Abstract
Direct transcutaneous prosthetic attachment (osseointegration) consists of implanting directly into the residuum bone a metal pylon whose external fraction connects the residuum to the external prosthesis. Since the introduction of osseointegration about 20 years ago, the obvious challenge associated with this technology has been the skin-pylon interface as a source of infections. In comparison, the bone-device interface was considered less problematic because of the knowledge and experience inherited from dental implantology and total joint replacement (arthroplasty). Current methods of pylon fixation in osseointegration follow arthroplasty’s paradigm of positioning the pylon’s shaft inside the bone’s medullary canal. However, adopting the medullary canal as a holding compartment for the pylon’s shaft creates the problem of shaft loosening, which has not yet been solved in arthroplasty.
INTRODUCTION
Two procedures, a two-step and a one-step, exist for direct transcutaneous prosthetic attachment (osseointegration). According to the two-step procedure, a titanium fixture is first fitted into the medullary canal of the residuum bone [1–3]. The implant is left inside the body for several months; the skin above the distal end of the fixture is then cut and an abutment is attached to the bottom of the fixture. The abutment penetrates the residuum’s skin and serves as the pylon connecting the residuum to the limb prosthesis.* According to the one-step procedure, the shaft of a pylon is implanted directly into the bone canal, and bone ossifies around the shaft concurrently with the skin’s integration with the pylon collar [4–5]. Rehabilitation outcomes of the direct transcutaneous prosthetic attachment under either scenario rely on the longevity and strength of the bond between the pylon and bone walls and on the infection-free seal of skin surrounding the pylon. Of those two conditions, the infection-free skin-pylon interface has been considered the challenge of the highest priority [6–14]. Design modifications and surface treatments of the pylons aimed at improving the skin-device interface have been analyzed elsewhere [15].
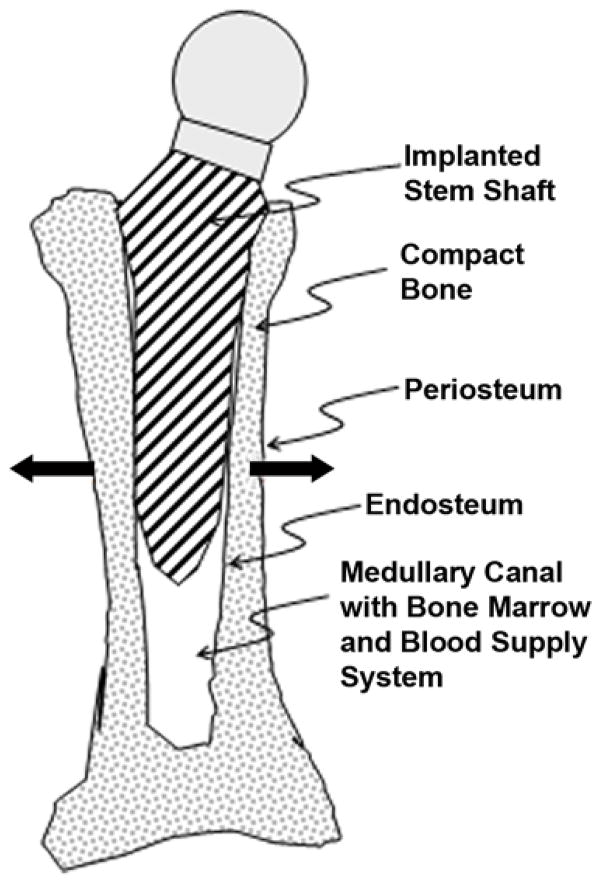
In the 1960s, Sir John Charnley pioneered modern total hip replacement (THR) [16]. A stem with an artificial femoral head is inserted into the prebored and cleaned medullary canal of a tube bone, with or without cement, as schematically shown in Figure 1. Porous or roughened surfaces are engineered to stimulate bone growth (ossification) into the stems. THR is widely used in many countries (in the United States, about 300,000 hip replacements are performed each year [17]) and has been proven to be effective, but up to 2 percent of patients still require surgical revision because of loosening of the prosthesis’ shaft relative to the bone [18]. Loosening occurs when surrounding bone tissues weaken and osteolysis (bone resorption) prevails over the process of ossification, with a consequent decrease in strength of the bond between the shaft and the surrounding bone walls. Another phenomenon that occurs following arthroplasty, which has not been conclusively explained, is that younger and more physically active patients encounter a higher risk of future prostheses loosening [19]. This fact contradicts the anticipated positive association of bone regeneration capability with younger age and higher activity level [20–21].
