (NC) with no personal or family history of allergic diseases or past history of ASA hypersensitivity was recruited from the general population. Informed consent was obtained from all subjects prior to enrollment, and the study was approved by the Ajou University Hospital Institutional Review Board. Skin prick testing was performed with 55 common aero-allergens (Bencard Co., West Sussex, UK). Atopy was defined as one or more positive reactions to common inhalant allergens. Total IgE concentration was measured using a UniCAP system (Pharmacia Diagnostics, Uppsala, Sweden), according to the manufacturer's instructions. SNPs in the promoter and/or exons of the CYP2C9, UGT1A6, and NAT2 genes of 40 healthy volunteers were sequenced using an ABI Prism 3100 DNA analyzer (Applied Biosystems, Foster City, CA, USA). We identified two SNPs in CYP2C9, one in the promoter (CYP2C9 -1188T>C) and one in an exon (CYP2C9*3A1075C). For UGT1A6, we selected two exonic SNPs (UGT1A6 T181A A>G and UGT1A6 R184S A>C). We confirmed five SNPs for NAT2 (NAT2 9796A>T, NAT2 197G>A, NAT2 286G>A, NAT2 9601A>G, and NAT2 9306A>G). These SNPs were genotyped using primer extension and a SNAPshot ddNTP primer extension kit (Applied Biosystems).
Differences in mean values of phenotypic characteristics among the patients were compared using a Student's t-test for continuous variables or the χ2 test for categorical variables. Differences in genotype frequency between the two groups were examined using the χ2 test. A logistic regression recessive analysis model was used after adjustment for age and gender covariates, followed by a multiple comparison test. All statistical analyses were performed using SPSS version 12.0 (SPSS Inc., Chicago, IL, USA). In all analyses, P<0.05 was considered statistically significant.
The mean age was significantly higher in the AIU group than in the NC group (35.1±11.5 vs. 32.5±12.6 years, respectively; P=0.041). The AIU patients exhibited a significantly higher rate of atopy than the NC group (69.6 vs. 13.2%, respectively; P<0.001) and higher serum total IgE (log transformed; 5.1±1.2 vs. 3.6±1.3 IU/mL respectively; P<0.001).
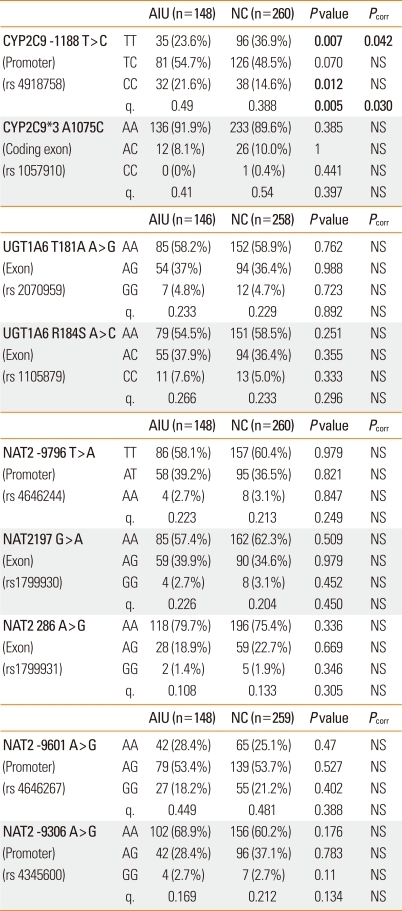
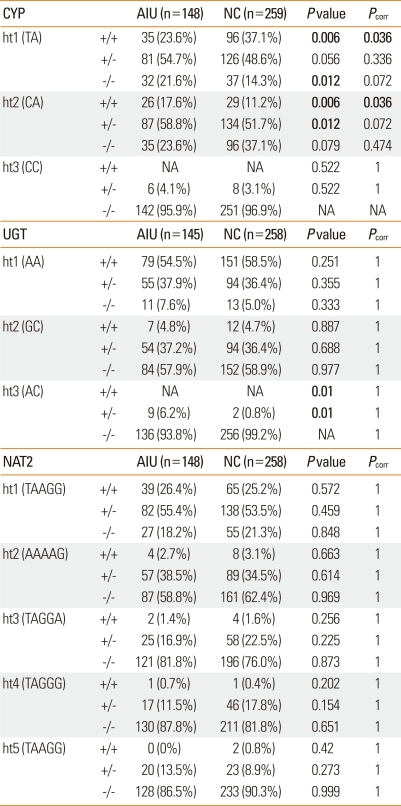
The genotype frequencies for the two polymorphisms in each of CYP2C9 and UGT1A6 and the five polymorphisms in NAT2 were analyzed and compared between AIU patients and NC subjects. The two polymorphisms in CYP2C9 were in linkage disequilibrium (/D'/=1, r2=0.067). The frequency of the minor C allele of CYP2C9 -1188T>C was significantly higher in the AIU group than in the NC group (P=0.005), and remained significant after a multiple comparison (Pcorr=0.03). The frequency of the variant genotype CC of CYP2C9 -1188T>C was also higher in the AIU patients compared with the controls in both the co-dominant (P=0.007) and recessive models (P=0.012). In addition, the relationship between the CYP2C9 -1188T>C polymorphism and AIU remained significant after a multiple comparison test (Pcorr=0.042 [co-dominant model] for AIU vs. NC; Table 1). Using Power Analysis and Sample Size (PASS, 2008), the power of this genetic association study was calculated to be 74% for detecting an effect size (W) of 0.1430 using 2 degrees of freedom with the χ2 test, at a significance level (alpha) of 0.05. The frequency of haplotype 2 [CA] was significantly higher in the AIU group compared with the NC group in both the co-dominant (P=0.006; Pcorr=0.036) and dominant models (P=0.012) (Table 2).