Figure 1.
Schematic of intramedullary implanted artificial hip prosthetic stem. Bold arrows indicate vector of bone wall growth with widening of medullary canal.
Although much is known about total joint replacement, research has had little success in elucidating the genesis of prosthetic stem loosening. Different theories, including the genome-based theory [22], try to explain loosening of implanted prostheses but none can be considered satisfactory [23–25]. A variety of design modifications of the stems has been introduced and examined, including taper slip stems with a polished surface, fixation by intramedullary nails, or use of high-pressure saline to inflate the diameter of a cylindrical implant [26]. However, all known approaches depend on the medullary canal’s ability to act as a holding cavity for the prosthesis’ shaft. We suggest that such use of the medullary canal contradicts the biological purpose of the canal, namely its role as a designated functional cavity for the bone marrow [27]. We note also that the insertion of a stem into the canal destroys the endosteum, a thin layer of connective tissue filled with cortical capillaries that lines the medullary cavity.
WHY CAN MEDULLARY CANAL NOT BOND WELL WITH IMPLANTED STEM?
The current philosophy of fixing the stem in the medullary canal presumes that the canal’s walls will eventually tighten around the inserted shaft, similarly to the tightening observed in jaw tissues around tooth implants. In a prospective study, the cumulative dental implant survival was found to be 99.4 percent (n = 835) [28]. We believe that an important difference exists between the interaction of a jawbone with a tooth implant and a tube bone with the prosthetic implant. Keeping a tooth root in a firm surrounding is a natural feature of the jawbone. Thus, when the dental implant replaces the missing root, the procedure does not evoke a new bone remodeling feature but rather utilizes an existing one. Namely, the remodeling of the jawbone after the implantation is directed toward the space occupied by the implant and is naturally stimulated in that direction by the loads transmitted from the implant to the bone.
Inserting the stem into the medullary canal, however, is preceded by boring the canal, partially destroying the endosteum lining the canal’s walls. Even without implanting the stem, the inward ossification in the process of repairing the damaged canal would not proceed beyond the limit defined by the former inner diameter of the canal (Figure 2). The inhibition of cell growth in a certain direction, while growth in another direction or directions continues, is an example of the well-recognized mechanism of anisotropy (directional dependence) in biology [29–30]. No comprehensive data yet exist on the mechanism that inhibits ossification beyond the limit of the bone wall thickness, but having such a mechanism in place to protect the integrity and normal functioning of the bone marrow seems reasonable.
Figure 2.
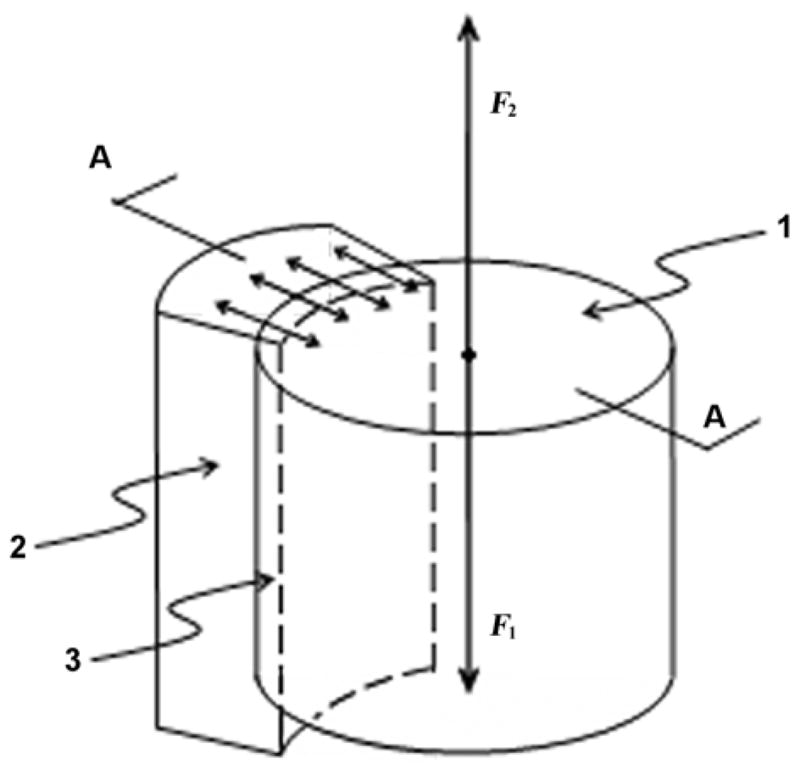
Schematic of implantation of stem’s shaft in medullary canal of a cortical tube bone. 1 = shaft of stem, 2 = cortical wall of bone, 3 = endosteum removed by boring medullary canal prior to installation, A-A = cross-section, F1 = vertical load to shaft, F2 = reaction from bone walls.