Table 1.
Genotype and allele frequencies of CYP2C9, UGT1A6 and NAT2 gene polymorphisms
P values were applied by binary logistic regression with sex and age. P<0.05 was considered to be significant. P value was further tested using multiple comparisons, as indicated, Pcorr for AIU vs. NC.
AIU, aspirin intolerant urticaria; NC, normal control; CYP2C9, cytochrome P450C9; UGT1A6, UDP-glucuronosyltransferase 1A6; NAT2, N-acetyl transferase 2.
Table 2.
Haplotype frequencies of CYP2C9, UGT1A6 and NAT2 gene polymorphisms
P values were applied by binary logistic regression with sex and age.
There was no significant difference in allele, genotype, or haplotype frequencies of the UGT1A6 and NAT2 genetic polymorphisms between the AIU and NC groups. Clinical parameters, including atopic status, serum total IgE levels, and autoantibody levels, did not differ between any of the genotypes for any of the polymorphisms.
We analyzed the genotype frequencies for two SNPs in both CYP2C9 and UGT1A6, and five SNPs in NAT2, and compared them between AIU patients and normal control subjects in a Korean population. This is the first study to investigate the SNPs of CYP2C9, UGT1A6, and NAT2 in AIU patients. Among the nine polymorphisms in the three metabolic enzyme genes analyzed, CYP2C9 -1188T>C showed a significant association with the AIU phenotype. The frequencies of the minor C allele and the CC genotype of CYP2C9 -1188T>C were significantly higher in the AIU group compared with the NC group. There was no significant difference in allele, genotype, or haplotype frequencies of the UGT1A6 and NAT2 polymorphisms between the AIU and NC groups.
CYP2C9 is involved in the metabolism of many therapeutic agents, including non-steroidal anti-inflammatory drugs, oral anticoagulants, and angiotensin receptor antagonists. The metabolism of non-steroidal anti-inflammatory drugs involves oxidation by CYP enzymes and conjugation, particularly glucuronidation, by phase II enzymes. Aspirin is deacetylated to salicylic acid and is further metabolized by glucuronidation, hydroxylation, and glycine conjugation, with CYP2C9 playing a major role in the metabolic process.5 Genetic polymorphisms in CYP2C9 have been associated with cutaneous adverse reactions induced by diphenylhydantoin.9 The effects of inter-individual differences in UGT1A6 and CYP2C9 genotypes on ASA metabolism have been described in colon adenoma.5 Recently, CYP2C19 and CYP2C9 genetic polymorphisms were shown to be significantly associated with an increased risk for the development of antituberculosis drug-induced maculopapular eruption in the Korean population.10 Collectively, these findings suggest that the C allele may be involved in the pathogenesis of AIU; however, further studies of CYP2 enzyme activity are needed.
The NAT2 gene variants and its subphenotypes have been linked to the development of asthma. Additionally, the NAT2 slow acetylator phenotype may be a determinant in a patient's susceptibility to asthma.11 Another study indicated that SNPs in the NAT2 gene are closely associated with asthma-related traits such as high serum IgE and blood eosinophil counts, and aspirin hypersensitivity in asthmatic subjects.7,8 Significant associations between UGT1A6 T181A gene polymorphisms and asthma susceptibility have also been reported.12 However, the results of this study did not show a relationship between NAT2 or UGTA6 polymorphisms and AIU in a Korean population.