Consider a schematic of resistance of the bone-implant interface to a vertical load (Figure 2). When the stem is implanted, its stable position is caused by the equality of the vertical load F1 and the reaction F2, which is a resultant friction force between the shaft and the walls. The maximal value of the reaction F2 and consequently the maximal load F1 at which the shaft of the stem does not slide down is a product of a coefficient of friction between the walls of the bone and the shaft and the sum of normal reactions applied to the shaft by the bone. These reactions are shown in cross-section A-A (Figure 2) as multiple arrows pointing to the center of the cross-section. Normal forces applied to the bone walls from the shaft are shown as multiple arrows pointing outward. These forces are circularly dispersed and cause ischemia (restriction of blood supply) of the bone tissues surrounding the implant [31]. Because of ischemia in the bone wall tissues and the damage to the medullary artery and cortical capillaries in the endosteum [27], ossification may not be completed in such a way as to reliably withstand the vertical load applied to the implant. We suggest that ischemia of the inner surface of the medullary canal may be a more serious cause of resorption of the bone cells surrounding the implant than the particles of plastic or metal from the wear of the artificial joint surfaces.
Another fundamental reason why the medullary canal is not a reliable holding cavity for the stem’s shaft is that it increases in diameter when a person is in a development age or has a high level of physical activity [32]. The pulling action of the skeletal muscles applied to the periosteum prompts outward bone ossification and an increase in the thickness of the bone walls (Figure 3(a)) with the concurrent increase of the canal’s diameter. A continued increase in the medullary canal’s diameter diminishes the reliability of the bond between the canal’s inner walls and the shaft, whether with or without cement.
Figure 3.
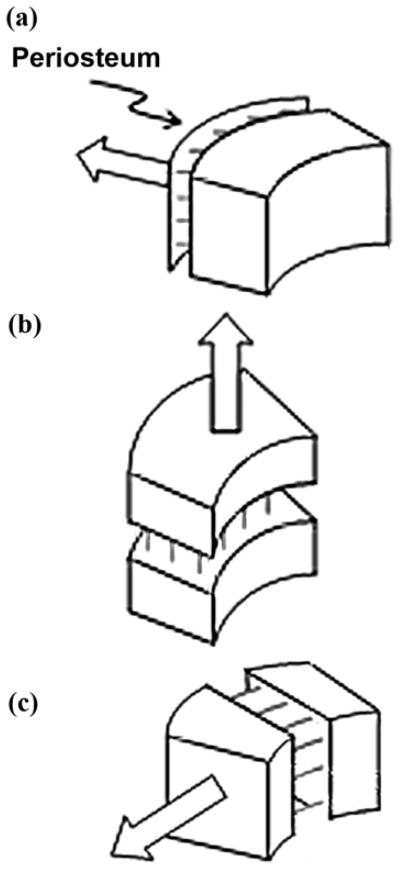
Ossification (indicated by multiple lines) in response to pulling forces: (a) from muscles applied to periosteum, (b) by lengthening, and (c) by widening.
We consider the process of canal widening the second reason for the loosening of the bond between the bone walls and the stem in THR patients. Therefore, the hypothesis is that ossification toward the center of the medullary canal cannot reliably lock the prosthetic stem.
OSSEOINTEGRATION CHALLENGE
A mechanical disadvantage exists in osseointegration compared with arthroplasty, elevating the prospects for pylon loosening. That disadvantage stems from the area of a bone’s contact with the pylon being significantly smaller than that with a shaft in the total joint replacement procedure, as well as the loads applied to the bone-device area being higher. The area of contact of an implant with the bone walls is proportional to the depth of insertion of the shaft into the bone canal; in direct skeletal attachment, the depth of insertion cannot be greater than the length of the residuum.
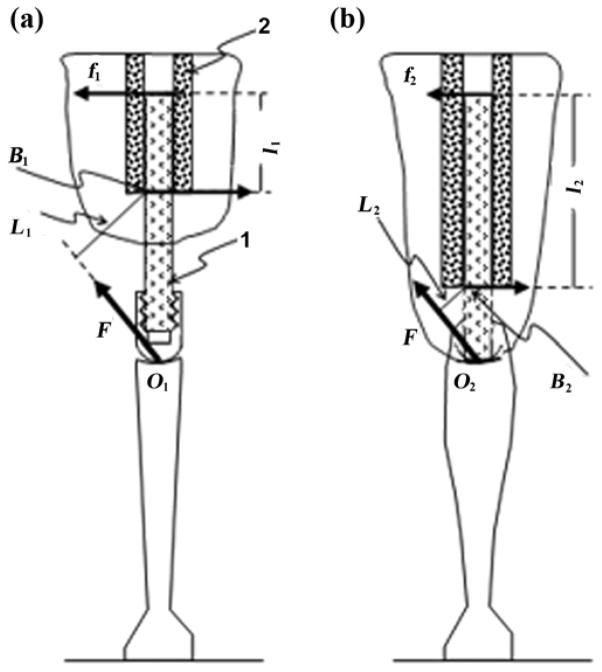
Osseointegration has reasonable indications for an amputee with a short residuum of one-third of a limb segment or shorter. Therefore, the depth of insertion in osseointegration is always smaller compared with those following arthroplasty. A model of loading/resistance with both methods is presented and depicted in Figure 4.
Figure 4.
Modeling of moment of resistance to bending of pylon (1) implanted to medullary canal (2) via (a) osseointegration and (b) arthroplasty. L1 = lever arm with respect to point B1 of bending force F applied to point O1, L2 = lever arm with respect to point B2 of force F applied to point O2, l1 = lever arm of reaction force f1 with respect to point B1, l2 = lever arm of reaction force f2 with respect to point B2.
Consider the pylon (1) implanted at a depth of l1 in the medullary canal (2) of a residuum bone during the osseointegration procedure (Figure 4(a)). Let force F be applied to the point O1 of the distal end of the thigh portion of prosthetic knee mechanism, and let the force of the same magnitude and direction as F be applied to the point O2 of the distal end of the prosthetic knee joint implanted at the depth of l2 during arthroplasty (Figure 4(b)).
The force F creates a bending moment M1 relative to point B1 defining the distal zone of a contact area of the implant and the bone wall. Similarly, the force F creates the moment M2 relative to point B2 with lever arm L2. The bending moment M1, which is a product of F and the lever arm L1, has to be balanced by a moment of resistance generated by the couple ±f1 of the bone walls’ reactions with the lever arm l1. Reactions f1 can be found from Equation (1):
| (1) |
Similarly, the reactions f2 on the walls of a medullary canal following the knee arthroplasty will be
| (2) |
As just discussed, L1 > L2, and l1 < l2. Combining this with Equations (1) and (2), we obtain
| (3) |
Because of Newton’s third law, normal loads from the implanted shaft to the bone walls following the procedure of direct skeletal attachment will also be greater when compared with the procedure of total joint replacement. A suggestion that would not be reasonable is that the damage to the bone walls and consequently the potential for further loosening should be expected in a higher percentage in osseointegration than in traditional arthroplasty if the medullary canal placement is in use.