In summary, we identified a statistically significant association between the C allele of CYP2C9 -1188T>C and AIU, suggesting that this allele may modulate the risk for, and contribute to, the development of the AIU phenotype.
ACKNOWLEDGMENTS
This study was supported by a grant of the Korean Health 21 R&D Project, Ministry of health & Welfare, ROK (A111218-11-PG01).
Footnotes
There are no financial or other issues that might lead to conflict of interest.
References
- 1.Palikhe NS, Kim SH, Choi GS, Ye YM, Park HS. No evidence of association between interleukin-13 gene polymorphism in aspirin intolerant chronic urticaria. Allergy Asthma Immunol Res. 2009;1:36–40. doi: 10.4168/aair.2009.1.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miners JO, Birkett DJ. Cytochrome P4502C9: an enzyme of major importance in human drug metabolism. Br J Clin Pharmacol. 1998;45:525–538. doi: 10.1046/j.1365-2125.1998.00721.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ciotti M, Marrone A, Potter C, Owens IS. Genetic polymorphism in the human UGT1A6 (planar phenol) UDP-glucuronosyltransferase: pharmacological implications. Pharmacogenetics. 1997;7:485–495. doi: 10.1097/00008571-199712000-00007. [DOI] [PubMed] [Google Scholar]
- 4.Lampe JW, Bigler J, Horner NK, Potter JD. UDP-glucuronosyltransferase (UGT1A1*28 and UGT1A6*2) polymorphisms in Caucasians and Asians: relationships to serum bilirubin concentrations. Pharmacogenetics. 1999;9:341–349. doi: 10.1097/00008571-199906000-00009. [DOI] [PubMed] [Google Scholar]
- 5.Bigler J, Whitton J, Lampe JW, Fosdick L, Bostick RM, Potter JD. CYP2C9 and UGT1A6 genotypes modulate the protective effect of aspirin on colon adenoma risk. Cancer Res. 2001;61:3566–3569. [PubMed] [Google Scholar]
- 6.Palikhe NS, Kim SH, Park HS. What do we know about the genetics of aspirin intolerance? J Clin Pharm Ther. 2008;33:465–472. doi: 10.1111/j.1365-2710.2008.00961.x. [DOI] [PubMed] [Google Scholar]
- 7.Kim JM, Park BL, Park SM, Lee SH, Kim MO, Jung S, Lee EH, Uh ST, Park JS, Choi JS, Kim YH, Kim MK, Choi IS, Cho SH, Choi BW, Park HS, Chang HS, Shin HD, Park CS. Association analysis of N-acetyl transferase-2 polymorphisms with aspirin intolerance among asthmatics. Pharmacogenomics. 2010;11:951–958. doi: 10.2217/pgs.10.65. [DOI] [PubMed] [Google Scholar]
- 8.Batra J, Sharma SK, Ghosh B. Arylamine N-acetyltransferase gene polymorphisms: markers for atopic asthma, serum IgE and blood eosinophil counts. Pharmacogenomics. 2006;7:673–682. doi: 10.2217/14622416.7.5.673. [DOI] [PubMed] [Google Scholar]
- 9.Lee AY, Kim MJ, Chey WY, Choi J, Kim BG. Genetic polymorphism of cytochrome P450 2C9 in diphenylhydantoin-induced cutaneous adverse drug reactions. Eur J Clin Pharmacol. 2004;60:155–159. doi: 10.1007/s00228-004-0753-0. [DOI] [PubMed] [Google Scholar]
- 10.Kim SH, Kim SH, Yoon HJ, Shin DH, Park SS, Kim YS, Park JS, Jee YK. NAT2, CYP2C9, CYP2C19, and CYP2E1 genetic polymorphisms in anti-TB drug-induced maculopapular eruption. Eur J Clin Pharmacol. 2011;67:121–127. doi: 10.1007/s00228-010-0912-4. [DOI] [PubMed] [Google Scholar]
- 11.Tamer L, Calikoğlu M, Aras Ateş N, Yildirim H, Karakaş S, Atik U. Relationship between N-acetyl transferase-2 gene polymorphism and risk of bronchial asthma. Tuberk Toraks. 2006;54:137–143. [PubMed] [Google Scholar]
- 12.Polonikov AV, Ivanov VP, Solodilova MA. Genetic variation of genes for xenobiotic-metabolizing enzymes and risk of bronchial asthma: the importance of gene-gene and gene-environment interactions for disease susceptibility. J Hum Genet. 2009;54:440–449. doi: 10.1038/jhg.2009.58. [DOI] [PubMed] [Google Scholar]