“OSSEO-LOCKING” HYPOTHESIS AND IMPLANT DESIGN RECOMMENDATIONS
According to the hypothesis stated, if circular cortical ossification instead of radial endosteum ossification is used, a more reliable fixation of joint prostheses may develop.
The phenomenon of circular lateral ossification is not new and constitutes a component of natural bone fracture healing. But the most powerful demonstration of the phenomenon can be seen in the bone distractional osteogenesis introduced by Ilizarov [33]. The method allows for bone lengthening (Figure 3(b)) and widening (Figure 3(c)) when the bone fragments are moved apart approximately 1 to 2 mm a day in a fixating apparatus [33]. Importantly, the volume of ossification in the circular lateral direction during bone widening is comparable to that during bone lengthening [34]. However, this technique of induced ossification has never been applied to lock devices implanted in the medullary canal.
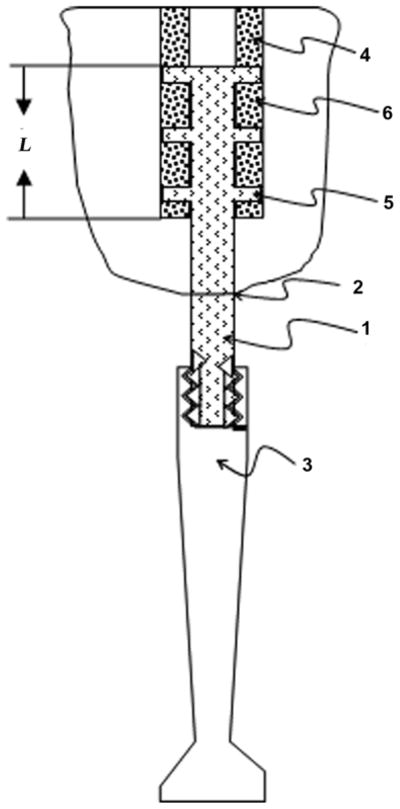
We suggest creating favorable conditions for the ingrowth of bone cells to lock the implant by employing the mechanism of distractional osteogenesis in the circular direction. Zones of ossification will presumably produce the effect of “osseo-locking” between and throughout the sides of the implanted part of the prosthesis’ stem (Figure 5). For the initiation of the effect of osseo-locking, bone preparation should include fashioned slots in the bone walls in the longitudinal direction [35]. The protruding sides of the installed implant are positioned in the slots and the ossification between and throughout the side elements will progress in the circular lateral direction. The process, as we anticipate it, will naturally lock the implant with an anchoring effect similar to that of the interlocking nailing but without its complications [36].
Figure 5.
Method of osseo-locking an implant (1) for transcutaneous (2) attachment of external limb prosthesis (3) to residuum bone (4). Side elements (5) are placed to slots of length L in bone walls for inducing creation of osseo-locks (6) between elements (5).
The partially sectioned side view of the bone with the implant containing side elements (Figure 5) shows newly ossified zones of the bone walls and demonstrates how the device is integrated with the bone at the end of healing.
DISCUSSION
This article notes that the medullary canal has natural limitations for reliable fixation in principle. We suggest inducing ossification in a different direction for pylon fixation, namely, circularly relative to the bone circumference. To induce circular ossification in the bone walls, we introduce here a pylon with new design characteristics to be implanted in specially prepared slots in the bone walls and hypothesize that the novel design will reduce the rate of the prosthetic shaft’s loosening. If verified, this hypothesis could have important implications for orthopedic surgery and rehabilitation.
Considering the gravity of the medical consequences of prosthetic implant loosening, we question the philosophy of the device-bone interface adopted from the total joint replacement procedure. We view the approaches as ineffective based on the placement of the stem in the bone’s medullary canal and relying on strong ossification inside the canal in the inward direction.
Because the medullary canal placement of the implant has been developed for the purpose of total joint replacement, a technology of direct transcutaneous prosthetic attachment (osseointegration) [1] has inherited the use the medullary placement of the implants, also inheriting the problem of loosening. The goal of osseointegration is to eliminate or avoid pain and discomfort associated with the traditional residuum-socket attachment of the external prosthesis to the body [2]. The skin-pylon interface presents the obvious challenge in osseointegration [4,15,37]; however, the bone-device interface is an issue no less critical, affecting the wide acceptance of osseointegration in the future. Currently, the shaft of a pylon is implanted into the medullary canal, and therefore the longevity of a bone-device bond depends on the inward ossification as in the total joint replacement technique. During direct transcutaneous prosthetic attachment, the skin-device zone can be an additional source for tracking infections, resulting in a higher percentage of loosening of the bone-device bond. Mechanical loading of the bone walls in osseointegration is also higher compared with arthroplasty. Therefore, the reliability of the in-bone implantation in direct transcutaneous prosthetic attachment must be a priori higher than in arthroplasty.
Bone has the capability to regenerate, forming new osseous tissue at locations that are damaged or missing after the removal of bone screws, fracture healing, distraction osteogenesis (during limb lengthening), and integration of orthopedic implants with the host bone [24]. The anisotropy of ossification is one of the fundamental features of bone regeneration. Of the different directions analyzed in this article, ossification toward the center of the medullary canal, which is employed in arthroplasty, appears the least effective. We hypothesize that the attachment of the implant should instead be based on ossification of the bone walls in the circular direction, thereby establishing natural osseo-locks.
Further studies are required to verify the hypothesis and to refine the methodology and instrumentation of the osseo-locking technique [35]. If proven, the recommendations presented in this article for a potentially more biological method of in-bone implantation could be useful also for total joint replacement and for other technologies based on the bone-device bond.
CONCLUSIONS
We hypothesize that if circular instead of inward ossification is employed, a more reliable bone-device bond can be achieved. The recommendations for an osseo-locking technique as a potentially more biological method of in-bone implantation could be useful not only in direct skeletal attachment of limb prostheses but also in total joint replacement.
Acknowledgments
This material was based on work supported in part by the National Institutes of Health, grant 1R43HD057492-01A1.
Biography

Footnotes
References
- 1.Eriksson E, Brånemark PI. Osseointegration from the perspective of the plastic surgeon. Plast Reconstr Surg. 1994;93(3):626–37. [PubMed] [Google Scholar]
- 2.Branemark R, Brånemark PI, Rydevik B, Myers RR. Osseointegration in skeletal reconstruction and rehabilitation: A review. J Rehabil Res Dev. 2001;38(2):175–81. [PubMed] [Google Scholar]
- 3.Manurangsee P, Isariyawut C, Chatuthong V, Mekraksawanit S. Osseointegrated finger prosthesis: An alternative method for finger reconstruction. J Hand Surg [Am] 2000;25(1):86–92. doi: 10.1053/jhsu.2000.jhsu025a0086. [DOI] [PubMed] [Google Scholar]
- 4.Pendegrass CJ, Goodship AE, Blunn GW. Development of a soft tissue seal around bone-anchored transcutaneous amputation prostheses. Biomaterials. 2006;27(23):4183–91. doi: 10.1016/j.biomaterials.2006.03.041. [DOI] [PubMed] [Google Scholar]
- 5.Pendegrass CJ, Goodship AE, Price JS, Blunn GW. Nature’s answer to breaching the skin barrier: An innovative development for amputees. J Anat. 2006;209(1):59–67. doi: 10.1111/j.1469-7580.2006.00595.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Holgers KM, Thomsen P, Tjellstrom A. Persistent irritation of the soft tissue around an osseointegrated titanium implant. Case report. Scand J Plast Reconstr Surg Hand Surg. 1994;28(3):225–30. doi: 10.3109/02844319409015984. [DOI] [PubMed] [Google Scholar]
- 7.Colon G, Ward BC, Webster TJ. Increased osteoblast and decreased Staphylococcus epidermidis functions on nanophase ZnO and TiO2. J Biomed Mater Res A. 2006;78(3):595–604. doi: 10.1002/jbm.a.30789. [DOI] [PubMed] [Google Scholar]
- 8.Holgers KM, Brånemark PI. Immunohistochemical study of clinical skin-penetrating titanium implants for orthopaedic prostheses compared with implants in the craniofacial area. Scand J Plast Reconstr Surg Hand Surg. 2001;35(2):141–48. doi: 10.1080/028443101300165273. [DOI] [PubMed] [Google Scholar]
- 9.Granstrom G, Bergstrom K, Odersjo M, Tjellstrom A. Osseointegrated implants in children: Experience from our first 100 patients. Otolaryngol Head Neck Surg. 2001;125(1):85–92. doi: 10.1067/mhn.2001.116190. [DOI] [PubMed] [Google Scholar]
- 10.Yang BC, Weng J, Li XD, Yang ZJ, Feng JM, Chen JY, Zhang XD. Preliminary study on HA coating percutaneously implanted in bone. Int J Artif Organs. 1999;22(10):713–18. [PubMed] [Google Scholar]
- 11.Lloyd S, Almeyda J, Sirimanna KS, Albert DM, Bailey CM. Updated surgical experience with bone-anchored hearing aids in children. J Laryngol Otol. 2007;121(9):826–31. doi: 10.1017/S0022215107003714. [DOI] [PubMed] [Google Scholar]
- 12.Aaron RK, Herr HM, Ciombor DM, Hochberg LR, Donoghue JP, Briant CL, Morgan JR, Ehrlich MG. Horizons in prosthesis development for the restoration of limb function. J Am Acad Orthop Surg. 2006;14(10 Suppl):S198–204. doi: 10.5435/00124635-200600001-00043. [DOI] [PubMed] [Google Scholar]
- 13.Pitkin M, Blinova MI, Yudintseva NV, Potokin IL, Raykhtsaum G, Pinaev GP. In: Ostrovskaya E, editor. Skin and bone integrated prosthetic technology. I. Characterization and morphology of human cells cultivated on titanium implants of different structures; 9th Russian National Congress, “People and Health”; 2004 Nov 22–26; St. Petersburg, Russia. St. Petersburg (Russia): People and Health; 2004. Oct 25, p. 217. [Google Scholar]
- 14.Pitkin M, Raykhtsaum G, inventors. Skin integrated device. 20070071788. United States patent. 2007 Mar 29;
- 15.Pitkin M, Raykhtsaum G, Pilling J, Galibin OV, Protasov MV, Chihovskaya JV, Belyaeva IG, Blinova MI, Yudintseva MN, Potokin IL, Pinaev GP, Moxson V, Duz V. Porous composite prosthetic pylon for integration with skin and bone. J Rehabil Res Dev. 2007;44(5):723–38. doi: 10.1682/jrrd.2006.12.0160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Charnley J. Arthroplasty of the hip. A new operation. Lancet. 1961;1(7187):1129–32. doi: 10.1016/s0140-6736(61)92063-3. [DOI] [PubMed] [Google Scholar]
- 17.National Institute of Arthritis and Musculoskeletal and Skin Diseases [homepage on the Internet] What is joint replacement? Do many people have joints replaced; [about 1 screen] Bethesda (MD): National Institutes of Health, Department of Health and Human Services; c2008. Joint replacement surgery and you: Information for multicultural communities. [updated 2005 Oct; cited 2007 Nov 20] Available from: http://www.niams.nih.gov/Health_Info/Joint_Replacement/default.asp#joint5. [Google Scholar]
- 18.Karachalios T, Hartofilakidis G, Zacharakis N, Tsekoura M. A 12- to 18-year radiographic follow-up study of Charnley low-friction arthroplasty. The role of the center of rotation. Clin Orthop Relat Res. 1993;(296):140–47. [PubMed] [Google Scholar]
- 19.Munger P, Roder C, Ackermann-Liebrich U, Busato A. Patient-related risk factors leading to aseptic stem loosening in total hip arthroplasty: A case-control study of 5,035 patients. Acta Orthop. 2006;77(4):567–74. doi: 10.1080/17453670610012629. [DOI] [PubMed] [Google Scholar]
- 20.Chao EY, Inoue N. Biophysical stimulation of bone fracture repair, regeneration and remodeling. Eur Cell Mater. 2003;6:72–85. doi: 10.22203/ecm.v006a07. [DOI] [PubMed] [Google Scholar]
- 21.Khan K, McKay H, Kannus P, Bailey D, Wark J, Bennell K. Physical activity and bone health. Champaign (IL): Human Kinetics; 2001. [Google Scholar]
- 22.Malik MH, Jury F, Bayat A, Ollier WE, Kay PR. Genetic susceptibility to total hip arthroplasty failure: A preliminary study on the influence of matrix metallo-proteinase 1, interleukin 6 polymorphisms and vitamin D receptor. Ann Rheum Dis. 2007;66(8):1116–20. doi: 10.1136/ard.2006.062018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.How good are knee replacements? Lancet. 1991;338(8765):477–78. [PubMed] [Google Scholar]
- 24.Prendergast PJ, Taylor D. Design of intramedullary prostheses to prevent bone loss: Predictions based on damage-stimulated remodelling. J Biomed Eng. 1992;14(6):499–506. doi: 10.1016/0141-5425(92)90103-r. [DOI] [PubMed] [Google Scholar]
- 25.Reininga IH, Wagenmakers R, Van den Akker-Scheek I, Stant AD, Groothoff JW, Bulstra SK, Zijlstra W, Stevens M. Effectiveness of computer-navigated minimally invasive total hip surgery compared to conventional total hip arthroplasty: Design of a randomized controlled trial. BMC Musculoskelet Disord. 2007;8:4. doi: 10.1186/1471-2474-8-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guyen O, Chen QS, Bejui-Hugues J, Berry DJ, An KN. Unconstrained tripolar hip implants: Effect on hip stability. Clin Orthop Relat Res. 2007;455:202–8. doi: 10.1097/01.blo.0000238796.59596.1f. [DOI] [PubMed] [Google Scholar]
- 27.Nagasawa T. Microenvironmental niches in the bone marrow required for B-cell development. Nat Rev Immunol. 2006;6(2):107–16. doi: 10.1038/nri1780. [DOI] [PubMed] [Google Scholar]
- 28.Khayat PG, Milliez SN. Prospective clinical evaluation of 835 multithreaded tapered screw-vent implants: Results after two years of functional loading. J Oral Implantol. 2007;33(4):225–31. doi: 10.1563/1548-1336(2007)33[225:PCEOMT]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 29.Green PB. Cell walls and the geometry of plant growth. Brookhaven Symp Biol. 1964;16:203–17. [PubMed] [Google Scholar]
- 30.Thompson DW. On growth and form. New York (NY): Dover; 1992. [Google Scholar]
- 31.He Y, Luo Y, Tang S, Rajantie I, Salven P, Heil M, Zhang R, Luo D, Li X, Chi H, Yu J, Carmeliet P, Schaper W, Sinusas AJ, Sessa WC, Alitalo K, Min W. Critical function of Bmx/Etk in ischemia-mediated arteriogenesis and angiogenesis. J Clin Invest. 2006;116(9):2344–55. doi: 10.1172/JCI28123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bowlby AA. Surgical pathology and morbid anatomy. 3. London (England): J & A Churchill; 1895. p. 329. [Google Scholar]
- 33.Ilizarov GA. The principles of the Ilizarov method. Bull Hosp Jt Dis Orthop Inst. 1988;48(1):1–11. [PubMed] [Google Scholar]
- 34.Ilizarov GA. The tension-stress effect on the genesis and growth of tissues. Part I. The influence of stability of fixation and soft-tissue preservation. Clin Orthop Relat Res. 1989;(238):249–81. [PubMed] [Google Scholar]
- 35.Pitkin M. In-bone implantable shaft for prosthetic joints or for direct skeletal attachment of external limb prostheses and method of its installation. 11/899068. United States patent application US. 2007 Sep 5;
- 36.Zalavras CG, Singh A, Patzakis MJ. Novel technique for medullary canal débridement in tibia and femur osteomyelitis. Clin Orthop Relat Res. 2007;461:31–34. doi: 10.1097/BLO.0b013e318098673f. [DOI] [PubMed] [Google Scholar]
- 37.Pitkin M, Raykhtsaum G, Galibin OV, Protasov MV, Chihovskaya JV, Belyaeva IG. Skin and bone integrated prosthetic pylon: A pilot animal study. J Rehabil Res Dev. 2006;43(4):573–80. doi: 10.1682/jrrd.2005.05.0160. [DOI] [PMC free article] [PubMed] [Google Scholar]